Abstract
Background
Stress is a well-known factor affecting cardiac contractility through the cardiac sympathetic nerves. A positive inotropic effect of the cardiac sympathetic nerves on the myocardium is reflected by preejection period (PEP) shortening. Patients with Parkinson disease (PD) and neurogenic orthostatic hypotension (NOH) (PD+NOH) or with pure autonomic failure (PAF) have markedly decreased myocardial 6-[18]F-fluorodopamine-derived radioactivity, reflecting cardiac sympathetic denervation. The functional effects of the cardiac sympathetic denervation have been unknown.
Methods
We measured PEP and heart rate-corrected PEP (PEPI) responses to i.v. tyramine (1 mg/min) in 13 patients (9 PD+OH and 4 PAF) with low 6-[18]F-fluorodopamine-derived radioactivity and in subjects with normal radioactivity (15 multiple system atrophy with NOS patients (MSA+NOS).
Results
Baseline PEP and PEPI did not differ between the groups. By 10 minutes after initiation of tyramine infusion, PEP and PEPI were significantly lower (p<0.01) in MSA+NOS, compared to baseline, whereas PEP and PEPI remained unchanged in the PD+NOH/PAF group. The PEP and PEPI decrease was larger in the MSA+NOS group than in the PD+NOH/PAF group (p<0.05).
Conclusion
One of the functional consequences of cardiac sympathetic denervation is failure to increase contractility in response to stimuli that depend on endogenous norepinephrine release.
Keywords: Parkinson disease, pure autonomic failure, multiple system atrophy, sympathetic denervation, preejection period
INTRODUCTION
Multiple system atrophy (MSA), pure autonomic failure (PAF) and Parkinson disease (PD) belong to a group of neurodegenerative disorders called synucleinopathies, because they are characterized by fibrillary aggregates of the protein alpha-synuclein in particular populations of neurons and glia.1 All three diseases feature autonomic dysfunction—in particular sympathetic neurocirculatory failure manifested by neurogenic orthostatic hypotension (NOH). Over the past decade compelling evidence has accrued for profound loss of cardiac sympathetic nerves in both PAF and in PD+NOH.2-4 In marked contrast, most patients with MSA have intact cardiac and overall sympathetic innervation.4 The functional effects of cardiac sympathetic denervation in synucleinopathies have been unknown.
Sympathetic noradrenergic activity profoundly affects systolic time intervals. Shortening of the preejection period (PEP) in response to peripheral adrenergic stimulation and its elongation in response to beta-1 adrenoceptor blockade indicate that PEP reduction reflects a positive inotropic effect of the cardiac sympathetic nerves on the myocardium.5 In the present study we examined functional consequences of cardiac sympathetic denervation in patients with alpha-synucleinopathies. We hypothesized that patients with NOH and cardiac sympathetic denervation (PD+NOH or PAF) have attenuated responses of inotropic indices to i.v. tyramine (TYR), an indirectly acting sympathomimetic amine. In contrast, patients with NOH and intact cardiac sympathetic innervation (MSA) would have normal responses to TYR.
SUBJECTS AND METHODS
The subjects were studied at the National Institutes of Health Clinical Center after giving informed, written consent to participate in one or more protocols approved by the Intramural Research Board of the National Institute of Neurological Disorders and Stroke. The study population consisted of a total of 28 patients with chronic autonomic failure, stratified into two groups. The first group consisted of 13 patients (9 PD+NOH, 4 PAF, aged 67± (SEM) 2 years old, 7 males, 6 females) who had neuroimaging evidence for cardiac sympathetic denervation (DEN group). The second group consisted of 15 MSA+NOH patients who had neuroimaging evidence of intact sympathetic innervation (INN group, 56±3 years old, 12 males, 3 females). Cardiac sympathetic denervation was defined by low concentrations of 6-[18F] fluorodopamine-derived radioactivity in the interventricular septum (less than 5000 Bq/mL per MBq/kg) left ventricular free wall (less than 4000 Bq/mL per MBq/kg) corresponding to about 2 standard deviations below the normal means, as described elswhere.4
Each patient had both electrocardiographic, impedance cardiographic monitoring (BioZ, Cardiodynamics, San Diego, CA, USA), beat-to-beat blood pressure measurements using a brachial arterial catheter. Continuous vital signs data were digitized and recorded using a PowerLab (AD Instruments Ltd., Castle Hill, Australia) data acquisition system.
On the day of infusion, TYR was dissolved in 5% dextrose and infused i.v. at a rate of 1 mg/min for 10 min. During the infusion patients were supine, except that in those with severe supine hypertension (systolic pressure more than 200 mm Hg), TYR was infused during head-up tilting (15-30 degrees), to decrease baseline pressure. Blood samples were drawn after the patient was at rest and then at 10 min during the infusion, transferred to heparinized sample tubes, and placed immediately on ice. The plasma was was separated by refrigerated centrifugation. A sample of the TYR infusate was also taken. The plasma and infusate samples were stored at −70 °C or colder until assayed for catechol contents in our laboratory by liquid chromatography with electrochemical detection after batch alumina extraction.6
PEP and left ventricular ejection time (LVET) were measured non-invasively using the BioZ impedance cardiographic device, before and during the TYR infusion. Heart rate-corrected indices including left ventricular ejection time index (LVETI) and preejection period index (PEPI) were calculated according to Weissler’s equations as follows: LVETI=1.7·HR+LVET and PEPI=0.4·HR+PEP.7 Systolic time ratio index (STRI) was calculated as follows: STRI=PEPI/LVETI.
Statistical analyses were performed using SPSS version 11.5 (SPSS Inc., Chicago, IL, USA). Changes associated with drug infusion were compared using paired t tests. Group differences in mean values at baseline and at the end of the tests and in mean responses were analyzed by means of independent means t tests (or the Mann-Whitney u test, depending on normality of the data distributions). Mean values were expressed ± standard error of mean. A p values less than 0.05 was considered statistically significant.
RESULTS
SBP, DBP and MAP tended to be higher (p=0.06, p=0.07, and p=0.03) in the DEN than INN group (Table 1). There were no group differences in the mean PEP, PEPI, LVET, LVETI or STRI (Table 1, Figure 1).
Table 1.
Hemodynamic and neurochemical parameters in patients with cardiac sympathetic denervation (DEN) or normal cardiac innervation (INN) at baseline and during i.v. tyramine infusion (TYR).
| DEN (n=13) | INN (n=15) | |||
|---|---|---|---|---|
| Baseline | TYR | Baseline | TYR | |
| HR (bpm) | 68.8±3.3 | 70±4.1 | 73.5±2.6 | 71.2±3.2 |
| SBP (mm Hg) | 181±6 | 213±10 | 160±8 | 202±(8) |
| DBP (mm Hg) | 86±3 | 101±4* | 79±3 | 88±4 |
| PEP (ms) | 104±6.9 | 94±7.9* | 99.4±6.9 | 71.8±5.8† |
| LVET (ms) | 316±7.0 | 324±5.6 | 300±6.4 | 317±7.5 |
| LVETI (ms) | 433±8.7 | 4436.9 | 431±7.3 | 443±8.7 |
| NE (pg/mL) | 195±36 | 245±34* | 305±41 | 456±66† |
| DHPG (pg/mL) | 593±52*** | 909±81*** | 1050±87 | 1713±135††† |
p<0.05, DEN vs. INN
p<0.05, DEN vs. INN for change from baseline
p<0.005 DEN vs. INN for change from baseline
Abbreviations: heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), preejection period (PEP), left ventricular ejection time (LVET), left ventricular ejection time index (LVETI), norepinephrine (NE), dihydroxyphenylglycol (DHPG).
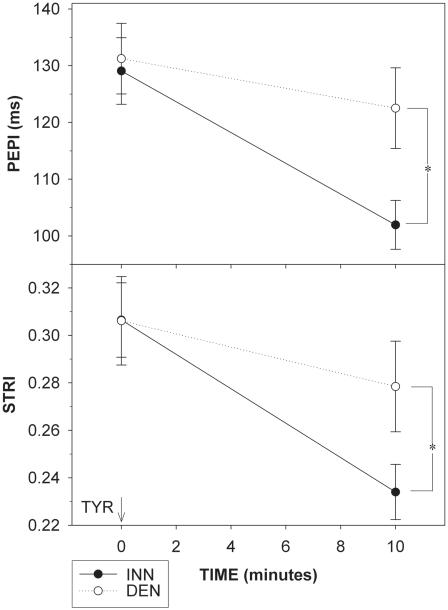
Figure 1.
Changes in preejection period index (PEPI; upper panel) and systolic time ratio index (STRI) defined as ratio of PEPI to left ventricular ejection time index (lower panel) in response to tyramine (TYR) infusion in patients with cardiac sympathetic denervation (DEN, white circles, dashed line) and in patients with normal cardiac innervation (INN, black circles, solid line). *p<0.05.
Plasma dihydroxyphenylglycol (DHPG) levels at baseline were lower in the DEN than the INN group (p<0.001, Table 1). Plasma norepinephrine tended to be lower (p=0.06) at baseline in the DEN group.
TYR infusion increased SBP (p<0.001), DBP (p<0.05), and MAP (p<0.001) in both patient groups (Table 1). DBP and MAP tended to be higher during TYR infusion in the DEN group (p=0.035, p=0.09), with similar DBP and MAP responses in the two groups, while no group difference in SBP was observed. Heart rate did not change during TYR infusion in either group (Table 1). LVET and LVETI tended to increase in response to TYR in both groups. In the INN group, TYR decreased both PEP (p<0.001, Table 1) and PEPI (p<0.001; Figure 1). In contrast, TYR failed to decrease PEP or PEPI in the DEN. During TYR infusion, PEP and PEPI were shorter in the INN than in the DEN group (p=0.03, p=0.02). The changes of PEP and PEPI were smaller in the DEN than in the INN group (p=0.02, p=0.013). In the INN group, STRI decreased (p<0.001), whereas in the DEN group they only tended to decrease (p=0.07, p=0.08). During TYR infusion, STRI was higher in the DEN group (p=0.05). The change of STRI was smaller in the DEN group (p=0.03).
TYR infusion increased in plasma DHPG (p<0.001) and norepinephrine (p=0.003) levels in both the INN and DEN groups. During TYR infusion DHPG responses were smaller in the DEN than the INN group (p<0.001, Table 1). Plasma norepinephrine was lower during TYR infusion (p=0.01) in the DEN than in the INN group (Table 1). Responses of plasma norepinephrine to TYR were also smaller (p=0.04) in the DEN group.
DISCUSSION
In this study, patients with neurogenic orthostatic hypotension (NOH) and cardiac sympathetic denervation, as indicated by low myocardial concentrations of 6-[18F] fluorodopamine-derived radioactivity (PD+NOH or PAF), had a failure to increase cardiac contractility, indicated by multiple parameters (PEP, PEPI, STRI) during i.v. TYR infusion, whereas by all these indices MSA patients with NOH and intact cardiac innervation had inotropic responses. These findings suggest that in patients with synucleinopathies, cardiac sympathetic denervation results in failure to increase contractility in response to a stimulus that normally increases release of norepinephrine from sympathetic nerves. By extension to other situations such as exercise, in which increases in cardiac sympathetic outflow augment delivery of norepinephrine to its receptors, the results may help explain common complaints of fatigue and exercise intolerance in patients with Parkinson disease.8, 9
Despite substantial alterations in neurochemical profiles reflecting generalized sympathetic denervation in PD+NOH and PAF, TYR infusion revealed only rather subtle differences in cardiac contractility and there was no difference in pressor responses between the DEN and INN groups. The compensatory adjustments seem to include augmented responses to occupation of cardiovascular adrenoceptors.
ACKNOWLEDGEMENTS
The research reported here was supported by the intramural research program of the NIH.
Footnotes
For submission to
Annals of New York Academy of Sciences
Poster presentation
REFERENCES
- 1.Marti MJ, Tolosa E, Campdelacreu J. Clinical overview of the synucleinopathies. Mov Disord. 2003;18(Suppl 6):S21–7. doi: 10.1002/mds.10559. [DOI] [PubMed] [Google Scholar]
- 2.Braune S, et al. Cardiac uptake of [123I]MIBG separates Parkinson's disease from multiple system atrophy. Neurology. 1999;53:1020–5. doi: 10.1212/wnl.53.5.1020. [DOI] [PubMed] [Google Scholar]
- 3.Takatsu H, et al. Cardiac sympathetic denervation from the early stage of Parkinson's disease: clinical and experimental studies with radiolabeled MIBG. J Nucl Med. 2000;41:71–7. [PubMed] [Google Scholar]
- 4.Goldstein DS, et al. Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–47. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 5.Schachinger H, et al. Cardiovascular indices of peripheral and central sympathetic activation. Psychosom Med. 2001;63:788–96. doi: 10.1097/00006842-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1994;653:131–8. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- 7.Weissler AM, Harris WS, Schoenfeld CD. Systolic time intervals in heart failure in man. Circulation. 1968;37:149–59. doi: 10.1161/01.cir.37.2.149. [DOI] [PubMed] [Google Scholar]
- 8.Friedman JH, et al. Fatigue in Parkinson's disease: a review. Mov Disord. 2007;22:297–308. doi: 10.1002/mds.21240. [DOI] [PubMed] [Google Scholar]
- 9.Werner WG, DiFrancisco-Donoghue J, Lamberg EM. Cardiovascular response to treadmill testing in Parkinson disease. J Neurol Phys Ther. 2006;30:68–73. doi: 10.1097/01.npt.0000282570.78544.00. [DOI] [PubMed] [Google Scholar]