Abstract
Activation of the inducible caspase 9 (iC9) safety gene by a dimerizing drug (chemical inducer of dimerization (CID) AP1903) effectively resolves the symptoms and signs of graft-versus-host disease (GvHD) in haploidentical stem cell transplant (HSCT) recipients. However, after CID treatment, 1% of iC9-T cells remain and can regrow over time; although these resurgent T cells do not cause recurrent GvHD, it remains unclear whether repeat CID treatments are a safe and feasible way to further deplete residual gene-modified T cells should any other adverse effects associated with them occur. Here, we report a patient who received an infusion of haploidentical iC9-T cells after HSCT and subsequently received three treatments with AP1903. There was a mild (grade 2) and transient pancytopenia following each AP1903 administration but no non-hematological toxicity. Ninety five percent of circulating iC9-T cells (CD3+CD19+) were eliminated after the first AP1903 treatment. Three months later, the residual cells had expanded more than eightfold and had a lower level of iC9 expression. Each repeated AP1903 administration eliminated a diminishing percentage of the residual repopulating cells, but elimination could be enhanced by T-cell activation. These data support the safety and efficiency of repeated CID treatments for persistent or recurring toxicity from T-cell therapies.
Introduction
Although adoptive cellular immunotherapy can be an effective therapeutic strategy to treat human malignancies, the adverse effects may be both severe and prolonged. After allogeneic stem cell transplantation, for example, adoptive transfer of T cells to accelerate immune reconstitution and antiviral immunity can produce progressive and fatal acute and chronic graft-versus-host disease (GvHD), while transfer of tumor-directed T cells can lead to a fatal cytokine release syndrome or to on-target/off-tumor or off-target events that may be both rapid in onset and fatal in outcome.1,2,3,4,5,6,7 As a consequence, there is increasing interest in developing safety or suicide systems that can address this panoply of adverse events by rapidly, reliably, and fully eliminating the T cells producing the unwanted events.
The herpes simplex virus thymidine kinase (HSV-tk) suicide gene system has previously been used as a safety system since it allows T cells to be ablated by the administration of prodrugs such as ganciclovir. However, activation of HSV-TK by ganciclovir is relatively slow, and administration of ganciclovir to treat cytomegalovirus (CMV) infections would result in unwanted destruction of HSV-TK expressing cells. We have previously described an approach based on the expression of an inducible human caspase 9 transgene (iC9), which is dimerized and hence activated by the administration of an otherwise bioinert small molecule drug, AP1903.8,9,10 Since no monoclonal antibody is available to detect iC9, we monitored the infused iC9-T cells in vivo by generating a retroviral vector encoding iC9 in combination with a truncated CD19 linked by a 2A sequence to use as a selectable and trackable marker. Our preclinical and clinical analyses have shown close concordance between changes in levels of CD3+CD19+ expression and iC9 transgene expression.11,12,13 This iC9 safety switch is human derived, has limited immunogenicity, and allows patients to receive ganciclovir and related drugs to treat viral infections without T-cell damage. Activation of iC9 eradicates up to 99% of iC9-expressing T cells (iC9-T cells) in vitro and in vivo within 2 hours of a single dose of the chemical inducer of dimerization (CID AP1903), and even a single dose of dimerizing drug to activate the iC9 transgene can produce sufficient in vivo allodepletion of GvHD-inducing T cells.11,13,14,15
Although a single dose of dimerizing drug can effectively control GvHD, we observed that the small remaining fraction of iC9-T cells can subsequently expand and repopulate patients. Although such resurgence does not lead to a recurrence of GvHD, it currently remains unclear whether we could continue to deplete cells in vivo should other adverse effects associated with adoptive transfer of T cells occur, and it is unknown whether multiple doses of CID could have unanticipated toxicity. Here, we evaluated the feasibility and safety of multiple treatments with dimerizing drug in vivo in a patient who received three doses of the drug.
Results
Patient details
The patient was an 8-year-old male who received a haploidentical stem cell transplant (HSCT) with CD34+ selected stem cells from his mother to treat acquired hemophagocytic lymphohistiocytosis with central nervous system involvement. Prior to HSCT, he had received long-term steroid treatment for his primary disease, and his early post-transplant course was complicated by reactivation of multiple viruses (CMV, human herpesvirus 6 (HHV6), adenovirus (AdV), BK virus (BKV), and Epstein–Barr virus (EBV)), persistent transaminitis, and steroid-induced hypertension and hyperglycemia. He was enrolled on the DOTTI (Administration of haploidentical DOnor T cells Transduced with the Inducible caspase-9 suicide gene) study in which patients received escalating doses of iC9-T cells post HSCT in order augment immune reconstitution.15 On day 47 post HSCT, he received 1 × 106 iC9-T cells/kg. He had no immediate adverse effects from the T-cell infusions, and within 1–10 weeks, reactivations of CMV, HHV6, AdV, and BKV were successfully controlled as judged by polymerase chain reaction (PCR) analyses for viral DNA in blood (Table 1).
Table 1. Viral reactivations and infections after iC9-T–cell infusion.
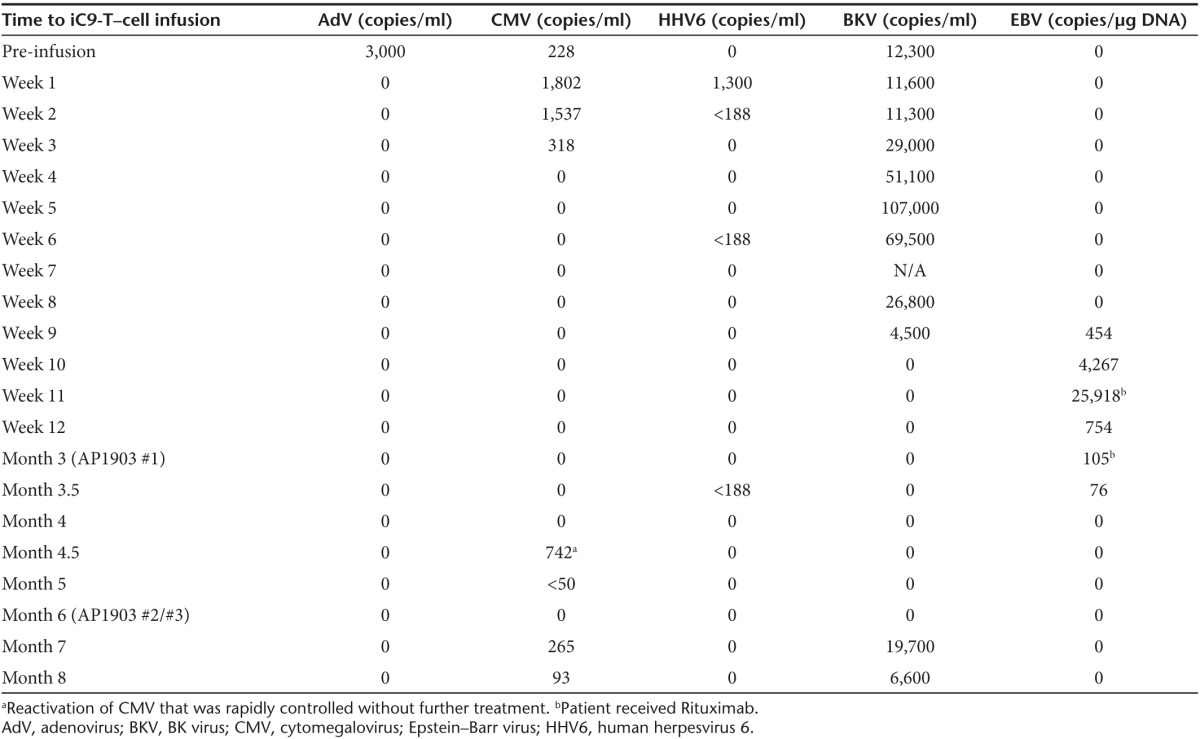
Three months after administration of iC9-T cells, however, the patient was admitted with gastritis. A gastric biopsy was positive by PCR for EBV, HHV6, and HHV7, but there was no histological evidence of GvHD. As his EBV load in blood was also elevated, he was treated with Rituximab (Table 1). During this admission, he developed a skin rash, and biopsy was consistent with GvHD (grade 2). He was given a single dose of iC9 dimerizer drug AP1903 (#1), and his rash had completely resolved within 24 hours. Over the next 4–6 weeks, his gastritis also resolved. However, 3 weeks later, his hepatic transaminases became elevated although bilirubin and alkaline phosphatase remained normal. Circulating iC9-T cells (detected as the CD3+CD19+ population) remained below pre-dimerizer drug levels (220/µl: Figure 1: #1), but the possibility that the infused T cells may have contributed to a possible flare of acute GvHD was nonetheless considered. He therefore received two additional doses of the dimerizing drug (Figure 1: #2, #3) at intervals of 48 hours. Retreatment had no effect on his transaminitis, which was also unresponsive to intensive subsequent immunosuppression. A repeat liver biopsy 5 weeks later showed macrovesicular steatosis compatible with drug therapy and parenteral nutrition to which his transaminitis was attributed since he had no evidence of acute or chronic GvHD. Unfortunately, in association with prolonged steroid therapy, the patient then developed pulmonary zygomycetes from which he died 2 months later.
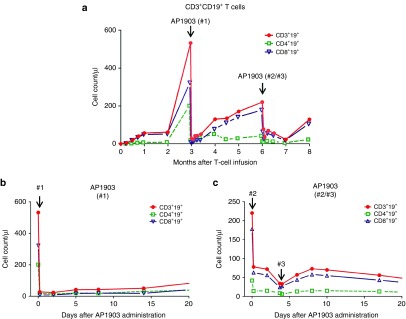
Figure 1.
Detection of circulating iC9-T cells before and after dimerizer treatment. (a) Counts of circulating iC9-T cells after infusion. Red line with filled circle represents CD3+CD19+ T cells, green dashed line with square represents CD4+CD19+ T cells, and blue line with empty triangle represents CD8+CD19+ T cells. Dynamics of iC9-T cells within 20 days after (b) first dimerizer treatment and (c) second/third dimerizer treatments.
Effects of multiple doses of dimerizing drug on serial depletion of iC9-T cells
After the initial dose of dimerizing drug (dose #1; 3 months after the initial iC9-T–cell infusion), circulating CD3+CD19+ T cells decreased from 532/µl to 28/µlL within 2 hours (Figure 1a,b). Residual CD3+CD19+ T cells expanded over the next 3 months (Figure 1a), and on administration of the second dose of dimerizing drug (#2), the circulating CD3+CD19+ T cells decreased from 220/µl to 78/µl; there was no substantive change in CD3+CD19+ T cells after the third dose of AP1903, 48 hours later (Figure 1c).
Safety of multiple doses of dimerizing drug
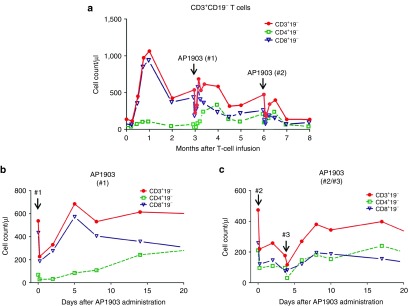
There were no non-hematological toxicities associated with administration of any of the doses of AP1903. However, following each dose, there was a transient decrease of peripheral blood cell counts in all lineages. Figure 2 shows the effects of the dimerizer on nontransduced (CD3+CD19−) T cells after each treatment with the dimerizing drug; counts returned to pretreatment levels within 72 hours (Figure 2b,c). The drug had similar effects on B lymphocytes and natural killer (NK) cells (see Supplementary Figure S1) and on neutrophils and platelets (see Supplementary Figure S2). Overall, we observed a transient hematological toxicity that was limited to grade 2.
Figure 2.
Counts of circulating CD3+CD19− T cells. (a) Counts of circulating endogenous T cells after iC9-T–cell infusion. Red line with filled circle represents CD3+CD19− T cells, green dashed line with square represents CD4+CD19− T cells, and blue line with empty triangle represents CD8+CD19− T cells. Counts of endogenous CD3+ T cells after (b) first dimerizer treatment and (c) second/third dimerizer treatments.
Mechanism of iC9 T-cell resistance/recovery
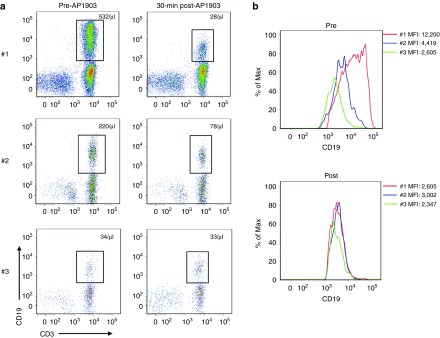
A subpopulation of iC9+ T cells may persist after exposure to the dimerizing drug because the surviving cells express lower levels of the transgene. We therefore examined the level of iC9-ΔCD19 expression in the T-cell population before and after each dimerizer treatment. Figure 3a shows the absolute number of CD3+CD19+ T cells detected in peripheral blood before each dimerizer treatment. Immediately prior to the first exposure to dimerizing drug, the mean fluorescence intensity (MFI) of CD19 in iC9-T cells was 12,200 (Figure 3b left panel). The recovering iC9+ cells, however, had a lower MFI, so that immediately prior to the second treatment, the value was 4,419. The cells surviving from the second treatment had still lower median expression, so that immediately prior to treatment 3 the MFI was just 2,605 (Figure 3b left panel). Of note, immediately following dimerizer treatment, the MFI of surviving cells was always less than 3,000, irrespective of the number of prior drug exposures (Figure 3b right panel), meaning that the highest expressing fraction of cells (>3,000 MFI) is removed at each drug exposure. Consistent with selective removal of the highest expressing cells, the iC9 transgene copy number detected before and after each dimerizer treatment by quantitative PCR (Q-PCR) showed a 90% decline after the first dimerizer treatment (from 11.5 to 1.1, normalized by β-actin), but limited further decline after exposure #2 and no discernible additional fall after exposure #3 (see Supplementary Figure S3). Hence, differences in the proportion of cells eliminated after each dose relate to a progressive destruction of the most highly transduced and most highly expressing cells.
Figure 3.
The expression of CD19 on iC9-T cells at each treatment. (a) Flow analysis shows iC9-T cells prior to and post each dimerizer treatment, the absolute number of iC9-T cells in peripheral blood is also shown. (b) The MFI in the gated CD3+CD19+ population. MFI, mean fluorescence intensity.
Lower levels of iC9 expression also reflect reduced T-cell activation
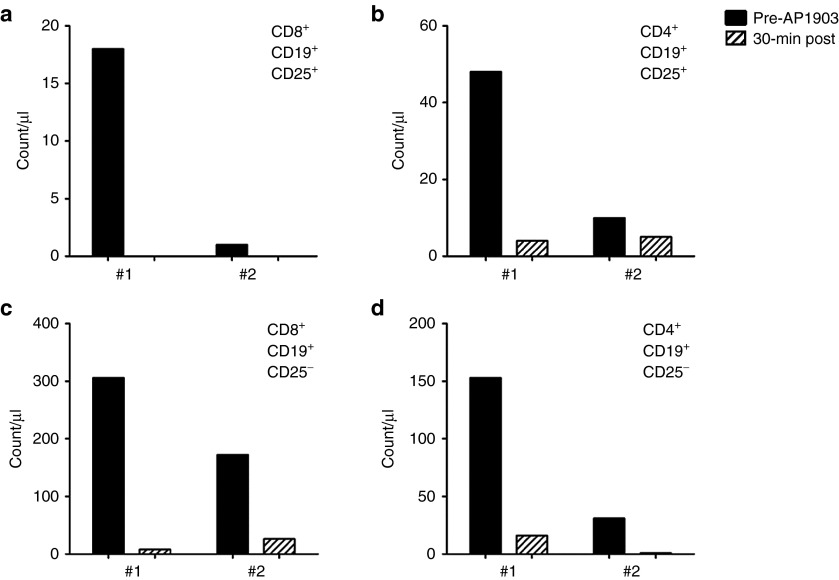
Although iC9 selectively eliminated the most highly expressing and most highly transduced T cells, the susceptibility to dimerizing drug may be further influenced by the activation status of the T cells. Transgene expression derived from retroviral integrants is highly dependent on the state of T-cell activation;16,17,18 during GvHD, for example, alloreactive cells are the most activated, express the highest level of iC9, and therefore are the most readily eliminated.10 To determine whether the reduced iC9-CD19 expression observed during subsequent exposure to dimerizing drug also correlated with the level of activation of the residual transduced T cells, we measured CD25 positivity in the T-cell population (Figure 4). All CD8+CD19+CD25+ T cells were eliminated after the first dimerizer treatment (18/µl and 0/µl, pre and post treatment, respectively) and, in contrast to the bulk population of T cells, had not returned in the 3 months prior to the second treatment (Figure 4a). Similarly, more than 90% of the CD4+CD19+CD25+ T-cell population was eliminated after the first dimerizer treatment (48/µl to 4/µl, pre and post treatment, respectively) and had minimal re-expansion during the next 3 months (10/µl and 7/µl, pre and post treatment, respectively) (Figure 4b).
Figure 4.
Activated T cells before and after dimerizer treatment. Count of circulating (a) CD8+CD19+CD25+ T cells; (b) CD4+CD19+CD25+ T cells; (c) CD8+CD19+CD25− T cells; and (d) CD4+CD19+CD25− T cells.
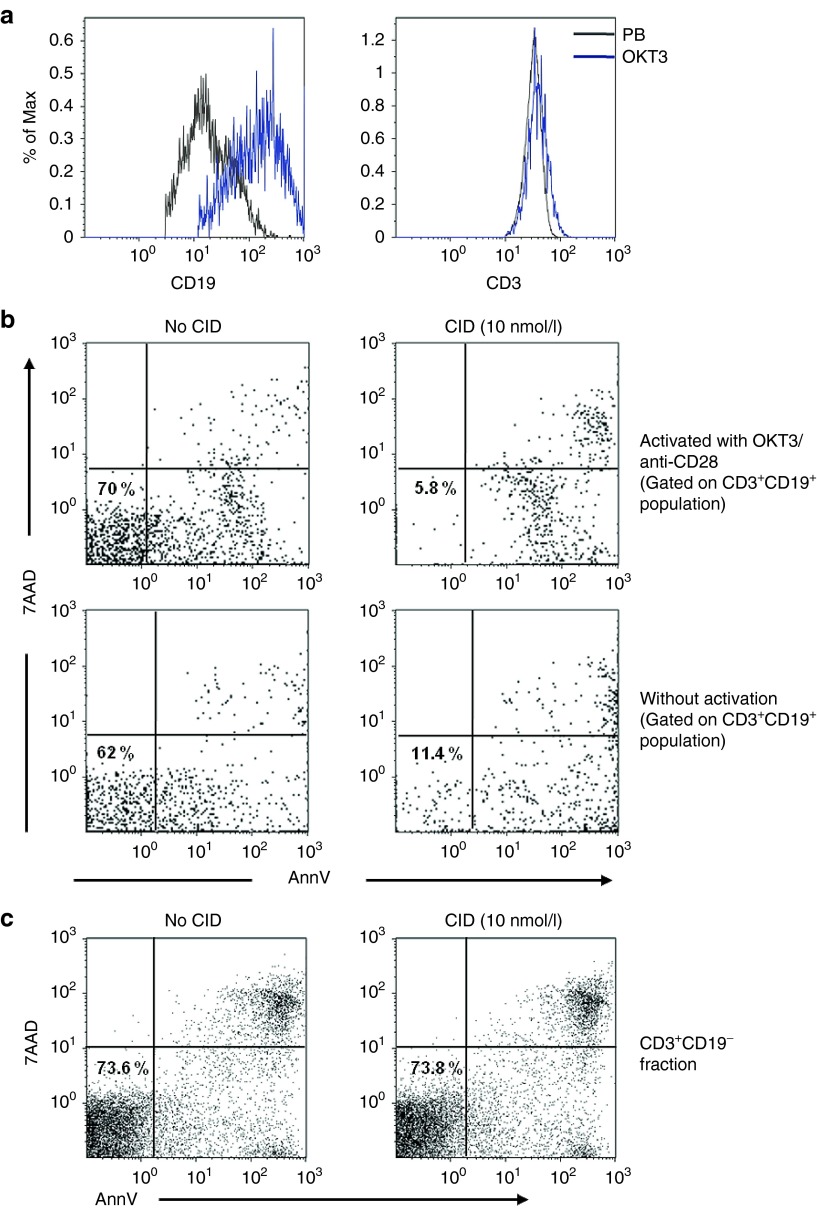
To further assess whether the reduced in vivo destruction of T cells after subsequent doses of the dimerizing agent predominantly reflected the lack of associated in vivo (allo)activation by GvHD, we collected T cells from the patient after his GvHD had been treated with dimerizing drug. These T cells were then exposed in vitro to CD3/CD28 monoclonal antibodies to simulate T-cell receptor (TCR) activation and co-stimulation. At 48–72 hours, CD3/CD28 reactivated CD3+CD19+ cells had significantly higher transgene expression than nonreactivated cells, with their CD19 MFI increasing from 4,603 to 30,720 (CD19+), while their CD3 MFI was unchanged (Figure 5a). Following exposure to CD3/CD28, we treated these cells with CID, and the percentage of CD3+CD19+ cells surviving exposure to CID was reduced by half, from 18 to 8% (Figure 5b). Killing of CD3+CD19− T cells following CID exposure was unaffected by prior anti-CD3/anti-CD28 activation (Figure 5c).
Figure 5.
Transgene expression could be upregulated by reactivation in vitro and makes the cells more sensitive to the dimerizer. (a) The expression of CD19 significantly increased (left panel) after ex vivo activation by OKT3/anti-CD28 antibody and CD3 (right panel), black line indicates the expression in peripheral blood and blue line indicates the expression in T cells reactivated by OKT3/anti-CD28. (b) In vitro drug sensitivity in CD3+CD19+ T cells with or without OKT3/anti-CD28 reactivation and (c) in vitro drug sensitivity in CD3+CD19− populations. The percentage indicates the live cells in the population evaluated by 7AAD−/AnnV−. Representative example of three experiments is shown. 7-AAD, 7-amino-actinomycin; AnnV, Annexin V.
Screening for mutations of the iC9 transgene
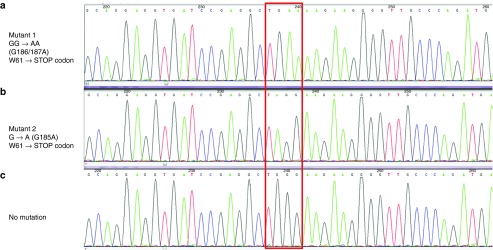
To evaluate whether resistance arose from functional mutations in the iC9 protein, we sequenced the iC9 transgene obtained after amplification of the DNA extracted from total peripheral blood mononuclear cells (PBMCs) collected at different time points before and after AP1903 treatments. We sequenced multiple individual clones derived from the subcloned PCR products. Nonsense mutations causing a premature stop codon in the iC9 transgene were detected in 2 out of 10 and 1 out of 10 subcloned PCR products in the DNA extracted from PBMCs collected 30 minutes and 4 days after the third administration of AP1903, respectively (Figure 6). Hence, sporadic mutations could contribute to the low responses but they are neither the sole nor the primary mechanism for dimerizer resistance.
Figure 6.
Mutations detected in DNA after the third dimerizer treatment. iC9 transgene was amplified from the DNA extracted from peripheral blood mononuclear cells collected after the third administration of AP1903; multiple individual clones derived from the subcloned polymerase chain reaction products were sequenced. Nonsense mutations that generated a premature stop codon in the transgene at amino acid 61 were detected in the 2 out of 10 DNA samples and 1 out of 10 sample obtained 30 minutes and 4 days post #3 treatment, respectively (indicated in the red box). The mutations generated a stop codon at amino acid 61 instead of tryptophan (W61), (a) GG→AA (186–187), (b) G185A, and (c) No mutation.
Discussion
Rapid control or elimination of cells expressing a therapeutic transgene may be required to overcome both short- and long-term toxicities.19,20,21,22 We have previously shown that the iC9 safety system can be activated by a single dose of 0.4 mg/kg AP1903, and iC9 activation can effectively and sustainably remove alloreactive T cells causing GvHD after haplo-HSCT. The system can immediately control the symptoms and signs of a systemic inflammatory response associated with T-cell activation.14,15,23 In the current report, we show that additional dimerizer treatments can eliminate up to 85% of the iC9-resistant T cells that survive after the first dose of dimerizer drug. In circumstances where persistence or regrowth of adoptively transferred T cells may be problematic, the administration of multiple doses of dimerizing drug appears to be safe and to induce further T-cell depletion.
A number of safety concerns are associated with adoptive T-cell therapies. Adverse effects include the acute toxicities of GvHD after transfer of allogeneic T cells2,12,15 and a cytokine release syndrome (or systemic inflammatory response) when adoptively transferred T cells become highly activated and expand in vivo, producing pro-inflammatory cytokines and inducing the release of additional inflammatory mediators from monocytes and other cells.4,24,25,26 In addition to these complications, there may be off-target damage to unrelated cells. For instance, fatal neurologic and cardiac toxicities can be caused by the cross-reactivity of high-affinity MAGE-A3 TCR with unrecognized expression of epitopes, even unrelated peptides in off-target organs or tissues.7,27,28 Finally, there may be on-target but off-tumor organ toxicities such as the hypogammaglobulinemia that follows the prolonged depletion of normal CD19+ B cells by long-lived CD19-CAR T cells intended to kill CD19+ malignancies.20
We have previously shown that a single dose of dimerizing drug produces sufficient iC9 activation to rapidly deplete adoptively transferred haploidentical donor T cells and to rapidly and completely resolve the symptoms and signs of GvHD. Importantly, this single-dose regimen is sufficient to permanently abrogate the GvHD even though a small number of remaining (in vivo dimerizer-resistant) T cells subsequently expand and repopulate the host, likely because these cells have been depleted of their alloreactive component. In other applications of adoptive T-cell therapy, however, resurgent T cells might again produce the adverse effects initially observed, including the elimination of CD19+ normal B cells by CD19-CAR T cells. The outcome we report here is the first to show that such resistant and then resurgent T cells nonetheless continue to be vulnerable to killing by re-exposure to additional doses of the dimerizing drug in vivo, although the level of killing is lower than on initial exposure (85 versus 95%).
One concern over repeated administration of dimerizing drug is that there may be unexpected toxicities. Although no evidence of treatment-related serious adverse events was reported in large animal models exposed either to a higher dosage of dimerizing drug for 30 days consecutively or to long-term administration of the agent,29,30 there are no phase 1 data to show that multidose administration is safe in humans.31 Our patient had evidence of liver toxicity after the first dose of AP1903, but we think this was likely not associated with CID administration since the onset was delayed and did not worsen with subsequent drug treatments. Moreover, no such toxicity was reported in a phase 1 study of single-dose administration of the dimerizing drug even when a higher dose was infused (1.0 mg/kg versus 0.4 mg/kg); of note, none of the seven other patients treated with the agent had hepatotxicity attributable to drug administration.12,14,15 We did, however, observe one potentially related toxicity in this patient, a mild and transient pancytopenia (grade 2) that was present immediately after each administration of AP1903 and resolved within 72 hours. This response was not observed in preclinical testing, or in the phase 1 study, or in any of seven other patients we treated. We do not know the mechanism underlying this idiosyncratic reaction, but since it lasted less than 72 hours after each administration of the drug we believe it is less likely attributable to direct toxicity to marrow or blood cells.
Although additional doses of the dimerizing drug continued to deplete T cells, a small proportion of iC9-T cells survived even after three treatments. Clinical studies using the herpes simplex virus thymidine kinase (HSV-TK) suicide gene system showed that more than 10% of the transduced T lymphocytes were resistant to killing even after multiple doses of ganciclovir.32 Although neither iC9 or HSV-TK can produce 100% elimination of transduced cells, the iC9 system may have advantages over HSV-TK. The iC9 system can control a broader range of toxicities from adoptively transferred T cells since it is activated by a drug that is otherwise bioinert rather than a therapeutically efficacious prodrug such as ganciclovir, and may be preferable for the treatment of some of the acute adverse events associated with adoptive transfer of T cells as the speed of action is faster. iC9 may also be less immunogenic than HSV-TK in immunocompetent T-cell recipients23 and therefore less likely to lead to unwanted elimination of the T cells, but this remains to be established.
While the ability to repeatedly reduce T-cell numbers may provide adequate control of many adverse events, some will require complete elimination of the transferred cells. Multiple mechanisms may contribute to dimerizer resistance of iC9-T cells, such as selection of a subset of T cells with proviral integration sites that favor low gene expression, transgene silencing by promoter hypermethylation, or constitutively high expression of anti-apoptotic proteins.18,33,34,35,36,37,38 Our earlier studies suggested that high levels of transcription of the iC9 transgene caused by TCR activation in alloreactive T cells explain the selective elimination of these cells by the dimerizer in patients who have clinical evidence of acute GvHD. Here, we found that surviving iC9-T cells continue to be eliminated by subsequent and delayed doses of the same drug. Although we cannot formally conclude that the iC9-T cell clones spared by the first dose of AP1903 are eliminated by subsequent doses of the drug, the continuous elimination of iC9-T cells may reflect dynamic changes in the levels of iC9 expression in vivo. This explanation reflects the existence either of alloreactive T cells with different thresholds for TCR activation or T cells that respond to other environmental factors in the infused polyclonal iC9-T cells. After the first dose of AP1903, residual alloreactive T cells with low TCR affinity and T cells responding to homeostatic stimuli rather than alloreactive antigens may emerge and become activated to express sufficient levels of iC9 to be eliminated by subsequent doses of AP1903. Although sporadic nonsense mutations of the iC9 transgene were observed, their frequency is too low for them to contribute substantively to the partial dimerizer resistance observed.
In conclusion, we show the feasibility, safety, and efficacy of serial activation of the iC9 safety switch, which may enable toxicities associated with the adoptive transfer of T cells to be sustainably reduced. It remains to be elucidated whether alternative vector design, such as the introduction of internal potent promoters into lentiviral vectors, or specific gene editing may allow higher constitutive expression of the iC9 transgene and even higher levels of sustained elimination of transgenic cells, should such activity be necessary.39,40
Materials and Methods
Patients and study design. This phase 1 clinical study was conducted in accordance with the Declaration of Helsinki and was approved by the institutional review board of Baylor College of Medicine. This trial was registered at www.clinicaltrials.gov as NCT01494103. The study was designed to assess the safety and efficacy of infusing escalating doses of donor-derived iC9-T cells in patients undergoing haplo-HSCT using Clinimacs selected CD34+ stem cells from an human leukocyte antigen haploidentical donor. Briefly, the patient reported here received iC9-T cells at day 47 after transplant at a dose of 1 × 106 cells/kg as previously described.15 The patient received three doses of AP1903 (0.4 mg/kg) at day 89, 175, and 178 after the infusion of iC9-T cells to deplete the iC9-T cells associated adverse events, the administration of AP1903 (Rimiducid, Bellicum Pharmaceuticals, Houston, TX) as 2-hour infusion.31
Monitoring of infections. Viral (AdV, BKV, CMV, and HHV6) reactivation or infections were monitored by Q-PCR assays (ViraCor-IBT Laboratories, Lee's Summit, MO) on plasma as noted. EBV-DNA viral load was determined by Q-PCR of PBMCs using specific primers and probes targeting the EBER gene.41
Generation of iC9-T cells. The iC9-T cells were generated as previously described.15 In brief, PBMCs from transplant donors were obtained by Ficoll density before they were activated by anti-CD3 antibody. Gene modification with iC9 followed the previously reported procedure.15
Flow cytometry analysis. The iC9-T cells were characterized using a panel of fluorochrome-conjugated monoclonal antibodies (BD Biosciences, San Jose, CA and Beckman Coulter, Brea CA). Cells were acquired on a FACS Calibur flow cytometer. Nontransduced control cells were used to set the negative gate for CD19 expression. Flow cytometry data were analyzed using Cell Quest software (Becton Dickinson) and Kaluza software (Beckman Coulter).
Real-time quantitative PCR of iC9. T2A.ΔCD19 transgene. The iC9 transgene was measured in PBMCs by Q-PCR as previously described.12,14,15
Assessment of transgene expression following reactivation. The PBMCs were collected after treatment of AP1903, a portion of the cells were reactivated on 24-well plates coated with 1 µg/ml OKT3 and 1 µg/ml anti-CD28 (Clone CD28.2, BD Pharmingen, San Diego, CA) for 48–72 hours. CD19 expression in both reactivated and nonreactivated cells were evaluated by flow cytometry and Q-PCR.
Induction of apoptosis with CID. Suicide gene functionality was assessed by CID (AP20187, Clontech Laboratories, Mountain View, CA), at 10 nM final concentration. Cells were stained with annexin V and 7-amino-actinomycin (7-AAD) (BD Pharmingen) for 22–30 hours and analyzed by flow cytometry. Cells negative for both annexin V and 7-AAD were considered viable, cells that were annexin V positive were apoptotic, and cells that were both annexin V and 7-AAD positive were necrotic. The percentage killing induced by dimerization was corrected for baseline viability as follows: Percentage killing = 100 − (%Viability in CID-treated cells ÷ %Viability in nontreated cells) × 100.
Screening for mutations of the iC9 transgene. DNA was extracted from PBMCs at different time points before and after AP1903 treatments. The iC9 transgene was amplified by high fidelity PCR and subcloned into the TOPO Blunt-End vector (ThermoFisher Scientific, Waltham, MA). The sequence of the primers used for amplification was forward 5′- TGTGGGTCAGAGAGCCAAAC -3′, reverse 5′- ctgcagcacagcgttatctc -3′. For each time point, we sequenced the iC9 transgene from DNA plasmid obtained from multiple individual subcloned colonies.
SUPPLEMENTARY MATERIAL Figure S1. Counts of circulating B cells and NK cells. Figure S2. Counts of neutrophil and platelets at each dimerizer treatment. Figure S3. The relative copy number of the iC9 transgene to β-actin of DNA obtained from PBMCs, evaluated by Q-PCR.
Acknowledgments
The authors thank the patient and family for their cooperation. They also thank David M. Spencer who provided the CID (AP1903/Rimiducid, Bellicum Pharmaceuticals), Yu-Feng Lin who coordinated the clinical study, Maksim Mamonkin for helpful discussion, and Catherine Gillespie for editing the manuscript. This clinical protocol (IND13813) was supported by NIH-NHLBI grant U54HL08100 and the development of the caspase system by P01CA094237 and P50CA126752. The clinical trial also received support from the Clinical Research Center at Texas Children's Hospital, the Institute for Clinical and Translational Research at Baylor College of Medicine and shared resources of the Dan L. Duncan Cancer Center support grant P30CA125123. X.Z. and M.K.B. conceived and designed study and wrote the manuscript; X.Z. performed experiments and analyzed data; S.N. enrolled patients and monitored clinical responses; O.D. performed Q-PCR; G.D. and H.E.H. contributed to the preparation of the manuscript; H.E.H. and M.K.B. are principal investigators and IND sponsors for the clinical trial. The Center for Cell and Gene Therapy has a collaborative research agreement with Celgene.
Supplementary Material
Counts of circulating B cells and NK cells.
Counts of neutrophil and platelets at each dimerizer treatment.
The relative copy number of the iC9 transgene to β-actin of DNA obtained from PBMCs, evaluated by Q-PCR.
References
- Cieri, N, Mastaglio, S, Oliveira, G, Casucci, M, Bondanza, A and Bonini, C (2014). Adoptive immunotherapy with genetically modified lymphocytes in allogeneic stem cell transplantation. Immunol Rev 257: 165–180. [DOI] [PubMed] [Google Scholar]
- Amrolia, PJ, Muccioli-Casadei, G, Huls, H, Adams, S, Durett, A, Gee, A et al. (2006). Adoptive immunotherapy with allodepleted donor T-cells improves immune reconstitution after haploidentical stem cell transplantation. Blood 108: 1797–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, RA, Yang, JC, Kitano, M, Dudley, ME, Laurencot, CM and Rosenberg, SA (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 18: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens, RJ, Davila, ML, Riviere, I, Park, J, Wang, X, Cowell, LG et al. (2013). CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5: 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer, JN, Dudley, ME, Feldman, SA, Wilson, WH, Spaner, DE, Maric, I et al. (2012). B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119: 2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, DW, Kochenderfer, JN, Stetler-Stevenson, M, Cui, YK, Delbrook, C, Feldman, SA et al. (2015). T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385: 517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, RA, Chinnasamy, N, Abate-Daga, D, Gros, A, Robbins, PF, Zheng, Z et al. (2013). Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother 36: 133–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer, DM, Wandless, TJ, Schreiber, SL and Crabtree, GR (1993). Controlling signal transduction with synthetic ligands. Science 262: 1019–1024. [DOI] [PubMed] [Google Scholar]
- Fan, L, Freeman, KW, Khan, T, Pham, E and Spencer, DM (1999). Improved artificial death switches based on caspases and FADD. Hum Gene Ther 10: 2273–2285. [DOI] [PubMed] [Google Scholar]
- Straathof, KC, Pulè, MA, Yotnda, P, Dotti, G, Vanin, EF, Brenner, MK et al. (2005). An inducible caspase 9 safety switch for T-cell therapy. Blood 105: 4247–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tey, SK, Dotti, G, Rooney, CM, Heslop, HE and Brenner, MK (2007). Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant 13: 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, CA, Asgari, Z, Liu, E, Yvon, E, Heslop, HE, Rooney, CM et al. (2010). An inducible caspase 9 suicide gene to improve the safety of mesenchymal stromal cell therapies. Stem Cells 28: 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Stasi, A, Tey, SK, Dotti, G, Fujita, Y, Kennedy-Nasser, A, Martinez, C et al. (2011). Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 365: 1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X, Di Stasi, A, Tey, SK, Krance, RA, Martinez, C, Leung, KS et al. (2014). Long-term outcome after haploidentical stem cell transplant and infusion of T cells expressing the inducible caspase 9 safety transgene. Blood 123: 3895–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X, Dotti, G, Krance, RA, Martinez, CA, Naik, S, Kamble, RT et al. (2015). Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood 125: 4103–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollok, KE, Hanenberg, H, Noblitt, TW, Schroeder, WL, Kato, I, Emanuel, D et al. (1998). High-efficiency gene transfer into normal and adenosine deaminase-deficient T lymphocytes is mediated by transduction on recombinant fibronectin fragments. J Virol 72: 4882–4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn, ER, Lum, LG and Trevor, KT (1998). T cell activation modulates retrovirus-mediated gene expression. Hum Gene Ther 9: 1457–1467. [DOI] [PubMed] [Google Scholar]
- Cattoglio, C, Maruggi, G, Bartholomae, C, Malani, N, Pellin, D, Cocchiarella, F et al. (2010). High-definition mapping of retroviral integration sites defines the fate of allogeneic T cells after donor lymphocyte infusion. PLoS One 5: e15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers, CH, Sleijfer, S, van Steenbergen, S, van Elzakker, P, van Krimpen, B, Groot, C et al. (2013). Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 21: 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalos, M, Levine, BL, Porter, DL, Katz, S, Grupp, SA, Bagg, A et al. (2011). T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3: 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens, RJ, Rivière, I, Park, JH, Davila, ML, Wang, X, Stefanski, J et al. (2011). Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118: 4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl, HC, Zaia, J, Rosenberg, SA, June, CH, Dotti, G, Kahn, J, et al. (2011). Considerations for the clinical application of chimeric antigen receptor T cells: observations from a recombinant DNA Advisory Committee Symposium. Cancer Res 71: 3175–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber, C, Abhyankar, H, Heslop, HE, Brenner, MK, Liu, H, Dotti, G et al. (2013). The immunogenicity of virus-derived 2A sequences in immunocompetent individuals. Gene Ther 20: 958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp, SA, Kalos, M, Barrett, D, Aplenc, R, Porter, DL, Rheingold, SR et al. (2013). Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila, ML, Riviere, I, Wang, X, Bartido, S, Park, J, Curran, K et al. (2014). Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6: 224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, DL, Levine, BL, Kalos, M, Bagg, A and June, CH (2011). Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linette, GP, Stadtmauer, EA, Maus, MV, Rapoport, AP, Levine, BL, Emery, L et al. (2013). Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 122: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, BJ, Gerry, AB, Dukes, J, Harper, JV, Kannan, V, Bianchi, FC et al. (2013). Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med 5: 197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, RE, De Claro, RA, Yan, J, Chien, S, Von Recum, H, Morris, J et al. (2004). Differences in F36VMpl-based in vivo selection among large animal models. Mol Ther 10: 730–740. [DOI] [PubMed] [Google Scholar]
- Okazuka, K, Beard, BC, Emery, DW, Schwarzwaelder, K, Spector, MR, Sale, GE et al. (2011). Long-term regulation of genetically modified primary hematopoietic cells in dogs. Mol Ther 19: 1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuliucci, JD, Oliver, SD, Morley, S, Ward, C, Ward, J, Dalgarno, D et al. (2001). Intravenous safety and pharmacokinetics of a novel dimerizer drug, AP1903, in healthy volunteers. J Clin Pharmacol 41: 870–879. [DOI] [PubMed] [Google Scholar]
- Garin, MI, Garrett, E, Tiberghien, P, Apperley, JF, Chalmers, D, Melo, JV et al. (2001). Molecular mechanism for ganciclovir resistance in human T lymphocytes transduced with retroviral vectors carrying the herpes simplex virus thymidine kinase gene. Blood 97: 122–129. [DOI] [PubMed] [Google Scholar]
- Priyadharshini, B, Thornley, TB, Daniels, KA, Cuthbert, A, Welsh, RM, Greiner, DL et al. (2013). Alloreactive CD8 T cells rescued from apoptosis during co-stimulation blockade by Toll-like receptor stimulation remain susceptible to Fas-induced cell death. Immunology 138: 322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barese, CN, Felizardo, TC, Sellers, SE, Keyvanfar, K, Di Stasi, A, Metzger, ME et al. (2015). Regulated apoptosis of genetically modified hematopoietic stem and progenitor cells via an inducible caspase-9 suicide gene in rhesus macaques. Stem Cells 33: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns, WR, Zheng, Z, Rosenberg, SA and Morgan, RA (2009). Lack of specific gamma-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation. Blood 114: 2888–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, AC, Ahlgren-Berg, A, Egan, JB, Dodd, IB and Shearwin, KE (2009). Potent transcriptional interference by pausing of RNA polymerases over a downstream promoter. Mol Cell 34: 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, AC, Egan, JB and Shearwin, KE (2011). Transcriptional interference by RNA polymerase pausing and dislodgement of transcription factors. Transcription 2: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, E, Liu, H, West, JA, Zhou, X, Dakhova, O, Wheeler, DA, et al. (2015). Clonal dynamics in vivo of virus integration sites of T cells expressing a safety switch. Mol Ther.doi: 10.1038/mt.2015.217 (epub ahead of print). [DOI] [PMC free article] [PubMed]
- Genovese, P, Schiroli, G, Escobar, G, Di Tomaso, T, Firrito, C, Calabria, A et al. (2014). Targeted genome editing in human repopulating haematopoietic stem cells. Nature 510: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini, L (2015). Gene therapy returns to centre stage. Nature 526: 351–360. [DOI] [PubMed] [Google Scholar]
- Kimura, H, Morita, M, Yabuta, Y, Kuzushima, K, Kato, K, Kojima, S et al. (1999). Quantitative analysis of Epstein–Barr virus load by using a real-time PCR assay. J Clin Microbiol 37: 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Counts of circulating B cells and NK cells.
Counts of neutrophil and platelets at each dimerizer treatment.
The relative copy number of the iC9 transgene to β-actin of DNA obtained from PBMCs, evaluated by Q-PCR.