Abstract
We describe for the first time the detection of Coxiella-like bacteria (CLB), Theileria luwenshuni, and T. ovis endosymbionts in blood-sucking deer keds. Eight deer keds attached to a Korean water deer were identified as Lipoptena fortisetosa (Diptera: Hippoboscidae) by morphological and genetic analyses. Among the endosymbionts assessed, CLB, Theileria luwenshuni, and T. ovis were identified in L. fortisetosa by PCR and nucleotide sequencing. Based on phylogeny, CLB 16S rRNA sequences were classified into clade B, sharing 99.4% identity with CLB from Haemaphysalis longicornis in South Korea. Although the virulence of CLB to vertebrates is still controversial, several studies have reported clinical symptoms in birds due to CLB infections. The 18S rRNA sequences of T. luwenshuni and T. ovis in this study were 98.8–100% identical to those in GenBank, and all of the obtained sequences of T. ovis and T. luwenshuni in this study were 100% identical to each other, respectively. Although further studies are required to positively confirm L. fortisetosa as a biological vector of these pathogens, strong genetic relationships among sequences from this and previous studies suggest potential transmission among mammalian hosts by ticks and keds.
Introduction
Deer keds (genus Lipoptena), also known as louse flies, are obligate, blood-feeding ectoparasites that belong to the Hippoboscidae family [1]. Deer keds typically parasitize deer, antelope, goat, and sheep [2]. After keds reach a suitable host, wings are broken off at the base, leaving behind a stump [1].
To date, ill effects by deer keds on hosts have not been well established [3]. Anemia and mechanical damage due to heavy infestation were suggested as clinical symptoms [3]. Recently, the importance of deer keds as a potential vector of various pathogens, including Anaplasma ovis [4], Bartonella spp. [5], Rickettsia spp. [4], and Trypanosoma spp. [6], was reported. Previous studies have generally investigated pathogens in L. cervi, another deer ked species, whereas pathogens in L. fortisetosa have not been well studied.
L. fortisetosa was first identified in Japan in 1965 [7], and since been identified in only a few other countries, including the Czech Republic [8], Poland [9], and Moldavia [10]. In South Korea, studies on the distribution of Hippoboscidae have identified two species of deer keds, L. cervi and L. fortisetosa, but L. fortisetosa was found only on Jeju island (33°29’N and 126°31’E), which has a warm oceanic climate [2,11,12]. However, pathogens carried by Lipoptena have not been well characterized.
In S. Korea, reports on vector-borne diseases and its pathogens are ubiquitous, which include anaplasmosis in human [13], Borrelia burgdorferi in human [14], Bartonella spp. in Korean water deer [15], Coxiella burnetii in raw milk [16], Hepatozoon spp. in leopard cat [17], and Theileria spp. in Chinese water deer [18]. Climate change, due to global warming, has engendered a more subtropical climate, which may increase the risk of vector-borne diseases nationally [19]. Warm summer seasons, in particular, provide an ideal environment for vectors throughout the country.
The objective of this study was to investigate the distribution of L. fortisetosa in inland regions of S. Korea, and to evaluate L. fortisetosa as a potential vector of pathogens including apicomplexans (Babesia spp., Theileria spp., Hepatozoon spp.), rickettsias (Anaplasma spp., Ehrlichia spp., Rickettsia spp.), Bartonella spp., Borrelia spp., and Coxiella spp.
Materials and Methods
Ethics statement
A wild Korean water deer in this study was road-killed and transferred to the Wildlife Treatment Center in Daegu. Ethical approval for the collection of keds and permission to conduct this study on this site were not required from any authority because the deer was dead when transferred to the center and removal of keds from deer was neither harmful nor against animal welfare. All the procedures regarding samplings and experiments were performed by veterinarians with appropriate handlings. The deer carcass was incinerated by an authorized company. While Korean water deer is designated as vulnerable species by International Union for Conservation of Nature and Natural Resources (http://www.iucnredlist.org/details/10329/0), the deer used in this study was dead. Thus, this study did not involve endangered or protected species.
Collection of ked samples and species identification
Eight keds were collected from a wild Korean water deer that was road-killed at 322 Gwahakbukro (35°39'50.00" N, 128°25'34.16" E), Dalsung, Gyeongbuk province, S. Korea, in 2015 and transferred to the Wildlife Treatment Center in Daegu, S. Korea, in 2015. Species of keds were identified using morphological characteristics [1,11,20], and through analysis of the cytochrome oxidase subunit I (cox-1) gene using primers in Table 1.
Table 1. Primers used in this study to detect pathogens in deer keds (Lipoptena fortisetosa) collected from Korean water deer.
| Species | Target gene | Name of primer | Primer sequence (5’−3’) | Expected size (bp) | Reference |
|---|---|---|---|---|---|
| L. fortisetosa | cox-1 | CI-J-1632 | TGA YCA AAT TTA YAA Y | 730 | Modified from [37] |
| CI-N-2329 | ACT GTA AAT ATR TGA TGA GCT CA | ||||
| CI-J-1718 | GGA TTT GGW AAT TGA YTA RTW CC | 519 | |||
| CI-N-2191 | GGT AAA ATT AAA ATA TAA ACT TC | ||||
| A. phagocytophilum | 16S rRNA | EE1 | TCC TGG CTC AGA ACG AAC GCT GGC GGC | 1433 | [38] |
| EE2 | AGT CAC TGA CCC AAC CTT AAA TGG CTG | ||||
| EE3 | GTC GAA CGG ATT ATT CTT TAT AGC TTG C | 928 | |||
| EE4 | CCC TTC CGT TAA GAA GGA TCT AAT CTC C | ||||
| Borrelia spp. | 5S–23S rRNA | Bb23S3 | CGA CCT TCT TCG CCT TAA AGC | 412 | [39] |
| Bb23Sa | TAA GCT GAC TAA TAC TAA TTA CCC | ||||
| Bb23S3nF | CTG CGA GTT CGC GGG AGA | 226–266 | |||
| Bb23SanR | TCC TAG GCA TTC ACC ATA | ||||
| Coxiella spp. | 16S rRNA | Cox16SF1 | CGT AGG AAT CTA CCT TRT AGW GG | 1321–1416 | [23] |
| Cox16SR2 | GCC TAC CCG CTT CTG GTA CAA TT | ||||
| Cox16SR1 | ACT YYC CAA CAG CTA GTT CTC A | 719–813 | |||
| Bartonella spp. | ITS-1 | QHVE-OF | TTC AGA TGA TGA TCC CAA GC | 736 | [15] |
| QHVE-OR | AAC ATG TCT GAA TAT ATC TTC | ||||
| QHVE-IF | CCG GAG GGC TTG TAG CTC AG | 484 | |||
| QHVE-IR | CAC AAT TTC AAT AGA AC | ||||
| Hepatozoon spp. | 18S rRNA | HepFc | ATA CAT GWG CAM AWT CTC AAC | 680 | [40] |
| HepRc | TTA TWA TTC CAT GCT GCA G | ||||
| T. ovis | 18S rRNA | To18F | CGA ATC GCG TCT TCG GAT GCG | 418 | In this study |
| To18R | GCC ACA ATG CAA AGA CTC G | ||||
| T. luwenshuni | 18S rRNA | Tlw18F | GAA TCG CAG CTT TTG CGG CG | 1061 | In this study |
| Tlw18R | ATA CCC GCA TCC TAT TTA GCA GG |
DNA extraction and PCR
DNA was extracted from whole deer keds using a DNeasy® Blood & Tissue Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions, and the quality and quantity of DNAs were estimated using an Infinite® 200 pro NanoQuant (Tecan, Männedorf, Switzerland).
PCR assays were performed using the AccuPower PCR Premix Kit (Bioneer, Daejeon, Korea) to detect: 16S rRNA sequences of the genera, Anaplasma and Coxiella; 18S rRNA sequences of the genera, Babesia, Theileria, and Hepatozoon; 5S-23S rRNA region sequences of Borrelia spp.; and an internal transcribed spacer region sequence of Bartonella spp. Commercial PCR kits were also adapted to detect Babesia spp. (AccuPower® Babesia PCR Kit, Bioneer), Theileria spp. (AccuPower® Theileria PCR Kit, Bioneer), and rickettsias (AccuPower® Rickettsiales 3-Plex PCR Kit, Bioneer) of genera, Anaplasma, Ehrlichia, and Rickettsia. PCR amplicons were estimated using gel electrophoresis with UV transillumination after ethidium bromide staining. The species of pathogens in deer keds were identified by designing species-specific primers, which are listed in Table 1, and comparing to expected amplicons.
DNA sequencing
Amplicons matching expected sizes were sequenced using BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, New York, USA) following the manufacturer’s instructions, and analyzed with ABI 3730XL DNA Analyzer (Applied Biosystems). Empirical sequences were compared with those deposited in GenBank using BLASTn.
Phylogenetic analysis
Phylogenetic relationships of L. fortisetosa, Coxiella-like bacteria (CLB), T. luwenshuni, and T. ovis investigated in this study were assessed using MEGA 6.06 based on a maximum likelihood method [21]. To estimate the reliability of constructed trees, bootstrap analysis was performed with 1,000 replicates. Host, country of isolation, and GenBank accession numbers are shown in the figure.
Results
Identification of ked species
Morphologically, keds collected in this study were flattened dorso-ventrally, with a depressed head and compound eyes (Fig 1A and 1B). The thorax and abdomen gives these insects their louse-like appearance; however, wing vestiges were observed at the margin of the thorax from the dorsal view (Fig 1B, yellow arrows). In addition, the lengths of the head and thorax regions ranged between 1.3–1.5 mm, and body lengths reached ~3.0 mm (Fig 1C and 1D). These results are consistent with characteristics of L. fortisetosa, but differ from those of L. cervi. Head and thorax region lengths of L. cervi are ~2.0 mm, and body lengths are 4–7 mm [1,11,20].
Fig 1. Morphology of the deer keds Lipoptena fortisetosa collected from a Korean water deer.
(A) Eight L. fortisetosa are 3.0 mm in length. (B) Close-up of the head of L. fortisetosa: protruded proboscis at the end of the head and compound eyes. Yellow bars indicate stump of wings. (C) Dorsal view of L. fortisetosa with strong claws at the end of six segmented legs. Bodies are coved by short hair. Head and thorax parts are nearly 1.4 mm in length. (D) Ventral view of L. fortisetosa. Marks on the ruler at the bottom of the insets in C and D are 1 mm apart.
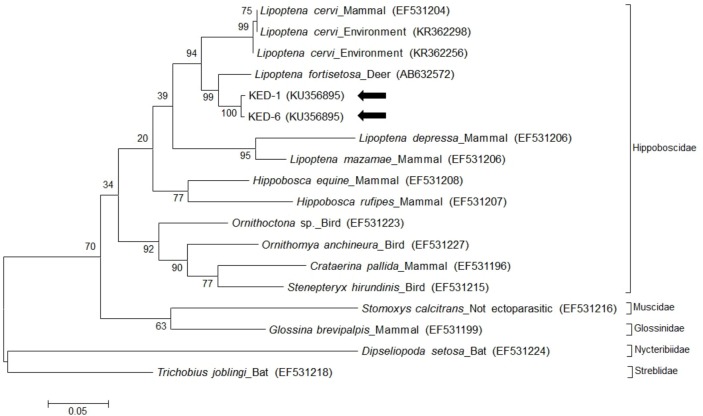
Moreover, universal primers for the cox-1 gene were used to amplify 472-bp fragments from ked samples. The sequences (accession nos. KU356895 and KU356896) of the cox-1 gene in two ked samples exhibited 95.4% and 96.0% identity, respectively, with the cox-1 gene of L. fortisetosa (accession no. AB632572) deposited in GenBank (Fig 2). Based on morphological characteristics and cox-1 sequence identity, keds collected from a Korean water deer were identified as L. fortisetosa.
Fig 2. Phylogenetic analysis of the cytochrome oxidase subunit I gene in Lipoptena fortisetosa.
The two ked sequences (KED-1, 6) are marked by arrows. Phylogenetic trees were constructed based on the maximum likelihood method with 1,000 replicates. Scale bar represents the phylogenetic distance between sequences. Species, host, and GenBank accession numbers are included in the figure.
PCR and phylogenetic analysis
In all, three species of endosymbionts in L. fortisetosa were identified by PCR. Coxiella spp. were detected in five keds, and T. luwenshuni and T. ovis were detected in six keds. None of the rickettsias, Babesia spp., Bartonella spp., Borrelia spp., and Hepatozoon spp. was detected (Table 2). Individual keds carried from zero to three endosymbionts.
Table 2. Pathogens detected in Lipoptena fortisetosa using PCR.
| ID of ked | KED-1 | KED-2 | KED-3 | KED-4 | KED-5 | KED-6 | KED-7 | KED-8 | Total |
|---|---|---|---|---|---|---|---|---|---|
| Coxiella spp. | + | + | + | + | + | - | - | - | 5/8 (62.5%) |
| Theileria ovis | + | + | + | + | - | - | + | + | 6/8 (75.0%) |
| Theileria luwenshuni | + | + | + | + | - | - | + | + | 6/8 (75.0%) |
| No. of pathogens detected | 3 | 3 | 3 | 3 | 1 | 0 | 2 | 2 |
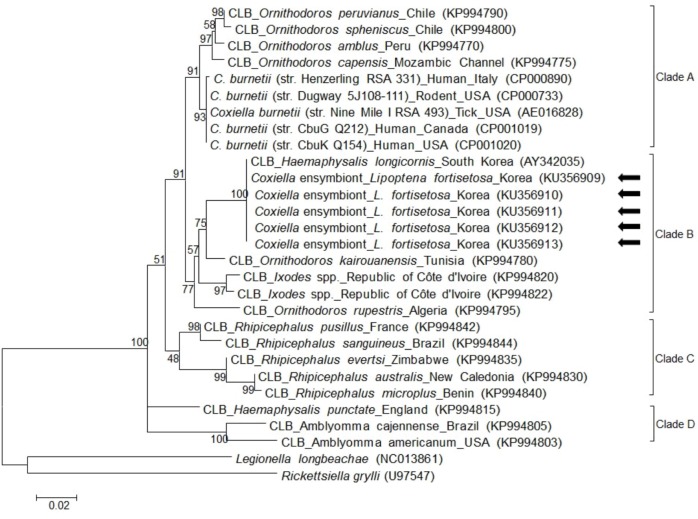
For Coxiella spp., 719-bp fragments of 16S rRNA were amplified. All five of the obtained sequences exhibited 100% identity to each other, and 99.4% identity with a Coxiella endosymbiont sequence from Haemaphysalis longicornis in S. Korea (AY342035) deposited in the GenBank database. Based on a previous study [22], CLB detected in this study were classified into clade B (Fig 3).
Fig 3. Phylogenetic analysis of Coxiella 16S rRNA in Lipoptena fortisetosa.
All 5 Coxiella-like bacteria (CLB) in this study showed 100% identity with one another and with the Coxiella endosymbiont (AY342035) of Haemaphysalis longicornis in South Korea. CLB in this study are marked with arrows. Phylogenetic trees were constructed based on the maximum likelihood method with 1,000 replicates. Scale bar represents the phylogenetic distance between sequences. Species, host, region of isolation, and GenBank accession numbers are included in figure.
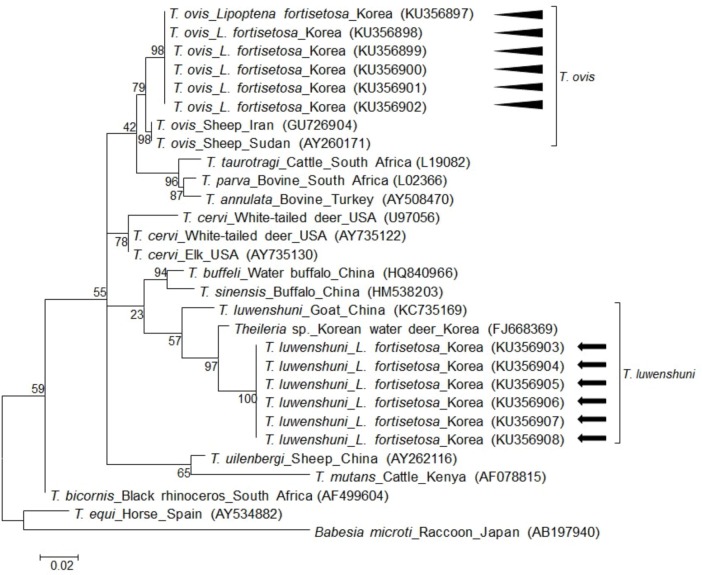
Using Theileria genus-specific primers, 259-bp fragments of Theileria 18S rRNA were amplified. However, the sequences of 259-bp amplicons were insufficient to differentiate among species since sequences were highly similar among T. cervi (100%, GU946217), T. luwenshuni (99.6%, KC769997), and T. ovis (100%, JX262363). After designing species-specific primers for T. cervi, T. luwenshuni, and T. ovis, 420-bp and 1058-bp gene fragments for 18S rRNA of T. ovis and T. luwenshuni were amplified, respectively, whereas no amplicons were detected for T. cervi. Both T. ovis and T. luwenshuni were detected in six keds. For each species, the sequences were 100% identical (Fig 4). When compared with sequences in GenBank, T. ovis shared 98.8% identity with T. ovis (KP019206), and T. luwenshuni shared 98.7% identity with T. luwenshuni (KC735157).
Fig 4. Phylogenetic analysis of the 18S rRNA in Theileria luwenshuni and T. ovis in Lipoptena fortisetosa.
All 6 T. luwenshuni detected in this study showed 100% identity with one another and with the Theileria sp. (FJ668369) identified from Korean water deer. All 6 T. ovis detected in this study showed 100% identity with one another and with T. ovis (GU726904) identified from a sheep in Iran. The sequences of T. luwenshuni and T. ovis are marked with an arrow and arrowhead, respectively. Phylogenetic trees were constructed based on the maximum likelihood method with 1,000 replicates. Scale bar represents the phylogenetic distance between sequences. Species, host, region of isolation, and GenBank accession numbers are included in the figure.
The obtained sequences in this study were submitted to GenBank. Accession numbers are KU356897–KU356902 (T. ovis), KU356903–KU356908 (T. luwenshuni), and KU356909–KU356913 (CLB).
Discussion
Coxiella genus includes C. burnetii, C. cheraxi, and unclassified CLB [22,23]. Among them, C. burnetii is a zoonotic pathogen that causes acute or chronic illness and flu-like symptoms in humans, and abortion in animals [23]. C. burnetii is shed in milk, feces, and urine from infected animals and can be transmitted by inhalation of aerosolized microorganisms [24]. Due to its environmental resistance, route of transmission, and difficulty to diagnose, C. burnetii is designated as a category B potential biological weapon by the United States [25].
According to a recent study, Coxiella spp. could be classified into four different clades (A–D), according to their genetic characteristics [22], with C. burnetii belonging to clade A. In this study, CLB detected from L. fortisetosa was classified into clade B through genetic analysis of 16S rRNA. In addition, CLB detected in our study exhibited 100% identity with Coxiella endosymbiont (AY342035) from H. longicornis in S. Korea. The perfect sequence identity of 16S rRNA between CLB in this study and AY342035 in a previous study suggests that CLB is potentially transmitted between mammals by ticks and keds.
In previous studies, C. burnetii and CLB were detected from various sources including ticks, flies, dairy cattle, raw milk, and aborted fetuses [23,24,26]. In 1958, C. burnetii was detected from Melophagus ovinus (Family: Hippoboscidae), also known as sheep ked, and confirmed as a bona fide vector of C. burnetii through animal experiments, clinical manifestations, and antigen-antibody tests [27]. Based on these results, we suggest that L. fortisetosa could act as a vector not only for CLB but also for C. burnetii, though further studies are required to confirm this hypothesis. Although the virulence of CLB to vertebrates is still controversial [23], several studies have reported clinical symptoms (systematic and even fatal) in birds due to CLB infections [28–30]. Therefore, further studies are required to assess the virulence of CLB to vertebrate.
Till now, different Theileria spp. including T. lestoquardi, T. ovis, T. uilenbergi, T. luwenshuni, T. separate, and T. recondite have been found in small ruminants [31]. Of these species, T. lestoquardi, T. luwenshuni, and T. uilenbergi are known to be highly pathogenic to sheep and goats [31]. In S. Korea, T. luwenshuni and T. ovis were reported in Chinese water deer [18], and T. luwenshuni was found in roe deer and H. longicornis [32]. In the past, T. luwenshuni was considered to be a Theileria sp. indistinct from T. ovis and T. lestoquardi that caused ovine and caprine theileriosis [33]; however, it can now be distinguished from T. ovis and T. lestoquardi based on their biological characteristics and 18S rRNA sequence [33]. This is particularly important as the pathogenicities of T. ovis and T. luwenshuni to ruminants vary. In this study, 259-bp of Theileria 18S rRNA was amplified by PCR but amplicon was insufficient to clearly differentiate the species, owing to the high sequence similarity among Theileria spp. Using species-specific primer sets, we revealed the presence of mixed infection of T. ovis and T. luwenshuni in L. fortisetosa.
The sequences of T. luwenshuni in this study showed 100% identity to a Theileria sp. (FJ668369) that was detected from a Chinese water deer in S. Korea [18], and 98.7% identity to T. luwenshuni (KC735157) from a goat in China. While the sequence FJ668369 was originally submitted simply as Theileria sp., we now propose that species is T. luwenshuni based on our phylogenetic analysis. The sequences of T. ovis in this study showed 98.8% identity to T. ovis (FJ668373) that was detected from a Chinese water deer in S. Korea [18]. The high sequence similarity between these sequences and sequences obtained in previous studies suggest the potential transmission of Theileria among mammals, ticks, and keds.
Deer, antelope, goats, and sheep are the main hosts of deer keds [2]. However, incidental infestation of deer keds in other animals, including dogs, horses, and other ruminants, has also been reported [34]. Moreover, human dermatitis caused by ked bites has been often reported in Finland [35].
Recently, due to global warming and an increasing number of wild animals in S. Korea, caution to vector-borne diseases has been raised. Vectors generally transmit pathogens mechanically or biologically [36]. The results of this study suggest that L. fortisetosa is a potential biological vector of CLB endosymbionts. However, ecological aspects, such as the life cycle and reproduction of deer keds, are yet to be determined.
In this study, we report the first identification of CLB, T. ovis and T. luwenshuni in L. fortisetosa. Further investigations are required to confirm L. fortisetosa as a biological vector of these pathogens. Moreover, owing to the possible transmission of vector-borne pathogens, members of the medical and veterinary field need to be cautious of potential contact with deer keds.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. NRF-2013R1A1A2013102). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lloyd JE. Louse flies, keds, and related flies (Hippoboscoidea) In: Mullen GR, Durden LA, editors. Medical and veterinary entomology. London: Academic press; 2009. pp. 339–352. [Google Scholar]
- 2.Yamauchi T, Tsurumi M, Kataoka N. Distributional records of Lipoptena species (Diptera: Hippoboscidae) in Japan and Jeju-do, Korea. Med Entomol Zool. 2009; 60: 131–133. [Google Scholar]
- 3.Allan SA. Biting flies (class Insecta: order Diptera) In: Samuel WM, Pybus MJ, Kocan A. Alan, editors. Parasitic diseases of wild mammals. Ames, Iowa: Iowa State University Press; 2001. pp. 18–45. [Google Scholar]
- 4.Hornok S, de la Fuente J, Biró N, Fernández de Mera IG, Meli ML, Elek V, et al. First molecular evidence of Anaplasma ovis and Rickettsia spp. in keds (Diptera: Hippoboscidae) of sheep and wild ruminants. Vector Borne Zoonotic Dis. 2011; 11: 1319–1321. 10.1089/vbz.2011.0649 [DOI] [PubMed] [Google Scholar]
- 5.Dehio C, Sauder U, Hiestand R. Isolation of Bartonella schoenbuchensis from Lipoptena cervi, a blood-sucking arthropod causing deer ked dermatitis. J Clin Microbiol. 2004; 42: 5320–5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Böse R, Petersen K. Lipoptena cervi (Diptera), a potential vector of Megatrypanum trypanosomes of deer (Cervidae). Parasitol Res. 1991; 77: 723–725. [DOI] [PubMed] [Google Scholar]
- 7.Maa TC. A synopsis of the Lipopteninae. J Med Entomol. 1965; 2: 233–248. [DOI] [PubMed] [Google Scholar]
- 8.Zeman P. Borrelia-infection rates in tick and insect vectors accompanying human risk of acquiring Lyme borreliosis in a highly endemic region in Central Europe. Folia Parasitol. 1998; 45: 319–325. [PubMed] [Google Scholar]
- 9.Kowal J, Nosal P, Rościszewska M, Matysek M. New records of Lipoptena fortisetosa Maa, 1965 (Diptera: Hippoboscidae) in Poland. Dipteron. 2009; 25: 27–29. [Google Scholar]
- 10.Metelitsa AK, Veselkin GA. Parasitism of the louse fly Lipoptena fortisetosa on cattle. Parazitologiia. 1989; 23: 276–277. [PubMed] [Google Scholar]
- 11.Choi C, Lee S, Moon K, Kang C, Yun Y. New record of Lipoptena fortisetosa (Diptera: Hippoboscidae) collected from siberian roe deer on Jeju island, Korea. J Med Entomol. 2013; 50: 1173–1177. [DOI] [PubMed] [Google Scholar]
- 12.Kim HC, Chong ST, Chae J, Lee H, Klein TA, Suh SJ, et al. New record of Lipoptena cervi and updated checklist of the louse flies (Diptera: Hippoboscidae) of the Republic of Korea. J Med Entomol. 2010; 47: 1227–1230. [DOI] [PubMed] [Google Scholar]
- 13.Kim KH, Yi J, Oh WS, Kim NH, Choi SJ, Choe PG, et al. Human granulocytic anaplasmosis, South Korea, 2013. Emerg Infect Dis. 2014; 20: 1708–1711. 10.3201/eid2010.131680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park KH, Chang WH, Schwan TG. Identification and characterization of lyme disease spirochetes, Borrelia burgdorferi sensu lato, isolated in Korea. J Clin Microbiol. 1993; 31: 1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko S, Kim S, Kang J, Won S, Lee H, Shin N, et al. Molecular detection of Bartonella grahamii and B. schoenbuchensis-related species in Korean water deer (Hydropotes inermis argyropus). Vector Borne Zoonotic Dis. 2013; 13: 415–418. 10.1089/vbz.2012.1105 [DOI] [PubMed] [Google Scholar]
- 16.Park JY, Lee WY, Cho SN, Park YS, Park KS, Youn HJ, et al. Isolation and cultivation of a Coxiella burnetii strain from raw milk of dairy cows in Korea. J Korean Soc Microbiol. 1993; 28: 285–293. [Google Scholar]
- 17.Kubo M, Jeong A, Kim S, Kim Y, Lee H, Kimura J, et al. The first report of Hepatozoon species infection in leopard cats (Prionailurus bengalensis) in Korea. J Parasitol. 2010; 96: 437–439. 10.1645/GE-2270.1 [DOI] [PubMed] [Google Scholar]
- 18.Han J, Jang H, Lee S, Na K. High prevalence of Theileria sp. in wild Chinese water deer (Hydropotes inermis argyropus) in South Korea. Vet Parasitol. 2009; 164: 311–314. 10.1016/j.vetpar.2009.05.027 [DOI] [PubMed] [Google Scholar]
- 19.Seo MG, Yun SH, Choi SK, Cho GJ, Park YS, Cho KH, et al. Molecular and phylogenetic analysis of equine piroplasms in the Republic of Korea. Res Vet Sci. 2013; 94: 579–583. 10.1016/j.rvsc.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 20.Maa TC. A synopsis of Diptera Pupipara of Japan. Pac Insects. 1967; 9: 727–760. [Google Scholar]
- 21.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013; 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duron O, Noël V, McCoy KD, Bonazzi M, Sidi-Boumedine K, Morel O, et al. The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii. PLoS Pathog. 2015; 11: e1004892 10.1371/journal.ppat.1004892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duron O, Jourdain E, McCoy KD. Diversity and global distribution of the Coxiella intracellular bacterium in seabird ticks. Ticks Tick Borne Dis. 2014; 5: 557–563. 10.1016/j.ttbdis.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 24.Ho T, Htwe KK, Yamasaki N, Zhang GQ, Ogawa M, Yamaguchi T, et al. Isolation of Coxiella burnetii from dairy cattle and ticks, and some characteristics of the isolates in Japan. Microbiol Immunol. 1995; 39: 663–671. [DOI] [PubMed] [Google Scholar]
- 25.Duron O, Sidi-Boumedine K, Rousset E, Moutailler S, Jourdain E. The importance of ticks in Q fever transmission: what has (and has not) been demonstrated? Trends Parasitol. 2015; 31: 536–552. 10.1016/j.pt.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 26.Nelder MP, Lloyd JE, Loftis AD, Reeves WK. Coxiella burnetii in wild-caught filth flies. Emerg Infect Dis. 2008; 14: 1002–1004. 10.3201/eid1406.071691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavilanis V, Duval L, Foley AR, L'heureux M. An epidemic of Q fever at Princeville, Quebec. Can J Public Health. 1958; 49: 520–529. [PubMed] [Google Scholar]
- 28.Woc-Colburn AM, Garner MM, Bradway D, West G, D'Agostino J, Trupkiewicz J, et al. Fatal coxiellosis in Swainson's Blue Mountain rainbow lorikeets (Trichoglossus haematodus moluccanus). Vet Pathol. 2008; 45: 247–254. 10.1354/vp.45-2-247 [DOI] [PubMed] [Google Scholar]
- 29.Shivaprasad H, Cadenas M, Diab S, Nordhausen R, Bradway D, Crespo R, et al. Coxiella-like infection in psittacines and a toucan. Avian Dis. 2008; 52: 426–432. [DOI] [PubMed] [Google Scholar]
- 30.Vapniarsky N, Barr BC, Murphy B. Systemic Coxiella-like infection with myocarditis and hepatitis in an eclectus parrot (Eclectus roratus). Vet Pathol. 2012; 49: 717–722. 10.1177/0300985811409251 [DOI] [PubMed] [Google Scholar]
- 31.Razmi G, Yaghfoori S. Molecular surveillance of Theileria ovis, Theileria lestoquardi and Theileria annulata infection in sheep and ixodid ticks in Iran. Onderstepoort J Vet Res. 2013; 80: 1–5. [DOI] [PubMed] [Google Scholar]
- 32.Moon K, Lee S, Choi C, Kim S, Kang C, Lee K, et al. Investigation of Theileria sp. from ticks and roe deer (Capreolus pygargus) in Jeju island. J Vet Clin. 2014; 31: 6–10. [Google Scholar]
- 33.Yin H, Schnittger L, Luo J, Seitzer U, Ahmed JS. Ovine theileriosis in China: a new look at an old story. Parasitol Res. 2007; 101: 191–195. [DOI] [PubMed] [Google Scholar]
- 34.Hermosilla C, Pantchev N, Bachmann R, Bauer C. Lipoptena cervi (deer ked) in two naturally infested dogs. Vet Rec. 2006; 159: 286–287. [DOI] [PubMed] [Google Scholar]
- 35.Härkönen S, Laine M, Vornanen M, Reunala T. Deer ked (Lipoptena cervi) dermatitis in humans–an increasing nuisance in Finland. Alces. 2009; 45: 73–79. [Google Scholar]
- 36.Thrusfield M. The transmission and maintenance of infection In: Thrusfield M, editor. Veterinary Epidemiology. Oxford: Blackwell Publishing; 2005. pp. 98–115. [Google Scholar]
- 37.Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 1994; 87: 651–701. [Google Scholar]
- 38.Barlough JE, Madigan JE, DeRock E, Bigornia L. Nested polymerase chain reaction for detection of Ehrlichia equi genomic DNA in horses and ticks (Ixodes pacificus). Vet Parasitol. 1996; 63: 319–329. [DOI] [PubMed] [Google Scholar]
- 39.Chu CY, Jiang BG, Liu W, Zhao QM, Wu XM, Zhang PH, et al. Presence of pathogenic Borrelia burgdorferi sensu lato in ticks and rodents in Zhejiang, south-east China. J Med Microbiol. 2008; 57: 980–985. 10.1099/jmm.0.47663-0 [DOI] [PubMed] [Google Scholar]
- 40.Otranto D, Dantas-Torres F, Weigl S, Latrofa MS, Stanneck D, Decaprariis D, et al. Diagnosis of Hepatozoon canis in young dogs by cytology and PCR. Parasit Vectors. 2011; 4: 55 10.1186/1756-3305-4-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.