Abstract
Background: We sought to compare mortality rates and related diagnoses in hospitalized patients with multiple sclerosis (MS), those with diabetes mellitus (DM), and the general hospitalized population (GHP).
Methods: Patients who died between 2007 and 2011 were identified in the US hospital–based Premier Healthcare Database. Demographic information was collected, mortality rates calculated, and principal diagnoses categorized.
Results: Of 55,152 unique patients with MS identified, 1518 died. Mean age at death was 10 years younger for the MS group (63.4 years) than for the DM (73.3 years) and GHP (73.1 years) groups. Age-adjusted mortality rates, based on the 2000 US Standard Million Population, were 1077, 1248, and 1133 per 100,000, respectively. Infection was the most common principal diagnosis at the hospital stay during which the patient died in the MS cohort (43.1% vs. 26.3% and 24.0% in the DM and GHP groups, respectively). Other common principal diagnoses in the MS group included pulmonary (17.5%) and cardiovascular (12.1%) disease. Septicemia/sepsis/septic shock was a secondary diagnosis for 50.7% of patients with MS versus 36.0% and 31.0% of patients in the DM and GHP cohorts, respectively.
Conclusions: Patients with MS had a shorter life span than patients with DM or the GHP and were more likely to have a principal diagnosis of infection at their final hospital stay. However, the database was limited to codes recorded in the hospital; diagnoses received outside the hospital were not captured.
Multiple sclerosis (MS) is a chronic neurodegenerative disorder with a clinical course that evolves over decades.1,2 Multiple prospective, retrospective cohort, and registry studies of varying durations have shown relatively high mortality rates in patients with MS compared with the general population.3–7 Standardized mortality rates for patients with MS have been shown to increase 2 years after diagnosis and to continue to rise for 10 years.8 Indeed, patients with MS have been shown to have a 2.9-fold increased risk of death and an approximate life span reduction of 10 years compared with age-matched controls.9
Although evidence is accumulating that patients with MS have a reduced life expectancy relative to non-MS populations,3–6,10–16 debate continues to surround the primary causes of death in patients with this disease. Previous research has shown high rates of death in this population, attributed to cardiovascular disease, infection, respiratory disease, and MS itself.3–6,12,15,16 Interpretation of these findings is complicated by the different—and subjective—determinants of causes of death (eg, death certificates and registry data) used across studies or the length of time for the analysis, as many longitudinal studies have spanned decades, with medical advances over time confounding interpretation.3,12,13,15,16 Studies examining large numbers of patients over a relatively short period using objective measures of mortality are, therefore, needed.
Clearly, identifying causes of death in this population is challenging. If the clinical presentation is benign, MS is unlikely to be recorded as a cause of death despite its potential contribution to mortality, although many studies have attributed more than 50% of deaths in this population to MS.3–6,12,15,16 Patients also often have multiple comorbid conditions that may overshadow the effects of MS on survival outcomes.5 In addition, only a few studies have focused on US-based cohorts,7,13,17,18 and none have reported in-hospital mortality using objective disease classification codes.
Aside from analyzing death certificates, examining the diagnoses recorded during the hospitalization in which the patient died could provide some understanding of the drivers behind the excess mortality in patients with MS. To this end, a retrospective analysis of records from patients hospitalized in the United States was conducted. The aim of the present study was to compare in-hospital mortality rates and related diagnoses in patients with MS with those with another well-documented chronic condition observed in hospitalized patients, diabetes mellitus (DM), and patients in the general hospitalized population (GHP).
Patients and Methods
Patient Population
Data were derived from the Premier Healthcare Database, a large US hospital–based database developed for quality and utilization benchmarking (Premier Healthcare Solutions, Inc, Charlotte, NC; available at https://www.premierinc.com). The Premier database contains information from more than 700 geographically diverse, nonprofit, nongovernment, community, teaching, and nonteaching hospitals, corresponding to approximately 20% of all annual acute-care hospitalizations in the United States. Information on more than 75 million discharged patients between 2000 and the present is included, with a mean of 6 million discharges per year. Data are submitted quarterly or monthly and undergo several quality checks by the hospital-based users.
Patient diagnoses and procedures were categorized according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding system. As recorded in the patient's hospital discharge record, the principal diagnosis code reflected the diagnosis that was responsible for the patient's hospitalization, and secondary diagnosis codes indicated all other (nonprincipal) diagnoses. The ICD-9-CM codes provided by the hospital were assumed to be correct and complete, as with all similarly derived administrative databases. Death certificates were not analyzed for this study. The database is compliant with the provisions of the Health Insurance Portability and Accountability Act of 1996. Patient data are de-identified to protect the patients whose data are represented. Consequently, this study did not require institutional review board waiver or approval.
In this retrospective database analysis, three mutually exclusive populations of patients 18 years or older were assembled according to the primary and secondary ICD-9-CM codes listed in the database: patients with MS, patients with DM, and the GHP. Patients with MS listed as a primary or secondary diagnosis were included in this analysis if the code for MS (ICD-9-CM code 340) was recorded for the hospitalization in which they died. Patients with DM listed as a primary or secondary diagnosis were included if they had at least one code for DM (ICD-9-CM code 250.00–250.93) for the hospitalization in which they died and if they did not have any codes for MS. The GHP included patients with any ICD-9-CM code other than 340 or 250.00 to 250.93 for the hospitalization in which they died. For all three patient groups, inclusion depended on their being discharged as an inpatient between January 1, 2007, and December 31, 2011.
Statistical Analysis
Descriptive statistics were used to describe hospital characteristics (eg, number of beds and geographic location), patient demographics, diagnosis category frequency, and age at death. Mortality outcomes were compared using unadjusted mortality rates. These outcomes were measured from birth (not from diagnosis because the database did not record information on age at disease onset) and were adjusted according to the 2000 US Standard Million Population.19 A t test at the P < .05 threshold of significance was used to analyze each continuous variable (ie, mean age at death) in the cohorts. A χ2 test was used to compare the distributions of categorical variables (sex, race/ethnicity, All Patient Refined Diagnosis Related Group [APR-DRG; 3M, St. Paul, MN] severity of illness, and hospital characteristics).20
To gain a better understanding of the types of diagnoses associated with hospitalization, diagnosis codes were grouped into categories. These categories were derived by an expert panel consisting of a physician with MS expertise, an epidemiologist, and a pharmacoeconomist. Principal diagnosis codes were grouped into six categories: infection (including pulmonary infection), pulmonary disease (excluding infection), cardiovascular disease, cancer, MS, and all other diagnoses. Secondary diagnosis codes were grouped into three categories for analysis because the following relevant categories are frequently not coded as principal diagnoses (except for septicemia): septicemia/sepsis/septic shock, accidental death, and suicide. Multiple secondary (nonprincipal) diagnoses were available for most patients, but the patient was counted only once if there were multiple codes indicating the same condition (eg, 038.xx and 995.92 are two diagnoses related to sepsis). Because a patient could have both a principal and a secondary diagnosis recorded in the database, these analyses were conducted separately to avoid counting a patient more than once.
Results
Patient Disposition
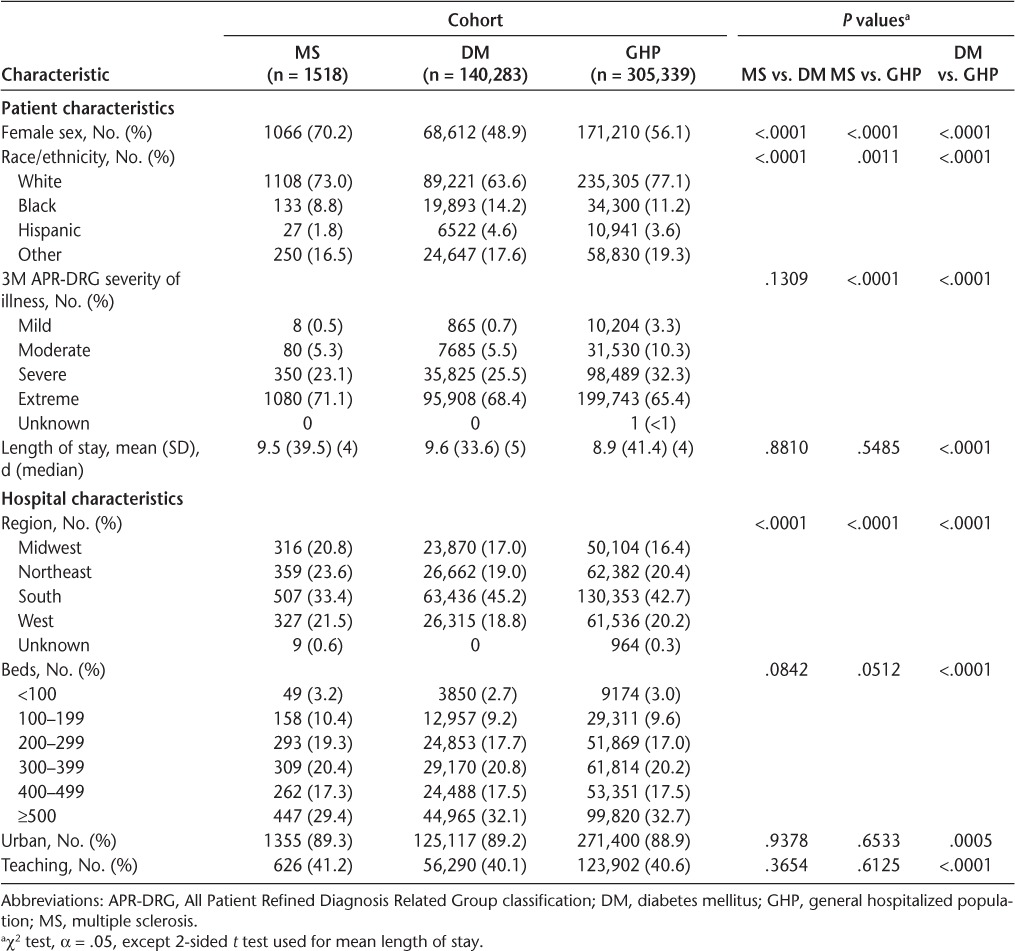
A total of 55,152 unique inpatients with MS were identified from the Premier database, 1518 of whom died in the hospital or after discharge to hospice care (Table 1). Of the patients with MS who died, 70.2% were female, 73.0% were white, and 71.1% had extreme illness according to the APR-DRG classification. Significant differences in sex, ethnicity, and illness severity were noted between deceased patients in the MS cohort and those who died in the DM group (n = 140,283) and the GHP (n = 305,339). The mean (SD) lengths of stay in the MS, DM, and GHP cohorts were somewhat similar: 9.5 (39.5) days, 9.6 (33.6) days, and 8.9 (41.4) days, respectively (P < .05 for DM vs. GHP only). Median lengths of stay were similar at 4, 5, and 4 days, respectively.
Table 1.
Patient and hospital characteristics
Mortality
Age-adjusted mortality rates, based on the 2000 US Standard Million Population, were 1077 per 100,000 for MS, 1248 per 100,000 for DM, and 1133 per 100,000 for GHP despite the generally younger age at death in the MS population (Table 2). In patients who died in the hospital, the mean age at death was approximately 10 years younger for patients with MS (63.4 years) than for patients with DM (73.3 years) and those in the GHP (73.1 years) (Figure 1). Median age at death was 63 years in the MS cohort, compared with 75 and 77 years in the DM and GHP cohorts, respectively. The differences in age at death between patients with MS and those in the DM and GHP cohorts were significant (P < .0001 for both comparisons). In an age group analysis, the MS cohort showed a peak mortality rate between 60 and 64 years of age (Figure 2). This contrasts with the DM and GHP cohorts, which showed a continual rise in the death rate as age increased. Because of the differences in distribution of deaths by age group among the different cohorts, proportional mortality rates were not calculated.
Table 2.
Age-adjusted mortality rates for patients in the MS, DM, and GHP cohorts who died a
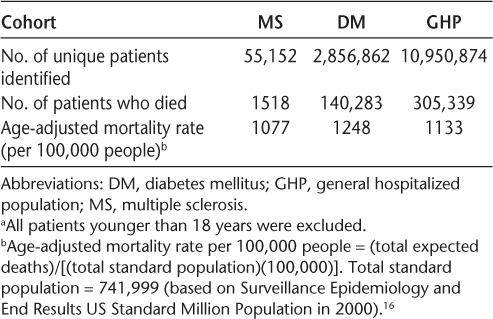
Figure 1.
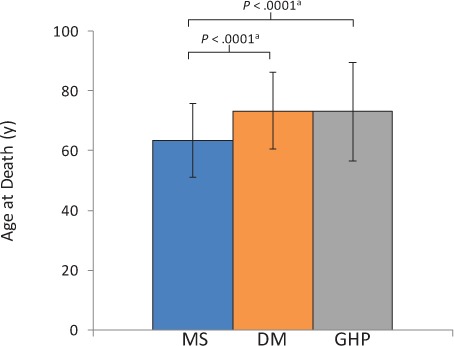
Mean (SD) age at death in the multiple sclerosis (MS), diabetes mellitus (DM), and general hospitalized population (GHP) cohorts
Patients in the MS cohort had a younger age at death than those in the DM and GHP cohorts. aT test, 2-sided, α = .05.
Figure 2.
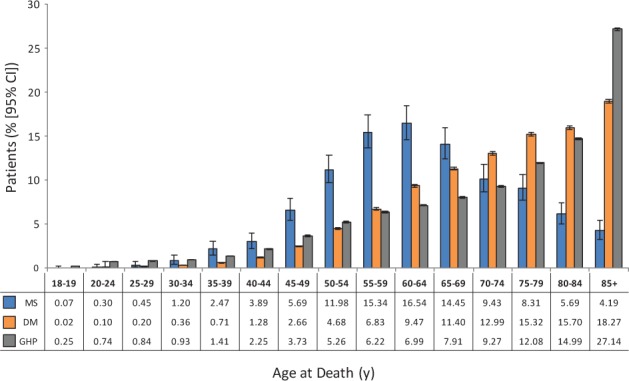
Percentage of deaths stratified by age
The highest proportion of deaths in the multiple sclerosis (MS) cohort occurred in the 60- to 64-year-old range. CI, confidence interval; DM, diabetes mellitus; GHP, general hospitalized population.
Infection (including pulmonary infection) was the most common principal diagnosis in deceased patients with MS, accounting for 43.1% of deaths (compared with 26.3% for the DM cohort and 24.0% for the GHP cohort) (Figure 3). Other common principal diagnosis categories associated with death in the MS cohort included pulmonary disease (excluding infection, 17.5%) and cardiovascular disease (12.1%). In the non-MS comparator cohorts, cardiovascular disease and infection were the most common principal diagnosis categories associated with death. Principal diagnoses of cancer were less common in patients with MS (5.2%) than in patients with DM (7.2%) or the GHP (11.7%).
Figure 3.
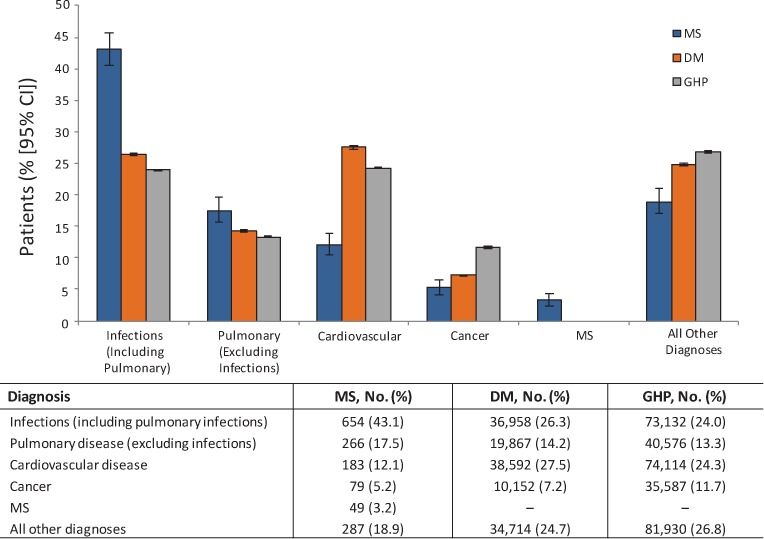
Proportion of patients with each principal diagnosis category at their final hospitalization
The most common principal diagnoses associated with death in the multiple sclerosis (MS) population were infections, pulmonary disease, and cardiovascular disease. CI, confidence interval; DM, diabetes mellitus; GHP, general hospitalized population.
The secondary diagnosis of septicemia/sepsis/septic shock was recorded in 773 patients (50.9%) in the MS cohort (Figure 4). In the DM and GHP cohorts, this secondary diagnosis was recorded less frequently (36.0% and 31.0%, respectively). Relatively little difference between cohorts was seen in rates of accidents or suicide, which were limited to those who presented to the hospital and were subsequently admitted.
Figure 4.
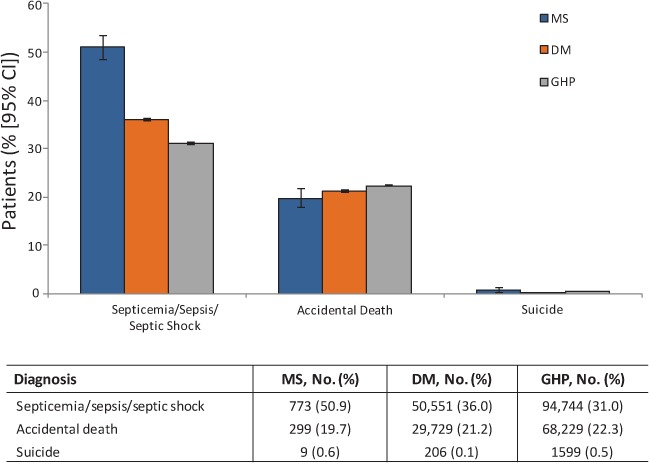
Proportion of patients with septicemia/sepsis/septic shock, accidents, or suicide listed as secondary diagnoses during their last hospital admission
A higher proportion of patients with multiple sclerosis (MS) had septicemia/sepsis/septic shock listed as a secondary diagnosis than those in the diabetes mellitus (DM) or general hospitalized population (GHP) cohorts. CI, confidence interval.
Discussion
Patients with MS in this study had an approximately 10-year lower mean age at death than those with DM or in the GHP, with most deaths occurring in individuals 55 to 69 years of age. In contrast, in the DM and GHP cohorts, most deaths occurred in those 70 years and older. The peak in mortality between 60 and 64 years of age in the MS population seems to suggest that mortality decreased with age, but this is likely not the case. Rather, it is more likely that deaths in the older patients were not captured in the database. This could have occurred if the patients died outside of the hospital or if they had switched to a different hospital that did not participate in the database. However, the proportion of deaths outside the hospital does not seem to be systematically different for patients with MS relative to those with DM or in the GHP; no data are known that suggest that more patients die at home with MS than with other disease states. The reduced life span in the MS population in this study is consistent with that in previous studies.4–6,9–11,14
The most common principal diagnosis (ie, the most common diagnosis responsible for the patient's hospitalization) in deceased patients with MS was infection, with septicemia/sepsis/septic shock commonly recorded as a secondary diagnosis. This finding is in agreement with several other studies in which infections/sepsis were commonly cited as the cause of death in patients with MS.4,5,12,13,15,17 Comparisons of causes of death across studies may, however, be confounded by methodological differences. For example, two studies that examined government records to determine causes of death differed in whether infections were considered independent of or related to MS.4,8 In a Finnish study, deceased patients were stratified into two categories: death from MS or MS-related causes (including infections related to underlying MS) and death due to any cause.16 In contrast, MS and infectious diseases were categorized as separate causes of death in a Norwegian study, regardless of the underlying cause of infection.4 Consequently, the proportion of deaths attributed to MS in the two studies differed: 70.3% in the Finnish study versus 56.5% in the Norwegian study. Such disparities may also exist among the hospitals supplying data to the Premier database.
Generally, the ICD-9-CM codes reported in the Premier database are considered to be associated with, rather than official causes of, death because of these potential reporting differences and because the information provided to the database could not be checked against official cause of death data sources, such as the National Death Index.21 The data in this article provide a fairly indirect measure of the cause of death as it may appear on a death certificate, as emphasized by the low rate at which MS was identified as the principal diagnosis.
The selection of a principal diagnosis may have been influenced by the coding system used on the death certificate. In January 1999, the United States began to use the ICD-10 coding system to classify mortality data from death certificates; however, hospitals in the United States have not yet adopted ICD-10 for their recordkeeping and reporting activities. Therefore, the Premier hospital database relies on the use of ICD-9-CM codes. Consequently, only one principal diagnosis code for each hospital stay is recorded, and it tends to capture the most prominent focus of treatment during the hospital stay as opposed to the underlying treated condition resulting in the outcome of the stay. Some patients with MS may also have had DM or other chronic diseases that were not analyzed; the comparator group of patients with DM included those having DM and possibly other chronic diseases but not MS. As such, the presence of DM as a comorbidity may increase the risk of infection in patients with MS, and possibly the risk of death.
Other potential limitations of this study must be noted. First, hospital-based data reflect care provided in the hospital setting only. Therefore, other factors outside of the patient's hospital stay that may have influenced survival outcomes (eg, medication adherence and over-the-counter drug use) were not captured. These missing data would be particularly important for the MS population because early initiation of disease-modifying therapy has been shown to prolong survival.22 Furthermore, the restriction to the hospital setting is likely to have excluded many cases of accident/suicide, and, hence, the interpretation of the findings for these causes of death is unclear. Another limitation is that different forms of MS were not distinguishable using ICD-9 coding, and patients with primary progressive MS are known to have a significantly shorter life span than those with relapsing-remitting MS,4 although a more recent publication found no difference.23
Additionally, as a comparison group, DM is common and chronic but is not primarily a neurologic condition and is often diagnosed at a later age than MS; thus, age at death might be expected to be greater for patients with DM than for patients with MS. However, this limitation does not diminish the relevance of the finding that age at death in patients with MS was found to be similar to findings in other studies.4–6,9–11,14 Moreover, DM is known to increase the risk of infection and possibly mortality, and the infection diagnosis findings in patients with MS may be related to the comorbid diabetes or another infection-prone status that was not identified.
One reason for earlier death in patients with MS, as found in this study, may be frailty secondary to severe neurologic disability; however, measures of the severity of MS disability were not available in the data. It has been recognized that physical or cognitive disability can predispose patients with MS to other illnesses, such as pneumonia, sepsis, and thrombosis, which can be fatal in and of themselves.24 Congruent with the findings of this study, a recent study using commercial insurance claims data also identified infections, aspiration pneumonias, and ischemic or embolic cardiovascular events, which are all often associated with immobility, as significant causes of death in patients with MS.25
Insofar as underrecognized deaths may have been a study limitation, it is possible that some patients in each of the comparison groups were discharged to hospice facilities (Table 1) that were not recorded by the hospital according to Centers for Medicare and Medicaid Services reporting standards. If such guidelines were not followed, it could have increased the sample size of one or more of the cohorts in this study and might have altered the distribution of other patient characteristics in one or more groups. However, it is unknown to what extent, and in what direction, any of the study results might have changed as a result of different discharge information. For example, older patients with MS may more often reside and die in nursing homes, without exposure to hospital settings, from which the study data originated.
This study has the advantage of capturing data from a large sample of patients collected over a short time frame (January 1, 2007–December 31, 2011). In contrast, previous studies reached large patient numbers only over decades-long observation periods,4,9,16 which presents the risk of introducing substantial time effects due to changes in the natural history,26 diagnostic criteria,27,28 and treatment of MS.29 Protracted time frames can, therefore, result in study populations that are not sufficiently homogeneous to draw meaningful conclusions.
In conclusion, use of the Premier database has permitted the identification of a large number of patients with MS who died in the hospital, indicating that this population has a 10-year lower mean age at death—but a lower age-adjusted mortality rate—than patients with DM and those in the GHP. Based on the analysis of objective ICD-9-CM codes, infection was the most common principal diagnosis in deceased patients with MS. Greater awareness of the pertinent diagnoses associated with the death of patients with MS during a hospitalization might help improve care for this population. Future research into differences between deaths in the hospital compared with other locations may be warranted, as may analyses of the linkages between clinically relevant issues, such as infection and other comorbid medical conditions, neurologic disability, or deaths occurring in hospitals compared with skilled nursing facilities or other locations.
PracticePoints.
Patients with MS have, on average, a shorter life span compared with non-MS populations, but the causes of in-hospital mortality in this population are not well understood.
In a retrospective analysis of a hospital discharge database, the most common principal diagnoses in the terminal hospitalization for patients with MS included infection (43.1%), pulmonary disease (17.5%), and cardiovascular disease (12.1%).
The most common secondary diagnoses were septicemia/sepsis/septic shock.
Better knowledge of the diagnoses associated with death during a hospitalization in patients with MS can improve the care of these patients.
Acknowledgments
We are grateful to the patients and hospital staff who provided the data used in this analysis. We also thank Robert C. Ristuccia, PhD, of Precept Medical Communications for medical writing assistance in the preparation of the manuscript and Volker Knappertz, MD, for contributions to earlier drafts of a similar manuscript and to related scientific presentations of early analyses of portions of the data.
Footnotes
Financial Disclosures: Dr. Ernst's former employer, Premier Healthcare Solutions Inc, contracted with Bayer HealthCare Pharmaceuticals to perform the analyses. Dr. Cutter has received compensation in the past 24 months for participation in Data and Safety Monitoring Committees for the following organizations focused on medical research: Apotek, Ascendis, Biogen Idec, Cleveland Clinic, GlaxoSmithKline Pharmaceuticals, Gilead Pharmaceuticals, Modigenetech/Prolor, Merck/Ono Pharmaceuticals, Merck, Neuren, PCT Bio, Teva, Vivus, National Heart, Lung, and Blood Institute (Protocol Review Committee), National Institute of Neurological Disorders and Stroke, National Multiple Sclerosis Society, and National Institute of Child Health and Human Development (Obstetric-Fetal Pharmacology Research Unit oversight committee). In the same period he received compensation for consulting, speaking, and advisory board membership from Alexion, Allozyne, Bayer, Consortium of Multiple Sclerosis Centers (grant), Klein Buendel Inc, Genzyme, MedImmune, Novartis, Nuron Biotech, Receptos, Revalesio, Sanofi-Aventis, Spinifex Pharmaceuticals, Somahlution, Teva Pharmaceuticals, and Xenoport. Dr. Cutter is president of Pythagoras Inc, a private consulting company in Birmingham, AL. Dr. Kaufman has received personal compensation for activities with Bayer HealthCare Pharmaceuticals, McNeil Consumer Healthcare, and UCB as a consultant; and research support from McNeil Consumer HealthCare, CSL Behring, Onyx Pharmaceuticals, Incyte Corp, and Sanofi-Aventis. Dr. Pleimes is managing director and a shareholder of Myelo Pharmaceuticals GmbH; was a salaried employee and is currently a paid consultant for Bayer Pharma AG/Bayer HealthCare Pharmaceuticals; and owns stock in Bayer AG, the owner of Bayer Pharma AG/Bayer HealthCare Pharmaceuticals.
Funding/Support: This study was funded by Bayer HealthCare Pharmaceuticals Inc.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Weinshenker BG, Bass B, Rice GP et al. The natural history of multiple sclerosis: a geographically based study, I: clinical course and disability. Brain. 1989;112(pt 1):133–146. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- 3.Bronnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain. 2004;127(pt 4):844–850. doi: 10.1093/brain/awh104. [DOI] [PubMed] [Google Scholar]
- 4.Grytten TN, Lie SA, Aarseth JH, Nyland H, Myhr KM. Survival and cause of death in multiple sclerosis: results from a 50-year follow-up in Western Norway. Mult Scler. 2008;14:1191–1198. doi: 10.1177/1352458508093890. [DOI] [PubMed] [Google Scholar]
- 5.Hirst C, Swingler R, Compston DA, Ben-Shlomo Y, Robertson NP. Survival and cause of death in multiple sclerosis: a prospective population-based study. J Neurol Neurosurg Psychiatry. 2008;79:1016–1021. doi: 10.1136/jnnp.2007.127332. [DOI] [PubMed] [Google Scholar]
- 6.Ragonese P, Aridon P, Mazzola MA et al. Multiple sclerosis survival: a population-based study in Sicily. Eur J Neurol. 2010;17:391–397. doi: 10.1111/j.1468-1331.2009.02814.x. [DOI] [PubMed] [Google Scholar]
- 7.Wynn DR, Rodriguez M, O'Fallon WM, Kurland LT. A reappraisal of the epidemiology of multiple sclerosis in Olmsted County, Minnesota. Neurology. 1990;40:780–786. doi: 10.1212/wnl.40.5.780. [DOI] [PubMed] [Google Scholar]
- 8.Sumelahti ML, Hakama M, Elovaara I, Pukkala E. Causes of death among patients with multiple sclerosis. Mult Scler. 2010;16:1437–1442. doi: 10.1177/1352458510379244. [DOI] [PubMed] [Google Scholar]
- 9.Bronnum-Hansen H, Stenager E, Hansen T, Koch-Henriksen H. Survival and mortality rates among Danes with MS. Int MS J. 2006;13:66–71. [PubMed] [Google Scholar]
- 10.Kaufman D, Reshef S, Golub H et al. Survival in commercially insured multiple sclerosis patients and comparator subjects in the U.S. Mult Scler Relat Disord. 2014;3:364–371. doi: 10.1016/j.msard.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Kingwell E, van der KM, Zhao Y et al. Relative mortality and survival in multiple sclerosis: findings from British Columbia, Canada. J Neurol Neurosurg Psychiatry. 2012;83:61–66. doi: 10.1136/jnnp-2011-300616. [DOI] [PubMed] [Google Scholar]
- 12.Koch-Henriksen N, Bronnum-Hansen H, Stenager E. Underlying cause of death in Danish patients with multiple sclerosis: results from the Danish Multiple Sclerosis Registry. J Neurol Neurosurg Psychiatry. 1998;65:56–59. doi: 10.1136/jnnp.65.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redelings MD, McCoy L, Sorvillo F. Multiple sclerosis mortality and patterns of comorbidity in the United States from 1990 to 2001. Neuroepidemiology. 2006;26:102–107. doi: 10.1159/000090444. [DOI] [PubMed] [Google Scholar]
- 14.Sadovnick AD, Ebers GC, Wilson RW, Paty DW. Life expectancy in patients attending multiple sclerosis clinics. Neurology. 1992;42:991–994. doi: 10.1212/wnl.42.5.991. [DOI] [PubMed] [Google Scholar]
- 15.Smestad C, Sandvik L, Celius EG. Excess mortality and cause of death in a cohort of Norwegian multiple sclerosis patients. Mult Scler. 2009;15:1263–1270. doi: 10.1177/1352458509107010. [DOI] [PubMed] [Google Scholar]
- 16.Sumelahti ML, Tienari PJ, Wikstrom J, Salminen TM, Hakama M. Survival of multiple sclerosis in Finland between 1964 and 1993. Mult Scler. 2002;8:350–355. doi: 10.1191/1352458502ms811oa. [DOI] [PubMed] [Google Scholar]
- 17.Kurtzke JF, Beebe GW, Nagler B, Nefzger MD, Auth TL, Kurland LT. Studies on the natural history of multiple sclerosis, V: long-term survival in young men. Arch Neurol. 1970;22:215–225. doi: 10.1001/archneur.1970.00480210025003. [DOI] [PubMed] [Google Scholar]
- 18.Wallin MT, Page WF, Kurtzke JF. Epidemiology of multiple sclerosis in US veterans, VIII: long-term survival after onset of multiple sclerosis. Brain. 2000;123(pt 8):1677–1687. doi: 10.1093/brain/123.8.1677. [DOI] [PubMed] [Google Scholar]
- 19.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People Statistical Notes, No. 20. http://www.cdc.gov/nchs/data/statnt/statnt20.pdf. Accessed May 2015. [PubMed]
- 20.Averill R, Goldfield N, Hughes J What Are APR-DRGs? An Introduction to Severity of Illness and Risk of Mortality Adjustment Methodology. Salt Lake City, UT: 3M Health Information Systems; 2003. [Google Scholar]
- 21.Centers for Disease Control and Prevention. National Death Index 2013. http://www.cdc.gov/nchs/ndi.htm. Accessed January 2013.
- 22.Goodin DS, Reder AT, Ebers GC et al. Survival in MS: a randomized cohort study 21 years after the start of the pivotal IFNβ-1b trial. Neurology. 2012;78:1315–1322. doi: 10.1212/WNL.0b013e3182535cf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harding KE, Wardle M, Moore P et al. Modelling the natural history of primary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:13–19. doi: 10.1136/jnnp-2014-307791. [DOI] [PubMed] [Google Scholar]
- 24.Goodin DS, Ebers GC, Cutter G et al. Cause of death in MS: long-term follow-up of a randomised cohort, 21 years after the start of the pivotal IFNβ-1b study. BMJ Open. 2012;2:e001972. doi: 10.1136/bmjopen-2012-001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodin DS, Corwin M, Kaufman D et al. Causes of death among commercially insured multiple sclerosis patients in the United States. PLoS One. 2014;9:e105207. doi: 10.1371/journal.pone.0105207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motheral B, Brooks J, Clark MA et al. A checklist for retrospective database studies: report of the ISPOR Task Force on Retrospective Databases. Value Health. 2003;6:90–97. doi: 10.1046/j.1524-4733.2003.00242.x. [DOI] [PubMed] [Google Scholar]
- 27.McDonald WI, Compston A, Edan G et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 28.Polman CH, Reingold SC, Banwell B et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The IFNB Multiple Sclerosis Study Group. Interferon β-1b is effective in relapsing-remitting multiple sclerosis, I: clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]


