Abstract
Background: Delayed-release dimethyl fumarate (DMF; also known as gastroresistant DMF) is indicated for relapsing multiple sclerosis (MS). The objective of this study was to explore the safety and tolerability of DMF when administered with interferon beta (IFNβ) or glatiramer acetate (GA).
Methods: Patients with relapsing-remitting MS receiving established therapy with the same dose of IFNβ or GA for at least 12 months continued their prescribed therapy for 2 months (monotherapy period) and then received DMF 240 mg three times daily in addition to their prescribed MS therapy for 6 months (add-on therapy period). Safety and magnetic resonance imaging outcomes were monitored monthly.
Results: During the add-on therapy period, in the DMF+IFNβ (n = 57) and DMF+GA (n = 47) groups, the overall incidence of adverse events was 95% and 100%, respectively; the most common adverse events were flushing, diarrhea, and abdominal pain. In both groups, mean lymphocyte counts decreased but remained within normal limits, and hepatic transaminase levels increased transiently; no case met Hy's law criteria. There was no overall increased risk of infection. In both groups, gadolinium-enhancing lesion activity and new/enlarging T2 lesions decreased compared with the monotherapy period (exploratory endpoints).
Conclusions: The safety profile of DMF taken with IFNβ or GA was acceptable and consistent with the known safety profile of DMF monotherapy.
Therapeutic options for patients responding suboptimally to multiple sclerosis (MS) disease-modifying therapy (DMT) include switching to a different DMT1–4 and using a second DMT as add-on therapy.5–9 When switching, overlapping exposure of the discontinued and newly initiated DMTs is sometimes used as a preventive measure against exacerbations of disease activity.10–12 Hence, it is important to establish that safety and tolerability are not compromised with concurrent exposure to DMTs.
Delayed-release dimethyl fumarate (DMF; also known as gastroresistant DMF) is indicated for the treatment of relapsing MS. In phase 3 clinical trials, 240 mg of DMF administered twice daily or three times daily showed significant efficacy on clinical and neuroradiologic measures, combined with an acceptable safety profile, in people with relapsing-remitting MS (RRMS).13,14 The most common adverse events (AEs) in DMF-treated patients were flushing and gastrointestinal (GI) events; other safety signals of note included a decrease from baseline in mean white blood cell (WBC) and lymphocyte counts and a transient increase from baseline in mean values for hepatic transaminases. Concurrent administration of DMF with a single administration of interferon beta (IFNβ)-1a (30 μg intramuscularly) or glatiramer acetate (GA) (20 mg subcutaneously) in completed phase 1 drug interaction studies showed no effect on the pharmacokinetic profile of DMF, and no new safety signals were detected (Biogen, unpublished data).
The phase 2, open-label EXPLORE study, described in this report, was designed to examine the safety profile of DMF administered concurrently with IFNβ or GA. The DMF was administered for 6 months in patients with RRMS receiving established therapy (≥12 months continuously taking the same dose) with commercially available IFNβ (Avonex [Biogen, Cambridge, MA], Betaseron [Bayer HealthCare Pharmaceuticals, Whippany, NJ], or Rebif [EMD Serono Inc, Rockland, MA]) or GA. Safety assessments included collection of AEs and laboratory values at monthly clinic visits. Magnetic resonance imaging (MRI) parameters (gadolinium-enhancing [Gd+] lesion activity and number of T2-hyperintense lesions) were monitored as part of the exploratory efficacy analyses.
Methods
Patients
Key eligibility criteria included age 18 to 55 years, RRMS diagnosis per the McDonald criteria,15 Expanded Disability Status Scale score16 of 0.0 to 5.0, established therapy with the same dose of a prescribed IFNβ or GA for at least 12 months consecutively at the time of enrollment, and at least one relapse within 12 months before enrollment or a Gd+ lesion(s) on brain MRI within 6 weeks before enrollment. Key exclusion criteria included progressive forms of MS, MS relapse within 50 days before enrollment, and a clinically significant medical history or laboratory test abnormalities.
Design
EXPLORE was a phase 2, open-label study conducted at 20 US sites from August 2010 to March 2012. A total of 108 patients were enrolled and grouped based on their currently prescribed MS therapy (IFNβ or GA). After screening and on enrollment, patients continued taking their prescribed MS therapy for 2 months (monotherapy period) and then took DMF concurrently with their prescribed MS therapy for 6 months (add-on therapy period). The DMF was dosed at 120 mg three times daily for 1 week and at 240 mg three times daily thereafter.
Study Procedures and Endpoints
Safety and MRI outcomes were monitored at monthly monotherapy visits (weeks −8, −4, and 0/baseline) and add-on therapy visits (weeks 4, 8, 12, 16, 20, and 24). A follow-up visit occurred within a mean (SD) of 14 (5) days after the last dose of DMF. In the event of relapse, an unscheduled relapse assessment visit occurred within 5 days after symptom onset.
Safety endpoints included AEs, serious AEs, discontinuation due to AEs, laboratory abnormalities, and MRI activity. Exploratory efficacy endpoints included Gd+ lesion activity and mean monthly number of new or newly enlarging T2-hyperintense lesions.
Statistical Analyses
Safety endpoints for the add-on therapy period were analyzed for the safety population, defined as any patient who took at least one capsule of DMF concurrently with background therapy.
The following highlighted AEs were defined based on preferred terms, Standardized Medical Dictionary for Regulatory Activities (MedDRA) Queries, or system organ classes: flushing and other related symptoms, GI tolerability events, hepatic disorders, ischemic cardiovascular disorders, infections, and potential malignancies. Flushing and other related symptoms were prespecified preferred terms. The GI tolerability events, hepatic disorders, ischemic cardiovascular disorders, and potential malignancies were based on Standardized MedDRA Queries. Infections were defined based on the Infections and Infestations system organ class.
Number of Gd+ lesions (including new and persistent lesions) was analyzed for the monotherapy and add-on therapy periods. The primary analysis was based on the Gd cohort, defined as the subset of DMF-dosed patients with at least one Gd+ lesion in any one of the three scans scheduled before DMF dosing (weeks −8, −4, and 0/baseline), and a sensitivity analysis was performed using the intention-to-treat (ITT) population, defined as patients who took at least one capsule of DMF concurrently with background therapy, and included patients with and without Gd+ lesions in the monotherapy period. The mean number of Gd+ lesions (hereafter referred to as “the number of Gd+ lesions”) was calculated as the total number of lesions on nonmissing scans divided by the number of nonmissing scans. Missing averages were not imputed. The analyses compared, for the same patient, the number of Gd+ lesions across three brain MRI scans during the monotherapy period (weeks −8, −4, and 0/baseline) versus the last 8 weeks of the add-on therapy period (weeks 16, 20, and 24) using the Wilcoxon signed rank test.
The number of new or newly enlarging T2 lesions was measured at week 0 (compared with week −8) and at either week 24 (compared with week 0) or the premature withdrawal visit (compared with week 0). The primary analysis was conducted using the ITT population; a sensitivity analysis was conducted using the Gd cohort. The monthly rate of new T2 lesions during the monotherapy and add-on therapy periods was calculated retrospectively. The difference in the monthly rates was compared using the Wilcoxon signed rank test.
All MRI data collected within 24 days after intravenous corticosteroid treatment were excluded from the analyses. The MRI analyses were limited to patients with analyzable data on Gd+ lesions or T2 lesions in both the monotherapy and add-on therapy periods.
Ethical Conduct of the Study
This study was performed in accordance with Title 21, US Code of Federal Regulations; the International Conference on Harmonisation Guideline on Good Clinical Practices17; the EU Clinical Trial Directive 2001/20/EC; and the ethical principles outlined in the Declaration of Helsinki.18 Written informed consent was obtained from patients before evaluations were performed to determine eligibility. The investigators obtained approval for the study protocol from the following ethics committees: Aurora institutional review board (IRB), Milwaukee, WI; Beth Israel Deaconess Medical Center Committee on Clinical Investigations, Boston, MA; Biomedical Research Alliance of New York LLC IRB, Lake Success, NY; Cleveland Clinic IRB WB-2, Cleveland, OH; Johns Hopkins Medicine IRB, Baltimore, MD; North Memorial Medical Center, Robbinsdale, MN; Providence Health & Services IRB, Portland, OR; Saint Joseph's Hospital and Medical Center, Institutional Review Board for Human Research, Phoenix, AZ; UT Southwestern Medical Center at Dallas IRB, Dallas, TX; and Western Institutional Review Board, Olympia, WA. Written informed consent was obtained from each patient before performing study-related activities.
Results
Patients
A total of 108 patients were enrolled at 20 US sites (Figure 1). Fifty-nine patients received IFNβ and 49 patients received GA as their prescribed MS therapy during the monotherapy period; 57 (DMF+IFNβ) and 47 (DMF+GA) of these patients, respectively, received DMF during the add-on therapy period. The percentage of patients completing DMF therapy was 79% (DMF+IFNβ) and 81% (DMF+GA), and the percentage completing the study was 79% in both treatment groups. Baseline (week 0) demographic and disease characteristics (Table 1) and MRI characteristics (Table 2) were generally similar between treatment groups.
Figure 1.
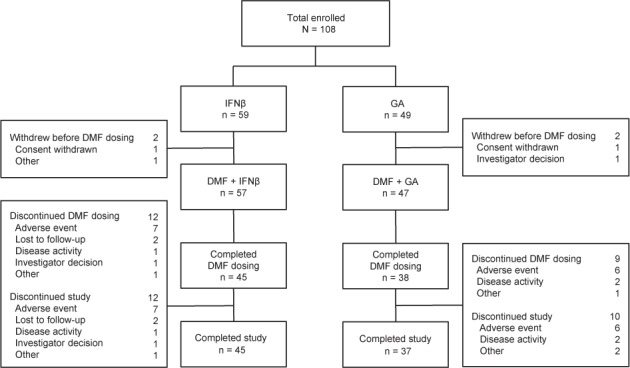
Patient disposition
DMF, delayed-release dimethyl fumarate (also known as gastroresistant DMF); GA, glatiramer acetate; IFNβ, interferon beta.
Table 1.
Baseline (week 0) demographic and MS disease characteristics of patients who completed the monotherapy period and were dosed with DMF+IFNβ or DMF+GA (ITT population)
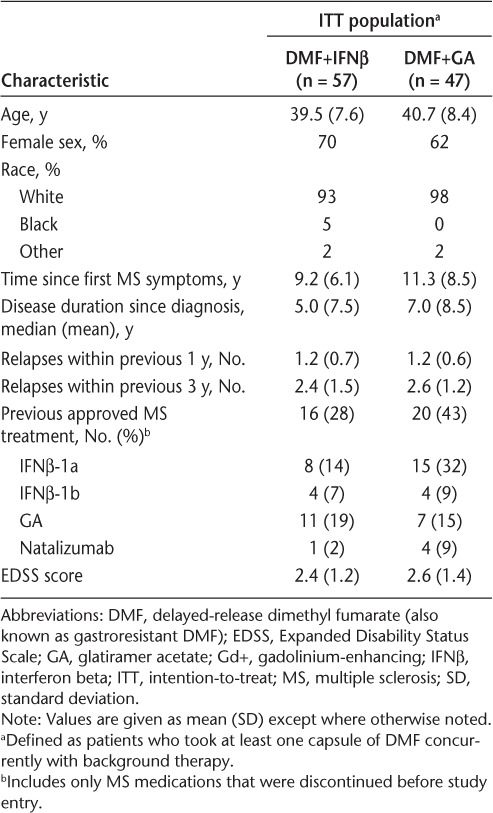
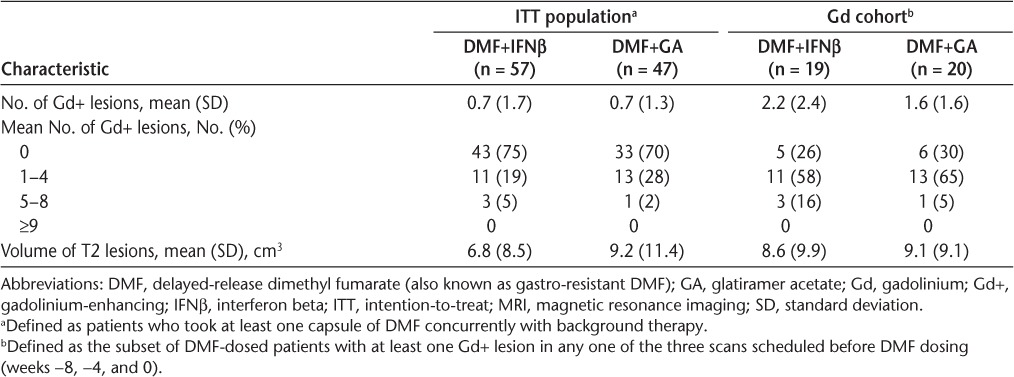
Table 2.
Baseline (week 0) MRI characteristics of the ITT population and the Gd cohort
Patients took one 120-mg DMF capsule three times daily for the first week of the add-on therapy period and two 120-mg DMF capsules three times daily thereafter. The percentage of patients who were at least 80% adherent to DMF treatment during the add-on study period was 79% (DMF+IFNβ) and 85% (DMF+GA). The total number of patient-years exposed to DMF was 22.4 (DMF+IFNβ) and 19.4 (DMF+GA).
The Gd cohort comprised 39 patients, including 19 in the DMF+IFNβ group and 20 in the DMF+GA group. Baseline MRI characteristics were generally similar between treatment groups in the Gd cohort (Table 2).
Safety Experience During the Add-on Therapy Period
During the add-on therapy period, the overall incidence of AEs was 95% (DMF+IFNβ) and 100% (DMF+GA). Most AEs were assessed as mild or moderate in severity. The most common AEs (reported by ≥20% of patients in the DMF+IFNβ or DMF+GA group) were flushing (42% and 53%, respectively), diarrhea (32% and 15%, respectively), and abdominal pain (21% and 6%, respectively) (Table 3).
Table 3.
Most common AEs (≥20% of patients in either treatment group), all serious AEs, and all AEs leading to the discontinuation of DMF use reported during the add-on therapy period
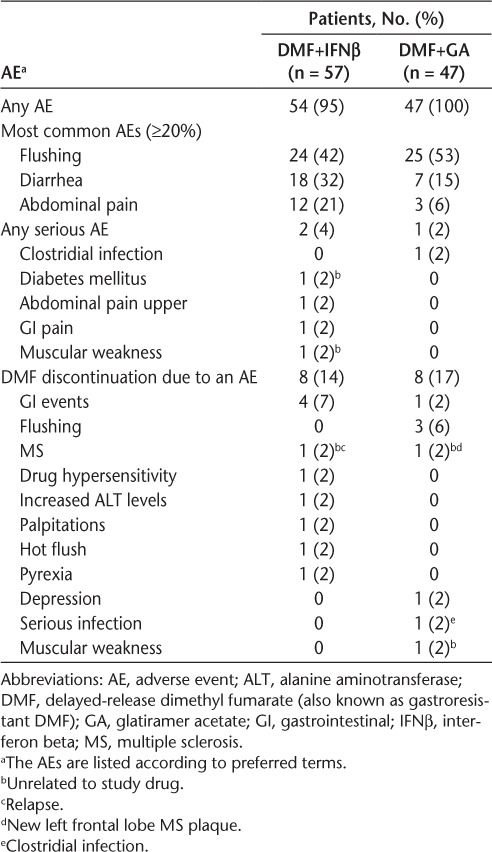
The incidence of serious AEs was 4% (DMF+IFNβ) and 2% (DMF+GA). Five serious AEs were reported in three patients, including muscular weakness and diabetes mellitus in one patient (DMF+IFNβ), both considered unrelated to study treatment; upper abdominal pain and GI pain in one patient (DMF+IFNβ), both considered potentially related to study treatment; and clostridial infection in one patient (DMF+GA), considered potentially related to study treatment (Table 3). No patients died during the study.
The incidence of DMF discontinuation due to AEs was 14% (DMF+IFNβ) and 17% (DMF+GA). The most common AEs leading to DMF discontinuation were GI disorders (7% [DMF+IFNβ] and 2% [DMF+GA]) and flushing (0% [DMF+IFNβ] and 6% [DMF+GA]) (Table 3).
The incidences of the highlighted AEs in the DMF+IFNβ and DMF+GA groups, respectively, were as follows: flushing and other related symptoms, 56% and 57%; GI tolerability events, 61% and 49%; hepatic disorders, 12% and 17%; infections, 56% and 62%; and ischemic cardiovascular disorders, 2% and 0%; there were no potential malignancies. The most common GI tolerability events (reported by ≥10% of patients) in the DMF+IFNβ group were diarrhea (32%), abdominal pain (21%), nausea (19%), upper abdominal pain (14%), and abdominal discomfort (12%) and in the DMF+GA group were diarrhea (15%) and flatulence (13%). Hepatic disorders in the DMF+IFNβ and DMF+GA groups, respectively, included increased alanine aminotransferase (ALT) (11% and 15%), aspartate aminotransferase (AST) (7% and 9%), γ-glutamyltransferase (4% and 6%), and blood bilirubin (0% and 2%) levels.
One patient in the DMF+IFNβ group reported an AE associated with ischemic cardiovascular disorders. The patient experienced nonserious events of electrocardiographic T-wave abnormal/sinus bradycardia on day 1 of the add-on therapy period, before the first dose of DMF was administered. The events were assessed as mild and unrelated to study treatment and resolved on day 29.
There were no abnormalities in hematologic values observed during the monotherapy period. Mean WBC and lymphocyte counts decreased from baseline to week 24 (add-on therapy period) but remained within normal limits throughout the study (Figure 2). At week 24, the mean percentage decrease from baseline in lymphocyte counts was 22% (DMF+IFNβ) and 7% (DMF+GA). At week 26 (a safety follow-up visit scheduled 2 weeks after the completion of treatment), there was a slight increase in lymphocyte counts, but counts did not return to baseline levels. In the DMF+IFNβ and DMF+GA groups, 18 (32%) and three (6%) patients, respectively, had a worst postbaseline lymphocyte count of less than 0.8 × 109/L (Common Terminology Criteria grade19 2, 3, or 4), and four (7%) and two (4%) patients had a worst postbaseline lymphocyte count of less than 0.5 × 109/L (Common Terminology Criteria grade 3 or 4).
Figure 2.
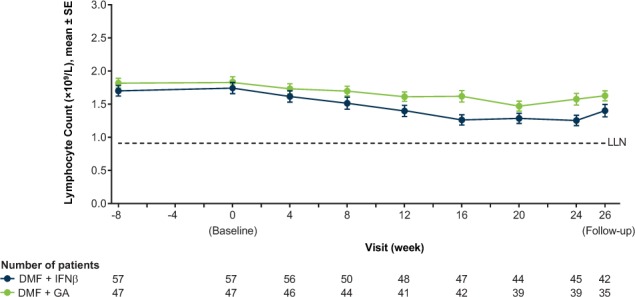
Mean ± SE lymphocyte counts by visit
The lower limit of normal (LLN) is in standard units. If there are multiple LLNs for a given parameter, the highest LLN is shown. DMF, delayed-release dimethyl fumarate (also known as gastroresistant DMF); GA, glatiramer acetate; IFNβ, interferon beta.
There was an increased incidence of elevated hepatic transaminase levels during the add-on therapy period. Most patients had elevations that were less than 3× the upper limit of normal (ULN). Three patients in the DMF+IFNβ group (5%) and one patient in the DMF+GA group (2%) experienced clinically relevant ALT elevations (≥3× ULN), and one patient in the DMF+IFNβ group (2%) experienced ALT elevation greater than 5× ULN. One patient (2%) receiving DMF+IFNβ withdrew due to an increased ALT level. One patient in the DMF+IFNβ group experienced a clinically relevant AST elevation (≥3× ULN). No case fulfilled Hy's law for drug-induced liver injury (transaminase elevations ≥3× ULN concurrent with bilirubin levels >2× ULN).
Exploratory Efficacy Endpoints
The number of patients with analyzable Gd+ lesion data in the monotherapy and add-on therapy periods was 16 (Gd cohort) and 47 (ITT population) in the DMF+IFNβ group and 18 (Gd cohort) and 43 (ITT population) in the DMF+GA group. For the primary analysis (Gd cohort), the mean number of Gd+ lesions during the monotherapy and add-on therapy periods was 1.06 and 0.17, respectively, in the DMF+IFNβ group, which was a significant mean reduction of 0.9 (P < .0001), and 1.72 and 0.83, respectively, in the DMF+GA group, which was a significant mean reduction of 0.89 (P = .0124) (Figure 3A). For the sensitivity analysis (ITT population, which included patients with or without Gd+ lesions in the monotherapy period), the mean number of Gd+ lesions during the monotherapy and add-on therapy periods was 0.36 and 0.09, respectively, in the DMF+IFNβ group (P = .0013 for the mean reduction) and 0.72 and 0.39, respectively, in the DMF+GA group (P = .0347 for the mean reduction).
Figure 3.
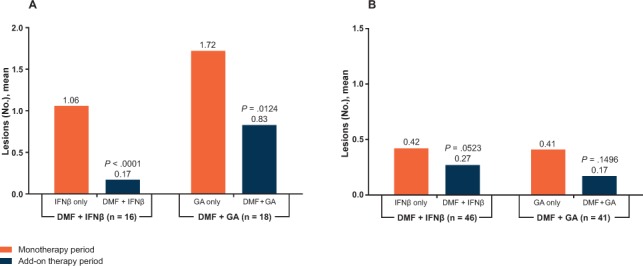
Mean number of gadolinium-enhancing (Gd+) lesions and number of new or newly enlarging T2 lesions during the monotherapy and add-on therapy periods
A, Mean numbers of Gd+ lesions during the monotherapy period (weeks −8, −4, and 0) and the add-on therapy period (weeks 16, 20, and 24) were examined for the gadolinium (Gd) cohort (primary analysis). The difference between the two periods (for each participant) was analyzed using the Wilcoxon signed rank test. B, The monthly rates of new T2 lesions during the monotherapy and add-on therapy periods were examined for the intention-to-treat population (primary analysis). The difference in the monthly rates was compared using the Wilcoxon signed rank test. Analyses were limited to patients with analyzable data on Gd+ lesions or T2 lesions in both periods. DMF, delayed-release dimethyl fumarate (also known as gastroresistant DMF); GA, glatiramer acetate; IFNβ, interferon beta.
The number of patients with analyzable T2 lesion data in the monotherapy and add-on therapy periods was 15 (Gd cohort) and 46 (ITT population) in the DMF+IFNβ group and 18 (Gd cohort) and 41 (ITT population) in the DMF+GA group. For the primary analysis (ITT population), the mean monthly number of new or newly enlarging T2 lesions during the monotherapy and add-on therapy periods was 0.42 and 0.27, respectively, in the DMF+IFNβ group (P = .0523 for the mean reduction) and 0.41 and 0.17, respectively, in the DMF+GA group (P = .1496 for the mean reduction) (Figure 3B). For the sensitivity analysis (Gd cohort), the mean monthly number of new or newly enlarging T2 lesions during the monotherapy and add-on therapy periods was 1.23 and 0.67, respectively, in the DMF+IFNβ group (P = .0040 for the mean reduction) and 0.89 and 0.25, respectively, in the DMF+GA group (P = .0356 for the mean reduction).
Discussion
The safety and tolerability of concomitant exposure to MS therapeutic agents is largely unknown, and guidance on whether or how to treat patients with more than one DMT is generally lacking. Overlapping exposure to DMTs is sometimes used during the transition from one therapeutic agent to another in an attempt to minimize potential exacerbations of disease activity.10–12 In addition, add-on therapy has attracted interest as a way to potentially target multiple elements of MS pathology using therapeutics with distinct mechanisms of action.20 Several clinical trials have examined the efficacy and safety of combination therapy, but the results were inconclusive.5–9
EXPLORE was conducted to examine the safety and tolerability of concurrent exposure to DMF and IFNβ or GA. The dose of DMF used (240 mg three times daily) was higher than the currently approved dose (240 mg twice daily) because, at the time EXPLORE was designed, phase 3 data indicating that 240 mg twice daily and three times daily dosing are comparable in terms of efficacy and safety13,14 were not yet available. Dosing in EXPLORE was based on a phase 2 study in people with RRMS in which DMF 240 mg three times daily significantly reduced brain MRI activity over 6 months, whereas DMF 120 mg once daily or three times daily had more modest effects that were not statistically significant.21 (The phase 2 study did not include a DMF 240 mg twice daily arm.) Nevertheless, the 240-mg three times daily dosing regimen is relevant and informative in an analysis of safety and tolerability.
The safety profile of DMF combined with IFNβ or GA was broadly similar to the safety profile of DMF 240 mg three times daily monotherapy in an integrated analysis of the phase 3 DEFINE (Determination of the Efficacy and Safety of Oral Fumarate in Relapsing-Remitting MS) and CONFIRM (Comparator and an Oral Fumarate in Relapsing-Remitting Multiple Sclerosis) trials,22 although comparisons among studies should be made with caution in light of differences in DMF treatment duration (up to 6 months in EXPLORE vs. up to 2 years in DEFINE and CONFIRM) and treatment regimen (DMF as add-on therapy in EXPLORE vs. DMF monotherapy in DEFINE and CONFIRM). In all three studies, AEs were generally reported as mild or moderate, and flushing and GI events were among the most common AEs associated with DMF treatment.22,23
In EXPLORE, DMF was associated with a decrease from baseline in mean WBC and lymphocyte counts. After 24 weeks of DMF treatment during the add-on therapy period, the percentage decrease in lymphocyte counts in both treatment arms was comparable with that seen after 24 weeks of DMF 240 mg twice daily and three times daily monotherapy in a pooled analysis of phase 2 and 3 clinical studies of DMF in RRMS.24 Mean WBC counts were lower in the DMF+IFNβ group than in the DMF+GA group at baseline, which could be due to the nonrandom assignment of patients to treatment groups; nevertheless, the overall shape of the curves was similar, suggesting a consistent change relative to the groups' respective baselines. The overall incidence of infections in EXPLORE was comparable with that seen in an integrated analysis of DEFINE and CONFIRM.25
There was an increased incidence of elevated hepatic transaminase levels after initiation of DMF treatment in EXPLORE, but no case met Hy's law criteria for drug-induced liver injury. Similar to the phase 3 trials, a small number of patients had clinically relevant elevations (≥3× ULN) in ALT and AST levels.25 There were no potential malignancies or deaths reported. Overall, there were no unexpected safety signals.
EXPLORE was not designed to examine the efficacy of DMF combined with IFNβ or GA, and MRI measures were included primarily to assess safety. However, MRI results during the add-on therapy period compared with the monotherapy period showed no worsening in MS disease activity but rather a decrease in Gd+ lesion activity and new or newly enlarging T2 lesions in both treatment arms. This finding is consistent with the results of post hoc analyses of DEFINE and CONFIRM showing that DMF 240 mg twice daily and three times daily significantly reduced the number of Gd+ lesions (integrated analysis) and the number of new or enlarging T2 lesions (DEFINE only) versus placebo after 6 months of treatment.26
The exploratory efficacy analysis is limited in several respects and should be interpreted with caution. EXPLORE was a nonrandomized open-label study with a limited sample size. There was no parallel control group treated with monotherapy throughout the study period, which would be necessary to rule out regression-to-the-mean phenomena in a longitudinal comparison (eg, patients with higher baseline disease activity might seem to improve over time with or without DMF add-on therapy). Finally, the monotherapy and add-on therapy periods were of different durations (2 months vs. 6 months), limiting the ability to draw comparisons between them.
PracticePoints.
Overlapping exposure to MS disease-modifying therapies is sometimes used during the transition from one therapeutic agent to another to minimize potential exacerbations of disease activity. Hence, it is important to establish that safety and tolerability are not compromised with concurrent exposure to disease-modifying therapies.
Delayed-release dimethyl fumarate (DMF) 240 mg three times daily was well tolerated as add-on therapy to established treatment with interferon beta or glatiramer acetate. No new safety signals were identified. The safety profile of concurrent exposure to DMF and interferon beta or glatiramer acetate is acceptable and consistent with the known safety profile of DMF monotherapy.
In summary, DMF was well tolerated as add-on therapy to established treatment with IFNβ or GA. No new safety signals were identified. The safety profile of concurrent exposure to DMF and IFNβ or GA is acceptable and consistent with the known safety profile of DMF monotherapy.
Acknowledgments
Karyn M. Myers, PhD (Biogen), wrote the first draft of the manuscript based on input from the authors.
Footnotes
Financial Disclosures: Dr. Calkwood has performed advisory, consultancy, and speaker activities for Acorda, Bayer HealthCare, Biogen, EMD Serono, Genzyme, Novartis, Questcor, and Teva; and grant/research activities for Biogen, Novartis, Roche, and Xenoport. Dr. Vollmer has performed advisory board, lecture, and consultancy activities for Cleveland Clinic, Novartis, Mandell Center for MS, Teva, Teva Canada Innovations, Questcor, Biogen, Xenoport, University of Florida PeerView, Krog & Partners, National MS Society, University of St. Louis, Acorda, Mylan, Global Prairie, and Deloitte Consulting; and received research support from Biogen, Teva Pharmaceutical Industries, Genzyme, Ono Pharmaceuticals, Elan Pharmaceuticals, Novartis, National Institutes of Health, Avanir Pharmaceuticals, and Janssen Research & Development. Dr. Fox has received consultant fees from Actelion, Biogen, Medday, Novartis, Questcor, Teva, and Xenoport; served on advisory committees for Actelion, Biogen, and Novartis; and received research grant funding from Novartis. Drs. Zhang, Novas, Sheikh, and Viglietta are employees of and hold stock/stock options in Biogen.
Funding/Support: This study was funded by Biogen, which reviewed the paper and provided feedback to the authors and provided medical writing support in the development of the manuscript. The authors had full editorial control of the manuscript and provided their final approval of all content.
References
- 1.Castillo-Trivino T, Mowry EM, Gajofatto A et al. Switching multiple sclerosis patients with breakthrough disease to second-line therapy. PLoS One. 2011;6:e16664. doi: 10.1371/journal.pone.0016664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gajofatto A, Bacchetti P, Grimes B, High A, Waubant E. Switching first-line disease-modifying therapy after failure: impact on the course of relapsing-remitting multiple sclerosis. Mult Scler. 2009;15:50–58. doi: 10.1177/1352458508096687. [DOI] [PubMed] [Google Scholar]
- 3.Jokubaitis VG, Li V, Kalincik T et al. Fingolimod after natalizumab and the risk of short-term relapse. Neurology. 2014;82:1204–1211. doi: 10.1212/WNL.0000000000000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rio J, Tintore M, Sastre-Garriga J et al. Change in the clinical activity of multiple sclerosis after treatment switch for suboptimal response. Eur J Neurol. 2012;19:899–904. doi: 10.1111/j.1468-1331.2011.03648.x. [DOI] [PubMed] [Google Scholar]
- 5.Freedman MS, Wolinsky JS, Wamil B et al. Teriflunomide added to interferon-beta in relapsing multiple sclerosis: a randomized phase II trial. Neurology. 2012;78:1877–1885. doi: 10.1212/WNL.0b013e318258f7d4. [DOI] [PubMed] [Google Scholar]
- 6.Goodman AD, Rossman H, Bar-Or A et al. GLANCE: results of a phase 2, randomized, double-blind, placebo-controlled study. Neurology. 2009;72:806–812. doi: 10.1212/01.wnl.0000343880.13764.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lublin FD, Cofield SS, Cutter GR et al. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann Neurol. 2013;73:327–340. doi: 10.1002/ana.23863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudick RA, Stuart WH, Calabresi PA et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–923. doi: 10.1056/NEJMoa044396. [DOI] [PubMed] [Google Scholar]
- 9.Vollmer TL, Phillips JT, Goodman AD et al. An open-label safety and drug interaction study of natalizumab (Antegren) in combination with interferon-beta (Avonex) in patients with multiple sclerosis. Mult Scler. 2004;10:511–520. doi: 10.1191/1352458504ms1084oa. [DOI] [PubMed] [Google Scholar]
- 10.La Mantia L, Prone V, Marazzi MR, Erminio C, Protti A. Multiple sclerosis rebound after fingolimod discontinuation for lymphopenia. Neurol Sci. 2014;35:1485–1486. doi: 10.1007/s10072-014-1800-y. [DOI] [PubMed] [Google Scholar]
- 11.Siger M, Durko A, Nicpan A, Konarska M, Grudziecka M, Selmaj K. Discontinuation of interferon beta therapy in multiple sclerosis patients with high pre-treatment disease activity leads to prompt return to previous disease activity. J Neurol Sci. 2011;303:50–52. doi: 10.1016/j.jns.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Sorensen PS, Koch-Henriksen N, Petersen T, Ravnborg M, Oturai A, Sellebjerg F. Recurrence or rebound of clinical relapses after discontinuation of natalizumab therapy in highly active MS patients. J Neurol. 2014;261:1170–1177. doi: 10.1007/s00415-014-7325-8. [DOI] [PubMed] [Google Scholar]
- 13.Gold R, Kappos L, Arnold DL et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- 14.Fox RJ, Miller DH, Phillips JT et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367:1087–1097. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Edan G et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 17.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH harmonized tripartite guideline: Guideline for Good Clinical Practice. J Postgrad Med. 2001;47:45–50. [PubMed] [Google Scholar]
- 18.World Medical Association. Declaration of Helsinki. Helsinki, Finland: World Medical Association; 1964. [Google Scholar]
- 19.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE): Version 4.0. Rockville, MD: National Cancer Institute; May 28, 2009. [Google Scholar]
- 20.Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89:225–240. doi: 10.1016/j.mayocp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Kappos L, Gold R, Miller DH et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet. 2008;372:1463–1472. doi: 10.1016/S0140-6736(08)61619-0. [DOI] [PubMed] [Google Scholar]
- 22.Bar-Or A, Gold R, Fox RJ Clinical efficacy and safety of oral BG-12 (dimethyl fumarate) in relapsing-remitting multiple sclerosis (RRMS): an integrated analysis of the phase 3 DEFINE and CONFIRM studies. Paper presented at: 7th Latin American Congress of Multiple Sclerosis; November 28–30, 2012; Rio de Janeiro, Brazil.
- 23.Phillips JT, Selmaj K, Gold R et al. Clinical significance of gastrointestinal and flushing events in patients with multiple sclerosis treated with delayed-release dimethyl fumarate. Int J MS Care. 2015;17:236–243. doi: 10.7224/1537-2073.2014-069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox RJ, Chan A, Gold R Lymphocyte count reductions with delayed-release dimethyl fumarate: integrated analysis of the phase 2, phase 3, and extension studies. Paper presented at: 2014 Joint ACTRIMS-ECTRIMS Meeting; September 10–13, 2014; Boston, MA.
- 25.Cambridge, MA: Biogen; 2013. Tecfidera [package insert] [Google Scholar]
- 26.Kappos L, Giovannoni G, Gold R et al. Time course of clinical and neuroradiological effects of delayed-release dimethyl fumarate in MS. Eur J Neurol. 2015;22:664–671. doi: 10.1111/ene.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]


