Abstract
Background
Variability in the management of glomerulonephritis may negatively impact efficacy and safety. However, there are little/no data on actual variability in the treatment of minimal change disease (MCD)/focal segmental glomerulosclerosis (FSGS) in adults. We assessed Spanish practice patterns for the management of adult nephrotic syndrome due to MCD or FSGS. The absence of reasonably good evidence on treatment for a disease often increases the variability substantially. Identification of evidence–practice gaps is the first necessary step in the knowledge-to-action cyclical process. We aim to analyse the real clinical practice in adults in hospitals in Spain and compare this with the recently released Kidney Disease: Improving Global Outcomes clinical practice guideline for glomerulonephritis.
Methods
Participating centres were required to include all adult patients (age >18 years) with a biopsy-proven diagnosis of MCD or FSGS from 2007 to 2011. Exclusion criteria included the diagnosis of secondary nephropathy.
Results
We studied 119 Caucasian patients with biopsy-proven MCD (n = 71) or FSGS (n = 48) from 13 Spanish hospitals. Of these patients, 102 received immunosuppressive treatment and 17 conservative treatment. The initial treatment was steroids, except in one patient in which mycophenolate mofetil was used. In all patients, the steroids were given as a single daily dose. The mean duration of steroid treatment at initial high doses was 8.7 ± 13.2 weeks and the mean global duration was 38 ± 32 weeks. The duration of initial high-dose steroids was <4 weeks in 41% of patients and >16 weeks in 10.5% of patients. We did find a weak and negative correlation between the duration of whole steroid treatment in the first episode and the number of the later relapses (r = −0.24, P = 0.023). There were 98 relapses and they were more frequent in MCD than in FSGs patients (2.10 ± 1.6 versus 1.56 ± 1.2; P = 0.09). The chosen treatment was mainly steroids (95%). Only seven relapses were treated with another drug as a first-line treatment: two relapses were treated with mycophenolate and five relapses were treated with anticalcineurinics. A second-line treatment was needed in 29 patients (24.4%), and the most frequent drugs were the calcineurin inhibitors (55%), followed by mycophenolate mofetil (31%). Although cyclophosphamide is the recommended treatment, it was used in only 14% of the patients.
Conclusions
We found variation from the guidelines in the duration of initial and tapered steroid therapy, in the medical criteria for classifying a steroid-resistant condition and in the chosen treatment for the second-line treatment. All nephrologists started with a daily dose of steroids as the first-line treatment. The most frequently used steroid-sparing drug was calcineurin inhibitors. Cyclophosphamide use was much lower than expected.
Keywords: clinical practice variability, glomerulonephritis, immunosuppression
Introduction
Variability in the management of glomerulonephritis could negatively impact efficacy and safety, increasing the risk of progression to end-stage renal disease [1]. More than 75% of Canadian IgA nephropathy patients have proteinuria >1 g/day and GFR >50 mL/min/1.73 m2, yet only 33% received steroid treatment despite evidence suggesting this would improve renal outcome [2–4]. Furthermore, 19% of patients with membranous nephropathy and subnephrotic proteinuria are treated with immunosuppression in spite of a good long-term renal outcome with renin–angiotensin system blockade [5]. Currently, there is little information on variability of clinical practice for the management of adult nephrotic syndrome due to minimal change disease (MCD) or focal segmental glomerulosclerosis (FSGS), which are considered rare diseases. Two nephrologist-level surveys have been published in the last 16 years, one on paediatric steroid-resistant FSGS patients and the other on overall glomerulonephritis management, both presenting only North America data [6, 7]. Only one study has assessed the homogeneity of treatment in adults [8], but it did not provide patient-level data, and gaps may exist between what nephrologists plan to do and what they actually do.
The Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for glomerulonephritis presents an excellent summary of the current state of knowledge on glomerular disease management and thus provides a useful framework for the clinician [9].
Childhood nephrotic syndrome is one of the most common childhood kidney diseases, affecting ∼16 per 100 000 children [10]. More than 90% of cases are due to MCD or FSGS. Nephrotic syndrome incidence is lower in adults than in children, and MCD and FSGS account for only 25–30% of adult nephrotic syndrome cases. There are scarce randomized clinical trials (RCTs) addressing therapy for these conditions [11]. As a consequence, most recommendations for the treatment of adult nephrotic syndrome are based largely on extrapolation from childhood studies, and the quality of evidence is low (‘the true effect may be substantially different from the estimate of the effect’) or very low (‘the estimate of effect is very uncertain, and often will be far from the truth’).
Under these circumstances, implementation of guidelines may be compromised if recommendations differ much from usual clinical practice. We aimed to assess Spanish practice patterns for the management of adult nephrotic syndrome due to MCD or FSGS, as a country representative of a public universal health care system, and to compare these with recommendations from the KDIGO clinical practice guideline for glomerulonephritis [9].
Methods
Study population and patient selection
We enrolled patients with a renal biopsy diagnosis of MCD or FSGS from 2007 to 2011 in 13 Spanish hospitals belonging to the Spanish group for the study of glomerular diseases (GLOSEN). Clinical records were retrospectively reviewed. Renal biopsies were processed for light microscopy, immunofluorescence and electron microscopy. Patients younger than 18 years at the time of the biopsy, with any cause of secondary forms or who had received immunosuppressive treatment prior to renal biopsy were excluded. Demographics, baseline characteristics, kind and length of treatment at the beginning and in relapses, frequency and timing of relapses, complications, outcome data and laboratory parameters were recorded.
Definitions
Complete remission was defined as a daily urine protein excretion <0.3 g/day, urine protein:creatinine ratio <0.3 g/g, normal serum creatinine and serum albumin >3.5 g/dL. Partial remission was defined as proteinuria reduction ≥50% from the baseline value with absolute proteinuria 0.3–3.5 g/day (urine protein:creatinine ratio 0.3–3.5 g/g) and stable serum creatinine (change in creatinine <25%). Frequent relapse was defined as two or more relapses within 6 months of initial response or four or more relapses in any 12-months period. Steroid dependence was defined as two consecutive relapses during steroid therapy. Steroid resistance was defined as persistence of proteinuria despite prednisone 1 mg/kg/day or 2 mg/kg every other day for >4 months.
Statistical analyses
Baseline characteristics are expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) for continuous variables and percentage for categorical variables. Data between groups were compared by χ2 test, paired t-test, one-way analysis of variance or Mann–Whitney U test, as appropriate. Statistics were calculated using SPSS for Windows, version 11 (SPSS, Chicago, IL, USA).
Results
We studied 119 adult patients with biopsy-proven MCD (71 patients) or FSGS (48 patients) from 13 GLOSEN hospitals. Baseline characteristics are shown in Table 1. Serum albumin and the number of sclerotic glomeruli were significantly lower in MCD than in FSGS patients (2.10 ± 0.65 versus 2.82 ± 0.99 g/dL and 4.94 ± 10.04 versus 18.20 ± 19.32; P < 0.0001, respectively).
Table 1.
Baseline clinical characteristics of 126 patients included in the study
| Total | MCD | FSGS | P-value | |
|---|---|---|---|---|
| Age (years) | 47.98 ± 19.85 | 50.54 ± 20.13 | 44.34 ± 19.06 | ns |
| BMI (kg/m2) | 26.85 ± 5.38 | 26.48 ± 4.85 | 27.32 ± 6.10 | ns |
| Serum creatinine (μmol/L) | 114.9 ± 70.7 | 109.6 ± 76.9 | 121.1 ± 61.8 | ns |
| Proteinuria (g/24 h) | 7.98 ± 6.73 | 8.71 ± 7.27 | 6.97 ± 5.83 | ns |
| UPCR (mg/g) | 4876 ± 3607 | 4454 ± 2963 | 5453 ± 4359 | ns |
| Albumin (g/dL) | 2.44 ± 0.90 | 2.10 ± 0.65 | 2.82 ± 0.99 | 0.0001 |
| Number of relapses | 1.75 ± 1.44 | 1.96 ± 1.62 | 1.45 ± 1.08 | 0.038 |
| Number of glomeruli | 15.05 ± 8.38 | 14.97 ± 8.98 | 15.08 ± 7.89 | ns |
| Sclerotic glomeruli (%) | 7.36 ± 13.42 | 4.94 ± 10.04 | 18.20 ± 19.32 | 0.0001 |
UPCR, urinary protein:creatinine ratio.
Of the 119 patients, 102 (82%) received steroids or immunosuppressive treatment (calcineurin inhibitors, mycophenolate mofetil or cyclophosphamide; active treatment group) and 17 received conservative treatment. Six of the 102 patients who received active treatment had proteinuria <3.5 g/day and serum albumin >3.5 g/dL. Two of the 17 patients on conservative treatment had proteinuria >3.5 g/day and serum albumin <3.5 g/dL, the rest not having nephrotic syndrome.
Median follow-up was 44 (IQR 22–56) months. At the end of follow-up, 3 (2.5%) patients had died, 20 (16.8%) were lost to follow-up, 71 (59.7%) attained complete remission, 18 (15.1%) achieved partial remission and 7 (5.9%) maintained nephrotic syndrome.
Initial therapy for the first episode
Almost all (101 of the 102) active treatment patients received steroids as first-line treatment. One FSGS patient initially received mycophenolate. The mean initial steroid dose was 63 ± 16 mg/day (0.89 ± 0.13 mg/kg/day) in MCD patients and 63.7 ± 11.79 (0.85 ± 0.14 mg/kg/day) in FSGS patients (ns).
The mean duration of steroids at initial high doses was 8.7 ± 13.2 weeks (median 5, IQR 3–9) and the mean global duration of steroid treatment was 38 ± 32 weeks (median 26, IQR 17–45). The mean duration of initial high-dose steroids was less for MCD than for FSGS patients (5.7 ± 3.5 versus 13.0 ± 19.4 weeks, P = 0.024). The entire steroid treatment duration was also significantly shorter for MCD than for FSGS patients (24.7 ± 20.7 versus 55.6 ± 39.4 weeks; P < 0.0001).
The duration of initial high-dose steroids was <4 weeks in 41% of patients (43% in MCD and 37.5% in FSGS; P = 0.548, NS), 4–8 weeks in 32.6% (40% in MCD versus 22.5% in FSGS; P = 0.017), 8–12 weeks in 8.4% (11% in MCD versus 5% in FSGS; P < 0.001), 12–16 weeks in 7.4% (5.4% in MCD versus 10% in FSGS; P < 0.0001) and >16 weeks in 10.5% of patients, all of them having FSGS (25% of FSGS patients).
Of the 102 active treatment patients, 21 were defined as steroid dependent [17/62 (27.4%) MCD, 4/40 (10%) FSGS; P = 0.03], 26 as steroid resistant [6/62 (9.6%) MCD, 20/40 (50%) FSGS; P < 0.0001, ns] and 55 responded to steroids [39/62 (63%) MCD versus 16/40 FSGS (40%) FSGS; P < 0.002].
The total duration of steroid treatment was longer in steroid-resistant (60.1 ± 36.2 weeks) than in steroid-dependent patients (28.7 ± 25.7 weeks) or responder patients (29.4 ± 27.7 weeks) (P < 0.0001) and we found no difference in high-dose length (9.5 ± 11.8 weeks in steroid dependent, 7.3 ± 5.0 weeks in steroid sensitive and 8.8 ± 15.8 weeks in steroid resistant; P = 0.86). In the steroid-resistant group (n = 26), 7 patients received steroids for <4 weeks and 6 patients for <8 weeks (Figure 1).
Fig. 1.
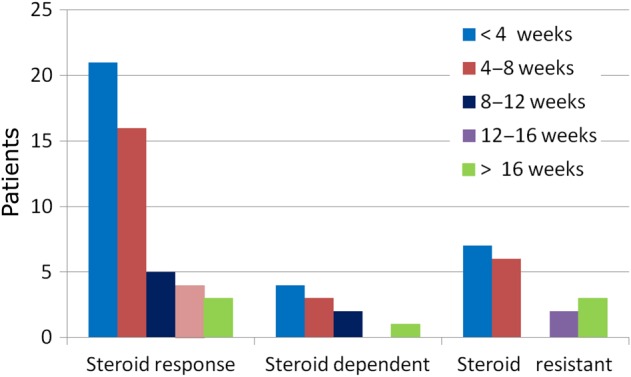
Duration of high-dose steroids according to response.
A negative correlation was found between the full duration of steroid treatment in the first episode and the number of later relapses (r = −0.24, P = 0.023). The average number of relapses was similar in patients who received steroids for 4 versus 4 weeks (1.95 ± 1.60 versus 1.88 ± 1.42). At the end of follow-up, patients who had attained complete remission had received steroid treatment for a shorter period in the first episode (33.9 ± 32 weeks) than patients in partial remission (64.6 ± 28.1 weeks; P = 0.012) or non-responder patients (46.2 ± 62.8 weeks; P = 0.024).
Second-line treatment for the first episode
A second-line treatment was prescribed for the first episode in 29 patients (24.4%): calcineurin inhibitors [n = 16 (55%)], mycophenolate [n= 7 (31%)] and cyclophosphamide [n= 4 (14%)]. This distribution was similar in steroid-dependent and in steroid-resistant patients. Indications for second-line treatment included steroid resistance or dependence, partial response to steroids, frequent relapses and toxicity or contraindications to steroids. Some patients received more than one agent (usually sequentially).
Initial therapy in relapses
There were 98 relapses and they tended to be more frequent in MCD than in FSGS patients (2.10 ± 1.6 versus 1.56 ± 1.2 relapses; P = 0.09) (Figure 2).
Fig. 2.
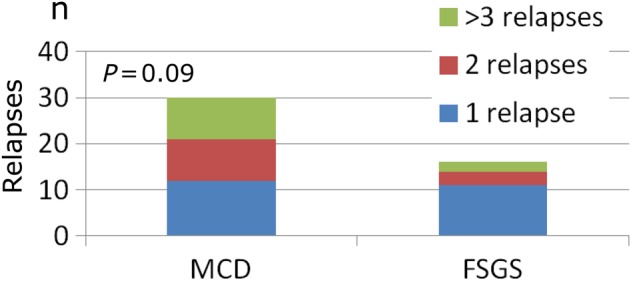
Number of relapses.
Relapses were treated initially with steroids in 91 patients (95%). Only seven relapses were treated initially with another drug: two with mycophenolate and five with calcineurin inhibitors (Figure 3).
Fig. 3.
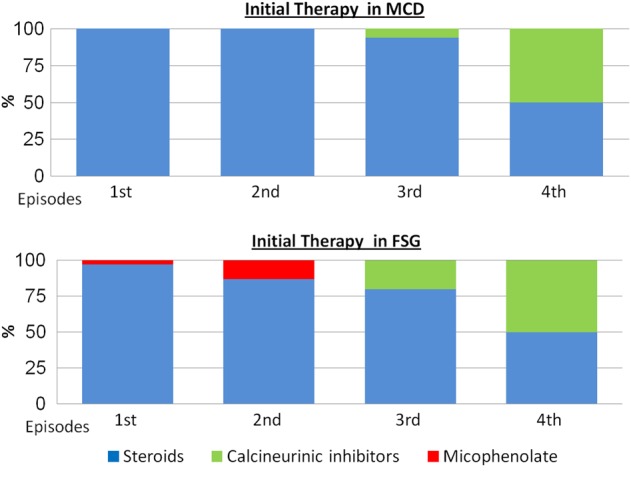
First-line treatment in the first episode and in relapses.
The average initial dose of steroids was significantly lower in each successive relapse: the average dose was 55.79 ± 18.34 mg/day in the first treatment, 55.7 ± 18.3 mg/day in the first relapse, 52.5 ± 17.9 mg/day in the second relapse, 47.0 ± 20.5 mg/day in the third relapse and 42.8 ± 19.7 mg/day in the fourth relapse (P < 0.0001). There was no difference in the duration of steroid treatment between the relapses.
Second-line treatment in relapses
Calcineurin inhibitors were more frequently used in relapses. Thus, they were the second-line treatment in 81% of patients in the first relapse and in 77.7% of patients in the second relapse.
Discussion
Although there is some uniformity, significant practice variability does exist in the management of adult idiopathic nephrotic syndrome, MCD and FSGS in Spain. Our study demonstrated variability in criteria for starting treatment, in duration of initial and tapered off steroid regimens, in medical criteria for classifying the steroid-resistant condition and in the second-line treatment.
Nephrotic syndrome secondary to MCD is associated with significant morbidity due to accelerated atherosclerosis, due in part to dyslipidemia [12], infections [13, 14] and thromboembolic events [15]. Patients with FSGS and persistent nephrotic syndrome are at increased risk of progressive CKD and its accompanying cardiovascular morbidity and mortality. Risks are dependent on the level of proteinuria and kidney function, so patients with non-nephrotic proteinuria have a good prognosis, with kidney survival rates of >95% after a mean follow-up of 6.5–9.3 years [7, 16], even in older studies. Hence, the KDIGO guideline recommends steroids and immunosuppressive therapy only in idiopathic nephrotic syndrome and not in patients with subnephrotic proteinuria. In our study, six patients received immunosuppressive treatment, although they only had subnephrotic proteinuria. In a Canadian survey, 9% of nephrologists would not prescribe immunosuppressive therapy to patients with FSGS and proteinuria >5 g/day and, conversely, 26% of nephrologists would treat FSGS and proteinuria of 2 g/day with prednisone or calcineurin inhibitor [6].
The overall grade of evidence to guide treatment of these diseases is based on and extrapolated from studies in children. The current KDIGO guideline suggests that the initial high dose of steroids should be maintained for a minimum of 4 weeks if complete remission is achieved and for a maximum of 16 weeks if complete remission is not attained. Steroids should be tapered slowly over a total period of up to 6 months after achieving remission [9]. There is some consensus that adults respond more slowly to therapy than children and they should not be considered to be steroid resistant until after 16 weeks of therapy. However, these timelines are arbitrary. A recent systematic review of the Cochrane Central Register of Controlled Trials, Medline and Embase reference articles and abstracts from conference proceedings for RCTs or quasi-RCTs identified only three RCTs with 68 participants >18 years of age. These data proved inadequate to draw any firm conclusions with respect to the utility or duration of prednisone therapy in adults. Nonetheless, in spite of the severe paucity of RCT data, other nephrology societies, such as the Canadian Society of Nephrology, agreed with the general principles of management proposed by the KDIGO guideline [17].
Using these guidelines as a comparison, Spanish nephrologists appear to be prescribing high-dose steroids for less time than recommended in the guideline but keep a similar duration of the tapering period. Treatment length variability was also found in a Canadian and North American practice variation study in children [1, 18].
In our study, nearly half of patients (41%) were treated with high steroid doses for less time than recommended in most guidelines (<16 weeks). The shorter duration was not associated with a higher number of subsequent relapses or with requiring second-line treatment.
In fact, the percentage of steroid-resistant patients in MCD (9.6%) was similar [13, 19–21] or even lower than in previous reports [22, 23]. In our study, the global steroid treatment was long (38 ± 32 weeks), and this may explain the good outcome. In a meta-analysis of published studies in childhood MCD, a longer course of initial steroid treatment (lower dose for longer time) was associated with a lower relapse rate [24]. We questioned whether it is necessary to maintain high-dose steroids for up to 16 weeks if a complete remission is not attained, given the potential for major side effects. In this regard, the addition of three new well-designed studies has changed previous Cochrane Collaboration conclusions on steroid treatment duration in children [24]. There was no significant difference in the risk of steroid dependence between prednisone for 8–12 weeks and longer duration or total dose of therapy, indicating that there is no benefit of increasing the duration of high-dose prednisone beyond 8–12 weeks in the initial episode.
The KDIGO guideline [9] recommends alkylating agents for steroid-dependent patients and suggests calcineurin inhibitors only for patients with relapse despite cyclophosphamide and for those who wish to preserve fertility [9]. This suggestion is based on studies that show that both drugs lead to remission in a significant number of adults [25–27], but the relapse-free interval appears to be longer with cyclophosphamide.
For steroid-resistant nephrotic syndrome, mainly FSGS patients, calcineurin inhibitors are suggested based on several studies [28–30]. The study by Gullatti et al. [29] compared tacrolimus for 12 months or 6 monthly infusion of intravenous cyclophosphamide, with both arms receiving equal amounts of alternate-day prednisolone. Complete remission was significantly higher with tacrolimus (52.4%) than with cyclophosphamide (14.8%).
In our study, the use of cyclophosphamide was the exception not the rule. Calcineurin inhibitors were the most commonly used drugs in steroid-dependent or steroid-resistant patients. In a Canadian variability study, there was uniformity in the use of an alkylating agent (cyclophosphamide) as a steroid-sparing therapy in children with biopsy-proven MCD and calcineurin inhibitors to reduce steroid exposure in children with FSGS. Despite a lack of evidence that kidney biopsy results infer the success for a specific therapy or disease outcome in steroid-dependent or steroid-resistant patients, in the Canadian study, histopathological findings appeared to influence treatment choices [1].
The Cochrane group identified no RCTs comparing regimens in adults with steroid-dependent or relapsing disease or comparing treatment regimens comprising alkylating agents, cyclosporine, tacrolimus, levamisole or mycophenolate mofetil [11].
The KDIGO guideline's preface states the primary goal of the guideline is ‘to improve quality of care … by helping clinicians know and better understand the evidence (or lack of evidence) that determines practice’. However, there is a strong body of literature suggesting that despite the presence of reasonably good evidence on treatment for a disease, current clinical practice often substantially deviates from the evidence [6]. In the present Spanish study, adherence to guidelines was higher for recommendations supported by good evidence, for instance, the use of steroids as first-line treatment, and has been more chaotic for other recommendations based on lower-quality evidence, e.g. the duration of steroid treatment and second-line treatment in patients with steroid dependence or steroid resistance.
Spain is a country with universal health care access and insurance coverage for immunosuppressive medications. Therefore, the observed variability would not be linked to poor access to medical care or immunosuppressive drugs.
Most (95%) recommendations for the management of MCD and FSGS in adults are Grade ‘C’ or lower and three recommendations are not graded. Systematic reviews usually conclude that new RCTs should be carried out and that extrapolation of the paediatric literature should be avoided, but the state of the art has not changed in recent decades.
These are orphan diseases, and the limited number of new cases seen yearly by individual nephrologists causes variability and limits our ability to advance therapy. Reference centres should be instituted that either care for these patients or provide and monitor advice to individual nephrologists. This kind of structure would allow the implementation of prospective homogeneous protocols with frequent assessment of results and facilitate enrolling patients in RCTs.
There are several limitations in our study design. The studied period preceded the KDIGO glomerulonephritis guideline publication in 2012. Thus, these results should not be interpreted as a failure of guideline implementation, but instead highlight the variability in practice patterns and current care gaps linked to literature evidence at that moment. On the other hand, most participant centres (but not all) are academic hospitals, so it is possible that regional hospitals present a different spectrum of variability. Finally, in some clinical scenarios (e.g. adverse reactions to drugs, unacceptable cosmetic side effects), deviations from published guidelines and therapy individualization are entirely justified, and we do not consider these particular situations. We should not forget that these diseases can affect old and very old patients. In the Spanish glomerular disease registry, 13.4 and 8.2% of renal biopsies in patients >65 years of age with nephrotic syndrome were diagnosed as MCD and FSGS, respectively. Intolerance to steroid or other immunosuppressive therapy tends to be more significant and severe in the presence of advanced age and other comorbid conditions, such as obesity and diabetes. Even evidence supported by good quality data may not suit an individual patient.
In summary, we have documented significant clinical practice variability and evidence of practice gaps in the management of adult nephrotic syndrome in Spain. Clinical practice variability is higher in items supported by low (Grade C) or very low grade evidence (Grade D or not graded). The high clinical practice variability and low-quality evidence to support recommendations argue for the establishment of reference centres where patients with these rare diseases can be treated following homogeneous protocols and enrolled in RCTs.
Conflict of interest statement
None declared.
Acknowledgements
A.O. received ISCII and FEDER funds (FIS PI13/00047ISCIII-RETIC REDinREN RD12/0021), Programa Intensificación Actividad Investigadora (ISCIII/Agencia Laín-Entralgo/CM).
References
- 1.Samuel S, Morgan CJ, Bitzan M et al. Substantial practice variation exists in the management of childhood nephrotic syndrome. Pediatr Nephrol Berl Ger 2013; 28: 2289–2298 [DOI] [PubMed] [Google Scholar]
- 2.Barbour SJ, Cattran DC, Kim SJ et al. Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int 2013; 84: 1017–1024 [DOI] [PubMed] [Google Scholar]
- 3.Manno C, Torres DD, Rossini M et al. Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant 2009; 24: 3694–3701 [DOI] [PubMed] [Google Scholar]
- 4.Pozzi C, Andrulli S, Del Vecchio L et al. Corticosteroid effectiveness in IgA nephropathy: long-term results of a randomized, controlled trial. J Am Soc Nephrol 2004; 15: 157–163 [DOI] [PubMed] [Google Scholar]
- 5.Hladunewich MA, Troyanov S, Calafati J et al. Metropolitan Toronto Glomerulonephritis Registry: the natural history of the non-nephrotic membranous nephropathy patient. Clin J Am Soc Nephrol 2009; 4: 1417–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour S, Beaulieu M, Gill J et al. The need for improved uptake of the KDIGO glomerulonephritis guidelines into clinical practice in Canada: a survey of nephrologists. Clin Kidney J 2014; 7: 538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velosa JA, Donadio JV, Holley KE. Focal sclerosing glomerulonephropathy: a clinicopathologic study. Mayo Clin Proc 1975; 50: 121–133 [PubMed] [Google Scholar]
- 8.Stirling CM, Mathieson P, Boulton-Jones JM et al. Treatment and outcome of adult patients with primary focal segmental glomerulosclerosis in five UK renal units. QJM 2005; 98: 443–449 [DOI] [PubMed] [Google Scholar]
- 9.Summary of recommendation statements. Kidney Int Suppl 2012; 2: 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gipson DS, Massengill SF, Yao L et al. Management of childhood onset nephrotic syndrome. Pediatrics 2009; 124: 747–757 [DOI] [PubMed] [Google Scholar]
- 11.Palmer SC, Nand K, Strippoli GF. Interventions for minimal change disease in adults with nephrotic syndrome. The Cochrane Collaboration (ed). Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, 2008. http://doi.wiley.com/10.1002/14651858.CD001537.pub4 (13 October 2015, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radhakrishnan J, Appel AS, Valeri A et al. The nephrotic syndrome, lipids, and risk factors for cardiovascular disease. Am J Kidney Dis 1993; 22: 135–142 [DOI] [PubMed] [Google Scholar]
- 13.Huang JJ, Hsu SC, Chen FF et al. Adult-onset minimal change disease among Taiwanese: clinical features, therapeutic response, and prognosis. Am J Nephrol 2001; 21: 28–34 [DOI] [PubMed] [Google Scholar]
- 14.McIntyre P, Craig JC. Prevention of serious bacterial infection in children with nephrotic syndrome. J Paediatr Child Health 1998; 34: 314–317 [DOI] [PubMed] [Google Scholar]
- 15.Mahmoodi BK, ten Kate MK, Waanders F et al. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation 2008; 117: 224–230 [DOI] [PubMed] [Google Scholar]
- 16.Cameron JS, Turner DR, Ogg CS et al. The long-term prognosis of patients with focal segmental glomerulosclerosis. Clin Nephrol 1978; 10: 213–218 [PubMed] [Google Scholar]
- 17.Cybulsky AV, Walsh M, Knoll G et al. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis: management of glomerulonephritis in adults. Am J Kidney Dis 2014; 63: 363–377 [DOI] [PubMed] [Google Scholar]
- 18.Lande MB, Leonard MB. Variability among pediatric nephrologists in the initial therapy of nephrotic syndrome. Pediatr Nephrol 2000; 14: 766–769 [DOI] [PubMed] [Google Scholar]
- 19.Szeto C-C, Lai FM-M, Chow K-M et al. Long-term outcome of biopsy-proven minimal change nephropathy in Chinese adults. Am J Kidney Dis 2015; 65: 710–718 [DOI] [PubMed] [Google Scholar]
- 20.Mak SK, Short CD, Mallick NP. Long-term outcome of adult-onset minimal-change nephropathy. Nephrol Dial Transplant 1996; 11: 2192–2201 [DOI] [PubMed] [Google Scholar]
- 21.Korbet SM, Schwartz MM, Lewis EJ. Minimal-change glomerulopathy of adulthood. Am J Nephrol 1988; 8: 291–297 [DOI] [PubMed] [Google Scholar]
- 22.Waldman M, Crew RJ, Valeri A et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol 2007; 2: 445–453 [DOI] [PubMed] [Google Scholar]
- 23.Nolasco F, Cameron JS, Heywood EF et al. Adult-onset minimal change nephrotic syndrome: a long-term follow-up. Kidney Int 1986; 29: 1215–1223 [DOI] [PubMed] [Google Scholar]
- 24.Hodson EM, Knight JF, Willis NS et al. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev 2015; 3: CD001533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ESCAPE Trial Group, Wühl E, Trivelli A et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med 2009; 361: 1639–1650 [DOI] [PubMed] [Google Scholar]
- 26.Rovin BH, McKinley AM, Birmingham DJ. Can we personalize treatment for kidney diseases? Clin J Am Soc Nephrol 2009; 4: 1670–1676 [DOI] [PubMed] [Google Scholar]
- 27.Upadhyay A, Earley A, Haynes SM et al. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med 2011; 154: 541–548 [DOI] [PubMed] [Google Scholar]
- 28.Ponticelli C, Villa M, Banfi G et al. Can prolonged treatment improve the prognosis in adults with focal segmental glomerulosclerosis? Am J Kidney Dis 1999; 34: 618–625 [DOI] [PubMed] [Google Scholar]
- 29.Gulati A, Sinha A, Gupta A et al. Treatment with tacrolimus and prednisolone is preferable to intravenous cyclophosphamide as the initial therapy for children with steroid-resistant nephrotic syndrome. Kidney Int 2012; 82: 1130–1135 [DOI] [PubMed] [Google Scholar]
- 30.Cattran DC, Appel GB, Hebert LA et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int 1999; 56: 2220–2226 [DOI] [PubMed] [Google Scholar]


