Abstract
In the last two decades, perceptions about the role of body fat have changed. Adipocytes modulate endocrine and immune homeostasis by synthesizing hundreds of hormones, known as adipocytokines. Many studies have been investigating the influences and effects of these adipocytokines and suggest that they are modulated by the nutritional and immunologic milieu. Kidney transplant recipients (KTRs) are a unique and relevant population in which the function of adipocytokines can be examined, given their altered nutritional and immune status and subsequent dysregulation of adipocytokine metabolism. In this review, we summarize the recent findings about four specific adipocytokines and their respective roles in KTRs. We decided to evaluate the most widely described adipocytokines, including leptin, adiponectin, visfatin and resistin. Increasing evidence suggests that these adipocytokines may lead to cardiovascular events and metabolic changes in the general population and may also increase mortality and graft loss rate in KTRs. In addition, we present findings on the interrelationship between serum adipocytokine levels and nutritional and immunologic status, and mechanisms by which adipocytokines modulate morbidity and outcomes in KTRs.
Keywords: adiponectin, kidney transplantation, leptin, resistin, visfatin
Introduction of adipocytokines
In healthy individuals, the role of adipocytokines on various outcomes has been widely studied. However, little is known about how impaired kidney function and renal replacement therapy modify these associations [1]. For example, few studies have examined the impact of adipocytokines on long-term outcomes, such as graft loss and death, in kidney transplant recipients (KTRs). In Table 1, we summarize all studies investigating the role of adipocytokines in KTR and chronic kidney disease (CKD) patients.
Table 1.
Studies evaluating adipocytokines in renal transplant recipients and patients with CKD
| Author (year) | Number of patients | RRT | Findings | Positive correlations | Negative correlations | Lack of correlations |
|---|---|---|---|---|---|---|
| (A) Leptin | ||||||
| Agras et al. (2005) [2] | 41 | Tx. | Leptin seems to increase bone mass. | BMI, BMD, z score of BMD | ||
| Agras et al. (2006) [3] | 63 | Tx. | Leptin has an effect on lymphoid stem cells. | CD34/7 | CD34/7/8/4 | |
| Baczkowsk et al. (2000) [4] | 28 | Tx. | Imbalance between leptin and body weight also persists after renal transplantation | Cortisol, BMI | ||
| El Haggan et al. (2004) [5] | 41 | Tx. | Pretransplant leptin levels reduced after transplantation | Fat mass, CRP | Dietary intake | |
| Fonseca et al. (2015) [6] | 40 | Tx. | Leptin levels are independently determined by graft function | Male, DGF | Acute rejection | |
| Kagan et al. (2002) [7] | 24 | Tx. | Leptin shows correlation with gender, BMI, insulin and cortisol levels | Gender, BMI, cortisol, insulin | ||
| Kayacan et al. (2003) [8] | 34 | Tx. | Pretransplant leptin levels reduced after transplantation and was not effected by alimentary intake | HOMA, fat mass | ||
| Kokot et al. (1998) [9] | 40 | Tx | Pretransplant leptin levels reduced after transplantation | BMI | Age | |
| Kokot et al. (1999) [10] | nd. | Tx | Elevated leptin levels not only modulated by BMI | BMI | ||
| Kovesdy et al. (2010) [11] | 978 | Tx | Leptin lowers the bone turnover independently from PTH | PTH | vitD | |
| Landt et al. (1998) [12] | 29 | Tx | Pretransplant leptin levels reduced after transplantation | BMI | Gender | |
| Lee et al. (2010) [13] | 55 | Tx. | Leptin correlates with metabolic syndrome | MetSy, waistCX, BMI, fat mass, CRP | ||
| Lee et al. (2014) [14] | 74 | Tx. | Leptin was positively associated with peripheral arterial stiffness among renal transplant recipients | |||
| Malyszko et al. (2005) [15] | 27 | Tx. | Leptin is associated with graft function, but not related to BMD and bone metabolism | Body mass/fat, creatinine | Nutrition, BMD | |
| Nicoletto et al. (2012) [16] | 32 | Tx. | Pretransplant leptin levels reduced after transplantation | Gender, BF, HOMA | ||
| Rafieian-Kopaei et al. (2013) [17] | 72 | Tx | Leptin levels and duration of kidney transplant shows strong negative correlation | Gender | Duration of kidney Tx | Age, BMI, creatinine |
| Souza et al. (2007) [18] | 32 | Tx. | Pretransplant leptin levels reduced after transplantation | HOMA, fat mass | GFR | |
| (B) Adiponectin | ||||||
| Adamczak et al. (2007) [19] | 228 | Tx | ADPN levels are higher in RTR than in healthy controls, but lower than in HD patients | BMI, GFR, HOMA-IR | ||
| Adamczak et al. (2011) [20] | 88 | Tx | Role of ADPN in LVH and atherosclerosis cannot be confirmed | |||
| Alam et al. (2012) [21] | 987 | Tx | Elevated levels of ADPN increase mortality | GFR, BMI, abd.circ., CRP | CCI | |
| Bayes et al. (2007) [22] | 68 | Tx | Atorvastatin therapy did not modulate ADPN levels | HDL | HOMA-IR, creatinin | |
| Bayes et al. (2005) [23] | 199 | Tx | ADPN were lower, BMI higher in patients who developed NODAT | TNF, BMI, PAPP-A | Insulin | Age, sex |
| Canas et al. (2012) [24] | 157 | Tx | ADPN has an inverse association with insulin resistance | HOMA-R, c-IMT | ||
| Chitalia et al. (2010) [25] | 43 | Tx | ADPN levels do not predict the CV risk in RTR | hsCRP | GFR, BMI, Hgb, waist circumference | BP, smoking, lipids, DM |
| Chudek et al. (2003) [26] | 44 | HD/Tx | Kidney plays an important role in biodegradation of ADPN | HOMA-IR | ||
| Chudek et al. (2013) [27] | 372 | Tx | ACE I/D polymorphism modulates ADPN levels | Female, ACE II genotype | BMI | |
| Fonseca et al. (2015) [6] | 40 | Tx | ADPN level is not only modified by early graft function | Male, DGF | Acute rejection | |
| Ho et al. (2015) [28] | 69 | Tx | ADPN has negative correlation with arterial stiffness | DM, smoking, BMI, waist CX, BP, arterial stiffness | ||
| Idorn et al. (2012) [29] | 57 | Tx | ADPN level decreases after transplantation and does not predict NODAT | GFR, BMI, insulin | ||
| Kaisar et al. (2009) [30] | 137 | Tx | Hypoadiponectinemia associated with CVD | HDL, female | BMI, MetSy, IGT, TG, CRP, GFR | |
| Kang et al. (2012) [31] | 575 | Tx | ADIPOQ rs1501299 is associated with PTDM in a sex-specific manner | |||
| Kulshrestha et al. (2013) [32] | 74 | Tx | Patients with metabolic syndrome have lower ADPN levels after transplantation | Clinical events | ||
| Lee et al. (2011) [33] | 55 | Tx | Body fat mass is an independent predictor of ADPN levels | Fat mass, waist CX, MetSy | ||
| Leibowitz et al. (2013) [34] | 35 | Tx | ADPN in hypertensive patients is not a predictive factor for CVD | BMI, TG | ||
| Malyszko et al. (2005) [35] | 82 | Tx | ADPN seems to have defense mechanism against endothelial damage | CD146, thrombomodulin, creatinine | BMI, protein Z | |
| Nicoletto et al. (2013) [36] | 270 | Tx | TT genotype of ADPN increases the prevalence of NODAT | |||
| Nishimura et al. (2009) [37] | 98 | Tx | TAC and ARB modulate ADPN levels and posttransplant ADPN levels correlate with NODAT | HOMA-IR | hsCRP | |
| Prasad et al. (2012) [38] | 129 | Tx | ADPN level lower in South African population | GFR | ||
| Roos et al. (2012) [39] | 206 | Tx | Pretransplant ADPN level predicts higher risk for graft loss | Graft loss | ||
| Sethna et al. (2009) [40] | 33 | Tx | Lower ADPN levels associate with higher ambulatory BP | HT | ||
| Shen et al. (2007) [41] | 54 | Tx | ADPN levels are higher in RTR than in healthy controls, but lower than in HD patients (AdipoR1/2) | HOMA-IR | ||
| Shu et al. (2012) [42] | 271 | Tx | ADPN level is lower in patients with metabolic syndrome, even with lower GFR | HDL | GFR, MetSy, BMI | |
| Taherimahmoudi et al. (2010) [43] | 67 | Tx | ADPN levels are higher in RTR than in healthy controls, but lower than in HD patients. ADPN did not decrease immediately after transplantation | BMI, HOMA-R, GFR | ||
| Teplan et al. (2007) [44] | 68 | Tx | Immunosuppressive therapy could decrease BMI | Leptin | BMI | |
| Teplan et al. (2008) [45] | 140 | Tx | In obese RTR, ADMA is increased and ADPN levels are decreased | BMI | ||
| Yilmaz et al. (2005) [46] | 27 | Tx | ADMA, hsCRP decreasing instantly after transplantation, not like FMD and ADPN (they change later on) | |||
| Yu et al. (2011) [47] | 398 | Tx | SNP-45/276 of the ADPN gene were significantly associated with an increased risk for NODAT | |||
| (C) Visfatin | ||||||
| Axelsson et al. (2007) [48] | 189 | CKD | Elevated with higher CKD stages and may predict mortality | IL-6, hsCRP, VCAM | GFR | |
| Bessa et al. (2010) [49] | 40 | CKD | Visfatin is strongly associated with ED and flow-mediated dilatation | ICAM, VCAM, CRP, IL-6 | FMD, GFR | |
| Carrero et al. (2009) [50] | 246 | CKD | Elevated visfatin is associated with anorexia | PEW | TG, Chol, albumin | BMI, leptin |
| Eleftheriadis et al. (2013) [51] | 33 | HD | Visfatin is elevated in HD patients and is connected with decreased demands for rHuEpo | TSAT, Hgb | DM, BMI, IL-6 | |
| Erten et al. (2008) [52] | 31/30 | HD/CAPD | In CAPD patients, visfatin is higher than in HD/healthy individuals | IL-6, TNF | Left ventricular diastolic function | Left ventricular mass index |
| Kato et al. (2009) [53] | 68 | HD | Visfatin shows a strong association with time spent on HD | Time on HD, hsCRP | Albumin | BMI, adiponectin, body fat |
| Lu et al. (2013) [54] | 173 | CKD | Visfatin level is significantly higher in CAD patients and correlates with E-selectin | CAD, hsCRP, BNP, WBC, LDL | GFR, albumin | |
| Mahmood et al. (2010) [55] | 50 | CKD | Higher than in healthy controls. No modulation by DM | Proteinuria | GFR | DM |
| Malyszko et al. (2009) [56] | 100 | Tx | Higher than in healthy controls | VCAM, CRP, PTH | GFR, albumin | Gender, comorbidities, medication |
| Malyszko et al. (2010) [57] | 75/40 | HD/CAPD | Clearance modulated by RRT type, visfatin could be the link between inflammation and adipocytokines | TG, hsCRP, IL-6, TNF, ICAM, VCAM, CD146, HD vin | GFR | |
| Mu et al. (2011) [58] | 117 | CKD | Visfatin may play an important role in uremia-related atherosclerosis | ED, hsCRP, TG, LDL | GFR, FMD, HDL | |
| Yilmaz et al. (2008) [59] | 58 | Tx | Visfatin levels decline after Tx | ED, hsCRP | GFR, FMD | Medication |
| Yilmaz et al. (2008) [60] | 406 | CKD | Visfatin is associated with ED independently from inflammation | ED, hsCRP | GFR, FMD | |
| (D) Resistin | ||||||
| Akagun et al. (2014) [61] | 69 | HD | Increased in HD patients with failed renal allografts | TNF, IL-6, hsCRP | Albumin | |
| Chung et al. (2012) [62] | 100 | HD | Low resistin levels independently predict poor hospitalization-free survival | IL-6 | ||
| Dan et al. (2014) [63] | 96 | CKD | Serum resistin is higher in the CKD population | |||
| Filippidis et al. (2005) [64] | 33 | HD | HD does not effect the resistin levels, kidney plays a role in elimination, did not reduce insulin sensitivity | BMI, body fat, HOMA-R, insulin | ||
| Kawamura et al. (2010) [65] | 3192 | CKD | Serum resistin is higher in CKD population. | hsCRP, TG, HOMA | GFR, HDL | BMI |
| Kaynar et al. (2014) [66] | 150 | Tx | Resistin is not elevated in Tx patients | PEW | ||
| Kielstein et al. (2003) [67] | 30 | HD | Resistin levels depend mainly on GFR and its levels do not modulate insulin sensitivity | Homocysteine, age | GFR | Insulin, leptin, BMI, waistCX |
| Malyszko et al. (2006) [68] | 96 | Tx | Kidney function is a major determinant of elevating resistin and inflammation | hsCRP, IL-6, RBCc, WBC, VCAM | GFR | |
| Marouga et al. (2013) [69] | 80 | CKD | Resistin may be a part of the reverse epidemiology phenomenon of CKD patients | TNF, hsCRP | Alb, GFR, Htc, BMI, leptin, HOMA | HOMA, BMI, cholesterine, leptin |
| Oltean et al. (2013) [70] | 63 | DBD | High resistin level in DABD causes delayed graft function | |||
| Spoto et al. (2013) [71] | 231 | HD | Resistin predicts death depending on ADPN level | hsCRP | ADPN | Leptin, HOMA |
abd.circ., abdominal circumference; ACE, angiotensin-converting enzyme; ADMA, asymmetric dimethylarginine; ADPN, adiponectin; Alb, albumin; ARB, angiotensin receptor blocker; BF, body fat; BMD, bone mineral density; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CCI, chronic coronary insufficiency; CKD, chronic kidney disease; CRP, C-reactive protein; CVD, cardiovascular disease; DABD, donation after brain death; DGF, delayed graft function; DM, diabetes mellitus; ED, endothelial dysfunction; FMD, fibromuscular dysplasia; GFR, glomerular filtration rate; HD, hemodialysis; HDL, high density lipoprotein; Hgb, hemoglobin; HOMA, homeostasis model assessment; hsCRP, high-sensitivity C-reactive protein; ICAM, intracellular adhesion molecule; IGT, impaired glucose tolerance; IL, interleukin; LDL, low-density lipoprotein; LVH, left ventricular hypertrophy; MetSy, metabolic syndrome; NODAT, new-onset diabetes after transplantation; PAPP-A, pregnancy-associated plasma protein A; PEW, protein energy wasting; PTH, parathyroid hormone; RBC, red blood cell; RRT, renal replacement therapy; RTR, renal transplant recipients; SNP, single nucleotide polymorphism; TSAT, transferrin saturation; TG, triglyceride; TNF, tumor necrosis factor; Tx, transplantation; vitD, vitamin D; VCAM, vascular cell adhesion molecule; waistCX, waist circumference; WBC, white blood cell.
Leptin was first described as a biomarker of obesity two decades ago; it is a 16-kDa peptide product of the obese gene predominantly secreted by white adipose tissue with the primary function of modulating hunger and satiety [72, 73]. Leptin crosses the blood–brain barrier and inhibits neuropeptide Y neurons. I also activates the sympathetic neuron system in the hypothalamus and increases circulating sympathetic hormone levels [74–76]. Leptin acts upon the Ob leptin receptors and activates tyrosine kinase and intracellular pathways [72, 75].
Leptin's counterpart is adiponectin, which negatively correlates with nutritional parameters, suggesting that higher adiponectin levels are observed in healthy individuals. It is a 30-kDa plasma protein synthetized within adipose tissue, and it circulates in the blood as low and high molecular weight isoforms [78]. It activates the downstream signaling pathways through the AdipoR1 receptor, found in kidney cells and skeletal muscle, as well as the AdipoR2 receptor, located in the liver. In the general population, it is considered to be an anti-inflammatory adipocytokine that improves insulin sensitivity and offers cardioprotective benefits [79].
One of the largest adipocytokines is visfatin (52 kDa), which is largely synthesized and secreted by granulocytes and has recently been found to be an insulin sensitizer [56, 80]. Interestingly, endothelial cells within the uremic milieu have also been found to secrete visfatin [81]. While the exact pathways and functions of visfatin are still under investigation, it most likely activates insulin-like receptors and the tyrosine kinase pathway and has insulin-like, pro-inflammatory and anti-apoptopic actions [56, 80].
Resistin is another adipocytokine with metabolic functions and is known to cause insulin resistance. It is a 12.5-kDa cysteine-rich protein that is synthesized in different isoforms by macrophages [82–85]. It acts as a pro-inflammatory factor that increases the production of inflammatory cytokines and elevates the expression of cell adhesion molecules. While its exact biologic functions are still being elucidated, higher resistin levels have been associated with chronic inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease, and resistin may play a role in the pathophysiology of atherosclerosis and endothelial cell injury [86, 87].
There are other adipocytokines, such as apelin, chemerin and omentin, that will not be discussed in detail in this article. These hormones have been recently discovered, and they are primarily expressed in visceral adipose tissue. For example, apelin synthesis is upregulated by nutritional and inflammatory markers, and it is negatively correlated with endothelial dysfunction [88]. In KTRs, lower apelin levels were associated with underlying cardiovascular disease (CVD) [89]. Moreover, lower apelin levels were observed and were found to be associated with endothelial damage and inflammation [89]. In addition, chemerin affects adipocyte differentiation and insulin signaling, and, despite positive correlations with inflammatory parameters and dyslipidemia, elevated levels have been associated with a survival advantage in dialysis patients [90]. Lastly, omentin's gene locus has been linked to diabetes and it is negatively correlated with inflammatory and nutritional parameters and positively correlated with plasma adiponectin levels, suggesting a protective role [91, 92].
Less is known about adipocytokines' functions and associations with outcomes within KTRs. To better inform the field, we analyzed these associations using one of the largest prevalent KTR cohorts (MINIT-HU) with measured adipocytokines [93–98]. In this study, we collected sociodemographic information, clinical parameters, medical and transplant history and laboratory data from 993 prevalent KTRs who were followed within a single ambulatory transplant clinic at the Department of Transplantation and Surgery, Faculty of Medicine, Semmelweis University in Budapest, Hungary, during the period of 31 December 2006–31 December 2007 [93–98]. Table 2 shows the correlations of the measured adipocytokines in this population, which will be discussed later in this article.
Table 2.
Correlations in 988 renal transplant recipients
| Leptin |
Adiponectin |
Resistin |
||||
|---|---|---|---|---|---|---|
| Correlation coefficient | P-value | Correlation coefficient | P-value | Correlation coefficient | P-value | |
| Demographic data | ||||||
| Age | 0.08 | 0.02 | 0.08 | <0.01 | −0.05 | 0.12 |
| Charlson comorbidity index | 0.03 | 0.43 | 0.13 | <0.01 | <0.01 | 0.89 |
| ESRD time | −0.02 | 0.58 | 0.09 | <0.01 | 0.1 | <0.01 |
| Kidney-related parameters | ||||||
| eGFR | −0.22 | <0.01 | −0.25 | <0.01 | −0.45 | <0.01 |
| Inflammatory markers | ||||||
| TNF-α | 0.06 | 0.08 | 0.07 | 0.04 | 0.21 | <0.01 |
| CRP | 0.09 | <0.01 | 0.02 | 0.55 | 0.18 | <0.01 |
| IL-6 | 0.09 | <0.01 | 0.08 | 0.01 | 0.11 | <0.01 |
| Nutritional parameters | ||||||
| BMI | 0.48 | <0.01 | −0.19 | <0.01 | −0.11 | <0.01 |
| Abdominal circumference | 0.31 | <0.01 | −0.22 | <0.01 | −0.08 | 0.02 |
| HDL cholesterol | −0.02 | 0.54 | 0.34 | <0.01 | −0.13 | <0.01 |
| LDL cholesterol | 0.02 | 0.44 | −0.01 | 0.70 | −0.12 | <0.01 |
| Cholesterol | 0.10 | 0.00 | 0.12 | <0.01 | −0.1 | <0.01 |
| Adipocytokines | ||||||
| Leptin | −0.04 | 0.18 | 0.0472 | 0.1402 | ||
| Resistin | 0.05 | 0.14 | 0.13 | <0.01 | ||
| Adiponectin | −0.04 | 0.18 | 0.13 | <0.01 | ||
| Transplantation-related data | ||||||
| Cold ischemic time | 0.06 | 0.07 | 0.07 | 0.03 | 0.07 | 0.04 |
| PRA mean | 0.04 | 0.24 | 0.05 | 0.14 | 0.06 | 0.06 |
| HLA mismatch | −0.07 | 0.03 | 0.02 | 0.45 | −0.04 | 0.17 |
ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate; CRP, C-reactive protein; TNF, tumor necrosis factor; IL, interleukin; BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PRA, panel reactive antibodies; HLA, human leukocyte antigen.
Levels of adipocytokines in the serum
Adipocytokine concentrations are typically measured in serum, although normal ranges have not yet been defined in KTRs. Inflammation may potentially induce production, and given that the kidneys play a primary role in many of these hormones' clearance, impaired renal function may lead to higher circulating adipocytokine levels.
Prior data have shown that the kidney function has a modulating effect on leptin levels. Cumin et al. [99] demonstrated that leptin clearance is eliminated following bilateral nephrectomy in rats. Additionally, studies also suggest that leptin is filtered through the glomerulus and degraded in the proximal tubule by megalin [73, 100, 101]. In addition, small proportions of leptin have been observed in urine in animals as well as humans [102–104]. These findings indicate that in KTRs, leptin levels may be elevated in the context of impaired renal function and possibly by the uremic milieu [105, 106]. In the non-obese population, levels typically range from 5 to 10 ng/mL, while in obese subjects levels may be 10-fold higher in healthy persons with normal kidney function [107, 108]. In patients with impaired kidney function, hyperleptinemia with levels up to 490 ng/mL have been observed [109]. Some studies suggest that uremia may be associated with higher leptin levels, while others suggest that only nutritional parameters modulate these hormones [110–112].
In contrast, leptin's counterpart, adiponectin, is downregulated in patients with obesity. In diabetic populations, plasma concentrations typically range between 5 and 30 µg/mL, with higher levels observed in those who are female and of older age [113, 114]. Adiponectin is metabolized by the liver, and metabolites are eliminated by the kidneys [115]. Adiponectin molecules may be filtered via the glomerulus, and they are detected in renal arteriole muscle and endothelial cells, as well as within proximal and distal tubules. Proximal tubule cells can synthesize and secrete adiponectin and can therefore be measured in urine [116, 117]. In patients with impaired kidney function, serum adiponectin levels are elevated. The uremic milieu and pro-inflammatory cytokines impair adiponectin synthesis as biofeedback for impaired renal elimination [118].
While the pathways by which visfatin is metabolized and eliminated are still under investigation, it is thought that the kidney plays an important role in its biodegradation. In a study of metabolic syndrome patients, the mean ± standard deviation of visfatin concentrations was 3.8 ± 8.8 ng/mL [119]. Visfatin levels are typically elevated in patients with CKD, including those undergoing hemodialysis, although a recent large cohort study suggested that levels only correlated with inflammation and insulin resistance and not with kidney function [50, 120]. Moreover, in a study of >3000 patients by Kocelak et al. [121], it was found that visfatin levels were not associated with kidney function, although the cohort was largely comprised of elderly patients. In a study of KTRs, higher visfatin concentrations were observed (37 ± 14 ng/mL), although higher levels were also observed in the healthy control group (28 ± 11 ng/mL) [56].
The normal range of resistin has not been defined, although in one study of healthy volunteers by Spoto et al. [122] it was found that levels ranged between 1.2 and 29.9 ng/mL. Some studies suggest that resistin functions as a pro-inflammatory cytokine and its serum level may be elevated by inflammatory parameters [123]. In CKD patients, strong correlations between resistin and kidney function have been observed [124]. Futhermore, Malyszko et al. [125] demonstrated that hemodialysis patients with residual kidney function had significantly lower resistin levels than those without residual kidney function, supporting the hypothesis that resistin is cleared via the kidney.
Based on these data (Table 1), there does not appear to be a strong, if any, correlation between leptin and glomerular filtration rate, although there does appear to be a correlation between leptin and nutritional parameters. In contrast, adiponectin levels are decreased in obese patients, but higher levels are observed with impaired kidney function. Kidney function, as well as inflammation, also appears to have a substantial impact on visfatin and resistin levels. Table 2 also indicates a correlation between adipocytokines and graft function.
Adipocytokines and nutritional status
Adipocytokines modulate appetite and nutritional status in KTR and CKD patients. Leptin is the primary hormone that regulates satiety and maintains body weight. In healthy subjects, an increase in body weight results in higher leptin levels and subsequent reduction in hunger [126]. Higher leptin levels may also lead to anorexia by binding to melanocortin-4 receptors (MC4-R), and inhibition of peripheral MC4-R can reduce muscle atrophy and improve anorexia via insulin-like growth factor-1 (IGF-1) [127]. As such, higher leptin concentrations due to impaired kidney function may contribute to protein energy wasting (PEW).
Adiponectin is considered to be an anti-inflammatory cytokine and also plays a key role in metabolic syndrome. In experimental studies, higher adiponectin levels have been shown to decrease high-density lipoprotein (HDL) levels, vis-à-vis apolipoprotein A1 and dysregulation of hepatocytes [128]. Elevated adiponectin levels may also lead to malnutrition via weight loss due to increased energy expenditure [129]. It has been observed that malnourished patients on renal replacement therapy have higher adiponectin levels [130].
Visfatin is a pro-inflammatory cytokine synthesized by macrophages embedded in visceral adipose tissue. In non-obese individuals, there is a similar degree of visfatin production in subcutaneous and visceral fat tissue, while in obese patients visceral synthesis is more prominent [131, 132]. At this time, the association between visfatin and nutritional status remains unclear [50, 132–136]. In a study by Carrero et al. [50], visfatin was not linked with nutritional parameters in Stage 5 CKD patients, although it was observed that higher serum visfatin levels were associated with increased satiety. Considering that CKD patients have higher visfatin levels, and that visfatin is associated with reduced appetite, it has been suggested that this hormone is part of the PEW syndrome.
Resistin was recently discovered as a hormone that causes insulin resistance in rodents, although these findings are still under investigation in humans. In obese rodents, higher resistin concentrations were observed, which positively correlated with dyslipidemia. It has also been suggested that a reduction of serum resistin levels with antibody neutralization might improve insulin sensitivity [137–139]. Obese individuals have higher resistin levels that persist even with weight gain or loss [140]. It has been suggested that resistin may link PEW to inflammation, since patients with a lower body mass index (BMI) have higher levels of inflammatory factors as well as resistin levels [66, 69].
Adipocytokines in inflammation and their effects on kidney cells
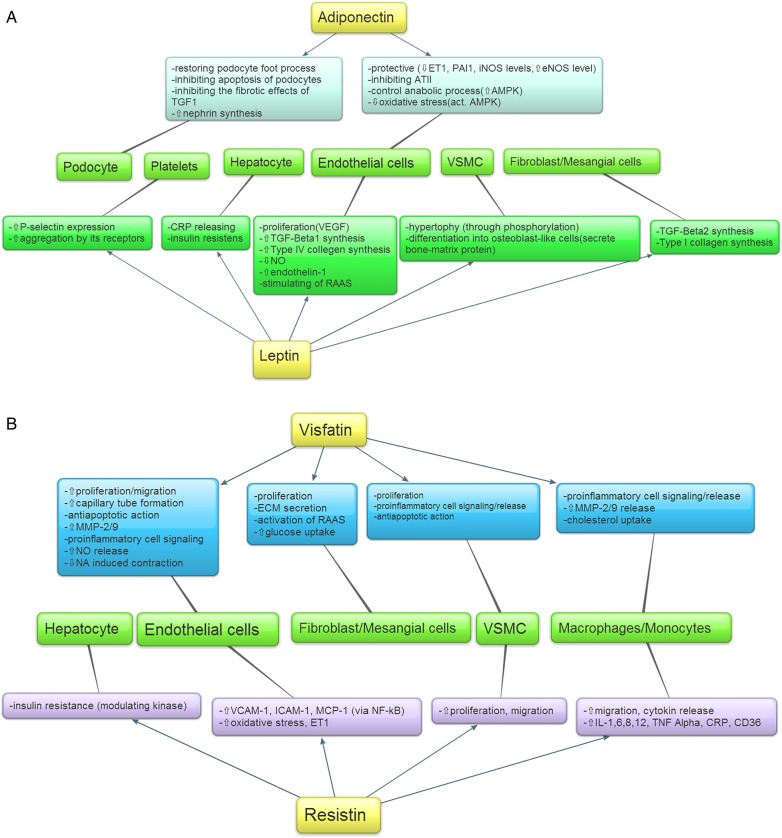
Figure 1 shows the potential mechanisms of adipocytokines on kidney cells. Leptin acts as a pro-inflammatory factor, stimulating the production of pro-inflammatory cytokines [141]. It activates neutrophil and monocyte cell phagocytosis and reactive oxygen species through natural killer cells [142, 143]. Leptin also alters the adaptive immune system by activating CD4+ T lymphocytes and signaling negatively for CD25+ T regulatory cells [144]. It has been hypothesized that the inactivity of regulatory T cells might lead to graft loss since stimulated CD4+CD25+Foxp3 regulatory T cells prevent rejection, although this has not yet been confirmed in studies [145]. In addition, some in vitro studies suggest that stem cell incubation in a leptin-rich environment induces granulocyte formation [146].
Fig. 1.
(A) Effects of leptin and adiponectin on different types of cells. (B) Effects of visfatin and resistin on different types of cells. ATII, angiotensin II; CRP, C-reactive protein; ECM, extracellular matrix; eNOS, extracellular nitric oxide synthetase; ET1, endothelin-1; ICAM, intracellular adhesion molecule; IL, interleukin; iNOS, inducible nitric oxide synthetase; MCP, methyl-accepting chemotaxis protein; MMP, matrix metalloproteinase; Na, sodium; NF-kB, nuclear factor κB; NO, nitric oxide; PAI1, plasminogen activator inhibitor-1; RAAS, renin-angiotensin-aldosterone system; RAAS, renin–angiotensin–aldosterone system; TGF, transforming growth factor; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor; VSMC, vascular smooth muscle cell.
The location of adipocytokine receptors bears particular relevance, given their central role in the activation of intracellular pathways. Leptin receptors are expressed in different areas of the kidney, including the inner medulla and vascular structures in the corticomedullary region [147–149]. Within endothelial cells, leptin increases sodium excretion and diuresis by decreasing sodium–potassium channel activity and reducing nitrogen monoxide (NO) production, inhibiting its protective role [150–154]. Within glomerular endothelial, mesangial and vascular smooth muscle cells, leptin induces proliferation and size increases by activating the mitogen-activated protein kinase (MAPK) pathways [155–157]. Moreover, it induces hypertrophy through different factors causing thickening of the basement membrane. Lastly, leptin also activates the renin–angiotensin–aldosterone system (RAAS) [156, 158, 159]. Observational studies have not found an association between leptin and inflammation in KTRs (Tables 1 and 2).
In contrast to leptin, adiponectin functions as an anti-inflammatory cytokine. Adiponectin enhances anti-inflammatory cytokine synthesis, inhibits angiotensin II–induced inflammation and decreases albuminuria. Recent findings suggest that adiponectin activates nuclear factor κB (NF-κB) transcription. NF-κB is a protein kinase that regulates the immune system through T-cell activity and plays an important role in acute rejection [160]. Furthermore, some but not all data suggest that adiponectin has regulatory effects on T cells. The anti- and pro-inflammatory effects may change according to the molecular isoform of adiponectin [161, 162].
Receptors of adiponectin are also present in kidney cells. Through AdipoR1 it activates the adenosine monophosphate (AMP)–activated protein kinase (AMPK) pathway, inducing protective processes. Within podocytes, adiponectin restores foot processes and inhibits apoptosis, fibrotic effects of TGF-1, nephrin and Nox4 synthesis. Within endothelial cells, it downregulates pro-inflammatory and vasoconstrictor factors and oxidative stress. Furthermore, it elevates endothelial nitric oxide synthase (NOS) concentration and controls anabolic processes [163]. In summary, adiponectin may have protective effects on kidney, although higher levels have not been associated with better graft survival in KTRs [21]. Indeed, adiponectin has demonstrated weak associations with inflammatory parameters in KTRs (Tables 1 and 2).
Resitin's and visfatin's exact receptors are still unknown. Both facilitate the synthesis of inflammatory cytokines within mononuclear and endothelial cells and inhibit the apoptosis of macrophages. They also promote the expression of cell adhesion molecules and chemotaxis [85, 164–168]. Within endothelial cells, they activate NF-κB transcription factors that augment inducible NOS concentrations and increase the release of endothelin-1 and NO. Visfatin also promotes proliferation and capillary tube formation [81, 168–173]. Moreover, visfatin activates superoxide production, inducing glomerular permeability and RAAS [174–176]. Visfatin and resistin are important factors of inflammation in KTRs (Tables 1 and 2).
It should be noted that strong associations have been observed between visfatin and CD68+ macrophages [135]. CD68+ macrophage accumulation has been observed in acute rejection, although further investigation is needed to determine this relationship. Specifically for resistin, Akagun et al. [61] demonstrated that patients receiving hemodialysis with failed kidney allografts had significantly higher resistin levels than those without allografts, suggesting that resistin may play a role in chronic inflammation.
In conclusion, higher adipocytokines observed in the context of impaired kidney function may be due to not only reduced clearance, but also to kidney degradation through inflammatory pathways leading to death and graft loss in KTRs.
Adipocytokines role in transplant comorbidities
Pro-inflammatory adipocytokines may lead to hypertension, insulin resistance, CVD and chronic or acute inflammation [177]. In KTRs with impaired allograft function receiving immunosuppression therapy, adipocytokines may contribute to the development of posttransplant bone disease, anemia, cardiovascular morbidity, new-onset diabetes after transplantation (NODAT) and cancer.
Given that the kidney is one of the major end-organs regulating mineral homeostasis, impaired kidney function may lead to metabolic bone disease. Leptin may in fact accelerate bone metabolism by activating and converting vascular muscle cells into osteoblasts [178]. In epidemiological studies, parathyroid hormone and leptin have shown a negative correlation, and higher leptin levels have been associated with reduced bone turnover [11, 179]. Additionally, leptin causes oversynthesis of fibroblast growth factor 23, subsequently altering the regulation of phosphate homeostasis [180].
Impaired kidney function is the main cause of secondary anemia because of reduced erythropoietin production. Interestingly, leptin receptors may be found in hematopoietic stem cells and higher leptin production has been observed in bone marrow [181]. In epidemiological studies, serum leptin levels are a stronger predictor of erythropoietin sensitivity than body fat and are strongly associated with hemoglobin [182–184]. Erythropoietin stimulating agents (ESAs) administered to CKD patients with higher leptin levels seem to be less effective [182]. A correlation was also observed between serum leptin and erythropoietin resistance in KTRs [96].
Cardiovascular disease is the leading cause of morbidity and mortality among KTRs. Adiponectin presumably has a protective role in the pathogenesis of CVD. Adiponectin receptors are present in endothelial and vascular muscle cells and may reduce oxidative stress, atherosclerosis, fibrosis and proliferation. In the general population, observational studies suggest that lower adiponectin levels are associated with higher cardiovascular mortality [185, 186]. For example, in the LANDMARK 2 study, there was an inverse correlation between adiponectin, inflammation and nutrition in KTRs; furthermore, male patients with lower adiponectin levels had higher CVD risk [30, 187]. However, other data in KTRs have shown positive correlations between higher adiponectin levels and lower survival rates [21]. In a study by Sahin et al. [188], adiponectin levels were significantly higher in patients receiving cyclosporine compared with those on tacrolimus. Malgorzewicz et al. [189] suggested that higher leptin levels in obese patients are a risk factor for CVD and chronic rejection. They also demonstrated that patients with a BMI >25 kg/m2 had significantly higher leptin:adiponectin ratios, and those obese patients with higher leptin:adiponectin ratios had a greater risk for cardiovascular events. According to Nicoletto et al. [16], 5 years after transplantation, patients' leptin, insulin resistance and lipid profiles were similar to the pretransplantation period. This metabolic profile is possibly associated with the higher incidence of CVD observed in the late posttransplantation period. Visfatin and resistin play an important role in the development of endothelial dysfunction that may lead to CVD. For example, higher visfatin levels have been observed in patients with coronary artery disease and atherosclerotic plaques [190–192]. Additionally, Weikert et al. [193] demonstrated that patients in the highest resistin quartile had a 2-fold higher risk of myocardial infarction than patients in the lowest quartile. In a prospective study of 2739 participants from the Framingham Offspring Study followed for 6 years, Frankel et al. [194] demonstrated that higher resistin levels were associated with a higher risk of incident heart failure.
Epidemiological data suggest that the prevalence of NODAT ranges from 4 to 25% in KTRs [195]. In one observational study, lower pretransplantation adiponectin levels were an independent predictor of NODAT [196]. Recently, Romanowski et al. [197] found a significant association between the leptin gene allele and posttransplant diabetes, and it is thought that the 276G/T adiponectin gene polymorphism is also responsible for NODAT. Shu et al. [198] also examined various adipocytokines in KTRs with and without metabolic syndrome and found that metabolic syndrome was associated with significantly higher leptin, adiponectin, resistin and visfatin levels. Observational studies have not yet demonstrated an association between visfatin and glucose metabolism, but in animal studies visfatin has been found to have a protective effect on pancreatic beta cells [119, 199–201]. While resistin's role in diabetes is still under investigation, Shu et al. [198] have demonstrated strong associations between the presence of the metabolic syndrome and higher serum resistin levels.
Microalbuminuria is an important hallmark of worsening kidney function. Yano et al. [202] found that adiponectin is associated with low-grade albuminuria in obese and lean non-diabetic patients. Following multivariable adjustment, urine albumin excretion was significantly higher among patients with lower versus higher adiponectin levels. Several other studies suggest that lower adiponectin levels correlate with albuminuria in obese and diabetic patients [203]. In an observational study including patients with type 1 diabetes mellitus, Prior et al. [204] demonstrated strong positive associations between serum adiponectin and plasma antioxidant status. This correlation was most relevant in patients with albuminuria, suggesting an association between higher adiponectin levels, antioxidant status and reduced oxidative cellular burden.
It is well established that immunosuppression therapy heightens the risk of malignant disorders [205]. In some forms of cancer, adiponectin receptors have been found within malignant cells [206]. Recent data have also shown that lower adiponectin levels are associated with a higher incidence of colorectal and prostate cancer [207]. In spite of these findings, locally elevated adiponectin levels have been observed in chondrosarcoma, where adiponectin has been shown to increase the expression of vascular endothelial growth factor [206].
Adipocytokines and outcomes in KTRs
There are limited data regarding the association between adipocytokines and outcomes in patients with end-stage renal disease. Epidemiological studies suggest that leptin declines after kidney transplantation and that it correlates with nutritional parameters. In CKD patients, lower leptin concentrations predict mortality, although there has been only one study examining the association between leptin, mortality and graft loss in KTRs [208, 209]. Similar to other nutritional factors, higher leptin levels are associated with higher mortality risk in patients undergoing hemodialysis [210]. Another notable finding is that leptin deficiency has been associated with prolonged graft survival, suggesting that lean individuals with lower leptin levels may benefit from kidney transplantation [209]. This hypothesis is supported by data from Fonseca et al. [6], who found that decreases in leptin levels pre- and posttransplantation were associated with less delayed graft function since higher levels may increase the risk of graft loss; however, these relations require more investigation.
There have been comparatively more observational data assessing the role of adiponectin in KTRs. Only a few articles have examined the association between adiponectin and mortality. Similar to studies of adiponectin and mortality in hemodialysis patients [211], we found that higher levels of adiponectin were associated with increased mortality, but not graft loss, despite the protective effects of adiponectin [21]. Roos et al. [39] found that low pretransplant adiponectin levels predicted risk of graft loss, which may be due to atherogenic pathways. It should also be highlighted that, according to Fonseca et al. [6], early graft function is not a primary modifier of adiponectin levels since there were no significant differences in serum adiponectin levels among those with preserved versus impaired graft function.
A number of studies have examined visfatin in patients with impaired renal function (Table 1). However, only two articles have investigated its association with outcomes in KTRs, suggesting that levels decline after transplantation and correlate with the parameters of endothelial dysfunction [56, 59]. Axelsson et al. [48] demonstrated that higher visfatin levels were associated with higher mortality in CKD.
Numerous studies have examined the role of resistin in mortality among patients with impaired kidney function, although few in the KTR population (Table 1). Spoto et al. [122] analyzed the association between resistin and all-cause and cardiovascular mortality among KTRs and found significant associations between serum resistin and death, although serum adiponectin appeared to be a potent effect modifier of this relationship. In contrast, Chung et al. [212] found that lower serum resistin concentrations were associated with lower survival rates in patients on hemodialysis. Our results also suggest that higher resistin levels have a dose-dependent relationship with risk of graft loss and mortality even after multivariable adjustment in KTRs [213]. We found that for every 10 ng/mL elevation of serum resistin level there is a 30% increased mortality risk and 73% increased risk of graft loss [213].
One of the major limitations of these collective studies is that they have typically examined adipocytokine concentrations at a single point in time; therefore, they cannot examine changes in levels over time. In addition, most were of small sample size and were unable to examine cause-specific mortality. At this time, further studies of adipocytokines are needed in the field of transplantation.
Conclusion
Adipocytokine levels are often elevated in patients with kidney disease, including KTRs with impaired graft function. These cytokines have been associated with endothelial dysfunction, inflammation and malnutrition, which may contribute to the degradation of kidney tissue. Higher leptin levels might play a role in rejection through the regulation of macrophages. Adiponectin also has important regulatory effects on the adaptive immune system and may have a role in rejection. In addition, few studies have shown that these adipocytokines are independent predictors of survival in KTRs. Further studies are needed to understand the role of adipocytokines in the nutritional status, graft function and survival of KTRs.
Conflict of interest statement
None declared.
References
- 1.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol 2006; 6: 772–783 [DOI] [PubMed] [Google Scholar]
- 2.Agras PI, Baskin E, Saatci U et al. Relationship between leptin and bone mineral density in renal transplant recipients. Transplant Proc 2005; 37: 3106–3108 [DOI] [PubMed] [Google Scholar]
- 3.Agras PI, Saatci U, Baskin E et al. Hyperleptinemia and its relation with peripheral C34(+)CD7(+) stem cells in renal transplant recipients. Transpl Immunol 2006; 15: 241–245 [DOI] [PubMed] [Google Scholar]
- 4.Baczkowska T, Soin J, Soluch L et al. The role of leptin in body mass index increase in renal allograft recipients. Transplant Proc 2000; 32: 1331–1332 [DOI] [PubMed] [Google Scholar]
- 5.El Haggan W, Chauveau P, Barthe N et al. Serum leptin, body fat, and nutritional markers during the six months post-kidney transplantation. Metabolism 2004; 53: 614–619 [DOI] [PubMed] [Google Scholar]
- 6.Fonseca I, Oliveira JC, Santos J et al. Leptin and adiponectin during the first week after kidney transplantation: biomarkers of graft dysfunction? Metabolism 2015; 64: 202–207 [DOI] [PubMed] [Google Scholar]
- 7.Kagan A, Haran N, Leschinsky L et al. Serum concentrations of leptin in heart, liver and kidney transplant recipients. Isr Med Assoc J 2002; 4: 213–217 [PubMed] [Google Scholar]
- 8.Kayacan SM, Yildiz A, Kazancioglu R et al. The changes in serum leptin, body fat mass and insulin resistance after renal transplantation. Clin Transplant 2003; 17: 63–68 [DOI] [PubMed] [Google Scholar]
- 9.Kokot F, Adamczak M, Wiecek A. Plasma leptin concentration in kidney transplant patients during the early post-transplant period. Nephrol Dial Transplant 1998; 13: 2276–2280 [DOI] [PubMed] [Google Scholar]
- 10.Kokot F, Adamczak M, Wiecedil et al. Plasma immunoreactive leptin and neuropeptide Y levels in kidney transplant patients. Am J Nephrol 1999; 19: 28–33 [DOI] [PubMed] [Google Scholar]
- 11.Kovesdy CP, Molnar MZ, Czira ME et al. Associations between serum leptin level and bone turnover in kidney transplant recipients. Clin J Am Soc Nephrol 2010; 5: 2297–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landt M, Brennan DC, Parvin CA et al. Hyperleptinaemia of end-stage renal disease is corrected by renal transplantation. Nephrol Dial Transplant 1998; 13: 2271–2275 [DOI] [PubMed] [Google Scholar]
- 13.Lee MC, Lee CJ, Ho GJ et al. Hyperleptinemia positively correlated with metabolic syndrome in renal transplant recipients. Clin Transplant 2010; 24: E124–E1249 [DOI] [PubMed] [Google Scholar]
- 14.Lee MC, Chen YC, Ho GJ et al. Serum leptin levels positively correlate with peripheral arterial stiffness in kidney transplantation patients. Transplant Proc 2014; 46: 353–358 [DOI] [PubMed] [Google Scholar]
- 15.Malyszko J, Malyszko JS, Pawlak K et al. Correlations between leptin, body composition, bone mineral density, and bone metabolism in kidney transplant recipients. Transplant Proc 2005; 37: 2151–2153 [DOI] [PubMed] [Google Scholar]
- 16.Nicoletto BB, Souza GC, Goncalves LF et al. Leptin, insulin resistance, and metabolic changes 5 years after renal transplantation. J Ren Nutr 2012; 22: 440–449 [DOI] [PubMed] [Google Scholar]
- 17.Rafieian-Kopaei M, Nasri H. Serum leptin in renal transplant patients. JRIP 2013; 2: 55–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souza GC, Costa CA, Goncalves LF et al. Leptin serum levels in the first year post-renal transplantation. Transplant Proc 2007; 39: 439–440 [DOI] [PubMed] [Google Scholar]
- 19.Adamczak M, Szotowska M, Chudek J et al. Plasma adiponectin concentration in patients after successful kidney transplantation–a single-center, observational study. Clin Nephrol 2007; 67: 381–390 [DOI] [PubMed] [Google Scholar]
- 20.Adamczak M, Blach A, Kolonko A et al. Plasma adiponectin concentration and left ventricular hypertrophy in kidney transplant patients. Clin Transplant 2011; 25: 561–568 [DOI] [PubMed] [Google Scholar]
- 21.Alam A, Molnar MZ, Czira ME et al. Serum adiponectin levels and mortality after kidney transplantation. Clin J Am Soc Nephrol 2013; 8: 460–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bayes B, Granada ML, Pastor MC et al. Obesity, adiponectin and inflammation as predictors of new-onset diabetes mellitus after kidney transplantation. Am J Transplant 2007; 7: 416–422 [DOI] [PubMed] [Google Scholar]
- 23.Bayes B, Granada ML, Lauzurica R et al. Effect of low doses of atorvastatin on adiponectin, glucose homeostasis, and clinical inflammatory markers in kidney transplant recipients. Transplant Proc 2005; 37: 3808–3812 [DOI] [PubMed] [Google Scholar]
- 24.Canas L, Bayes B, Granada ML et al. Is adiponectin a marker of preclinical atherosclerosis in kidney transplantation? Clin Transplant 2012; 26: 259–266 [DOI] [PubMed] [Google Scholar]
- 25.Chitalia N, Raja RB, Bhandara T et al. Serum adiponectin and cardiovascular risk in chronic kidney disease and kidney transplantation. J Nephrol 2010; 23: 77–84 [PubMed] [Google Scholar]
- 26.Chudek J, Adamczak M, Karkoszka H et al. Plasma adiponectin concentration before and after successful kidney transplantation. Transplant Proc 2003; 35: 2186–2189 [DOI] [PubMed] [Google Scholar]
- 27.Chudek J, Szotowska M, Karkoszka H et al. Genotypes of renin-angiotensin system and plasma adiponectin concentration in kidney transplant patients. Ann Transplant 2013; 18: 593–603 [DOI] [PubMed] [Google Scholar]
- 28.Ho GJ, Lee MC, Lee CJ et al. Hypoadiponectinemia correlates with arterial stiffness in kidney transplantation patients. Clin Exp Nephrol 2015; 19: 534–541 [DOI] [PubMed] [Google Scholar]
- 29.Idorn T, Hornum M, Bjerre M et al. Plasma adiponectin before and after kidney transplantation. Transpl Int 2012; 25: 1194–1203 [DOI] [PubMed] [Google Scholar]
- 30.Kaisar MO, Armstrong K, Hawley C et al. Adiponectin is associated with cardiovascular disease in male renal transplant recipients: baseline results from the LANDMARK 2 study. BMC Nephrol 2009; 10: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang ES, Magkos F, Kim BS et al. Variants of the adiponectin and adiponectin receptor-1 genes and posttransplantation diabetes mellitus in renal allograft recipients. J Clin Endocrinol Metab 2012; 97: E129–E135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulshrestha S, Ojo AO, Luan FL.. Metabolic syndrome, vitamin D deficiency and hypoadiponectinemia among nondiabetic patients early after kidney transplantation. Am J Nephrol 2013; 37: 399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee MC, Lee CJ, Chou KC et al. Hypoadiponectinemia correlates with metabolic syndrome in kidney transplantation patients. Transplant Proc 2011; 43: 2601–2605 [DOI] [PubMed] [Google Scholar]
- 34.Leibowitz A, Peleg E, Ben-David A et al. Normal adiponectin levels in kidney transplant patients with hypertension. Clin Transplant 2013; 27: 562–566 [DOI] [PubMed] [Google Scholar]
- 35.Malyszko J, Malyszko JS, Brzosko S et al. Markers of endothelial cell activation/injury: CD146 and thrombomodulin are related to adiponectin in kidney allograft recipients. Am J Nephrol 2005; 25: 203–210 [DOI] [PubMed] [Google Scholar]
- 36.Nicoletto BB, Souza GC, Fonseca NK et al. Association between 276G/T adiponectin gene polymorphism and new-onset diabetes after kidney transplantation. Transplantation 2013; 96: 1059–1064 [DOI] [PubMed] [Google Scholar]
- 37.Nishimura K, Kishikawa H, Kato T et al. Tacrolimus and angiotensin receptor blockers associated with changes in serum adiponectin level in new-onset diabetes after renal transplantation: single-center cross-sectional analysis. Transpl Int 2009; 22: 694–701 [DOI] [PubMed] [Google Scholar]
- 38.Prasad GV, Vorobeichik L, Nash MM et al. Lower total and percent of high-molecular-weight adiponectin concentration in South Asian kidney transplant recipients. Clin Kidney J 2012; 5: 124–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roos M, Baumann M, Liu D et al. Low pre-transplant adiponectin multimers are associated with adverse allograft outcomes in kidney transplant recipients a 3-year prospective study. Regul Pept 2012; 178: 11–15 [DOI] [PubMed] [Google Scholar]
- 40.Sethna CB, Leonard MB, Gallagher PR et al. Serum adiponectin levels and ambulatory blood pressure monitoring in pediatric renal transplant recipients. Transplantation 2009; 88: 1030–1037 [DOI] [PubMed] [Google Scholar]
- 41.Shen YY, Charlesworth JA, Kelly JJ et al. The effect of renal transplantation on adiponectin and its isoforms and receptors. Metabolism 2007; 56: 1201–1208 [DOI] [PubMed] [Google Scholar]
- 42.Shu KH, Tsai IC, Ho HC et al. Serum adiponectin levels in renal transplant recipients with and without metabolic syndrome. Transplant Proc 2012; 44: 676–679 [DOI] [PubMed] [Google Scholar]
- 43.Taherimahmoudi M, Ahmadi H, Mehrsai A et al. Plasma adiponectin concentration and insulin resistance: role of successful kidney transplantation. Transplant Proc 2010; 42: 797–800 [DOI] [PubMed] [Google Scholar]
- 44.Teplan V, Schuck O, Stollova M et al. Obesity and adiponectin after kidney transplantation. Acta Physiol Hung 2007; 94: 149–157 [DOI] [PubMed] [Google Scholar]
- 45.Teplan V, Schuck O, Racek J et al. Asymmetric dimethylarginine and adiponectin after renal transplantation: role of obesity. J Ren Nutr 2008; 18: 154–157 [DOI] [PubMed] [Google Scholar]
- 46.Yilmaz MI, Saglam M, Caglar K et al. Endothelial functions improve with decrease in asymmetric dimethylarginine (ADMA) levels after renal transplantation. Transplantation 2005; 80: 1660–1666 [DOI] [PubMed] [Google Scholar]
- 47.Yu AR, Xin HW, Wu XC et al. Adiponectin gene polymorphisms are associated with posttransplantation diabetes mellitus in Chinese renal allograft recipients. Transplant Proc 2011; 43: 1607–1611 [DOI] [PubMed] [Google Scholar]
- 48.Axelsson J, Witasp A, Carrero JJ et al. Circulating levels of visfatin/pre-B-cell colony-enhancing factor 1 in relation to genotype, GFR, body composition, and survival in patients with CKD. Am J Kidney Dis 2007; 49: 237–244 [DOI] [PubMed] [Google Scholar]
- 49.Bessa SS, Hamdy SM, El-Sheikh RG. Serum visfatin as a non-traditional biomarker of endothelial dysfunction in chronic kidney disease: an Egyptian study. Eur J Intern Med 2010; 21: 530–535 [DOI] [PubMed] [Google Scholar]
- 50.Carrero JJ, Witasp A, Stenvinkel P et al. Visfatin is increased in chronic kidney disease patients with poor appetite and correlates negatively with fasting serum amino acids and triglyceride levels. Nephrol Dial Transplant 2010; 25: 901–906 [DOI] [PubMed] [Google Scholar]
- 51.Eleftheriadis T, Pissas G, Remoundou M et al. Increased visfatin in hemodialysis patients is associated with decreased demands for recombinant human erythropoietin. Ren Fail 2013; 35: 1399–1403 [DOI] [PubMed] [Google Scholar]
- 52.Erten Y, Ebinc FA, Ebinc H et al. The relationship of visfatin levels to inflammatory cytokines and left ventricular hypertrophy in hemodialysis and continuous ambulatory peritoneal dialysis patients. Ren Fail 2008; 30: 617–623 [DOI] [PubMed] [Google Scholar]
- 53.Kato A, Odamaki M, Ishida J et al. Relationship between serum pre-B cell colony-enhancing factor/visfatin and atherosclerotic parameters in chronic hemodialysis patients. Am J Nephrol 2009; 29: 31–35 [DOI] [PubMed] [Google Scholar]
- 54.Lu YC, Hsu CC, Yu TH et al. Association between visfatin levels and coronary artery disease in patients with chronic kidney disease. Iran J Kidney Dis 2013; 7: 446–452 [PubMed] [Google Scholar]
- 55.Mahmood N, Junejo AM, Jamal Q et al. Association of visfatin with chronic kidney disease in a cohort of patients with and without diabetes. J Pak Med Assoc 2010; 60: 922–926 [PubMed] [Google Scholar]
- 56.Malyszko J, Malyszko JS, Mysliwiec M. Visfatin, a new adipocytokine, is predominantly related to inflammation/endothelial damage in kidney allograft recipients. Transplant Proc 2009; 41: 150–153 [DOI] [PubMed] [Google Scholar]
- 57.Malyszko J, Malyszko JS, Mysliwiec M. Visfatin and endothelial function in dialyzed patients. Nephrology 2010; 15: 190–196 [DOI] [PubMed] [Google Scholar]
- 58.Mu J, Feng B, Ye Z et al. Visfatin is related to lipid dysregulation, endothelial dysfunction and atherosclerosis in patients with chronic kidney disease. J Nephrol 2011; 24: 177–184 [DOI] [PubMed] [Google Scholar]
- 59.Yilmaz MI, Saglam M, Carrero JJ et al. Normalization of endothelial dysfunction following renal transplantation is accompanied by a reduction of circulating visfatin/NAMPT. A novel marker of endothelial damage? Clin Transplant 2009; 23: 241–248 [DOI] [PubMed] [Google Scholar]
- 60.Yilmaz MI, Saglam M, Carrero JJ et al. Serum visfatin concentration and endothelial dysfunction in chronic kidney disease. Nephrol Dial Transplant 2008; 23: 959–965 [DOI] [PubMed] [Google Scholar]
- 61.Akagun T, Caliskan Y, Yazici H et al. Elevated resistin levels are associated with inflammation in hemodialysis patients with failed renal allografts. Int J Artif Organs 2014; 37: 358–363 [DOI] [PubMed] [Google Scholar]
- 62.Chung W, Jung ES, Shin D et al. Low resistin level is associated with poor hospitalization-free survival in hemodialysis patients. J Korean Med Sci 2012; 27: 377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dan S, Aditya P, Banerjee P et al. Effect of chronic kidney disease on serum resistin level. Niger J Clin Pract 2014; 17: 735–738 [DOI] [PubMed] [Google Scholar]
- 64.Filippidis G, Liakopoulos V, Mertens PR et al. Resistin serum levels are increased but not correlated with insulin resistance in chronic hemodialysis patients. Blood Purif 2005; 23: 421–428 [DOI] [PubMed] [Google Scholar]
- 65.Kawamura R, Doi Y, Osawa H et al. Circulating resistin is increased with decreasing renal function in a general Japanese population: the Hisayama Study. Nephrol Dial Transplant 2010; 25: 3236–3240 [DOI] [PubMed] [Google Scholar]
- 66.Kaynar K, Kural BV, Ulusoy S et al. Is there any interaction of resistin and adiponectin levels with protein-energy wasting among patients with chronic kidney disease. Hemodial Int 2014; 18: 153–162 [DOI] [PubMed] [Google Scholar]
- 67.Kielstein JT, Becker B, Graf S et al. Increased resistin blood levels are not associated with insulin resistance in patients with renal disease. Am J Kidney Dis 2003; 42: 62–66 [DOI] [PubMed] [Google Scholar]
- 68.Malyszko J, Malyszko JS, Pawlak K et al. Resistin, a new adipokine, is related to inflammation and renal function in kidney allograft recipients. Transplant Proc 2006; 38: 3434–3436 [DOI] [PubMed] [Google Scholar]
- 69.Marouga A, Dalamaga M, Kastania AN et al. Correlates of serum resistin in elderly, non-diabetic patients with chronic kidney disease. Clin Lab 2012; 59: 1121–1128 [DOI] [PubMed] [Google Scholar]
- 70.Oltean S, Pullerits R, Floden A et al. Increased resistin in brain dead organ donors is associated with delayed graft function after kidney transplantation. J Transl Med 2013; 11: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spoto B, Mattace-Raso F, Sijbrands E et al. Resistin and all-cause and cardiovascular mortality: effect modification by adiponectin in end-stage kidney disease patients. Nephrol Dial Transplant 2013; 28(Suppl 4): iv181–iv187 [DOI] [PubMed] [Google Scholar]
- 72.Attoub S, Noe V, Pirola L et al. Leptin promotes invasiveness of kidney and colonic epithelial cells via phosphoinositide 3-kinase-, Rho-, and Rac-dependent signaling pathways. FASEB J 2000; 14: 2329–2338 [DOI] [PubMed] [Google Scholar]
- 73.Hama H, Saito A, Takeda T et al. Evidence indicating that renal tubular metabolism of leptin is mediated by megalin but not by the leptin receptors. Endocrinology 2004; 145: 3935–3940 [DOI] [PubMed] [Google Scholar]
- 74.Yang R, Barouch LA. Leptin signaling and obesity cardiovascular consequences. Circ Res 2007; 101: 545–559 [DOI] [PubMed] [Google Scholar]
- 75.Bjørbæk C, Buchholz RM, Davis SM et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem 2001; 276: 4747–4755 [DOI] [PubMed] [Google Scholar]
- 76.Simonds SE, Cowley MA, Enriori PJ. Leptin increasing sympathetic nerve outflow in obesity: a cure for obesity or a potential contributor to metabolic syndrome? Adipocyte 2012; 1: 177–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhillon SS, Belsham DD. Leptin differentially regulates NPY secretion in hypothalamic cell lines through distinct intracellular signal transduction pathways. Regul Pept 2011; 167: 192–200 [DOI] [PubMed] [Google Scholar]
- 78.Shen YY, Hughes JT, Charlesworth JA et al. Adiponectin is present in the urine in its native conformation, and specifically reduces the secretion of MCP-1 by proximal tubular cells. Nephrology (Carlton) 2008; 13: 405–410 [DOI] [PubMed] [Google Scholar]
- 79.Liu M, Liu F. Regulation of adiponectin multimerization, signaling and function. Best Pract Res Clin Endocrinol Metab 2014; 28: 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stastny J, Bienertova-Vasku J, Vasku A. Visfatin and its role in obesity development. Diabetes Metab Syndr 2012; 6: 120–124 [DOI] [PubMed] [Google Scholar]
- 81.Romacho T, Villalobos LA, Cercas E et al. Visfatin as a novel mediator released by inflamed human endothelial cells. PLoS One 2013; 8: e78283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aruna B, Ghosh S, Singh AK et al. Human recombinant resistin protein displays a tendency to aggregate by forming intermolecular disulfide linkages. Biochemistry 2003; 42: 10554–10559 [DOI] [PubMed] [Google Scholar]
- 83.Patel SD, Rajala MW, Rossetti L et al. Disulfide-dependent multimeric assembly of resistin family hormones. Science 2004; 304: 1154–1158 [DOI] [PubMed] [Google Scholar]
- 84.Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and type II diabetes. Clin Sci (Lond) 2005; 109: 243–256 [DOI] [PubMed] [Google Scholar]
- 85.Reilly MP, Lehrke M, Wolfe ML et al. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 2005; 111: 932–939 [DOI] [PubMed] [Google Scholar]
- 86.Fadda SM, Gamal SM, Elsaid NY et al. Resistin in inflammatory and degenerative rheumatologic diseases. Relationship between resistin and rheumatoid arthritis disease progression. Z Rheumatol 2013; 72: 594–600 [DOI] [PubMed] [Google Scholar]
- 87.Karmiris K, Koutroubakis IE, Xidakis C et al. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis 2006; 12: 100–105 [DOI] [PubMed] [Google Scholar]
- 88.Fujie S, Sato K, Miyamoto-Mikami E et al. Reduction of arterial stiffness by exercise training is associated with increasing plasma apelin level in middle-aged and older adults. PLoS One 2014; 9: e93545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malyszko J, Malyszko JS, Pawlak K et al. Apelin, a novel adipocytokine, in relation to endothelial function and inflammation in kidney allograft recipients. Transplant Proc 2008; 40: 3466–3469 [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto T, Qureshi AR, Anderstam B et al. Clinical importance of an elevated circulating chemerin level in incident dialysis patients. Nephrol Dial Transplant 2010; 25: 4017–4023 [DOI] [PubMed] [Google Scholar]
- 91.Ruster C, Wolf G. Adipokines promote chronic kidney disease. Nephrol Dial Transplant 2013; 28(Suppl 4): iv8–iv14 [DOI] [PubMed] [Google Scholar]
- 92.Miyamoto S, Sharma K. Adipokines protecting CKD. Nephrol Dial Transplant 2013; 28(Suppl 4): iv15–iv22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molnar MZ, Czira ME, Rudas A et al. Association of the malnutrition-inflammation score with clinical outcomes in kidney transplant recipients. Am J Kidney Dis 2011; 58: 101–108 [DOI] [PubMed] [Google Scholar]
- 94.Molnar MZ, Tabak AG, Alam A et al. Serum erythropoietin level and mortality in kidney transplant recipients. Clin J Am Soc Nephrol 2011; 6: 2879–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kovesdy CP, Mucsi I, Czira ME et al. Association of serum phosphorus level with anemia in kidney transplant recipients. Transplantation 2011; 91: 875–882 [DOI] [PubMed] [Google Scholar]
- 96.Molnar MZ, Czira ME, Rudas A et al. Association between the malnutrition-inflammation score and post-transplant anaemia. Nephrol Dial Transplant 2011; 26: 2000–2006 [DOI] [PubMed] [Google Scholar]
- 97.Kovesdy CP, Czira ME, Rudas A et al. Body mass index, waist circumference and mortality in kidney transplant recipients. Am J Transplant 2010; 10: 2644–2651 [DOI] [PubMed] [Google Scholar]
- 98.Molnar MZ, Keszei A, Czira ME et al. Evaluation of the malnutrition-inflammation score in kidney transplant recipients. Am J Kidney Dis 2010; 56: 102–111 [DOI] [PubMed] [Google Scholar]
- 99.Cumin F, Baum HP, Levens N. Leptin is cleared from the circulation primarily by the kidney. Int J Obes Relat Metab Disord 1996; 20: 1120–1126 [PubMed] [Google Scholar]
- 100.Zou Z, Chung B, Nguyen T et al. Linking receptor-mediated endocytosis and cell signaling evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem 2004; 279: 34302–34310 [DOI] [PubMed] [Google Scholar]
- 101.Shah M, Baterina OY, Taupin V. ARH directs megalin to the endocytic recycling compartment to regulate its proteolysis and gene expression. J Cell Biol 2013; 202: 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyer C, Robson D, Rackovsky N. Role of the kidney in human leptin metabolism. Am J Physiol 1997; 273: E903–E907 [DOI] [PubMed] [Google Scholar]
- 103.Cumin F, Baum HP, Levens N. Mechanism of leptin removal from the circulation by the kidney. J Endocrinol 1997; 155: 577–585 [DOI] [PubMed] [Google Scholar]
- 104.Lönnqvist F, Nordfors L, Jansson M et al. Leptin secretion from adipose tissue in women. Relationship to plasma levels and gene expression. J Clin Invest 1997; 99: 2398–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aminzadeh MA, Pahl MV, Barton CH et al. Human uraemic plasma stimulates release of leptin and uptake of tumour necrosis factor-alpha in visceral adipocytes. Nephrol Dial Transplant 2009; 24: 3626–3631 [DOI] [PubMed] [Google Scholar]
- 106.Kalbacher E, Koppe L, Zarrouki B et al. Human uremic plasma and not urea induces exuberant secretion of leptin in 3T3-L1 adipocytes. J Ren Nutr 2011; 21: 72–75 [DOI] [PubMed] [Google Scholar]
- 107.Boden G, Chen X, Mozzoli M et al. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab 1996; 81: 3419–3423 [DOI] [PubMed] [Google Scholar]
- 108.Garibotto G, Russo R, Franceschini R et al. Inter-organ leptin exchange in humans. Biochem Biophys Res Commun 1998; 247: 504–509 [DOI] [PubMed] [Google Scholar]
- 109.Dagogo-Jack S, Ovalle F, Landt M. Hyperleptinemia in patients with end-stage renal disease undergoing continuous ambulatory peritoneal dialysis. Perit Dial Int 1998; 18: 34–40 [PubMed] [Google Scholar]
- 110.Silva MI, Vale BS, Lemos CC et al. Body adiposity index assess body fat with high accuracy in nondialyzed chronic kidney disease patients. Obesity (Silver Spring) 2013; 21: 546–552 [DOI] [PubMed] [Google Scholar]
- 111.Ho KJ, Xue H, Mauro CR et al. Impact of uremia on human adipose tissue phenotype. J Surg Res 2013; 179: 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ruhl CE, Harris TB, Ding J et al. Body mass index and serum leptin concentration independently estimate percentage body fat in older adults. Am J Clin Nutr 2007; 85: 1121–1126 [DOI] [PubMed] [Google Scholar]
- 113.Sharma K, Ramachandrarao S, Qiu G et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 2008; 118: 1645–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Obata Y, Yamada Y, Takahi Y et al. Relationship between serum adiponectin levels and age in healthy subjects and patients with type 2 diabetes. Clin Endocrinol (Oxf) 2013; 79: 204–210 [DOI] [PubMed] [Google Scholar]
- 115.Tacke F, Wustefeld T, Horn R et al. High adiponectin in chronic liver disease and cholestasis suggests biliary route of adiponectin excretion in vivo. J Hepatol 2005; 42: 666–673 [DOI] [PubMed] [Google Scholar]
- 116.Perri A, Vizza D, Lofaro D et al. Adiponectin is expressed and secreted by renal tubular epithelial cells. J Nephrol 2013; 26: 1049–1054 [DOI] [PubMed] [Google Scholar]
- 117.von Eynatten M, Liu D, Hock C et al. Urinary adiponectin excretion a novel marker for vascular damage in type 2 diabetes. Diabetes 2009; 58: 2093–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bruun JM, Lihn AS, Verdich C et al. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab 2003; 285: E527–E533 [DOI] [PubMed] [Google Scholar]
- 119.Chedraui P, Perez-Lopez FR, Escobar GS et al. Circulating leptin, resistin, adiponectin, visfatin, adipsin and ghrelin levels and insulin resistance in postmenopausal women with and without the metabolic syndrome. Maturitas 2014; 79: 86–90 [DOI] [PubMed] [Google Scholar]
- 120.Nusken KD, Petrasch M, Rauh M et al. Active visfatin is elevated in serum of maintenance haemodialysis patients and correlates inversely with circulating HDL cholesterol. Nephrol Dial Transplant 2009; 24: 2832–2838 [DOI] [PubMed] [Google Scholar]
- 121.Kocelak P, Olszanecka-Glinianowicz M, Owczarek A et al. Plasma visfatin/nicotinamide phosphoribosyltransferase (visfatin/NAMPT) concentration is not related to kidney function in elderly subjects. Clin Chem Lab Med 2015; 53: 793–799 [DOI] [PubMed] [Google Scholar]
- 122.Spoto B, Mattace-Raso F, Sijbrands E et al. Resistin and all-cause and cardiovascular mortality: effect modification by adiponectin in end-stage kidney disease patients. Nephrol Dial Transplant 2013; 28(Suppl 4): iv181–iv187 [DOI] [PubMed] [Google Scholar]
- 123.Fargnoli JL, Sun Q, Olenczuk D et al. Resistin is associated with biomarkers of inflammation while total and high-molecular weight adiponectin are associated with biomarkers of inflammation, insulin resistance, and endothelial function. Eur J Endocrinol 2010; 162: 281–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Axelsson J, Bergsten A, Qureshi AR et al. Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int 2006; 69: 596–604 [DOI] [PubMed] [Google Scholar]
- 125.Malyszko J, Malyszko JS, Kozminski P et al. Elevated resistin is related to inflammation and residual renal function in haemodialysed patients. Nephrology (Carlton) 2007; 12: 246–253 [DOI] [PubMed] [Google Scholar]
- 126.Friedman JM. Leptin and the regulation of body weigh. Keio J Med 2011; 60: 1–9 [DOI] [PubMed] [Google Scholar]
- 127.Scholze A, Tepel M. Role of leptin in reverse epidemiology in chronic kidney disease. Semin Dial 2007; 20: 534–538 [DOI] [PubMed] [Google Scholar]
- 128.Liu Q, Yuan B, Lo KA et al. Adiponectin regulates expression of hepatic genes critical for glucose and lipid metabolism. Proc Natl Acad Sci USA 2012; 109: 14568–14573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qi Y, Takahashi N, Hileman SM et al. Adiponectin acts in the brain to decrease body weight. Nat Med 2004; 10: 524–529 [DOI] [PubMed] [Google Scholar]
- 130.Ekramzadeh M, Sohrabi Z, Salehi M et al. Adiponectin as a novel indicator of malnutrition and inflammation in hemodialysis patients. Iran J Kidney Dis 2013; 7: 304–308 [PubMed] [Google Scholar]
- 131.Curat CA, Wegner V, Sengenes C et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006; 49: 744–747 [DOI] [PubMed] [Google Scholar]
- 132.Berndt J, Kloting N, Kralisch S et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes 2005; 54: 2911–2916 [DOI] [PubMed] [Google Scholar]
- 133.Pagano C, Pilon C, Olivieri M et al. Reduced plasma visfatin/pre-B cell colony-enhancing factor in obesity is not related to insulin resistance in humans. J Clin Endocrinol Metab 2006; 91: 3165–3170 [DOI] [PubMed] [Google Scholar]
- 134.Varma V, Yao-Borengasser A, Rasouli N et al. Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipids, and inflammation. J Clin Endocrinol Metab 2007; 92: 666–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chang YC, Chang TJ, Lee WJ et al. The relationship of visfatin/pre-B-cell colony-enhancing factor/nicotinamide phosphoribosyltransferase in adipose tissue with inflammation, insulin resistance, and plasma lipids. Metabolism 2010; 59: 93–99 [DOI] [PubMed] [Google Scholar]
- 136.Wang P, van Greevenbroek MM, Bouwman FG et al. The circulating PBEF/NAMPT/visfatin level is associated with a beneficial blood lipid profile. Pflugers Arch 2007; 454: 971–976 [DOI] [PubMed] [Google Scholar]
- 137.Rajala MW, Qi Y, Patel HR et al. Regulation of resistin expression and circulating levels in obesity, diabetes, and fasting. Diabetes 2004; 53: 1671–1679 [DOI] [PubMed] [Google Scholar]
- 138.Sato N, Kobayashi K, Inoguchi T et al. Adenovirus-mediated high expression of resistin causes dyslipidemia in mice. Endocrinology 2005; 146: 273–279 [DOI] [PubMed] [Google Scholar]
- 139.Steppan CM, Bailey ST, Bhat S et al. The hormone resistin links obesity to diabetes. Nature 2001; 409: 307–312 [DOI] [PubMed] [Google Scholar]
- 140.Park HK, Ahima RS. Resistin in rodents and humans. Diabetes Metab J 2013; 37: 404–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Loffreda S, Yang SQ, Lin HZ et al. Leptin regulates proinflammatory immune responses. FASEB J 1998; 12: 57–65 [PubMed] [Google Scholar]
- 142.Lord GM, Matarese G, Howard JK et al. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 1998; 394: 897–901 [DOI] [PubMed] [Google Scholar]
- 143.Zhao Y, Sun R, You L et al. Expression of leptin receptors and response to leptin stimulation of human natural killer cell lines. Biochem Biophys Res Commun 2003; 300: 247–252 [DOI] [PubMed] [Google Scholar]
- 144.De Rosa V, Procaccini C, Cali G et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity 2007; 26: 241–255 [DOI] [PubMed] [Google Scholar]
- 145.Joffre O, Santolaria T, Calise D et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med 2008; 14: 88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Umemoto Y, Tsuji K, Yang FC et al. Leptin stimulates the proliferation of murine myelocytic and primitive hematopoietic progenitor cells. Blood 1997; 90: 3438–3443 [PubMed] [Google Scholar]
- 147.Martinez-Anso E, Lostao MP, Martinez JA. Immunohistochemical localization of leptin in rat kidney. Kidney Int 1999; 55: 1129–1130 [DOI] [PubMed] [Google Scholar]
- 148.Elis Yildiz S, Deprem T, Karadag Sari E et al. Immunohistochemical distribution of leptin in kidney tissues of melatonin treated diabetic rats. Biotech Histochem 2015; 90: 270–277 [DOI] [PubMed] [Google Scholar]
- 149.Serradeil-Le Gal C, Raufaste D, Brossard G et al. Characterization and localization of leptin receptors in the rat kidney. FEBS Lett 1997; 404: 185–191 [DOI] [PubMed] [Google Scholar]
- 150.Bełtowski J, W jcicka G, Górny D et al. Human leptin administered intraperitoneally stimulates natriuresis and decreases renal medullary Na+, K+-ATPase activity in the rat—impaired effect in dietary-induced obesity. Med Sci Monit 2002; 8: BR221–BR229 [PubMed] [Google Scholar]
- 151.Jackson EK, Li P. Human leptin has natriuretic activity in the rat. Am J Physiol 1997; 272: F333–F338 [DOI] [PubMed] [Google Scholar]
- 152.Vecchione C, Maffei A, Colella S et al. Leptin effect on endothelial nitric oxide is mediated through Akt–endothelial nitric oxide synthase phosphorylation pathway. Diabetes 2002; 51: 168–173 [DOI] [PubMed] [Google Scholar]
- 153.Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol Ren 2002; 282: F777–F784 [DOI] [PubMed] [Google Scholar]
- 154.Bełtowski J, Jamroz-Wiśniewska A, Borkowska E et al. Up-regulation of renal Na+, K+-ATPase: the possible novel mechanism of leptin-induced hypertension. Pol J Pharmacol 2004; 56: 213–222 [PubMed] [Google Scholar]
- 155.Lee MP, Orlov D, Sweeney G. Leptin induces rat glomerular mesangial cell hypertrophy, but does not regulate hyperplasia or apoptosis. Int J Obes (Lond) 2005; 29: 1395–1401 [DOI] [PubMed] [Google Scholar]
- 156.Zeidan A, Purdham DM, Rajapurohitam V et al. Leptin induces vascular smooth muscle cell hypertrophy through angiotensin II- and endothelin-1-dependent mechanisms and mediates stretch-induced hypertrophy. J Pharmacol Exp Ther 2005; 315: 1075–1084 [DOI] [PubMed] [Google Scholar]
- 157.Allison MA, Jenny NS, McClelland RL et al. The associations of adipokines with selected markers of the renin–angiotensinogen–aldosterone system: the multi-ethnic study of atherosclerosis. J Hum Hypertens 2015; 29: 127–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee MP, Madani S, Sekula D et al. Leptin increases expression and activity of matrix metalloproteinase-2 and does not alter collagen production in rat glomerular mesangial cells. Endocr Res 2005; 31: 27–37 [DOI] [PubMed] [Google Scholar]
- 159.Han DC, Isono M, Chen S et al. Leptin stimulates type I collagen production in db/db mesangial cells: glucose uptake and TGF-beta type II receptor expression. Kidney Int 2001; 59: 1315–1323 [DOI] [PubMed] [Google Scholar]
- 160.Vu D, Tellez-Corrales E, Sakharkar P et al. Impact of NF-kappaB gene polymorphism on allograft outcome in Hispanic renal transplant recipients. Transpl Immunol 2013; 28: 18–23 [DOI] [PubMed] [Google Scholar]
- 161.Fantuzzi G. Adiponectin in inflammatory and immune-mediated diseases. Cytokine 2013; 64: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Scotece M, Conde J, Lopez V et al. Adiponectin and leptin: new targets in inflammation. Basic Clin Pharmacol Toxicol 2014; 114: 97–102 [DOI] [PubMed] [Google Scholar]
- 163.Christou GA, Kiortsis DN. The role of adiponectin in renal physiology and development of albuminuria. J Endocrinol 2014; 221: R49–R61 [DOI] [PubMed] [Google Scholar]
- 164.Burnett MS, Lee CW, Kinnaird TD et al. The potential role of resistin in atherogenesis. Atherosclerosis 2005; 182: 241–248 [DOI] [PubMed] [Google Scholar]
- 165.Adya R, Tan BK, Punn A et al. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res 2008; 78: 356–365 [DOI] [PubMed] [Google Scholar]
- 166.Wang P, Xu TY, Guan YF et al. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res 2009; 81: 370–380 [DOI] [PubMed] [Google Scholar]
- 167.Moschen AR, Kaser A, Enrich B et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol 2007; 178: 1748–1758 [DOI] [PubMed] [Google Scholar]
- 168.Pirvulescu MM, Gan AM, Stan D et al. Subendothelial resistin enhances monocyte transmigration in a co-culture of human endothelial and smooth muscle cells by mechanisms involving fractalkine, MCP-1 and activation of TLR4 and Gi/o proteins signaling. Int J Biochem Cell Biol 2014; 50: 29–37 [DOI] [PubMed] [Google Scholar]
- 169.Li Y, Zhang Y, Dorweiler B et al. Extracellular Nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem 2008; 283: 34833–34843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kim SR, Bae YH, Bae SK et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochim Biophys Acta 2008; 1783: 886–895 [DOI] [PubMed] [Google Scholar]
- 171.Maggio AB, Wacker J, Montecucco F et al. Serum resistin and inflammatory and endothelial activation markers in obese adolescents. J Pediatr 2012; 161: 1022–1027 [DOI] [PubMed] [Google Scholar]
- 172.Romacho T, Azcutia V, Vazquez-Bella M et al. Extracellular PBEF/NAMPT/visfatin activates pro-inflammatory signalling in human vascular smooth muscle cells through nicotinamide phosphoribosyltransferase activity. Diabetologia 2009; 52: 2455–2463 [DOI] [PubMed] [Google Scholar]
- 173.Calabro P, Samudio I, Willerson JT et al. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation 2004; 110: 3335–3340 [DOI] [PubMed] [Google Scholar]
- 174.Huang Q, Guo Y, Zeng H et al. Visfatin stimulates a cellular renin-angiotensin system in cultured rat mesangial cells. Endocr Res 2011; 36: 93–100 [DOI] [PubMed] [Google Scholar]
- 175.Song HK, Lee MH, Kim BK et al. Visfatin: a new player in mesangial cell physiology and diabetic nephropathy. Am J Physiol Renal Physiol 2008; 295: F1485–F1494 [DOI] [PubMed] [Google Scholar]
- 176.Boini KM, Zhang C, Xia M et al. Visfatin-induced lipid raft redox signaling platforms and dysfunction in glomerular endothelial cells. Biochim Biophys Acta 2010; 1801: 1294–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Briffa JF, McAinch AJ, Poronnik P et al. Adipokines as a link between obesity and chronic kidney disease. Am J Physiol Renal Physiol 2013; 305: F1629–F1636 [DOI] [PubMed] [Google Scholar]
- 178.Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos Int 2008; 19: 905–912 [DOI] [PubMed] [Google Scholar]
- 179.Zhang J, Wang N. Leptin in chronic kidney disease: a link between hematopoiesis, bone metabolism, and nutrition. Int Urol Nephrol 2014; 46: 1169–1174 [DOI] [PubMed] [Google Scholar]
- 180.Tsuji K, Maeda T, Kawane T et al. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin D3 synthesis in leptin-deficient mice. J Bone Miner Res 2010; 25: 1711–1723 [DOI] [PubMed] [Google Scholar]
- 181.Gainsford T, Alexander WS. A role for leptin in hemopoieses? Mol Biotechnol 1999; 11: 149–158 [DOI] [PubMed] [Google Scholar]
- 182.Axelsson J, Qureshi AR, Heimburger O et al. Body fat mass and serum leptin levels influence epoetin sensitivity in patients with ESRD. Am J Kidney Dis 2005; 46: 628–634 [DOI] [PubMed] [Google Scholar]
- 183.Nasri H. Association of serum leptin with anemia in maintenance hemodialysis patients. Saudi J Kidney Dis Transpl 2006; 17: 521–525 [PubMed] [Google Scholar]
- 184.Stenvinkel P, Heimburger O, Lonnqvist F et al. Does the ob gene product leptin stimulate erythropoiesis in patients with chronic renal failure? Kidney Int 1998; 53: 1430–1431 [DOI] [PubMed] [Google Scholar]
- 185.Kumada M, Kihara S, Sumitsuji S et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 2003; 23: 85–89 [DOI] [PubMed] [Google Scholar]
- 186.Tamura T, Furukawa Y, Taniguchi R et al. Serum adiponectin level as an independent predictor of mortality in patients with congestive heart failure. Circ J 2007; 71: 623–630 [DOI] [PubMed] [Google Scholar]
- 187.Gnacinska M, Malgorzewicz S, Lysiak-Szydlowska W et al. The serum profile of adipokines in overweight patients with metabolic syndrome. Endokrynol Pol 2010; 61: 36–41 [PubMed] [Google Scholar]
- 188.Sahin G, Akay OM, Uslu S et al. Association between endothelial and platelet function markers and adiponectin in renal transplanted recipients on cyclosporine and tacrolimus immunosuppression based therapy. Nephrology (Carlton) 2015; 20: 392–398 [DOI] [PubMed] [Google Scholar]
- 189.Malgorzewicz S, Debska-Slizien A, Czajka B et al. Adipokines and nutritional status in kidney transplant recipients. Transplant Proc 2014; 46: 2622–2626 [DOI] [PubMed] [Google Scholar]
- 190.Wang XH, Dou LZ, Gu C et al. Plasma levels of omentin-1 and visfatin in senile patients with coronary heart disease and heart failure. Asian Pac J Trop Med 2014; 7: 55–62 [DOI] [PubMed] [Google Scholar]
- 191.Hung WC, Yu TH, Hsu CC et al. Plasma visfatin levels are associated with major adverse cardiovascular events in patients with acute ST-elevation myocardial infarction. Clin Invest Med 2015; 38: E100–E109 [DOI] [PubMed] [Google Scholar]
- 192.Dahl TB, Yndestad A, Skjelland M et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation 2007; 115: 972–980 [DOI] [PubMed] [Google Scholar]
- 193.Weikert C, Westphal S, Berger K et al. Plasma resistin levels and risk of myocardial infarction and ischemic stroke. J Clin Endocrinol Metab 2008; 93: 2647–2653 [DOI] [PubMed] [Google Scholar]
- 194.Frankel DS, Vasan RS, D'Agostino RB Sr et al. Resistin, adiponectin, and risk of heart failure the Framingham offspring study. J Am Coll Cardiol 2009; 53: 754–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pham PT, Pham PM, Pham SV et al. New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab Syndr Obes 2011; 4: 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bayes B, Lauzurica R, Granada ML et al. Adiponectin and risk of new-onset diabetes mellitus after kidney transplantation. Transplantation 2004; 78: 26–30 [DOI] [PubMed] [Google Scholar]
- 197.Romanowski M, Dziedziejko V, Maciejewska-Karlowska A et al. Adiponectin and leptin gene polymorphisms in patients with post-transplant diabetes mellitus. Pharmacogenomics 2015; 16: 1–9 [DOI] [PubMed] [Google Scholar]
- 198.Shu KH, Wu MJ, Chen CH et al. Serum adipokine levels in renal transplant recipients. Transplant Proc 2014; 46: 381–384 [DOI] [PubMed] [Google Scholar]
- 199.Olszanecka-Glinianowicz M, Owczarek A, Bozentowicz-Wikarek M et al. Relationship between circulating visfatin/NAMPT, nutritional status and insulin resistance in an elderly population—results from the PolSenior substudy. Metabolism 2014; 63: 1409–1418 [DOI] [PubMed] [Google Scholar]
- 200.Xiang RL, Mei M, Su YC et al. Visfatin protects rat pancreatic beta-cells against IFN-gamma-induced apoptosis through AMPK and ERK1/2 signaling pathways. Biomed Environ Sci 2015; 28: 169–177 [DOI] [PubMed] [Google Scholar]
- 201.Kim da S, Kang S, Moon NR et al. Central visfatin potentiates glucose-stimulated insulin secretion and beta-cell mass without increasing serum visfatin levels in diabetic rats. Cytokine 2014; 65: 159–166 [DOI] [PubMed] [Google Scholar]
- 202.Yano Y, Hoshide S, Ishikawa J et al. Differential impacts of adiponectin on low-grade albuminuria between obese and nonobese persons without diabetes. J Clin Hypertens (Greenwich) 2007; 9: 775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Othman M, Kawar B, El Nahas AM. Influence of obesity on progression of non-diabetic chronic kidney disease: a retrospective cohort study. Nephron Clin Pract 2009; 113: c16–c23 [DOI] [PubMed] [Google Scholar]
- 204.Prior SL, Tang TS, Gill GV et al. Adiponectin, total antioxidant status, and urine albumin excretion in the low-risk “golden years” type 1 diabetes mellitus cohort. Metabolism 2011; 60: 173–179 [DOI] [PubMed] [Google Scholar]
- 205.Kasiske BL, Snyder JJ, Gilbertson DT et al. Cancer after kidney transplantation in the United States. Am J Transplant 2004; 4: 905–913 [DOI] [PubMed] [Google Scholar]
- 206.Scheid MP, Sweeney G. The role of adiponectin signaling in metabolic syndrome and cancer. Rev Endocr Metab Disord 2014; 15: 157–167 [DOI] [PubMed] [Google Scholar]
- 207.Hebbard L, Ranscht B. Multifaceted roles of adiponectin in cancer. Best Pract Res Clin Endocrinol Metab 2014; 28: 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Scholze A, Rattensperger D, Zidek W et al. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity (Silver Spring) 2007; 15: 1617–1622 [DOI] [PubMed] [Google Scholar]
- 209.Moraes-Vieira PM, Bassi EJ, Larocca RA et al. Leptin deficiency modulates allograft survival by favoring a Th2 and a regulatory immune profile [corrected] Am J Transplant 2013; 13: 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zoccali C, Postorino M, Marino C et al. Waist circumference modifies the relationship between the adipose tissue cytokines leptin and adiponectin and all-cause and cardiovascular mortality in haemodialysis patients. J Intern Med 2011; 269: 172–181 [DOI] [PubMed] [Google Scholar]
- 211.Rhee CM, Nguyen DV, Moradi H et al. Association of adiponectin with body composition and mortality in hemodialysis patients. Am J Kidney Dis 2015; 66: 313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chung W, Jung ES, Shin D et al. Low resistin level is associated with poor hospitalization-free survival in hemodialysis patients. J Korean Med Sci 2012; 27: 377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nagy K, Ujszaszi A, Czira ME et al. Association between serum resistin level and outcomes in kidney transplant recipients. Transpl Int 2016; 29: 352–361 [DOI] [PubMed] [Google Scholar]