Abstract
Background: Multiple sclerosis (MS) is an inflammatory and neurodegenerative disease of the central nervous system. Fatigue is a common and disabling symptom, often causing decreased quality of life, social withdrawal, and unemployment. We developed and studied the feasibility of a cognitive-behavioral group intervention to manage fatigue in MS. We aimed to integrate the concepts of cognitive-behavioral therapy and evidence-based patient information.
Methods: We conducted patient interviews and a focus group to assess patients' interest in and need for fatigue self-management training and developed the program accordingly. The program consists of six 90-minute modules, which were structured with the use of moderation cards, helping to guarantee treatment fidelity. The program was tested on three pilot groups (N = 16) in a rehabilitation center. Fatigue, depression, and coping self-efficacy were assessed at baseline and after the intervention. Acceptance and general satisfaction with the program were also evaluated.
Results: Patient interviews elicited different characteristics of fatigue, suggesting that patients had different requirements. The program was very acceptable to patients. Pre-post assessments of the pilot study showed significantly improved scores on the Coping Self-Efficacy Scale (P = .013) but not on the Fatigue Scale for Motor and Cognitive Functions and the 30-item Inventory of Depressive Symptomatology.
Conclusions: These preliminary results suggest that this program is a feasible cognitive-behavioral group training program that may improve coping self-efficacy and has the potential to subsequently reduce fatigue. The next step is evaluation of the program in a randomized controlled trial.
Multiple sclerosis (MS) is a poorly understood neurodegenerative disease that usually starts in young adulthood. Disease progression is unpredictable, and patients experience a wide range of symptoms, including visual impairment, muscle weakness, sensory problems, and pain.1 Fatigue is a common symptom that 75% to 95% of patients struggle with at some point in the course of the disease.2 Many patients consider fatigue to be their most disabling symptom, even more so than weakness or pain.3 Fatigue is a common reason for unemployment and early retirement4 and often negatively affects activities of daily living, such as doing housework and attending social events.5,6 Fatigue can be triggered by mental or physical exertion, but it can also occur without a trigger.7
Although there are several theories of fatigue in MS, the causes of fatigue are still poorly understood. It has been postulated that there are primary and secondary mechanisms that lead to fatigue.8 Primary fatigue is defined as a direct sequel of physical changes to the body caused by the disease process. Primary proposed mechanisms include axonal loss or injury,9 functional reorganization of damaged brain areas, and increased functional recruitment of other brain areas,10 as well as immunologic and neuroendocrine factors.11 Secondary fatigue is due to other factors that can cause or worsen fatigue, including sleep problems, depression, stress, MS-specific drugs, and reduced physical activity.12,13 All these factors have explained only a small percentage of the variance in the presentation of fatigue. Van Kessel and Moss-Morris14 developed a biopsychosocial model of MS fatigue that proposes that primary factors trigger fatigue. Patients' emotional, behavioral, cognitive, and physiological responses worsen and perpetuate fatigue. Changes to circadian rhythms through lack of routine and disrupted sleep-wake patterns also perpetuate fatigue.14
Currently, no approved medical treatment is available for MS-related fatigue in Germany,15 and drug trials to date suggest poor efficacy.16 However, behavioral treatment approaches have shown encouraging effects. The most promising results have been shown by various patient education programs that teach patients ways of managing daily life with fatigue.17 Most studies were based on cognitive-behavioral therapy (CBT)18 and energy conservation techniques.19 The former is based on the premise that cognitions, behaviors, emotions, and physiology all interact and that changes in one of these systems can affect another. Techniques for CBT include exposure, behavioral experiments, relaxation, and social skills training.20
Despite the existence of a couple of patient education programs and CBT approaches, currently no highly standardized group intervention is available in German. Van Kessel et al.18 conducted a randomized controlled study of 72 participants that showed a significant reduction in fatigue levels in patients with MS compared with a relaxation group below those of the control group. Follow-up data demonstrated sustained fatigue levels after 6 months. We aimed to develop and pilot a program based on the CBT intervention described by Van Kessel et al. to be implemented in rehabilitation clinics in Germany even if the clinics do not specialize in MS. The program was highly standardized and structured by means of moderation cards, containing detailed instructions for the facilitator, to make it easily implemented by trained caregivers such as nurses.
Materials and Methods
The study was approved by the Ethics Committee of the Hamburg Chamber of Physicians. All the participants received information about and an explanation of the study and signed an informed consent form before participation.
Patient Interviews/Focus Group
To tailor the fatigue-management program to patients' needs, we conducted four single face-to-face patient interviews. The interviews took 17 to 43 minutes to conduct and contained open-ended questions on the topics of 1) individual characteristics of fatigue, 2) patients' strategies for managing fatigue, 3) needs and expectations regarding the content of a program targeting fatigue management, and 4) patients' wishes concerning training time scheduling and travel expenses as well as homework load. All the participants were asked to speak freely to enable us to assess their personal situations as closely as possible. The interviews were audio-recorded and transcribed verbatim.
Inclusion criteria for the patient interviews were a diagnosis of MS by McDonald criteria,21 age of 18 years or older, and a score of 4 or higher on the fatigue subscale of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis.22 There were no exclusion criteria. Eligible patients with MS were identified via the MS outpatient clinic database of University Medical Center Hamburg-Eppendorf (Hamburg, Germany) and then were contacted by telephone and invited to participate.
In addition to the interviews, a focus group (n = 12, same inclusion criteria) addressed the same questions as the interviews. Focus group participants had not previously taken part in the patient interviews. We chose a roundtable setting for the group discussion that invited participants to feel relaxed and to facilitate the expression of ideas and thoughts. The discussion was audio-recorded and transcribed. We used framework analysis methods according to Mayring23 to categorize all the collected data systematically. The qualitative content analysis was primarily inductive. Theoretical and practical interpretations of the findings were developed, and a summary table was compiled. One researcher (MJW) transcribed and categorized the statements. This list was then discussed by two of us (SL and JP) based on our views of the transcripts. The transcripts were double-checked, and negotiations of conflicts were discussed with the senior author (CH). The same inclusion criteria and procedures were applied for the focus group as for the interviews. The major aim of the qualitative work was to clarify the relevance of concepts drawn from the literature as well as provide input for offering fatigue-management approaches in a group setting.
Description of the Intervention
The “Fatigue Management in MS” program (FatiMa) was developed based on an existing CBT manual obtained from Van Kessel et al.18 and on results from the patient interviews and the focus group. Findings from the interviews/focus group that concerned time issues (ie, travel, session length, and homework) were considered, and the program was shaped accordingly. We added information on evidence concerning MS-related fatigue and findings from a recently performed meta-analysis on fatigue-management interventions (MJ Wendebourg, C Heesen, J Poettgen, S Köpke, unpublished data). The FatiMa includes six highly structured and documented modules based on Microsoft PowerPoint (Microsoft Corp, Redmond, WA) presentations. To enhance interaction with and learning from peers, FatiMa is presented in small-group sessions of six to eight patients. The facilitator uses printed cards that give instructions as to what the facilitator should say and do at different points of the session to ensure standardization of the conduct of the program. The key content includes information about causes of fatigue and treatment evidence, sleep, activity and rest, symptom management, stress, and managing thoughts and behavior. A fundamental component of FatiMa is working with examples and transferring knowledge to enhance self-reflection abilities. In each session, participants are asked to freely engage in conversations. Each session begins with a recapitulation of the previous session to clarify possible questions, followed by a discussion of the homework. All the sessions are facilitated by a health-care professional, who can be an MS-specialized nurse or an occupational therapist. Based on previous education programs with trained trainers, we are confident that different health-care professionals can conduct the program.24,25
Patients are also provided with tools—for example, diaries, timetables, and activity lists—to support the transfer of goals to daily life. For homework, patients are invited to develop their own fatigue model and to figure out personal triggers to develop coping strategies.
A comprehensive manual gives the trainer instructions for facilitation of the group, as well as elaborate background knowledge for each module, its scientific basis, and explanations of the mode of action required by each part of the program.
At the beginning of the group, participants are asked to present parts of their homework to the group to share mutual findings and experiences but also to discuss differences. After this introduction, the topic of the respective session is introduced. Two fictional characters with MS demonstrate unhelpful ways of managing fatigue, for example, lack of physical activity, excessive daytime napping, and negative thoughts. Participants are invited to share their own experiences. Sharing experiences and strategies with peers facilitates discussion of more helpful alternatives in a supportive atmosphere. In the following practical part of each session, participants try out newly acquired strategies. At the end, the homework for the next session is explained (only in sessions 1–5).
At the end of each session, patients receive a summary of the content and the homework sheet. Each session lasts approximately 90 minutes, including a 10-minute break.
Session 1 is education oriented and gives an overview of current knowledge on causes of and treatment options for MS fatigue based on a systematic literature review, indicating that the actual program is based on one of the most convincing studies (MJ Wendebourg, C Heesen, J Poettgen, S Köpke, unpublished data). Homework includes answering questions on personal fatigue characteristics. Participants are encouraged to think about which aspects of their life are limited owing to fatigue.
In session 2, participants use the answers from the homework sheet to build their own fatigue model that lists potential causes and fatigue-perpetuating factors, such as negative thinking and over-resting. Every participant develops his or her own fatigue model individually, depending on which factors apply to him or her. The facilitator helps when questions or difficulties arise.
The first part of session 3 focuses on sleep hygiene and the importance of a balance between activity and rest. Patients are encouraged to set personal goals concerning activity and rest during preplanned times of the day. These plans are transferred to daily life as homework.
The educational part of session 4 explains MS symptoms and how the way patients think about their symptoms may worsen fatigue; some thoughts about fatigue symptoms can be unhelpful and can negatively influence patients' perceived feeling of control over their lives, thereby perpetuating fatigue. As homework, patients use a symptom diary in which they write down every discomfort they experience during the day and reflect about reasons for the discomfort—both MS related and not.
In session 5, participants are taught the concept of identifying unhelpful thoughts and behavior patterns and actively finding alternative thoughts and behavior patterns. In the exercise part, participants are encouraged to look for their own negative thoughts or unhelpful behaviors. As homework, they are encouraged to find alternative thoughts and to practice implementing these new thoughts in their daily routine.
Session 6 focuses on stress-relieving techniques and the importance of social support that patients can use in times of need (eg, friends, household helpers, and professional medical help).
All homework is subject to group discussion in the next session, thereby promoting the exchange of thoughts, perceived difficulties, and solutions by learning from peers. It is emphasized that daily practice during and after the program is crucial to reducing fatigue. The contents of the sessions are summarized in Table 1.
Table 1.
Overview of the contents of the FatiMa sessions

Feasibility Groups
The study aimed to evaluate the feasibility of FatiMa in a rehabilitation context to assess participants' acceptance of the training curriculum and to pilot possible outcome parameters for a subsequent trial. We invited patients from an associated inpatient and outpatient rehabilitation center to participate in the program because in a pure outpatient setting it might be difficult for patients to participate after work over a 3-week period.
The inclusion criteria were a definite diagnosis of MS according to the McDonald criteria21 and self-perceived problems with fatigue as assessed by a standard admission questionnaire that is administered to all patients when enrolling in the rehabilitation program. It asks whether the participant struggles with fatigue at the time of admission to the rehabilitation center. Patients with MS were contacted by the staff of the rehabilitation center.
Outcome Measures
To assess changes in fatigue, we used the Fatigue Scale for Motor and Cognitive Functions (FSMC).26 To assess changes in coping behavior, we applied the 13-item short form of the Coping Self-Efficacy Scale,27 which provides a measure of a person's perceived ability to select the adequate coping strategy for a given challenge and has recently been applied in MS.28
Depression is a common correlate of fatigue. Questionnaires help distinguish between these two frequent MS symptoms that may present in a similar way. A change in cognition and behavior as it is taught in CBT approaches may ameliorate depression as well as fatigue. To assess depressive symptoms, participants filled out the 30-item version of the Inventory of Depressive Symptomatology (IDS-30).29
All the data were collected by the staff of the rehabilitation center. Baseline data were collected on the day that patients entered the rehabilitation center after consenting to participate in the pilot study. Postintervention data were collected on the last day of the FatiMa sessions (session 6).
Patient Evaluation
To assess satisfaction with the program, we used a 17-item self-developed questionnaire that was previously used in a pilot trial of metacognitive training.30 Items are rated on a 5-point Likert scale ranging from 0 (“not at all”) to 4 (“maximal”). Additional open-format items asked for positive and negative thoughts about the program and recommendations for improvements. Finally, patients were asked to give an overall score for the whole program (ranging from 1 = very good to 6 = unsatisfactory).
Statistical Analysis
Statistical analysis was completed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp, Armonk, NY). Considering the small sample size and the lack of normally distributed data, we conducted nonparametric descriptive statistics and tests. We report aggregated questionnaire sum scores using the median. Testing for differences between pre-post measures, we conducted Wilcoxon signed rank tests. No calculation for a saturated sample size was conducted. Analysis of the evaluation is based on 16 patients. There were no missing data.
Results
Patient Interviews/Focus Group
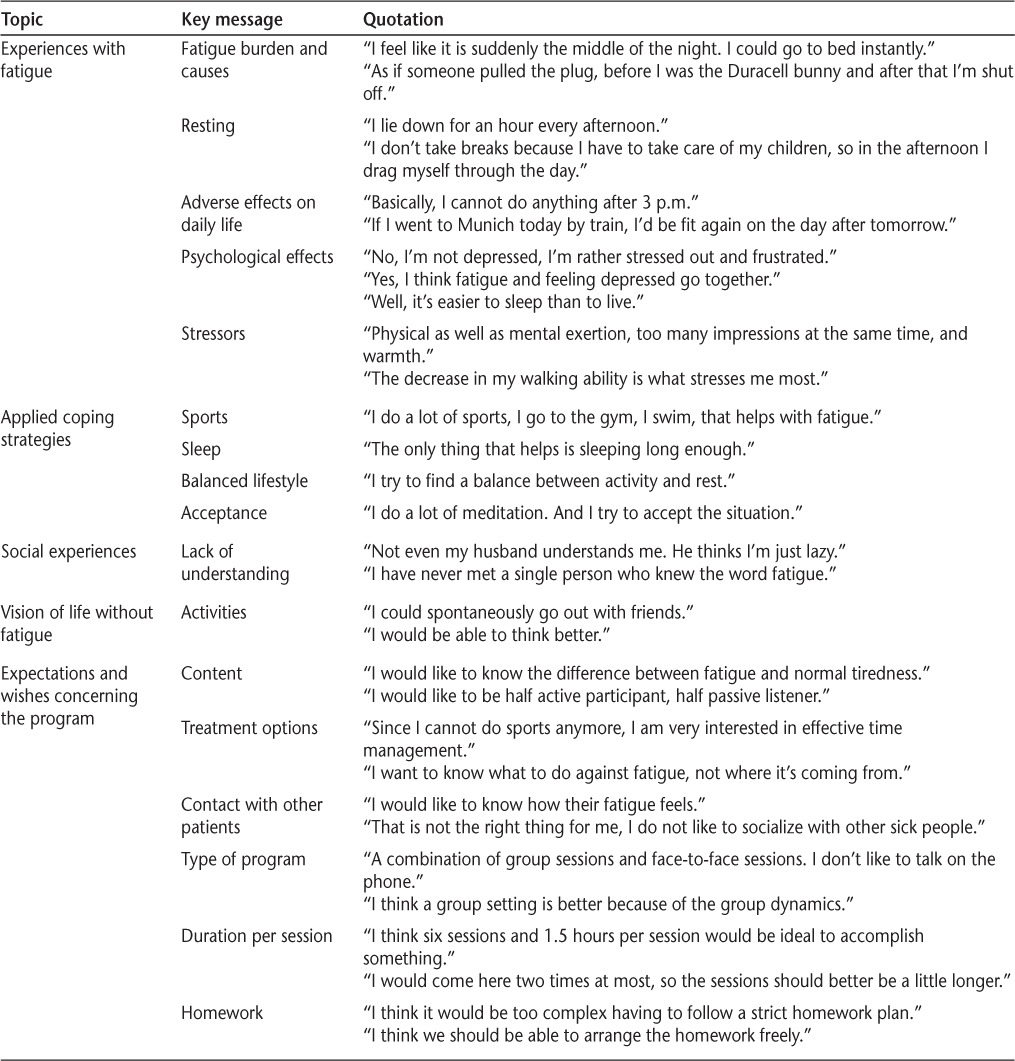
Overall, 16 patients were included in the qualitative part of the study, consisting of face-to-face interviews (n = 4; three women and one man) and a focus group (n = 12; ten women and two men). Patients described a variety of symptoms and characterized fatigue differently. However, there was also substantial agreement: all the patients expressed an unmet need for fatigue-management training. All but one patient preferred a group setting to individual face-to-face training. None wanted to use telephone counseling as a means of communication in the training. Four patients indicated a preference to have a passive role in the program (listen to the facilitator or other participants only, not be actively involved in any discussions); all others wanted to participate actively (tell other participants about their personal experiences, present their homework, and try activities such as diaphragmatic breathing). All the patients were interested in receiving guidance to reduce fatigue during the day and improve sleep hygiene. Two patients wanted information about treatment options. Some patients expressed worries that training might worsen fatigue because of long travel distances to the treatment center and too much homework. Table 2 gives an overview of the topics discussed and statements given by patients during the interviews and the focus group.
Table 2.
Topics, key messages, and quotations from face-to-face interviews and the focus group
Pilot Study
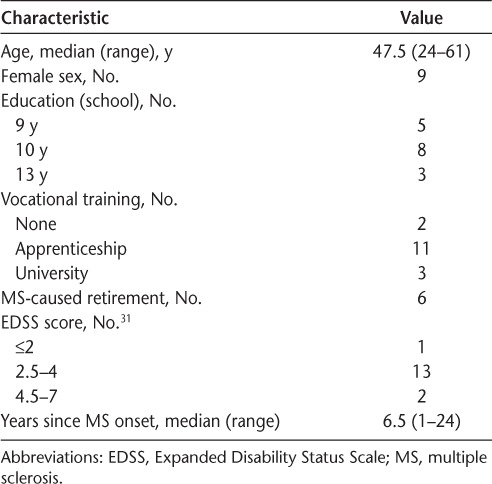
Between January and May 2013, 16 patients from an MS inpatient and outpatient rehabilitation center in Hamburg who had not previously taken part in the focus group participated in the pilot study. Participants were recruited by the rehabilitation center staff. Overall, patients participated in one of three FatiMa pilot trainings, with five to six patients per training. See Table 3 for pilot study patient characteristics. The training was conducted by a specially trained final-year medical student (MJW).
Table 3.
Sample characteristics of the 16 participants in the pilot study
The pilot study took place on Monday and Thursday mornings at 10:00 for 3 weeks. During the standard rehabilitation process, patients took part in regular activities, such as physical exercise and relaxation programs, together with all the other rehabilitation patients. The FatiMa sessions were easily integrated into patients' weekly plans based on the biweekly 1.5-hour sessions. Altogether, 11 inpatients and 5 outpatient rehabilitation patients were included. By implementing the FatiMa sessions in the rehabilitation process, long extra travel was avoided and attendance was ensured.
Effects of the Program
Baseline fatigue scores were high (Table 4). Patients' median score on the FSCM total was 78.00 (range, 20–100) at baseline and 81.00 after the intervention. Total score as well as subscale scores did not change significantly during the intervention.
Table 4.
Baseline and postintervention outcome measure scores
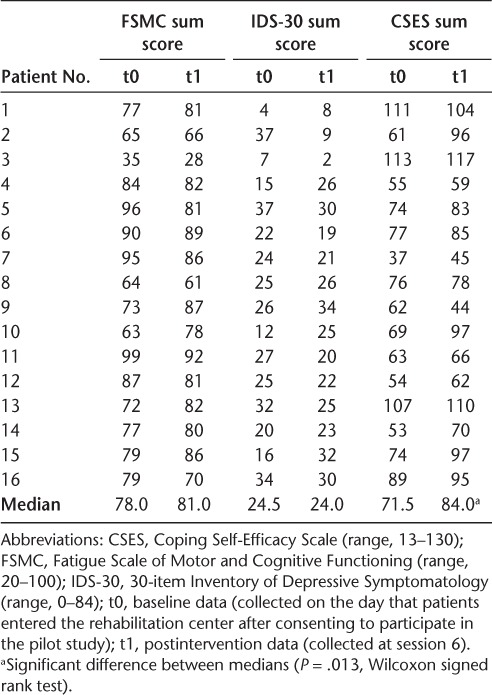
At baseline, 13 patients (81.25%) had IDS-30 scores of 14 or higher. The median baseline IDS-30 score was 24.50 (range, 0–84). The median postintervention IDS-30 score was 24.00, indicating no major effect of the training on depressive symptoms.
The baseline Coping Self-Efficacy Scale median score was 71.50, and the postintervention median score was 84.00, indicating a significant improvement in coping (P = .013). All but one patient showed improved coping self-efficacy.
Patient compliance was high, as only two participants missed one session each (attendance rate, 96.6%). There were no dropouts. On the evaluative questionnaire, patients assessed the training program positively, with a median of 56.2 of 68.0 points. The median overall program score was 2.1, that is, “good” (range, 1–3).
Discussion
We developed a group training program to treat fatigue in MS based on an established CBT face-to-face/telephone intervention.18 Results of patient interviews showed that there is a demand for patient education programs because patients were interested in learning coping strategies and other ways of managing fatigue. Because of the big differences in fatigue characteristics and patients' needs, developing a program that meets all patients' requirements proved to be a challenge.
Substantial sources of concern for the interviewed patients were long homework tasks and long travel distances that might induce fatigue and too many sessions. Thus, implementation in the neurorehabilitation setting ensured that participants had no extra travel time or additional burden of activity. When preparing homework sheets, we also made sure that homework did not take up too much time. We changed the face-to-face/telephone approach to a group session approach, which improves accessibility for patients and is in accordance with patients' wishes. Furthermore, there was a desire for communication with other patients experiencing fatigue. Regarding the content, the most relevant change was the emphasis on evidence-based patient information in session 1, educating participants on the origin of MS-related fatigue and current therapy approaches. A pilot study of patients from a collaborating outpatient rehabilitation center showed the program's feasibility. Although we found no improvement in fatigue and depression, coping self-efficacy was changed. There are some possible explanations for both findings. First, the pilot study was not powered to show significant treatment effects but to show feasibility. Second, 3 weeks might not be enough time for patients to integrate new behavior patterns into their daily routine and, therefore, may not be an adequate follow-up assessment time point to clarify effectiveness. A 3- or 6-month follow-up, as used by Van Kessel et al.,18 might show reduced fatigue levels due to better implementation in patients' daily life. Third, biweekly sessions give patients little time to do their homework, which might also negatively affect adherence to newly obtained fatigue-management strategies. Moreover, patients currently participating in a neurorehabilitation program, especially in an outpatient setting, might be too busy to keep up with their homework, for example, repeating exercises, working on alternative thoughts, or keeping a balanced schedule. Also, the travel to and from the rehabilitation center might have burdened patients. Furthermore, group training may be less effective than individual training. This may explain the discrepancy between improved coping strategies and no effect on perceived fatigue.
Similar to the present program, the pilot study by Thomas et al.,32 including 16 participants, found significantly improved levels of perceived self-efficacy but no change in fatigue severity. In a recent randomized controlled trial, patients showed significantly improved fatigue levels.18
Contrary to Van Kessel et al., who used the Chalder Fatigue Scale,33 we used the FSMC to measure fatigue because the Chalder Fatigue Scale is not currently available in German. There are no data available concerning sensitivity to change in FSMC scores over time. Thus, more discrete changes in fatigue may not have been detected by the FSMC. A larger study using a translated version of the Chalder Fatigue Scale may clarify whether treatment effects are comparable with the telephone-administered and face-to-face program. A validation study of the German version has recently been performed.34
The current literature on fatigue-management programs for MS-related fatigue demonstrates mostly moderate short-term effects in reducing fatigue. Still, many of these programs demand a substantial amount of time from both the patient and the facilitator by being face-to-face or telephone-based programs. In addition, trainer expertise is needed. Therefore, it might be hard to implement these concepts in regular patient care. We aimed to develop a program that could be implemented in standard German rehabilitation centers if effectiveness can be demonstrated in a randomized controlled trial. This cost-effectiveness and implementation perspective is a special feature of this approach.
PracticePoints.
Fatigue is a common and disabling symptom in MS, often causing decreased quality of life, social withdrawal, and unemployment.
We developed a highly standardized and documented fatigue-management group education program to be implemented in rehabilitation centers.
A feasibility study of the program showed positive effects on coping self-efficacy.
Evaluation of the program showed that most participants were overall highly satisfied with the length of sessions, the amount of information given per session, and the homework. Furthermore, participants were satisfied with their learning process.
Based on the present data, we decided not to change the approach but rather to conduct a larger trial for the following reasons. First, the program is based on changes in patients' thoughts and behaviors. For that reason, effects might be shown once participants have had time to practice new strategies to implement new thoughts and change well-established patterns to new ones. Therefore, follow-up data for a longer period may be needed to show intervention effects. Second, a change in the length of the program or the biweekly format would enhance travel time and increase the treatment time burden. In addition, a longer program would not be implementable in the rehabilitation context. Third, the current negative data might be due to the fact that the primary fatigue scale, the FSMC, is possibly not responsive enough to detect change. In fact, only a few studies have applied the FSMC as an intervention outcome. Another study might, therefore, apply another instrument as the primary endpoint.
In this pilot study, we demonstrated the feasibility of FatiMa. However, the effectiveness of FatiMa for fatigue has to be shown in a larger randomized controlled trial.
Acknowledgments
We thank Dr. Iris-Katharina Penner for her support and guidance.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Patti F, Vila C. Symptoms, prevalence and impact of multiple sclerosis in younger patients: a multinational survey. Neuroepidemiology. 2014;42:211–218. doi: 10.1159/000360423. [DOI] [PubMed] [Google Scholar]
- 2.Braley TJ, Chervin RD. Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment. Sleep. 2010;33:1061–1067. doi: 10.1093/sleep/33.8.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vucic S, Burke D, Kiernan MC. Fatigue in multiple sclerosis: mechanisms and management. Clin Neurophysiol. 2010;121:809–817. doi: 10.1016/j.clinph.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 4.Strober LB, Christodoulou C, Benedict RH et al. Unemployment in multiple sclerosis: the contribution of personality and disease. Mult Scler. 2012;18:647–653. doi: 10.1177/1352458511426735. [DOI] [PubMed] [Google Scholar]
- 5.Amato MP, Ponziani G, Rossi F, Liedl CL, Stefanile C, Rossi L. Quality of life in multiple sclerosis: the impact of depression, fatigue and disability. Mult Scler. 2001;7:340–344. doi: 10.1177/135245850100700511. [DOI] [PubMed] [Google Scholar]
- 6.Smith MM, Arnett PA. Factors related to employment status changes in individuals with multiple sclerosis. Mult Scler. 2005;11:602–609. doi: 10.1191/1352458505ms1204oa. [DOI] [PubMed] [Google Scholar]
- 7.Mills RJ, Young CA. A medical definition of fatigue in multiple sclerosis. QJM. 2008;101:49–60. doi: 10.1093/qjmed/hcm122. [DOI] [PubMed] [Google Scholar]
- 8.Krupp LB, Serafin DJ, Christodoulou C. Multiple sclerosis-associated fatigue. Expert Rev Neurother. 2010;10:1437–1447. doi: 10.1586/ern.10.99. [DOI] [PubMed] [Google Scholar]
- 9.Tartaglia MC, Narayanan S, Arnold DL. Mental fatigue alters the pattern and increases the volume of cerebral activation required for a motor task in multiple sclerosis patients with fatigue. Eur J Neurol. 2008;15:413–419. doi: 10.1111/j.1468-1331.2008.02090.x. [DOI] [PubMed] [Google Scholar]
- 10.Colombo B, Martinelli Boneschi F et al. MRI and motor evoked potential findings in nondisabled multiple sclerosis patients with and without symptoms of fatigue. J Neurol. 2000;247:506–509. doi: 10.1007/s004150070148. [DOI] [PubMed] [Google Scholar]
- 11.Heesen C, Nawrath L, Reich C, Bauer N, Schulz KH, Gold SM. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? J Neurol Neurosurg Psychiatry. 2006;77:34–39. doi: 10.1136/jnnp.2005.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kos D, Duportail M, D'hooghe M, Nagels G, Kerckhofs E. Multidisciplinary fatigue management programme in multiple sclerosis: a randomized clinical trial. Mult Scler. 2007;13:996–1003. doi: 10.1177/1352458507078392. [DOI] [PubMed] [Google Scholar]
- 13.Bol Y, Duits AA, Hupperts RM, Vlaeyen JW, Verhey FR. The psychology of fatigue in patients with multiple sclerosis: a review. J Psychosom Res. 2009;66:3–11. doi: 10.1016/j.jpsychores.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Van Kessel K, Moss-Morris R. Understanding multiple sclerosis fatigue: a synthesis of biological and psychological factors. J Psychosom Res. 2006;61:583–585. doi: 10.1016/j.jpsychores.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Pucci E, Brañas Tato P, D'Amico R, Giuliani G, Solari A, Taus C. Amantadine for fatigue in multiple sclerosis. Cochrane Database Syst Rev. 2007;(1):CD002818. doi: 10.1002/14651858.CD002818.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asano ML, Finlayson M. Meta-analysis of three different types of fatigue management interventions for people with multiple sclerosis: exercise, education, and medication. Mult Scler Int. 2014;2014:798285. doi: 10.1155/2014/798285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas S, Thomas PW, Kersten P et al. A pragmatic parallel arm multi-centre randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2013;84:1092–1099. doi: 10.1136/jnnp-2012-303816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Kessel K, Moss-Morris R, Willoughby E, Chalder T, Johnson MH, Robinson E. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosom Med. 2008;70:205–213. doi: 10.1097/PSY.0b013e3181643065. [DOI] [PubMed] [Google Scholar]
- 19.Mathiowetz VG, Finlayson ML, Matuska KM, Chen HY, Luo P. Randomized controlled trial of an energy conservation course for persons with multiple sclerosis. Mult Scler. 2005;11:592–601. doi: 10.1191/1352458505ms1198oa. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann S, Smits J. Cognitive-behavioural therapy for anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry. 2008;69:621–632. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polman CH, Reingold SC, Banwell B et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold SM, Heesen C, Schulz H et al. Disease specific quality of life instruments in multiple sclerosis: validation of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS) Mult Scler. 2001;7:119–130. doi: 10.1177/135245850100700208. [DOI] [PubMed] [Google Scholar]
- 23.Mayring P. Qualitative content analysis: theoretical foundation, basic procedures and software solution. http://nbn-resolving.de/urn:nbn:de:0168-ssoar-395173. Published 2014. Accessed June 24, 2014.
- 24.Köpke S, Richter T, Kasper J, Mühlhauser I, Flachenecker P, Heesen C. Implementation of a patient education program on multiple sclerosis relapse management. Patient Educ Couns. 2012;86:91–97. doi: 10.1016/j.pec.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Köpke S, Kasper J, Mühlhauser I, Nübling M, Heesen C. Patient education program to enhance decision autonomy in multiple sclerosis relapse management: a randomized-controlled trial. Mult Scler. 2009;15:96–104. doi: 10.1177/1352458508095921. [DOI] [PubMed] [Google Scholar]
- 26.Penner IK, Raselli C, Stöcklin M, Opwis K, Kappos L, Calabrese P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): the validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler. 2009;15:1509–1517. doi: 10.1177/1352458509348519. [DOI] [PubMed] [Google Scholar]
- 27.Chesney A, Neilands T, Chambers B, Taylor J, Folkman S. A validity and reliability study of the coping self-efficacy scale. Br J Health Psychol. 2006;11:421–437. doi: 10.1348/135910705X53155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikula P, Nagyova I, Krokavcova M et al. Coping and its importance for quality of life in patients with multiple sclerosis. Disabil Rehabil. 2014;36:732–736. doi: 10.3109/09638288.2013.808274. [DOI] [PubMed] [Google Scholar]
- 29.Fischer S, Fischer M, Nicholls RA et al. Diagnostic accuracy for major depression in multiple sclerosis using self-report questionnaires. Brain Behav. 2015;5:e00365. doi: 10.1002/brb3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pöttgen J, Lau S, Penner I, Heesen C, Moritz S. Managing neuropsychological impairment in multiple sclerosis: pilot study on a standardized metacognitive intervention. Int J MS Care. 2015;17:130–137. doi: 10.7224/1537-2073.2014-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 32.Thomas S, Thomas PW, Nock A et al. Development and preliminary evaluation of a cognitive behavioural approach to fatigue management in people with multiple sclerosis. Patient Educ Couns. 2010;78:240–249. doi: 10.1016/j.pec.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Chalder T, Berelowitz G, Pawlikowska T et al. Development of a fatigue scale. J Psychosom Res. 1993;37:147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 34.Feddersen LK, Heesen C, Poettgen J. German validation of the Chalder Fatigue Scale. Poster presented at: 19th Annual Conference of RIMS (Rehabilitation in Multiple Sclerosis); June 6–7, 2014; Brighton, UK.


