Abstract
Background
There is growing evidence that adrenocorticotropic hormone (ACTH) may be effective in treating various forms of glomerular diseases. However, the efficacy of treatment and frequency of adverse effects associated with the use of ACTH in glomerular diseases are unknown. A systematic review and meta-analysis of the literature was performed.
Methods
A literature search was performed using Medline, Embase, Google Scholar and the Cochrane Database of Systematic Reviews from inception through 18 July 2015. Studies assessing the efficacy and safety of ACTH treatment in adults with glomerular diseases were included.
Results
Of the 343 identified citations, 18 evaluated the drug efficacy and 12 evaluated the adverse effects. The most common glomerular diseases were membranous nephropathy (MN), primary focal segmental glomerulosclerosis (FSGS) and minimal change disease (MCD). The overall rate of complete remission in MN was 80% at 0–6 months, 69% at >6–12 months, 90% at >12–24 months and 95% beyond 24 months of follow-up. Fifty percent of primary FSGS and MCD patients treated with ACTH were in remission at 6 months, but the relapse rate was high after ACTH discontinuation (17%). Evidence of ACTH efficacy for other glomerular diseases was scarce. Edema was the most commonly reported adverse effect {incidence rate [IR] 0.10 [95% confidence interval (CI) 0.04–0.18]} followed by insomnia [IR 0.08 (95% CI 0.03–0.15)]. The dropout rate due to adverse events was 7%, mostly due to edema and weight gain.
Conclusions
ACTH is a well-tolerated therapy and is most promising when treating patients with MN. There may be a potential role for ACTH in patients with MCD and FSGS, but data are lacking.
Keywords: ACTH, adverse effects, glomerular diseases, meta-analysis, systematic review
INTRODUCTION
Adrenocorticotropic hormone (ACTH) was one of the first therapies widely used several decades ago for the treatment of childhood nephrotic syndrome [1]. It fell out of favor after easy-to-use synthetic oral glucocorticoids became available [2]. Recently, ACTH has been resurrected as a potential therapeutic option for a variety of glomerular diseases [3–7]. In addition to its steroidogenesis effects, ACTH acts as an agonist of the melanocortin system, which plays a role in various physiologic functions, including melanin synthesis, immunomodulation, anti-inflammation, lipolysis stimulation and modulation of exocrine function [8]. Animal studies have suggested that the antiproteinuric effect of ACTH might in fact be mediated through the melanocortin receptors that are expressed on glomerular podocytes and renal parenchymal cells [9, 10]. This was shown in the rat model of passive Heymann nephritis [10]. The rats that were treated with specific melanocortin 1 receptor agonist had a significantly lower degree of proteinuria compared with the untreated rats. Studies in humans have also shown ACTH to be effective in reducing proteinuria in patients with nephrotic syndrome who have not responded to corticosteroid treatment, thereby suggesting that noncorticosteroid mechanisms may play a role [3–6].
Since the therapeutic effect of ACTH on nephrotic syndrome was first reexamined by Berg et al. [3] in 1999, the literature on its use in various glomerular diseases has rapidly expanded [4–7, 11–13]. However, the exact efficacy of ACTH in inducing remission in patients with glomerular diseases and the frequency of adverse effects associated with the use of ACTH remain largely unknown. Given the absence of data, we undertook this systematic review and meta-analysis to evaluate the efficacy and establish the incidence of adverse effects associated with ACTH in treating various types of glomerular diseases.
METHODS
This systematic review and meta-analysis is reported in accordance with previously published guidelines [14, 15].
Search strategy
Published studies in the Cochrane Database of Systematic Reviews, Embase, Medline and Google Scholar were evaluated by two investigators (W.K. and W.C.) from inception through 18 July 2015 as outlined in Item S1 in Supplementary data. Additional pertinent studies were obtained by performing a manual search using references from the articles that were retrieved from the search strategy noted above.
Selection criteria
The inclusion criteria were as follows: (i) the studies were randomized controlled trials (RCTs) or observational studies (case–control, cross-sectional, cohort studies or case series), (ii) data on either efficacy or safety of ACTH treatment in glomerular diseases were provided and (iii) the primary study patients had reached adulthood (age >18 years old). The search was limited to English-language articles. Both published articles and conference abstracts were included. Study eligibility was independently determined by the two investigators noted above. Differing decisions were resolved by mutual consensus.
Data extraction
A standardized data collection form was used to extract the following information: last name of the first author, study design, publication year, country where the study was conducted, number of patients studied, characteristics of included participants, period of follow-up, type of glomerular disease, form of ACTH preparation, dosing, duration of treatment, treatment response and type and number of adverse effects following ACTH treatment.
Statistical analysis
MetaXL software (EpiGear International, Sunrise Beach, QLD, Australia) [16] was used for data analysis. The incidence rates (IRs) and 95% confidence intervals (CIs) of adverse effects were reported using a DerSimonian–Laird random-effects model [17]. A random-effects model was used for data analysis due to the high likelihood of interstudy variances and the Cochran Q test was performed to assess statistical heterogeneity. The I2 statistic was added to evaluate the degree of variation across studies related to heterogeneity instead of chance. ‘An I2 of 0–25% represents insignificant heterogeneity, 26–50% low heterogeneity, 51–75% moderate heterogeneity and >75% high heterogeneity’ [18]. The frequencies of each type of adverse effect were presented as a crude percentage. To assess for publication bias funnel plots were used.
RESULTS
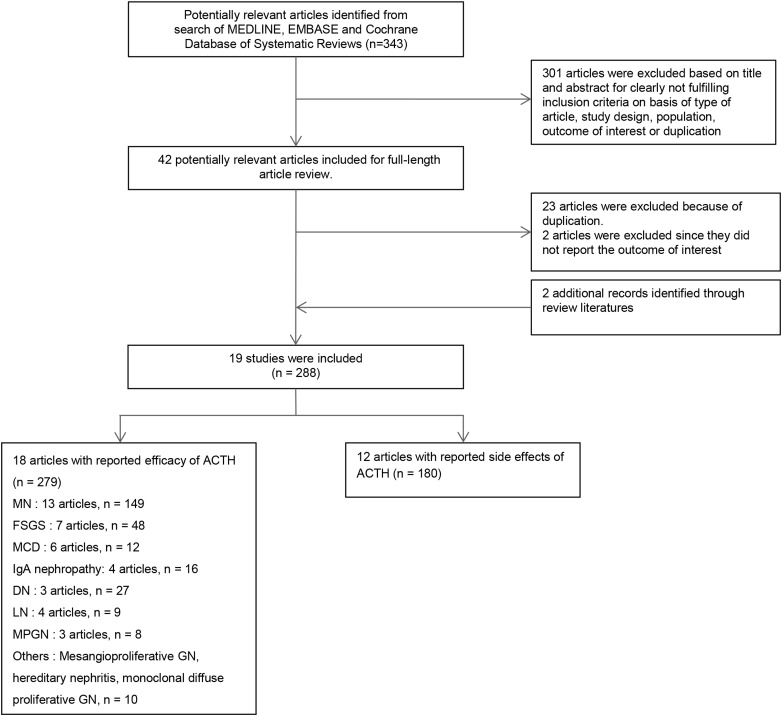
A flow diagram for retrieval and inclusion of studies is shown in Figure 1. Our search strategy yielded 343 potentially relevant articles. Three hundred and one articles were excluded after the initial screening and 42 articles were included for full-length review. Eventually, 19 articles met all the inclusion criteria [3–7, 11–13, 19–29]. Details of these studies are outlined in Table 1.
Fig. 1.
Study selection flow chart.
Table 1.
Main characteristics of the included studies
| Author | Type of publication | Study design | n | MN | FSGS | MCD | IgA | DN | LN | MPGN | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Khastgir et al. [25] | Conference abstract | Retrospective cohort | 9 | – | – | 2 | 5 | – | 2 | – | – |
| Lorusso et al. [7] | Article | Prospective cohort | 18 | 10 | 2 | 3 | – | – | – | 3 | – |
| Hladunewich et al. [13] | Article | Prospective cohort | 20 | 20 | – | – | – | – | – | – | – |
| Finocchietti et al. [12] | Conference abstract | Prospective cohort | 19 | 19 | – | – | – | – | – | – | – |
| Madan et al. [24] | Conference abstract | Retrospective cohort | 22 | 4 | 7 | 2 | 5 | 2 | 1 | 1 | – |
| Tumlin et al. [23] | Article | Prospective cohort | 14 | – | – | – | – | 14 | – | – | – |
| Hogan et al. [6] | Article | Retrospective cohort | 24 | – | 24 | – | – | – | – | – | – |
| Berg et al. [22] | Conference abstract | Retrospective cohort | 10 | – | 10 | – | – | – | – | – | – |
| Berg and Back (2013) [21] | Conference abstract | Retrospective cohort | 5 | – | – | – | – | – | 5 | – | – |
| Bomback et al. [5] | Article | Prospective cohort | 15 | 5 | 3* | 2 | 5 | – | – | ||
| Bomback et al. [4] | Article | Retrospective cohort | 21 | 11 | 1* | 1 | 1 | – | 1 | 4 | 2 |
| Hofstra et al. [20] | Conference abstract | Prospective cohort | 14 | 14 | – | – | – | – | – | – | – |
| Rauen et al. [19] | Article | Retrospective cohort | 4 | 4 | – | – | – | – | – | – | – |
| Ponticelli et al. [11] | Article | Randomized controlled trial | 16 | 16 | – | – | – | – | – | – | – |
| Berg et al. [29] | Conference abstract | Randomized controlled trial | 15 | 15 | – | – | – | – | – | – | – |
| Picardi et al. [28] | Letter to the editor | Retrospective cohort | 7 | 7 | – | – | – | – | – | – | – |
| Berg and Arnadottir [27] | Article | Retrospective cohort | 23 | 10 | 1 | 2 | – | 2 | – | – | 8 |
| Berg et al. [3] | Article | Prospective cohort | 14 | 14 | – | – | – | – | – | – | – |
| Berg and Nilsson-Ehle [26] | Article | Prospective cohort | 9 | – | – | – | 1 | – | 1 | – | 7 |
| Total | 279 | 149 | 48 | 12 | 17 | 18 | 10 | 8 | 17 |
DN, diabetic nephropathy; FSGS, focal segmental glomerulosclerosis; IgA, IgA nephropathy; LN, lupus nephritis; MCD, minimal change disease; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis.
*Patients were also included in Hogan et al. [6].
Efficacy of ACTH in the treatment of glomerular diseases
Eighteen studies [3–7, 11–13, 19–25, 27–29] were included for the evaluation of drug efficacy (n = 270), consisting of 10 published articles, 1 letter to the editor and 7 conference abstracts. There were two RCTs, seven prospective cohorts and nine retrospective cohorts. These studies included participants with membranous nephropathy (MN; n= 149), focal segmental glomerulosclerosis (FSGS) and minimal change disease (MCD; n = 60), IgA nephropathy (n = 16), lupus nephritis (n = 9), diabetic nephropathy (n = 18) and other glomerular diseases (n = 18).
The included studies were heterogeneous in regard to patient characteristics, disease severity, the form of ACTH preparations, ACTH dosing, treatment duration, follow-up duration and response criteria. Therefore, the treatment responses were reevaluated based on the information provided on individual patients using the same response criteria. Complete remission was defined as a final urinary protein excretion ≤0.3 g/day. Partial remission was defined as a ≥50% reduction in urinary protein excretion and urinary protein excretion <3.5 g/day. All patients failing to meet these criteria were deemed as nonresponders. However, response reevaluation is not applicable to the abstracts due to the limited data available on each individual patient.
Membranous nephropathy
A total of nine published studies [3–5, 7, 11, 13, 19, 27, 28] with 97 MN patients and four abstracts [12, 20, 24, 29] with 52 MN patients were included. We grouped patients based on the duration of follow-up, with a summary of baseline characteristics and response rates as presented in Tables 2 and 3. One hundred and nine patients from nine studies [3, 7, 11, 12, 19, 20, 27–29] were treated with synthetic ACTH (tetracosactide), whereas 40 patients from four studies [4, 5, 13, 24] were treated with natural ACTH (H.P. Acthar gel; Mallinckrodt). The dose of synthetic ACTH ranged from 0.25 to 2 mg/week and natural ACTH was 80–160 units/week. The duration of treatment ranged from 2 to 24 months and maximum follow-up duration was 82 months. The overall response rate (complete and partial remission) for MN was 80% at 0–6 months, 69% at 6–12 months, 90% at 12–24 months and 95% in those with >24 months of follow-up (Table 2). There were four relapses reported after discontinuation of ACTH (3%).
Table 2.
Summary of published articles on adrenocorticotropic hormone therapy in membranous nephropathy
| Author | Number of patients | Immunosuppression response category | ACTH preparation | Total dose (per week) | Duration of treatment (months) | Baseline proteinuria (g or g/g) | Baseline GFR (mL/min/1.73 m2) | Baseline Cr (mg/dL) | Baseline serum albumin (g/dL) | CR | PR | NR | Relapse |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up duration 0–6 months | |||||||||||||
| Bomback et al. [5] | 5 | 5 IR | Natural | 160 U | 6 | 3.8 (2.2–12.5) | 34 (21–44) | 2 (1.6–2.9) | NA | 0 | 2 | 3 | 0 |
| Bomback et al. [4] | 5 | 5 IR | Natural | 120–160 U | 6 | 6.7 (4.6–9) | 57 (21 to >60) | NA | NA | 0 | 5 | 0 | 0 |
| Ponticelli et al. [11] | 16 | 16 naïve | Synthetic | 2 mg | 6 | 6 (4.4–8.5)a | NA | 1 (3.6)b | NA | 3 | 8 | 5 | 0 |
| Berg et al. [3] | 14 | 4 IR/7 SR/3 naïve | Synthetic | 1.6 mg | 2 | 4.8 (3.7–15.9) | 43 (20–78) | 1.5 (1.1–3.6) | 2.3 (1.2–3.2) | 1 | 13 | 0 | 0 |
| Total patients | 40 | 4 (10%) | 28 (70%) | 8 (20%) | 0 (0%) | ||||||||
| Follow-up duration >6–12 months | |||||||||||||
| Lorusso et al. [7] | 9 | NA | Synthetic | 1 mg | 12 | 4 (3–10) | NA | NA | 3.2 (2.2–4.1) | 4 | 0 | 5 | 0 |
| Hladunewich et al. [13] | 20 | Excluded IR or SR | Natural | 80–160 U | 3 | 9 (3.4)b | 77 (30)b | NA | 2.7 (0.8)b | 2 | 10 | 8 | 0 |
| Bomback et al. [4] | 4 | 3 IR/1 naïve | Natural | 120–160 U | 6 (5–11) | 9 (4.9–11.9) | 42.5 (20 to >60) | NA | NA | 1 | 1 | 2 | 0 |
| Ponticelli et al. [11] | 16 | 16 naïve | Synthetic | 2 mg | 12 | 6 (4.4–8.5)a | NA | 1 (3.6)b | NA | 10 | 4 | 2 | 0 |
| Berg et al. [27] | 1 | NA | Synthetic | 0.5–2 mg | 7 | 7.8 | NA | NA | NA | 1 | 0 | 0 | 0 |
| Berg et al. [3] | 5 | 4 IR/1 SR | Synthetic | 2 mg | 12 | 8.8 (7.1–13.7) | 45 (20–61) | 1.5 (1.1–3.6) | 1.7 (1.2–1.9) | 4 | 1 | 0 | 0 |
| Total patients | 55 | 22 (40%) | 16 (29%) | 17 (31%) | 0 (0%) | ||||||||
| Follow-up duration >12–24 months | |||||||||||||
| Lorusso et al. [7] | 2 | NA | Synthetic | 1 mg | 12 | 6.6 (3.2–10) | NA | NA | 2.4 (2.2–2.6) | 0 | 0 | 2 | 1 |
| Bomback et al. [4] | 2 | 2 IR | Natural | 80–160 U | 12 | 3 (2.5–3.6) | 60 (40 to >60) | NA | NA | 2 | 0 | 0 | 0 |
| Ponticelli et al. [11] | 9 | 9 naïve | Synthetic | 2 mg | 12 | NA | NA | NA | NA | 6 | 3 | 0 | 0 |
| Berg et al. [27] | 2 | NA | Synthetic | 0.5–2 mg | 9 (7–11) | 5.4 (3.5–7.3) | NA | NA | NA | 1 | 1 | 0 | 0 |
| Berg et al. [3] | 5 | 4 IR/1 SR | Synthetic | 2 mg | 12 | 8.8 (7.1–13.7) | 45 (20–61) | 1.5 (1.1–3.6) | 1.7 (1.2–1.9) | 4 | 1 | 0 | 0 |
| Total patients | 20 | 13 (65%) | 5 (25%) | 2 (10%) | 1 (5%) | ||||||||
| Follow-up duration >24 months | |||||||||||||
| Lorusso et al. [7] | 5 | NA | Synthetic | 1 mg | 12 | 4 (3–8.6) | NA | NA | 3.7 (2.6–4.1) | 2 | 2 | 1 | 0 |
| Rauen et al. [19] | 4 | 4 IRc | Synthetic | 0.25–2.25 mg | 13 (3–24) | 9.6 (6–20) | 39.5 (20–62) | NA | NA | 2 | 2 | 0 | 0 |
| Berg et al. [27] | 7 | NA | Synthetic | 0.5–2 mg | 2–11 | 7.5 (3.2–26.7) | NA | 1.2 (0.7–5.4) | 1.9 (1–2.3) | 3 | 4 | 0 | 1 |
| Berg et al. [3] | 5 | 4 IR/1 SR | Synthetic | 2 mg | 12 | 8.8 (7.1–13.7) | 45 (20–61) | 1.5 (1.1–3.6) | 1.7 (1.2–1.9) | 4 | 1 | 0 | 0 |
| Total patients | 21 | 11 (52%) | 9 (43%) | 1 (5%) | 1 (5%) | ||||||||
ACTH, adrenocorticotropic hormone; CR, complete response; GFR, glomerular filtration rate; IR, immunosuppression resistant (other than steroids); NA, data not available; naïve, never received immunosuppression; NR, no response; PR, partial response; SR, steroid resistant.
Data presented as median and range;
amedian (IQR);
bmean (SD);
ctwo patients received other immunosuppressive agents concomitant with ACTH.
Table 3.
Summary of conference abstracts and letters to the editor on adrenocorticotropic hormone therapy in membranous nephropathy
| Author | Number of patients | Immunosuppression response category | ACTH preparation | Total dose (per week) | Duration of treatment (months) | Follow-up duration (months) | CR | PR | NR | Early termination |
|---|---|---|---|---|---|---|---|---|---|---|
| Finocchietti et al. [12]a,b | 19 | NA | Synthetic | 1 mg | 12 | 12 | NA | NA | NA | 0 |
| Madan et al. [24]c | 4 | 3 SR or IR, 1 naïve | Natural | 160 U | >6 | NA | 1 | 1 | 1 | 1 |
| Hofstra et al. [20]a | 14 | NA | Synthetic | Max 2 mg | 9 | 10–21 | 0 | 4 | 8 | 2 |
| Berg et al. [29]d | 15 | 15 naïve | Synthetic | 1–2 mg | 9 | 21 | 11 | 3 | 1 | 0 |
| Picardi et al. [28] | 7 | NA | Synthetic | 2 mg | 12 | 12 | 5 | 0 | 0 | 2 |
ACTH, adrenocorticotropic hormone; CR, complete response; IR, immunosuppression resistant (other than steroids); NA, data not available; naïve, never received immunosuppression; NR, no response; PR partial response; SR, steroid resistant.
aCR defined as urine protein excretion <500 mg/day, PR defined as >50% reduction of proteinuria and urine protein excretion <3.5 g/day.
bCR defined as urine protein excretion <200 mg/day, PR defined as >50% reduction of proteinuria and urine protein excretion <2 g/day.
cResponse criteria were not provided.
dNo reported treatment response, but significant reductions of urine protein excretion <1 g/day were observed in most patients at 6 and 12 months.
Of the 13 published studies and abstracts, only 2 were RCTs comparing ACTH injections with other therapies in MN. One was presented as an abstract comparing ACTH plus an angiotensin-converting enzyme inhibitor to an angiotensin-converting enzyme inhibitor only [29]. Thirty patients with MN were randomized into each arm. ACTH induced remission in all 15 patients who received synthetic ACTH for 9 months, compared with 1 of 15 patients in the control group. The other RCT compared the efficacy of 6 months of methylprednisolone alternating with alkylating agents to 1 year of synthetic ACTH 1 mg twice weekly [11]. There were no significant differences between the two treatment groups in terms of the number of remissions at 12 months (93 versus 87%), median time to response (2 versus 3 months), number of relapse (7 versus 3 patients) or the decrease in the degree of proteinuria (5.1–2.1 g/day versus 6.0–0.3 g/day). However, in patients treated with ACTH, time spent without nephrotic syndrome was longer and more complete remissions were achieved.
Only one study, by Hladunewich et al. [13], compared the efficacy of different doses of natural ACTH (H.P. Acthar gel) in 20 patients with MN. Nine patients were randomly assigned to receive ACTH 40 units twice weekly and 11 were assigned to receive 80 units twice weekly. At the end of a 12-week treatment period, none of the patients in the 40-unit arm achieved a meaningful change in proteinuria, whereas 5 of 11 patients in the 80-unit arm had at least a 30% reduction in proteinuria. The authors suggested that the cumulative dose of natural ACTH (80 units twice weekly) for at least 3 months appeared to be necessary for a response. No studies have directly compared the efficacy of different doses of synthetic ACTH when treating patients. The majority of the synthetic ACTH studies have used 2-mg weekly dose, but a case series using low-dose synthetic ACTH (1 mg weekly) also reported a reasonable response rate (44% at 12 months) [7]. The equivalent dosage of natural versus synthetic ACTH is not known.
Of the nine published studies that included patients with MN, only four studies included patients (n = 29) who had previously failed other immunosuppressive therapy such as cyclophosphamide, mycophenolate mofetil, calcineurin inhibitor, rituximab or corticosteroids [3–5, 19]. Twenty-five of the 29 (86%) achieved remission after 6 months of follow-up.
FSGS and MCD
There were five published studies (n = 35) [4–7, 27] and three abstracts (n = 21) [22, 24, 25] that included patients with FSGS or MCD (excluding 4 FSGS patients from Bomback et al. [4, 5] who were included in the study by Hogan et al. [6]) (Tables 4 and 5). Treatment duration ranged from 2 to 56 months. Of the 56 patients with FSGS or MCD, 38 (68%) were treated with natural ACTH [4–6, 24, 25] and 18 (32%) were treated with synthetic ACTH [7, 21, 27]. Of the 35 patients (from the published articles), 27 had failed previous immunosuppressive therapy including steroids [4–7, 27]. The overall response rate was 50% after 6 months of follow-up. Six of 35 patients (17%) experienced relapse after ACTH discontinuation. According to the largest study with 24 FSGS patients by Hogan et al. [6], patients who experienced remission were either steroid resistant or dependent. The remitters tended to have a lower serum creatinine at baseline. However, no associations were observed between age, ethnicity, FSGS subtype, use of additional immunosuppression during ACTH treatment, accumulative dose or duration of ACTH therapy and the rate of remission.
Table 4.
Summary of published articles on adrenocorticotropic hormone therapy in focal segmental glomerulosclerosis and minimal change disease
| Author | Number of patients | Immunosuppression response category | ACTH preparation | Total dose (per week) | Duration of treatment (month) | Baseline proteinuria (g or g/g) | Baseline GFR (mL/min/1.73 m2) | Baseline Cr (mg/dL) | Baseline serum albumin (g/dL) | CR | PR | NR | Relapse |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow-up duration 6–12 months | |||||||||||||
| Bomback et al. [5] | 2 | 2 IR | Natural | 160 U | 6 | 4 (3.2–4.8) | 120 (117–123) | 0.65 (0.6–0.7) | 3 (2.5–3.4) | 0 | 1 | 1 | 1 |
| Bomback et al. [4] | 1 | 1 IR | Natural | 160 U | 4 | 18.6 | 15 | NA | NA | 0 | 0 | 1 | 0 |
| Berg et al. [27] | 1 | 1 SR | Synthetic | 0.5–2 mg | 7 | 9.6 | NA | NA | NA | 0 | 1 | 0 | 0 |
| Total patients | 4 | 0 (0%) | 2 (50%) | 2 (50%) | 1 (25%) | ||||||||
| Follow-up duration >12–24 months | |||||||||||||
| Lorusso et al. [7]a | 4 | NA | Synthetic | 1 mg | 12 | 7.7 (3–13) | NA | NA | 2.9 (1.7–3.4) | 2 | 1 | 1 | 2 |
| Hogan et al. [6]b | 17 | 15 SR or SD, 2 naïve | Natural | 160 U | 16 (12–24) | 4.6 (1.6–23.8) | 47 (23–124) | 1.5 (0.6–3.3) | 3.2 (1.6–4.8) | 2 | 3 | 12 | 2 |
| Berg et al. [27] | 1 | 1 SR | Synthetic | 0.5–2 mg | 2 | 3.9 | NA | NA | NA | 1 | 0 | 0 | 1 |
| Total patients | 22 | 5 (23%) | 4 (18%) | 13 (59%) | 5 (23%) | ||||||||
| Follow-up duration >24 months | |||||||||||||
| Lorusso et al. [7] | 1 | NA | Synthetic | 1 mg | 12 | 3 | NA | NA | 2.4 | 0 | 1 | 0 | 0 |
| Hogan et al. [6] | 6 | 6 SD or SR | Natural | 160 U | 36 (28–56) | 9.6 (2.3–15.2) | 29 (17–67) | 2.5 (1.1–3.6) | 2.1 (1.3–2.9) | 0 | 2 | 4 | 0 |
| Berg et al. [27] | 1 | 1 SR | Synthetic | 0.5–2 mg | 7 | 3.4 | NA | NA | NA | 0 | 1 | 0 | 0 |
| Total patients | 8 | 0 (0%) | 4 (50%) | 4 (50%) | 0 (0%) | ||||||||
ACTH, adrenocorticotropic hormone; CR, complete response; GFR, glomerular filtration rate; IR, immunosuppression resistant (other than steroids); NA, data not available; naïve, never received immunosuppression; NR, no response; PR partial response; SD, steroid dependent; SR, steroid resistant.
Data presented as median and range;
aone patient dropped out of the study (not included in the table);
btwo patients received other immunosuppressive agents concomitant with ACTH.
Table 5.
Summary of abstracts on adrenocorticotropic hormone therapy in focal segmental glomerulosclerosis and minimal change disease
| Author | Number of patients | Immunosuppression response category | ACTH preparation | Total dose (per week) | Duration of treatment (months) | Follow-up duration (months) | CR | PR | NR | Early termination |
|---|---|---|---|---|---|---|---|---|---|---|
| Khastgir et al. [25]a | 2 | 2 IR | Natural | 160 U | >6 | >6 | 2 | 0 | 0 | 0 |
| Madan et al. [24]b | 8 | NA | Natural | 160 U | >6 | >6 | 2 | 3 | 3 | 1 |
| Berg et al. [22]c,d | 10 | NA | Synthetic | 2 mg | 18 (7–48) | 0–29e | 3 | 5 | 2 | 0 |
ACTH, adrenocorticotropic hormone; CR, complete response; IR, immunosuppression resistant (other than steroids); NA, data not available; NR, no response; PR partial response.
aResponse criteria were not provided.
bCR defined as urine protein excretion <500 mg/day; PR defined as >50% reduction of proteinuria and urine protein excretion <3.5 g/day.
cCR defined as urine protein excretion <200 mg/day; PR defined as >50% reduction of proteinuria and urine protein excretion <2 g/day.
dAll patients received other immunosuppressive agents concomitant with ACTH.
eMonths after ACTH discontinuation.
Other glomerular diseases
The evidence for ACTH treatment in other glomerular diseases is summarized in Supplementary data, Table S1. Most studies were small and heterogeneous and therefore it was difficult to draw any conclusions on the effectiveness of ACTH therapy.
Adverse effects
There were 12 studies that reported adverse effects associated with ACTH in a total of 171 patients with underlying glomerular diseases including MN (n = 87), FSGS (n = 30), MCD (n = 8), IgA nephropathy (n = 12), lupus nephritis (n = 4), diabetic nephropathy (n = 14) and other (n = 16) [3–7, 11, 13, 19, 23, 25, 26, 28]. The dose of synthetic ACTH ranged from 0.25 to 3.3 mg/week and natural ACTH was 80 to 224 units/week. Treatment duration ranged from 2 to 48 months. Table 6 summarizes the major adverse effects of ACTH. Supplementary data, Figure S1 shows the forest plot of the included studies. Edema was the most common adverse effect (IR 0.10), followed by insomnia (IR 0.08), hyperglycemia (IR 0.07) and mood swings (IR 0.07). The dropout rate due to adverse events was 7%, mostly due to edema and weight gain (5 of 12 patients). No severe adverse reactions or deaths associated with ACTH injections were reported (Table 7). The adverse effect profiles of natural and synthetic ACTH were similar (Table 6).
Table 6.
Adverse effects of ACTH
| Adverse effects | All |
Natural ACTH |
Synthetic ACTH |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence rate | 95% CI | I2 | P-value | Incidence rate | 95% CI | I2 | P-value | Incidence rate | 95% CI | I2 | P-value | |
| Edema | 0.10 | 0.04–0.18 | 52 | 0.02 | 0.09 | 0.01–0.21 | 66 | 0.01 | 0.10 | 0.02–0.22 | 40 | 0.14 |
| Insomnia/increased alertness | 0.08 | 0.03–0.15 | 53 | 0.02 | 0.12 | 0.03–0.25 | 65 | 0.01 | 0.03 | 0.00–0.09 | 0 | 0.58 |
| Mood swings | 0.07 | 0.02–0.14 | 57 | 0.01 | 0.06 | 0.00–0.17 | 69 | 0.01 | 0.07 | 0.00–0.18 | 46 | 0.10 |
| Hyperglycemia | 0.07 | 0.04–0.11 | 0 | 0.52 | 0.10 | 0.05–0.16 | 0 | 0.52 | 0.04 | 0.00–0.09 | 0 | 0.59 |
| Hyperpigmentation | 0.06 | 0.02–0.12 | 44 | 0.05 | 0.06 | 0.02–0.12 | 12 | 0.34 | 0.06 | 0.00–0.18 | 64 | 0.02 |
| Hypertension | 0.06 | 0.02–0.11 | 36 | 0.11 | 0.05 | 0.01–0.11 | 25 | 0.24 | 0.06 | 0.00–0.17 | 51 | 0.07 |
| Weight gain | 0.06 | 0.03–0.11 | 26 | 0.19 | 0.10 | 0.04–0.18 | 26 | 0.24 | 0.03 | 0.00–0.07 | 0 | 0.64 |
| Gastrointestinal symptoms | 0.06 | 0.01–0.12 | 55 | 0.01 | 0.06 | 0.00–0.19 | 73 | <0.001 | 0.04 | 0.01–0.11 | 0 | 0.42 |
| Pain | 0.05 | 0.01–0.13 | 67 | <0.001 | 0.09 | 0.00–0.24 | 82 | <0.001 | 0.03 | 0.00–0.08 | 0 | 0.95 |
| Dizziness | 0.04 | 0.01–0.08 | 11 | 0.34 | 0.04 | 0.00–0.12 | 52 | 0.06 | 0.03 | 0.00–0.08 | 0 | 0.92 |
| Upper respiratory tract symptoms | 0.04 | 0.01–0.08 | 28 | 0.17 | 0.03 | 0.00–0.09 | 34 | 0.18 | 0.05 | 0.00–0.13 | 31 | 0.20 |
| Fatigue | 0.04 | 0.01–0.11 | 60 | <0.001 | 0.06 | 0.00–0.19 | 78 | <0.001 | 0.03 | 0.00–0.07 | 0 | 0.74 |
| Muscle cramps | 0.04 | 0.01–0.08 | 28 | 0.17 | 0.03 | 0.00–0.09 | 34 | 0.18 | 0.05 | 0.00–0.13 | 31 | 0.20 |
| Flushing | 0.03 | 0.01–0.06 | 0 | 0.83 | 0.04 | 0.01–0.09 | 9 | 0.36 | ||||
| Cushingoid appearance | 0.03 | 0.01–0.06 | 0 | 0.74 | 0.04 | 0.00–0.09 | 25 | 0.24 | ||||
| Rash | 0.03 | 0.01–0.06 | 0 | 0.86 | 0.02 | 0.00–0.06 | 0 | 0.69 | 0.04 | 0.00–0.10 | 0 | 0.72 |
| Respiratory tract infection | 0.02 | 0.01–0.05 | 0 | 0.99 | 0.02 | 0.00–0.05 | 0 | 0.94 | 0.03 | 0.00–0.08 | 0 | 0.89 |
| Bone loss | 0.02 | 0.00–0.04 | 0 | >0.99 | 0.02 | 0.00–0.05 | 0 | 0.92 | ||||
| Tremor | 0.02 | 0.01–0.05 | 0 | 0.83 | 0.03 | 0.00–0.07 | 18 | 0.30 | ||||
| Hoarseness | 0.02 | 0.00–0.05 | 0 | 0.97 | 0.02 | 0.00–0.06 | 0 | 0.60 | ||||
| Acne | 0.02 | 0.00–0.05 | 0 | >0.99 | 0.04 | 0.01–0.09 | 0 | 0.47 | ||||
| Blurred vision | 0.02 | 0.00–0.05 | 0 | 0.97 | 0.02 | 0.00–0.06 | 0 | 0.60 | ||||
| Polyuria | 0.02 | 0.01–0.05 | 0 | >0.99 | 0.02 | 0.00–0.05 | 0 | 0.94 | 0.03 | 0.00–0.08 | 0 | 0.95 |
| Palpitations | 0.02 | 0.01–0.05 | 0 | >0.99 | 0.02 | 0.00–0.05 | 0 | 0.94 | 0.03 | 0.00–0.08 | 0 | 0.95 |
| Increased appetite | 0.02 | 0.00–0.05 | 0 | >0.99 | 0.02 | 0.00–0.05 | 0 | 0.94 | ||||
| Delayed wound healing | 0.02 | 0.00–0.05 | 0 | >0.99 | 0.02 | 0.00–0.05 | 0 | 0.94 | ||||
ACTH, adrenocorticotropic hormone.
Table 7.
Potential future considerations of ACTH in glomerular diseases
| Glomerular disease | Indication | Treatment regimen |
|---|---|---|
| MN | As first-line therapy or in resistant MN | ACTH alone or in a combination with other immunosuppressive therapies. Response may depend on cumulative dose of ACTH [13] |
| Suggested dose: | ||
| FSGS and MCD | Resistant FSGS or MCD (data are limited) | ACTH alone or in a combination with another immunosuppressive therapy Suggested dose: |
| IgA nephropathy | Proteinuria >1 g/day despite maximally tolerated RAAS blockade (data are limited) | Suggested dose:
|
ACTH, adrenocorticotropic hormone; FSGS, focal segmental glomerulosclerosis; MCD, minimal change disease; MN, membranous nephropathy; RAAS, renin–angiotensin–aldosterone system.
Data are lacking to make any recommendations for lupus nephritis, MN and diabetic nephropathy.
Evaluation for publication bias
Funnel plots to evaluate publication bias regarding the incidence of adverse effects of ACTH treatment in glomerular diseases are summarized in Supplementary data, Figure S2. The graphs for assessing publication bias are slightly asymmetric and suggest the presence of publication in favor of negative studies evaluating the incidence of acne, blurred vision, bone loss, muscle cramps, delayed wound healing, hoarseness, gastrointestinal tract symptoms, respiratory tract infection, polyuria and rash. The slight asymmetry of the graphs also suggests the presence of publication in favor of positive studies regarding the incidence of hyperpigmentation, cushingoid appearance, dizziness, fatigue, flushing, hypertension, insomnia, mood swings, pain, tremor, upper respiratory tract symptoms and weight gain. Otherwise, the graphs for assessing publication bias are insignificant in the selected studies for incidence of edema, hyperglycemia, increased appetite and palpitation.
DISCUSSION
In this study, we present the first systematic review of ACTH effectiveness and meta-analysis of its adverse effects in glomerular diseases. According to the current evidence, ACTH seems to be a promising treatment for nephrotic syndrome, especially MN. ACTH was shown to reduce proteinuria in patients with MN who previously failed to respond to oral corticosteroids, suggesting ACTH may have antiproteinuric effects besides its steroidogenic properties. However, the precise dose and duration of therapy required to produce a sustained response remain unknown. A study by Berg et al. [3] showed that a quick relapse of proteinuria and dyslipidemia occurred after 2 months of therapy with ACTH, whereas remission was sustained up to 30 months in a subgroup of patients who received ACTH for 12 months [3]. Hladunewich et al. [13] also pointed out that the degree of proteinuria was inversely related to the cumulative dose of ACTH. These data suggest that a higher cumulative dose of ACTH may be associated with more complete and sustained remission. ACTH was shown to reduce or completely clear antiphospholipase A2 receptor (PLA2R) in most patients who had a positive anti-PLA2R at baseline, suggesting that ACTH therapy may also exert therapeutic value by suppressing autoantibody production [5, 13].
In patients with FSGS and MCD, the treatment failure rate beyond 6 months of follow-up was high (∼50%) compared with patients with MN (5–31%). Reported relapses were also frequent after stopping treatment (17%). However, this may be due to the resistant nature of patients' disease since the majority of included patients had failed to respond to two to three immunosuppressive agents in the past. Berg et al. [22] reported that 10 patients with FSGS had a reduction in proteinuria (5–1 g) after replacing oral corticosteroids with synthetic ACTH while continuing a second immunosuppressive agent. These data suggest that ACTH may have a potential role in treating patients with resistant FSGS or MCD. However, due to the small and heterogeneous nature of these studies, the evidence for efficacy of ACTH in FSGS and MCD is limited at the present time.
There are currently two forms of ACTH injections commercially available. One is natural ACTH (H.P. Acthar gel; Mallinckrodt), a 39-amino-acid peptide isolated from highly purified porcine pituitary extract, which is currently the only ACTH-containing product approved by the US Food and Drug Administration for treatment of patients with nephrotic syndrome in the USA. The second is a synthetic form of ACTH, containing only the first 24 amino acids from the 39-amino-acid ACTH peptide [30]. Up until now, there has been no head-to-head comparison between these two forms of ACTH. On the basis of the current literature, patients with nephrotic syndrome responded to both forms of ACTH equally, and the adverse effect profiles were similar (Table 6).
There are no reported studies in the literature that directly compare ACTH with oral glucocorticoids in adults with glomerular diseases. As mentioned above, ACTH may be more advantageous than oral glucocorticoids since it may have direct protective effects on the podocyte [10]. Similarly, ACTH seems to have a better adverse effect profile compared with oral glucocorticoids. There was only 1 patient who reported bone loss among the 167 nephrotic patients treated with ACTH (0.5%). The low rate of bone loss in patients treated with ACTH may be due to the anabolic effect of ACTH, which has been shown to prevent bone loss [30, 31]. Therefore, ACTH could be more beneficial in patients who have osteoporosis and in whom the use of steroids is contraindicated [31–33]. The insulinogenic effect of the natural ACTH [8] may also decrease the incidence of glucose intolerance, considering only 2 of the 18 patients with diabetic nephropathy treated with natural ACTH required a reduction in their ACTH dose due to hyperglycemia [23, 30]. In this meta-analysis, ACTH was generally well tolerated. Edema was the most common adverse effect, which was managed with diuretics. Insomnia and mood swings were other common adverse effects. This could be due to the direct neurobiological effects of ACTH through melanocortin system activation [32]. Studies comparing the safety of ACTH with that of oral glucocorticoids are needed to better delineate the pros and cons of ACTH as a replacement for steroids when treating patients with glomerular diseases.
There are several limitations to our study. First, several factors decreased the quality of the evidence. About 40% of included citations were abstracts or letters to the editor, thus minimal details on patients' baseline characteristics, treatment regimen and response criteria were provided. Most studies were small observational studies with limited controls and short-term follow-up. Second, there were statistical heterogeneities in the analysis of the incidence of adverse effects. The potential sources of these heterogeneities included differences in the types of glomerular diseases, baseline characteristics of patients and different formulations of ACTH. Third, the lack of consistent treatment effect was most likely due to heterogeneous baseline characteristics, specifically pretreatment, with other immunosuppressive therapies.
In conclusion, ACTH is a promising therapy for nephrotic syndrome in patients with MN. Overall it is well tolerated and has a more favorable adverse effect profile compared with corticosteroids. An RCT with extended follow-up is warranted to examine the efficacy and safety of ACTH therapy in nephrotic patients.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
AUTHOR CONTRIBUTIONS
All authors had access to the data and a role in writing the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1.van Acker KJ, Hooft C. The influence of hormone treatment on the natural evolution of the idiopathic nephrotic syndrome in childhood. Acta Paediatr Scand 1968; 57: 479–486 [DOI] [PubMed] [Google Scholar]
- 2.Lauson HD, Forman CW, McNamara H et al. The effect of corticotropin (ACTH) on glomerular permeability to albumin in children with the nephrotic syndrome. J Clin Invest 1954; 33: 657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg AL, Nilsson-Ehle P, Arnadottir M. Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney Int 1999; 56: 1534–1543 [DOI] [PubMed] [Google Scholar]
- 4.Bomback AS, Tumlin JA, Baranski J et al. Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Des Devel Ther 2011; 5: 147–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bomback AS, Canetta PA, Beck LH Jr et al. Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trial. Am J Nephrol 2012; 36: 58–67 [DOI] [PubMed] [Google Scholar]
- 6.Hogan J, Bomback AS, Mehta K et al. Treatment of idiopathic FSGS with adrenocorticotropic hormone gel. Clin J Am Soc Nephrol 2013; 8: 2072–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lorusso P, Bottai A, Mangione E et al. Low-dose synthetic adrenocorticotropic hormone-analog therapy for nephrotic patients: results from a single-center pilot study. Int J Nephrol Renovasc Dis 2015; 8: 7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong R. The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol 2012; 8: 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong R, Ge Y, Tolbert E et al. Renoprotection by adrenocorticotropin in experimental acute kidney injury. J Am Soc Nephrol 2009; 20: 510A [Google Scholar]
- 10.Lindskog A, Ebefors K, Johansson ME et al. Melanocortin 1 receptor agonists reduce proteinuria. J Am Soc Nephrol 2010; 21: 1290–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponticelli C, Passerini P, Salvadori M et al. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis 2006; 47: 233–240 [DOI] [PubMed] [Google Scholar]
- 12.Finocchietti D, Cantaluppi V, Medica D et al. The anti-proteinuric effect of adrenocorticotropic hormone in patients with resistant nephrotic syndrome is related to its direct activity on glomerular and tubular epithelial cells. Nephrol Dial Transplant 2014; Abstract 29: p. 194 [Google Scholar]
- 13.Hladunewich MA, Cattran D, Beck LH et al. A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant 2014; 29: 1570–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012 [DOI] [PubMed] [Google Scholar]
- 15.STROBE statement—checklist of items that should be included in reports of observational studies (STROBE initiative). Int J Public Health 2008; 53: 3–4 [DOI] [PubMed] [Google Scholar]
- 16.Barendregt J, Doi S. MetaXL User Guide: Version 1.0. Wilston, QLD, Australia: EpiGear International, 2010 [Google Scholar]
- 17.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; 28: 105–114 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rauen T, Michaelis A, Floege J et al. Case series of idiopathic membranous nephropathy with long-term beneficial effects of ACTH peptide 1-24. Clin Nephrol 2009; 71: 637–642 [DOI] [PubMed] [Google Scholar]
- 20.Hofstra J, Brink H, Van de Kerkhof J. Treatment with synthetic ACTH in patients with idiopathic membranous nephropathy and high risk for renal failure. J Am Soc Nephrol 2010; 21: 680A [Google Scholar]
- 21.Berg A-L, Back SE. ACTH treatment in patients with lupus nephritis. Am J Kidney Dis 2013: Abstract 61: p. A25 [Google Scholar]
- 22.Berg A-L, Dolinina J, Back SE. Steroid replaced with ACTH treatment in FSGS patients. Am J Kidney Dis 2013; Abstract 61: p. A25 [Google Scholar]
- 23.Tumlin JA, Galphin CM, Rovin BH. Advanced diabetic nephropathy with nephrotic range proteinuria: a pilot study of the long-term efficacy of subcutaneous ACTH gel on proteinuria, progression of CKD, and urinary levels of VEGF and MCP-1. J Diabetes Res 2013; 2013: 489869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madan A, Milward AS, Khastgir A. Treatment of nephrotic syndrome with Acthar® gel: a retrospective case series. Am J Kidney Dis 2014; Abstract 63: p. A75 [Google Scholar]
- 25.Khastgir A, Mijovic-Das S, Stankovic A et al. HP Acthar® gel in patients with IgA nephropathy, membranous lupus nephritis and minimal change disease: a retrospective case series. Am J Kidney Dis 2015; Abstract 65: p. A49 [Google Scholar]
- 26.Berg AL, Nilsson-Ehle P. ACTH lowers serum lipids in steroid-treated hyperlipemic patients with kidney disease. Kidney Int 1996; 50: 538–542 [DOI] [PubMed] [Google Scholar]
- 27.Berg A-L, Arnadottir M. ACTH-induced improvement in the nephrotic syndrome in patients with a variety of diagnoses. Nephrol Dial Transplant 2004; 19: 1305–1307 [DOI] [PubMed] [Google Scholar]
- 28.Picardi L, Villa G, Galli F et al. ACTH therapy in nephrotic syndrome induced by idiopathic membranous nephropathy. Clin Nephrol 2004; 62: 403–404 [DOI] [PubMed] [Google Scholar]
- 29.Berg A, Stefánsson B, Arnadottir M. A randomized, controlled study on treatment with adrenocorticotropic hormone in idiopathic membranous nephropathy. American Society of Nephrology. Abstract Book; poster no. F-PO1112.
- 30.Gong R. Leveraging melanocortin pathways to treat glomerular diseases. Adv Chronic Kidney Dis 2014; 21: 134–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaidi M, Sun L, Robinson LJ et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci USA 2010; 107: 8782–8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab 2003; 284: E468–E474 [DOI] [PubMed] [Google Scholar]
- 33.Minetto M, Reimondo G, Osella G et al. Bone loss is more severe in primary adrenal than in pituitary-dependent Cushing's syndrome. Osteoporos Int 2004; 15: 855–861 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.