Abstract
Background
Globally there is an increase in incidence of chronic kidney diseases (CKDs). Diabetes mellitus (DM), hypertension and stone diseases are the major risk factors for CKD. We organized kidney disease screening camps in a semi-urban population of Gujarat, India on the occasion of World Kidney Day (WKD).
Methods
Voluntary participants from six towns were screened. Estimated glomerular filtration rate (eGFR) was calculated by the Modification of Diet in Renal Disease formula and CKD was defined as an eGFR <60 mL/min/1.73 m2 or albuminuria ≥1+. Urogenital ultrasonography was performed with emphasis on stone burden. Participants with known diabetes, stone diseases, hypertension, kidney/liver/cardiac disease, hepatitis, HIV, transplant recipients, pregnant women and those <18 years were excluded from the study.
Results
Of the 2350 participants (1438 men), CKD was found in 20.93% and eGFR <60 mL/min/1.73 m2 was noted in 8.29% of participants. The prevalence of CKD peaked after the seventh decade of life in both genders. There was no significant difference in the prevalence of CKD between coastal and non-coastal regions, however, obesity, hypertension and diabetes were more common in the coastal belt, whereas stone burden was greater in the non-coastal region.
Conclusions
The prevalence of CKD in a semi-urban apparently healthy Indian population was higher than the reported prevalence in developed countries. Significant differences between regions point to the need to evaluate and correctregion-specific risk factors.
Keywords: chronic kidney disease, diabetes, hypertension, prevalence, stone burden
Background
The World Health Organization (WHO) co-sponsored an international conference on primary health care in Alma-Ata, USSR (now in Kazakhstan) in September 1978. The Declaration of Alma-Ata stated ‘the need for urgent action by all governments, all health and development workers, and the world community to protect and promote the health of all the people of the world’. Unfortunately, at least in India, although progress has been achieved in different sectors, much needs to be done to achieve good health for its citizens. Chronic kidney disease (CKD) belongs to the category of non-communicable diseases (NCDs). According to a report, NCDs were responsible for 63% of deaths worldwide in 2008, and in India alone they were responsible for 53% of deaths [1]. According to this report, causes of mortality were cardiovascular diseases in 24%, chronic respiratory diseases in 11%, cancer in 6% and diabetes in 2% population. There is limited information available about the ‘state of kidney health’ in India, and especially the rural and semi-urban population, who have limited access to health services for various reasons. The age-adjusted incidence rate of end-stage renal disease (ESRD) in India was reported to be 229 per million population (pmp) and >100 000 new patients enter renal replacement programs annually in India [2, 3]. In a recent multicenter study in India, the prevalence of CKD was observed to be 17.2%, with about 6% having CKD stage 3 or worse [4]. These reports suggest that much needs to be done to focus on kidney diseases in India. Kidney diseases may become a major threat to the health of the population of developing nations and particularly India in the near future.
Hypertension, diabetes mellitus (DM), obesity and stone diseases are the common risk factors for development of CKD, as well as less common causes such as infections and congenital anomalies of the urogenital tract.
World Kidney Day (WKD) is has been celebrated every year in many parts of the world since 2006 [5]. Our aim in celebrating this day is to increase awareness of kidney diseases in the general population so that we can prevent the progression of kidney diseases and help suffering patients with therapeutic strategies. However, there is limited benefit of such celebrations to the people in less developed areas of the world due to the lack of awareness about kidney diseases, a lack of commitment and will of the personnel engaged in health practices and the paucity of resources to address this issue. We therefore decided to address the ‘kidney health’ of the semi-urban population of Gujarat on the occasion of WKD in March 2014.
We screened the population of six towns where we had established dialysis centers to serve the local population by organizing a kidney disease screening program as a pilot project. All willing participants were included in the study except pregnant women, persons <18 years of age and those with hypertension, DM, CKD, hepatitis B or C, HIV, cardiac/liver pathology or if they had undergone kidney transplantation.
Materials and methods
This study was conducted in 2014 on Sundays from the end of February to June to ensure that maximum participants could take advantage of these free services. The Institutional Ethics Committee approved the study forms and design. Participants were informed about these camps by advertising in local newspapers and on television and by placing billboards in the villages for 1 week before the scheduled date of the camp. Every camp was open from 8 a.m. to 8 p.m. A team of nephrologists, pathologists, urologists, radiologists, medical officers, nurses, technicians and clerical staff trained to conduct interviews participated in the camps. A structured questionnaire in the local language was developed and volunteer participants were requested to complete it on arrival and after enrollment (Appendix) with the help of our staff. It included history of illness, diabetes, hypertension, swelling of the face/eyelids in the morning, urinary complaints, medications and any significant family history related to kidney diseases.
Demographic studies included height, weight, temperature, pulse rate, respiratory rate, blood pressure (BP) and body mass index. Lab investigations included complete blood counts, random blood sugar, serum creatinine (SCr) and urinalysis. Ultrasonography of the abdomen was carried out and any anomaly related to the urogenital tract was noted. Particular emphasis was placed on the presence of calculi in the urogenital tract. After the reports were printed, each participant was examined by a nephrologist and urologist and advised for further management according to examination and reports.
Overweight was defined as a BMI between 25 and 30 kg/m2 and BMI >30 kg/m2 was considered obese. Hypertension was defined as systolic/diastolic blood pressure >140/90 mmHg. Blood samples were collected and sent to nearby labs accredited under the National Accreditation Board for Testing and Calibration Laboratories (NABL) or to our own lab. The specimens were stored at 4–6°C. Complete blood counts were performed by an automated hematology analyzer (Sysmex XN series, Sysmex, Kobe, Japan). Reference ranges were hemoglobin 12–14 g/dL for women and 13–17 g/dL for men, and total leukocyte count 4–11 × 103/µL. Biochemistry was performed on a Roche Hitachi 912 analyzer calibrated with commercially available controls. SCr was measured using a modified Jaffe colorimetric method, with a reference range was 0.5–1.4 mg/dL. Plasma glucose was measured by the glucose oxidase peroxidase method, with a reference range of 70–140 mg/dL. Standardization was carried out using a chemistry calibrator traceable to an isotope dilution mass spectrometry (IDMS) reference method using the National Institute of Standards and Technology (NIST) Standard Reference Material 967. Urinalysis was performed using a fully automated urinalysis system (Urisys 2400, Roche Diagnostics India) from midstream urine samples from all participants in the same season and similar weather conditions for all the centers. Estimated glomerular filtration rate (eGFR) was calculated using the modified Modification of Diet in Renal Disease 3 equation using standardized SCr, age, race and gender [6]. DM was defined as random blood sugar ≥200 mg/dL as per the American Diabetes Association guidelines [7]. Proteinuria was considered for urine albumin ≥1+, which is equivalent to 25 mg/dL. All participants received their reports along with instructions from a nephrologist and urologist before they left the camp site. They were advised to come for follow-up to our center for further care or if they needed any additional consultation.
Further comparisons of towns from North Gujarat were made with those of the coastal region of Saurashtra to determine the prevalence of hypertension, diabetes and stone burden in these areas. This comparison was made because North Gujarat is a distance from coast and has hard water compared with other parts of the state.
Statistical analysis
Creatinine clearance was measured by Cockroft–Gault formula and was included in the study. SPSS 20 (IBM, Armonk, NY) was used for statistical analyses. Continuous variables were expressed as mean ± SD and categorical variables were expressed as number (%). Data followed normal and non-normal distributions; continuous variables were tested for normally distributed variables using independent unpaired Student's t-test, and Mann–Whitney U-test was used for non-normally distributed continuous variables. For categorical variables, Pearson χ2 test was used. Fisher's exact test was used when appropriate. P-values <0.05 were considered statistically significant.
Results
Of the 2800 participants screened from six towns in Western India, 2350 fulfilling the selection criteria were included in the study. The mean distance of camp venues from our premises was 132.33 ± 82.48 km (range 61–311). The demographics and results are summarized in Table 1. None of the participants had a history of illness, diabetes, hypertension, swelling of the face/eyelids or urinary complaints. Family history regarding kidney diseases was insignificant. This included a history of diagnosed kidney failure or any visit to the doctor for urinary problems, swelling of the face/eyelids, weakness, hypertension, diabetes or hereditary diseases related to kidney disease or birth defects. Of the studied participants, 16.1% had a positive history and hence were removed from the study. BMI <25 kg/m2 was observed in 1141 (48.55%) participants and BMI ≥25 kg/m2 was observed in 1209 (51.45%) participants. DM was observed in 82 (7.19%) participants of the lower BMI group versus 146 (12.08%) in the higher BMI group (P < 0.01). Males had a higher incidence of DM than females in both groups, constituting 74.39% in the former and 65.07% in the latter. Hypertension was observed in 207 (18.14%) participants belonging to lower BMI group versus 427 (35.32%) in the higher BMI group (P < 0.01). Males had a higher incidence than females in both groups, constituting 73.43% in the former and 65.07% in the latter. Thus DM and hypertension were more common with higher BMI (P < 0.01). All the participants with stone burden had multiple calculi and concretions in the kidneys with/without calculi in the ureter/bladder. The size of the smallest calculus was 3 mm and the largest was 45 mm.
Table 1.
Results of screening of participants for kidney diseases
| Parameters evaluated | Total | Males | Females | P-value |
|---|---|---|---|---|
| Subjects, n | 2350 | 1438 | 912 | <0.01 |
| Age (years), mean ± SD | 48.16 ± 14 | 49.77 ± 14.47 | 46.77 ± 13 | <0.01 |
| BMI (kg/m2), mean ± SD | 25.61 ± 5.25 | 25.11 ± 5 | 26.41 ± 5.53 | <0.01 |
| Overweight | 772 (32.85%) | 469 (32.61%) | 303 (33.22%) | 0.79 |
| Obesity | 423 (18%) | 218 (15.16%) | 205 (22.48%) | <0.01 |
| Hypertension | 631 (26.85%) | 385 (26.78%) | 246 (26.97%) | 0.91 |
| Diabetes mellitus | 230 (9.79%) | 151 (10.50%) | 79 (8.66%) | 0.16 |
| Serum creatinine (mg/dL), mean ± SD | 1.02 ± 0.42 | 1.11 ± 0.45 | 0.87 ± 0.3 | <0.01 |
| eGFR (mL/min/1.73 m2), mean ± SD | 87.84 ± 21 | 88.19 ± 20.91 | 87.30 ± 21.24 | 0.32 |
| Urine albumin ≥1+ | 324 (13.79%) | 208 (14.46%) | 116 (12.72%) | 0.23 |
| Stone burden | 404 (17.19%) | 277 (19.26%) | 127 (13.92%) | <0.01 |
| Hemoglobin <12 g/dL | 638 (27.15%) | 207 (14.4%) | 431 (47.26%) | <0.01 |
| eGFR <60 mL/min/1.73 m2 | 195 (8.29%) | 108 (7.5%) | 87 (9.5%) | 0.38 |
| CKD eGFR <60 mL/min/1.73 m2 ± urine albumin ≥1+ | 492 (20.93%) | 302 (21.0%) | 190 (20.8%) | 0.96 |
On comparing the towns from North Gujarat with those of Saurashtra, the prevalence of hypertension, diabetes and stone burden was different in the two areas. Their demographics, clinical, radiological and laboratory data along with gender distribution are summarized in Table 2. The incidence of obesity, overweight, hypertension and DM was greater in Saurashtra whereas the incidence of stone burden, elevated SCr and proteinuria was higher in North Gujarat (P < 0.01). Hemoglobin <12 g/dL was observed in both regions without any significant difference between them (P = 0.30).
Table 2.
Comparison of demographics and results of screening participants for kidney diseases from two regions of Western India
| Parameter | Total |
Males |
Females |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Region 1 | Region 2 | P-value | Region 1 | Region 2 | P-value | Region 1 | Region 2 | P-value | |
| Total, n | 1213 | 1137 | 0.98 | 743 | 695 | 0.98 | 470 | 442 | 0.98 |
| Age (years), mean ± SD | 49.38 ± 13.34 | 47.8 ± 14.68 | 0.01 | 49.25 ± 15.42 | 50.24 ± 13.53 | 0.20 | 45.37 ± 12.98 | 48.04 ± 12.9 | <0.01 |
| BMI (kg/m2), mean ± SD | 26.53 ± 5.24 | 24.59 ± 5.03 | <0.01 | 24.08 ± 4.65 | 26.02 ± 5.12 | <0.01 | 25.39 ± 5.52 | 27.32 ± 5.38 | <0.01 |
| Overweight | 445 (36.60%) | 327 (28.75%) | <0.01 | 269 (36.2%) | 200 (28.77%) | <0.01 | 130 (27.56%) | 173 (39.14%) | <0.01 |
| Obesity | 278 (22.91%) | 145 (12.75%) | <0.01 | 140 (18.84%) | 78 (11.22%) | <0.01 | 79 (16.74%) | 126 (28.51%) | <0.01 |
| Hypertension | 372 (30.66%) | 259 (22.77%) | <0.01 | 206 (27.72%) | 166 (23.88%) | 0.11 | 87 (18.44%) | 159 (35.97%) | <0.01 |
| Diabetes mellitus | 129 (10.63%) | 101 (8.88%) | 0.70 | 78 (10.49%) | 73 (10.50%) | 0.93 | 29 (6.14%) | 50 (11.31%) | 0.01 |
| Serum creatinine (mg/dL), mean ± SD | 1.04 ± 0.43 | 0.99 ± 0.4 | <0.01 | 1.15 ± 0.47 | 1.08 ± 0.43 | 0.01 | 0.87 ± 0.28 | 0.87 ± 0.32 | 1 |
| Urinary albumin ≥1+ | 106 (8.73%) | 218 (19.17%) | <0.01 | 64 (8.61%) | 144 (20.71%) | <0.01 | 42 (8.93%) | 74 (16.74%) | <0.01 |
| CKD | 190 (15.66%) | 302 (26.0%) | <0.01 | 116 (15.61%) | 186 (26.76%) | <0.01 | 73 (15.53%) | 117 (26.47%) | <0.01 |
| eGFR (mL/min/1.73 m2), mean ± SD | 78.84 ± 21.95 | 89.34 ± 20.76 | <0.01 | 87.22 ± 21.22 | 89.09 ± 20.61 | 0.10 | 84.64 ± 21.21 | 89.73 ± 21.0 | <0.01 |
| Stone burden | 118 (9.72%) | 286 (25.15%) | <0.01 | 114 (15.34%) | 163 (23.45%) | <0.01 | 57 (12.12%) | 70 (15.84%) | 0.13 |
| Hemoglobin ≤12 g/dL | 341 (27.96%) | 297 (26.12%) | 0.30 | 116 (15.61%) | 91 (13.09%) | 0.20 | 225 (47.7%) | 206 (46.61%) | 0.75 |
| eGFR <60 mL/min/1.73 m2 | 91 (7.46%) | 104 (9.14%) | 0.17 | 54 (7.26%) | 54 (7.76%) | 0.79 | 37 (7.84%) | 50 (11.31%) | 0.10 |
| ADPKD | 5 (0.41%) | 3 (0.26%) | 0.79 | 5 (0.67%) | 3 (0.43%) | 0.79 | 0 (0%) | 0 (0%) | – |
Region 1: coastal region (Saurashtra), Region 2: non-coastal region (North Gujarat). ADPKD, autosomal dominant polycystic kidney disease.
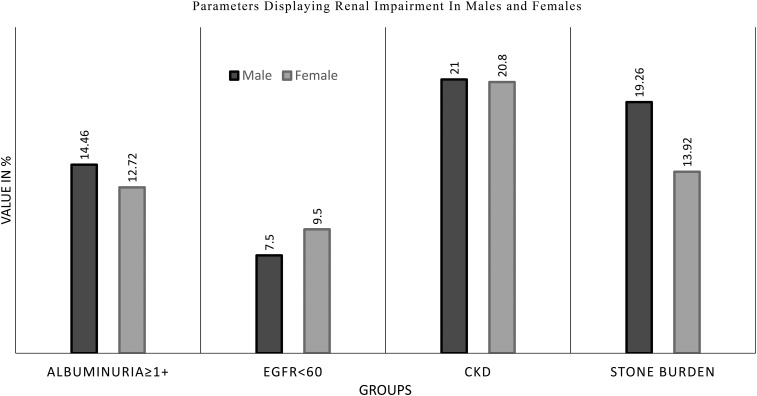
Table 3 describes renal impairment observed in different age groups from 18 to >70 years. This includes eGFR <60 mL/min/1.73 m2, albuminuria ≥25 mg/dL, with CKD stages 3, 4 and 5 and stone burden. It was observed that overall CKD peaked in the seventh decade of life in both genders. Autosomal dominant polycystic kidney disease was incidentally noted in eight men. Significant albuminuria was observed in 291 (16.77%) participants with an eGFR >60 mL/min/1.73 m2. Of 492 participants with CKD greater than stage 2, 32.52% were found to have CKD stage 3A, 4.87% were found to have CKD stage 3B, 2.03% were found to have CKD stage 4 and 0.20% were found to have CKD stage 5. Figure 1 depicts the findings of our study regarding kidney diseases in this population.
Table 3.
Renal impairment in different age groups
| Age group (years) | eGFR <60 mL/min/1.73 m2 (N = 195), /total | Albuminuria (N = 324), /total | CKD (N = 492), /total | Stone burden (N = 404), /total | Creatinine clearance, mean ± SD |
|---|---|---|---|---|---|
| Total | |||||
| 18–30 | 17 (5.94%)/286 | 36 (12.59%)/286 | 53 (18.53%)/286 | 39 (13.64%)/286 | 89.90 ± 32.30 |
| 31–40 | 19 (4.58%)/415 | 41 (9.88%)/415 | 57 (13.74%)/415 | 78 (18.8%)/415 | 91.17 ± 33.12 |
| 41–50 | 52 (8.47%)/614 | 86 (14.01%)/614 | 126 (20.52%)/614 | 122 (19.87%)/614 | 86.48 ± 30.33 |
| 51–60 | 46 (7.96%) /578 | 78 (13.49%)/578 | 121 (20.93%)/578 | 94 (16.26%)/578 | 80.78 ± 28.57 |
| 61–70 | 34 (10.46%)/325 | 58 (17.85%)/325 | 87 (26.77%)/325 | 51 (15.69%)/325 | 75.56 ± 27.29 |
| >70 | 27 (20.45%)/132 | 25 (18.93%)/132 | 48 (36.36%)/132 | 20 (15.15%)/132 | 66.23 ± 25.99 |
| Males | |||||
| 18–30 | 10 (5.78%)/173 | 22 (12.72%)/173 | 32 (18.5)%/173 | 23 (13.29%)/173 | 89.96 ± 33.59 |
| 31–40 | 8 (3.72%)/215 | 18 (8.37%)/215 | 25 (11.63%)/215 | 47 (21.86%)/215 | 93.92 ± 32.15 |
| 41–50 | 24 (6.88%)/349 | 53 (15.19%)/349 | 70 (20.06%)/349 | 87 (24.93%)/349 | 84.50 ± 29.57 |
| 51–60 | 28 (7.61%)/368 | 58 (15.76%)/368 | 84 (22.83%)/368 | 71 (19.3%)/368 | 81.96 ± 29.12 |
| 61–70 | 21 (9.33%)/225 | 39 (17.33%)/225 | 57 (25.33%)/225 | 36 (16%)/225 | 76.05 ± 27.84 |
| >70 | 17 (15.74%)/108 | 19 (17.59%)/108 | 34 (31.48%)/108 | 14 (12.96%)/108 | 68.94 ± 26.69 |
| Females | |||||
| 18–30 | 7 (6.19%)/113 | 14 (12.39%)/113 | 21 (18.58%)/113 | 16 (14.16%)/113 | 89.80 ± 30.33 |
| 31–40 | 11 (5.5%)/200 | 23 (11.5%)/200 | 32 (16%)/200 | 31 (15.5%)/200 | 88.64 ± 34 |
| 41–50 | 28 (10.57%)/265 | 33 (12.45%)/265 | 56 (21.13%)/265 | 35 (13.2%)/265 | 89.12 ± 31.17 |
| 51–60 | 18 (8.57%)/210 | 20 (9.52%)/210 | 37 (17.62%)/210 | 23 (10.96%)/210 | 78.68 ± 27.52 |
| 61–70 | 13 (13%)/100 | 19 (19%)/100 | 30 (30%)/100 | 15 (15%)/100 | 74.44 ± 26.12 |
| >70 | 10 (41.67%)/24 | 6 (25%)/24 | 14 (58.33%)/24 | 6 (25%)/24 | 59.55 ± 21.37 |
Fig. 1.
Bar diagram depicting the parameters of renal impairment in the form of albuminuria >1+, eGFR <60 ml/min/1.73 m2, CKD and stone burden.
Discussion
The risk of developing CKD is increasing globally [8–18]. Its complications include cardiovascular mortality, kidney disease progression, cognitive decline, anemia, mineral and bone disorders and fractures. Screening and timely intervention can prevent CKD and its complications [18]. In our study of a semi-urban population we observed a relatively high prevalence of hypertension, diabetes and stone burden. Interestingly, we also found a high stone burden in the non-coastal area as compared with the coastal area. Thus both regions showed a high risk of their populations progressing to CKD, but for different reasons.
Zhang and Rothenbacher [19] studied the prevalence of CKD worldwide in 2008. They reviewed 26 studies from the USA, Europe, Asia and Australia. They observed that the median prevalence of CKD was 7.2% in persons ≥30 years old, whereas in persons ≥64 years, the prevalence varied from 23.4 to 35.8%. According to the Kidney Dialysis Outcomes Quality Initiative (KDOQI) practice guidelines published in 2002 by the National Kidney Foundation (NKF), CKD is defined as creatinine clearance or GFR <60 mL/min/1.73 m2 [20, 21]. These guidelines were adopted by all studies to arrive at the prevalence of CKD. In the cross-sectional multicenter Screening and Early Evaluation of Kidney Disease (SEEK) Indian study by Singh et al. [4] on 6120 subjects, the prevalence of CKD was 16.4%. In this study, CKD stages were defined as per the KDOQI guidelines (eGFR <60 mL/min/1.73 m2 or proteinuria >1+ on dipstick) [22]. SEEK studies of Thailand and Saudi Arabia reported CKD prevalences of 17.5 and 5.7%, respectively [23, 24]. The present study shows that the prevalence of CKD is 18.60% in our semi-urban population who are unaware of their present kidney Health status. About 400 participants from the total population studied were removed from the analysis since they were already suffering from DM/hypertension/stone disease/other sickness and are under treatment from local medical practitioners.
These figures indicate the significance of WKD to focus attention on prevention and cure of kidney diseases globally. The theme of WKD-2014 was ‘Kidney Health for All’. Urban populations have easy access to medical care compared with rural and semi-urban populations of developing countries. We decided to organize a screening program in a semi-urban population where we could easily set up the required basic facilities for a screening camp.
In India, as in other developing nations that have adopted the lifestyle of developed nations, the population has started becoming more sedentary and eating a high fat and low fiber diet, giving rise to obesity and increases in BMI, which is associated with hypertension and diabetes [9, 25]. In India itself, which has geographically varied areas, the prevalence of hypertension and diabetes are different in different areas, varying from 13 to 58% and 6 to 20% [26, 27]. In the SEEK-India study, hypertension was noted in 64.5% and DM was noted in 31.6% of the CKD group. In that study, self-reported kidney stone disease was observed in 4.5%. In our study, the mean eGFR of our population was 87.84 ± 21 mL/min/1.73 m2. Finding hypertension in 26.85% and diabetes in 9.79% of a population that is otherwise ‘healthy’ according to their own belief suggests that this population is living on the brink of CKD, warranting immediate measures to improve their health status. We compared two regions of this state that occupies the western part of India: North Gujarat, which is inland, and Saurashtra, which is on the coast. Interestingly, although the populations in both areas belong to the middle class income group as per the prevailing social norms, there was substantial difference in the patterns of affection. North Gujarat emerges as the stone belt, with most of the population harboring multiple calculi. This population also had associated proteinuria and elevated SCr, whereas hypertension and diabetes, which are believed to be afflictions of affluence, were observed in Saurashtra. Both areas have populations progressing to CKD, but for different reasons.
The answer to this mammoth problem is timely intervention. In 2003, Mani [28] reported that diabetes accounts for ~30% of chronic renal failure and hypertension for 10% of the patient load on waiting lists for kidney transplantation. In a house-to-house survey of 25 000 in South India, he found an incidence of 6% for hypertension and 4% for diabetes. He used only reserpine, hydralazine and hydrochlorothiazide for hypertension, and glibenclamide and metformin for diabetes, the cheapest agents available, and was able to control blood pressure to ≤140/90 mmHg in 96% of cases and reduce glycosylated hemoglobin (HbA1C) by ≥10% of the original reading in 77% and HbA1C of 7% in 50% of the diabetic subjects. This study suggests that early screening and intervention can control the progress of CKD in our semi-urban population.
This was a single-point cross-sectional study carried out to screen a population who was willing to participate. Hence the possibility of under-/overestimation cannot be ruled out. The persons suffering from diabetes, hypertension or stone burden were excluded from the study. In the population studied, women do not go out unless there is specific purpose. They shy away even for a routine health check-up, unless forced to by the men of the house or that area, which rarely happens. Thus more males participated in this study and the actual prevalence of CKD may be higher. The equation for CKD employed here followed the KDOQI guidelines, which may not be ideal for our population. This study does not elaborate on the observation of hemoglobin <12 g/dL in ~28% of the population, which also could be a contributory factor of ill health. We did not adhere to the criterion of persistence for 3 months to label this population as CKD. Hence, larger studies involving more participants from other parts of the state as well as the country need to be carried out.
In conclusion, in a semi-urban population of India that does not have the facilities for screening and early intervention for kidney diseases, the prevalence of CKD was higher than that reported in developed nations. This study may represent the tip of the iceberg since participants considered themselves healthy and individuals with risk factors for CKD were excluded. A relatively high prevalence of undiagnosed hypertension, diabetes and stone burden indicates that a larger population is likely to develop CKD if timely intervention is not provided. In addition, there are geographical differences, as stone burden associated with increased SCr and proteinuria was more prevalent in the non-coastal region, whereas diabetes and hypertension were more prevalent in the coastal region. There is an urgent need to draft preventive health policies and plan for the allocation of more resources to improve kidney health in India. This study also suggests that the populations of other developing nations, especially in Asia, are also likely to be progressing toward CKD due to hypertension, DM and obesity.
Conflict of interest statement
None declared.
References
- 1.Sharma K. Burden of non-communicable diseases in India: setting priority for action. Int J Med Sci Public Health 2013; 2: 7–11 [Google Scholar]
- 2.Modi GK, Jha V. The incidence of end-stage renal disease in India: a population-based study. Kidney Int 2006; 70: 2131–2133 [DOI] [PubMed] [Google Scholar]
- 3.Kher V. End-stage renal disease in developing countries. Kidney Int 2002; 62: 350–362 [DOI] [PubMed] [Google Scholar]
- 4.Singh AK, Farag YMK, Mittal BV et al. Epidemiology and risk factors of chronic kidney disease in India—results from the SEEK (Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol 2013; 14: 114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah PR, Modi PR, Kute VB et al. World Kidney Day 2010: medical aspects of 10 live donor renal transplantations in a single center from a developing country. Transplant Proc 2012; 44: 47–48 [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Greene T, Kusek JW et al. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 2000; 11: 155A. [Google Scholar]
- 7.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003; 26 (Suppl 1): S5–S20 [DOI] [PubMed] [Google Scholar]
- 8.Meguid El Nihas A, Bello AK. Chronic kidney disease: the global challenge. Lancet 2005; 365: 331–340 [DOI] [PubMed] [Google Scholar]
- 9.Jafar TH. Hypertension and kidney disease in Asia. Curr Opin Nephrol Hypertens 2006; 15: 291–295 [DOI] [PubMed] [Google Scholar]
- 10.Wen CP, Cheng TY, Tsai MK et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008; 371: 2173–2182 [DOI] [PubMed] [Google Scholar]
- 11.Safarinejad MR. The epidemiology of adult chronic kidney disease in a population-based study in Iran: prevalence and associated risk factors. J Nephrol 2009; 22: 99–108 [PubMed] [Google Scholar]
- 12.Imai E, Horio M, Watanabe T et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol 2009; 13: 621–630 [DOI] [PubMed] [Google Scholar]
- 13.Shan Y, Zhang Q, Liu Z et al. Prevalence and risk factors associated with chronic kidney disease in adults over 40 years: a population study from Central China. Nephrology 2010; 15: 354–361 [DOI] [PubMed] [Google Scholar]
- 14.Varma PP, Raman DK, Ramakrishnan TS et al. Prevalence of early stages of chronic kidney disease in apparently healthy central government employees in India. Nephrol Dial Transplant 2010; 25: 3011–3017 [DOI] [PubMed] [Google Scholar]
- 15.Sharma SK, Ghimire A, Carminati S et al. Management of chronic kidney disease and its risk factors in eastern Nepal. Lancet Glob Health 2014; 2: e506–e507. [DOI] [PubMed] [Google Scholar]
- 16.Jafar TH. The growing burden of chronic kidney disease in Pakistan. N Engl J Med 2006; 354: 995–997. [DOI] [PubMed] [Google Scholar]
- 17.Jha V, Garcia-Garcia G, Iseki K et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013; 382: 260–272. [DOI] [PubMed] [Google Scholar]
- 18.Rajapurkar M, Dabhi M. Burden of disease—prevalence and incidence of renal disease in India. Clin Nephrol 2010; 74(Suppl 1): 9–12. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Q-L, Rothenbacher D. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 2008; 8: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 (2 Suppl 1): S1–S266 [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 22.Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the national kidney foundation. Am J Kidney Dis 2007; 50: 169–180 [DOI] [PubMed] [Google Scholar]
- 23.Ingsathit A, Thakkinstian A, Chaiprasert A et al. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol Dial Transplant 2010; 25: 1567–1575 [DOI] [PubMed] [Google Scholar]
- 24.Alsuwaida AO, Farag YM, Al Sayyari AA et al. Epidemiology of chronic kidney disease in the kingdom of Saudi Arabia (SEEK-Saudi investigators)—a pilot study. Saudi J Kidney Dis Transpl 2010; 21: 1066–1072 [PubMed] [Google Scholar]
- 25.Dans A, Ng N, Varghese C et al. The rise of chronic non-communicable diseases in southeast Asia: time for action. Lancet 2011; 377: 680–689 [DOI] [PubMed] [Google Scholar]
- 26.Prasad DS, Kabir Z, Dash AK et al. Prevalence and risk factors for diabetes and impaired glucose tolerance in Asian Indians: a community survey from urban Eastern India. Diabetes Metab Syndr 2012; 6: 96–101 [DOI] [PubMed] [Google Scholar]
- 27.Devi P, Rao M, Sigamani A et al. Prevalence, risk factors and awareness of hypertension in India: a systematic review. J Hum Hypertens 2013; 27: 281–287 [DOI] [PubMed] [Google Scholar]
- 28.Mani MK. Prevention of chronic renal failure at the community level. Kidney Int Suppl 2003; 83: S86–S89 [DOI] [PubMed] [Google Scholar]