Abstract
Background
Patients with end-stage renal disease are at high risk for medical errors given their comorbidities, polypharmacy and coordination of care with other hospital departments. We previously developed a hemodialysis safety checklist (Hemo Pause) to be jointly completed by nurses and patients. Our objective was to determine the feasibility of using this checklist during every hemodialysis session for 3 months.
Methods
We conducted a single-center, prospective time series study. A convenience sample of 14 nurses and 22 prevalent in-center hemodialysis patients volunteered to participate. All participants were trained in the administration of the Hemo Pause checklist. The primary outcome was completion of the Hemo Pause checklist, which was assessed at weekly intervals. We also measured the acceptability of the Hemo Pause checklist using a local patient safety survey.
Results
There were 799 hemodialysis treatments pre-intervention (13 January–5 April 2014) and 757 post-intervention (5 May–26 July 2014). The checklist was completed for 556 of the 757 (73%) treatments. Among the hemodialysis nurses, 93% (13/14) agreed that the checklist was easy to use and 79% (11/14) agreed it should be expanded to other patients. Among the hemodialysis patients, 73% (16/22) agreed that the checklist made them feel safer and should be expanded to other patients.
Conclusions
The Hemo Pause safety checklist was acceptable to both nurses and patients over 3 months. Our next step is to spread this checklist locally and conduct a mixed methods study to determine mechanisms by which its use may improve safety culture and reduce adverse events.
Keywords: checklist, hemodialysis, patient safety, quality improvement
Introduction
Hemodialysis is a complex and invasive procedure that affords risk to vulnerable patients. It has been estimated that 2–4% of end-stage renal disease (ESRD) patient deaths may be attributed to a hemodialysis-related complication [1]. A survey carried out in 2006 that elicited opinions from patients and health care providers about safety concerns in the hemodialysis unit reported that ∼50% of the patients fear that an error will be made during their hemodialysis treatment [2]. Moreover, hemodialysis nurses have reported a lack of compliance with established hemodialysis policies and procedures and a feeling of ‘always needing to rush’ to get patients in and out for hemodialysis treatment as ongoing barriers to patient safety [3].
Dialysis unit staff (nurses, nephrologists, technicians) have called for action to improve the delivery of hemodialysis care and reduce risk. Several nephrology leaders have provided recommendations for strategies that should be considered to achieve these goals [4, 5]. Improving the patient safety culture in the hemodialysis unit is one such strategy [6, 7]. Studies have demonstrated that incorporating a culture of trust, transparency and discipline is associated with sustainable improvements in patient safety [8]. The use of checklists in surgical and intensive care settings is one patient safety strategy that may improve safety culture by promoting communication, teamwork and consistency of care through standardization of protocols and procedures [9].
Checklists may represent a solution to improving the safety culture in the hemodialysis unit. Indeed, checklists have been piloted in two European hemodialysis units, which demonstrated an improvement in safety culture and quality of care [10, 11]. Despite the improvements, the aforementioned checklists focused primarily on nurse–physician communication and the patient experience. Neither checklist was designed to be used collaboratively between nurses and patients, which is an important aspect of the hemodialysis procedure.
Accordingly, to increase patient involvement and empowerment in hemodialysis, we recently developed a hemodialysis safety checklist (Hemo Pause) using a structured panel process and principles of human factors engineering [12]. In brief, the Hemo Pause checklist was designed by a multidisciplinary team of physicians, nurses and administrators using an iterative process that consisted of literature reviews, surveys and an in-person consensus meeting. This method is a proven technique for developing quality and patient safety measures in health care [13–18]. In the next phase, we now describe the results from a small-scale pilot quality improvement program to determine the feasibility of using the Hemo Pause checklist during every hemodialysis session for 3 months before widescale adoption to the entire hemodialysis unit.
Materials and methods
Study design
We conducted a single-center, prospective time series study at St Michael's Hospital in Toronto, ON, Canada. The pre-intervention period was from 13 January to 5 April 2014 and the post-intervention period was from 5 May to 26 July 2014. During the intervention period, the Hemo Pause checklist was used during every hemodialysis session. A convenience sample of 14 nurses (out of 60) and 22 prevalent in-center hemodialysis patients (out of 72) volunteered to participate. The study was limited to English-speaking nurses and patients. The study adhered to the Declaration of Helsinki, was approved by the Research Ethics Board of St Michael's Hospital, and all nurses and patients gave written informed consent. This quality improvement initiative was also approved by the Division of Nephrology and Diabetes Comprehensive Care Program at St Michael's Hospital in Toronto, ON, Canada.
Hemo Pause intervention
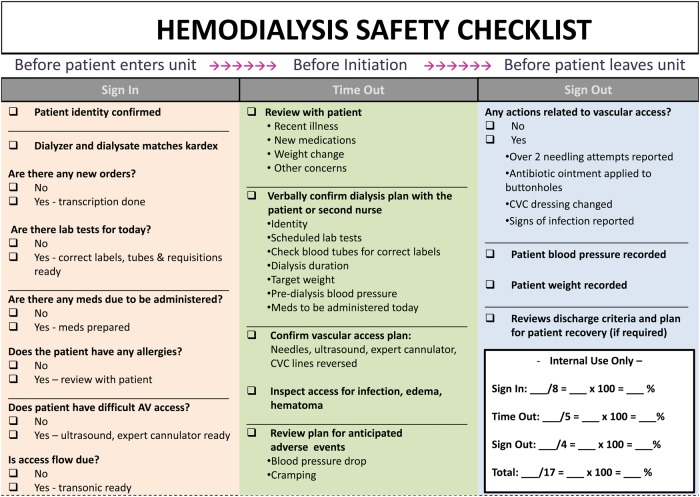
The Hemo Pause checklist is meant to be used at three different intervals: (i) before the patient arrives (‘sign in’), (ii) prior to cannulation and hemodialysis initiation (‘time out’) and (iii) after hemodialysis completion (‘sign out’). The specific checklist elements are outlined in Figure 1. A key element of the checklist is the ‘time out’ section, whereby nurses engage in a conversation with the patient and the patient has an opportunity to ask questions about their treatment plan and correct any errors or omissions. All participants received training on the proper administration of the Hemo Pause checklist prior to its implementation. Nurses and patients were also encouraged to offer suggestions throughout the implementation period to improve the performance of the Hemo Pause checklist, consistent with quality improvement methodology.
Fig. 1.
Hemodialysis safety checklist (Hemo Pause). CVC, central venous catheter.
Outcomes
The primary outcome was completion of the Hemo Pause checklist, which was assessed at weekly intervals. A random sample of 20 encounters between nurses and patients while using the Hemo Pause checklist were also observed to document the duration and quality of the interaction (i.e. participants were using the Hemo Pause checklist as intended, and not haphazardly checking off items).
We assessed the effect of the Hemo Pause checklist on patient safety and the patient experience using a local patient safety survey adapted from the Renal Physicians' Association survey [2]. This was conducted before and after implementation of the Hemo Pause checklist. A 5-point Likert scale from strongly agree [5] to strongly disagree [1] was used to score the patient safety survey.
We also collected data on pre-specified quality of care items and adverse events to guide outcome selection for future studies. These included pre-/post-hemodialysis weights missed, pre-/post-hemodialysis blood pressure missed, incorrect hemodialysis prescription, missed hemodialysis sessions, missed medication administration, missed physician orders, more than two cannulation attempts, needle dislodgements, access infections and interventions, blood transfusions, systolic blood pressure <90 mmHg during hemodialysis treatment, falls, hospitalizations and death.
Statistical analysis
We expressed continuous variables as mean [standard deviation (SD)] or median [interquartile range (IQR)] as appropriate and categorical variables as a percentage. We used statistical process control (SPC) charts to analyze checklist completion [19]. The SPC charts combine chronological analysis with tests of statistical significance, which allow them to evaluate the effectiveness and sustainability of a process over time. This approach is particularly useful for quality improvement interventions [20]. Control limits were plotted 3 SDs from the mean; this is typical for SPC charts since almost all data will fall within ±3 SDs of the mean if the underlying process is in statistical control [19, 20]. We compared variables using Student's t-test, the Mantel–Haenszel χ2 test or Fisher's exact test, as appropriate. We considered a two-sided P-value <0.05 as statistically significant.
Results
There were 799 hemodialysis treatments pre-intervention and 757 post-intervention. Table 1 describes the characteristics of the patients enrolled in the study.
Table 1.
Baseline characteristics of the Hemo Pause patients
| Characteristic | Hemo Pause cohort (n = 22) |
|---|---|
| Mean age, years (SD) | 59 (11) |
| Male, n (%) | 15 (68) |
| Mean duration on dialysis, years (SD) | 6.9 (5.9) |
| Mean number of medications (SD) | 13.6 (4.1) |
| Number of dialysis days per week, | |
| median (IQR) | 3.0 (2.3) |
| Dialysis access, n (%) | |
| Fistula | 13 (59) |
| Graft | 3 (14) |
| Central venous catheter | 6 (27) |
| Comorbidities, n (%) | |
| Diabetes | 9 (41) |
| Hypertension | 20 (91) |
| Coronary artery disease | 9 (41) |
| Congestive heart failure | 1 (5) |
| Chronic obstructive pulmonary disease | 2 (9) |
| Peripheral vascular disease | 2 (9) |
| Cerebrovascular disease | 1 (5) |
| Cancer | 0 (0) |
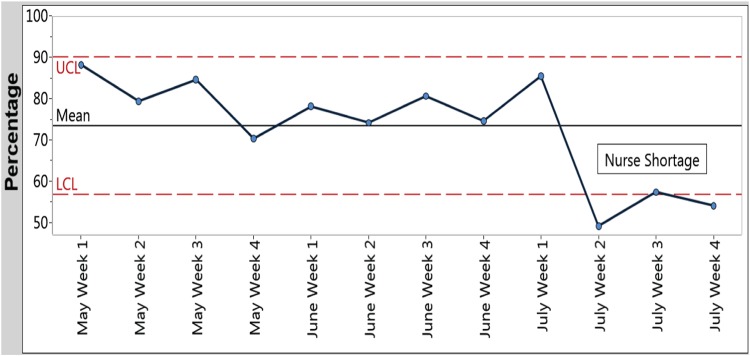
During the 3-month implementation period, the Hemo Pause checklist was completed for 556 of 757 (73%) possible hemodialysis treatments. The most common reasons for non-completion of the checklist were a shortage of nursing staff trained in the use of the checklist during the summer vacation months and patient admission to the hospital. Of the 20/757 (3%) observed encounters, all were conducted with sufficient quality and in <5 min. Figure 2 demonstrates usage of the Hemo Pause checklist throughout the implementation period.
Fig. 2.
The percentage of the Hemo Pause checklists in the patient chart completed on a weekly basis. Upper and lower control limits (UCL and LCL) are plotted at ±3 SDs from the mean. A shortage of nurses trained in administration of the Hemo Pause checklist started the second week of July.
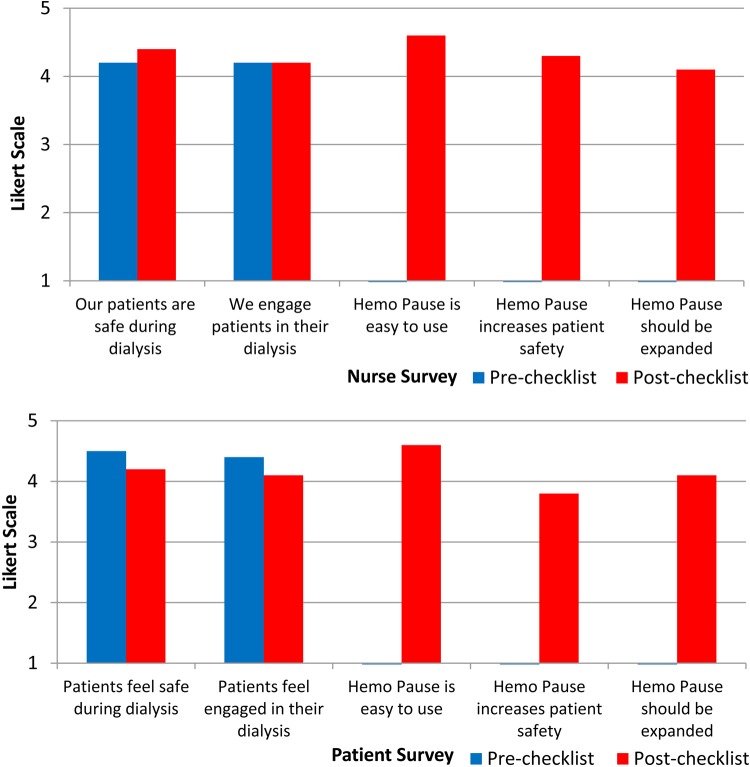
There were no significant differences between the pre-intervention and post-intervention scores on the patient safety surveys (Figure 3). Among hemodialysis nurses, 93% (13/14) agreed that the Hemo Pause checklist was easy to use, 79% (11/14) agreed it should be expanded to other patients and 93% (13/14) would want the Hemo Pause checklist used during their hemodialysis sessions if they developed ESRD. Among hemodialysis patients, 73% (16/22) agreed that the Hemo Pause checklist made them feel safer and 73% (16/22) agreed it should be expanded to other patients. Of the 200 total survey questions asked to nurses and patients, negative comments occurred on 4% (7/200) of survey responses.
Fig. 3.
Patient safety survey results. The top panel represents nurse responses and the bottom panel patient responses. A 5-point Likert scale from strongly agree (5) to strongly disagree (1) was used to score the surveys.
Table 2 compares the safety parameters collected during the pre-intervention and post-intervention periods. The changes in pre-dialysis weights, pre-dialysis blood pressure and intradialytic hypotension were statistically significant, but the effect sizes were small and the direction of the changes inconsistent (both in favor of and in opposition to the Hemo Pause checklist). The most common quality of care problems were (i) missed blood pressure before hemodialysis [385/757 (51%)], (ii) missed blood pressure after hemodialysis [276/757 (36%)] and (iii) missed weight measurements after hemodialysis [27/757 (4%)].
Table 2.
Quality of care and adverse events before and after the Hemo Pause implementation
| Pre-checklist |
Post-checklist |
P-value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Hemodialysis treatments | 799 | 757 | |||
| Pre-weights missed | 1 | 0 | 12 | 2 | 0.01 |
| Post-weights missed | 18 | 2 | 27 | 4 | 0.13 |
| Pre-blood pressures missed | 452 | 57 | 385 | 51 | 0.03 |
| Post-blood pressures missed | 320 | 40 | 276 | 36 | 0.16 |
| Incorrect hemodialysis prescription | 16 | 2 | 13 | 2 | 0.71 |
| Missed hemodialysis session | 24 | 3 | 24 | 3 | 0.88 |
| Missed medication administration | 8 | 1 | 14 | 2 | 0.19 |
| Missed physician orders | 8 | 1 | 5 | 1 | 0.58 |
| More than two cannulation attempts | 0 | 0 | 0 | 0 | N/A |
| Needle dislodgements | 0 | 0 | 0 | 0 | N/A |
| Access infections | 0 | 0 | 0 | 0 | N/A |
| Access interventions | 2 | 0 | 2 | 0 | 1.00 |
| Blood transfusions | 8 | 1 | 2 | 0 | 0.11 |
| Falls | 0 | 0 | 0 | 0 | N/A |
| Systolic blood pressure <90 mmHg during hemodialysis | 5 | 1 | 22 | 3 | 0.01 |
| Hospitalizations | 3 | 0 | 1 | 0 | 0.62 |
| Deaths | 0 | 0 | 0 | 0 | N/A |
Discussion
In our pilot quality improvement study, we found that the Hemo Pause checklist was completed for 556 of the 757 (73%) treatments over 3 months. The majority of nurses and patients involved in this pilot study agreed that the Hemo Pause checklist was easy to use and should be expanded to other patients.
The results of our study are in keeping with the results of two other hemodialysis checklist feasibility studies [10, 11]. The checklists by Marcelli et al. [10] and Galland et al. [11] were found to be feasible and acceptable for use among their study participants. The acceptance of our checklist by nursing staff and patients may be related to the common features it shares with the aforementioned checklists, with 70–80% of the items similar across all three checklists. Specifically, all three checklists incorporate three phases of safety checks: pre-session, session initiation and post-session. Common checklist items at each time point include the following:
Pre-session: confirmation of patient identity and a review of patient-reported problems and the dialysis access (including infection prevention and cannulation plan)
Session initiation: a review of the dialysate prescription, treatment plan (including blood pressure, target weight, treatment time and possible complications) and dialysis access difficulties (including needle size and cannulation attempts)
Post-session: a review of vital signs, blood loss and dialysis access complications, target weight and treatment time.
These checklist similarities provide face validity for the items included on all three checklists. Therefore, these elements should be strongly considered for inclusion on current and future hemodialysis safety checklists.
However, our checklist differs from those of Marcelli et al. and Galland et al. in its intended purpose and format. The checklist by Marcelli et al. focused on the patient experience and allowed nurses to complete hemodialysis sessions independently with minimal physician input required due to the reorganization of dialysis services, while the checklist by Galland et al. focused on communication between nurses and physicians. In contrast, our checklist focused on communication between nurses and patients. These differences emphasize the importance of local context in quality improvement [21]. Indeed, although checklists may have common elements and can be adapted between dialysis units, each dialysis unit must tailor the checklist to their own environment, work processes and needs. Another important difference is the format of the checklist. The Hemo Pause checklist was designed to be completed manually as the hemodialysis session progresses, whereas the checklist by Marcelli et al. [10] was completed automatically from device monitors and electronic records rather than being physically checked by a person. This difference may be important, since it is the act of completing the checklist that affects behavior, not using the checklist as a means of data collection or assurance [22, 23].
Feasibility testing is an important part of any quality improvement effort since it allows for a change to be incrementally accepted by staff and patients and modified by end users on a small scale before widespread dissemination [24]. There are a number of observations noted in our study that relate to the feasibility of the Hemo Pause checklist and have implications regarding its future modification and implementation. First, checklist completion declined at the same time as a nurse shortage during the summer vacation period. This observation suggests that all dialysis unit staff should be trained in checklist administration since it is spread throughout the unit. Second, nurse and patient perceptions of safety climate and engagement did not improve at the end of the study. This result may have been due to these items being rated highly prior to the checklist, which did not leave much room for scores to increase. These positive safety ratings may have been due to the highly selected convenience sample of nurses and patients who participated in the study. Additional considerations are that a more sensitive and validated patient safety measurement tool may be needed or improvements in safety culture require a series of interventions rather than a single intervention. Published strategies to strengthen safety culture include structured educational programs, leadership walk rounds and team training [25, 26]. Comprehensive Unit-Based Safety Programs (CUSPs) combine all of these elements along with specific strategies to promote best practices, and they have shown promise in two systematic reviews on safety culture promotion methods [25, 26]. Recent opinion leaders also suggest that team training is a prerequisite for checklist effectiveness [27]. These interventions were not used along with the Hemo Pause checklist and may explain why the unit safety culture did not improve despite highly motivated nurses and patients. Third, the checklist did not affect the rate of quality of care deficiencies or adverse events. Several items reached statistical significance, but these likely represent false-positive results due to the number of hypothesis-generating tests performed, the small effect sizes and the inconsistent relationship both in favor of and against the checklist. Moreover, the short duration of the follow-up period makes it unlikely that a change in organizational safety culture would occur at such a rapid pace to cause a change in quality of care and adverse events. Lastly, Table 2 should be interpreted with caution because this study was not powered to detect an effect on hard clinical outcomes, and we also found it challenging to measure so many processes simultaneously that may have resulted in measurement and ascertainment bias. For example, it is very unlikely that no patient required more than two cannulation attempts over the complete 6-month study period, which suggests a problem with the ascertainment of this outcome. Instead, we reinforce that these quality of care data are intended to guide future studies so that we can focus on the common quality of care problems in our hemodialysis unit.
Even though this study did not include a formal qualitative component, several comments by nurses and patients warrant mention. Nurses noted that work duplication was a significant barrier to checklist implementation and preferred the study to have been organized according to dialysis shift rather than self-selection. In this way, nurses working on the same dialysis shift would have more flexibility to change care processes in order to integrate the checklist into usual workflow and minimize duplication of work. Several nurses commented that the checklist being spread across so many different dialysis shifts limited its effectiveness, which is consistent with observations that there can be wide variations in safety culture within a single institution [28]. Patients noted that their initial reluctance to participate in safety initiatives was not substantiated at the end of the study. Their concerns centered on how their feedback would be perceived by nurses, as well as treatment delays from the new process. At the end of the study, the patients appreciated the opportunity to work collaboratively with nurses on a shared purpose. They felt their input was valued and important, which outweighed any small disruptions to treatment duration. These comments from nurses and patients suggest some concerns and strategies to consider when involving nurse and patient stakeholders in patient safety and quality improvement activities.
Our findings have several implications. First, they provide support that a hemodialysis safety checklist is a feasible patient safety tool that can be integrated into every hemodialysis session. The exact design of the checklist should be modified to the policies and practices of the local hemodialysis unit and its patient safety objectives, keeping in mind that hemodialysis is a fairly stereotyped process such that the core features of our checklist should be considered in the design of other hemodialysis checklists. Second, our findings highlight the importance of patient safety to nursing staff and patients, as both groups agreed that this initiative improved patient safety and should be expanded to other patients. This result supports local, provincial and national health care mandates to improve patient safety and may serve as the initial impetus to consider a policy to use checklists in the hemodialysis unit as a measure to promote best practices and organizational safety culture. In Canada, governments have already started to incorporate checklists and their compliance as a quality metric in other medical disciplines [29].
The strengths of our study include its practical quality improvement approach, which incorporated real-time feedback from nurses and patients to improve the checklist. We also performed a small random audit of checklist encounters to document the quality of the interaction, which is often omitted in other checklist studies [27]. Our checklist was designed using a structured panel process, which is a proven technique for developing quality and patient safety measures in health care. We also involved human factors engineers to address safety problems that the checklist could introduce as a result of interactions between people, technology and work environments [30]. Even though the hard copy format added time to the nurses' workload, we believe that this step may have helped integrate the checklist into normal hemodialysis workflow. Some integration challenges still remain with our checklist, given a completion rate of <100%. Our greatest challenges going forward are to further minimize duplication of work and ensure that the checklist provides an immediate advantage to staff to compensate for their upfront time commitment. It is not enough to develop a fast and simple checklist since staff must also feel that the checklist makes their work easier and helps patients for it to become usual care. This combination has been achieved with some surgical and intensive care unit checklists [9, 31], which suggests similar checklist integration may be possible with hemodialysis.
Our study also has several limitations. First, the checklist testing period was only 3 months and therefore it is possible that the observed checklist completion rate may not be sustainable. This drop-off was encountered by Marcelli et al. [10], where checklist usage was compromised by the opening of a new dialysis shift. Second, the generalizability of our findings is limited by the single-center design involving a small number of self-selected nurses and patients. While these latter two limitations can be addressed by studying the Hemo Pause checklist for additional time and in different clinical settings, the purpose of this study was to demonstrate feasibility rather than sustainability and spread. Moreover, as hemodialysis is a fairly stereotyped process, many of the components of our checklist could be incorporated into future hemodialysis checklists. Third, Table 2 should be interpreted with caution since the objective was to inform outcome selection for future studies. We suspect some measurement and ascertainment bias given the number of outcomes that were recorded in this feasibility study. Finally, there is no high-quality evidence to prove that the Hemo Pause checklist improves patient safety culture, the patient experience or patient outcomes.
We are currently addressing these questions in the next phase of our quality improvement program. First, the Hemo Pause checklist will be expanded locally at St Michael's Hospital. We will randomize different hemodialysis shifts to the Hemo Pause checklist or usual care for 3 months. The primary outcome will be safety culture and patient experience, as measured by validated Agency for Healthcare Research and Quality tools [32, 33]. Secondary outcomes will include intradialytic hypotension, access infections, hospital admissions and death. We will also include a formal qualitative interview component to try and identify the mechanisms by which the Hemo Pause checklist may improve patient safety and reduce adverse events. If the survey and qualitative data support the Hemo Pause checklist, we would then engage other hemodialysis units to conduct a cluster randomized controlled trial that is adequately powered for the composite outcome of intradialytic hypotension, access infections, hospital admissions and death.
Conclusion
In summary, our study shows that the Hemo Pause safety checklist was acceptable to both nurses and patients when integrated into usual care over a 3-month period. Further research is needed to determine the role of checklists in hemodialysis and their impact on safety culture, the patient experience and clinical outcomes.
Authors' contributions
S.S., A.T., R.W., Z.H. and C.B. contributed to the research idea and study design; A.T., A.R. and P.R. participated in data acquisition; S.S., A.T., A.R., P.R., Z.H. and C.B. carried out data analysis/interpretation; statistical analysis was done by S.S. and A.R.; and Z.H. and C.B were involved in supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors approved the final version of the submitted manuscript. Z.H. takes responsibility that this study has been reported honestly, accurately and transparently and that no important aspects of the study have been omitted.
Conflict of interest statement
None declared.
Acknowledgements
The authors would like to thank Jill Campbell, the hemodialysis nurses, the hemodialysis staff and the hemodialysis patients at St Michael's Hospital for their support of the Hemo Pause patient safety initiative. S.S. is supported by a Kidney Research Scientist Core Education and National Training Program Post-Doctoral Fellowship (co-funded by the Kidney Foundation of Canada, Canadian Society of Nephrology and Canadian Institutes of Health Research).
References
- 1.Bray BD, Boyd J, Daly C et al. How safe is renal replacement therapy? A national study of mortality and adverse events contributing to the death of renal replacement therapy recipients. Nephrol Dial Transplant 2014; 29: 681–687 [DOI] [PubMed] [Google Scholar]
- 2.Renal Physicians Association: Health and safety survey to improve patient safety in end stage renal disease. http://www.kidneypatientsafety.org/about.aspx (10 November 2015, date last accessed)
- 3.Page A. Keeping Patients Safe: Transforming the Work Environment of Nurses. Washington, DC: National Academies Press, 2004 [PubMed] [Google Scholar]
- 4.Pippias M, Tomson CR. Patient safety in chronic kidney disease: time for nephrologists to take action. Nephrol Dial Transplant 2014; 29: 473–475 [DOI] [PubMed] [Google Scholar]
- 5.Kliger AS. Maintaining safety in the dialysis facility. Clin J Am Soc Nephrol 2015; 10: 688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulrich B, Kear T. Patient safety culture in nephrology nurse practice settings: initial findings. Nephrol Nurs J 2014; 41: 459–475 [PubMed] [Google Scholar]
- 7.Ulrich B, Kear T. Patient safety and patient safety culture: foundations of excellent health care delivery. Nephrol Nurs J 2014; 41: 447–456 [PubMed] [Google Scholar]
- 8.Garrick R, Kliger A, Stefanchik B. Patient and facility safety in hemodialysis: opportunities and strategies to develop a culture of safety. Clin J Am Soc Nephrol 2012; 7: 680–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Treadwell JR, Lucas S, Tsou AY. Surgical checklists: a systematic review of impacts and implementation. BMJ Qual Saf 2014; 23: 299–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcelli D, Matos A, Sousa F et al. Implementation of a quality and safety checklist for haemodialysis sessions. Clin Kidney J 2015; 8: 265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galland R, Hallonet P, Pachot M et al. Interests of advanced systematic evaluation of dialysis session. Nephrol Ther 2013; 9: 215–221 [DOI] [PubMed] [Google Scholar]
- 12.Silver SA, Thomas A, Rathe A et al. Development of a hemodialysis safety checklist using a structured panel process. Can J Kidney Health Dis 2015; 2: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guttmann A, Razzaq A, Lindsay P et al. Development of measures of the quality of emergency department care for children using a structured panel process. Pediatrics 2006; 118: 114–123 [DOI] [PubMed] [Google Scholar]
- 14.Kroger E, Tourigny A, Morin D et al. Selecting process quality indicators for the integrated care of vulnerable older adults affected by cognitive impairment or dementia. BMC Health Serv Res 2007; 7: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindsay P, Schull M, Bronskill S et al. The development of indicators to measure the quality of clinical care in emergency departments following a modified-Delphi approach. Acad Emerg Med 2002; 9: 1131–1139 [DOI] [PubMed] [Google Scholar]
- 16.Bell CM, Brener SS, Comrie R et al. Quality measures for medication continuity in long-term care facilities, using a structured panel process. Drugs Aging 2012; 29: 319–327 [DOI] [PubMed] [Google Scholar]
- 17.Morris AM, Brener S, Dresser L et al. Use of a structured panel process to define quality metrics for antimicrobial stewardship programs. Infect Control Hosp Epidemiol 2012; 33: 500–506 [DOI] [PubMed] [Google Scholar]
- 18.Jeffs L, Law MP, Straus S et al. Defining quality outcomes for complex-care patients transitioning across the continuum using a structured panel process. BMJ Qual Saf 2013; 22: 1014–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Provost LP, Murray SK. The Health Care Data Guide: Learning From Data for Improvement. San Francisco, CA: Jossey-Bass, 2011 [Google Scholar]
- 20.Benneyan JC, Lloyd RC, Plsek PE. Statistical process control as a tool for research and healthcare improvement. Qual Saf Health Care 2003; 12: 458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan HC, Provost LP, Froehle CM et al. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf 2012; 21: 13–20 [DOI] [PubMed] [Google Scholar]
- 22.Bray BD, Metcalfe W. Improving patient safety in haemodialysis. Clin Kidney J 2015; 8: 262–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leape LL. The checklist conundrum. N Engl J Med 2014; 370: 1063–1064 [DOI] [PubMed] [Google Scholar]
- 24.Ogrinc GS, Headrick LA, Moore SM et al. Fundamentals of Health Care Improvement: A Guide to Improving Your Patient's Care. Cambridge, MA: Institute for Healthcare Improvement, 2012 [Google Scholar]
- 25.Morello RT, Lowthian JA, Barker AL et al. Strategies for improving patient safety culture in hospitals: a systematic review. BMJ Qual Saf 2013; 22: 11–18 [DOI] [PubMed] [Google Scholar]
- 26.Weaver SJ, Lubomksi LH, Wilson RF et al. Promoting a culture of safety as a patient safety strategy: a systematic review. Ann Intern Med 2013; 158(5 Pt 2): 369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clay-Williams R, Colligan L. Back to basics: checklists in aviation and healthcare. BMJ Qual Saf 2015; 24: 428–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang DT, Clermont G, Sexton JB et al. Perceptions of safety culture vary across the intensive care units of a single institution. Crit Care Med 2007; 35: 165–176 [DOI] [PubMed] [Google Scholar]
- 29.Urbach DR, Govindarajan A, Saskin R et al. Introduction of surgical safety checklists in Ontario, Canada. N Engl J Med 2014; 370: 1029–1038 [DOI] [PubMed] [Google Scholar]
- 30.Agency for Healthcare Research and Quality. Human Factors Engineering. http://psnet.ahrq.gov/primer.aspx?primerID=20 (9 November 2015, date last accessed) [DOI] [PubMed]
- 31.Berenholtz SM, Pronovost PJ, Lipsett PA et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med 2004; 32: 2014–2020 [DOI] [PubMed] [Google Scholar]
- 32.Agency for Healthcare Research and Quality. Surveys on Patient Safety Culture. http://www.ahrq.gov/professionals/quality-patient-safety/patientsafetyculture/index.html (20 January 2016, date last accessed)
- 33.Agency for Healthcare Research and Quality. CAHPS In-Center Hemodialysis Survey. https://cahps.ahrq.gov/surveys-guidance/ich/index.html (20 January 2016, date last accessed)