Abstract
Background: Fatigue is one of the most common and debilitating symptoms of multiple sclerosis (MS). The program “Minimise Fatigue, Maximise Life: Creating Balance with Multiple Sclerosis” (MFML) was created in New Zealand because of the lack of a fatigue management program for people with MS in that country. This program aims to empower individuals with MS to manage their own symptoms of fatigue. The objective of this study was to evaluate the MFML fatigue self-management program.
Methods: Self-report questionnaires were used to measure impact of fatigue (5-item Modified Fatigue Impact Scale), self-efficacy (MS Self-efficacy Scale), and quality of life (12-item Short Form Health Status Survey [SF-12]) 1 month before (T1), at commencement of (T2) (to investigate the stability of questionnaire scores before the intervention), and at the end of (T3) the 6-week group-based program. Increased self-efficacy and quality of life scores and a decrease in reported impact of fatigue were the anticipated primary outcomes, with participants acting as their own controls.
Results: Twenty-five women (aged 37–63 years) participated. Stability of scores for all the questionnaires was evidenced between T1 and T2. After the intervention (T3), scores showed a significant improvement in self-efficacy and impact of fatigue, with large effect sizes, but no change in either the physical or mental component summary of the SF-12.
Conclusions: Despite the small sample size, this study showed an organized approach to the management of fatigue for people living with MS, and its outcomes demonstrated benefits for participants.
The onset of multiple sclerosis (MS) usually occurs in the most productive years of young adult lives, with twice as many females being diagnosed as males.1 The cause of MS is still unknown, with evidence suggesting that both genetic and environmental factors play a role.1 MS is more common in those of Northern European ancestry. New Zealand (NZ), which was settled by Northern Europeans, therefore has a high incidence of MS, with an age-standardized prevalence rate of 73.1 per 100,000 population.2
Disease advancement can take a relapsing-remitting or a progressive course, with some people experiencing mild symptoms and others higher levels of disability.1 One of the most common yet least understood symptoms of MS is fatigue that interferes with activities of daily living and participation in life.3 It affects approximately 80% of people who have the disease.4 Fatigue is multifactorial and independent of age and disease duration.5
Investigations into the management of fatigue in MS have provided several strategies for amelioration.6 A recent review7 suggests that nonpharmacologic interventions are considered to be the first step in managing fatigue. These interventions include exercise, education about energy conservation strategies, and behavioral/lifestyle modifications.8 For such interventions to be sustained and, therefore, effective, people with MS need to be able to incorporate them into their daily activities themselves. Self-management of symptoms in other areas of MS has been successful9; therefore, it is important to provide those living with MS with a way to develop strategies for behavior change to reduce fatigue and allow participation in daily life.
Approaches to fatigue management have been reported in the literature. One such approach incorporates energy conservation education.10–12 The other uses a cognitive-behavioral approach to provide knowledge about fatigue.13 Such programs were, however, unavailable in clinical practice to people with MS in NZ. This lack of availability in NZ led to development of the program “Minimise Fatigue, Maximise Life: Creating Balance with Multiple Sclerosis” (MFML). The program incorporates the self-management principles of prioritizing resources, building partnerships with health-care providers, using a group setting with others with whom one identifies, and problem solving, decision making, and action taking with others,14–17 with the aim of empowering individuals with MS to build self-efficacy to manage their own symptoms of fatigue. Unlike other fatigue management programs for people with MS, MFML was developed using participatory action research. This methodological approach facilitated partnering between health researchers and people with MS to ensure ownership of and relevance for people living with MS in NZ.18,19 Development of MFML is described elsewhere.19
This study formed part of a mixed-methods evaluation of the MFML fatigue self-management program.20 Using a mixed-methods approach facilitated a more comprehensive evaluation of the program and contributed to greater understanding21–23 of the impact of MFML on the daily lives of individuals living with MS. This article reports the outcomes of the standardized questionnaires for fatigue, self-efficacy, and quality of life used in a component of the evaluation of the program.
Methods
In 2013, five health professionals (NZ-registered physiotherapists and occupational therapists with previous experience working with people with MS in community settings), selected based on their geographic spread throughout NZ, were trained to deliver the program. Training was provided by one of us (JS) in an intensive 2-day face-to-face course. Training role modeled the delivery of the program and included education about principles of self-management and working with groups.
In 2014, these health professional facilitators then recruited 4 to 6 people with MS each from their local area to attend the program. Recruitment was via advertisements in local MS society newsletters or via word of mouth through outreach personnel of the local MS society. Attendees were adult volunteers (age >18 years) diagnosed as having MS of any form or stage who felt they had sufficient cognitive and communication ability to participate in group discussions in English. Participants in this study volunteered from those who attended the MFML program in their local areas during the first half of 2014. Ethical approval was gained from the relevant institutional ethics committee, the University of Otago Human Ethics Committee (Health) H13/112, before commencement of the study. Participants provided written consent to participate in this study.
The program ran for approximately 2 hours, once per week, for 6 weeks. Each participant in the program received a workbook. Topics for discussion and reflection included MS-related fatigue, energy conservation strategies, exercise, medication and diet, and strategies for maximizing life via planning and prioritization or delegation of tasks. The workbook includes reflection and planning forms (a fatigue diary, weekly planner sheet, and weekly meal planner), which participants completed as homework. Participants were also encouraged to draw on the personal experiences of other people with MS, written as case illustrations in the workbook, and on other attendees' experiences to reflect on their own experiences of life with MS and to identify and trial personal strategies to better manage their fatigue.
Data Collection
On behalf of the researchers, the health professional facilitators handed participants a set of written self-report questionnaires to be completed independently by each participant at three different time points: 1) 1 month before the program commenced (time 1 [T1]), 2) at the start of the program (time 2 [T2]), and 3) at the conclusion of the program (time 3 [T3]). These time points were chosen to investigate the stability of the questionnaire scores before the intervention and change in score after the intervention. Thereby, participants acted as their own controls. The questionnaires included the 5-item Modified Fatigue Impact Scale (MFIS-5),24 the MS Self-efficacy Scale (MSSES),25 and the 12-item Short Form Health Status Survey (SF-12).26 These questionnaires were chosen because they measure outcomes of interest (ie, fatigue, self-efficacy, and quality of life) and for their brevity.
Impact of fatigue was measured using the MFIS-5, identified by the Multiple Sclerosis Quality of Life Inventory.27 This is a compact 5-item version shown to correlate well with the original 40-item Fatigue Impact Scale24 and its shortened 21-item scale.28 Both of these scales have shown excellent reliability and validity.29,30 Each item ranges from 0 (never) to 4 (almost always), with higher scores indicating greater fatigue.
The MSSES is a 14-item questionnaire generated as a disease-specific measure of self-efficacy. This outcome measure is completed based on the individual's disagreement or agreement with 14 statements using a 6-point scale ranging from strongly disagree to strongly agree.31 The scale is reliable internally, with a Cronbach alpha of 0.81.31
The SF-1226 is a commonly used quality of life questionnaire. It was developed as a shorter version of the Medical Outcomes Study SF-36.32 The SF-12 is a 12-question assessment that addresses the eight domains covered in the SF-36. However, due to the measure being abbreviated, there are two composite scores that represent the physical component summary and the mental component summary derived from the SF-36.33 Physical and mental component summary measures can be compared with US norm-based scores, with scores greater than 50 indicating better health.26 Participants in this study completed the SF-12 (v1), modified slightly in its font to make it easier for people with MS with visual impairment to use.
Data Analysis
Data were screened and cleaned, and reverse coding of negatively worded items in the MSSES was undertaken. Demographic data were analyzed descriptively. Paired t tests were used to compare the results between T1 and T2 and between T2 and T3. Any difference with a probability value less than .05 was considered to be statistically significant, with standardized effect sizes reported. Data analysis was undertaken using IBM SPSS Statistics for Windows, version 22-x (IBM Corp, Armonk, NY). The mean difference in scores, and the standard deviation of those differences, yielded a measure of the stability of the scales before the intervention began (T1 and T2) and allowed for comparing the scores immediately before and after the program (T2 and T3).
Results
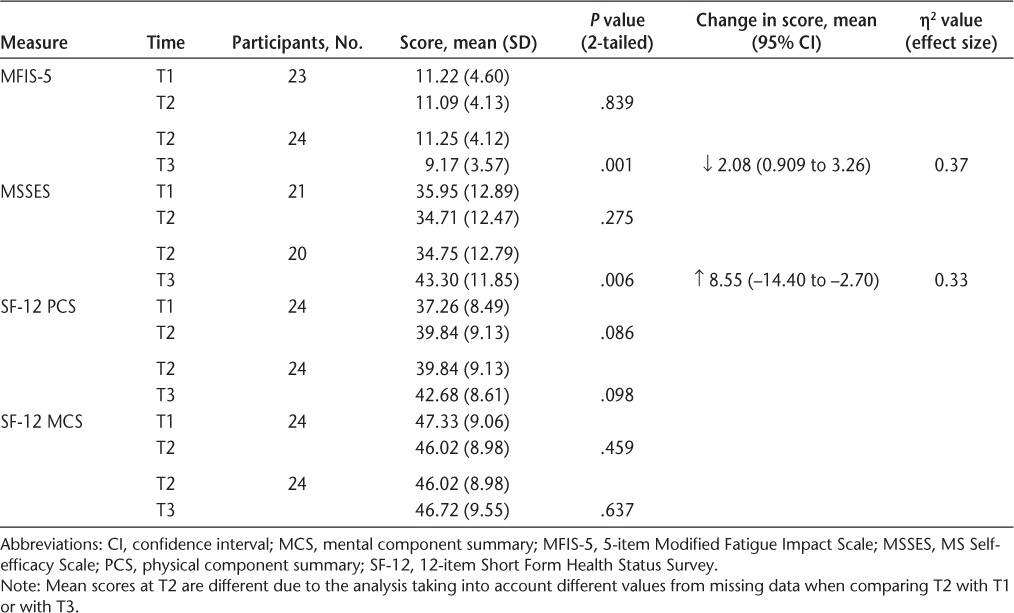
A total of 26 individuals participated in the program, and 25 consented to be involved in this study. One participant did not complete the full range of questionnaires, and these data are not included, leaving 24 participants' data for analysis. A few of these 24 participants did not answer all the questions on the MFIS-5 and the MSSES, resulting in different n values in the respective analyses of these measures. Participants were all women and ranged in age from 37 to 63 years (mean [SD], 49.29 [8.12] years). More than 60% of the sample reported their diagnosis as relapsing-remitting MS. The range of time since diagnosis was large (2–40 years), with a mean of nearly 12 years. Participants had a range of mobility impairments, and some required assistance with transport due to difficulty driving. Most identified as NZ European (n = 21; 87.5%). Other participant characteristics are summarized in Table 1. Evidence of stability of scores was noted between T1 and T2, with no statistically significant change in score for any of the measures. After the intervention, participants' scores showed a significant improvement in perceived impact of fatigue (P = .001) and self-efficacy (P = .006), with large effect sizes (Cohen's d) (η2 = 0.37 and 0.33, respectively),34 but no changes were seen in the physical and mental component summary scores of the SF-12 quality of life measure (Table 2).
Table 1.
Participant characteristics (N = 24)
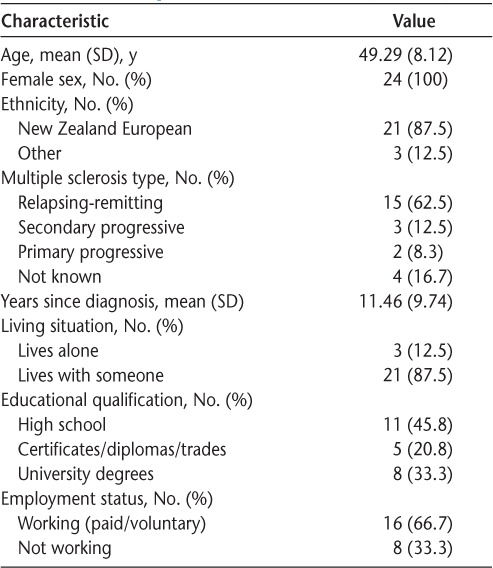
Table 2.
Results of paired t tests for impact of fatigue, self-efficacy, and quality of life measures between time 1 (T1) and T2 (before the intervention) and between T2 and T3 (after the intervention)
Discussion
A point of difference for the MFML program compared with other fatigue management programs was that it was developed using a participatory action research method, partnering with individuals living with MS.18,19 This newly developed fatigue self-management program reduced the perceived impact of fatigue and improved self-efficacy for the study participants and, therefore, warrants further research. The intent of the program was to build self-efficacy through education and support from others who understand living with MS and, thereby, help attendees develop their own strategies to manage their fatigue on a daily basis. The improvements in perceived impact of fatigue and self-efficacy reflect these aspects.
No change was shown in participants' physical or mental component summary scores of the quality of life measure (SF-12). Quality of life is a broad concept. Although the SF-12 contains questions related to physical function, it includes only one question related specifically to an individual's energy levels. Because MS is a long-term and progressive condition, participants' level of physical function would not, therefore, be expected to improve. In addition, having only one question about energy in the SF-12 does not necessarily capture the multifaceted nature of the fatigue experienced by people with MS. Both of these dimensions seem to be borne out by the results of the SF-12 in the present study, suggesting that the SF-12 was not a useful measure for this study. Mental component summary scores were, however, comparable with the average for the general population in the United States35 as well as in the 1996–1997 NZ Health Survey,36 suggesting that the fatigue experienced in MS is not necessarily indicative of depression. We suggest, therefore, that the SF-12 was not a useful measure for this population.
The literature identifies other fatigue management programs, some of which10–12,37 seem to be modified versions of an energy conservation course by Packer et al.38 Others have used a cognitive-behavioral approach to fatigue management.13,39 In addition, a group of researchers published their protocol for a study examining fatigue management using a cognitive-behavioral approach.40 Although these studies reported the efficacy of fatigue management programs via reductions in the impact of fatigue and improvements in self-efficacy or quality of life, the actual measures used were not the same as those used in the present study. Therefore, it is difficult to directly compare the results.
In future studies and to compare results internationally, we recommend that outcome measures be selected from the newly established Multiple Sclerosis Task Force.41 For example, a measure that may have been useful in the present study is the MS Quality of Life-54 (MSQOL-54) questionnaire. This is an MS-specific, health-related quality of life measure and is composed of the SF-36 and 18 other disease-specific items.42,43 The Task Force highly recommends this measure for its excellent psychometrics and clinical utility in the MS population across all levels of disability and disease severity.41 It has also been recommended for use in MS research.41 In a recent study,42 the potential prognostic value of this measure was investigated. It was compared with the outcomes of the Expanded Disability Status Scale, the Fatigue Severity Scale, and the Hamilton Rating Scale for Depression at two time points 3 years apart. It was found that the MSQOL-54 predicted changes in Expanded Disability Status Scale and Hamilton Rating Scale for Depression scores over the 3 years.42 Another study of the MSQOL-54 prognostic capabilities showed that the various domains of the questionnaire can be used to predict a change in fatigue status.44 However, although this measure seems useful in that it measures different aspects of life with MS, because of its length, we suggest that a shortened version would have the potential to provide a standardized measure for use in research and clinical practice with less burden of fatigue to individuals who already experience fatigue.
The MSSES measure used in this study contains double-negative questions that participants reported as challenging (in individual interviews after the study). For a questionnaire to accurately reflect the impact of a study, it is important that participants understand the questions. Cognitive impairment can be a feature of MS. It may, therefore, be worth considering cognitive screening for participants in future research of this nature. However, level of cognition should not be used as an exclusion criterion for participation in the program (outside of research) for individuals who want to participate. Individuals with MS are likely to gain some benefit from attending the program regardless of cognitive deficit as long as they are able to participate.
One factor that may have influenced the effectiveness of MFML was its effect on participants with varying time since diagnosis. It would be interesting to see whether participants with a longer time since diagnosis would gain more benefit than those with a shorter time since diagnosis, or vice versa. However, the sample was too small for this sort of comparison. Furthermore, it may be of benefit to establish whether people with differing lengths of diagnosis would place importance on different aspects of the program.
Other limitations of this study included that all the participants were women and, therefore, were not representative of those with MS in general. Indeed, it could be argued that the results may be attributable to positive social interaction arising from participants simply attending the program rather than from the intervention itself. In addition, participants were not randomly selected but volunteered to take part in the study. This may also have contributed to the positive results of this study because volunteers are more likely to be receptive to behavior change. However, by measuring at two different time points before the intervention (T1 and T2), participants effectively acted as their own controls. That the questionnaire scores showed a stable state between these two time points suggests that volunteering for the study did not influence the results of the study. Although there are validated ways to ascertain readiness for behavior change, and a program such as MFML could measure potential attendees' readiness for change, it could be argued that people who volunteer to receive a fatigue management program do so because they are ready and willing to address their fatigue. Therefore, we suggest that the study results reflect the benefits of the MFML program to the participants, adding to the existing literature on fatigue management for people with MS. Future work should incorporate randomization and some form of control group, such as a wait-list control group.
Conclusion
Despite the relatively small sample size, this study demonstrated the benefits of the MFML fatigue self-management program. This program provides an organized approach to the management of fatigue for people living with MS in NZ. For future studies and in clinical practice, it is recommended that outcome measures be selected from the Multiple Sclerosis Task Force to provide a basis for comparison with other interventions for fatigue management. Furthermore, because of the participatory approach used in the development of MFML, it has the potential to be adapted using the same approach for people living with other long-term conditions who experience fatigue.
PracticePoints.
Accessible and organized approaches to the management of fatigue for people living with MS are required.
A group-based program using the principles of self-management can empower patients with MS to have confidence in their ability to manage the impact of fatigue on their lives.
Researchers and clinicians, where applicable, should choose outcome measures from the MS Task Force recommendations to facilitate international comparison of an intervention's efficacy.
Acknowledgments
We thank Dr. Jonathan Williman, biostatistician, for statistical advice. Special thanks go also to the health professional facilitators for delivering the program and to the participants for taking part in the study.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: This work was supported by a University of Otago research grant.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Alla S, Mason DF. Multiple sclerosis in New Zealand. J Clin Neurosci. 2014;21:1288–1291. doi: 10.1016/j.jocn.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Navipour H, Madani H, Mohebbi MR, Navipour R, Roozbayani P, Paydar A. Improved fatigue in individuals with multiple sclerosis after participating in a short-term self-care program. NeuroRehabilitation. 2006;21:37–41. [PubMed] [Google Scholar]
- 4.Cook KF, Bamer AM, Roddey TS, Kraft GH, Kim J, Amtmann D. Multiple sclerosis and fatigue: understanding the patient's needs. Phys Med Rehabil Clin N Am. 2013;24:653–661. doi: 10.1016/j.pmr.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flachenecker P, Kumpfel T, Kallmann B et al. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler. 2002;8:523–526. doi: 10.1191/1352458502ms839oa. [DOI] [PubMed] [Google Scholar]
- 6.Bakshi R. Fatigue associated with multiple sclerosis: diagnosis, impact and management. Mult Scler. 2003;9:219–227. doi: 10.1191/1352458503ms904oa. [DOI] [PubMed] [Google Scholar]
- 7.Asano M, Finlayson ML. Meta-analysis of three different types of fatigue management interventions for people with multiple sclerosis: exercise, education, and medication. Mult Scler Int. 2014;2014 doi: 10.1155/2014/798285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacAllister WS, Krupp LB. Multiple sclerosis-related fatigue. Phys Med Rehabil Clin N Am. 2005;16:483–502. doi: 10.1016/j.pmr.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Shevil E, Finlayson M. Process evaluation of a self-management cognitive program for persons with multiple sclerosis. Patient Educ Couns. 2009;76:77–83. doi: 10.1016/j.pec.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Finlayson M. Pilot study of an energy conservation education program delivered by telephone conference call to people with multiple sclerosis. NeuroRehabilitation. 2005;20:266–277. [PubMed] [Google Scholar]
- 11.Mathiowetz V, Finlayson ML, Matuska KM, Chen HY, Luo P. Randomized controlled trial of an energy conservation course for persons with multiple sclerosis. Mult Scler J. 2005;11:592–601. doi: 10.1191/1352458505ms1198oa. [DOI] [PubMed] [Google Scholar]
- 12.Mathiowetz V, Matuska KM, Murphy ME. Efficacy of an energy conservation course for persons with multiple sclerosis. Arch Phys Med Rehabil. 2001;82:449–456. doi: 10.1053/apmr.2001.22192. [DOI] [PubMed] [Google Scholar]
- 13.Moss-Morris R, McCrone P, Yardley L, van Kessel K, Wills G, Dennison L. A pilot randomised controlled trial of an Internet-based cognitive behavioural therapy self-management programme (MS Invigor8) for multiple sclerosis fatigue. Behav Res Ther. 2012;50:415–421. doi: 10.1016/j.brat.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 15.Jones H, Berard LD, MacNeill G, Whitham D, Yu C. Self-management education. Can J Diabetes. 2013;37(suppl 1):S26–S30. doi: 10.1016/j.jcjd.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Lorig K, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 17.Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001;24:561–587. doi: 10.2337/diacare.24.3.561. [DOI] [PubMed] [Google Scholar]
- 18.Mulligan H, Snowdon J, Tapper L. Consumer involvement in the development of a programme to support behaviour change: the Canterbury Fatigue Project. Paper presented at: Rehabilitation in Multiple Sclerosis (RIMS) 19th Annual Conference; June 6–7, 2014; Brighton, UK.
- 19.Mulligan H, Lusty A, Delorme A, Bong S, Wilkinson A. Consumers and health professionals' perceptions of Participatory Action Research in developing a health resource. N Z J Physiother. 2015;43:93–97. [Google Scholar]
- 20.Mulligan H, Wilkinson A, Snowdon J. Perceived impact of a self-management program for fatigue in multiple sclerosis: a qualitative study. Int J MS Care. 2016;18:27–32. doi: 10.7224/1537-2073.2014-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene J. Mixed Methods in Social Inquiry. San Francisco, CA: Jossey-Bass; 2007. [Google Scholar]
- 22.Greene J, Benjamin L, Goodyear L. The merits of mixing methods in evaluation. Evaluation. 2001;7:25–44. [Google Scholar]
- 23.Greene J, Caracelli V, Graham W. Toward a conceptual framework for mixed-method evaluation designs. Educ Eval Policy Analysis. 1989;11:255–274. [Google Scholar]
- 24.Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(suppl 1):S79–S83. doi: 10.1093/clinids/18.supplement_1.s79. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz CE, Coulthard-Morris L, Zeng Q, Retzlaff P. Measuring self-efficacy in people with multiple sclerosis: a validation study. Arch Phys Med Rehabil. 1996;77:394–398. doi: 10.1016/s0003-9993(96)90091-x. [DOI] [PubMed] [Google Scholar]
- 26.Jakobsson U, Westergren A, Lindskov S, Hagell P. Construct validity of the SF-12 in three different samples. J Eval Clin Pract. 2012;18:560–566. doi: 10.1111/j.1365-2753.2010.01623.x. [DOI] [PubMed] [Google Scholar]
- 27.Consortium of Multiple Sclerosis Centers Health Services Research Subcommittee. MSQLI: Multiple Sclerosis Quality of Life Inventory: A User's Manual. New York, NY: National Multiple Sclerosis Society; 1997. [Google Scholar]
- 28.Tellez NJR, Tintore M, Nos C, Galan I, Montalban X. Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? Mult Scler J. 2005;11:198–202. doi: 10.1191/1352458505ms1148oa. [DOI] [PubMed] [Google Scholar]
- 29.Osborne LA, Noble GJ, Maramba IDC et al. Outcome measures for multiple sclerosis. Phys Ther Rev. 2014;19:24–38. [Google Scholar]
- 30.Kos D, Kerckhofs E, Ketelaer P et al. Self-report assessment of fatigue in multiple sclerosis: a critical evaluation. Occup Ther Health Care. 2004;17:45–62. doi: 10.1080/J003v17n03_04. [DOI] [PubMed] [Google Scholar]
- 31.Rigby SA, Domenech C, Thornton EW, Tedman S, Young CA. Development and validation of a self-efficacy measure for people with multiple sclerosis: the Multiple Sclerosis Self-efficacy Scale. Mult Scler. 2003;9:73–81. doi: 10.1191/1352458503ms870oa. [DOI] [PubMed] [Google Scholar]
- 32.Ware JE., Jr. SF-36 health survey update. Spine. 2000;25:3130–3139. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 33.Rehabilitation Institute of Chicago. Rehabilitation measures database: short form 12 item (version 2) health survey. http://www.rehabmeasures.org/Lists/RehabMeasures/PrintView.aspx?ID=1149. Published 2010. Accessed December 2014.
- 34.Pallant JF. SPSS Survival Manual: A Step-by-Step Guide to Data Analysis Using SPSS for Windows (Version 15) 3rd ed. Sydney, Australia: Allen & Unwin; 2007. [Google Scholar]
- 35.Ware JE, Snow KK, Kosinski M. Health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute; 1993. [Google Scholar]
- 36.Ministry of Health. Taking the Pulse: 1996–97 New Zealand Health Survey SF-36 Health Status Questionnaire: Demographic and Socioeconomic Variables. http://www.moh.govt.nz/notebook/nbbooks.nsf/0/B5DEDA9A12DACE3B4C25677D00720599/$file/ttp1.pdf. Accessed December 2014.
- 37.Vanage SM, Gilberston KK, Mathiowetz V. Effects of an energy conservation course on fatigue impact for persons with progressive multiple sclerosis. Am J Occup Ther. 2003;57:315–323. doi: 10.5014/ajot.57.3.315. [DOI] [PubMed] [Google Scholar]
- 38.Packer TL, Brink N, Sauriol A. Managing Fatigue: A Six Week Course for Energy Conservation. Tucson, AZ: Therapy Skill Builders; 1995. [Google Scholar]
- 39.van Kessel K, Wouldes T, Moss-Morris R. A New Zealand pilot randomised controlled trial of a web-based interactive self-management programme (MSInvigor8) with and without email support for the treatment of multiple sclerosis fatigue [published online May 7, 2015] Clin Rehabil. doi: 10.1177/0269215515584800. [DOI] [PubMed] [Google Scholar]
- 40.Thomas PW, Thomas S, Kersten P et al. Multi-centre parallel arm randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based cognitive behavioural approach to managing fatigue in people with multiple sclerosis. BMC Neurol. 2010;10:43. doi: 10.1186/1471-2377-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Potter K, Cohen ET, Allen DD et al. Outcome measures for individuals with multiple sclerosis: recommendations from the American Physical Therapy Association Neurology Section Task Force. Phys Ther. 2014;94:593–608. doi: 10.2522/ptj.20130149. [DOI] [PubMed] [Google Scholar]
- 42.Kisic Tepavcevic D, Pekmezovic T, Stojsavljevic N et al. Predictive value of health-related quality of life in progression of disability and depression in persons with multiple sclerosis: a 3-year study. Acta Neurol Belg. 2013;113:403–409. doi: 10.1007/s13760-013-0191-9. [DOI] [PubMed] [Google Scholar]
- 43.Preedy V. Multiple Sclerosis Quality of Life-54 Questionnaire. In: Kreutzer J, DeLuca J, Caplan B, editors. Encyclopedia of Clinical Neuropsychology. New York, NY: Springer; 2011. pp. 1684–1685. [Google Scholar]
- 44.Drulovic J, Bursac LO, Milojkovic D, Tepavcevic DK, Gazibara T, Pekmezovic T. MSQoL-54 predicts change in fatigue after inpatient rehabilitation for people with multiple sclerosis. Disabil Rehabil. 2013;35:362–366. doi: 10.3109/09638288.2012.704122. [DOI] [PubMed] [Google Scholar]


