Abstract
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder of unknown etiology. Although relatively common in children, how this condition affects brain structure and function in a pediatric population remains unclear. Here, we investigate brain changes in adolescents with IBS and healthy controls. Imaging was performed with a Siemens 3 Tesla Trio Tim MRI scanner equipped with a 32-channel head coil. A high-resolution T1-weighted anatomical scan was acquired followed by a T2-weighted functional scan. We used a surface-based morphometric approach along with a seed-based resting-state functional connectivity (RS-FC) analysis to determine if groups differed in cortical thickness and whether areas showing structural differences also showed abnormal RS-FC patterns. Patients completed the Abdominal Pain Index and the GI Module of the Pediatric Quality of Life Inventory to assess abdominal pain severity and impact of GI symptoms on health-related quality of life (HRQOL). Disease duration and pain intensity were also assessed. Pediatric IBS patients, relative to controls, showed cortical thickening in the posterior cingulate (PCC), whereas cortical thinning in posterior parietal and prefrontal areas were found, including the dorsolateral prefrontal cortex (DLPFC). In patients, abdominal pain severity was related to cortical thickening in the intra-abdominal area of the primary somatosensory cortex (SI), whereas HRQOL was associated with insular cortical thinning. Disease severity measures correlated with cortical thickness in bilateral DLPFC and orbitofrontal cortex. Patients also showed reduced anti-correlations between PCC and DLPFC compared to controls, a finding that may reflect aberrant connectivity between default mode and cognitive control networks. We are the first to demonstrate concomitant structural and functional brain changes associated with abdominal pain severity, HRQOL related to GI-specific symptoms, and disease-specific measures in adolescents with IBS. It is possible such changes will be responsive to therapeutic intervention and may be useful as potential markers of disease progression or reversal.
Introduction
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder (FGID) characterized by chronic abdominal pain and/or discomfort, and accompanied by altered bowel patterns [1, 2]. Although more frequently studied and reported in adults, this syndrome also affects children and adolescents and is associated with the occurrence of early, adverse life events, and the development of mood and somatoform disorders into adulthood [3–5]. In Western societies alone, the reported incidence of pediatric IBS is not uncommon, with an estimated 8–23% diagnosed between 4 and 18 years (yrs.) of age [6, 7]. Furthermore, the presentation of IBS symptoms can be debilitating for a child, interfering with school and other social activities, leading to impairments in daily functioning and decreasing overall well-being and quality of life [8, 9]. Therefore, IBS represents an important clinical problem in children that needs to be addressed since identifying effective treatments for this syndrome would be life-altering for many.
IBS is considered a ‘functional disorder’ given that alterations in bowel function and associated symptoms manifest in absence of any apparent structural or biochemical disease process. Despite the fact that IBS diagnosis has evolved from one entirely based on exclusion to a symptom-based approach currently embodied by Rome III criteria [10, 11], diagnosis still remains descriptive and without a definitive or objective biomarker. Undoubtedly, the availability of such markers would form a basis of enhanced phenotyping of the disease state, provide an earlier diagnosis, reduce costs associated with unnecessary and expensive testing, and provide valuable information to researchers and clinicians in search of novel and effective treatments.
To date, the pathophysiology of IBS is not well understood. The etiology of this syndrome is considered multifactorial, with central sensitization a likely contributing factor [12–15]. A number of putative mechanisms have been described in an attempt to explain the neurobiology underlying IBS, including disruptions in gut-brain axis signaling [12, 14, 16]; a hypothesis now made more tractable to scientific inquiry with the advent of magnetic resonance imaging (MRI). Collectively, neuroimaging studies performed in adults with IBS have reported structural and functional brain changes that were associated with (i) increased visceral sensitivity [17]; (ii) altered affective processes that modulate visceral pain [18]; and (iii) disruptions in endogenous descending pain inhibitory mechanisms [19, 20]. In contrast to the adult literature, our understanding of how IBS affects brain structure and function in children is less well delineated. Furthermore, while an IBS diagnosis in children shares similar diagnostic criteria to that in adults, the early structural and functional brain changes associated with the onset and progression of this syndrome may differ dramatically in the developing brain and warrants investigation.
The primary aim of this study was to identify concurrent structural and functional brain changes in adolescents with a clinical diagnosis of IBS relative to healthy controls using MRI. A secondary aim was to examine whether alterations in brain morphology were related to abdominal pain severity, health-related quality of life (HRQOL) as it pertains to GI-specific symptoms, and clinical measures of disease duration and pain intensity (over the past week). Specifically, we asked the following questions: (1) Are there significant structural and functional changes in the brains of pediatric patients with IBS compared to healthy cohorts? (2) If so, do these brain changes in adolescents with IBS parallel those reported in adults? and lastly, (3) To what extent are these changes associated with the severity of current abdominal pain, impact of GI-related symptoms on health and well-being, and clinical variables such as disease duration and retrospective pain intensity ratings?
Materials and Methods
Participants
Nineteen adolescents (mean age ± SD = 16.00 ± 2.09; Females = 15) diagnosed with IBS (based on Rome III criteria; see below) were prospectively enrolled in this study. All patients were frequency matched to healthy controls on the basis of age and gender (see Results section for final sample demographics). For the healthy controls, all demographic, psychometric, and neuroimaging data were obtained from our existing P.A.I.N. Group database. In both groups, scanning parameters and procedures for image acquisition were identical and carried out using the same scanner. All other study-related procedures were the same with the exception of psychometric questionnaire administration; only psychometric questionnaire data were available for the patient group.
General inclusion criteria required that all participants were right-handed, between the age of 8 and 21 yrs., had no indication of claustrophobia or suicidal ideation, passed MRI safety screening, and had negative urine tests for drugs of abuse and/or pregnancy. In addition, healthy controls were deemed eligible for inclusion in the current study as long as they reported no history of a psychiatric disorder, neurological disease, or serious medical condition and were not currently taking any medications with CNS effects. Prior to commencement of study-related procedures, written informed consent or assent was given by each participant (with parental permission for children < 18 yrs.). All study-related procedures and materials were approved by the Boston Children’s Hospital (BCH) Institutional Review Board (IRB-P00007161 initial approval date was 10/02/2013) and conducted in accordance with the Declaration of Helsinki.
Pediatric patients with IBS were recruited from the Motility and Functional Gastrointestinal Disorders Center and the General GI Clinics at BCH. Diagnoses were made by a gastroenterologist (S.N.) using pediatric Rome III criteria [11, 21]. All bowel habit subtypes were included in this study. Exclusionary factors for the patient group included any evidence of organic gastrointestinal disease, hepatic disorders, positive laboratory test results including abnormalities in complete blood count, erythrocyte sedimentation rate, albumin, serum amylase, lipase, liver enzymes, urine analysis, celiac screening, and stool examination for occult blood. Exclusion based on medication usage in patients was determined on a case-by-case basis. Proton pump inhibitors and antispasmodics were allowed as long as patient had been on a stable dose for at least 4 weeks. In general, patients were never asked to refrain from taking their prescribed medication and in some cases, were included if they were on a stable (at least 3 months), low to moderate dose of a psychotropic medication (e.g., anti-depressant or anxiolytics).
Psychometric and Clinical Measures
All patients fulfilled diagnostic criteria for IBS using The Questionnaire on Pediatric Gastrointestinal Symptoms—Rome III Version [21]. In addition, patients completed a series of self-report questionnaires to assess characteristics of their abdominal pain severity, impact of GI-related symptoms on HRQOL, extent of functional disability, mood disturbances and degree of pain catastrophizing. Questionnaires administered included The Abdominal Pain Index (API) [22, 23], the Pediatric Quality of Life Inventory (PedsQL; version 4.0) (Child report, ages 8–12; Teen Report, ages 13–18; Young Adult Report, ages 18–25) [24], the PedsQL Gastrointestinal (GI) Symptoms Module (PedsQL GI module; version 3.0) [25] and the Functional Disability Inventory (FDI) [26]. Other self-report measures included the Revised Children’s Anxiety and Depression Scale (RCADS) [27], and the Pain Catastrophizing Scale−Child Version (PCS-C) [28].
The API was used to provide an overall measure of abdominal pain severity and consisted of five items assessing the frequency, duration, and intensity of abdominal pain in the past two weeks. The PedsQL is a 23-item questionnaire, using a 5-point Likert scale (0 = never a problem, 1 = almost never a problem, 2 = sometime a problem, 3 = often a problem, 4 = almost always a problem), that measures the core dimensions of health and well-being in terms of physical (8-items), emotional (5-items), social (5-items) and school functioning (5-items). This measure yields a PedsQL total score with lower scores representing greater GI related symptoms and decreased health-related quality of life. The PedsQL GI Symptoms Module contains 74-items from 14 different scales describing both GI and non-GI related symptoms. All items on this measure, like the core PedsQL, use the same 5-point Likert response scale. The PedsQL GI Symptoms Total Score (58-items) was calculated by reverse scoring and averaging responses from the ten GI-specific symptom scales (i.e., stomach pain and hurt, stomach discomfort when eating, food and drink limits, nausea and vomiting, gas and bloating, constipation, blood in poop, diarrhea, worry about going poop, worry about stomach aches). This yielded a measure of the impact of IBS-related symptoms on overall health and well-being across the last month, with lower scores reflecting greater impairments in quality of life. In addition, impairments in physical functioning and limitations due to symptoms were assessed using the FDI, a 15-item measure that asks participants’ to rate the level of difficulty associated with performing routine daily activities on a 5-point Likert scale ranging from 0 (“no trouble”) to 4 (“impossible”), with higher scores reflecting greater functional disability. The presence of mood disorders, including depression and anxiety was evaluated using the RCADS. The RCADS is a 47-item, self-report questionnaire with subscales that include separation anxiety disorder, social phobia, generalized anxiety disorder, panic disorder, obsessive-compulsive disorder, and major depressive disorder. It also yields a Total Anxiety Score (sum of the 5 anxiety subscales). Items are rated on a 4-point Likert-scale from 0 (“never”) to 3 (“always”). Lastly, the PCS-C is a 13-item questionnaire used to examine how catastrophic thinking is related to the experience of pain in children. The pain catastrophizing construct is comprised of three dimensions: rumination, magnification, and helplessness. Patients are asked to rate how strongly the thought or feeling prevails when experiencing pain. Items are rated from 0 (“not at all”) to 4 (“all the time”).
In addition, clinical outcome measures including disease duration (duration of IBS symptoms in yrs.) and pain intensity (over the past week) in relation to IBS symptoms were evaluated by a gastroenterologist at the time of the clinical visit, with the latter assessed verbally using a 0 to 10 numerical rating scale (NRS; 0 = no pain and 10 = worst pain imaginable).
MRI Data Acquisition and Image Preprocessing
Imaging was performed with a Siemens 3 Tesla Trio Tim MRI scanner equipped with a 32-channel head coil. A high-resolution, T1-weighted magnetization-prepared rapid gradient-echo sequence was acquired for each participant [slices = 176, field of view = 220 x 220, echo time = 1.74, repetition time = 2520, flip angle = 7°, resolution = 1 x 1 mm, slice thickness = 1 mm, no gap]. The anatomical scan was followed by a functional T2-weighted echo-planar imaging scan during ‘resting-state’ wherein participant was instructed to relax with eyes open (slices = 34, 300 volumes, field of view = 224 x 224 mm, echo time = 30 ms, repetition time = 2010 ms, flip angle = 90°, resolution = 3.5 x 3.5, slice thickness = 5 mm). Diffusion tensor imaging and pseudo-continuous arterial spin labeling scans were also acquired following the resting-state functional MRI (fMRI) scan (data to be presented in a separate report). Total scan time was approximately 50 min.
The preprocessing pipeline for our cortical thickness analysis was performed using FreeSurfer (version 5.3.0) (http://surfer.nmr.mgh.harvard.edu), a semi-automated toolbox for reconstruction and visualization of the cortical surface [29–31]. The pipeline consisted of affine registration of the T1-weighted volume to Talairach space, skull stripping, white matter (WM) segmentation and tessellation of the gray/white matter boundary. At each step, visual inspection and manual correction of topological errors were performed. Following reconstruction of the cortical surface, brains were inflated, averaged across participants to produce a study-specific brain, and then smoothed using a 10 mm full-width at half maximum Gaussian kernel. Smoothing was performed on the surface tessellation using an iterative nearest-neighbor procedure, avoiding averaging of data across sulci or outside gray matter (GM). Each hemisphere was then parcellated into 34 distinct regions using the Desikan-Killany atlas [32]. A direct measure of cortical thickness was calculated using the shortest distance (mm) between the pial surface and gray-white matter boundary at each point or vertex of the cortical mantle, using spatial intensity gradients without limitation of individual voxel intensities, and therefore allowing for subvoxel resolution.
For the resting-state fMRI (rs-fMRI) data, all preprocessing was performed using Statistical Parametric Mapping version 8 (SPM8; Wellcome Institute of Cognitive Neurology, London) in Matlab (R2015a). Preprocessing steps included slice timing correction, motion correction, coregistration of the anatomical image to the mean functional image, segmentation of the anatomical image into GM, WM, and CSF, and normalization of anatomical and functional images to the standard Montreal Neurologic Institute (MNI) 152 brain template (voxel size = 2 mm3). Normalized images were then smoothed with an 8 mm isotropic full-width at half maximum Gaussian Kernel.
Single-subject first level resting-state functional connectivity (RS-FC) analysis was performed with the Functional Connectivity toolbox (CONN toolbox; version 15.g) in Matlab [33] (www.nitrc.org/projects/conn). Each subject’s warped anatomical and smoothed preprocessed functional images were specified along with regions of interest (ROIs) for GM, WM, CSF and seeds. At the first-level, covariate regressors were entered into the model including segmented WM and CSF, realignment parameters obtained during the motion correction preprocessing step in SPM8, and transient spikes identified in the fMRI time series representing motion outliers obtained from the Artifact Detection Tools software program (www.nitrc.org/projects/artifact_detect/). A denoising step was conducted using the principal components based ‘aCompCor’ method [34] which does not rely on global signal regression but instead identifies principal components from segmented WM and CSF [33, 35, 36]. Resting-state fMRI data were bandpass filtered (0.008 to 0.09 HZ) and signal associated with the six motion parameters, motion outliers, and those from WM and CSF seed regions were regressed from the fMRI time series.
Statistical Analysis for Demographic, Psychometric and Clinical Measures
All statistical analyses for demographic, psychometric, and clinical measures were performed in SPSS version 21. Group comparisons using parametric or nonparametric tests were conducted as deemed necessary. To examine whether groups differed in age or eTIV, a series of one-way analysis of variance (ANOVAs) were performed. Additionally, a Chi-square (χ2) test of independence (Fisher’s Exact Test) was also conducted to examine whether groups differed proportionally for the gender variable. Differences in both age and gender were not expected since groups were matched on both variables. We also tested the extent to which psychometric and clinical measures were related using bivariate correlational analysis (Pearson’s r; 2-tailed) for the patient group.
Surface-based Cortical Thickness Analysis
Cortical thickness analysis for each hemisphere was conducted using FreeSurfer’s Query, Design, Estimate, Contrast (QDEC) graphical interface (version 1.5). Group comparisons were performed using a general linear model (GLM; Design Matrix: Different Offset; Different Slope) with diagnosis specified as the fixed factor and age designated as a nuisance variable. Estimated total intracranial volume (eTIV) was not entered into the model as a nuisance variable since this metric has been shown to be both genetically and cellularly independent of cortical thickness unlike surface area which is known to depend on cortical volume [37–39]. Results for each analysis were overlaid onto the average pial and inflated surface maps using QDEC. An initial cluster forming significance threshold of p ≤ 0.005 with a cluster extent of 100 was used. Correction for multiple comparisons was performed using random-field-theory-based significant clusters at p < 0.05. Talairach coordinates for peak vertices yielding significant between-subject effects were converted to Montreal Neurological Institute (MNI) space using GingerALE (version 2.3.4) (www.brainmap.org/ale/). In addition, we also examined post-hoc, group differences in other surface-based metrics including surface area (pial) and cortical volume. For these analyses, eTIV was entered as a nuisance variable along with age since both variables are known to be potential confounders, especially during brain development. Results for surface area and cortical volume analyses were supplemental (S1 Table).
Cortical Thickness, Psychometric and Clinical Variables
We performed a series of linear regression GLM analyses in QDEC (with age specified as a nuisance variable) to examine the relationship between cortical thickness and psychometric and clinical measures related to IBS-symptoms and clinical metrics, including abdominal pain severity (API), the impact of GI-specific symptoms on HRQOL (Total Symptoms scores from the PedsQL GI Symptoms Module), disease duration and pain intensity ratings, which were assessed at the time of the clinical visit. We also explored, post-hoc, the relationship between cortical thickness and functional disability (FDI), Total Anxiety (from the RCADS) and pain catastrophizing scores (PCS). Results for each analysis were overlaid onto the average pial and inflated surface using QDEC. An initial cluster forming significance threshold of p ≤ 0.005 and a cluster extent of 100 was used. Correction for multiple comparisons was performed using random-field-theory-based significant clusters at p < 0.05. Coordinates for peak vertices yielding significant between-subject effects were converted from Talairach to MNI space using GingerALE.
Seed-based Resting-state Functional Connectivity Analysis
A series of seed-to-voxel whole brain analyses using 10 mm diameter spherical ROIs as seeds were performed in CONN toolbox. All seeds were generated in MarsBar (http://marsbar.sourforge.net) using peak voxel coordinates (i.e., MNI) for clusters demonstrating significant group differences in cortical thickness from the surface-based analysis. These seeds were all lateralized to the right hemisphere and included the dorsomedial prefrontal cortex (DMPFC: 19, 41, 43), the dorsolateral prefrontal cortex (DLPFC: 43, 27, 38), the posterior parietal cortex (PPC: 21, -57, 60) and the posterior cingulate cortex (PCC: 15, -29, 38). Two additional seeds (10 mm diameter spheres) were chosen based on significant negative correlations obtained between our cortical thickness analysis and measures of abdominal pain severity (API scores) and the impact of GI-specific symptoms on HRQOL (Total Symptom scores from the PedsQL GI Symptoms Module). These included seeds for the left anterior insula (aINS: -32, 21, -5) and the inferolateral cluster in the right primary somatosensory cortex (SI: 67, -10, 27).
At the second-level, a series of voxel-wise analyses were performed and functional connectivity maps obtained for each of the six seeds. An initial height threshold level of p < 0.005 was used. Correction for multiple comparisons was accomplished with 3dClustSim using Analysis of Functional NeuroImages (AFNI; http://afni.nimh.nih.gov/afni/) [40] software which calculated the minimum number of contiguous voxels required for a cluster to be deemed significant (2-sided threshold for t-tests) at an FDR of p < 0.05 (5000 iterations). The final functional connectivity maps were projected onto a study-specific brain generated by averaging all participants’ warped T1-weighted images using Ged Ridgway’s Masking toolbox in SPM8 (http://www0.cs.ucl.ac.uk/staff/g.ridgway/masking/) [41].
Results
Demographic and Sample Characteristics
Out of the 19 adolescent patients enrolled in the study, two were excluded from all statistical analyses; one patient was omitted due to being asymptomatic (no longer presenting with IBS symptoms) at the time of scanning, while another patient’s structural data was deemed unusable due to excessive motion artifacts. Thus, the final study sample consisted of 17 adolescents with IBS (Females = 13; mean age ± SD = 16.44 ± 1.73; range, 12.43–18.5) that were age and gender matched to 17 healthy cohorts (Females = 13; mean age ± SD = 16.29 ± 1.83; range, 11.73–20.3). The demographic distribution for ethnicity of the entire sample was 88.24% Caucasian (30/34), 8.82% Asian (3/34), and 2.94% African American (1/34) (IBS patients: Caucasian = 16/17; African American = 1/17; Asian = 0/17). One-way ANOVAs reveled no significant group differences between age [F (1,32) = 0.06, p = 0.8] or eTIV [F (1, 32) = 3.94, p = 0.06]. Furthermore, the Chi-square test showed no difference in the proportion of males to females between groups [χ2 (1) = 0, p = 1.0].
Data for individual patient characteristics such as age, gender, bowel habit subtype, disease duration, and severity measures (i.e., pain intensity) are shown in Table 1. Our patient sample was predominantly female and heterogeneous with regard to bowel habit subtype, with the majority reporting constipation predominant symptoms (IBS with constipation, IBS-C = 9, IBS with diarrhea, IBS-D = 5, mixed IBS, IBS-M = 2, unspecified IBS = 1).
Table 1. Individual Patient Characteristics.
| Patient | Age (years) | Gender | Bowel Habit | Disease Duration (years) | Pain Intensity (NRS) |
|---|---|---|---|---|---|
| 1 | 18.41 | Female | IBS-C | 2 | 10 |
| 2 | 16.43 | Female | IBS-C | 3 | 8 |
| 3 | 16.9 | Female | IBS-D | 1.5 | 7 |
| 4 | 13.28 | Female | IBS-C | 2 | 7 |
| 5 | 17.59 | Male | IBS-D | 5 | 7 |
| 6 | 17.07 | Male | IBS-D | 3.5 | 9 |
| 7 | 17.55 | Female | IBS-C | 1 | 7 |
| 8 | 16.46 | Female | IBS-C | 2 | 8 |
| 9 | 18.5 | Female | IBS-U | 2 | 8 |
| 10 | 15.86 | Female | IBS-C | 6 | 8 |
| 11 | 16.67 | Female | IBS-M | 5 | 8 |
| 12 | 12.43 | Female | IBS-D | 1.5 | 8 |
| 13 | 18.3 | Female | IBS-C | 6 | 10 |
| 14 | 16.87 | Male | IBS-D | 3 | 9 |
| 15 | 15.06 | Female | IBS-M | 1 | 9 |
| 16 | 14.65 | Female | IBS-C | 7 | 9 |
| 17 | 17.5 | Male | IBS-C | 10 | 8 |
Abbreviations: Numerical rating scale; IBS-C = constipation predominant IBS; IBS-D = diarrhea predominant; IBS-M = IBS mixed; IBS-U = IBS unspecified.
Descriptive statistics for psychometric and clinical variables for the entire patient sample are displayed in Table 2. Overall, patients had an average disease duration of 3.62 yrs. (SD ± 2.52; range, 1–10 yrs.) and reported their pain intensity associated with IBS symptoms in the moderate to severe range, with an average NRS of 8.24 (SD ± .97; range, 7–10 NRS).
Table 2. Descriptives for Psychometric and Clinical Measures in IBS Patients.
| IBS Patients | N | ||
|---|---|---|---|
| Mean | SD | ||
| Disease Duration (yrs.) | 3.62 | 2.52 | 17 |
| Pain Intensity | 8.24 | 0.97 | 17 |
| Abdominal Pain Index | 2.70 | 0.74 | 15 |
| Functional Disability Inventory | 21.13 | 10.92 | 15 |
| PedsQL | 60.33 | 14.98 | 12 |
| PedsQL GI Module (Total Score) | 54.36 | 11.37 | 12 |
| PedsQL GI Module (Total Symptoms) | 48.67 | 12.89 | 12 |
| Worry about poop | 81.67 | 11.15 | 12 |
| Worry about abdominal pain | 52.08 | 33.78 | 12 |
| RCADS | |||
| Total Anxiety T Score | 44.36 | 11.72 | 14 |
| Total Anxiety Depression T score | 47.07 | 12.38 | 14 |
| Separation Anxiety T Score | 49.64 | 12.16 | 14 |
| General Anxiety T Score | 39.43 | 6.61 | 14 |
| Panic T Score | 53.14 | 12.59 | 14 |
| Social Phobia T Score | 43.93 | 11.46 | 14 |
| Obsessive-Compulsive T Score | 42.29 | 11.32 | 14 |
| Total Anxiety Depression Score | 34.57 | 18.74 | 14 |
| Total Depression Score | 57.23 | 12.21 | 14 |
| Total Anxiety Score | 23.50 | 14.88 | 14 |
| PCS-C | 24.82 | 10.33 | 11 |
| Rumination | 10.73 | 3.32 | 11 |
| Magnification | 3.36 | 2.46 | 11 |
| Helplessness | 10.73 | 5.83 | 11 |
Abbreviations: IBS = irritable bowel syndrome; SD = standard deviation; N = Total number of subjects with questionnaire data; yrs. = years; PedsQL = Pediatric Quality of Life Inventory; PedsQL GI Module = Pediatric Quality of Life Inventory Gastrointestinal Symptoms Module; RCADS = Revised Children’s Anxiety and Depression Scale; PCS-C = Pain Catastrophizing Scale−Child version.
Relationship between Psychometric and Clinical Measures
Summary of results for the bivariate correlational analysis are reported in the supplemental section (S2 Table). Briefly, in patients, functional disability (measured using the FDI) was positively correlated with Total Anxiety scores (r = .62, p = .02) from the RCADS. Conversely, the PedsQL was negatively correlated with FDI (r = -.73, p = .007) and Total Anxiety (r = -.79, p = .002) scores. The PedsQL was also negatively correlated with pain catastrophizing scores from the PCS (r = -.62, p = .04). These findings indicate that pediatric IBS patients reporting lower HRQOL, also reported greater functional disability associated with their symptoms, greater overall mood disturbances, and a greater propensity to catastrophize about their pain.
Group Differences in Cortical Thickness
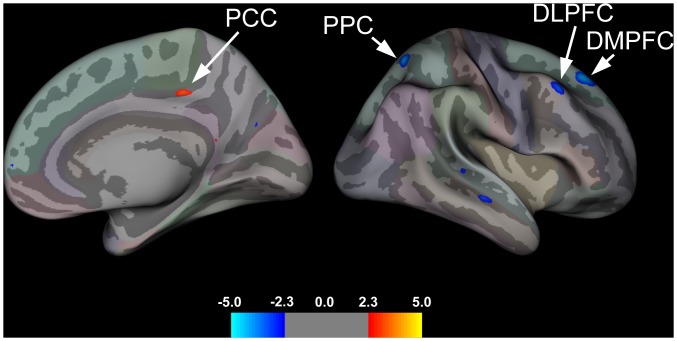
A summary of group effects are displayed in Table 3 and illustrated in Fig 1. Relative to controls, pediatric patients with IBS showed significant cortical thickening in the right PCC, whereas cortical thinning in the right DMPFC, DLPFC, and PPC were found.
Table 3. Summary of Group Differences in Cortical Thickness.
| Area | Side | NVtxs | Cluster Size (mm2) | Fvalue | MNI coordinates | Talairach coordinates | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | |||||
| DMPFC | R | 233 | 186.77 | -4.1805 | 19.38 | 40.95 | 43.48 | 16.5 | 32.5 | 46.8 |
| PPC | R | 196 | 111.35 | -4.1601 | 21.37 | -57.28 | 60.44 | 17.9 | -60.5 | 53.3 |
| DLPFC | R | 170 | 108.91 | -3.3002 | 44.31 | 27.06 | 38.26 | 38.6 | 19.9 | 41.3 |
| PCC | R | 146 | 56.44 | 3.1398 | 15.18 | -29.22 | 38.39 | 12.5 | -32.4 | 36.0 |
Abbreviations: NVtxs = number of vertices; R = right; MNI = Montreal Neurological Institute; DMPFC = dorsomedial prefrontal cortex; PPC = posterior parietal cortex; DLPFC = dorsolateral prefrontal cortex; PCC = posterior cingulate cortex.
Fig 1. Group Effects for Cortical Thickness.
Brain areas showing group differences in cortical thickness rendered onto the right hemisphere (medial and lateral view) of a semi-inflated averaged brain. Pediatric patients with IBS, compared to healthy cohorts, showed cortical thickening in the right posterior cingulate cortex (PCC) and cortical thinning in the right posterior parietal cortex (PPC), dorsomedial (DMPFC) and dorsolateral (DLPFC) prefrontal cortices.
Relationship Between Cortical Thickness and Abdominal Pain Severity
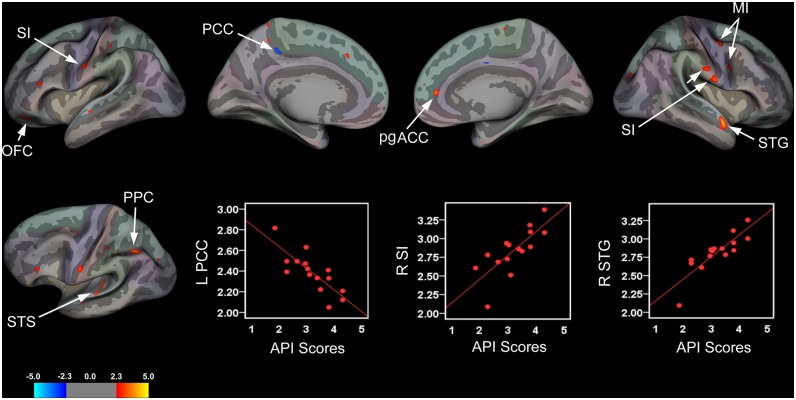
Surface-based analysis revealed significant associations between cortical thickness and abdominal pain severity as assessed by the API (Fig 2 and Table 4). In patients abdominal pain severity was correlated with cortical thinning in the left PCC and cortical thickening in the right DLPFC, superior temporal gyrus (STG), and two clusters in the right primary motor cortex (MI). In addition, greater severity of abdominal pain complaints was strongly correlated with cortical thickening in the left OFC, bank of the superior temporal sulcus (STS), PPC, and bilateral SI (Fig 2 and Table 4). With respect to the latter finding, more severe abdominal pain in patients was associated with cortical thickening localized to two distinct clusters located in the ‘intra-abdominal area’ of the right SI; an inferior and a superior cluster (Fig 2). Greater abdominal pain severity was also associated with cortical thickening in the right perigenual anterior cingulate cortex (pgACC; see Fig 2). Although this cluster passed the primary vertice-level threshold (p < .005), it did not survive the secondary cluster-extent threshold [F value = 3.5182, number of vertices = 63, cluster size = 35.83, MNI coordinates (x, y, z) = 16.02, 49.10, 60.88] and therefore will not be discussed further.
Fig 2. Brain Areas Showing Significant Associations between Cortical Thickness and Abdominal Pain Severity.
Brain areas showing changes in cortical thickness that were associated with abdominal pain severity rendered onto an inflated averaged brain for the left and right hemispheres (lateral and medial views) with corresponding scatter plots for selected clusters. Patients with greater abdominal pain complaints exhibited significant cortical thinning in the left posterior cingulate cortex (PCC) and cortical thickening in the left orbitofrontal cortex (OFC), bank of the superior temporal sulcus (STS), and posterior parietal cortex (PPC). The latter two clusters are displayed on an inflated surface of the left hemisphere that was tilted approximately 15° to highlight areas exhibiting cortical thickening. In the right hemisphere, abdominal pain severity was associated with cortical thickening in the superior temporal gyrus (STG), primary motor cortex (MI), and perigenual anterior cingulate cortex (pgACC), although the latter cluster did not pass the defined cluster-extent threshold. Patients also showed cortical thickening in the bilateral primary somatosensory cortices (SI) corresponding to the intra-abdominal or ‘viscerotopic’ area, with two clusters in the right hemisphere; an the inferiorly- and a superiorly-placed cluster. Scatter plots depicting correlations between cortical thickness values (mm) for significant clusters in the left (L) PCC, right (R) inferior SI intra-abdominal area, and R STG with respective abdominal pain severity scores.
Table 4. Summary of Significant Clusters for Brain Areas Showing a Relationship between Cortical Thickness, Abdominal Pain Severity and Impact of GI-specific Symptoms on Health-Related Quality of Life.
| Side | NVtxs | ClusterSize (mm2) | F value | MNI coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Abdominal Pain Index | ||||||||
| PPC (inferior parietal lobule) | L | 347 | 173.72 | 3.9802 | -36.74 | -56.91 | 45.82 | |
| OFC | L | 184 | 159.16 | 2.9112 | -29.94 | 42.36 | -19.03 | |
| STS | L | 172 | 85.81 | 3.2437 | -62.51 | -26.22 | 5.8 | |
| STG | R | 204 | 168.44 | 3.9534 | 53.11 | -3.04 | -23.57 | |
| SI | L | 179 | 86.38 | 3.0979 | -55.15 | -8.95 | 27.05 | |
| SI (superior cluster) | R | 205 | 65.13 | 3.3444 | 71.07 | -7.47 | 10.74 | |
| SI (inferior cluster) | R | 173 | 78.33 | 3.0020 | 66.64 | -10.33 | 27.46 | |
| MI (superior cluster) | R | 171 | 94.68 | 3.4159 | 45.89 | -2.25 | 46.07 | |
| MI (inferior cluster) | R | 100 | 52.47 | 3.2263 | 45.16 | 4.94 | 24.3 | |
| PCC | L | 124 | 44.34 | -3.3364 | -14.6 | -35.34 | 41.07 | |
| DLPFC | R | 110 | 53.32 | 2.9164 | 29.53 | 1.15 | 43.21 | |
| PedsQL GI Module (Total Symptoms) | ||||||||
| STS | L | 174 | 90.81 | -3.2230 | -53.11 | -35.56 | 4.1 | |
| aINS | L | 142 | 54.98 | -4.4277 | -31.66 | 21.19 | -5.47 | |
| Fusiform | L | 111 | 85.11 | -3.3793 | -30.04 | -73.7 | -7.88 | |
| PCC | R | 83 | 42.46 | -5.8281 | 6.88 | -24.96 | 32.73 | |
Abbreviations: NVtxs = number of vertices; R = right; L = left; MNI = Montreal Neurological Institute; PPC = posterior parietal cortex; OFC = orbitofrontal cortex; STG = superior temporal gyrus; SI = primary somatosensory cortex; MI = primary motor cortex; PCC = posterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; STS = superior temporal sulcus; aINS = anterior insula.
Relationship Between Cortical Thickness and Health-related Quality of Life
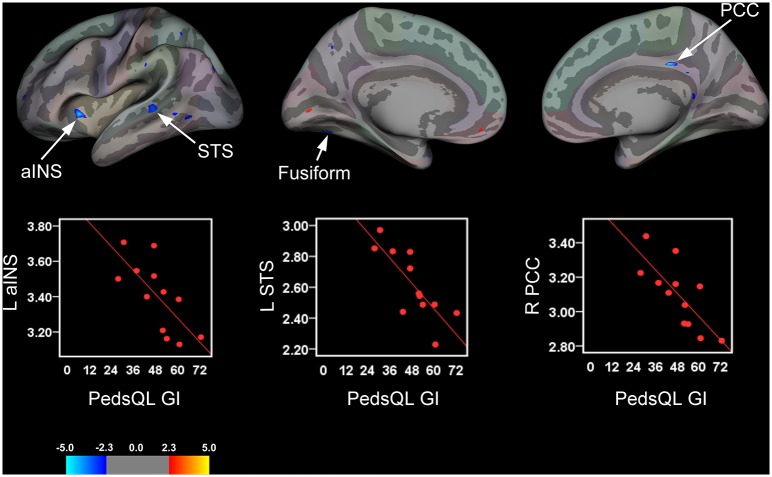
Pediatric patients with greater HRQOL and reduced GI-specific symptoms (indexed by the PedsQL GI Total Symptom scores), exhibited significant cortical thinning in the right PCC and left anterior insula (aINS) (Fig 3 and Table 4). Negative associations were also observed between PedsQL GI Symptoms Module (Total Symptom scores) and cortical thickness in the left STS and fusiform gyrus
Fig 3. Brain Areas Showing Significant Associations between Cortical Thickness and Impact of GI-specific Symptoms on Health-related Quality of Life.
Brain areas showing changes in cortical thickness that were associated with Total Symptoms scores from the PedsQL GI Symptoms Module rendered onto an inflated averaged study-specific brain for the left (lateral and medial views) and right hemisphere (medial view only) with corresponding scatter plots for selected significant clusters. For the PedsQL GI Symptoms Module (Total Symptom scores), greater reported HRQOL and lower GI-specific symptoms were associated with cortical thinning in the right (R) posterior cingulate cortex (PCC), left (L) anterior insula (aINS), L superior temporal sulcus (STS), and L fusiform gyrus.
Relationship Between Cortical Thickness and Clinical Measures
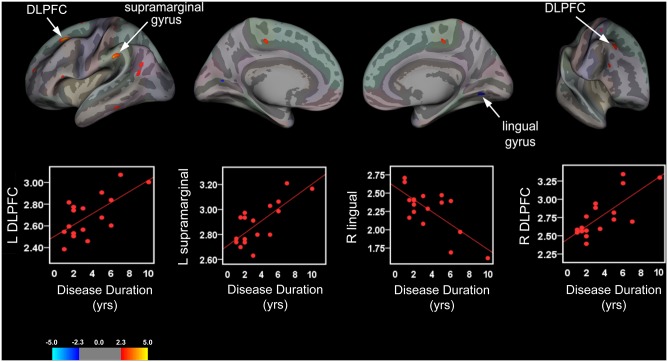
Results from the cortical thickness linear regression analysis with clinical variables are presented in Table 5 and illustrated in Figs 4 and 5, respectively. Analyses revealed significant positive correlations between disease duration and cortical thickness in bilateral DLPFC and left supramarginal gyri (Fig 4 and Table 5). A negative correlation between disease duration and cortical thickness in the right lingual gyrus was also found. Additionally, patients reporting higher levels of pain intensity associated with their IBS symptoms showed significant cortical thickening in bilateral OFC (Fig 5 and Table 5). Adolescent patients’ reporting greater pain intensity also exhibited cortical thinning in the left parsorbitalis and cortical thickening in the right MI.
Table 5. Summary of Significant Clusters for Brain Areas Showing a Relationship between Cortical Thickness, Disease Duration and Pain Intensity.
| Side | NVtxs | ClusterSize (mm2) | F value | MNI coordinates | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Disease Duration | |||||||
| Supramarginal gyrus | L | 185 | 90.03 | 4.4821 | -63.36 | -32.31 | 31.4 |
| R | 120 | 52.20 | 3.1117 | 57.56 | -18 | 17.06 | |
| DLPFC | L | 181 | 128.99 | 4.0740 | -20.32 | 25.24 | 45.26 |
| R | 116 | 77.36 | 3.0727 | 34.07 | 28.97 | 40.82 | |
| Lingual gyrus | R | 110 | 110.07 | -2.7540 | 18.94 | -69.95 | -4.82 |
| Pain Intensity | |||||||
| OFC | L | 267 | 112.35 | 3.8616 | 18.17 | 21.53 | -29.77 |
| R | 194 | 98.54 | 4.6369 | -6.06 | 23.94 | -31.39 | |
| Parsorbitalis | L | 110 | 92.47 | -3.9611 | 46.85 | 36.44 | -23.45 |
| MI | R | 195 | 79.54 | 2.9343 | -46.42 | -6.73 | 26.23 |
Abbreviations: NVtxs = number of vertices; R = right; L = left; MNI = Montreal Neurological Institute; DLPFC = dorsolateral prefrontal cortex; OFC = orbitofrontal cortex; MI = primary motor cortex.
Fig 4. Cortical Thickness and Disease Duration in Pediatric IBS Patients.
Brain areas showing a significant relationship between cortical thickness and disease duration rendered onto an inflated averaged study-specific brain for the left (L) and right (R) hemispheres (lateral and medial views) with corresponding scatter plots for selected clusters. Top panel: Longer disease durations (yrs) were correlated with cortical thickening (warm colors) in the L and R DLPFC, and L supramarginal gyrus. In contrast, cortical thinning (cool colors) in the R lingual gyrus was correlated with disease durations in IBS patients. Bottom panel: Scatter plots illustrating positive and negative correlations between cortical thickness values (mm) for significant clusters and duration of IBS symptoms.
Fig 5. Cortical Thickness and Pain Intensity in Pediatric IBS Patients.
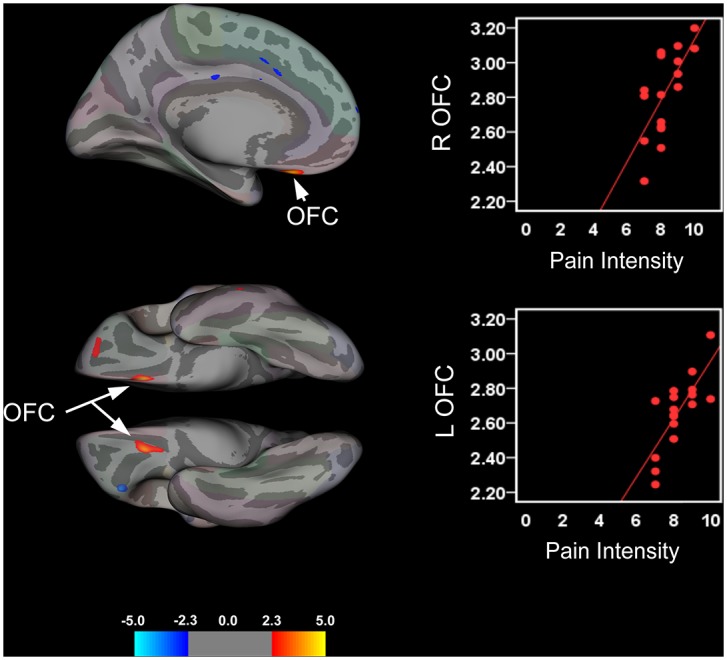
Brain areas showing changes in cortical thickness that were associated with retrospective pain intensity ratings (over past week) rendered onto an inflated averaged study-specific brain with corresponding scatter plots for selected clusters. In patients, greater pain intensity ratings were correlated with cortical thickening in the bilateral orbitofrontal cortices (OFC). Top panel shows the medial aspect of the right hemisphere with a cluster in the OFC along with the corresponding scatter plot. Bottom panel shows the ventral aspect of the OFC for both hemispheres with scatter plot showing the relationship between cortical thickness and pain intensity ratings for the OFC cluster in the left hemisphere.
Relationship between Cortical Thickness, Functional Disability, Total Anxiety and Pain Catastrophizing
Our exploratory analysis revealed that patients’ functional disability scores as assessed by the FDI were positively correlated with cortical thickness in the right PCC and intraoral/face area of SI and the left lingual and supramarginal gyri. Conversely, decreased cortical thickness in left MI was associated with greater reported functional disability. For Total Anxiety scores, greater reported anxiety was associated with cortical thickening in the left PPC and supramarginal gyrus as well as in the right inferior temporal and fusiform gyri. We also observed a strong negative correlation between cortical thickness in the right DLPFC and pain catastrophizing. Due to the exploratory nature of these analyses and the lack of a priori hypotheses, we limit the presentation of these findings to the results section; however, supplementary information including a table (S3 Table) and figure (S1 Fig) are made available for the interested reader.
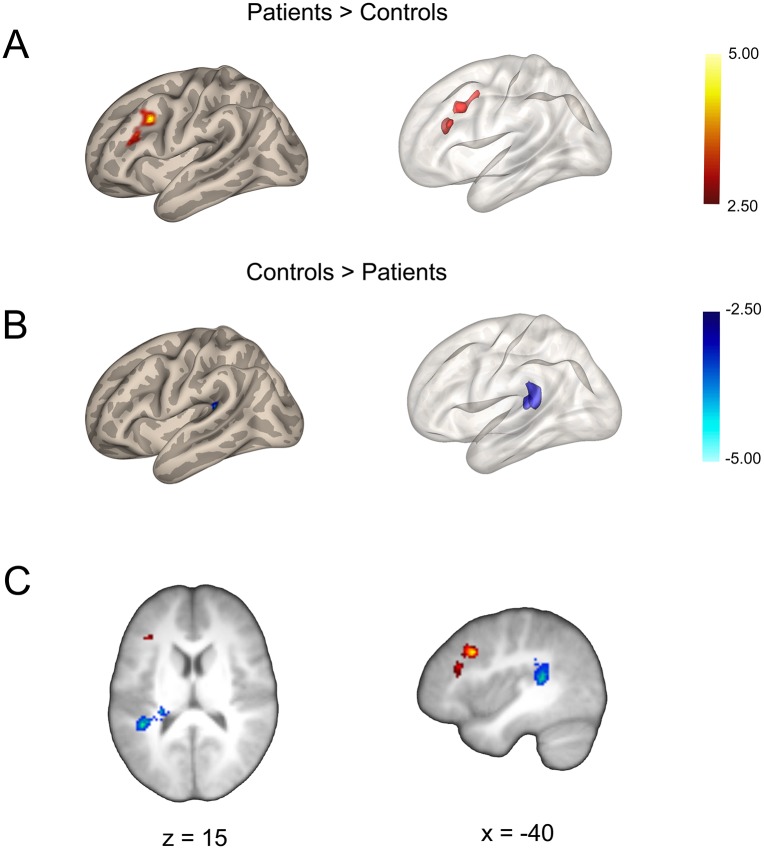
Group Differences in Resting-state Functional Connectivity
We examined whether cortical areas showing structural brain changes in adolescents with IBS also showed functional impairments in intrinsic network connectivity. Results from our seed-to-voxel whole brain analysis are displayed in Table 6. No suprathreshold clusters were found for the DMPFC or PPC seeds for either contrast (i.e., patient > controls; controls > patient). For the right DLPFC seed, RS-FC analysis revealed increased connectivity in patients for a cluster with a peak voxel in right medial occipital lobe corresponding to the cuneus, and extending medially into the left hemisphere, whereas controls showed anti-correlated RS-FC patterning. Relative to healthy controls, pediatric patients with IBS showed greater connectivity between the right PCC seed and left DLPFC (Fig 6). Inspection of the normalized z-score connectivity values (Fisher transformed) revealed that the aforementioned result was due to an attenuation in anti-correlated RS-FC between the PCC-DLPFC in patients relative to controls, with controls showing increased anti-correlated RS-FC patterning compared to patients. In contrast, controls compared to patients showed greater connectivity or correlated patterning between the right PCC seed and the left parietal operculum (Fig 6). For the DLPFC seed, patients showed increased functional connectivity or correlated RS-FC with the right cuneus, whereas controls showed anti-correlated patterning. For L aINS seed, patients showed increased RS-FC compared to controls, for two large clusters in the occipital region with peak voxels in the right hemisphere. The largest cluster had a peak voxel placed medially, corresponding to the cuneus and which extended laterally into the right hemisphere and medially into the left hemisphere. The second smaller cluster also had a peak voxel in right lateral occipital cortex that was restricted to the right hemisphere. Interestingly, with respect to the aINS RS-FC, controls showed greater anti-correlations in both instances. Lastly, for the right SI seed that corresponded to the inferior portion of the intra-abdominal area, patients showed significant increases in correlated RS-FC compared to controls, whereas controls showed greater anti-correlations for a large cluster in the left cerebellum.
Table 6. Summary of Group Effects for Seed-based Functional Connectivity Analysis.
| Seed | Region | Side | Ke | Z value | T value | p | MNI coordinates | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| PAT > CTL | |||||||||
| R DMPFC | No suprathreshold clusters | ||||||||
| R DLPFC | Cuneus | R | 494 | 3.58 | 4.01 | < 0.001 | 2 | -94 | 6 |
| R PPC | No suprathreshold clusters | ||||||||
| R PCC | DLPFC | L | 330 | 4.01 | 4.62 | 0.002 | -38 | 16 | 34 |
| L aINS | Cuneus/Lateral Occipital Cortex | R | 2109 | 3.95 | 4.53 | < 0.001 | 10 | -102 | 18 |
| Lateral Occipital Cortex | R | 1218 | 3.42 | 3.79 | < 0.001 | 28 | -96 | 4 | |
| R SIa | Cerebellum | L | 693 | 4.16 | 4.84 | < 0.001 | -2 | -54 | -32 |
| CTL > PAT | |||||||||
| R DMPFC | No suprathreshold clusters | ||||||||
| R DLPFC | No suprathreshold clusters | ||||||||
| R PPC | No suprathreshold clusters | ||||||||
| R PCC | Parietal Operculum | L | 338 | 4.73 | 5.74 | 0.002 | -24 | -32 | 10 |
| L aINS | No suprathreshold clusters | ||||||||
| R SIa | No suprathreshold clusters | ||||||||
Abbreviations: PAT = patients; CTL = controls; R = right; L = left; DMPFC = dorsomedial prefrontal cortex; DLPFC = dorsolateral prefrontal cortex; PPC = posterior parietal cortex; PCC = posterior cingulate cortex; aINS = anterior insula;
aSI = primary somatosensory cortex (intra-abdominal area), inferior cluster;
MNI = Montreal Neurological Institute.
Fig 6. Group Effects for the Seed-based Functional Connectivity Analysis for the Posterior Cingulate Cortex.
Seed-to-voxel whole-brain resting-state functional connectivity (RS-FC) results in pediatric IBS patients versus healthy controls for the left posterior cingulate cortex (PCC) seed rendered onto a semi-inflated brain showing cluster-corrected voxels (left panel) and the same cluster displayed in volume space rendered onto a semi-inflated glass brain (right panel) for the (A) dorsolateral prefrontal cortex (DLPFC) and the (B) parietal operculum. Statistical T maps showing results for RS-FC analysis for the PCC seed rendered onto the averaged study-specific brain (C). Hot voxels represent areas showing increased functional connectivity in patients relative to controls (Patients > Controls) for the PCC-DLPFC, whereas cool voxels represent decreased functional connectivity in patients versus controls for the PCC-Parietal Operculum (Controls > Patients).
Discussion
While a number of neuroimaging studies exist in the extant adult IBS literature [15, 42], we are aware of only one report on functional imaging brain changes in a pediatric population and that is in response to liminal and subliminal rectal distention [43]. In adults with IBS, numerous structural and functional brain changes have been reported [15], including alterations in cortical thickness and gray matter volume [44–47], white matter integrity [48], and disturbances in intrinsic network connectivity [49, 50]. In contrast, little is known about the disease-related structural and functional brain changes that occur in the pediatric brain. Here, we report for the first time concomitant alterations in brain morphology and RS-FC patterns in adolescents who met Rome III criteria for IBS. The main findings observed were: (1) IBS patients, compared to a healthy cohort, showed significant cortical thickening in the right dorsal PCC, and cortical thinning in the right DMPFC, DLPFC and PPC; (2) In patients, structural alterations, including cortical thickening in the bilateral ‘viscerotopic’ portion of SI, were related to greater abdominal pain severity scores, whereas cortical thinning in the left aINS was associated with greater HRQOL and a reduction in GI-specific symptoms; (3) Clinical characteristics including disease duration and pain intensity (related to IBS symptoms) were correlated with cortical thickening in the DLPFC and OFC, bilaterally; and (4) During resting-state, patients exhibited less anti-correlated RS-FC compared to controls between the right PCC seed and the left DLPFC, indicating aberrant connectivity patterns in key nodes of the default mode and cognitive control networks. Together, these findings indicate extensive structural changes in the brains of pediatric IBS patients compared to healthy controls that encompassed areas important for higher-order cognitive functioning, as well as pain processing, interoceptive awareness and integration of viscerosensory information. A subset of these structural brain changes were correlated with disease severity measures and disrupted intrinsic network connectivity patterns. Understanding the structural and functional brain alterations that occur at earlier stages of this syndrome may provide novel insights into the evolution of IBS and possibly lay the groundwork for the development of future biomarkers that will enhance our ability to monitor brain changes following therapeutic interventions and that may precede the clinical/subjective benefits.
Structural Brain Changes in Pediatric IBS
Our surface-based analysis revealed significant differences in cortical thickness in adolescents with IBS compared to healthy cohorts. Notably, patients showed a thickening in the right dorsal aspect of the PCC (dPCC), buried deep within the ventral bank of the posterior cingulate sulcus. The PCC is putatively involved in a number of processes [51]: (1) it is regarded as a core node of the default mode network (DMN) [52]; (2) it has been implicated in internally directed cognition [53] [54, 55]; (3) conscious awareness [56, 57]; (4) working memory [58]; (5) visuospatial orientation of the body in space or topokinesis [59], and (6) attention [60]. Moreover, there is a link between chronic pain conditions and abnormal task-dependent and intrinsic dPCC functioning. In visceral pain syndromes such as IBS increased activation in the dPCC to painful stimuli [61, 62], increased DMN connectivity with pain-related regions after lidocaine treatment [63] and diminished PCC functional connectivity during resting-state [64] have been found. The aforementioned functional changes in the PCC are in accord with our results demonstrating cortical thickening in this region in pediatric IBS patients. Although the causal factors underlying these disease-related structural changes in the dPCC cannot be determined here, they are unlikely due to simple age- or gender-related effects since IBS and healthy controls groups were matched on both variables. Further, age was controlled for in our analyses (specified as a nuisance variable). More parsimonious is an explanation that is viewed in context of the disease state. The cortical thickening of the dPCC in pediatric IBS patients observed in the present study may arise from a myriad of events that transpire as a result of IBS-related symptoms and progression of the disease over time. Alternatively, cortical thickness changes in the dPCC could result from different cortical developmental trajectories in patients relative to controls, wherein existing brain vulnerabilities or preconditions predispose certain individuals toward the development of aberrant structural brain changes during childhood and/or adolescence which then evolve and ultimately lead to the chronic disease state. Possible mechanisms for these neuroanatomical changes include increased dendritic aborization, spine density, synaptogenesis, unmasking of silent synapses, and/or use-dependent cortical restructuring (long-term potentiation; LTP) resulting from central sensitization of pathways conveying visceral nociceptive afferent signals from the gut to the brain.
In addition to increased cortical thickness in the dPCC, significant cortical thinning was also observed in pediatric IBS patients, relative to controls, in fronto-parietal areas such as the DMPFC, DLPFC, and PPC. The latter two areas comprise nodes of the cognitive control network (CCN) [65] and have been implicated in the integration of executive functions [66] such as attention [67], visuospatial working memory [68], decision-making, and cognitive inhibitory control processes [69], including descending pain modulation [70]. The DMPFC is also involved in mediating higher-order executive functions, but more in relation to social cognition, self-monitoring, affect regulation, and retrieval of autobiographical memories with social-emotional components. This area is also considered a key node of the DMN. All three of these areas share anatomical connections with the dPCC [54] [69, 71, 72]. Neuroimaging studies conducted in adults with IBS have shown disruptions in some of these same areas during both resting-state [64] and cognitively-demanding tasks [73], and in response to cued or uncued expectation of noxious stimulation to the abdomen [74]. In a previous study [75] we showed greater activation in the DMPFC in adult IBS patients compared to controls during an executive task used to assess global attentional network functioning. It is interesting to note that greater DMPFC activity was positively associated with GI symptom severity as well as pain catastrophizing and fear of uncertainty in these patients. The current findings demonstrating cortical thinning in the DMPFC in pediatric IBS patients may reflect the development of altered cognitive control functioning, and may over time, manifest as disruptions in corticolimbic inhibitory processes involved in attention or perhaps even lead to impairments in the ability to effectively regulate negative emotions and cognitions related to visceral sensations and symptoms (GI symptom specific anxiety).
In a study conducted by Seminowicz et al. (2010) gray matter volume (GMV) decreases were also observed in the PPC and rostral areas of the DLPFC (BA 46) in adult IBS patients compared to controls, whereas increases in the caudal portion of the DLPFC (BA 9) were found [47]. Thus, our cortical thickness changes in the DLPFC and PPC in pediatric IBS patients replicate, in part, their VBM findings. Numerous studies utilizing morphometric approaches have shown diminution of cortical thickness or GMV in prefrontal and posterior parietal areas such as the DLPFC and PPC in other chronic pain conditions including chronic low back pain [76–78], hip osteoarthritis [79] and migraine [80]. Furthermore, reversal in GMV reductions in the DLPFC following intervention with cognitive behavioral therapy [81] or hip replacement surgery [79] have also been reported and demonstrate the malleability of these morphological changes. In sum, the altered structural brain changes in fronto-parietal areas observed in the present study may reflect impairments in cognitive control functions previously reported in IBS patients [73, 82–86], and that may be evident early in evolution of this syndrome and possibly amenable to treatment.
Relationship between cortical thickness, abdominal pain, and GI-specific symptoms
Another important finding to emerge from this study was that pediatric IBS patients reporting greater abdominal pain severity, assessed using the API, showed significant bilateral cortical thickening in the inferolateral areas of SI, corresponding to the intra-abdominal or ‘viscerotopic’ homunculus and extending into the intraoral area. Histochemical tract-tracing and extracellular recording studies performed in monkeys and cats have shown that the intra-abdominal area of SI receives viscero-viscero and viscero-somatic inputs from third-order neurons arising predominantly via the spinothalamic tract, however, dorsal-column medial lemniscal [87, 88] involvement has also been identified. Furthermore, human studies utilizing neuroimaging and evoked potential techniques have shown activation of the intra-abdominal area following stimulation to the upper and lower GI tracts [89–96]. Our findings of cortical thickening in the viscerotopic portion of SI in adolescent patients with IBS reporting greater abdominal pain are in accord with these results and are also consistent, in part, with a recent report by Irimia and colleagues [48] demonstrating greater mean fractional anisotropy (FA) in white matter connections to this area using diffusion tensor imaging in adult IBS patients. It should be noted that similar somatotopic findings have been reported for headache (SI; first division of the trigeminal representation of the face) suggesting a common underlying nociceptive drive in these conditions [97]. However, despite the fact that the locations for reported differences in WM connectivity as measured by mean FA overlap remarkably with clusters displaying increased cortical thickness reported here, Irmia et al. (2015) found no evidence of group differences in cortical thickness nor did they find any relationship between mean FA and a measure of usual IBS severity (assessed using the Bowel Symptom Questionnaire). This difference in findings may be due to the population studied since we investigated cortical thickness in pediatric patients with IBS, whereas their study was performed in adults. Other methodological differences include the measures administered to assess symptom severity. Regardless of these differences, we are the first to report structural brain changes coincident with a behavioral report of abdominal pain severity in pediatric IBS patients. These findings may shed new light on the cortical reorganization that accompanies behavioral changes specific to this syndrome. Other areas that showed positive correlations between abdominal pain severity and cortical thickness included bilateral MI, PPC, and superior temporal gyri/sulci, while a decrease in cortical thickness was identified in the dPCC. Although interpreting these findings with regard to the underlying etiology is complicated by the fact that causal relationships cannot be inferred, possibilities include but are not limited to changes in dendritic morphology previously mentioned above, including the recruitment and subsequent unmasking of so-called ‘silent nociceptors’, a premise put forth by Cervero and Jänig [98], and reviewed by Derbyshire [99]. For example, these changes could be due to the repetitive barrage of nociceptive visceral input, perhaps as a result of peripheral factors such as inflammation or ischemic events over time, which in turn, lead to the central sensitization of second-order afferents and synaptic remodeling in thalamic pathways providing input (via third-order neurons) to the intra-abdominal area of SI. Future work is needed to determine whether these morphological changes are a consequence or a causal factor driving the emergence of IBS symptoms.
We also identified cortical thinning in the left aINS and right dPCC that was associated with greater HRQOL and a lessening of GI-specific symptoms in pediatric patients. These findings are intriguing given the role that the insula and PCC plays in pain processing and interoceptive awareness and in context of previous neuroimaging studies demonstrating abnormal functioning of these areas in patients with IBS and other chronic pain conditions [43, 46, 61, 62, 64, 100–106]. However, our aINS findings are somewhat at odds with previous morphometric studies including results reported by Davis and others demonstrating cortical thinning in this subregion in adult IBS patients [101, 107]. For example, we found no significant group differences for the aINS in our sample of adolescent patients relative to controls, although there was a small cluster in the left aINS that did not pass threshold correction (F = 2.45, Cluster size = 4.89 mm2; NVtxs = 12), but this was in the opposite direction. Furthermore, Davis et al. did not examine cortical thickness in relation to behavioral indices, making direct comparisons between studies untenable. Jiang et al. (2013) did examine disease severity and other psychometric measures in adult patients versus controls in relation to cortical thickness, however they report no significant correlations between cortical thickness in the aINS and their disease severity measures. Others have reported aINS thickening that was associated with longer disease durations [45] in adult IBS patients. Given that this is the first study to examine and identify structural alterations in a pediatric IBS population, and in relation to a measure of health-related well-being in context of GI-specific symptoms, it is not surprising that our results differed with respect to the adult IBS literature. A possible explanation for the disparate findings reported here and previous studies conducted with adult IBS patients may be simply attributable to age and therefore, differences in brain developmental trajectories. Other alternate explanations include but are not limited to differences in length (disease duration) and severity of the disease state (see next section). Clearly, more studies are needed in a larger sample of pediatric patients at discrete developmental stages in order to begin to delineate the morphometric brain changes related to brain development and those driven by the time course and severity of the disease.
Additionally, our finding of cortical thickness changes in the superior temporal sulcus (STS) (cortical thinning) in relation to improved HRQOL and a lessening of GI-specific symptoms is also of interest. While little is known about the role of this (non-hippocampal) region of the temporal lobe in relation to chronic pain, previous reports indicate that there may be a consistent contribution to pain processing. Alterations in functional responses to noxious heat [97] in an overlapping area of the temporal lobe have been reported in migraine, which shares some similarities to IBS (viz., central sensitization, visceral-type pain). This region is purportedly involved in sensory discriminative functions, including speech and biological motion perception [108–110]. In addition to sensory discriminative functions, the STS has also been reported to be involved in processing nonverbal social cues and emotional perception [111, 112]. Given that the pathophysiology of IBS has been linked to impaired quality of life, psychosocial functioning, and emotional processing deficits such as GI-specific anxiety, alexithymia and depression, these changes are of clinical importance [13, 113, 114]. While the functional significance of these results are not yet known, the fact that they were related to improved HRQOL indicate that these brain-behavioral changes may be useful as a metric for gauging the efficacy of therapeutic interventions in this clinical population.
Relationship between cortical thickness and disease-related clinical variables
Disease duration was correlated with cortical thickening in the bilateral DLPFC, an area which has been implicated in higher-order functions including cognitive control processes such as decision making, attention and descending pain modulation. It is noteworthy that cortical thickening in the supramarginal gyrus and cortical thinning in the lingual gyrus were also significantly related to duration of IBS symptoms. We have previously reported increased functional changes in the supramarginal gyrus in response to noxious heat in migraine patients and suggested a putative role for this region in pain may relate to cognitive interpretive processes [115]. This region has also been shown to be activated by both painful and non-painful aversive stimuli [116]. Thus, it is possible that these disease-related changes in brain architecture reflect a disruption in network processes associated with preservation of self in relation to perceived or actual harmful stimuli, although the exact meaning of these results remain to be determined.
In contrast to previous findings reported by Blankstein et al. (2010) demonstrating cortical thinning in the aINS associated with longer disease durations in adult patients with IBS, we found no significant relationship between cortical thickness and disease duration for this brain region [46]. However, we did find a small cluster in the right aINS that passed initial cluster thresholding, but failed to pass the cluster extent threshold determined a priori using 3dClustSim (F = 2.41, Cluster size = 5.77 mm2; NVtxs = 14). Nevertheless, the discrepancy in findings between our study conducted in adolescents and previous studies conducted in adults may relate to a number of factors, including differences in age, severity of IBS symptoms, and duration of disease. Clearly, the pediatric brain is not mature and therefore highly mutable in comparison to the adult brain. Although age was specified as regressor of no interest in the present study and therefore controlled for in the analysis, these differences in findings could be due to the developing nature of the pediatric brain relative to the more mature, adult brain. Moreover, as one might expect our adolescent IBS patients had much shorter disease durations (1–10 yrs.) compared to adult IBS patients (2–20 yrs.) sampled in the study by Blankstein et al. (2010) and this difference may underlie the observed differences in aINS cortical thickness since longer disease durations may produce more profound structural and functional brain changes that are not readily observed in the early stages of this syndrome. The notion that early brain changes may produce secondary effects with time has not been evaluated in adult or pediatric patients (viz., early vs. late onset IBS in either group), but should be an area of future study.
We also report an association between pain intensity scores and structural changes in the OFC. Specifically, patients reporting greater pain intensity associated with their IBS symptoms also showed significant cortical thickening localized to the bilateral OFC. The OFC is highly interconnected to limbic structures including areas involved in autonomic and stress-related physiological and behavioral responses [117]. This region receives visceral input from sensory areas as well as provides output to hypothalamic and midbrain structures involved in regulation of homeostatic functions [118, 119]. In addition, the OFC is considered important for regulation of affect, decision making [120], reward expectation, descending pain modulation [121, 122], integration of visceral sensorimotor information [117, 123] and interoceptive awareness [106]. Our findings of bilateral OFC in pediatric IBS are consistent with previous neuroimaging studies demonstrating functional and structural brain alterations in this region in adult IBS patients. For example, Jarcho et al. (2008) showed symptom improvement with a 5-HT3 receptor antagonist that was predicted by attenuated activity in the bilateral OFC and left medial temporal gyrus in response to rectal distention prior to drug treatment [124]. Additionally, Piché et al. (2009) showed cortical thickening in the OFC predicted reductions in pain inhibition in both IBS patients and healthy controls [122]. Using VBM, Seminowicz et al. (2010) also reported increased gray matter density in the left OFC in adult IBS patients compared to controls, indicating that these changes may be common across age for the disease state.
Functional Brain Changes in Pediatric IBS
Here we used resting-state fMRI and the regions noted above, that exhibited significant group differences in cortical thickness presumably due to underlying pathophysiology of IBS, as seeds in our RS-FC analysis (viz., PCC, DMPFC, DLPFC, and PPC). We also examined seeds in areas that showed structural changes that correlated with measures of abdominal pain severity and GI-specific symptoms in relation to HRQOL in the right intra-abdominal area of SI and the left anterior insula. In this way insights into how structural changes may be linked to reorganization of functional brain networks can be ascertained, although causal inferences still cannot be made.
Our seed-based analysis revealed altered RS-FC within key nodes of the DMN and CCN networks in adolescent patients with IBS. Specifically, we identified significant differences in RS-FC between the right PCC seed and the left DLPFC in patients relative to healthy cohorts of similar age. Inspection of the data revealed that this difference in RS-FC was not simply due to greater increases in RS-FC between the PCC-DLPFC in patients versus controls, but rather resulted from a diminution in anti-correlated connectivity patterns in the former group relative to the latter. Previous reports of alterations in canonical nodes of intrinsic connectivity networks such as the DMN have been described in clinical pain conditions [84, 125–128], including adults and adolescents with IBS during resting-state [64] and liminal rectal distension [43]. Structural and functional connections between the PCC and medial temporal and prefrontal (mPFC) and the inferior parietal cortices [129] have also been described using streamline and probabilistic tractography, and RS-FC methods [130]. Furthermore, the DLPFC has been implicated in chronic pain across numerous studies [70, 76, 81, 131, 132] and altered PCC-DLPFC functioning has been reported in fibromyalgia and migraine [84], suggesting that this area is involved across a spectrum of chronic pain conditions. The consequences of such changes in PCC-DLPFC connectivity could include alterations in attention [75, 133–137], arousal [54, 138] and cognitive control functions [139–141], a finding frequently reported for various chronic pain conditions. Although the exact functional significance of our RS-FC analysis are not known, the atypical connectivity patterns consisting of reduced PCC-DLPFC anti-correlations in patients compared to controls may reflect an attentional shift [54] of cognitive control areas toward viscerosensory and interoceptive processes related to chronic, ongoing abdominal pain and/or discomfort in these patients.
Study Caveats and Limitations
Several limitations should be mentioned in context of the present study findings. First, our sample of adolescents with IBS diagnosis was heterogeneous with respect to bowel habit subtype, with the majority of patients classified as constipation predominant, therefore limiting the generalizability of our results. A second major limitation was that medications with psychotropic effects were not controlled for in the patient group and this could have negatively influenced our results. However, it should be noted that inclusion of patients on psychotropic medications was restricted to those patients on low to moderate, stable doses. Third, a lack of psychometric measures for the healthy control group limits our ability to determine whether depression or anxiety influenced our results since we had little to no data with regard to these metrics between groups and therefore could not control for these variables in our analyses. Lastly, our study design was cross-sectional and therefore prevents us from making causal inferences. Future studies utilizing a longitudinal design in pediatric IBS patients are needed in order to determine whether the observed alterations in brain architecture and intrinsic network functioning are a consequence of IBS symptoms over time, or represent an antecedent, premorbid brain state that renders the brain vulnerable to the development of gut-brain signaling abnormalities, which in turn may lead to IBS.
Conclusions
In the present study, we evaluated concurrent structural and functional brain changes in adolescents with a diagnosis of IBS relative to a healthy cohort. We report both increases and decreases in cortical thickness in areas involved in cognitive inhibitory control, pain-related processes, viscerosensory motor and interoceptive functions, that were correlated with measures of abdominal pain severity, HRQOL in relation to GI-specific symptoms and disease severity measures such as duration of IBS symptoms and pain intensity. In addition, we also found atypical intrinsic RS-FC patterns between DMN and cognitive control network nodes, a finding which may reflect a shifting of attentional resources towards viscerosensory and interoceptive processes related to ongoing, abdominal pain. Whether these structural and functional brain changes are disease-driven or antecedent to the onset of childhood IBS remains to be determined. These brain changes (sensory, cognitive and emotional) may have long-term consequences on future behavior (e.g., anxiety, depression, psychosocial functioning, propensity to develop chronic pain, etc.), health and well-being, of individuals affected by IBS during their childhood years.
Supporting Information
(TIF)
Abbreviations: NVtxs = number of vertices; R = right; L = left; PPC = posterior Parietal Cortex; PCC = posterior cingulate cortex; SI = primary somatosensory cortex; MNI = Montreal Neurological Institute.
(DOCX)
Abbreviations: PedsQL = Pediatric Quality of Life Inventory; PedsQL GI Module = Pediatric Quality of Life Inventory Gastrointestinal Symptoms Module; API = Abdominal Pain Index; FDI = Functional Disability Inventory PCS-C = Pain Catastrophizing Scale−Child version.
(DOCX)
Abbreviations: NVtxs = number of vertices; R = right; L = left; MNI = Montreal Neurological Institute; FDI = Functional Disability Inventory; SI = primary somatosensory cortex; PCC = posterior cingulate cortex; PPC = posterior parietal cortex; PCS-C = Pain Catastrophizing Scale−Child version; DLPFC = dorsolateral prefrontal cortex.
(DOCX)
Acknowledgments
We would like to extend our appreciation to Duncan Hodkinson for his assistance with our resting-state functional connectivity analysis and our participants for their contributions to this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by K24 NS064050 NINDS (DB), the Mayday/Louis Herlands Chair for Pain Systems Neuroscience (DB), the Anesthesia Foundation (DB), K24 DK082792 NIDDK (SN), and the Behrakis Foundation (SN).
References
- 1.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123(6):2108–31. Epub 2002/11/28. 10.1053/gast.2002.37095 . [DOI] [PubMed] [Google Scholar]
- 2.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–91. Epub 2006/05/09. 10.1053/j.gastro.2005.11.061 . [DOI] [PubMed] [Google Scholar]
- 3.Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, et al. Association between early adverse life events and irritable bowel syndrome. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2012;10(4):385–90 e1–3. Epub 2011/12/20. 10.1016/j.cgh.2011.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sykes MA, Blanchard EB, Lackner J, Keefer L, Krasner S. Psychopathology in irritable bowel syndrome: support for a psychophysiological model. Journal of behavioral medicine. 2003;26(4):361–72. Epub 2003/08/19. . [DOI] [PubMed] [Google Scholar]
- 5.Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. The American journal of gastroenterology. 2008;103(3):765–74; quiz 75. Epub 2008/01/08. 10.1111/j.1572-0241.2007.01722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyams JS, Burke G, Davis PM, Rzepski B, Andrulonis PA. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. The Journal of pediatrics. 1996;129(2):220–6. Epub 1996/08/01. . [DOI] [PubMed] [Google Scholar]
- 7.Miele E, Simeone D, Marino A, Greco L, Auricchio R, Novek SJ, et al. Functional gastrointestinal disorders in children: an Italian prospective survey. Pediatrics. 2004;114(1):73–8. Epub 2004/07/03. . [DOI] [PubMed] [Google Scholar]
- 8.Varni JW, Bendo CB, Nurko S, Shulman RJ, Self MM, Franciosi JP, et al. Health-related quality of life in pediatric patients with functional and organic gastrointestinal diseases. The Journal of pediatrics. 2015;166(1):85–90. Epub 2014/09/23. 10.1016/j.jpeds.2014.08.022 . [DOI] [PubMed] [Google Scholar]
- 9.Varni JW, Lane MM, Burwinkle TM, Fontaine EN, Youssef NN, Schwimmer JB, et al. Health-related quality of life in pediatric patients with irritable bowel syndrome: a comparative analysis. Journal of developmental and behavioral pediatrics: JDBP. 2006;27(6):451–8. Epub 2006/12/14. . [DOI] [PubMed] [Google Scholar]
- 10.van Tilburg MA, Squires M, Blois-Martin N, Leiby A, Langseder A. Test of the child/adolescent Rome III criteria: agreement with physician diagnosis and daily symptoms. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2013;25(4):302–e246. Epub 2012/12/12. 10.1111/nmo.12056 . [DOI] [PubMed] [Google Scholar]
- 11.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130(5):1527–37. Epub 2006/05/09. 10.1053/j.gastro.2005.08.063 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain, behavior, and immunity. 2011;25(3):386–94. Epub 2010/11/26. 10.1016/j.bbi.2010.11.010 . [DOI] [PubMed] [Google Scholar]
- 13.Naliboff BD, Munakata J, Chang L, Mayer EA. Toward a biobehavioral model of visceral hypersensitivity in irritable bowel syndrome. Journal of psychosomatic research. 1998;45(6):485–92. Epub 1998/12/22. . [DOI] [PubMed] [Google Scholar]
- 14.Schwetz I, Bradesi S, Mayer EA. Current insights into the pathophysiology of irritable bowel syndrome. Current gastroenterology reports. 2003;5(4):331–6. Epub 2003/07/17. . [DOI] [PubMed] [Google Scholar]
- 15.Mayer EA, Gupta A, Kilpatrick LA, Hong JY. Imaging brain mechanisms in chronic visceral pain. Pain. 2015;156 Suppl 1:S50–63. Epub 2015/03/20. 10.1097/j.pain.0000000000000106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montiel-Castro AJ, Gonzalez-Cervantes RM, Bravo-Ruiseco G, Pacheco-Lopez G. The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Frontiers in integrative neuroscience. 2013;7:70 Epub 2013/10/11. 10.3389/fnint.2013.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118(5):842–8. Epub 2000/04/28. . [DOI] [PubMed] [Google Scholar]
- 18.Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2010;59(4):489–95. Epub 2009/08/05. 10.1136/gut.2008.175000 . [DOI] [PubMed] [Google Scholar]
- 19.King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL III. Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain. 2009;143(3):172–8. Epub 2009/03/13. 10.1016/j.pain.2008.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heymen S, Maixner W, Whitehead WE, Klatzkin RR, Mechlin B, Light KC. Central processing of noxious somatic stimuli in patients with irritable bowel syndrome compared with healthy controls. The Clinical journal of pain. 2010;26(2):104–9. Epub 2010/01/22. 10.1097/AJP.0b013e3181bff800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, et al. Rome III: The Functional Gastrtointestinal Disorders. 3rd ed: McLean, Virginia: Degnon Associ-ates; 2006. [Google Scholar]
- 22.Walker L, Smith CA, Garber J, Van Slyke DA. Development and validation of the pain response inventory for children. Psychological Assessment. 1997;9:392–405. [Google Scholar]
- 23.Laird KT, Sherman AL, Smith CA, Walker LS. Validation of the Abdominal Pain Index using a revised scoring method. Journal of pediatric psychology. 2015;40(5):517–25. Epub 2015/01/27. 10.1093/jpepsy/jsu118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical care. 2001;39(8):800–12. Epub 2001/07/27. . [DOI] [PubMed] [Google Scholar]
- 25.Varni JW, Bendo CB, Denham J, Shulman RJ, Self MM, Neigut DA, et al. PedsQL gastrointestinal symptoms module: feasibility, reliability, and validity. Journal of pediatric gastroenterology and nutrition. 2014;59(3):347–55. Epub 2014/05/09. 10.1097/mpg.0000000000000414 . [DOI] [PubMed] [Google Scholar]
- 26.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. Journal of pediatric psychology. 1991;16(1):39–58. Epub 1991/02/01. . [DOI] [PubMed] [Google Scholar]
- 27.Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE. Assessment of symptoms of DSM-IV anxiety and depression in children: a revised child anxiety and depression scale. Behaviour research and therapy. 2000;38(8):835–55. Epub 2000/08/11. . [DOI] [PubMed] [Google Scholar]
- 28.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, et al. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104(3):639–46. Epub 2003/08/21. . [DOI] [PubMed] [Google Scholar]
- 29.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–94. Epub 1999/02/05. 10.1006/nimg.1998.0395 . [DOI] [PubMed] [Google Scholar]
- 30.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. Epub 1999/02/05. 10.1006/nimg.1998.0396 . [DOI] [PubMed] [Google Scholar]
- 31.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(20):11050–5. Epub 2000/09/14. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–80. Epub 2006/03/15. 10.1016/j.neuroimage.2006.01.021 . [DOI] [PubMed] [Google Scholar]
- 33.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain connectivity. 2012;2(3):125–41. Epub 2012/05/31. 10.1089/brain.2012.0073 . [DOI] [PubMed] [Google Scholar]
- 34.Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. Epub 2007/06/15. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44(3):893–905. Epub 2008/11/04. 10.1016/j.neuroimage.2008.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59(2):1420–8. Epub 2011/09/06. 10.1016/j.neuroimage.2011.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral cortex (New York, NY: 1991). 2009;19(11):2728–35. Epub 2009/03/21. 10.1093/cercor/bhp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. Genetic influences on human brain structure: a review of brain imaging studies in twins. Human brain mapping. 2007;28(6):464–73. Epub 2007/04/07. 10.1002/hbm.20398 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53(3):1135–46. Epub 2009/12/17. 10.1016/j.neuroimage.2009.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and biomedical research, an international journal. 1996;29(3):162–73. Epub 1996/06/01. . [DOI] [PubMed] [Google Scholar]
- 41.Ridgway GR, Omar R, Ourselin S, Hill DL, Warren JD, Fox NC. Issues with threshold masking in voxel-based morphometry of atrophied brains. NeuroImage. 2009;44(1):99–111. Epub 2008/10/14. 10.1016/j.neuroimage.2008.08.045 . [DOI] [PubMed] [Google Scholar]
- 42.Petzke F. CNS processing of pain in functional somatic syndromes. Schmerz (Berlin, Germany). 2010;24(2):146–55. Epub 2010/04/09. 10.1007/s00482-010-0903-5 . [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Silverman A, Kern M, Ward BD, Li SJ, Shaker R, et al. Excessive coupling of the salience network with intrinsic neurocognitive brain networks during rectal distension in adolescents with irritable bowel syndrome: a preliminary report. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2015. Epub 2015/10/16. 10.1111/nmo.12695 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Labus JS, Dinov ID, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, et al. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. 2014;155(1):137–49. Epub 2013/10/01. 10.1016/j.pain.2013.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138(5):1783–9. Epub 2010/01/05. 10.1053/j.gastro.2009.12.043 . [DOI] [PubMed] [Google Scholar]
- 46.Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, Diamant NE. Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology. 2008;70(2):153–4. Epub 2007/10/26. 10.1212/01.wnl.0000295509.30630.10 . [DOI] [PubMed] [Google Scholar]
- 47.Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139(1):48–57 e2 Epub 2010/03/30. 10.1053/j.gastro.2010.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Irimia A, Labus JS, Torgerson CM, Van Horn JD, Mayer EA. Altered viscerotopic cortical innervation in patients with irritable bowel syndrome. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2015;27(8):1075–81. Epub 2015/05/09. 10.1111/nmo.12586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma X, Li S, Tian J, Jiang G, Wen H, Wang T, et al. Altered brain spontaneous activity and connectivity network in irritable bowel syndrome patients: A resting-state fMRI study. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2015;126(6):1190–7. Epub 2014/12/03. 10.1016/j.clinph.2014.10.004 . [DOI] [PubMed] [Google Scholar]
- 50.Hong JY, Kilpatrick LA, Labus JS, Gupta A, Katibian D, Ashe-McNalley C, et al. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34(43):14252–9. Epub 2014/10/24. 10.1523/jneurosci.1683-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(1):215–22. Epub 2012/01/06. 10.1523/jneurosci.3689-11.2012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. Epub 2008/04/11. 10.1196/annals.1440.011 . [DOI] [PubMed] [Google Scholar]
- 53.Chen L, Malarick C, Seefeld L, Wang S, Houghton M, Mao J. Altered quantitative sensory testing outcome in subjects with opioid therapy. Pain. 2009;143(1–2):65–70. Epub 2009/02/25. 10.1016/j.pain.2009.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain: a journal of neurology. 2014;137(Pt 1):12–32. Epub 2013/07/23. 10.1093/brain/awt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. Journal of cognitive neuroscience. 1999;11(1):80–95. Epub 1999/02/09. . [DOI] [PubMed] [Google Scholar]
- 56.Zhang Y, Ahmed S, Vo T, St Hilaire K, Houghton M, Cohen AS, et al. Increased pain sensitivity in chronic pain subjects on opioid therapy: a cross-sectional study using quantitative sensory testing. Pain medicine (Malden, Mass). 2015;16(5):911–22. Epub 2014/11/08. 10.1111/pme.12606 . [DOI] [PubMed] [Google Scholar]
- 57.Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Progress in brain research. 2005;150:205–17. Epub 2005/09/28. 10.1016/s0079-6123(05)50015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(51):13338–43. Epub 2006/12/22. 10.1523/jneurosci.3408-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Olson CR, Musil SY. Posterior cingulate cortex: sensory and oculomotor properties of single neurons in behaving cat. Cerebral cortex (New York, NY: 1991). 1992;2(6):485–502. Epub 1992/11/01. . [DOI] [PubMed] [Google Scholar]
- 60.Hahn B, Ross TJ, Stein EA. Cingulate activation increases dynamically with response speed under stimulus unpredictability. Cerebral cortex (New York, NY: 1991). 2007;17(7):1664–71. Epub 2006/09/12. 10.1093/cercor/bhl075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, et al. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103(1–2):99–110. Epub 2003/05/17. . [DOI] [PubMed] [Google Scholar]
- 62.Ringel Y, Drossman DA, Leserman JL, Suyenobu BY, Wilber K, Lin W, et al. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134(2):396–404. Epub 2008/02/05. 10.1053/j.gastro.2007.11.011 . [DOI] [PubMed] [Google Scholar]
- 63.Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ, Bushnell MC. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. NeuroImage. 2009;47(3):1007–14. Epub 2009/06/06. 10.1016/j.neuroimage.2009.05.068 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qi R, Liu C, Ke J, Xu Q, Zhong J, Wang F, et al. Intrinsic brain abnormalities in irritable bowel syndrome and effect of anxiety and depression. Brain imaging and behavior. 2015. Epub 2015/11/12. 10.1007/s11682-015-9478-1 . [DOI] [PubMed] [Google Scholar]
- 65.Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage. 2007;37(1):343–60. Epub 2007/06/08. 10.1016/j.neuroimage.2007.03.071 . [DOI] [PubMed] [Google Scholar]
- 66.Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Annals of the New York Academy of Sciences. 1995;769:71–83. Epub 1995/12/15. . [DOI] [PubMed] [Google Scholar]
- 67.Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annual review of neuroscience. 2010;33:1–21. Epub 2010/03/03. 10.1146/annurev-neuro-060909-152823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, affective & behavioral neuroscience. 2003;3(4):255–74. Epub 2004/03/26. . [DOI] [PubMed] [Google Scholar]
- 69.Katsuki F, Constantinidis C. Unique and shared roles of the posterior parietal and dorsolateral prefrontal cortex in cognitive functions. Frontiers in integrative neuroscience. 2012;6:17 Epub 2012/05/09. 10.3389/fnint.2012.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain: a journal of neurology. 2003;126(Pt 5):1079–91. Epub 2003/04/12. . [DOI] [PubMed] [Google Scholar]
- 71.Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. The Journal of comparative neurology. 1989;287(4):422–45. Epub 1989/09/22. 10.1002/cne.902870403 . [DOI] [PubMed] [Google Scholar]
- 72.Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1988;8(11):4049–68. Epub 1988/11/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aizawa E, Sato Y, Kochiyama T, Saito N, Izumiyama M, Morishita J, et al. Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology. 2012;143(5):1188–98. Epub 2012/07/31. 10.1053/j.gastro.2012.07.104 . [DOI] [PubMed] [Google Scholar]
- 74.Hong JY, Naliboff B, Labus JS, Gupta A, Kilpatrick LA, Ashe-McNalley C, et al. Altered brain responses in subjects with irritable bowel syndrome during cued and uncued pain expectation. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2016;28(1):127–38. Epub 2015/11/04. 10.1111/nmo.12710 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hubbard CS, Hong J, Jiang Z, Ebrat B, Suyenobu B, Smith S, et al. Increased attentional network functioning related to symptom severity measures in females with irritable bowel syndrome. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2015;27(9):1282–94. Epub 2015/06/20. 10.1111/nmo.12622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(46):10410–5. Epub 2004/11/19. 10.1523/jneurosci.2541-04.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buckalew N, Haut MW, Morrow L, Weiner D. Chronic pain is associated with brain volume loss in older adults: preliminary evidence. Pain medicine (Malden, Mass). 2008;9(2):240–8. Epub 2008/02/27. 10.1111/j.1526-4637.2008.00412.x . [DOI] [PubMed] [Google Scholar]
- 78.Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, et al. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125(1–2):89–97. Epub 2006/06/06. 10.1016/j.pain.2006.05.004 . [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(44):13746–50. Epub 2009/11/06. 10.1523/jneurosci.3687-09.2009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hu W, Guo J, Chen N, Guo J, He L. A meta-analysis of voxel-based morphometric studies on migraine. International journal of clinical and experimental medicine. 2015;8(3):4311–9. Epub 2015/06/13. [PMC free article] [PubMed] [Google Scholar]
- 81.Seminowicz DA, Shpaner M, Keaser ML, Krauthamer GM, Mantegna J, Dumas JA, et al. Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. The journal of pain: official journal of the American Pain Society. 2013;14(12):1573–84. Epub 2013/10/19. 10.1016/j.jpain.2013.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Icenhour A, Langhorst J, Benson S, Schlamann M, Hampel S, Engler H, et al. Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2015;27(1):114–27. Epub 2015/01/06. 10.1111/nmo.12489 . [DOI] [PubMed] [Google Scholar]
- 83.Labus JS, Hubbard CS, Bueller J, Ebrat B, Tillisch K, Chen M, et al. Impaired emotional learning and involvement of the corticotropin-releasing factor signaling system in patients with irritable bowel syndrome. Gastroenterology. 2013;145(6):1253–61 e1–3. Epub 2013/08/21. 10.1053/j.gastro.2013.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hubbard CS, Khan SA, Keaser ML, Mathur VA, Goyal M, Seminowicz DA. Altered Brain Structure and Function Correlate with Disease Severity and Pain Catastrophizing in Migraine Patients. eNeuro. 2014;1(1):e20 14. Epub 2015/04/22. 10.1523/eneuro.0006-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kennedy PJ, Clarke G, O'Neill A, Groeger JA, Quigley EM, Shanahan F, et al. Cognitive performance in irritable bowel syndrome: evidence of a stress-related impairment in visuospatial memory. Psychological medicine. 2014;44(7):1553–66. Epub 2013/08/30. 10.1017/s0033291713002171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hauser G, Pletikosic S, Tkalcic M. Cognitive behavioral approach to understanding irritable bowel syndrome. World journal of gastroenterology. 2014;20(22):6744–58. Epub 2014/06/20. 10.3748/wjg.v20.i22.6744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Willis WD, Al-Chaer ED, Quast MJ, Westlund KN. A visceral pain pathway in the dorsal column of the spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(14):7675–9. Epub 1999/07/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Palecek J. The role of dorsal columns pathway in visceral pain. Physiological research / Academia Scientiarum Bohemoslovaca. 2004;53 Suppl 1:S125–30. Epub 2004/05/04. . [PubMed] [Google Scholar]
- 89.Drewes AM, Dimcevski G, Sami SA, Funch-Jensen P, Huynh KD, Le Pera D, et al. The "human visceral homunculus" to pain evoked in the oesophagus, stomach, duodenum and sigmoid colon. Experimental brain research. 2006;174(3):443–52. Epub 2006/05/06. 10.1007/s00221-006-0480-0 . [DOI] [PubMed] [Google Scholar]
- 90.Derbyshire SW. A systematic review of neuroimaging data during visceral stimulation. The American journal of gastroenterology. 2003;98(1):12–20. Epub 2003/01/16. 10.1111/j.1572-0241.2003.07168.x . [DOI] [PubMed] [Google Scholar]
- 91.Aziz Q, Thompson DG, Ng VW, Hamdy S, Sarkar S, Brammer MJ, et al. Cortical processing of human somatic and visceral sensation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20(7):2657–63. Epub 2000/03/24. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Binkofski F, Schnitzler A, Enck P, Frieling T, Posse S, Seitz RJ, et al. Somatic and limbic cortex activation in esophageal distention: a functional magnetic resonance imaging study. Annals of neurology. 1998;44(5):811–5. Epub 1998/11/18. 10.1002/ana.410440516 . [DOI] [PubMed] [Google Scholar]
- 93.Furlong PL, Aziz Q, Singh KD, Thompson DG, Hobson A, Harding GF. Cortical localisation of magnetic fields evoked by oesophageal distension. Electroencephalography and clinical neurophysiology. 1998;108(3):234–43. Epub 1998/06/02. . [DOI] [PubMed] [Google Scholar]
- 94.Ladabaum U, Minoshima S, Hasler WL, Cross D, Chey WD, Owyang C. Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology. 2001;120(2):369–76. Epub 2001/02/13. . [DOI] [PubMed] [Google Scholar]
- 95.Aziz Q, Andersson JL, Valind S, Sundin A, Hamdy S, Jones AK, et al. Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology. 1997;113(1):50–9. Epub 1997/07/01. . [DOI] [PubMed] [Google Scholar]
- 96.Hobday DI, Aziz Q, Thacker N, Hollander I, Jackson A, Thompson DG. A study of the cortical processing of ano-rectal sensation using functional MRI. Brain: a journal of neurology. 2001;124(Pt 2):361–8. Epub 2001/02/07. . [DOI] [PubMed] [Google Scholar]
- 97.Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia: an international journal of headache. 2012;32(8):607–20. Epub 2012/05/25. 10.1177/0333102412445622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cervero F, Janig W. Visceral nociceptors: a new world order? Trends in neurosciences. 1992;15(10):374–8. Epub 1992/10/01. . [DOI] [PubMed] [Google Scholar]
- 99.Derbyshire SW. Visceral afferent pathways and functional brain imaging. TheScientificWorldJournal. 2003;3:1065–80. Epub 2003/11/13. 10.1100/tsw.2003.93 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hemington KS, Wu Q, Kucyi A, Inman RD, Davis KD. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain structure & function. 2015. Epub 2015/12/17. 10.1007/s00429-015-1161-1 . [DOI] [PubMed] [Google Scholar]
- 101.Hong JY, Labus JS, Jiang Z, Ashe-Mcnalley C, Dinov I, Gupta A, et al. Regional neuroplastic brain changes in patients with chronic inflammatory and non-inflammatory visceral pain. PloS one. 2014;9(1):e84564 Epub 2014/01/15. 10.1371/journal.pone.0084564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vogt BA, Derbyshire S, Jones AK. Pain processing in four regions of human cingulate cortex localized with co-registered PET and MR imaging. The European journal of neuroscience. 1996;8(7):1461–73. Epub 1996/07/01. . [DOI] [PubMed] [Google Scholar]
- 103.Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, Gonzalez-Roldan A, et al. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosomatic medicine. 2012;74(1):55–62. Epub 2012/01/03. 10.1097/PSY.0b013e3182408f04 . [DOI] [PubMed] [Google Scholar]
- 104.Jarrahi B, Mantini D, Balsters JH, Michels L, Kessler TM, Mehnert U, et al. Differential functional brain network connectivity during visceral interoception as revealed by independent component analysis of fMRI time-series. Human brain mapping. 2015;36(11):4438–68. Epub 2015/08/08. 10.1002/hbm.22929 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peltz E, Seifert F, DeCol R, Dorfler A, Schwab S, Maihofner C. Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. NeuroImage. 2011;54(2):1324–35. Epub 2010/09/21. 10.1016/j.neuroimage.2010.09.012 . [DOI] [PubMed] [Google Scholar]
- 106.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature reviews Neuroscience. 2002;3(8):655–66. Epub 2002/08/03. 10.1038/nrn894 . [DOI] [PubMed] [Google Scholar]
- 107.Jiang Z, Dinov ID, Labus J, Shi Y, Zamanyan A, Gupta A, et al. Sex-related differences of cortical thickness in patients with chronic abdominal pain. PloS one. 2013;8(9):e73932 Epub 2013/09/17. 10.1371/journal.pone.0073932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hein G, Knight RT. Superior temporal sulcus—It's my area: or is it? Journal of cognitive neuroscience. 2008;20(12):2125–36. Epub 2008/05/07. 10.1162/jocn.2008.20148 . [DOI] [PubMed] [Google Scholar]
- 109.Robins DL, Hunyadi E, Schultz RT. Superior temporal activation in response to dynamic audio-visual emotional cues. Brain and cognition. 2009;69(2):269–78. Epub 2008/09/24. 10.1016/j.bandc.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Annals of the New York Academy of Sciences. 2008;1145:283–99. Epub 2008/12/17. 10.1196/annals.1416.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Narumoto J, Okada T, Sadato N, Fukui K, Yonekura Y. Attention to emotion modulates fMRI activity in human right superior temporal sulcus. Brain research Cognitive brain research. 2001;12(2):225–31. Epub 2001/10/06. . [DOI] [PubMed] [Google Scholar]
- 112.Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends in cognitive sciences. 2000;4(7):267–78. Epub 2000/06/22. . [DOI] [PubMed] [Google Scholar]
- 113.Portincasa P, Moschetta A, Baldassarre G, Altomare DF, Palasciano G. Pan-enteric dysmotility, impaired quality of life and alexithymia in a large group of patients meeting ROME II criteria for irritable bowel syndrome. World journal of gastroenterology. 2003;9(10):2293–9. Epub 2003/10/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Surdea-Blaga T, Baban A, Dumitrascu DL. Psychosocial determinants of irritable bowel syndrome. World journal of gastroenterology. 2012;18(7):616–26. Epub 2012/03/01. 10.3748/wjg.v18.i7.616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moulton EA, Pendse G, Becerra LR, Borsook D. BOLD responses in somatosensory cortices better reflect heat sensation than pain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32(17):6024–31. Epub 2012/04/28. 10.1523/jneurosci.0006-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hayes DJ, Northoff G. Common brain activations for painful and non-painful aversive stimuli. BMC neuroscience. 2012;13:60 Epub 2012/06/09. 10.1186/1471-2202-13-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Annals of the New York Academy of Sciences. 2007;1121:54–71. Epub 2007/08/19. 10.1196/annals.1401.008 . [DOI] [PubMed] [Google Scholar]
- 118.Price JL. Prefrontal cortical networks related to visceral function and mood. Annals of the New York Academy of Sciences. 1999;877:383–96. Epub 1999/07/23. . [DOI] [PubMed] [Google Scholar]
- 119.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral cortex (New York, NY: 1991). 2000;10(3):206–19. Epub 2000/03/24. . [DOI] [PubMed] [Google Scholar]
- 120.Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Progress in neurobiology. 2008;86(3):216–44. Epub 2008/10/01. 10.1016/j.pneurobio.2008.09.001 . [DOI] [PubMed] [Google Scholar]
- 121.Moont R, Crispel Y, Lev R, Pud D, Yarnitsky D. Temporal changes in cortical activation during conditioned pain modulation (CPM), a LORETA study. Pain. 2011;152(7):1469–77. Epub 2011/02/23. 10.1016/j.pain.2011.01.036 . [DOI] [PubMed] [Google Scholar]
- 122.Piche M, Arsenault M, Rainville P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(45):14236–46. Epub 2009/11/13. 10.1523/jneurosci.2341-09.2009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in neurobiology. 2004;72(5):341–72. Epub 2004/05/26. 10.1016/j.pneurobio.2004.03.006 . [DOI] [PubMed] [Google Scholar]
- 124.Jarcho JM, Chang L, Berman M, Suyenobu B, Naliboff BD, Lieberman MD, et al. Neural and psychological predictors of treatment response in irritable bowel syndrome patients with a 5-HT3 receptor antagonist: a pilot study. Alimentary pharmacology & therapeutics. 2008;28(3):344–52. Epub 2008/12/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chanda ML, Alvin MD, Schnitzer TJ, Apkarian AV. Pain characteristic differences between subacute and chronic back pain. The journal of pain: official journal of the American Pain Society. 2011;12(7):792–800. Epub 2011/04/19. 10.1016/j.jpain.2011.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis and rheumatism. 2010;62(8):2545–55. Epub 2010/05/28. 10.1002/art.27497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, et al. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34(11):3969–75. Epub 2014/03/14. 10.1523/jneurosci.5055-13.2014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xue T, Yuan K, Zhao L, Yu D, Zhao L, Dong T, et al. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PloS one. 2012;7(12):e52927 Epub 2013/01/04. 10.1371/journal.pone.0052927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. NeuroImage. 2008;42(3):1178–84. Epub 2008/07/05. 10.1016/j.neuroimage.2008.05.059 . [DOI] [PubMed] [Google Scholar]
- 130.Khalsa S, Mayhew SD, Chechlacz M, Bagary M, Bagshaw AP. The structural and functional connectivity of the posterior cingulate cortex: comparison between deterministic and probabilistic tractography for the investigation of structure-function relationships. NeuroImage. 2014;102 Pt 1:118–27. Epub 2013/12/25. 10.1016/j.neuroimage.2013.12.022 . [DOI] [PubMed] [Google Scholar]
- 131.Bruehl S, Apkarian AV, Ballantyne JC, Berger A, Borsook D, Chen WG, et al. Personalized medicine and opioid analgesic prescribing for chronic pain: opportunities and challenges. The journal of pain: official journal of the American Pain Society. 2013;14(2):103–13. Epub 2013/02/05. 10.1016/j.jpain.2012.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee MC, Wanigasekera V, Tracey I. Imaging opioid analgesia in the human brain and its potential relevance for understanding opioid use in chronic pain. Neuropharmacology. 2014;84:123–30. Epub 2013/07/31. 10.1016/j.neuropharm.2013.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Apkarian AV. Human Brain Imaging Studies of Chronic Pain: Translational Opportunities Translational Pain Research: From Mouse to Man. Kruger L, Light AR, editors. Boca Raton, FL: Llc; 2010. [PubMed] [Google Scholar]
- 134.Beck AT S R, Brown GK. Beck Depression Inventory Manual. 2nd Edition ed1996. [Google Scholar]
- 135.Dick BD, Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesthesia and analgesia. 2007;104(5):1223–9, tables of contents. Epub 2007/04/26. 10.1213/01.ane.0000263280.49786.f5 . [DOI] [PubMed] [Google Scholar]
- 136.Eccleston C. Chronic pain and attention: a cognitive approach. The British journal of clinical psychology / the British Psychological Society. 1994;33 (Pt 4):535–47. Epub 1994/11/01. . [DOI] [PubMed] [Google Scholar]
- 137.Mao CP, Zhang QL, Bao FX, Liao X, Yang XL, Zhang M. Decreased activation of cingulo-frontal-parietal cognitive/attention network during an attention-demanding task in patients with chronic low back pain. Neuroradiology. 2014;56(10):903–12. Epub 2014/07/06. 10.1007/s00234-014-1391-6 . [DOI] [PubMed] [Google Scholar]
- 138.Roth RS, Geisser ME, Theisen-Goodvich M, Dixon PJ. Cognitive complaints are associated with depression, fatigue, female sex, and pain catastrophizing in patients with chronic pain. Archives of physical medicine and rehabilitation. 2005;86(6):1147–54. Epub 2005/06/15. 10.1016/j.apmr.2004.10.041 . [DOI] [PubMed] [Google Scholar]
- 139.Baker KS, Gibson S, Georgiou-Karistianis N, Roth RM, Giummarra MJ. Everyday Executive Functioning in Chronic Pain: Specific Deficits in Working Memory and Emotion Control, Predicted by Mood, Medications, and Pain Interference. The Clinical journal of pain. 2015. Epub 2015/12/03. 10.1097/ajp.0000000000000313 . [DOI] [PubMed] [Google Scholar]
- 140.Martinsen S, Flodin P, Berrebi J, Lofgren M, Bileviciute-Ljungar I, Ingvar M, et al. Fibromyalgia patients had normal distraction related pain inhibition but cognitive impairment reflected in caudate nucleus and hippocampus during the Stroop Color Word Test. PloS one. 2014;9(9):e108637 Epub 2014/10/03. 10.1371/journal.pone.0108637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weissman-Fogel I, Moayedi M, Tenenbaum HC, Goldberg MB, Freeman BV, Davis KD. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011;152(2):384–96. Epub 2010/12/21. 10.1016/j.pain.2010.10.046 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Abbreviations: NVtxs = number of vertices; R = right; L = left; PPC = posterior Parietal Cortex; PCC = posterior cingulate cortex; SI = primary somatosensory cortex; MNI = Montreal Neurological Institute.
(DOCX)
Abbreviations: PedsQL = Pediatric Quality of Life Inventory; PedsQL GI Module = Pediatric Quality of Life Inventory Gastrointestinal Symptoms Module; API = Abdominal Pain Index; FDI = Functional Disability Inventory PCS-C = Pain Catastrophizing Scale−Child version.
(DOCX)
Abbreviations: NVtxs = number of vertices; R = right; L = left; MNI = Montreal Neurological Institute; FDI = Functional Disability Inventory; SI = primary somatosensory cortex; PCC = posterior cingulate cortex; PPC = posterior parietal cortex; PCS-C = Pain Catastrophizing Scale−Child version; DLPFC = dorsolateral prefrontal cortex.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.