Abstract
The probiotic function to impact human health is thought to be related to their ability to alter the composition of the gut microbiota and modulate the human innate immune system. The ability to function as a probiotic is believed to be strain specific. Strains of Lactobacillus casei are commonly utilized as probiotics that when consumed alter the composition of the gut microbiota and modulate the host immune response. L. casei strains are known to differ significantly in gene content. The objective of this study was to investigate seven different L. casei strains for their ability to alter the murine gut microbiota and modulate the murine immune system. C57BL/6 mice were fed L. casei strains at a dose of 108 CFU/day/mouse for seven days and sacrificed 3.5h after the last administration. The cecal content and the ileum tissue were collected for microbiota analysis and immune profiling, respectively. While 5 of the L. casei strains altered the gut microbiota in a strain specific manner, two of the strains did not alter the overall cecal microbiota composition. The observed changes cluster into three groups containing between 1 and 2 strains. Two strains that did not affect the gut microbiota composition cluster together with the control in their impact on pattern recognition receptors (PRRs) expression, suggesting that the ability to alter the cecal microbiota correlates with the ability to alter PRR expression. They also cluster together in their impact on the expression of intestinal antimicrobial peptides (AMPs). This result suggests that a relationship exists between the capability of a L. casei strains to alter the composition of the gut microbiota, PRR regulation, and AMP regulation.
Introduction
Probiotics are live microorganisms, which when administered in adequate amounts, confer a health benefit on the host [1]. A diverse and rapidly expanding set of health benefits have been ascribed to probiotics including: improved ability to tolerate lactose; reduction in gastrointestinal pathogens; reduction in colorectal cancer; decrease in incidence of cold and flu; and a reduction in the symptoms associated with the inflammation-related disorders, such as ulcerative colitis [2–4]. One way to improve health of the host has been thought to be via altering the gut microbiota [5,6]. Although health benefits of probiotics are known to be strain specific [7–10], strain specificity of probiotics in their capability of modulating the composition of the gut microbiota has not been well studied.
The human gastrointestinal tract hosts over 1014 cells, with hundreds of different species collectively known as the microbiota [11–13]. Gut microbiota has been shown to be a major determinant in health and disease with its impact on immunity, nutrition, and pathogenesis [14]. The relationship between the complex and dynamic community of microorganisms in the gut and host immune system functions is bidirectional. This interaction is well balanced in healthy individuals and a break down can lead to gastrointestinal inflammations and metabolic disorders [15,16].
Lactobacillus are the most common genera from which probiotics have been derived and L casei is a commonly utilized probiotic species [17]. L. casei strains have been shown to alter the microbiota in the gut and influence the host immune response [18–20]. In our previous study we investigated the relationship between probiotic dose, time since probiotic consumption, changes in the gut microbiota, and immune health [20]. We have shown that L. casei 32G administration was capable of altering the murine cecal microbiota and that the alterations were dose and time dependent. We also found that the light/dark cycle has a significant impact on the composition of the cecum microbiota, hence must be taken into consideration when designing experiments that follow microbiota composition. Additionally, we demonstrated that the increase in prevalence of Lactobacillus in the intestinal microbiota was not directly due to the fed microorganism, L. casei 32G.
L. casei inhabits a diverse set of environmental habitats such as cheese, wine, pickle, reproductive and gastrointestinal tracts of humans and animals [21]. The population structure within the L. casei species has been analyzed by Multilocus Sequence Typing (MLST) and determined to diverge into three major lineages approximately 1.5 million years ago [22]. Subsequently, comparative genome analysis demonstrates that genome content can vary by as much as 32–45% between different strains of L. casei [23].
Since L. casei strains contain large genetic variation [23], we chose to study strain specificity of L. casei. We examined the ability of seven well characterized L. casei strains [23] to alter the composition of the gut microbiota and modulate the murine innate immune system. Additionally, we examined the relationships between pattern recognition receptors (PRRs), antimicrobial peptides (AMPs), and the gut microbiota.
Material and Methods
Bacterial strains
A total of seven previously described L. casei strains isolated from different ecological niches with known genome sequences were used in this study (L. casei 12A, ATCC 334, 32G, CRF28, UW-1, BL23 and M36) [23]. Stock cultures were maintained at -80°C in MRS broth (BD Difco, Sparks, MD) with 25% (v/v) glycerol (Sigma-Aldrich, St. Louis, MO). Working cultures were prepared from frozen stocks by two sequential transfers in MRS broth and incubations were conducted statically at 37°C for 24 h and 18 h, respectively. The culture was harvested by centrifugation at 5,000 rpm for 10 min at room temperature. The pellet was re-suspended in 0.85% NaCl (w/v) and the optical density at 600 nm (OD600) determined. A volume of washed cells (based upon the OD600) sufficient to yield a 25 ml cell suspension with an OD600 of 6.0 was harvested by centrifugation at 5,000 rpm and washed with 25 ml of 0.85% NaCl. The resulting pellet was suspended in 25 ml of 0.85% NaCl to obtain a final concentration of 109 CFU/ml. The final culture solution was enumerated daily on MRS agar to confirm the dose administered to the mice.
Animals
All procedures involving mice were conducted under the protocol #V01548 approved by the Animal Care and Use Committee of University of Wisconsin-Madison. Healthy, male C57BL/6 mice aged 8 weeks were obtained from Jackson Laboratories (Bar Harbor, ME) and group housed at University of Wisconsin-Madison Animal Health and Biomedical Science facility. Housing conditions were controlled at 25°C, 20–44% relative humidity with a 12 h light/dark cycle. Mice were fed ad libitum water and mouse chow (Harlan Teklad 7964 rodent diet, Madison, WI) throughout the study. The sample size in each group was estimated to be 6 by a sample size calculation (http://www.biomath.info/power/index.htm) with 80% power (unpaired t-test; α = 0.05) to detect a significant difference between the treatments and the control. The animals (n:48) included in this study were divided into 8 groups; each group (n:6) was administered daily 100 μl of either 0.85% NaCl (control) or one of the L. casei strains at 109 CFU/ml by oral gavage for seven days. Therefore, the delivered dose was 108 CFU/day/mouse.
Sample collection
Six mice from each group were euthanized by CO2 asphyxiation at 3.5h after administration of the last probiotic dose. Immediately after euthanasia, the intestinal tract was removed for analysis. The cecum content was collected and the samples were immediately put on ice, and then frozen at -20°C until processed for microbial DNA extraction. Approximately 2 cm-tissue from the distal ileum was collected for RNA isolation and preserved in RNAlater (Ambion, Carlsbad, CA) overnight at 4°C. After the overnight treatment, the samples were stored at -80 °C until processing.
DNA extraction
The cecum digesta was homogenized in 1.5ml of PBS and total DNA from 200 μl of the homogenate was isolated using the QIAamp DNA Stool Mini Kit (Qiagen Sciences, MD) with modifications to the manufacturer’s instructions. These modifications included an initial mechanical cell disruption step by inclusion of 0.1 mm glass beads (Sigma-Aldrich) followed by exposure to six 1 min beating at maximum speed in a Mini-beadbeater-96 (Biospec Products, Inc., Bartlesville, OK) with intervals of 2 min on ice. Subsequently, a heat treatment step was performed for 5 min at 95°C. The DNA was further purified by phenol:chloroform:isoamyl alcohol (25:24:1, pH 8) extraction, phase separation using Phase Lock Gels (5 PRIME) and ethanol precipitation using pellet paint co-precipitant (EMD Millipore). DNA was quantified by Qubit® 2.0 Fluorometer (Invitrogen, Carlsbad, CA). Extracted DNA was used to perform 16S rRNA sequencing.
Ion Torrent PGM Sequencing and Microbiota Analysis
Partial 16S rRNA sequences were determined on a 318 v2 chip using the Ion Torrent Personal Genome Machine System at University of Wisconsin-Madison, Biotechnology Center. Briefly, the V1-V2 region was amplified using forward primers that contained a sample-specific bar-code with an Ion A adapter and a key sequence, while the associated reverse primer contained a truncated P1 (trP1) adapter. The sequence of these primers were: forward (8FM—5'–CCA TCT CAT CCC TGC GTG TCT CCG ACT CAG BBB BBB BBB BBB BAG AGT TTG ATC MTG GCT CAG—3') with the Ion A adapter in italics, the key sequence in italics and underlined, the 13 bp bar code designated as Bs, and the 16S primer sequence in capital letters; reverse (357R - 5'–CCT CTC TAT GGG CAG TCG GTG ATC TGC TGC CTY CCG TA- 3') with the trP1 adapter in italics and the 16S primer sequence in capital letters. All PCR reactions were quality-controlled for amplicon saturation by gel electrophoresis. Equal quantities of each of the amplicons were pooled and purified using AxyPrep Mag PCR beads (Corning, Inc.). The resulting products were quantified using PicoGreen (Invitrogen) and Qubit fluorometer (Invitrogen) before sequencing. The data processing pipeline removed low-quality reads that: 1) did not completely match the PCR primer and barcode; 2) were shorter than 300 bp or longer than 400 bp in length; or 3) had an average quality score <22. Data analysis was performed in QIIME 1.8 framework [24]. Operational Taxonomic Units (OTUs) were generated with Uclust and chosen with QIIME picking OTU workflow based upon sequence similarity with a 97% similarity threshold. Taxonomy assignments were performed with RDP Classifier and sequences were aligned by PyNAST. Taxonomic identities were assigned using greengenes version 13_5 [25].
RNA isolation and Gene Expression Analysis
Tissue samples from the distal small intestine were homogenized in UltraPure guanidine isothiocyanate solution (Invitrogen) using a tissue grinder with a smooth pestle (Thomas Scientific, Swedesboro, NJ). RNA was isolated using PureLink RNA mini kit (Invitrogen) as recommended by the supplier. Concentrations and purity of RNA samples were determined with a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA). Total RNA was treated with DNase I (Invitrogen) to remove DNA contamination and subsequently converted into cDNA using iScriptTM cDNA synthesis kit (Bio-Rad, Hercules, CA) according to manufacturer’s protocol. qPCR was performed using the primers shown at S2 Table and the customized 96-well prime PCR assays (Bio-rad) were used to screen 29 different genes of interest. SsoFast™ EvaGreen® Supermix (Bio-Rad) was used under the following conditions: initial denaturation at 95°C for 2 min, followed by 40 cycles of 5 sec at 95°C and 30 sec at 60°C. Data were acquired in the final step at 95°C for 5 sec and melting curves (65 to 95°C) were generated at the end for each set of primers. Gene expression was normalized to β-actin and relative gene expression was calculated by 2-ΔΔCt method [26].
Statistical analysis
For microbiota data, the statistical difference between treatments was examined using the Monte-Carlo test in package ade4 [27,28] of R 2.14.0 [29] as described by de Carcer et al [30]. The Monte Carlo test is a non-parametric test based on random permutations. The statistical differences of the between treatments was evaluated with the function of ade4::randtest.between. The values of zero were replaced with the detection limit, which is determined by the ratio of one to the lowest read number in the data set. The Benjamini-Hochberg procedure was applied to control the false discovery rate. The dominant genera that increased or decreased in abundance were identified by correspondence analysis in package ade4 of R 2.14.0 as described by de Carcer et al [30]. Statistical difference for relative gene expression was assessed with the Wilcoxon rank sum test (Mann–Whitney test) using JMP version 10 (SAS Institute Inc., Cary, NC) and was presented as mean ± SEM. Statistical difference was determined at a P value of 0.05 or less. Bacterial genera detected in cecum content of mice administered saline (control) or L. casei strains and fold change in the expression of the targeted genes in the ileum of mice administered L. casei were used to generate a dendrograms by the Ward method of hierarchical clustering (JMP version 10, SAS Institute Inc., Cary, NC).
Results and Discussion
Alteration of cecal microbiota by Lactobacillus casei administration is strain specific
Interactions between the GI microbiota and the innate immune system results in homeostasis that is critical to human health and disease [14]. Probiotics have been shown to alter the composition of the GI microbiota and is thought to be a possible mechanism by which they impact human health [6]. We previously demonstrated that L. casei 32G alters the composition of gut microbiota in mice and piglets [19,20]. Additionally we have demonstrated that L. casei strains differ 32–45% in gene content [23], hence significant strain-to-strain differences in their ability to alter the gut microbiota is likely. To evaluate the strain-to-strain differences for their ability to alter the cecal microbiota, we fed mice 1 dose (108 CFU/day/mouse) daily of one of the seven previously described L. casei strains (12A, 32G, ATCC 334, BL23, CRF28, M36, and UW1). These strains were chosen based on characteristics such as ecological niche of isolation and evolutionary distance. Ion Torrent PGM sequencing of cecal content was conducted to assess the influence of L. casei strains on the murine GI microbiota. The sequencing resulted in a total of 1,546,910 filtered reads from 48 mice cecum digesta samples; the number of reads varied from 10,899 to 50,113 with an average of 32,227.3 reads per sample. To assess whether sufficient sequence reads had been collected to accurately determine the diversity of organisms present, shannon and chao1 index were examined; the results of this analysis are presented in S1 Fig. These results indicate that sufficient sequence reads were obtained to accurate describe the diversity present in these samples. After the taxonomic status of each read was assigned, 11 phyla, 20 classes, 36 orders, 61 families, 94 genera were identified.
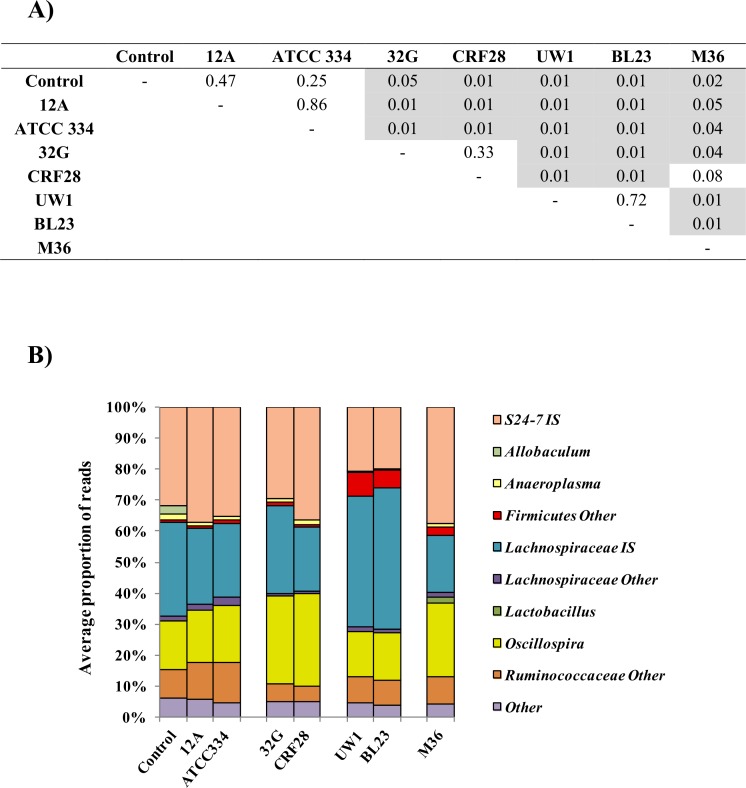
The cecum microbiota of the mice fed with different L. casei strains at 108 CFU/day dose were evaluated at the 3.5h time point, as this was the time we previously demonstrated that the 32G bolus reached the cecum [20]. To identify treatments that were significantly (p≤0.05) different from each other a Monte-Carlo test with 10,000 replicates was utilized. Treatments that are not significantly (p≤0.05) different are clustered together. The overall OTUs detected in the cecal microbiota at the genus level for 32G, CRF28, UW1, BL23 and M36-fed mice differed significantly (p<0.05) from the control mice; while, 12A and ATCC 334-fed mice did not differ significantly from the control mice, as determined by Monte-Carlo analysis (Fig 1A). Cluster analysis and Monte-Carlo test performed with OTUs at genus level revealed that L. casei strains constitute four groups based on their influence on overall microbiota composition (Fig 1). The overall cecal microbial composition of mice fed with the strain 12A or ATCC 334 clustered with the control. The 32G and CRF28-fed mice clustered together as did UW1 and BL23; while M36 did not cluster with any of the other strains. When the samples were compared at the genus level using correspondence analysis, significant (p<0.05) changes were observed between all samples and the controls, except 12A-fed mice, with 2, 4, 6, 7, 5, and 6 changes at the genera level for ATCC 334, 32G, CRF28, UW1, BL23, and M36 respectively (Table 1). These results demonstrate that most L. casei strains are capable of altering the gut microbiota and do so in a strain specific manner.
Fig 1. Comparison of the impact of Lactobacillus casei strains on the composition of cecal microbiome.
Mice were administered 1 dose (108 CFU/mouse/day) daily of L. casei 12A, ATCC334, 32G, CRF28, UW1, BL23 or M36 for 1 week and sacrificed 3.5h after the last dose. (A) Pair wise comparison of the OTUs at genus level detected in the cecal microbiome of each treatment. A Monte-Carlo test with 10,000 replicates was utilized to identify treatments that were significantly (p≤0.05) different. The values with p≤0.05 are highlighted. (B) Predominant genera in the cecum microbiome of mice administered L. casei strains. Treatments that are not significantly (p≤0.05) different are clustered together. Only genera that comprise greater than 5% of the total microbiome in at least one treatment are presented (n: 6 for each bar).
Table 1. Bacterial genera detecteda in cecum content of mice administered saline (control) or Lactobacillus casei strainsb.
| Taxon | Percentage (mean ± SE)cd | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | 12A | ATCC334 | 32G | CRF28 | UW1 | BL23 | M36 | |
| Bacteroidales S24-7 IS | 31.8±8.6 | 37.0±14.7 | 35.2±7.3 | 29.3±4.9 | 36.3±9.2 | 20.4±6.2 | 19.8±10.0 | 37.6±9.5 |
| Lachnospiraceae IS | 30.4±9.4 | 24.5±6.4 | 24.2±7.4 | 28.3±10.3 | 20.7±5.8 | 42.3±10.9 | 45.5±11.0 | 18.6±6.3 |
| Oscillospira | 15.5±10.8 | 16.8±5.2 | 18.7±5.4 | 28.5±11.8 | 30.0±7.6 | 14.8±4.0 | 15.2±5.1 | 24.1±5.3 |
| Ruminococcaceae;Other | 9.6±4.1 | 11.7±3.3 | 13.0±6.2 | 5.5±2.2 | 5.2±1.3 | 8.4±1.6 | 8.2±1.7 | 8.8±2.6 |
| Allobaculum | 2.7±6.4 | BQL | BQL | 0.2±0.4 | BQL | 0.0±0.0 | BQL | 0.0±0.1 |
| Clostridiales;Other;Other | 2.0±0.6 | 1.8±0.4 | 1.0±0.0 | 2.0±0.6 | 1.7±0.5 | 2.0±0.8 | 2.2±0.8 | 1.2±0.7 |
| Anaeroplasma | 1.8±1.8 | 1.3±0.5 | 1.3±0.5 | 1.0±1.5 | 1.5±1.0 | 0.6±0.5 | 0.3±0.5 | 1.2±1.4 |
| Lachnospiraceae;Other | 1.7±0.5 | 2.2±0.8 | 2.3±1.5 | 0.8±0.4 | 0.7±0.5 | 1.5±0.3 | 1.5±0.5 | 1.5±0.8 |
| Clostridia;Other | 1.3±1.1 | 1.0±0.6 | 1.0±0.6 | BQL | 0.2±0.4 | 0.4±0.1 | 0.3±0.5 | 0.4±0.2 |
| Ruminococcus | 0.9±0.4 | 1.2±0.4 | 1.3±0.5 | 1.0±0.0 | 1.0±0.0 | 0.7±0.2 | 0.7±0.5 | 0.9±0.1 |
| Firmicutes;Other | 0.6±0.2 | 0.7±0.8 | 0.8±0.4 | 1.3±1.5 | 0.8±0.4 | 7.5±6.7 | 5.8±5.7 | 2.4±2.7 |
| Bacteria;Other | 0.4±0.1 | 0.2±0.4 | BQL | 1.2±0.4 | 1.2±0.4 | 1.0±0.2 | 0.2±0.4 | 0.9±0.2 |
| Ruminococcaceae IS | 0.3±0.1 | BQL | 0.3±0.5 | 0.3±0.5 | BQL | 0.2±0.1 | BQL | 0.3±0.1 |
| Akkermansia | 0.1±0.2 | BQL | BQL | BQL | BQL | BQL | BQL | BQL |
| Lactobacillus | 0.0±0.0 | BQL | BQL | BQL | BQL | BQL | BQL | 1.8±4.3 |
| Number of alterationse | - | - | 2 | 4 | 6 | 7 | 5 | 6 |
aOnly genera that were present at ≥1% in a sample are included in this table.
bMice were administered 1 dose (108 CFU/mouse/day) daily of L. casei strains for 1 week and sacrificed 3.5h after the last dose.
cThe detection limit was 0.00009 and this value was used to calculate the p-value.
dGenera that differ from control within each group are shown in bold (p≤0.05). The statistical difference was examined using the Monte-Carlo test.
eThe number of genera that differed from the control for that treatment.
IS: Incertae Sedis.
BQL: Below quantifiable limit.
The overall microbiota at the phylum level differed significantly (p<0.05) from the controls in the mice fed BL23 or UW1 (S1 Table). The dominant phyla in rank order of the cecum microbiota in all of the samples were Firmicutes and Bacteriodetes. The Firmicutes and Bacteroidetes are the two major phyla found in the human and murine gut microbiota [31]. The Firmicutes/Bacteroidetes ratio has been found to have relevance to human health. For example, it has been shown that the Firmicutes/Bacteroidetes ratio was reduced in patients with Crohn’s disease, ulcerative colitis, and infectious colitis [32]. The percentage of phylum Firmicutes increased significantly (p < 0.05) in BL23 and UW1 groups of mice (from 65.7% to 79.5% and 78.0%) (S1 Table). These results indicate that some L. casei strains are capable of altering the gut microbiota at the phylum level.
The dominant genera in rank order of the cecum microbiota at genus level in all of the samples were Bacteroidales S24-7 Incertae Sedis (IS), Lachnospiraceae IS, and Oscillospira, together these genera comprise 77.5%-87% of the total microbiota (Table 1). The predominance of S24-7 IS decreased significantly (p < 0.05), from 31.8% to 19.8% and 20.4%, respectively, in the mice receiving the BL23 and UW1 strains. These strains were the only ones to result in a significant (p < 0.05) change in the level of S24-7 IS, a poorly characterized genus. The abundance of Lachnospiraceae IS was significantly (p < 0.05) different only in mice fed strains BL23, CRF28, M36, or UW1. While the abundance of Lachnospiraceae IS increased in the BL23 and UW1 group from 30.4% to 45.5% and 42.3% respectively, it decreased to 20.7% and 18.6% in the cecum of mice fed with CRF28 or M36, respectively. In contrast, Yin et al. reported a reduction in the predominance of Lachnospiraceae in fecal samples of BALB/c mice fed with BL23 in milk suspension compared to the mice fed with milk [33]. These differences could be due to the microbiota examined (cecum vs fecal), strain of mice examined, or administration of the bacterial culture as a milk suspension. Lachnospiraceae have been associated with butyrate production, which is important for epithelial cell growth [34] and has been found to be depleted in IBD patients [35]. It has been also shown that Clostridium difficile colonization can be controlled by a Lachnospiraceae isolate in germ free mice [36]. The other highly abundant genus in the cecum digesta was Oscillospira. Meta-analyses of human gut microbiota looking at the taxa associated with IBD have reported that Oscillospira abundance decreases in the subjects with Chron’s disease. Similarly, Oscillospira have found to be diminished in obese gut microbiota [37,38]. In our study, Oscillospira was significantly (p < 0.05) different only in the mice fed with 32G, CRF28 or M36 and the prevalence increased from 15.5% to 28.5%, 30% and 24.1%, respectively. Furthermore, 32G and CRF28 decreased the prevalence of Ruminococcaceae; Other from 9.6% to 5.5% and 5.2%, respectively. Predominance of genus determined as Firmicutes; Other was increased in mice cecum, after BL23, M36 and UW1 administration, from 0.6% to 5.8%, 2.4% and 7.5%, respectively. There were also marginal but significant (p<0.05) changes observed in the other genera including; Clostridiales;Other;Other, Anaeroplasma, Lachnospiraceae;Other, Clostridia;Other, Ruminococcus, Bacteria;Other, and Ruminococcaceae IS, which are presented in Table 1. Although we fed the mice with L. casei strains, the Lactobacillus genus was not one of the predominant bacterial genera detected in the cecum. This result suggests that the abundance of Lactobacillus was lower than the detection limit of Ion Torrent PGM sequencing. In our previous study, we demonstrated that the lactobacilli that increased in prevalence in the cecum microbiota of the mice fed L. casei 32G was not L. casei, rather it was L. johnsonii, a commensal lactobacilli present in the murine gut [39,40]. These results indicate that some strains are capable of altering the gut microbiota at the genus level. The observed changes cluster into three groups containing between 1 and 2 strains. The ability to alter the composition of the gut microbiota is likely to influence health, as numerous publications indicate that the gut microbiota impacts the health of the host.
Effect of Lactobacillus casei strain on intestinal barrier and innate immune system is strain specific
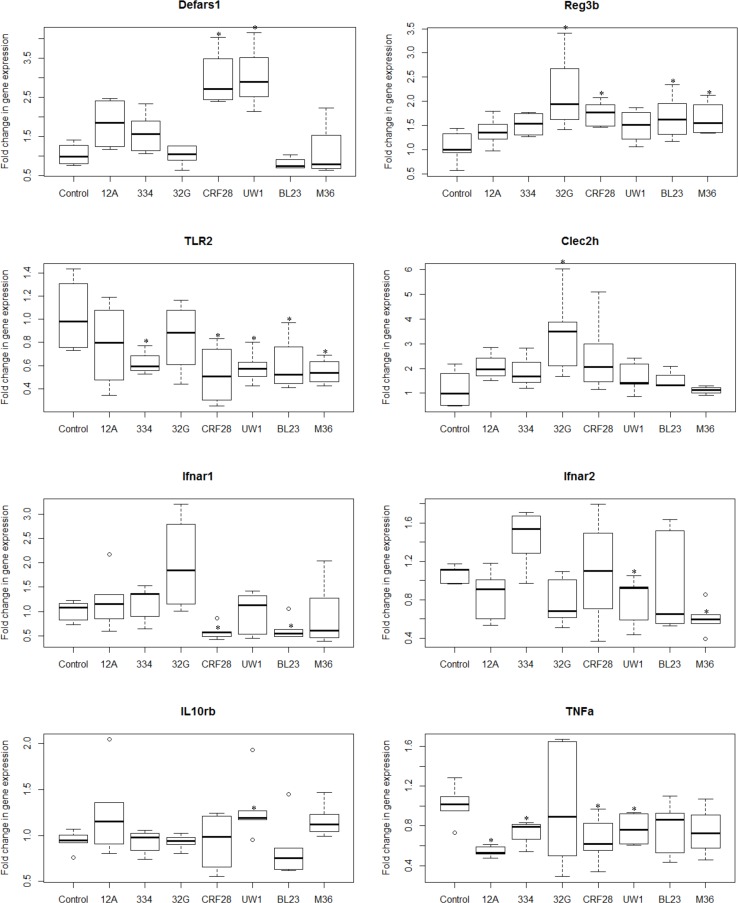
There is a well-balanced relationship between microbiota and the immune system in healthy individuals. A disruption in this balance can lead to gastrointestinal inflammation and metabolic disorders [16]. Probiotics have been shown to alter the composition of the gut microbiota and the expression of genes involved in host innate immunity [41,42]. In this study, we have demonstrated that L. casei strains alter the gut microbiota in a strain specific manner. Since there is a bidirectional interaction between the gut microbiota and host immunity, variation in the immunomodulatory capacity of individual L. casei strains is also likely. To evaluate the effect of different strains on mice intestinal barrier and innate immunity, we isolated total RNA from ileal tissue to establish a gene expression profile in mice subjected to one of the L. casei strains. We targeted 26 genes associated with the innate immune functioning and intestinal barrier. Expression of IL-12p35 and IL-12p40 genes were below the limit of detection in all samples evaluated. Eleven of the targeted genes (Lyz2, Defa3, Defa20, Defa21, Defa22, Defa23, Defa24, Defa-rs7, Pigr, ZO-1, IL-10ra,) showed no statistical differences from the control in all samples evaluated (data not shown). Thirteen of the targeted genes (Defa-rs1, Lyz1, Reg3β, Reg3γ, Clec2h, TLR2, TLR4 Ifnar1, Ifnar2, IL-10rb, Tnf-α, Occludin, and ZO-2) were expressed statistically (p<0.05) different that the expression in control mice in some samples and these results are presented in Fig 2 and S2 Fig.
Fig 2. Fold change in gene expression of antimicrobials, pattern recognition receptors, and cytokines in the ileum of mice administered L. casei 12A, ATCC 334, 32G, CRF28, UW-1, BL23 or M36.
The strains were administered 1 dose (108 CFU/ mouse) daily for 1 week and sacrificed 3.5h after the last dose; * p<0.05: significant differences from the control, (n: 6/group).
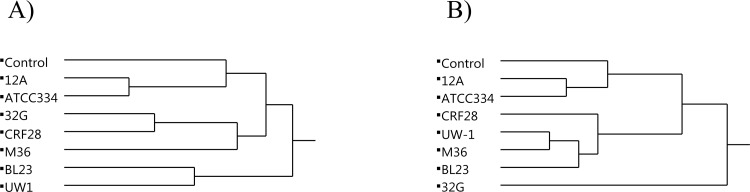
Intestinal cells recognize microbial ligands via pattern recognition receptors (PRRs) that contribute to the interaction between the gut microbiota and the innate immune system [43]. We screened intestinal PRRs including Toll-like receptors (TLRs) and C-type lectin like receptor 2h (Clec2h) to evaluate the effect of different L. casei strains on the expression of PRRs. TLRs induce the secretion of inflammatory cytokines, are involved in the maintenance of tight junctions between intestinal epithelial cells and the production of AMPs [44]. TLR4 is required for recognition of Gram (-) bacteria whereas TLR2 is the main receptor that recognizes Gram (+) bacteria [45]. TLR2 expression was significantly (p<0.05) decreased in mice fed strains ATCC 334, CRF28, UW1, BL23, and M36 whereas TLR4 expression was decreased only in mice fed BL23 and M36 (Fig 2 and S2 Fig). However, strains 12A and 32G did not have any significant effect on TLR expression. These results suggest that alteration in the expression of TLRs is a strain specific trait. The strains might have an indirect mechanism of action in suppressing the expression of TLRs. For example, L. casei strains might compete with commensals that stimulate expression of TLRs, thereby reducing TLR gene expression [46–48]. Another PRR we examined, Clec2h, was significantly (p < 0.05) up-regulated only in the mice fed-32G. A significant increase in Clec2h expression was also observed in our previous study with 32G fed mice. The function of Clec2h is poorly defined; however, it is thought to have a role in regulating innate immune responses [49]. These results demonstrate that administration of some of the L. casei strains modify the expression of TLRs and strain 32G consistently up-regulates the Clec2h expression. Additionally, cluster analysis of overall changes in expression of PRRs examined in this study revealed that ATCC 334 and 12A cluster together with the control; the clustering of the control with ATCC 334 and 12A also occurred in the composition of the cecal microbiota analysis (Fig 3). These results indicate that the ability of a strain to alter the cecal microbiota correlates with the ability to alter PRR expression.
Fig 3.
Hierarchical clustering of seven Lactobacillus casei strains and the control based on their impact on mouse cecal microbiota (A) and pattern recognition receptors in the murine ileum (B). The results presented in average.
AMPs are crucial components of innate immune system as an active intestinal mucosal defense and have an important role in shaping the composition of the intestinal microbiota [50–52]. Some AMPs, such as REG3g, require signals from commensal bacteria to be expressed, while others like lysozymes and some defensins are expressed independent of bacterial signals [53–55]. The bidirectional relationship between defensins and gut microbiota has been supported in previous studies. Menendez et al. reported that the host microbiota regulated ileal alpha defensin expression in mice exposed to oral antibiotic administration [56]. In addition, paneth cells alpha defensins have been shown to be essential in homeostatic control and shaping of the composition of intestinal microbiota [57]. In this study we examined the expression of different AMPs in the mouse small intestine to evaluate the influence of different L. casei strains on the expression of genes encoding AMPs. Strains 12A and ATCC 334 had no significant effect on the expression of AMPs in the murine small intestine (Fig 2). The other L. casei strains examined in this study varied in their effect on AMP gene expression (Fig 2). Administration of CRF28 and UW1 resulted in a significant (p < 0.05) increase in Defa-rs1 expression compared to the control mice. The expression of Reg3b was increased by strains 32G, CRF28, BL23 and M36. Lysozyme expression was decreased significantly (p < 0.05) only by M36. Expression of Reg3g was increased significantly (p < 0.05) only by strain 32G (S2 Fig). L. casei ATCC 334 and 12A were the only strains examined which did not alter the expression of any of the AMPs examined in this study (Table 2). Additionally, these two strains were the only ones that did not alter the murine gut microbiota. The other five strains alter the expression of the AMPs in a strain specific manner. Remarkably, even though we examined only a subset of the intestinal AMPs, the other 5 strains could be differentiated from each other based solely upon their influence on the expression of Defa-rs1, Reg3β, Reg3g, and lysozyme (Table 2). These results suggest a relationship exist between L. casei strains, AMP regulation, and cecal microbial composition.
Table 2. Change in gene expression of AMPs of mouse ileum fed with Lactobacillus casei strains.
| ATCC 334 | 12A | 32G | CRF28 | UW-1 | BL23 | M36 | |
|---|---|---|---|---|---|---|---|
| Defa-rs1 | NSC | NSC | NSC | ↑ | ↑ | NSC | NSC |
| Reg3β | NSC | NSC | ↑ | ↑ | NSC | ↑ | ↑ |
| Reg3g | NSC | NSC | ↑ | NSC | NSC | NSC | NSC |
| Lysozyme | NSC | NSC | NSC | NSC | NSC | NSC | ↓ |
↑: significant increase in the gene expression. ↓: significant decrease in the gene expression. NSC: No significant change in the gene expression.
PRRs regulate cytokine activity in response to microbial surface patterns. Cytokines are critical in the regulation and development of the innate immune system [58]. The effect of L. casei strains on both inflammatory and anti-inflammatory cytokines was analyzed and the strain-to-strain differences evaluated in this study. A significant (P<0.05) decrease in expression of TNF-α, a pro-inflammatory cytokine, was observed in mice fed-12A, ATCC 334, CRF28 and UW1, relative to the control mice (Fig 2). Interferons (IFN) are another group of inflammatory cytokines which signal via interferon receptors (IFNar) [59]. A reduction in the expression of the interferon receptor 1 (IFNar1) in mice fed-CRF28 and BL23 was observed. Additionally, a reduction in the expression of Interferon receptor 2 (IFNar2) was observed in mice fed-UW1 and M36. These results are significant as interferon receptors (IFNar) are required for IFNs to mediate inflammatory responses. Additionally, expression of Il-10rb, an anti-inflammatory marker, was significantly (p<0.05) increased by UW1 (Fig 2). IL-10r is required to activate members of the IL-10 subfamily of cytokines and a deficiency in IL-10rb leads to inflammatory bowel disease [60]. IL-10 suppresses the production of pro-inflammatory cytokines such as TNF-α and regulates inflammatory responses in the host [61]. Alternatively, the reduction in TNF-α expression could be due to the reduction in TLR2 expression, as TLR2 has been demonstrated to induce the production of inflammatory cytokines like TNF-α [62,63]. These results suggest that administration of L. casei strains tends to have an anti-inflammatory effect on the murine immune system that the extent and mechanism are strain specific.
Interactions between the host intestinal epithelial barrier and the gut commensal microbiota has a significant role in the health of the host in the gut [64,65]. Host intestinal epithelial barrier function is dependent on both the level and the distribution of tight junction proteins (TJPs) [66,67]. To evaluate the impact of different L. casei strains on TJPs production, we examined ileal expression of Occludin and Zonula Occludens (ZO) after administration of L. casei strains. We observed a significant (p < 0.05) increase in Occludin expression in mice fed-ATCC 334, CRF28, UW1, BL23 and M36 and a marginal increase in expression of ZO-2 noted in mice fed-12A and BL23 (S2 Fig). These results suggest that most L. casei strains are capable of strengthening intestinal epithelial barrier function via an increase in TJP gene expression.
Conclusion
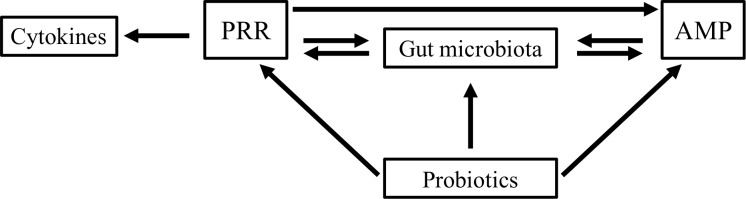
Variation in L. casei gene content is known to be large [23]; therefore, we chose to examine L. casei strain-to-strain variation in the ability to alter the gut microbiota and modulate the host immune system. This study has demonstrated that large strain-to-strain variation does exist with the L. casei species with regard to their ability to modulate the host gut microbiota and the host immune system. Additionally, our results indicate that there is a relationship between a strain’s ability to alter the composition of the gut microbiota, PRR regulation, and AMP regulation (Fig 4).
Fig 4. Proposed interaction between probiotics and the gut microbiota.
PRR, pattern recognition receptor; AMP, antimicrobial peptide.
Supporting Information
Alpha rarefaction plots based on Chao1 (A) and Shannon index (B).
(PDF)
The strains were administered 1 dose (108 CFU/ mouse) daily for 1 week and sacrificed 3.5h after the last dose; * p<0.05: significant differences from the control, (n: 6/group).
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by DuPont Inc. (Grant no. PRJ19MQ) and by the WARF (Grant no. PRJ66JZ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.FAO/WHO. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Córdoba, Argentina; 2001.
- 2.Salminen SJ, Gueimonde M, Isolauri E. Probiotics That Modify Disease Risk. J Nutr. 2005; 1294–1298. [DOI] [PubMed] [Google Scholar]
- 3.Leyer GJ, Li S, Mubasher ME, Reifer C, Ouwehand AC. Probiotic effects on cold and influenza-like symptom incidence and duration in children. Pediatrics. 2009;124: e172–e179. 10.1542/peds.2008-2666 [DOI] [PubMed] [Google Scholar]
- 4.Hörmannsperger G, Clavel T, Hoffmann M, Reiff C, Kelly D, Loh G, et al. Post-translational inhibition of IP-10 secretion in IEC by probiotic bacteria: impact on chronic inflammation. PLoS One. 2009;4: e4365 10.1371/journal.pone.0004365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owyang C, Wu GD. The gut microbiome in health and disease. Gastroenterology. 2014;146: 1433–6. 10.1053/j.gastro.2014.03.032 [DOI] [PubMed] [Google Scholar]
- 6.Ceapa C, Wopereis H, Rezaïki L, Kleerebezem M, Knol J, Oozeer R. Influence of fermented milk products, prebiotics and probiotics on microbiota composition and health. Best Pract Res Clin Gastroenterol. 2013;27: 139–155. 10.1016/j.bpg.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 7.Macho Fernandez E, Fernandez EM, Valenti V, Rockel C, Hermann C, Pot B, et al. Anti-inflammatory capacity of selected lactobacilli in experimental colitis is driven by NOD2-mediated recognition of a specific peptidoglycan-derived muropeptide. Gut. 2011;60: 1050–9. 10.1136/gut.2010.232918 [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Fatheree NY, Mangalat N, Rhoads JM. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299: G1087–G1096. 10.1152/ajpgi.00124.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, et al. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J Gastroenterol. 2007;13: 236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FAO/WHO. Guidelines for the evaluation of probiotics in food. Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food. London Ontario, Canada; 2002. pp. 1–11.
- 11.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312: 1355–9. 10.1126/science.1124234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proctor LM. The human microbiome project in 2011 and beyond. Cell Host Microbe. Elsevier; 2011;10: 287–291. 10.1016/j.chom.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 13.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. Nature Publishing Group; 2012;489: 220–30. 10.1038/nature11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milani C, Hevia A, Foroni E, Duranti S, Turroni F, Lugli GA, et al. Assessing the fecal microbiota: an optimized ion torrent 16S rRNA gene-based analysis protocol. PLoS One. 2013;8: e68739 10.1371/journal.pone.0068739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamada N, Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology. 2014;146: 1477–88. 10.1053/j.gastro.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iliev ID, Funari V a, Taylor KD, Nguyen Q, Reyes CN, Strom SP, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336: 1314–7. 10.1126/science.1221789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yakult Annual Report. An Unrivaled Advantage for Global Growth [Internet]. Tokyo, Japan; 2014. Available: http://www.yakult.co.jp/english/ir/management/pdf/ar2014.pdf.
- 18.Ivory K, Chambers SJ, Pin C, Prieto E, Arqués JL, Nicoletti C. Oral delivery of Lactobacillus casei Shirota modifies allergen-induced immune responses in allergic rhinitis. Clin Exp Allergy. 2008;38: 1282–1289. 10.1111/j.1365-2222.2008.03025.x [DOI] [PubMed] [Google Scholar]
- 19.Tandee K. Evaluation of potential probiotic Lactobacillus casei strains PhD. Thesis. University of Wisconsin-Madison. 2013. Available: http://depot.library.wisc.edu/repository/fedora/1711.dl:LS3GUH4GRCPJD8Y/datastreams/REF/content.
- 20.Aktas B, Wolfe TJ De, Tandee K, Safdar N, Darien BJ, Steele JL. The Effect of Lactobacillus casei 32G on the Mouse Cecum Microbiota and Innate Immune Response Is Dose and Time Dependent. PLoS One. 2015;10: e0145784 10.1371/journal.pone.0145784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kandler O, Weiss N. Genus Lactobacillus In: Sneath PHA, Mair NS, Sharpe ME, Holt JG, editors. Bergey’s Manual of Systematic Bacteriology. 9th ed. Baltimore: Williams & Wilkins; 1986. pp. 1063–1065. [Google Scholar]
- 22.Cai H, Rodríguez BT, Zhang W, Broadbent JR, Steele JL. Genotypic and phenotypic characterization of Lactobacillus casei strains isolated from different ecological niches suggests frequent recombination and niche specificity. Microbiology. 2007;153: 2655–65. 10.1099/mic.0.2007/006452-0 [DOI] [PubMed] [Google Scholar]
- 23.Broadbent JR, Neeno-Eckwall EC, Stahl B, Tandee K, Cai H, Morovic W, et al. Analysis of the Lactobacillus casei supragenome and its influence in species evolution and lifestyle adaptation. BMC Genomics. 2012;13: 533 10.1186/1471-2164-13-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high- throughput community sequencing data. Nat Methods. 2010;7: 335–336. 10.1038/nmeth0510-335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72: 5069–72. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods. 2001;25: 402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 27.Dray S, Dufour A-B. Journal of Statistical Software. J Stat Softw Sept. 2007;22: 1–20. [Google Scholar]
- 28.Chessel D, Dufour AB, Thioulouse J. The ade4 package—I: One-table methods. R News. 2004;4: 5–10. [Google Scholar]
- 29.TheRCoreTeam. R: A Language and Environment for Statistical Computing. 2013.
- 30.Aguirre de Cárcer D, Cuív PO, Wang T, Kang S, Worthley D, Whitehall V, et al. Numerical ecology validates a biogeographical distribution and gender-based effect on mucosa-associated bacteria along the human colon. ISME J. 2011;5: 801–9. 10.1038/ismej.2010.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen TLA, Vieira-Silva S, Liston A, Raes J. How informative is the mouse for human gut microbiota research? Dis Model Mech. 2015;8: 1–16. 10.1242/dmm.017400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, et al. Low counts of faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15: 1183–1189. 10.1002/ibd.20903 [DOI] [PubMed] [Google Scholar]
- 33.Yin X, Yan Y, Kim EB, Lee B, Marco ML. Short communication: effect of milk and milk containing Lactobacillus casei on the intestinal microbiota of mice. J Dairy Sci. Elsevier; 2014;97: 2049–55. 10.3168/jds.2013-7477 [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Dibaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106: 2365–2370. 10.1073/pnas.0812600106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank DN, St Amand AL, Feldman R a, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104: 13780–5. 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeves AE, Koenigsknecht MJ, Bergin IL, Young VB. Suppression of Clostridium difficile in the gastrointestinal tracts of germfree mice inoculated with a murine isolate from the family Lachnospiraceae. Infect Immun. 2012;80: 3786–94. 10.1128/IAI.00647-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57: 601–9. 10.1002/hep.26093 [DOI] [PubMed] [Google Scholar]
- 38.Walters W a, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588: 4223–4233. 10.1016/j.febslet.2014.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koonin E, et al. Comparative genomics of lactic acid bacteria reveals a niche-specific gene set. Proc Natl Acad Sci USA. 2006;103: 15611–15616. 10.1186/1471-2180-9-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pridmore RD, Berger B, Desiere F, Vilanova D, Barretto C, Pittet A-C, et al. The genome sequence of the probiotic intestinal bacterium Lactobacillus johnsonii NCC 533. Proc Natl Acad Sci USA. 2004;101: 2512–2517. 10.1073/pnas.0307327101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Guo X, Guo J, He Q, Li H, Song Y, et al. Lactobacillus casei reduces susceptibility to type 2 diabetes via microbiota-mediated body chloride ion influx. Sci Rep. 2014;4: 5654 10.1038/srep05654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bron PA, van Baarlen P, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Immunol. Nature Publishing Group; 2012;10: 66–78. 10.1038/nrmicro2690 [DOI] [PubMed] [Google Scholar]
- 43.Chu H, Mazmanian SK. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat Immunol. 2013;14: 668–675. 10.1038/ni.2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. Nature Publishing Group; 2010;10: 131–144. 10.1038/nri2707 [DOI] [PubMed] [Google Scholar]
- 45.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4: 499–511. 10.1038/nri1391 [DOI] [PubMed] [Google Scholar]
- 46.Kekkonen R a., Lummela N, Karjalainen H, Latvala S, Tynkkynen S, Järvenpää S, et al. Probiotic intervention has strain-specific anti-inflammatory effects in healthy adults. World J Gastroenterol. 2008;14: 2029–2036. 10.3748/wjg.14.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christensen HR, Frokiaer H, Pestka JJ, Alerts E. Lactobacilli Differentially Modulate Expression of Cytokines and Maturation Surface Markers in Murine Dendritic Cells. J Immunol. 2002;168: 171–178. 10.4049/jimmunol.168.1.171 [DOI] [PubMed] [Google Scholar]
- 48.Schuijt TJ, van der Poll T, de Vos WM, Wiersinga WJ. The intestinal microbiota and host immune interactions in the critically ill. Trends Microbiol. 2013;21: 221–229. 10.1016/j.tim.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 49.Vogler I, Steinle A. Vis-à-vis in the NKC: genetically linked natural killer cell receptor/ligand pairs in the natural killer gene complex (NKC). J Innate Immun. 2011;3: 227–35. 10.1159/000324112 [DOI] [PubMed] [Google Scholar]
- 50.Cunliffe R. α-Defensins in the gastrointestinal tract. Mol Immunol. 2003;40: 463–467. 10.1016/S0161-5890(03)00157-3 [DOI] [PubMed] [Google Scholar]
- 51.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3: 710–20. 10.1038/nri1180 [DOI] [PubMed] [Google Scholar]
- 52.Ostaff MJ, Stange EF, Wehkamp J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med. 2013;5: 1–19. doi: 10.1002/emmm.201201773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Natividad JMM, Hayes CL, Motta J-P, Jury J, Galipeau HJ, Philip V, et al. Differential induction of antimicrobial REGIII by the intestinal microbiota and Bifidobacterium breve NCC2950. Appl Environ Microbiol. 2013;79: 7745–54. 10.1128/AEM.02470-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallo RL, Hooper L V. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. Nature Publishing Group; 2012;12: 503–516. 10.1038/nri3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pütsep K, Axelsson LG, Boman A, Midtvedt T, Normark S, Boman HG, et al. Germ-free and colonized mice generate the same products from enteric prodefensins. J Biol Chem. 2000;275: 40478–40482. 10.1074/jbc.M007816200 [DOI] [PubMed] [Google Scholar]
- 56.Menendez A, Willing BP, Montero M, Wlodarska M, So CC, Bhinder G, et al. Bacterial stimulation of the TLR-MyD88 pathway modulates the homeostatic expression of ileal Paneth cell α-defensins. J Innate Immun. 2013;5: 39–49. 10.1159/000341630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11: 76–83. 10.1038/ni.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. 2011;118: 9–18. 10.1182/blood-2010-08-265892 [DOI] [PubMed] [Google Scholar]
- 59.de Weerd N a, Vivian JP, Nguyen TK, Mangan NE, Gould J a, Braniff S-J, et al. Structural basis of a unique interferon-β signaling axis mediated via the receptor IFNAR1. Nat Immunol. 2013;14: 901–7. 10.1038/ni.2667 [DOI] [PubMed] [Google Scholar]
- 60.Gertz EM, Ph D, Schäffer AA, Noyan F, Perro M, Sc M, et al. Inflammatory Bowel Disease and Mutations Affecting the Interleukin-10 Receptor. N Engl J Med. 2009;361: 2033–2045. 10.1056/NEJMoa0907206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Batista ML, Rosa JC, Lopes RD, Lira FS, Martins E, Yamashita a S, et al. Exercise training changes IL-10/TNF-alpha ratio in the skeletal muscle of post-MI rats. Cytokine. 2010;49: 102–8. 10.1016/j.cyto.2009.10.007 [DOI] [PubMed] [Google Scholar]
- 62.Kirschning CJ, Schuman RR. Toll-Like Receptor Family Members and Their Ligands Beutler B, Wagner H, editors. Germany: Springer-Verlag Berlin Heidelberg; 2002. [Google Scholar]
- 63.Paul WE, editor. Fundamental Immunology 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 64.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474: 307–17. 10.1038/nature10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barbara G, Zecchi L, Barbaro R, Cremon C, Bellacosa L, Marcellini M, et al. Mucosal Permeability and Immune Activation as Potential Therapeutic Targets of Probiotics in Irritable Bowel Syndrome. J Clin Gastroenterol. 2012;46: 52–55. [DOI] [PubMed] [Google Scholar]
- 66.Hwang I, An BS, Yang H, Kang HS, Jung EM, Jeung EB. Tissue-specific expression of occludin, Zona occludens-1, and junction adhesion molecule a in the duodenum, ielum, colon, kidney, liver, lung, brain, and skeletal muscle of C57BL mice. J Physiol Pharmacol. 2013;64: 11–18. [PubMed] [Google Scholar]
- 67.Patel RM, Myers LS, Kurundkar AR, Maheshwari A, Nusrat A, Lin PW. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. Elsevier Inc.; 2012;180: 626–635. 10.1016/j.ajpath.2011.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alpha rarefaction plots based on Chao1 (A) and Shannon index (B).
(PDF)
The strains were administered 1 dose (108 CFU/ mouse) daily for 1 week and sacrificed 3.5h after the last dose; * p<0.05: significant differences from the control, (n: 6/group).
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.