Abstract
Background
Management trends in early chronic kidney disease (CKD) and their associations with clinical outcomes have not previously been reported.
Methods
We evaluated incident (Stage G3A) CKD patients from an integrated health care system in 2004–06, 2007–09 and 2010–12 to determine adjusted trends in screening (urinary protein quantification), treatment [prescription for angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), and statin] and nephrology referral. For the same time periods, adjusted rates for mortality, progression to Stage G4 CKD and hospitalization for myocardial infarction or heart failure were calculated and compared across time periods.
Results
There were 728, 788 and 956 patients with incident CKD in 2004–06, 2007–09 and 2010–12, respectively. Adjusted rates of proteinuria quantification (31, 39 and 51 screens/100 person-years), statin prescription (53, 63 and 64 prescriptions/100 person-years) and nephrology referral (2, 3 and 5 referrals/100 person-years) all increased over time (P for trend <0.001 in all cases). ACEI/ARB prescription rates did not change (88, 83 and 80 prescriptions/100 person-years, P = 0.68). Adjusted death rates (7, 5 and 6 deaths/100 person-years), CKD progression (9, 10 and 7 progressors/100 person-years) and cardiovascular hospitalization (10, 8 and 9 hospitalizations per 100/person-years) did not change (P for trend >0.4 in all cases).
Conclusion
In this integrated health care system, management of incident CKD over the past decade has intensified.
Keywords: cardiovascular, chronic renal insufficiency, epidemiology, proteinuria, renin-angiotensin system
Introduction
Over the past decade, several consensus panels and workgroups have published position statements promoting a more aggressive approach to the screening and treatment of chronic kidney disease (CKD) [1–3]. Studies of management patterns in prevalent CKD patients are limited, but suggest that the uptake of evidence-based screening and treatment recommendations is low [4–6]. For example, a retrospective cohort study of 11 000 primary care patients with prevalent Stage G3 or G4 CKD identified proteinuria screening in only a third of the population, and fewer than half had controlled blood pressure [4]. Trends in screening and treatment of patients with CKD over the past decade are not well characterized, and it is therefore difficult to assess the impact of efforts to promote a more aggressive management approach in this population.
Additionally, information on the impact of changes in screening and treatment on patient outcomes is also limited. Careful assessment of patient outcomes is important given the potential risks, burdens and costs for patients and health care systems of more intensive screening and treatment programs. Moreover, the evidence used to formulate current consensus guidelines for patients with CKD is largely cited as weak-to-moderate [7]. Evaluation of outcomes in populations receiving guideline-based care can help confirm the benefit of recommended screening and treatment strategies.
In order to assess trends in CKD management, we evaluated screening, treatment and outcomes from 2004 to 2012 among incident CKD (Stage G3) patients in the Geisinger Health System, a large integrated health care system in central Pennsylvania. We chose to evaluate practice patterns in incident rather than prevalent patients since analyses of prevalent populations can be complicated by variable CKD duration and a greater likelihood of prior CKD-specific treatment, which could impact subsequent screening and treatment decisions by health care providers.
Materials and methods
This retrospective cohort study was reviewed and approved under ‘exempt’ status by the Geisinger Medical Center Institutional Review Board, based on established Geisinger criteria for the use of de-identified health information. Data were abstracted from EpicCare, Geisinger Medical Center's electronic health record, which contains detailed demographic, lifestyle (e.g. smoking), procedural, laboratory, radiographic, vital and other clinical data for more than 3.5 million patients receiving care in 45 outpatient clinics and 5 inpatient facilities in central Pennsylvania.
Study population
Geisinger primary care patients between 18 and 88 years of age were considered for inclusion. To meet the criteria of incident CKD Stage G3, a minimum of two outpatient estimated glomerular filtration rate (eGFR) values between 30 and 59 mL/min/1.73 m2 were required, with no prior values less than (and at least one value greater than) 60 mL/min/1.73 m2. Individuals having received a prior kidney transplant were excluded, as were those with a prior outpatient eGFR value <30 mL/min/1.73 m2. The study index date was the date of the first of the two qualifying eGFR values. Incident CKD Stage G3 patients were then grouped into three mutually exclusive cohorts according to study index date: 1 January 2004 through 31 December 2006; 1 January 2007 through 31 December 2009; and 1 January 2010 through 31 December 2012. The definition of each cohort period was arbitrary, and based on the presumption that a minimum of three distinct time periods would be necessary to verify trends in screening and outcomes. Patients were followed until death or the end of each cohort study period.
Measurements
GFR was estimated from serum creatinine using the CKD-EPI estimating equation [8]. Serum creatinine was measured at a single Geisinger lab using the isotope dilution/mass spectroscopy—traceable Roche enzymatic method throughout the entirety of the study period [9]. Instrument calibration at Geisinger labs is performed according to manufacturer's specifications. No changes in calibration techniques occurred during the study period.
Screening and treatment
We compared screening (urinary protein quantification), treatment [prescription for angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) for proteinuric patients, and prescription for HMG co-A Reductase Inhibitor (statin) for patients 50 years of age or older] and nephrology referral across the three cohorts. Urinary protein quantification was defined as a lab order (irrespective of completion) for any quantitative assessment of urinary protein excretion, to include a 24 h urine protein, a urine albumin-to-creatinine ratio (UACR) or urine protein-to-creatinine ratio (UPCR). Determination of ACEI or ARB treatment rates was limited to those with an indication based on proteinuria (for diabetics, UACR >30 mg/g creatinine, UPCR >150 mg/g creatinine or 24 h urine protein >300 mg; for non-diabetics, UACR >300 mg/g creatinine, UPCR >1000 mg/g or 24 h urine >1.0 g).
Outcomes
Study outcomes included death, CKD progression to Stage G4 (defined as the first outpatient eGFR value <30 mL/min/1.73 m2 after the index date) and hospitalization for myocardial infarction or heart failure (defined as a primary or secondary hospital discharge diagnosis). Information on vital status for Geisinger primary care recipients is updated monthly by query of the National Death Index [10].
Statistical analysis
Data are presented as mean and standard deviation (SD), or median and interquartile range (IQR), as appropriate for continuous variables, and as frequency and percentage for categorical variables. Baseline comparisons among the three cohorts were made using the Kruskal–Wallis non-parametric and Pearson's Chi-square tests, as appropriate. Screening and treatment rates were defined for each group and reported as the number of screens/treatments per 100 person-years. Rates of CKD progression, death and hospitalization for cardiovascular events were determined for each group and expressed as the number of events per 100 person-years. Rates were gender-, age- and baseline eGFR-adjusted in order to compare results across time periods. Adjusted incident rate ratios were determined using Poisson regression, using the 2004–06 cohort as the reference. All analyses were performed using Stata® 13 (Stata Corp, College Station, TX, USA).
Results
During Period 1 (1 January 2004–31 December 2006), Period 2 (1 January 2007–31 December 2009) and Period 3 (1 January 2010–31 December 2012), 728, 788 and 956 patients developed CKD, respectively. Characteristics of the populations (Table 1) were similar, with two exceptions: GFR was slightly higher at entry over time (49, 49 and 50 mL/min/1.73 m2) and systolic blood pressure was lower (135, 130 and 130 mmHg).
Table 1.
Characteristics of incident CKD patients at index date, by time period
| Characteristic | Period 1 (2004–06) (N = 728) |
Period 2 (2007–09) (N = 788) |
Period 3 (2010–12) (N = 956) |
|---|---|---|---|
| Follow-up, years; median (IQR) | 1.9 (1.5–2.4) | 1.9 (1.5–2.5) | 2.0 (1.4–2.4) |
| Age, years; mean (SD) | 73.7 (9.7) | 74.0 (9.6) | 73.4 (11.0) |
| Female gender; % | 60.9 | 59.8 | 61.0 |
| Never-smoker; % | 54.2 | 50.2 | 54.7 |
| Diabetes; % | 30.8 | 32.5 | 32.4 |
| History of myocardial infarction; % | 3.4 | 4.1 | 2.7 |
| History of heart failure; % | 15.4 | 12.2 | 12.0 |
| History of stroke; % | 7.0 | 6.4 | 5.3 |
| ACEI prescription at cohort entry; % | 32.6 | 34.5 | 33.9 |
| ARB prescription at cohort entry; % | 7.4 | 9.0 | 8.6 |
| Number of primary care visits during 12 months prior to cohort entry*; median (IQR) | 3 (2–5) | 3 (2–5) | 3 (2–4) |
| Systolic*/diastolic blood pressure, mmHg; mean (SD) | 135 (15)/72 (8) | 130 (13)/71 (8) | 130 (13)/71 (8) |
| eGFR*, mL/min/1.73 m2; mean (SD) | 49 (8) | 49 (7) | 50 (7) |
*P < 0.01.
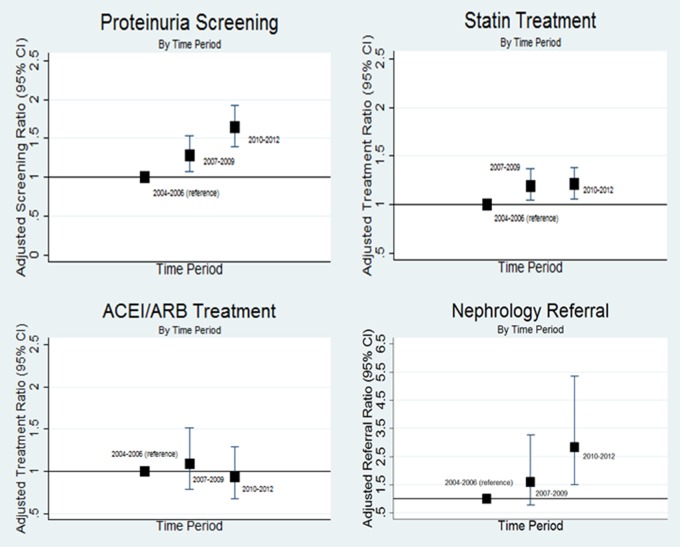
Follow-up time for screening and treatment endpoints, number of events, and crude and adjusted rates are shown in Table 2. During Periods 1, 2 and 3, both unadjusted and adjusted screening rates for proteinuria increased, as did statin treatment rates and nephrology referral rates. ACEI/ARB treatment rates were relatively high throughout all periods, and there was no statistically significant change in ACEI/ARB treatment rates across the time periods analyzed (Figure 1).
Table 2.
Crude and adjusteda screening and treatment rates among incident CKD patients, by time period
| 2004–06 | 2007–09 | 2010–12 | P for trend | |
|---|---|---|---|---|
| Proteinuria quantification | ||||
| Person-years follow-up | 1185 | 1308 | 1369 | |
| # Screened patients | 211 | 289 | 449 | |
| Screening rate (95% CI), per 100 py | 17.8 (15.6–20.4) | 22.1 (19.7–24.8) | 32.8 (29.9–36.0) | <0.001 |
| Adjusted screening rate (95% CI), per 100 py | 30.8 (27.0–58.3) | 39.2 (35.0–43.9) | 50.7 (46.3–55.5) | <0.001 |
| Statin treatment | ||||
| Person-years follow-up | 1073 | 1229 | 1366 | |
| # Treated patients | 371 | 480 | 590 | |
| Treatment rate (95% CI), per 100 py | 34.6 (31.2–38.3) | 39.1 (35.7–42.7) | 43.2 (39.8–46.8) | 0.09 |
| Adjusted treatment rate (95% CI), per 100 py | 52.6 (47.5–58.3) | 62.7 (57.3–68.5) | 63.5 (58.6–68.9) | <0.001 |
| ACEI or ARB treatment | ||||
| Person-years follow-up | 106 | 153 | 137 | |
| # Treated patients | 68 | 80 | 84 | |
| Treatment rate (95% CI), per 100 py | 64.3 (50.7–81.5) | 52.3 (42.0–65.1) | 61.3 (49.5–76.0) | 0.84 |
| Adjusted treatment rate (95% CI), per 100 py | 88.2 (67.6–115.0) | 82.8 (64.3–106.8) | 79.9 (61.8–103.2) | 0.82 |
| Nephrology referral | ||||
| Person-years follow-up | 1378 | 1521 | 1748 | |
| # Referred patients | 12 | 20 | 48 | |
| Referral rate (95% CI), per 100 py | 0.9 (0.5–1.5) | 1.3 (0.8–2.0) | 2.7 (2.0–3.6) | <0.001 |
| Adjusted referral rate (95% CI), per 100 py | 1.7 (1.0-3.0) | 2.6 (1.7–4.1) | 5.2 (3.9–6.9) | <0.001 |
Proteinuria screening and nephrology referral: n = 728, 788 and 956 during Periods 1, 2 and 3, respectively. Statin treatment was limited to those aged 50 years and older at entry: n = 713, 773 and 930 during Periods 1, 2 and 3, respectively. ACEI or ARB treatment was limited to those with an indication (proteinuria): n = 82, 102 and 115 during periods 1, 2 and 3, respectively. CI, confidence interval; py, person-years.
aStandardized to a 73-year-old male with baseline eGFR of 49 mL/min/1.73 m2.
Fig. 1.
Adjusted rate ratios for screening and treatment among incident CKD patients. Analyses are adjusted for age, gender and baseline eGFR. The y-axis scale for ‘Nephrology Referral’ differs from the scale used for the other three figures for purposes of legibility.
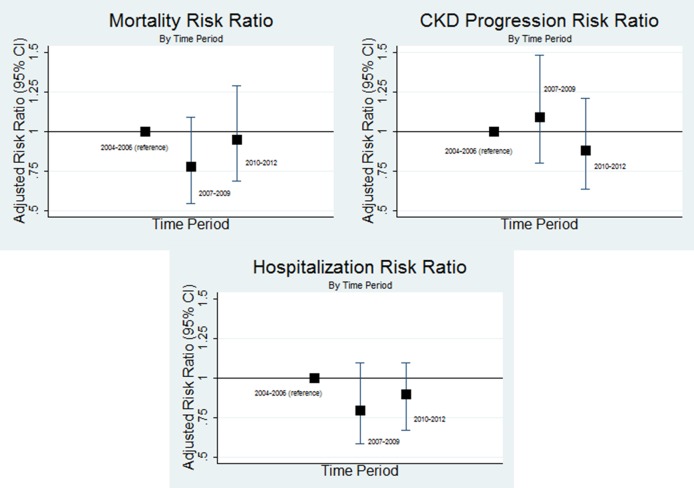
Follow-up time for each study endpoint, number of outcome events and standardized incidence rates are shown in Table 3. In both unadjusted and adjusted analyses, rates of all-cause mortality, CKD progression, and cardiovascular hospitalization did not change over time (adjusted rates: 6.6, 5.1 and 6.2 deaths per 100 person-years; 8.7, 9.8 and 7.4 CKD progressors per 100 person-years; 9.9, 8.0 and 8.9 cardiovascular hospitalizations per 100 person-years). For each outcome, rates were lower (albeit not significantly) in 2010–12 relative to 2004–06 (Figure 2).
Table 3.
Crude and adjusteda event rates among incident CKD patients, by time period
| 2004–06 | 2007–09 | 2010–12 | P for trend | |
|---|---|---|---|---|
| Mortality | ||||
| Person-years follow-up | 1378 | 1521 | 1748 | |
| # Deaths | 73 | 63 | 89 | |
| Mortality rate (95% CI), per 100 py | 5.3 (4.2–6.7) | 4.1 (3.2–5.3) | 5.1 (4.1–6.3) | 0.67 |
| Adjusted mortality rate (95% CI), per 100 py | 6.6 (4.9–8.7) | 5.1 (3.8–6.9) | 6.2 (4.8–8.1) | 0.97 |
| CKD progression | ||||
| Person-years follow-up | 1311 | 1472 | 1664 | |
| # CKD progressions | 75 | 86 | 75 | |
| Incident rate (95% CI), per 100 py | 5.7 (4.6–7.2) | 5.8 (4.7–7.2) | 4.5 (3.6–5.7) | 0.07 |
| Adjusted incident rate (95% CI), per 100 py | 8.7 (6.7–11.4) | 9.8 (7.7–12.5) | 7.4 (5.7–9.5) | 0.43 |
| Cardiovascular hospitalization | ||||
| Person-years follow-up | 1379 | 1522 | 1750 | |
| # Hospitalizations | 86 | 76 | 100 | |
| Hospitalization rate (95% CI), per 100 py | 6.2 (5.0–7.7) | 5.0 (4.0–6.3) | 5.7 (4.7–7.0) | 0.45 |
| Adjusted hospitalization rate (95% CI), per 100 py | 9.9 (7.7–12.6) | 8.0 (6.2–10.3) | 8.9 (7.0–11.2) | 0.72 |
aStandardized to a 73-year-old male with baseline eGFR of 49 mL/min/1.73 m2. CI, confidence interval; py, person-years.
Fig. 2.
Adjusted rate ratios for mortality, CKD progression and cardiovascular hospitalization among incident CKD patients. Analyses are adjusted for age, gender and baseline eGFR.
Discussion
In this single integrated health care system, rates of proteinuria screening, statin treatment and nephrology referral among incident CKD patients increased between 2004 and 2012, a trend suggestive of a more aggressive approach to CKD management. Despite these trends, no significant improvement in rates of CKD progression, mortality or cardiovascular hospitalization was observed.
Urinary protein quantification is recommended for all patients with reduced eGFR [7]. Screening rates among those with prevalent Stages 3 and 4 CKD vary widely, between 10 and 45% depending on diabetic status and screening definitions [5, 6, 11, 12]. In our population of incident CKD Stage G3 patients, quantification rates of urinary protein excretion doubled over the study period, but remained low in absolute terms; less than half of the incident CKD population in 2010–12 underwent urinary protein quantification. This rate is similar to an Australian cohort of patients with established CKD in which 43% of patients had undergone proteinuria assessment at the time of nephrology referral [5].
In contrast to our study, which found increasing rates of proteinuria screening but stable rates of ACEI/ARB treatment, others have identified changes in ACEI or ARB treatment patterns over time. Although not limited to those with CKD, Tomlinson et al. identified a 16% increased rate of ACEI or ARB prescribing over the period 2007–11 in patients covered by the UK's National Health Service [13]. Differences may reflect the high baseline rate of ACEI/ARB use in our health system, which has implemented a number of provider-targeted quality initiatives through the electronic medical record to encourage optimization of clinical care. These initiatives may also explain some of the increases in proteinuria screening and nephrology referral, despite a concomitant increase in eGFR level.
Several factors may explain why more aggressive CKD management has not improved outcomes. First, longer follow-up may be needed to capture the impact of more intensive management at the population level. The median follow-up in this analysis—1.9–2.0 years—may not be of sufficient duration to detect a positive impact on clinical outcomes such as mortality and CKD progression. With the exception of CKD progression, four to five times the population sizes would have been needed to power the mortality and cardiovascular endpoints for the modest differences in endpoint incidence we observed (post hoc sample size estimation). Second, differences in characteristics across the three cohorts not accounted for in our analyses may explain some of the outcome trends. Third, more intensive screening and treatment—derived largely from rigorously controlled clinical trials—may not necessarily translate to real-world clinical environments such as this one.
Primary care physicians and nephrologists differ in their characterization of risk among those with CKD, and this may influence practice patterns [14]. While nephrology referral has been shown to improve adherence to CKD screening and treatment guidelines [15, 16], and referral rates increased over the three time periods, only a small fraction of these incident populations was seen by a nephrologist, and this is not likely a contributing factor to the improvements in management observed.
This study has several important limitations. First, the relatively brief follow-up may not have been sufficient to detect meaningful associations between screening and treatment and clinical outcomes. Second, unaccounted for differences in population characteristics across the three time periods may confound the results; in a similar fashion, temporally evolving care patterns not tracked in this study may have also influenced the outcomes of interest. With respect to nephrology referrals, the data used for these analyses did not distinguish between completed and uncompleted referrals; while we expect from historical data a low rate of uncompleted referrals, we cannot exclude the possibility that uncompleted referrals biased the findings. It is recognized that late referrals to nephrologists may negatively impact clinically relevant outcomes, and we have not accounted for CKD progression at the time of referral in this analysis. While the study population was receiving care in a large health care system representative of the geographic region it serves, the results from this single-center study may not be generalizable to other populations. Screening rates may have been underestimated, as information on laboratory testing just prior to the onset of CKD was not included in the analyses. Finally, serum cystatin C was not readily available as a routine clinical laboratory test; the lack of its use may call into question the accuracy of identification of Stage 3A CKD patients.
We chose to limit our analyses to incident CKD patients. These observations are not generalizable to the larger prevalent CKD population. Targeted identification and treatment of risk factors among prevalent CKD patients may be an effective and important risk reduction strategy; however, comparing temporal management trends among prevalent populations is difficult, due to factors like disease duration and stability, which may influence care decisions made by providers. By focusing on incident CKD patients, this study provides information about care patterns at the onset of disease, when interventions may allow for even more effective prevention of cardiovascular and renal complications, and lead-time bias is less influential.
In summary, we demonstrate increased rates of proteinuria screening, statin treatment and nephrology referral among incident CKD patients between 2004 and 2012. The testing of interventions to enhance the kidney and cardiovascular risk profiles of patients with early CKD should be coupled with longitudinal assessment of clinical outcomes in order to determine the efficacy of management strategies in real-world settings.
Conflict of interest statement
R.M.P., A.R.C., K.E.W., K.M. and M.G. report no conflicts. J.C. has received investigator initiated grant support from the NIH, NKF and Amgen. Provisional patent has been submitted for glomerular filtration rate estimation using a panel of biomarkers. This study was presented in modified form as a poster at the American Society of Nephrology's annual conference in Philadelphia, PA, on 15 November 2014.
Acknowledgements
The authors thank Meredith Lewis for assistance with data extraction.
References
- 1.Levey AS, de Jong PE, Coresh J et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011; 80: 17–28 [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Merz CN et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110: 227–239 [DOI] [PubMed] [Google Scholar]
- 3.Eckardt KU, Berns JS, Rocco MV et al. Definition and classification of CKD: the debate should be about patient prognosis—a position statement from KDOQI and KDIGO. Am J Kidney Dis 2009; 53: 915–920 [DOI] [PubMed] [Google Scholar]
- 4.Philipneri MD, Rocca Rey LA, Schnitzler MA et al. Delivery patterns of recommended chronic kidney disease care in clinical practice: administrative claims-based analysis and systematic literature review. Clin Exp Nephrol 2008; 12: 41–52 [DOI] [PubMed] [Google Scholar]
- 5.Thorp ML, Smith DH, Johnson ES et al. Proteinuria among patients with chronic kidney disease: a performance measure for improving patient outcomes. Jt Comm J Qual Patient Saf 2012; 38: 277–282 [DOI] [PubMed] [Google Scholar]
- 6.Mendu ML, Schneider LI, Aizer AA et al. Implementation of a CKD checklist for primary care providers. Clin J Am Soc Nephrol 2014; 9: 1526–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.KDIGO Workgroup. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int 2012; Suppl 2013: 1–150 [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers GL, Miller WG, Coresh J et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 2006; 52: 5–18 [DOI] [PubMed] [Google Scholar]
- 10.National Center for Health Statistics. National Death Index. Atlanta, GA: Centers for Disease Control and Prevention, 2015 [Google Scholar]
- 11.Stevens PE, O'Donoghue DJ, de Lusignan S et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int 2007; 72: 92–99 [DOI] [PubMed] [Google Scholar]
- 12.Allen AS, Forman JP, Orav EJ et al. Primary care management of chronic kidney disease. J Gen Intern Med 2011; 26: 386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomlinson LA, Abel GA, Chaudhry AN et al. ACE inhibitor and angiotensin receptor-II antagonist prescribing and hospital admissions with acute kidney injury: a longitudinal ecological study. PLoS One 2013; 8: e78465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulware LE, Troll MU, Jaar BG et al. Identification and referral of patients with progressive CKD: a national study. Am J Kidney Dis 2006; 48: 192–204 [DOI] [PubMed] [Google Scholar]
- 15.Avorn J, Bohn RL, Levy E et al. Nephrologist care and mortality in patients with chronic renal insufficiency. Arch Intern Med 2002; 162: 2002–2006 [DOI] [PubMed] [Google Scholar]
- 16.Kinchen KS, Sadler J, Fink N et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med 2002; 137: 479–486 [DOI] [PubMed] [Google Scholar]