Abstract
AIM
To evaluate the inhibitive effect of olmesartan to fibroblast proliferation and the anti-scarring effect in Tenon's capsule, both in vitro and in vivo.
METHODS
Human primary Tenon's capsule fibroblasts were cultured in vitro, treated with up titrating concentrations of olmesartan. The rate of inhibition was tested with methyl thiazol tetrazolium (MTT) method. Real-time PCR was performed to analyze changes in mRNA expressions of the fibrosis-related factors: matrix metalloproteinase-2 (MMP-2), tissue inhibitor of metalloproteinase (TIMP-1,2) and proliferating cell nuclear antigen (PCNA). Thirty rabbits were divided into 5 groups (3, 7, 14, 21, and 28d). A rabbit conjunctiva flap model was created in each eye. Olmesartan solution was injected subconjunctivally and then evaluated its anti-proliferation and anti-fibrosis effects through the histological morphology and immunohistochemistry of MMP-2 and PCNA in each group. Only the 7d group was treated with Masson's trichrome to compare the neovascularization in the subconjunctiva area.
RESULTS
In vitro, cultured Tenon's capsule human fibroblasts showed a dose dependent inhibition by olmesartan in MTT. Olmesartan reduced mRNA expressions of MMP-2 and PCNA but increased mRNA expressions of TIMP-1 and TIMP-2. In vivo, the rabbit eyes treated with olmesartan at 3rd, 7th, 14th and 21st days demonstrated a significant reduced expressions of MMP-2 and PCNA compared with control eye, no significant difference observed in 28th day group. The cellular proliferation and neovascularization was suppressed by olmesartan in Masson's trichrome observation.
CONCLUSION
By inhibiting fibroblasts in vitro and in vivo, olmesartan prevents the proliferation and activity of fibroblasts in scar tissue formation, which might benefit glaucoma filtering surgery.
Keywords: olmesartan, trabeculectomy, anti-proliferative, matrix metalloproteinase-2, proliferating cell nuclear antigen
INTRODUCTION
Glaucoma filtration surgery (GFS, trabeculectomy) is a classic operation to treat glaucoma. The surgery itself can damage the structures of the conjunctiva and subconjunctival tissue, which stimulates fibroblasts, as mediated by macrophages, neutrophils and inflammatory cytokines (such as transforming growth factor-beta, insulin-like growth factor-1, IL-6)[1]–[2]. These molecules are involved in complex processes, leading to further extracellular matrix (ECM) secretion and remodeling. Any overwhelming activity within this process can cause overstimulation of fibroblasts, formation and accumulation of extraneous ECM. With the participation of matrix metalloproteinases (MMPs) in ECM remodeling and the subsequent matrix contraction, scarring results[3].
MMPs are a superfamily of zinc-dependent proteases, which has more than 20 members, acted through cleaving and degrading the ECM. Activated fibroblasts and macrophages can secrete MMPs during tissue repair. Tumor cells can also induce the secretion of MMPs to facilitate tumor cell migration and new blood vessels formation to support tumor growth. The activity of MMPs can be regulated and balanced by the endogenous inhibitor tissue inhibitor of matrix metalloproteinases (TIMPs)[4]–[5].
Status post GFS, some patients experience excessive tissue repair of the Tenon's capsule. The unrestrained proliferation of fibroblasts induces a strong inflammatory reaction with abundant ECM (including collagen fibers) formation. Meanwhile, MMPs and TIMPs are stimulated: MMPs promote the contraction of the ECM, which results in fibrosis and scarring surrounding the surgical canal created for aqueous humor drainage. Consequently, the intraocular pressure rebounds and indicates the failure of the GFS. Therefore, many topical medications have been studied to reduce the fibrosis and scarring after surgery so that the filtration bleb survives. MMC and 5-FU have been applied clinically for this purpose[6]. Although these drugs may achieve the desired inhibition of fibrosis, some obvious complications occur when these medications are applied, such as bleb leakage, infectious blebitis, low intraocular pressure associated with maculopathy and even endophthalmitis[7]–[8].
Olmesartan, a potent angiotensin II receptor blocker (ARB), is used in cardiovascular medicine for the treatment of hypertension. Angiotensin II, in addition to its potent vasoconstrictive effect, acts via its type 1(AT1) receptor to promote the generation of reactive oxygen species (ROS), vascular inflammation, fibrosis, and cell proliferation[9]- [12].
The emerging role of angiotensin II as a pro-inflammatory factor has been studied in cardiology, pulmonology, dermatology, hepatology, and nephrology[13]–[15]. This effect may be mediated by the stimulation of receptor AT1-activated second messengers, such as diacylglycerol and 1-4-5-inositol triphosphate, as well as the activation of C protein[16]. Angiotensin II can also activate the Ras/p38 MAPK/CREB pathway and ERK1/2 by regulating TGF-β1/Smad signaling in cardiac fibroblasts, enhancing the release of MMP-2 by endothelial cells via the pro-inflammatory factor, tumor necrosis factor (TNF)-α (as a mechanism of vasoconstriction and inflammation) [17]–[18]. Antagonists of the angiotensin II type 1 receptor inhibit this pro-inflammatory and fibrotic effect. Olmesartan effectively prevents nonalcoholic steatohepatitis and liver fibrosis, pulmonary fibrosis, and scarring and remolding of the heart[19]–[21].
Do similar effects of angiotensin and its antagonist exist in the eyes? The presence of angiotensin II and its receptors has been confirmed in the ocular system, and their distribution demonstrated the existence of a local renin-angiotensin system (RAS) instead of just diffusion from the system[22]–[23]. The correlation of the angiotensin system with glaucoma has been explored, and pilot studies evaluating the potential utility of angiotensin-converting-enzyme inhibitor (ACEI) and ARB on the control of ocular pressure were performed in animal models[24]. However, to our knowledge, research regarding the effects of ARB in the fibrosis of the Tenon's capsule has not been reported; this may represent a new therapeutic target for safely improving the success rate of GFS in the future.
MATERIALS AND METHODS
Primary Subconjunctiva Fibroblast Cell Culture
Human subconjunctival fibroblasts were isolated from patients with their informed oral consent(without stipend) and the approval of our institutional ethics committee. The tenets of the Declaration of Helsinki were followed. Tissue biopsies were cut into pieces, 0.5-1 mm, then treated as previously described[25]. Primary cell cultures from these tissues were maintained in culture medium [DMEM with 10% (vol/vol) fetal bovine serum (FBS), penicillin (100 units/mL), streptomycin (100 µnits/mL; Sigma Aldrich)]. The cells were passaged when they achieved 80% confluence, and the cells from the 3rd to the 7th passage were used for experiments.
Methyl Thiazol Tetrazolium Cytotoxicity Assay of Cultured Cells
Five thousand fibroblasts per well were seeded into 96-well plates. After the adhesion of cells to the bottom of plates after 12h, a gradient of concentration of olmesartan [dissolved in phosphate buffer solution (PBS)] was added to complete culture medium at a final concentrations of 2 µmol/mL, 1.5 µmol/mL, 1.25 µmol/mL, 1 µmol/mL, 0.75 µmol/mL, 0.5 µmol/mL, or 0 µmol/mL (control) and incubated for 48h. The colorimetric test with tetrazolium salt methyl thiazol tetrazolium [MTT, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was performed[26]. After dissolving the purple reaction product in DMSO, the optical density of each well was measured using an automatic plate reader at 490 nm wavelength (Bio-tek, USA). The rate of cell inhibition was calculated using the following equation to establish the concentration of olmesartan to use for further experiments.
Inhibition rate = (ODC−ODO)/ODC×100% (ODC: OD of the blank control; ODO: OD of olmesartan treated)[27].
Real-time Polymerase Chain Reaction
A concentration of olmesartan of 0.75 µmol/mL (inhibition rate of 11% in MTT) was selected for PCR, to choose an inhibition rate approximate 10% has reduced the cell numbers related influence to the results. Six flasks of cells newly seeded at the same density were divided into 2 groups, 3 as the olmesartan-treated group, and the rest as control. After incubating the cells with dulbecco's modified eagle medium (DMEM) without FBS for 24h to synchronize their growth, the medium was replaced with fresh complete medium (control) or complete medium with 0.75 µmol/mL of olmesartan as the experimental group. After an incubation for 48h, total RNA was isolated from the cells using RNAiso (Takara Bio, Kyoto, Japan), and its quality and concentration was measured using Thermo Scientific NanoDrop. One microgram of total RNA from each samples was used for reverse transcription using a real time (RT) reagent kit with a gDNA Eraser, followed by amplification using the Applied Biosystems step one detection system (Applied Biosystems STEP ONE, NY, USA) and SYBR Remix Ex Taq (Takara Bio, Kyoto, Japan). A ROX reference dye was applied to correct the fluorescence signal. The thermal cycle protocol consisted of: 30s at 95°C for 1 cycle, 5s at 95°C and 30s at 60°C with for 40 cycles, 15s at 95°C, 1min at 60°C, 15s at 95°C. The sequences of the PCR primers used were as follows: β-actin upper: CCCTGAAGTACCCCATCG, β-actin lower: GCTGGGTGTTTGAAGGTC; MMP-2 upper: AGTGGATGATGCCTTTGCTC, MMP-2 lower: GAGTCCGTCCTTACCGTCA; TIMP-1 upper: TCTGGCATCCTGTTGTTG, TIMP-1 lower: GGTCTGGTTGACTTCTGG; TIMP-2 upper: TCTGTGACTTCATCGTGCC, TIMP-2 lower: TGACCCAGTCCATCCAGAG; PCNA upper: GGCACTCAAGGACCTCAT, PCNA lower: CATACTGGTGAGGTTCACG.
Ct values were used for further analysis, and differences in the total amount of RNA present in each sample were normalized to β-actin. The ΔΔCt method was used to calculate the levels of gene expression relative to the expression levels of β-actin.
Rabbit Conjunctival Flap Model
Thirty New Zealand albino rabbits, weighting 2.2-2.5 kg each, of either sex were housed and fed separately and were randomly divided into 5 groups (3rd, 7th, 14th, 21st, and 28th day group which correspond to the day the rabbits' eyes would be harvest after the surgery), with 6 animals in each group. For each rabbit, one eye was used as the control eye while the other eye defined as the experimental eye. After anesthetized rabbit with phenobarbital (30 mg/kg, IV), a rabbit conjunctival flap model was created in both eyes through the incision of the conjunctiva between 10 o'clock and 2 o'clock in the upper limbus of the cornea with the fornix as the base, as previously described[28]. The scleral tissue under the conjunctiva flap was exposed thoroughly first and then pressed with a cotton swab to stop bleeding. Four to six stitches with 8-0 nylon threads were applied to suture the wound and restore the consistency of the eye. A 500 µL solution of 1% sterilized olmesartan (olmesartan acid-active metabolite, later referred to as olmesartan) in PBS was injected to subconjunctival (at 12 o' clock to the base of the conjunctiva flap) to the experimental eye, while only PBS was injected to subconjunctival to the other eye (control) in exactly the same location by the end of the surgery. Topical antibiotic eye drops were applied to both eyes and then during the following 3d. The same subconjunctival injection of olmesartan and PBS was repeated on the 5th postoperative day. On the 7th day postoperatively, the stitches were removed under topical anesthetic eye drops. On the 3rd, 7th, 14th, 21st and 28th days after surgery, all 6 rabbits from each group were firstly observed under slit-lamp for anterior segment of eyes, fundoscopy for retina, and then euthanized, and the eyes were enucleated and washed with icy PBS. All of the animal procedures complied with the ARVO Statement and were approved by our Institutional Review Board.
Hematoxylin and Eosin, Masson's Trichrome Stain and Immunohistochemistry
Biopsies of the conjunctiva, subconjunctiva and sclera were obtained from the surgical site and the adjacent area within 5 mm. Maintain the integrity of the structure for the cross-section of the tissues from the outermost surface of conjunctiva to the innermost sclera to guarantee the density of conjunctiva fixed. Tissues were fixed in a 4% paraformaldehyde solution in PBS, and sections were routinely stained with hematoxylin and eosin. Masson's trichrome staining was performed only on the day 7 group. For the immunohistochemistry stains, each 5-µm thick section was treated sequentially with PBS, 3% H2O2 in distilled water and a bovine serum albumin (BSA) solution were and incubated with primary antibodies to either MMP-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:200 dilution) or PCNA ( Santa Cruz Biotechnology, 1:400 dilution) for 24h in a humidified chamber at 4°C. After washing with PBS, the sections were incubated with a secondary antibody for 40min, were washed, and were then reacted with diaminobenzidine (DAB) until a brownish stain developed. The sections were rinsed in distilled water, dehydrated with ascending concentrations of alcohol, cleared in xylene and mounted in Permount.
Observation and Quantitation of Histology-stained Sections
All of the sections were observed by light microscopy and photographed with a digital camera (Nikon Eclipse, Tokyo, Japan). Pictures were taken at 100×, 200× and 400× magnification. The relative abundance and morphology of cells (including fibroblasts, inflammatory cells, epithelial cells) were observed on the HE-stained sections. The presence of new collagen and neovascularization were examined on Masson's trichrome stained sections (only the 7th day group eyes were chosen for this Masson's trichrome stain as mentioned before). The expression of MMP-2 by immunohistochemistry was analyzed using the Image-Pro Plus analysis system. For each section, 5 visual fields at 400× magnification were selected, and the mean density of the immunostain signal was recorded. The numbers of PCNA positive cells were calculated using Image-Pro Plus. The areas analyzed for either MMP-2 or PCNA expression from different experimental groups were from areas of comparable sections.
Statistical Analysis
Relative quantification of mRNA expression rectified by control and β-actin gene with ΔΔCt method (n=6). MTT and real-time PCR data were expressed as the mean±SD. Data from the different groups were compared using a paired t-test or a one way ANOVA. P<0.05 was with significant difference.
The results of immunofluorescence were expressed as the mean±SEM; the mean density of MMP-2 analyzed by Image-Pro Plus software. Positive PCNA expression cell nuclear numbers were analyzed by Image-Pro Plus software, data expressed as mean±SEM, paired t-test applied, P<0.05 as with significant difference.
RESULTS
Cell Culture
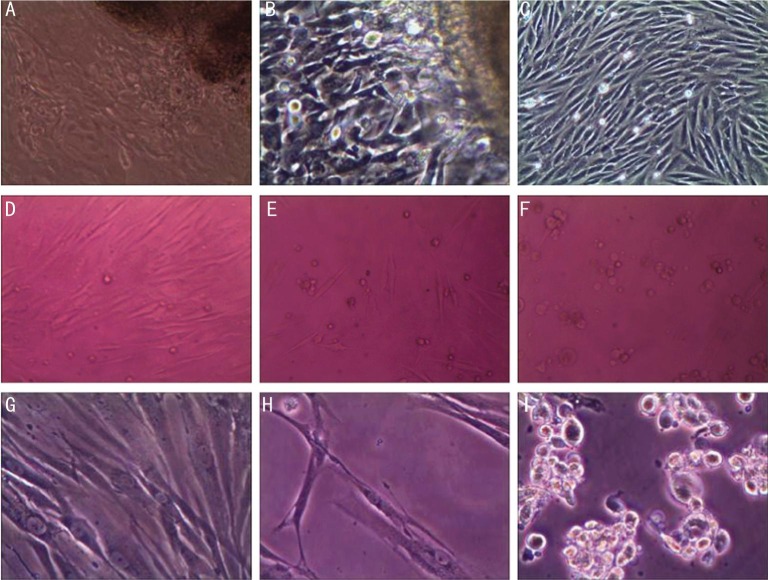
In primary cultures of human Tenon's fibroblast in vitro 7d after seeding, cells started to migrate from the tissue and grew vigorously (Figure 1). Two to three days later, the cells reached 80% confluence.
Figure 1. Fibroblast culture with different olmesartan concentration interference.
A,B,C: Cultured human Tenon's fibroblasts growing out of the primary tenon's tissue under 100× magnification; D,E,F: Fibroblasts treated with increased concentration of olmesartan (0.75 µmol/mL, 1.5 µmol/mL, 2 µmol/mL) in MTT with 100× magnification; G,H,I: Fibroblasts in control, 1.25 µmol/mL, 1.5 µmol/mL olmesartan treated fibroblasts in MTT with 200× magnification.
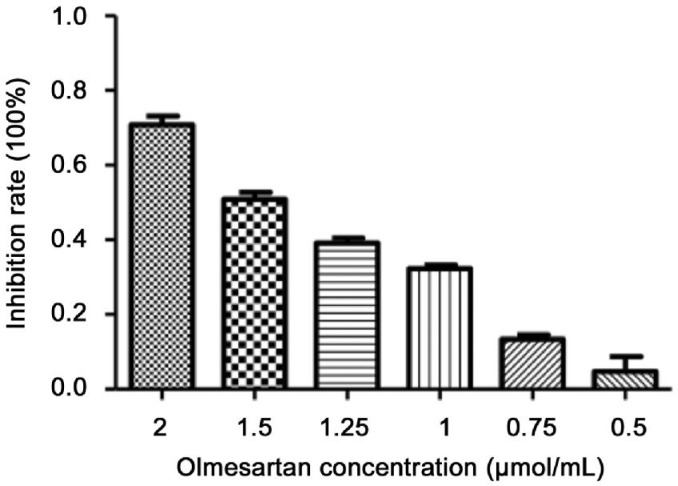
Methyl Thiazol Tetrazolium Assay of Cell Inhibition and Drug Concentration
In the MTT assay (Figure 2), the calculated inhibition rate was as reported previously[28]. Olmesartan (2.0 µmol/mL) generated a strong suppression of cells; lesser inhibition was observed at lower concentrations. Olmesartan (0.75 µmol/mL) with an 11% inhibition rate was chosen for subsequent experiments with cultured fibroblasts.
Figure 2. MTT result for inhibition rate of olmesartan with different concentration from 0.5 to 2 µmol/mL, increasing inhibition rate of fibroblasts with higher olmesartan concentration.
n=6 wells for each concentration.
Real-time Polymerase Chain Reaction
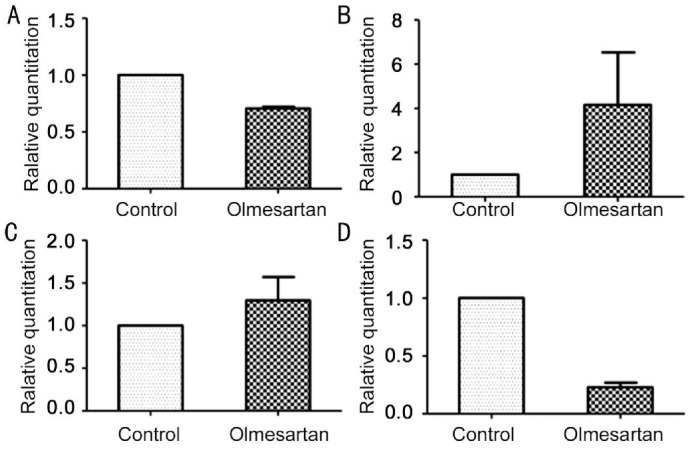
The inhibition effect of olmesartan on fibroblast cultures in vitro were analyzed by real-time PCR. The ΔΔCt method was used to calculate the relative gene expression for MMP-2, TIMP-1, TIMP-2, and PCNA (Figure 3). The expression of TIMP-1 and TIMP-2 increased in the presence of olmesartan, while MMP-2 and PCNA decreased compared with the control.
Figure 3. PCR of mRNA expression in vitro.
A: MMP-2 mRNA; B: TIMP-2 mRNA; C: TIMP-1mRNA; D: PCNA mRNA.
Observation of Rabbits' Eyes with Slit-lamp and Fundoscopy
Rabbits' eyes were observed under slit-lamp and fundoscopy before euthanasia. The corneal epithelia was intact with no spotting lesions, the anterior chambers were clear without obvious inflammatory reaction, the lenses were intact and translucent, and no abnormalities of the retina were detected by fundoscopy.
Haematoxylin and Eosin, Masson's Staining and Immunohistochemistry for Matrix Metalloproteinase-2 and Proliferating Cell Nuclear Antigen Expression
The subconjunctival injection of olmesartan in the surgical area induced less fibroblasts proliferation (Figure 4), neovascularization (Figure 5), ECM deposition (Figure 5) and epithelial proliferation (Figures 4 and 5) in conjunctiva of the rabbits eyes at each time group as compared with the control eye.
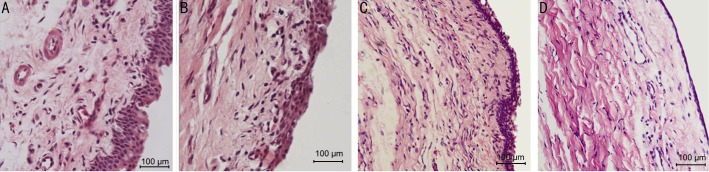
Figure 4. Hemotoxylin and eosin stain of rabbits conjunctiva, subconjunctiva with 10×40 magnification.
A: 7d group control eye with abundant epithelial cells proliferation and inflammatory cells migration in subconjunctiva; B: 7d group olmesartan eye with active proliferation both epithelial cells and fibroblasts in subconjunctiva; C: 14d group control eye with more fibroblasts; D: 14d group olmesartan eye.
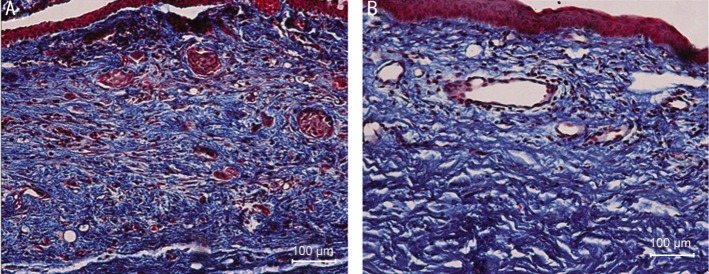
Figure 5. Masson's trichrome stain of rabbits conjunctiva, subconjunctiva and sclera tissue on the 7d group.
A: Control eye treated with PBS only, tight sclera tissue and very crowed neovasculature and fibroblasts in red; B: Olmesartan treated eye with less crowded neovasculature.
The inhibitive effects of olmesartan on the conjunctival and subconjunctival tissue proliferation were examined. Via haematoxylin and eosin staining (Figure 4), more conjunctival epithelial cells were observed in the control eye compared with the olmesartan-treated eye in day 3 group; more inflammatory cells migrated to or proliferated in the subconjunctival tissue of the control group. In the day 7 group, abundant fibroblast cells were observed in the subconjunctiva tissue in the control eye, while less fibroblasts were observed in the olmesartan-treated eye. From day 14 to day 21, cells either in the conjunctiva epithelium or subconjunctival tissue decreased; by day 28, the cells returned to baseline both in the control and olmesartan-treated eyes.
Masson's staining in the day 7 group (Figure 5) showed that neovascularization was suppressed by olmesartan in the treated eyes compared with the control eyes; also, collagen fibers and fibroblasts showed reduced density in the olmesartan group.
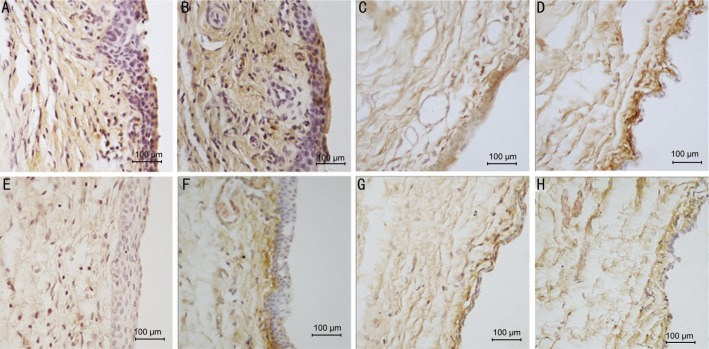
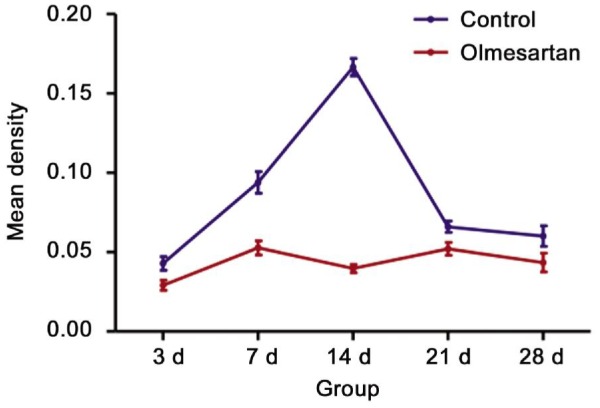
The analysis of the expression of MMP-2 by immunohistochemistry with the Image-Pro Plus software showed that the mean density of MMP-2 decreased with injections of olmesartan in the day 3, 7, 14 and 21 groups; however, no significant difference in the day 28 group was observed (Figures 6 and 7).
Figure 6. The immunohistochemical pictures show the expression of MMP-2 in conjunctiva and subconjunctival tissue of 10×40 magnification.
A: 3d group control eye, over-proliferative epithelial cells and fibroblasts in subconjunctiva, brown granules show the MMP-2 expression in all layers of tissues; B: 7d group control eye, brown staining in all layers; C: 21d group control eye, darker brown in subconjunctiva; D: 28d group control eye; E: 3d group olmesartan eye with milder brown in the same areas as the control; F: 7d group olmesartan eye, lighter brown mostly in the subconjunctival area right beneath the epithelial; G: 21d group olmesartan eye, lighter brown in all layers; H: 28d group olmesartan eye.
Figure 7. Olmesartan treated-eye presents with lower MMP-2 expression by means of mean density in each group comparing with the controlled eye.
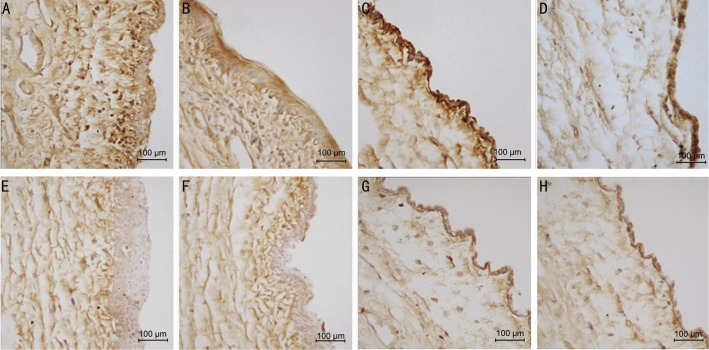
Given that PCNA were expressed in the cell nucleus, the number of positive cells decreased significantly with olmesartan injections in the day 3, 7, 14 and 21 groups but not in day 28 group (Figures 8 and 9).
Figure 8. Immunohistochemistry staining of PCNA, 10×40 magnification.
A: 3d group control eye, dark brown granules deposits in nuclear of cells either in epithelial or subconjunctiva fibroblasts; B: 14d group control eye, dark brown scattered in different layers cell nuclear; C: 21d group control eye with dark brown nuclear mainly in epithelial cells; D: 28d group control eye; E: 3d group olmesartan treated eye with less brown granules in nuclear; F: 14d group olmesartan eye with less PCNA positive cell nuclear; G: 21d group olmesartan eye with milder brown and less positive cells; H: 28d group olmesartan eye lighter brown granules.
Figure 9. Positive PCNA expression cell nuclear number.

Olmesartan-treated eye with lower PCNA expression in day 3, 7, 14 and 21 group, day 28 group has no significant difference.
DISCUSSION
Studies of MMPs in the cardiovascular system demonstrated their roles as a pro-proliferation factor and a member of signaling pathways, inducing cascades of tissue remodeling and contraction[29]. MMP-2 has a pivotal role in the MMP family through mutual regulation with the other members in the family[30]. In Tenon's fibroblasts, MMP-2 may be involved, as a pro-proliferation factor, in the molecular signaling to enhance cell proliferation activity. This is in accordance with the study of Yang et al's[31], which showed a higher MMP-2 expression in the fibroblasts of the more active stage of the pterygium. Moreover, targeting in MMP-2 inhibition can induce tumor cell apoptosis and reduce scar formation[32]–[33]. No matter olmesartan suppressed MMP-2 expression through fibroblasts inhibition or the effects on cross-talking of cell molecules it participates can restrain fibroblast proliferation, fibroblast proliferation and MMP-2 expression altered in parallel. Simultaneously, with the increased expression of TIMP-1 and TIMP-2, the endogenous inhibitors of MMPs, further suppressed the activity of MMPs.
In vivo, olmesartan suppressed the inflammatory reaction in the rabbit eye surgical model. The number of inflammatory cells decreased with the subconjunctival injection of olmesartan. These cells include fibroblasts macrophages and neutrophils. Excessively fibroblast proliferation and activated macrophage migration can interact with multiple inflammatory factors, which strengthens the inflammatory effects and induces fibrosis[34]. Therefore, as a key role in inflammatory reaction, fibroblasts are targeted for inflammation suppression. Decreasing fibroblasts either in vivo or in vitro has been demonstrated by our studies with the administration of olmesartan.
Neovascularization also plays an important role in inflammatory reaction, neo-vessels generated in the course of reaction bridge the inflammatory factors cascade. Through in vivo study of morphology on HE staining of Tenon's capsule in different groups, it is observed that neovascularization changed dynamically. They appear from 3d postoperatively, after day 7, less neo-vessels could be observed. Therefore, the 7d group was selected for Masson's trichrome stain for neovascularization comparison. Reduced angiogenesis in the presence of olmesartan may account for the suppression of the inflammatory response. As angiogenesis is closely associated with MMPs and VEGF, in our Masson's stained sections, we observed decreased neovascularization corresponding to weaker expression of MMP-2, as observed by immunohistochemistry, after treatment with olmesartan, which could be explained by the alteration of MMP-2 and angiogenesis related cytokines[35]–[37].
Because endothelial cells of blood vessels adapt to both physiological and pathological (inflammation and tumorigenesis) environments continuously, tissue repair processes begin with the injury-activated acute inflammation reaction, during this process, cytokines related to angiogenesis are released from the vessels adjacent to the injury area, which induces vascular sprouting and the production of granulation tissue that is highly vascularized. Without appropriate balance mechanisms, newly formed blood vessels do not regress. On the contrary, a positive feedback loop is formed to enhance further inflammatory responses. With the participation of MMP-2, membrane-type matrix metalloproteinase (MT1-MMP), the ECM was degraded, enabling new vascular sprouts to spread and progress. After GFS, similar processes occurred, as mediated by VEGF, inducing scar formation[38]–[39]. Therefore, olmesartan dampened this course via the inhibition of MMP-2 and correspondingly decreases angiogenesis.
Another proliferative related factor-PCNA was also examined during the study. Its expression was suppressed by olmesartan both in vivo and in vitro. PCNA, a protein present in eukaryotic cells, acts as a DNA clamp, encircles DNA, and promotes DNA synthesis and repair; meanwhile, PCNA can bind to CDK/cyclin and modulate the CDK2 and substrate reaction, with a negative effect on cell apoptosis. Therefore, PCNA also inhibits cell apoptosis. In the studies of tumor cells and fibroblasts, PCNA was widely used to monitor cell proliferation, especially the status of the proliferative activity of fibroblasts[40]–[41].
Although detailed molecular talk and cross-linking mechanisms require further study, we present evidence that olmesartan can effectively inhibit fibroblast proliferation in the conjunctiva flap model, which approximates the effects of GFS to the conjunctiva and subconjunctival tissue. Fibroblast proliferation and angiogenesis have been suppressed with olmesartan, resulting in decreased fibrosis and scarring, which should improve the results of surgery.
As with the first evaluation of olmesartan in Tenon's capsule in the eye, we observed no obvious toxic effect of olmesartan to the rabbit eye; its inhibitory effect on the fibroblasts and the related scarring has been shown in this study. We hope to offer further options for safer adjuvant medications for GFS surgery.
Acknowledgments
The authors thank the Scientific Research and Laboratory Center of the Second Affiliated Hospital of Xi'an Jiaotong University for the technical support.
Conflicts of Interest: Wang X, None; Fan YZ, None; Yao L, None; Wang JM, None.
REFERENCES
- 1.Eren K, Turgut B, Akin MM, Demir T. The suppression of wound healing response with sirolimus and sunitinib following experimental trabeculectomy in a rabbit model. Curr Eye Res. 2015:1–10. doi: 10.3109/02713683.2015.1023460. [DOI] [PubMed] [Google Scholar]
- 2.Xue H, McCauley RL, Zhang W, Martin DK. Altered interleukin-6 expression in fibroblasts from hypertrophic burn scars. J Burn Care Rehabil. 2000;21(2):142–146. doi: 10.1097/00004630-200021020-00010. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura-Shibasaki M, Ko JA, Takenaka J, Chikama T, Sonoda KH, Kiuchi Y. Matrix metalloproteinase and cytokine expression in Tenon fibroblasts during scar formation after glaucoma filtration or implant surgery in rats. Cell Biochem Funct. 2013;31(6):482–488. doi: 10.1002/cbf.2923. [DOI] [PubMed] [Google Scholar]
- 4.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20(3):161–168. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujiwara M, Muragaki Y, Ooshima A. Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol. 2005;153(2):295–300. doi: 10.1111/j.1365-2133.2005.06698.x. [DOI] [PubMed] [Google Scholar]
- 6.Lanigan L, Stuirmer J, Baez KA, Hitchings RA, Khaw PT. Single intraoperative applications of 5-fluorouracil during filtration surgery: early results. Br J Ophthalmol. 1994;78(1):33–37. doi: 10.1136/bjo.78.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casson R, Rahman R, Salmon JF. Long term results and complications of trabeculectomy augmented with low dose mitomycin C in patients at risk for filtration failure. Br J Ophthalmol. 2001;85(6):686–688. doi: 10.1136/bjo.85.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bindlish R, Condon GP, Schlosser JD, D'Antonio J, Lauer KB, Lehrer R. Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology. 2002;109(7):1336–1341. doi: 10.1016/s0161-6420(02)01069-2. [DOI] [PubMed] [Google Scholar]
- 9.Ball KJ, Williams PA, Stumpe KO. Relative efficacy of an angiotensin II antagonist compared with other antihypertensive agents. Olmesartan medoxomil versus antihypertensives. J Hypertens Suppl. 2001;19(1):S49–56. doi: 10.1097/00004872-200106001-00007. [DOI] [PubMed] [Google Scholar]
- 10.Anand N, Arora S, Clowes M. Mitomycin C augmented glaucoma surgery: evolution of filtering bleb avascularity, transconjunctival oozing, and leaks. Br J Ophthalmol. 2006;90(2):175–180. doi: 10.1136/bjo.2005.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Giusti VC, Garciarena CD, Aiello EA. Role of reactive oxygen species (ROS) in angiotensin II-induced stimulation of the cardiac Na+/HCO3- cotransport. J Mol Cell Cardiol. 2009;47(5):716–722. doi: 10.1016/j.yjmcc.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Pacurari M, Kafoury R, Tchounwou PB, Ndebele K. The Renin-Angiotensin-Aldosterone System in vascular inflammation and remodeling. Int J Inflam. 2014;2014:689360. doi: 10.1155/2014/689360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada K, Sugaya T, Murakami K, Yazaki Y, Komuro I. Angiotensin II type 1A receptor knockout mice display less left ventricu- lar remodeling and improved survival after myocardial infarction. Circulation. 1999;100(20):2093–2099. doi: 10.1161/01.cir.100.20.2093. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Ohta K, Hamaguchi A, et al. Contribution of renal angiotensin II type I receptor to gene expressions in hypertension-induced renal injury. Kidney Int. 1994;46(5):1346–1358. doi: 10.1038/ki.1994.404. [DOI] [PubMed] [Google Scholar]
- 15.Yuko W, Waseda Y, Yasui M, Nishizawa Y, Inuzuka K, Takato H, Ichikawa Y, Tagami A, Fujimura M, Nakao S. Angiotensin II type 2 receptor antagonist reduces bleomycin-induced pulmonary fibrosis in mice. Respir Res. 2008;9:43. doi: 10.1186/1465-9921-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escobar E, Rodríguez-Reyna TS, Arrieta O, Sotelo J. Angiotensin II, cell proliferation and angiogenesis regulator: biologic and therapeutic implications in cancer. Curr Vas Pharmacol. 2004;2(4):385–399. doi: 10.2174/1570161043385556. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-β1 pathways in cardiac fibroblasts. Cardiovasc ReS. 2011;91(1):80–89. doi: 10.1093/cvr/cvr067. [DOI] [PubMed] [Google Scholar]
- 18.Arenas IA, Xu Y, Lopez-Jaramillo P, Davidge ST. Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-α. Am J Physiol. 2004;286(4):C779–784. doi: 10.1152/ajpcell.00398.2003. [DOI] [PubMed] [Google Scholar]
- 19.Hirose A, Ono M, Saibara T, Nozaki Y, Masuda K, Yoshioka A, Takahashi M, Akisawa N, Iwasaki S, Oben JA, Onishi S. Angiotensin II type 1 receptor blocker inhibits fibrosis in rat nonalcoholic steatohepatitis. Hepatology. 2007;45(6):1375–1381. doi: 10.1002/hep.21638. [DOI] [PubMed] [Google Scholar]
- 20.Shaaban AA, Shaker ME, Zalata KR, El-kashef HA, Ibrahim TM. Modulation of carbon tetrachloride-induced hepatic oxidative stress, injury and fibrosis by olmesartan and omega-3. Chem Biol Interact. 2014;207:81–91. doi: 10.1016/j.cbi.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Kanamori H, Takemura G, Li Y, Okada H, Maruyama R, Aoyama T, Miyata S, Esaki M, Ogino A, Nakagawa M, Ushikoshi H, Kawasaki M, Minatoguchi S, Fujiwara H. Inhibition of Fas-associated apoptosis in granulation tissue cells accompanies attenuation of postinfarction left ventricular remodeling by olmesartan. Am J Physiol Heart Circ Physiol. 2007;292(5):H2184–2194. doi: 10.1152/ajpheart.01235.2006. [DOI] [PubMed] [Google Scholar]
- 22.Danser AH, Derkx FH, Admiraal PJ, Deinum J, de Jong PT, Schalekamp MA. Angiotensin levels in the eye. Invest Ophthalmol Vis Sci. 1994;35(3):1008–1018. [PubMed] [Google Scholar]
- 23.Vaajanen A, Vapaatalo H. Local ocular renin-angiotensin system-a target for glaucoma therapy? Basic Clin Pharmacol Toxicol. 2011;109(4):217–224. doi: 10.1111/j.1742-7843.2011.00729.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Runyan SA, Robinson MR. Novel ocular antihypertensive compounds in clinical trials. Clin Ophthalmol. 2011;5:667–677. doi: 10.2147/OPTH.S15971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seet LF, Su R, Toh LZ, Wong TT. In vitro analyses of the anti-fibrotic effect of SPARC silencing in human Tenon's fibroblasts: comparisons with mitomycin C. J Cell Mol Med. 2012;16(6):1245–1259. doi: 10.1111/j.1582-4934.2011.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cetin Y, Bullerman LB. Cytotoxicity of Fusarium mycotoxins to mammalian cell cultures as determined by the MTT bioassay. Food and Chem Toxicol. 2005;43(5):755–764. doi: 10.1016/j.fct.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Ren JH, Lin JS, He WS, Sun XM, Li PY, Chang Y, Liu Y, Li C, Gao XS. RASSF2 induces activated ras-dependent cell growth inhibition and apoptosis in human pancreatic cancer cells. J Cancer Mol. 2006;2(3):117–122. [Google Scholar]
- 28.Maruichi M, Takai S, Sugiyama T, Ueki M, Oku H, Sakaguchi M, Okamoto Y, Muramatsu M, Ikeda T, Miyazaki M. Role of chymase on growth of cultured canine Tenon's capsule fibroblasts and scarring in a canine conjunctival flap model. Exp Eye Res. 2004;79(1):111–118. doi: 10.1016/j.exer.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Bosonea AM, Wang X, Odenbach J, Fernandez-Patron C. Metalloproteinases in hypertension and cardiac disease: differential expression and mutual regulation. Drug Discov Today Dis Models. 2011;8(1):29–35. doi: 10.1016/j.ddmod.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253(1–2):269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 31.Yang SF, Lin CY, Yang PY, Chao SC, Ye YZ, Hu DN. Increased expression of gelatinase (mmp-2 and mmp-9) in pterygia and pterygium fibroblasts with disease progression and activation of protein kinase C. Invest Ophthalmol Vis Sci. 2009;50(10):4588–4596. doi: 10.1167/iovs.08-3147. [DOI] [PubMed] [Google Scholar]
- 32.Kargiotis O, Chetty C, Gondi CS, Tsung AJ, Dinh DH, Gujrati M, Lakka SS, Kyritsis AP, Rao JS. Adenovirus-mediated transfer of siRNA against MMP-2 mRNA results in impaired invasion and tumor-induced angiogenesis, induces apoptosis in vitro and inhibits tumor growth in vivo in glioblastoma. Oncogene. 2008;27(35):4830–4840. doi: 10.1038/onc.2008.122. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Wong TT, Mead AL, Khaw PT. Matrix metalloproteinase inhibition modulates postoperative scarring after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2003;44(3):1097–1103. doi: 10.1167/iovs.02-0366. [DOI] [PubMed] [Google Scholar]
- 34.Adegunsoye A, Balachandran J. inflammatory response mechanisms exacerbating hypoxemia in coexistent pulmonary fibrosis and sleep apnea. Mediators Inflamm. 2015;2015:510105. doi: 10.1155/2015/510105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sang QX. Complex role of matrix metalloproteinase in angiogenesis. Cell Res. 1998;8(3):171–177. doi: 10.1038/cr.1998.17. [DOI] [PubMed] [Google Scholar]
- 36.Chetty C, Lakka SS, Bhoopathi P, Rao JS. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int J Cancer. 2010;127(5):1081–1095. doi: 10.1002/ijc.25134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song H, Pan D, Sun W, Gu C, Zhang Y, Zhao P, Qi Z, Zhao S. SiRNA directed against annexin II receptor inhibits angiogenesis via suppressing MMP2 and MMP9 expression. Cell Physiol Biochem. 2015;35(3):875–884. doi: 10.1159/000369745. [DOI] [PubMed] [Google Scholar]
- 38.Arroyo AG, Iruela-Arispe ML. Extracellular matrix,inflammation,a nd the angiogenic response. Cardiovascular Res. 2010;86(2):226–235. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Bergen T, Vandewalle E, Van de Veire S, Dewerchin M, Stassen JM, Moons L, Stalmans I. The role of different VEGF isoforms in scar formation after glaucoma filtration surgery. Exp Eye Res. 2011;93(5):689–699. doi: 10.1016/j.exer.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Savio M, Stivala LA, Bianchi L, Vannini V, Prosper E. Involvement of the proliferating cell nuclear antigen (PCNA) in DNA repair induced by alkylating agents and oxidative damage in human fibroblasts. Carcinogenesis. 1998;19(4):591–596. doi: 10.1093/carcin/19.4.591. [DOI] [PubMed] [Google Scholar]
- 41.Kojima S, Sugiyama T, Takai S, Jin D, Ueki M, Oku H, Tabata Y, Ikeda T. Effects of gelatin hydrogel loading mitomycin C on conjunctival scarring in a canine filtration surgery model. Invest Ophthalmol Vis Sci. 2015;56(4):2601–2605. doi: 10.1167/iovs.15-16486. [DOI] [PubMed] [Google Scholar]