Abstract
AIM
To design, optimize and validate a rapid, internally controlled real-time polymerase chain reaction (RT-PCR) test for herpes simplex virus (HSV) in the diagnosis of necrotizing herpes stromal keratitis.
METHODS
Tears alone or together with corneal epithelium scrapings from 30 patients (30 eyes) suspected of necrotizing herpes stromal keratitis were tested for HSV DNA by RT-PCR. The samples were collected during the first visit and then on the subsequent 7, 14, 28, 42, and 56d. The symptoms of the patients were scored before treatment to determine the correlation between HSV concentration in the corneal epithelium scrapings and clinical scores.
RESULTS
The positive rate (46.4%) in the corneal epithelium group before the therapy was significantly higher than that (13.3%) in the tears group (P=0.006). There were 13 positive HSV patients before the therapy, the concentration of HSV DNA in corneal epithelium scrapings group was significantly higher than that in the tears group (paired t-test, P=0.0397). Multilevel mixed-effects model analysis showed that the difference between the corneal epithelium scrapings group and the tears group was statistically significant (P=0.0049). The Spearman rank correlation analysis indicated a positive correlation between the HSV concentration in the corneal epithelium scrapings and clinical scores before the treatment (r=0.844, P<0.0001).
CONCLUSION
RT-PCR appears to be a powerful molecular tool for the diagnosis of necrotizing herpes stromal keratitis.
Keywords: necrotizing herpes stromal keratitis, real-time polymerase chain reaction, corneal epithelium scrapings, tears
INTRODUCTION
Herpes stromal keratitis (HSK), which is subdivided into necrotizing herpes stromal keratitis and immune herpes stromal keratitis[1], is a leading cause of corneal blindness that accompanies herpes simplex virus (HSV) infection of the eye[2]. Many studies[3]–[5] have demonstrated that HSV can establish latency in either the trigeminal ganglia or the cornea after primary infection and can eventually be reactivated by fever, exposure to ultraviolet rays[6], general ill-health, emotional stress, physical exhaustion, mild trauma, menstrual stress[7] and so on[8].
Previously the diagnosis of HSK relied on a history of recurrent keratitis, as well as typical clinical manifestations in the infected eye[9]. However, after therapy, HSK does not have specific clinical features, so the disease remains a diagnostic and therapeutic challenge to ophthalmologists. Shimeld et al[10] successfully isolated HSV from the cornea in patients with chronic stromal keratitis, and virus isolation is considered the “golden standard” in laboratory diagnosis. However, this technique is time-consuming, has low sensitivity, and requires a special laboratory for viral processing. Techniques that rely on immunofluorescence are adversely influenced by false-positive and false-negative results, small sample size, and subjective variation in the interpretation of data[11]. The polymerase chain reaction (PCR), which is sensitive and has a relatively rapid processing time[12], can also be used to detect HSV DNA. However, the theoretically high sensitivity of PCR is offset by a high cost and the need for dedicated laboratory space (three separate areas) and trained technicians[13].
Real-time polymerase chain reaction (RT-PCR), considered a powerful molecular tool for the diagnosis of necrotizing herpes stromal keratitis, was developed as an alternative approach. RT-PCR is a variant of PCR that is performed in a closed system and does not require post-amplification sample manipulation. Importantly, the ability to quantify the DNA by this method allows the measurement of as little as several hundred DNA molecules to as much as hundreds of millions of DNA molecules[14]. RT-PCR overcomes the drawbacks of conventional PCR, reduces the risk for carry-over contamination, and eliminates the time-consuming detection step.
Here we report the results of a study testing the use of RT-PCR in the diagnosis of necrotizing herpes stromal keratitis. We analyzed the variation trend of the positive rate and concentration of HSV DNA in corneal epithelial scrapings and tears before and after the therapy, and we also examined the correlation between the HSV concentration and clinical scores before the treatment.
SUBJECTS AND METHODS
Subjects
A total of 272 specimens (105 corneal epithelium scrapings and 167 tears) were collected from 30 patients (30 eyes, 18 right and 12 left) with clinically diagnosed necrotizing herpes stromal keratitis. These patients were treated at the Department of Ophthalmology, Nanjing First Hospital between September 2012 and September 2013. The patients included 13 males and 17 females, and their ages ranged from 20 to 56y, with a mean age of 38.5y. Because the patients had normal kidney functions, were neither pregnant nor breast feeding, or did not have severe heart, lung, liver, or kidney dysfunctions or a history of diabetes and malignant tumors, the wide range in the age of the subjects did not significantly influence the study. This study was conducted in accordance with the Declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Nanjing Medical University. Written informed consent was obtained from all participants.
Inclusion and Exclusion Criteria
Selection of patients was based on the following criteria[15]: 1) history of recurrent keratitis; 2) presence of deep stromal infiltration; 3) typical dendritic or geographical configuration noted during one or more of the previous attacks; 4) corneal anesthesia; and 5) negative for bacterial and fungal ulcer (verified by cultivation). If one or more of the five criteria were not fulfilled or a secondary bacterial or fungal infection was found, the patient was excluded from the study. Patients recruited in this study were not undergoing treatment with any drug, including systemic antiviral drugs, or had stopped antiviral drugs treatment for at least 1wk. Patients were strictly prohibited from taking other antiviral drugs during this trial, and they had no other eye problems and had normally functioning kidneys (creatinine clearance rate ≥70 mL/min). Patients were excluded from the study if they were pregnant or breastfeeding or if they had severe heart, lung, liver, or kidney dysfunctions or a history of diabetes or malignant tumors. Three patients were excluded without normal functioning.
Treatment
Patients were treated with 0.15% ganciclovir (GCV) gel solution (one drop each time, 4 times per day, dripped into the conjunctival sac of the eye) and 0.1% fluorometholone eye drops (1 drop each time, 3 times a day, dripped into the conjunctival sac of the eye) until complete recovery, in combination with oral GCV (1000 mg per dose, 3 times per day for 8wk) followed by oral ACV at a dose of 400 mg twice per day for 6mo.
Specimen Collection
Specimens were collected during the first visit and then on the subsequent 7, 14, 28, 42, and 56d. Corneal epithelium scrapings were collected[12] by debriding the edge of an ulcer with sterile needles, then were stored in 100 µL of sterile saline at -70°C until processed. Tears (100 µL) were collected by stimulating the conjunctival fornix with minuscule sterile glass capillaries, and then were stored in the same conditions as the corneal epithelium scrapings.
There were 28 samples collected from the corneal epithelium scrapings on the initial visit, and 28, 23, 17, 8, and 1 (105 totally) collected on the respective follow-up visits. Two of the 30 patients refused to submit to the corneal epithelium scrapings during the first visit. Corneal epithelium scrapings collection stopped when the corneal epithelium was intact. The numbers of samples in the tears group were 30 from the first visit, and 30, 28, 27, 26, and 26 (167 totally) on the respective follow-up visits. With treatment, the volume of the tears was too small to collect from four patients.
DNA Extraction and Real-time Polymerase Chain Reaction
All samples obtained from the subjects were stored at -70°C before DNA extraction. RT-PCR [9] for the detection of HSV DNA was carried out using the Artus HSV-1 QS-RGQ kit (Qiagen, China). Reactions were set-up and performed according to the manufacturer's instructions, and were executed by the vitro medical diagnostic device intended for use on the Loche 480 instrument. All reactions were performed in a total volume of 40 µL. The reaction conditions were as follows: pre-denaturation at 37°C for 5min, followed by 40 cycles of denaturation at 94°C for 1min, annealing at 95°C for 5s and extension at 60°C for 30s.
Scoring of Symptoms
Patients were examined by slit lamp before and after the cornea was stained with fluorescein and then were instructed to grade each of his/her symptoms [(visual deterioration, redness, ophthalmalgia, lachrymation, photophobia, secretion, conjunctiva injection, ciliary injection, folliculosis, cornea inflammation, Keratic precipitate (kp), Tyndall phenomenon, posterior synechia of the iris, uncomfortable)] by using the numbers 0-4 (0=lowest, 4=highest). The patients were also asked to indicate the total scores in all follow-up windows. During follow-up, all subjects were asked about whether any discomfort had occurred during therapy. All patients orally administered with drugs underwent routine blood and urine examinations as well as liver and kidney function examinations to monitor adverse reactions of drugs.
Statistical Analysis
All data were analyzed by the SPSS19.0 and MLwiN2.28 software using the Chi-squared test, paired t-test, Spearman rank correlation analysis and multilevel mixed-effects model. Values on this study are reported as mean ±SD or P50 (P25-P75). Results were considered statistically significant for two-sided P<0.05.
RESULTS
Amplification Curves and Standard Curves of Herpes Simplex Virus Transcripts
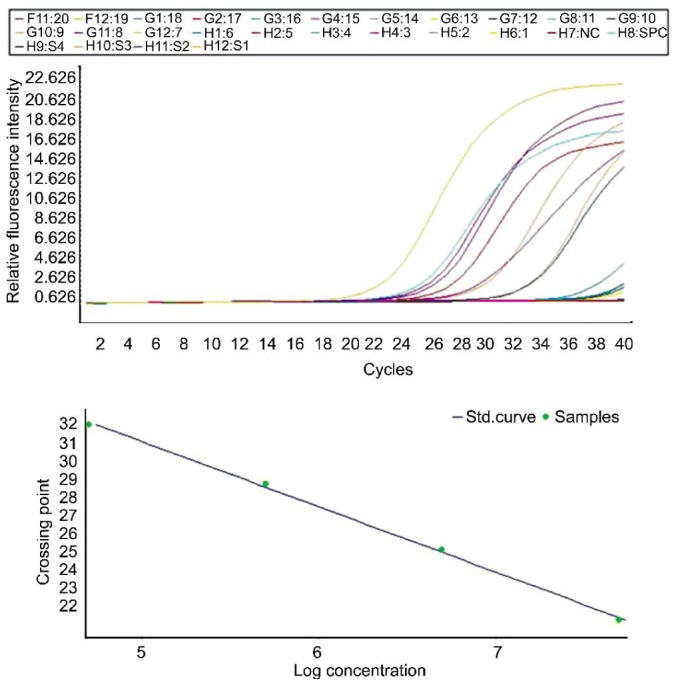
Amplification curves and the standard curve of HSV transcripts are shown in Figure 1. Values of RT-PCR were normalized to calculate copy numbers of HSV transcripts in the samples according to the standard curve with LightCycler Software, Version 3.5 (Roche Diagnostic, Inc.)[12].
Figure 1. Amplification curves and standard curve of HSV transcripts.
Variation Trend of Positive Rate
The comparison of the percentage of positive samples in the corneal epithelial scrapings and in the tears after each follow-up visit as detected by RT-PCR is shown in Table 1. The highest positive percentage was obtained in the corneal epithelium scrapings on the first visit. The positive percentage was significantly reduced in the corneal epithelium scrapings samples from day 1 to day 56 (8th week), while there was only marginally decline in the positive percentage of tears samples at the same time. Corneal epithelium scrapings yielded noticeably higher positive rate (46.4%) than in tears (13.3%) before the treatment (P=0.006), but there was no difference between corneal epithelium scrapings samples and tears samples during the progression.
Table 1. The comparison of positive rate in two groups with each follow-up point by RT-PCR.
| Group | Time | Pre-treatment | 1st week | 2nd week | 4th week | 6th week | 8th week |
| Corneal epithelium scrapings | Positive samples | 13/28 | 2/28 | 2/23 | 1/17 | 0/8 | 0/1 |
| 1P | 0.001 | 0.003 | 0.004 | 0.032 | 1.000 | ||
| Tears | Positive samples | 4/30 | 2/30 | 1/28 | 0/27 | 0/26 | 0/26 |
| 1P | 0.671 | 0.354 | 0.114 | 0.115 | 0.115 | ||
| P | 0.006 | 1.000 | 0.583 | 0.386 | — | — |
1P: Comparison of 1st week, 2nd week, 4th week, 6th week and 8th week to the pre-treatment (Chi-squared test).
Variation Trend of Concentration of Herpes Simplex Virus DNA
Prior to treatment, 13 (43.3%) of the 30 subjects were positive for HSV either in the corneal epithelium scrapings or the tears as measured by RT-PCR. Correlation of the quantitative viral DNA analysis to the positive patients is summarized in Table 2. The amount of HSV DNA in the corneal epithelium scrapings was significantly higher than in tears on the first visit (paired t-test, P=0.0397). Multilevel mixed-effects model analysis indicated that the difference between the corneal epithelium scrapings samples and the tears samples was statistically significant (P=0.0049); the concentration was undetectable at 28d after the treatment in the two groups (P=0.0007) and the decline rate of two groups was significantly different (P=0.0494).
Table 2. Results of the quantitative viral DNA analysis to the positive patients copies/mL.
| Groups | Statistical index | Pre-treatment | 7th day | 14th day | 28th day |
| Corneal epithelium scrapings | Sample amount | 13 | 13 | 13 | 13 |
| x±s | 390420.77±610715.88 | 529.23±1291.86 | 176.54±465.70 | 44.23±159.48 | |
| P50 (P25-P75) | 2460 (2165-636500) | 0 (0-0) | 0 (0-0) | 0 (0-0) | |
| Min. | 2030 | 0 | 0 | 0 | |
| Max. | 2020000 | 3460 | 1580 | 575 | |
| Incidence No. (rate) | 13 (100%) | 2 (15.38%) | 2 (15.38%) | 1 (7.69%) | |
| Tears | Sample amount | 13 | 13 | 13 | 13 |
| x±s | 893.85±2201.78 | 140.15±344.99 | 48.00±173.07 | 0.00±0.00 | |
| P50 (P25-P75) | 0 (0-1150) | 0 (0-0) | 0 (0-0) | 0 (0-0) | |
| Min. | 0 | 0 | 0 | 0 | |
| Max. | 8010 | 1020 | 624 | 0 | |
| Incidence No. (rate) | 4 (30.77%) | 2 (15.38%) | 1 (7.69%) | 0 (0.00%) |
Correlation Between Herpes Simplex Virus Concentration and Clinical Scores
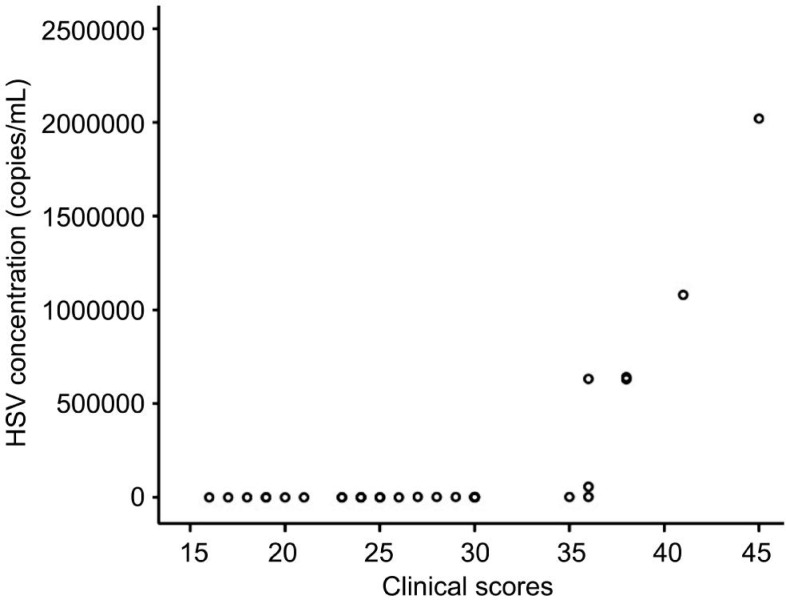
Scatter plot of the HSV concentration in the corneal epithelium scrapings (non-normal distribution) and clinical scores before the treatment are shown in the Figure 2. Spearman rank correlation analysis indicated that there was a positive correlation between HSV concentration and clinical score (r=0.844, P<0.0001). In all cases where lower concentrations of HSV were detected, the clinical scores decreased as well. The symptoms corresponded to the results obtained from the laboratory.
Figure 2. Scatter plot of the HSV concentration in the corneal epithelium scrapings and before the treatment.
DISCUSSION
In recent years, the incidence of HSK, which has already reached 31.5/105, has shown an increasing trend, both in China and other countries. Recurrent cases account for the majority of HSK cases, the incidence of which is 18.3/105[16]. Little statistical data is available on HSK in developing countries; however, the prevalence and incidence in developing countries are higher than those in developed countries. The people in developing countries tend to develop HSK at an earlier age. Viruses are usually latent in the trigeminal ganglia[17] after primary infection. With repeated reactivation cycles, viruses can also be found in corneal epithelial scrapings, stroma, or tears[18]. Necrotizing stromal keratitis is a relatively serious type of HSK, recurrent attacks of which may lead to blindness. Therefore, rapid and accurate laboratory diagnosis is quite important. The modified PCR, RT-PCR[19]–[20], is more specific and sensitive to viruses than normal PCR, and can be used to detect the virus more quickly at lower concentration to help make diagnosis sooner.
A multicenter, prospective, randomized, single-blind, and controlled clinical trial was conducted by the EYE and ENT Hospital of Fudan University, Hangzhou First People's Hospital, and by our lab. We recently published the results from that study, and here, we used the same criteria to select patients for this study. In the previous studies, we only used RT-PCR to diagnose HSK, but we did not monitor the variation in HSV concentration during the treatment. The objectives of this study were to develop an optimum laboratory test for the diagnosis of necrotizing herpes stromal keratitis and to determine if there is a correlation between HSV concentration and clinical scores.
Before the onset of therapy, the percentage of viral positive (46.4%) corneal epithelial scrapings and the concentration of virus in those scrapings were relatively high; however, patients occasionally experienced a mild trauma or complained about foreign body sensation after sampling. The percentage of viral positive (13.3%) tear samples and the concentration of virus in tears were relatively low; however, the sampling of tears does not cause appreciable discomfort in most patients. Some documents reported that positive rate of HSK in tears of asymptomatic patients from the American areas (most of them are white) was between 33.5% and 49%[21]–[22], while others have shown that the positive rate of HSK in the tears of asymptomatic patients in Japan was only 8.5%[23]. It is possible that many of the asymptomatic patients in Japan were HSK carriers after primary infection[21]. In our study, the positive rate of HSK in the tears of patients was 13.3%. Therefore, we infer that positive rate of HSK in the tears of patients is related to, not only population demographics, but also to geographical position.
HSV was detected in the corneal epithelial scrapings or tears of 13 of 30 patients when first examined. Although HSV was detected in the tears of only four of the 13 HSV positive patients, it was detected in the corneal epithelial scrapings of all 13 patients. When the concentration of virus in the corneal epithelial scrapings was lower than 105 copies/mL, HSV was not detected in the tears. When concentration of virus in the corneal epithelial scrapings was higher than 105 copies/mL, HSV was detected in the tears of 80% of those patients and the concentration of HSK in the corneal epithelial scrapings was significantly higher than in the tears.
Spearman rank correlation analysis indicated a positive correlation between the HSV concentration in the corneal epithelium scrapings (non-normal distribution) and clinical scores before the treatment. Patients with high clinical scores also had high concentrations of HSV in the corneal epithelium scrapings before the treatment, and vice versa. This result suggests that clinical manifestation before the treatment correlates with laboratory results. Our current research was done to understand on the relation between clinical manifestations and viral concentration at the first visit, and to establish a basis for diagnosis of herpes stromal keratitis.
Based on these results, we propose that while it is easier to detect virus in the corneal epithelial scrapings than in the tears, HSV becomes detectable in tears after replication when the viral concentration is high enough in the corneal epithelial scrapings to become sufficiently abundant in tears. We propose the use of tears from patients that refuse to allow collection of corneal epithelial scrapings or from whom collection of corneal epithelial scrapings would not be technically feasible. Whenever collecting corneal epithelial scrapings is feasible, tears cannot replace scrapings for diagnosis, but tears may act as a substitute for definitive diagnosis.
In conclusion, RT-PCR is a new and effective laboratory diagnostic technique that can be used to quantitatively test the concentration of HSV, observe transformation of HSV and make correct diagnosis for herpes stromal keratitis. The sites from where medical samples were collected before the treatment clearly affect the percentage of positive results and the concentration of HSV. Therefore, the accuracy of the diagnosis is improved if the medical material is collected from the corneal epithelium scrapings. After the treatment, the percentage of HSK positive samples is low in both corneal epithelium scrapings and in tears; therefore, the site of sample collection does not significantly affect the results. Using RT-PCR, we could monitor the changes in HSV concentration, which enabled us to assess the development of the disease.
Acknowledgments
Conflicts of Interest: Ma JX, None; Wang LN, None; Zhou RX, None; Yu Y, None; Du TX, None.
REFERENCES
- 1.Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea. 1999;18(2):144–154. doi: 10.1097/00003226-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Morris J, Stuart PM, Rogge M, Potter C, Gupta N, Yin XT. Recurrent herpetic stromal keratitis in mice, a model for studying human HSK. J Vis Exp. 2012;18(70):e4276. doi: 10.3791/4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaye SB, Lynas C, Patterson A, Risk JM, McCarthy K, Hart CA. Evidence for herpes simplex viral latency in the human cornea. Br J Ophthalmol. 1991;75(4):195–200. doi: 10.1136/bjo.75.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohrs RJ, Randall J, Smith J, Gilden DH, Dabrowski C, van Der Keyl H, Tal-Singer R. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74(24):11464–11471. doi: 10.1128/jvi.74.24.11464-11471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill JM, Ball MJ, Neumann DM, Azcuy AM, Bhattacharjee PS, Bouhanik S, Clement C, Lukiw WJ, Foster TP, Kumar M, Kaufman HE, Thompson HW. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J Virol. 2008;82(16):8230–8234. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludema C, Cole SR, Poole C, Smith JS, Schoenbach VJ, Wilhelmus KR. Association between unprotected ultraviolet radiation exposure and recurrence of ocular herpes simplex virus. Am J Epidemiol. 2014;179(2):208–215. doi: 10.1093/aje/kwt241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuokko H, Bloiqu R, Hukkanen V. Herpes simplex virus type 1 genital herpes in young women: current trend in Northern Finland. Sex Transm Infect. 2014;90(2):160. doi: 10.1136/sextrans-2013-051453. [DOI] [PubMed] [Google Scholar]
- 8.Farooq AV, Shukla D. Corneal latency and transmission of herpes simplex virus-1. Future Virol. 2011;6(1):101–108. doi: 10.2217/fvl.10.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon KC, Im SK, Park HY. Recurrent herpes simplex keratitis after verteporfin photodynamic therapy for corneal neovascularization. Cornea. 2010;29(4):465–467. doi: 10.1097/ICO.0b013e3181b53310. [DOI] [PubMed] [Google Scholar]
- 10.Shimeld C, Tullo AB, Easty DL, Thomsitt J. Isolation of herpes simplex virus from the cornea in chronic stromal keratitis. Br J Ophthalmol. 1982;66(10):643–647. doi: 10.1136/bjo.66.10.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elnifro EM, Cooper RJ, Klapper PE, Bailey AS, Tullo AB. Diagnosis of viral and chlamydial keratoconjunctivitis: which laboratory test? Br J Ophthalmol. 1999;83(5):622–627. doi: 10.1136/bjo.83.5.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakimaru-Hasegawa A, Kuo CH, Komatsu N, Komatsu K, Miyazaki D, Inoue Y. Clinical application of real-time polymerase chain reaction for diagnosis of herpetic diseases of the anterior segment of the eye. Jpn J Ophthalmol. 2008;52(1):24–31. doi: 10.1007/s10384-007-0485-7. [DOI] [PubMed] [Google Scholar]
- 13.Hlinomazová Z, Loukotová V, Horácková M, Sery O. The treatment of HSV1 ocular infections using quantitative real-time PCR results. Acta Ophthalmol. 2012;90(5):456–460. doi: 10.1111/j.1755-3768.2010.01933.x. [DOI] [PubMed] [Google Scholar]
- 14.Subhan S, Jose R J, Duggirala A, Hari R, Krishna P, Reddy S, Sharma S. Diagnosis of herpes simplex virus-1 keratitis: comparison of Giemsa stain, immunofluorescence assay and polymerase chain reaction. Current Eye Res. 2004;29(2–3):209–213. doi: 10.1080/02713680490504911. [DOI] [PubMed] [Google Scholar]
- 15.Oosterhuis JA, van Ganswijk R, Versteeg J. Acyclovir treatment in stromal herpetic keratitis. Doc Ophthalmol. 1983;56(1–2):81–88. doi: 10.1007/BF00154713. [DOI] [PubMed] [Google Scholar]
- 16.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20(1):1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Huang FF, Wang ZJ, Zhang CR. Tear HSV-specific secretory IgA as a potential indicator for recurrent stromal herpes simplex keratitis: a preliminary study. Cornea. 2013;32(7):987–991. doi: 10.1097/ICO.0b013e31828a8b96. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy DP, Clement C, Arceneaux RL, Bhattacharjee PS, Huq TS, Hill JM. Ocular herpes simplex virus type 1: is the cornea a reservoir for viral latency or a fast pit stop? Cornea. 2011;30:251–259. doi: 10.1097/ICO.0b013e3181ef241d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall D S, Linfert D R, Draghi A, McCarter YS, Tsongalis GJ. Identification of herpes simplex virus genital infection: comparison of a multiplex PCR assay and traditional viral isolation techniques. Mod Pathol. 2001;14(3):152–156. doi: 10.1038/modpathol.3880273. [DOI] [PubMed] [Google Scholar]
- 20.Jazeron JF, Barbe C, Frobert E, Renois F, Talmud D, Brixi-Benmansour H, Brodard V, Andréoletti L, Diebold MD, Lévêque N. Virological diagnosis of herpes simplex virus 1 esophagitis by quantitative real-time PCR assay. J Clin Microbiol. 2012;50(3):948–952. doi: 10.1128/JCM.05748-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46(1):241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar M, Hill JM, Clement C, Varnell ED, Thompson HW, Kaufman HE. A double-blind placebo-controlled study to evaluate valacyclovir alone and with aspirin for asymptomatic HSV-1 DNA shedding in human tears and saliva. Invest Ophthalmol Vis Sci. 2009;50(12):5601–5608. doi: 10.1167/iovs.09-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimomura Y, Higaki S. The kinetics of herpes virus on the ocular surface and suppression of its reactivation. Cornea. 2011;30 Suppl. 1:S3–7. doi: 10.1097/ICO.0b013e3182282005. [DOI] [PubMed] [Google Scholar]