Abstract
C3 glomerulopathy, a newly designated entity, is characterized by glomerular disease associated with dysregulation of the alternative complement pathway and is a rare cause of end-stage kidney disease. Overall disease characteristics that include clinical presentation, laboratory assessment, histopathology and genetic background have only been unravelled in recent years and have led to the development of anti-complement therapies targeting different levels of the alternative pathway. We describe the long-term outcomes following kidney transplantation in an Irish family with familial C3 glomerulopathy due to a hybrid CFHR3-1 gene.
Keywords: complement, graft function, graft survival, kidney transplantation
Introduction
C3 glomerulopathy is a new clinical entity describing complement-mediated glomerular pathology as a result of genetic or acquired defects in the regulation of the alternative complement pathway and is pathologically characterized by the deposition of C3 in the glomerulus in the absence of significant immunoglobulin [1]. The term encompasses dense deposit disease (DDD), previously known as membranoproliferative glomerulonephritis (MPGN) type II, C3 glomerulonephritis (C3GN) and complement factor H-related protein 5 (CFHR5) nephropathy. Electron dense deposits are seen within the glomerulus in all forms of C3 glomerulopathy. DDD is distinguished by the presence of intensely osmiophilic, ribbon-like electron dense material in the intramembranous location with associated transformation of the glomerular basement membrane [2]. In C3GN, the deposits are less discrete, more ill-defined and found in the mesangium and capillary walls in various combinations including subendothelial, intramembranous and subepithelial locations, with a similar description designated for MPGN type III [3, 4].
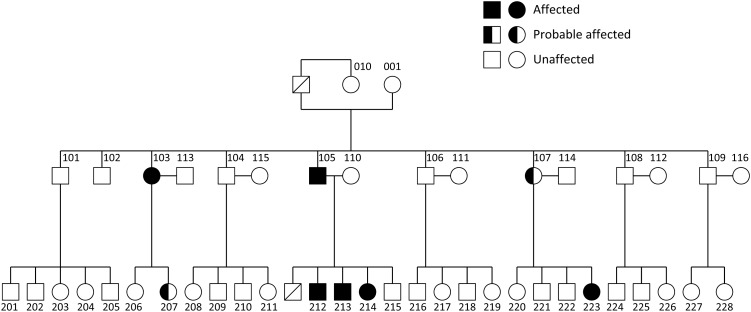
The age of presentation for C3 glomerulopathy can vary widely. DDD is frequently diagnosed in children and young adults while the other forms are found in an older age group. Presenting features consist of proteinuria, haematuria, hypertension and progressive renal failure, leading to end-stage kidney disease (ESKD) in 36–50% of patients [3, 5]. Genetic factors have been identified in cohorts of patients with C3 glomerulopathy and these include mutations in the complement regulatory protein factor H (CFH), factor I (CFI), CD46 (also known as membrane cofactor protein) [3], C3 [6], as well as genomic rearrangements within the complement factor H-related (CFHR) genes, such as internal duplication of the CFHR5 gene [7] and CFHR1 gene [8] and chromosomal deletion of the CFHR gene cluster leading to expression of a CFHR2-CFHR5 hybrid plasma protein [9]. An Irish family was first reported with autosomal dominant MPGN type III and linkage to the regulators of complement activation locus (chromosome 1q) was demonstrated more than a decade ago [10, 11]. A hybrid CFHR3-1 gene was subsequently identified, encoding an abnormal CFHR3-1 protein in eight affected family members over three generations (Figure 1) [12], of which five received kidney transplants. The outcomes of kidney transplantation in patients with C3G are generally favourable, although recurrence of disease in the allograft is common, reducing the overall allograft survival [13–16]. We describe the outcomes of kidney transplantation in this family (Table 1).
Fig. 1.
Pedigree with familial C3 glomerulopathy. Affected individuals were confirmed on renal biopsy and probable affected members had abnormal urinalysis with either significant proteinuria (at least 3+ or >300 mg over 24 h) or haematuria (at least 3+ on two occasions).
Table 1.
Characteristics of all five affected family members who underwent kidney transplantation
| Case index |
|||||
|---|---|---|---|---|---|
| Characteristics | 103 | 105 | 212 | 213 | 214 |
| Age of diagnosis (years) | 25 | 51 | 28 | 4 | 21 |
| Number of kidney transplants | 2 | 1 | 1 | 3 | 1 |
| Age at 1st tx (years) | 51 | 59 | 34 | 11 | 31 |
| Status of 1st tx | NF | F | F | NF | F |
| Age at 2nd tx (years) | 72 | – | – | 24 | – |
| Status of 2nd tx | F | – | – | NF | – |
| Age at 3rd tx (years) | – | – | – | 41 | – |
| Status of 3rd tx | – | – | – | F | – |
| 1st graft survival (months) | 116 | 129 (follow-up) | 105 (follow-up) | 79 | 49 (follow-up) |
| 2nd graft survival (months) | 9 (follow-up) | – | – | 170 | – |
| 3rd graft survival (months) | – | – | – | 5 (follow-up) | – |
| Recurrence in graft | Yes (bx proven) | – | Yes (bx proven) | Yes (bx proven) | – |
| Time to recurrence (months) | 101 | – | 93 | 0.5 | – |
| Creatinine (µmol/L) | 90 | 137 | 105 | 120 | 74 |
| UPCR (mg/mmol) | 39 | 10 | 179 | 10 | 13 |
bx, biopsy; tx, transplant; Status of transplant: NF, non-functioning; F, functioning.
Case presentation
Case index 103
She was first diagnosed with C3GN at the age of 25 years and progressed to end-stage kidney disease (ESKD) when she was 47 years old. She was on haemodialysis for 4 years prior to her first deceased donor kidney transplant [human leucocyte antigen mismatch (HLA MM) 1-1-0; panel reactive antibody (PRA) 5%; donation after brain death (DBD); donor age 20 years; donor creatinine 139 µmol/L]. She was noted to have slowly rising creatinine and proteinuria (3.2 g/24 h) approximately 8 years post-transplant. Transplant biopsy revealed features of a membranoproliferative pattern of injury. A large number of electron dense deposits were seen both in the mesangial regions and in the subendothelial aspect of the capillary walls that correlated with the findings of significant amounts of C3 by the direct immunofluorescence (DIF) technique, confirming disease recurrence in the allograft. Her allograft continued to deteriorate and she eventually returned to haemodialysis 9 years and 8 months post-transplant. The time from transplant to disease recurrence in the allograft was 101 months. Following this, she underwent a second deceased donor kidney transplant at the age of 72 years (HLA MM 0-1-2; PRA 100%; DBD; donor age 44 years; donor creatinine 60 µmol/L). At her most recent follow-up, her serum creatinine was 93 µmol/L (eGFR 51 mL/min) and urine protein:creatinine ratio (UPCR) was 39 mg/mmol. Her current immunosuppressive regimen consists of tacrolimus, mycophenolate mofetil (MMF) and prednisolone (Table 2).
Table 2.
Characteristics of the donor kidneys
| Case index | Transplant | HLA MM | PRA (%) | Donor type | Donor age (years) | Donor creatinine (µmol/L) |
|---|---|---|---|---|---|---|
| 103 | 1st | 1-1-0 | 5 | DBD | 20 | 139 |
| 103 | 2nd | 0-1-2 | 100 | DBD | 44 | 60 |
| 105 | 1st | 1-2-1 | 10 | DBD | 52 | 68 |
| 212 | 1st | 0-1-0 | 5 | DBD | 22 | 105 |
| 213 | 1st | 1-1-1 | 84 | LD | 39 | Normal rangea |
| 213 | 2nd | 1-1-2 | 85 | DBD | 43 | 76 |
| 213 | 3rd | 0-0-0 | 100 | LD | 33 | 77 |
| 214 | 1st | 1-2-2 | 5 | DBD | 18 | 48 |
HLA MM, human leucocyte antigen mismatch; PRA, panel reactive antibody; DBD, donation after brain death; LD, living donation.
aActual figure is not available.
Case index 105
He was first diagnosed with C3GN at the age of 51 years when he presented with hypertension, proteinuria, haematuria and renal impairment. He had previously donated a kidney to his son (case index 213) 12 years prior to presentation. A native kidney biopsy showed a membranoproliferative pattern of injury with large amounts of capillary wall C3 by DIF. On electron microscopy, immune deposits were seen in the distribution of the mesangial regions as well as the subendothelial, intramembranous and subepithelial aspects of the capillary loops. Despite treatment with prednisolone and pulsed cyclophosphamide, he progressed to ESKD and began peritoneal dialysis (PD). He received a deceased donor kidney transplant 1 year later (HLA MM 1-2-1; PRA 10%; DBD; donor age 52 years; donor creatinine 68 µmol/L). His graft function remains stable at his most recent follow-up (129 months post-transplantation) with a serum creatinine of 137 µmol/L (eGFR 43 mL/min) and UPCR of 10 mg/mmol. His immunosuppressive regimen consists of tacrolimus, MMF and prednisolone.
Case index 212
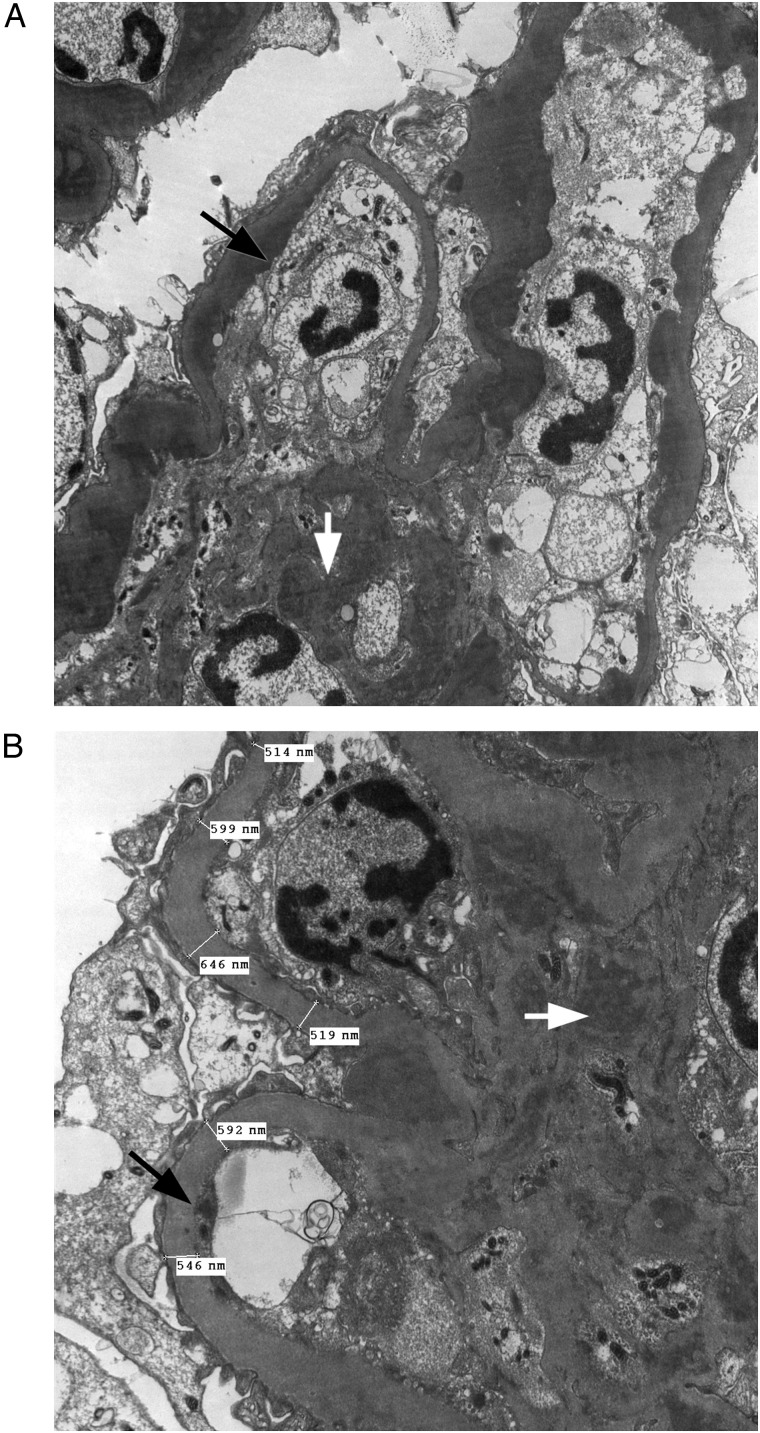
He was first diagnosed with C3GN at the age of 28 years. He progressed to ESKD within a 5-year period and was commenced on PD. He subsequently received a deceased donor kidney transplant (HLA MM 0-1-0; PGEN 5%; DBD; donor age 22 years; donor creatinine 105 µmol/L). His immunosuppressive regimen consists of tacrolimus and MMF. He developed significant proteinuria (1.8 g/24 h) a few years following transplant and a transplant biopsy showed features of recurrence of MPGN. This was confirmed by electron microscopy, with electron dense deposits seen in many capillary loops, predominantly in a subendothelial location with some also seen in a subepithelial location (Figure 2A and B). There were no clinical or pathologic features of allograft rejection. The time from kidney transplant to disease recurrence was 93 months. His most recent serum creatinine at follow-up was 105 µmol/L (eGFR >60 mL/min).
Fig. 2.
The electron micrographs illustrate features in keeping with recurrence of C3 MPGN in the allograft of case index 212. They show the presence of electron dense deposits in many capillary loops in a predominantly subendothelial location (black arrows) and mesangium (white arrows) under direct (A) ×6000 magnification and (B) ×10 000 magnification. In addition, there appears to be global increase in thickness of the basement membranes, most probably related to calcineurin inhibitor.
Case index 213
He presented at the age of 4 years with steroid-resistant nephrotic syndrome and MPGN type III was demonstrated on initial kidney biopsy. He progressed to ESKD 4 years later when he presented with malignant hypertension and stroke resulting in left-sided hemiparesis. He received a living donor kidney transplant from case index 105. Two weeks post-transplant he had a transplant biopsy that showed an acute diffuse proliferative glomerulonephritis, with the presence of subendothelial as well as subepithelial deposits suggestive of early recurrence of his original disease. A repeat biopsy was performed 2 years following transplant due to a slow but progressive rise in serum creatinine and proteinuria. This showed a C3 membranoproliferative pattern of injury, with a large number of deposits seen both in the mesangial regions and in the capillary loops, which confirmed recurrence of disease. There was no evidence of graft rejection. He progressed to allograft failure 6.5 years post-transplant and was commenced on PD. He received a second deceased donor kidney transplant at the age of 24 years that lasted for 14 years (HLA MM 1-1-2; PRA 85%; DBD; donor age 43 years; donor creatinine 76 µmol/L). In addition, he has a significant cardiac history with an episode of myocardial infarction, previous coronary artery bypass grafting and an implantable cardioverter defibrillator inserted. More recently, he received a third kidney transplant from his brother who was genetically screened and was negative for the familial mutation (HLA MM 0-0-0; PRA 100%; living donor; donor age 33 years; donor creatinine 77 µmol/L). His graft function remains stable with a creatinine of 120 µmol/L (eGFR 55 mL/min).
Case index 214
She was first diagnosed with C3GN at the age of 21 years. She progressed to ESKD and received a deceased donor kidney transplant 10 years later (HLA MM 1-2-2; PRA 5%; DBD; donor age 18 years; donor creatinine 48 µmol/L). Her graft function has been very stable over the years, with a serum creatinine of 74 µmol/L (eGFR >60 mL/min) and UPCR of 13 mg/mmol at her most recent follow-up (49 months post-transplantation). Her maintenance immunosuppressive regimen consists of tacrolimus, azathioprine and prednisolone. She has no clinical or biochemical evidence of disease recurrence.
Discussion
The CFHR3-1 hybrid gene is a unique cause of C3 glomerulopathy, most probably due to an abnormal crossover event during meiosis, as there are frequent interspersed repeat elements within the CFH-CFHR locus [12]. This is inherited in an autosomal dominant fashion and the exact cause in this family remains to be elucidated. In a previous study, a chromosomal deletion in the CFHR2 gene led to production of a hybrid CFHR21,2-CFHR5 plasma protein that stabilized C3 convertase and reduced the factor H-mediated convertase decay, enhancing alternative pathway activation and ultimately an increase in C3b deposition along the glomerular basement membrane and glomerular damage [9]. In addition, Gale et al. [7] also demonstrated that the mutant CHFR5 protein was associated with reduced affinity towards surface-bound complement. Thus, it was speculated that the mutant CFHR3-1 protein might have caused an abnormal accumulation of C3 within the kidneys through disruption of alternative pathway regulation by CFH and CFHR [12].
To our knowledge, this is the first description of renal transplant outcomes of hybrid CFHR3-1 gene–associated C3 glomerulopathy. In our cohort, five patients received a total of eight kidney transplants. Four renal allografts had disease recurrence (50%), of which three had biopsy-proven recurrence in the allografts, with time to recurrence ranging from as early as 2 weeks following living related donor transplantation (case index 213) to 93 and 101 months for the two remaining allografts, respectively. Similarly, Vernon et al. [17] previously reported one case of histological recurrence of CFHR5 nephropathy as early as 46 days after deceased donor kidney transplantation. In contrast, Andresdottir et al. [14] showed a biopsy-proven recurrence rate of 100% in DDD, with one recurrence occurring 12 days post-transplantation. There is inevitably an inherent bias in reporting the time of recurrence, as it is dependent on the timing of transplant biopsy, which may not be performed if the allograft remained stable with no clinical signs of acute or chronic rejection. The longest graft survival in our series was 170 months and the median graft survival was 92 months. A retrospective review of paediatric transplant recipients reported graft survival rates of 50% at 5 years in a cohort of 75 children with DDD, which was significantly lower than in transplanted children with all-cause ESKD and non-DDD forms of glomerulonephritis (74 and 72% 5-year graft survival rates, respectively) [15]. Moreover, the outcome of kidney transplantation in the Irish cohort with primary idiopathic MPGN was previously reported by Little et al. [16], where 49% of transplant recipients had evidence of disease recurrence after a median of 4.7 years (with a trend towards a greater likelihood of recurrence in DDD than MPGN), and within this group, 67% eventually lost their graft after a median time of 7.5 years.
There is always a concern over the use of a living related donor as an organ source in familial renal disease, owing to an increased lifetime risk to a genetically related potential donor of developing significant renal disease. In this large kindred, familial MPGN was first seen when case index 105 developed renal impairment following living donor nephrectomy. By recognizing that this familial renal disease is inherited in an autosomal dominant fashion with a wide range of disease onset, many of the first-degree relatives would have been precluded as potential living kidney donors. Fortunately, the underlying familial mutation was identified in recent years and we were able to provide genetic screening to case index 215, who was clinically asymptomatic. He successfully donated a kidney to his HLA-identical brother (case index 214), who has a PRA of 100%, and therefore a very low probability of receiving a third transplant from the deceased donor pool. Our study serves to highlight how advances in the genomic sciences can influence clinical practice and patient care at an individual level. Presently, it is recommended by the C3 glomerulopathy consensus group that all patients, regardless of being diagnosed with disease affecting the native or transplant kidney, should have serological investigations comprising measurements of serum C3, C4, factor H, paraprotein and C3 nephritic factor. Other tests that should be considered on an individual basis include measurement of serum factor B, C5, markers of C3/C5 activation, anti-factor H antibodies, anti-factor B antibodies and mutation screening of complement regulatory genes (such as CFH, CFI and CD46), activation protein gene (C3, CFB) and copy number variation across the CFH-CFHR locus [4]. These investigations should also be considered in potential living kidney donors with a strong family history.
To date, no treatment has been proven to be beneficial in C3 glomerulopathy. The Kidney Disease: Improving Global Outcomes clinical practice guideline for glomerulonephritis recommend that adults or children with presumed idiopathic MPGN accompanied by nephrotic syndrome and progressive decline in kidney function should receive oral cyclophosphamide or MMF with low-dose alternate day or daily corticosteroids with initial therapy limited to <6 months, which is based on level 2D evidence [18]. Recent advancements in our understanding of complement-mediated kidney injury have prompted the use of eculizumab, an anti-C5 humanized monoclonal antibody that inhibits formation of membrane attack complex, as a disease modifying agent. Results from anecdotal cases and open-label studies have shown modest benefits in some patients, although further evidence is awaited [19–22]. In conclusion, recurrence of disease is common in all forms of C3 glomerulopathy following kidney transplantation. Although disease recurrence was high in our cohort, overall transplant graft survival was good and transplantation remains a viable treatment option for ESKD secondary to C3 glomerulopathy.
Conflict of interest statement
None declared.
References
- 1.Fakhouri F, Fremeaux-Bacchi V, Noel LH et al. . C3 glomerulopathy: a new classification. Nat Rev Nephrol 2010; 6: 494–499 [DOI] [PubMed] [Google Scholar]
- 2.Smith RJ, Alexander J, Barlow PN et al. . New approaches to the treatment of dense deposit disease. J Am Soc Nephrol 2007; 18: 2447–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Servais A, Noel LH, Roumenina LT et al. . Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int 2012; 82: 454–464 [DOI] [PubMed] [Google Scholar]
- 4.Pickering MC, D'Agati VD, Nester CM et al. . C3 glomerulopathy: consensus report. Kidney Int 2013; 84: 1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appel GB, Cook HT, Hageman G et al. . Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol 2005; 16: 1392–1403 [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Barricarte R, Heurich M, Valdes-Canedo F et al. . Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest 2010; 120: 3702–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gale DP, de Jorge EG, Cook HT et al. . Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet 2010; 376: 794–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tortajada A, Yebenes H, Abarrategui-Garrido C et al. . C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation. J Clin Invest 2013; 123: 2434–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Wiesener M, Eberhardt HU et al. . Complement factor H-related hybrid protein deregulates complement in dense deposit disease. J Clin Invest 2014; 124: 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neary J, Dorman A, Campbell E et al. . Familial membranoproliferative glomerulonephritis type III. Am J Kidney Dis 2002; 40: E1. [DOI] [PubMed] [Google Scholar]
- 11.Neary JJ, Conlon PJ, Croke D et al. . Linkage of a gene causing familial membranoproliferative glomerulonephritis type III to chromosome 1. J Am Soc Nephrol 2002; 13: 2052–2057 [DOI] [PubMed] [Google Scholar]
- 12.Malik TH, Lavin PJ, Goicoechea de Jorge E et al. . A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol 2012; 23: 1155–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelo JR, Bell CS, Braun MC. Allograft failure in kidney transplant recipients with membranoproliferative glomerulonephritis. Am J Kidney Dis 2011; 57: 291–299 [DOI] [PubMed] [Google Scholar]
- 14.Andresdottir MB, Assmann KJ, Hoitsma AJ et al. . Renal transplantation in patients with dense deposit disease: morphological characteristics of recurrent disease and clinical outcome. Nephrol Dial Transplant 1999; 14: 1723–1731 [DOI] [PubMed] [Google Scholar]
- 15.Braun MC, Stablein DM, Hamiwka LA et al. . Recurrence of membranoproliferative glomerulonephritis type II in renal allografts: the North American Pediatric Renal Transplant Cooperative Study experience. J Am Soc Nephrol 2005; 16: 2225–2233 [DOI] [PubMed] [Google Scholar]
- 16.Little MA, Dupont P, Campbell E et al. . Severity of primary MPGN, rather than MPGN type, determines renal survival and post-transplantation recurrence risk. Kidney Int 2006; 69: 504–511 [DOI] [PubMed] [Google Scholar]
- 17.Vernon KA, Gale DP, de Jorge EG et al. . Recurrence of complement factor H-related protein 5 nephropathy in a renal transplant. Am J Transplant 2011; 11: 152–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int 2012; 2(Suppl 2): 198–199 [Google Scholar]
- 19.Bomback AS, Smith RJ, Barile GR et al. . Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol 2012; 7: 748–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daina E, Noris M, Remuzzi G. Eculizumab in a patient with dense-deposit disease. N Engl J Med 2012; 366: 1161–1163 [DOI] [PubMed] [Google Scholar]
- 21.Radhakrishnan S, Lunn A, Kirschfink M et al. . Eculizumab and refractory membranoproliferative glomerulonephritis. N Engl J Med 2012; 366: 1165–1166 [DOI] [PubMed] [Google Scholar]
- 22.Le Quintrec M, Lionet A, Kandel C et al. . Eculizumab for treatment of rapidly progressive C3 glomerulopathy. Am J Kidney Dis 2015; 65: 484–489 [DOI] [PubMed] [Google Scholar]