Abstract
The global primary literature on Zika virus (ZIKV) (n = 233 studies and reports, up to March 1, 2016) has been compiled using a scoping review methodology to systematically identify and characterise the literature underpinning this broad topic using methods that are documented, updateable and reproducible. Our results indicate that more than half the primary literature on ZIKV has been published since 2011. The articles mainly covered three topic categories: epidemiology of ZIKV (surveillance and outbreak investigations) 56.6% (132/233), pathogenesis of ZIKV (case symptoms/ outcomes and diagnosis) 38.2% (89/233) and ZIKV studies (molecular characterisation and in vitro evaluation of the virus) 18.5% (43/233). There has been little reported in the primary literature on ZIKV vectors (12/233), surveillance for ZIKV (13/233), diagnostic tests (12/233) and transmission (10/233). Three papers reported on ZIKV prevention/control strategies, one investigated knowledge and attitudes of health professionals and two vector mapping studies were reported. The majority of studies used observational study designs, 89.7% (209/233), of which 62/233 were case studies or case series, while fewer (24/233) used experimental study designs. Several knowledge gaps were identified by this review with respect to ZIKV epidemiology, the importance of potential non-human primates and other hosts in the transmission cycle, the burden of disease in humans, and complications related to human infection with ZIKV. Historically there has been little research on ZIKV; however, given its current spread through Australasia and the Americas, research resources are now being allocated to close many of the knowledge gaps identified in this scoping review. Future updates of this project will probably demonstrate enhanced evidence and understanding of ZIKV and its impact on public health.
Introduction
Zika virus (ZIKV) was first identified in rhesus monkeys in the Zika forest of Uganda in 1947 and has circulated in Africa and Asia relatively unnoticed for sixty years [1]. It is a Flavivirus transmitted by Aedes spp. mosquitoes, particularly Aedes aegypti, and infection is frequently asymptomatic. Clinical manifestations include rash, mild fever, arthralgia, conjunctivitis, myalgia, retro-orbital pain, headache and cutaneous maculopapular rash [2]. The epidemiology of ZIKV changed in 2007 when an outbreak occurred on Yap Island of the Federated States of Micronesia; this was the first report of infection outside of Africa or Asia. In 2013–2014 outbreaks occurred in New Caledonia, French Polynesia, the Cook Islands, Easter Island, Vanuatu, Samoa, Brazil (2015) and currently (March 2016) 31 countries in the Americas have reported autochthonous transmission [3]. With the geographic spread, a previously unreported clinical pattern began to emerge with an increase in cases of Guillain-Barré syndrome (French Polynesia) and a rise in infants born with microcephaly in Brazil [4].
A systematic summary of the global scientific knowledge regarding ZIKV and effective prevention and control measures is required to support evidence-informed decision making concerning this emerging public health issue. Scoping reviews are a synthesis method designed to address broad, often policy-driven research questions [5–7] by identifying all the relevant evidence concerning the issue and producing summaries of the findings [5,6,8]. Scoping reviews, similar to systematic reviews, follow a structured protocol for the identification and characterisation of the literature in a manner that is both reproducible and updateable [5,6,9]. Unlike a systematic review, a scoping review is well-suited to the identification of evidence on a broad topic, but does not include a quality assessment or in depth data extraction stage that would be required for meta-analysis of studies. Systematic reviews may be prioritized as a next step based on the results of a scoping review if adequate research exists on specific questions of interest [9,10]. Another important output of a scoping review is the identification of where evidence is lacking or non-existent to help direct future research and use of resources.
Objective
In response to the current ZIKV outbreaks and changes in its epidemiology, a scoping review was conducted to capture all published literature addressing the following aspects of ZIKV: 1) ZIKV infection in humans, any host or vector—pathogenesis, epidemiology, diagnosis, conditions for virus transmission, and surveillance for ZIKV, 2) studies on ZIKV—pathogenesis, transmission and molecular mechanisms, 3) prevention strategies to prevent ZIKV infections and/or control of ZIKV harbouring vectors, and 4) societal knowledge, perception and attitudes towards ZIKV.
Methods
Review protocol, team and expertise
To ensure the scoping review methods were reproducible, transparent and consistent, a scoping review protocol was developed a priori. The protocol, list of definitions, search algorithms, abstract screening form and data characterization and utility form (DCU) are available upon request. The location of the repository of relevant articles and the dataset resulting from this review is available upon request.
The review team consisted of individuals with multi-disciplinary expertise in epidemiology, microbiology, veterinary public health, knowledge synthesis and information science.
Review question and scope
This scoping review was conducted to answer the following question: What is the current state of the evidence on ZIKV pathogenesis, epidemiology, risk factors, diagnosis, surveillance methods, prevention and control strategies and, knowledge, societal attitudes and perceptions towards infections in humans, mosquito vectors and animal reservoirs?
Search strategy
To ensure the search was comprehensive the term “zika” was implemented in the following bibliographic databases on January 27 2016 and updated March 1, 2016: Scopus, PubMed/MEDLINE, Embase, CINAHL (Cumulative Index to Nursing & Allied Health), CAB, LILACS (South American), Agricola and the COCHRANE library for any relevant trials in the trial registry. No limits were placed on the search and it was pretested in Scopus.
The capacity of the electronic search to identify all relevant primary research was verified by hand searching the reference lists of three ZIKV risk assessments, 19 literature reviews. Reference lists of the 3 risk assessments [11–13] and 19 selected relevant literature reviews were evaluated for additional research that had been omitted by the bibliographic database search [14–29]. From this exercise 34 grey literature reports with primary information and peer-reviewed primary literature articles were identified and added to the scoping review process.
Additional grey literature was identified by hand-searching the websites of the World Health Organisation (http://www.who.int/csr/disease/zika/en/), Pan American Health Organisation (http://www.paho.org/hq/index.php?option=com_content&view=article&id=11585&Itemid=41688&lang=en), Center for Disease Control and Prevention (http://www.cdc.gov/zika/index.html), Morbidity and Mortality Weekly Report (http://www.cdc.gov/mmwr/zika_reports.html), European Center for Disease Control (http://ecdc.europa.eu/en/healthtopics/zika_virus_infection/Pages/index.aspx) and ProMed-mail (http://www.promedmail.org/) for primary research reports, guidelines, epidemiological alerts, situation reports, surveillance bulletins and referenced publications that were not already captured. One hundred and eleven additional references were added to the project, many of these were guidelines / government reports and new articles that have not been indexed in the bibliographic databases yet.
Relevance screening and inclusion criteria
A screening form was developed a priori to screen abstracts, titles and keywords of identified citations. Primary peer-reviewed articles were considered relevant if they addressed one or more aspects of the research question, conducted anywhere and anytime. Presently only articles in English and French are included but articles in Spanish and Portuguese are identified and can be included when resources are available. Primary research was defined as original research where authors generated and reported their own data.
Study Characterisation and extraction
Complete articles of potentially relevant citations were reviewed using a data characterization and utility (DCU) form consisting of potentially 52 questions designed a priori to confirm article relevance, data utility and extraction of the main characteristics including reported information, study methodology, populations, intervention strategies, sampling, laboratory tests and outcome characteristics.
Scoping review management, data charting and analysis
All potentially relevant citations identified by the literature search were imported into reference management software (RefWorks 2.0, ProQuest LLC, Bethesda, Maryland, USA), where duplicates were manually removed; the list of unique citations was then imported into a web-based electronic systematic review management platform (DistillerSR, Evidence Partners, Ottawa, Canada). All stages of the scoping review from relevance screening to data extraction were conducted within this software. Two reviewers independently completed all steps of the scoping review.
Data collected in the DCU form were exported into Excel spreadsheets (Microsoft Corporation, Redmond, WA), formatted, and analyzed descriptively (frequencies and percentages) to facilitate categorization, charting and discussion.
Results
Scoping review descriptive statistics
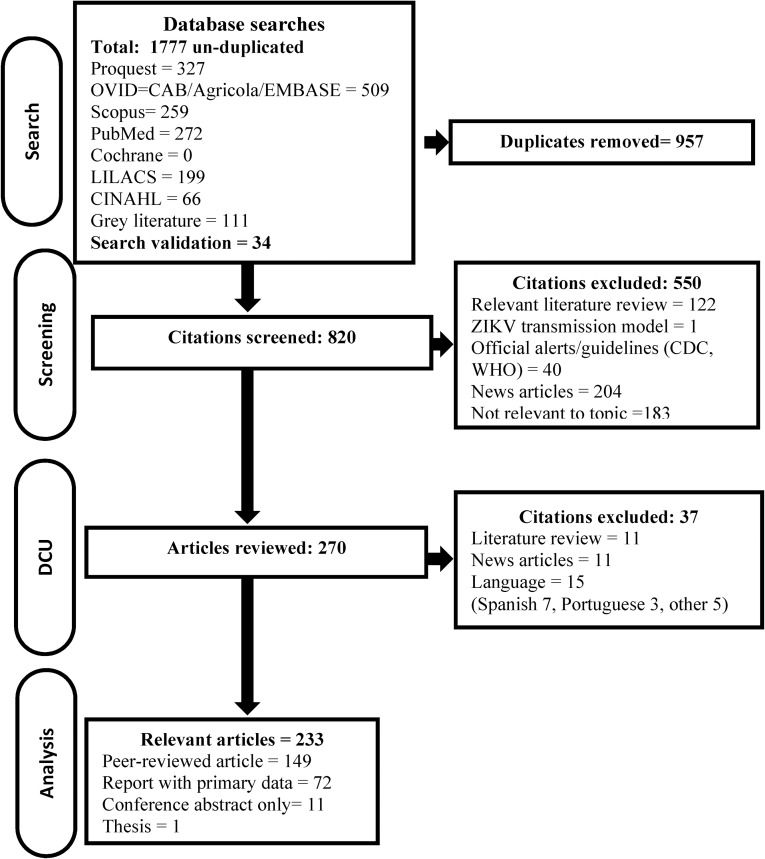
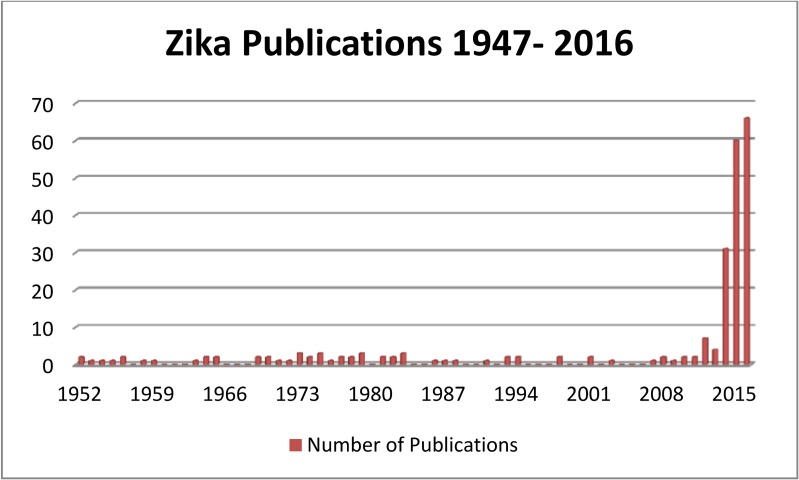
Of the 820 abstracts and titles screened for relevance, 270 were considered potentially relevant primary research/data and the full article was obtained, data was extracted and categorized for 233 relevant primary research articles in English or French, Fig 1. Research was scarce and sporadic after the virus was first isolated in 1947, until the outbreak on Yap Island, Micronesia in 2007; the majority of primary research on ZIKV has been published since 2011, 73.0% (170/233), Fig 2.
Fig 1. Flow of citations and articles through the scoping review process last updated March 1, 2016.
Fig 2. Number of primary literature publications on ZIKV by year of publication since its discovery in 1947 to search date March 1, 2016.
The general characteristics of the included articles are described in Table 1. Most included articles were peer-reviewed 63.9% (149/233) and in English 91.0% (212/233). Fifteen articles were in languages other than French and English (Spanish (7), Portuguese (3), Chinese (3), Russian (1), and German (1)) and Fig 1. The largest body of research is now from the Americas 34.87% (81/233), followed by Australasia 24.9% (58/233) and Africa, 24.0% (56/233), the remaining research was fairly evenly distributed over the other continents. The articles mainly covered three topic categories: epidemiology of ZIKV (surveillance and outbreak investigations) 56.6% (132/233), pathogenesis of ZIKV (case symptoms/ outcomes and diagnosis) 38.2% (89/233) and ZIKV studies (molecular characterisation and in vitro evaluation of the virus) 18.5% (43/233). There has been little reported in the primary literature on ZIKV vectors (12/233), ZIKV surveillance (13/233), diagnostic tests (12/233) and transmission (10/233). Three papers reported on ZIKV prevention/control strategies [30–32], one investigated knowledge and attitudes of health professionals [33] two vector mapping studies [34,35] and one ZIKV transmission model were reported [36]. The majority of studies used observational study designs, 89.7% (209/233) of which 62/233 were case studies or case series, while fewer (24/233) used experimental study designs.
Table 1. General characteristics of 233 included primary research publications.
| Category | Count | |
|---|---|---|
| Type of citation | ||
| Primary peer-reviewed | 149 | |
| Grey literature with primary data | 72 | |
| Conference proceeding | 11 | |
| Thesis | 1 | |
| Language | ||
| English | 212 | |
| French | 21 | |
| Continent | ||
| Central America/South America/Caribbean | 64 | |
| Australasia | 58 | |
| Africa | 56 | |
| Europe | 25 | |
| Asia | 20 | |
| North America | 17 | |
| Date of publication | ||
| Pre 1960 | 11 | |
| 1960–1970 | 8 | |
| 1971–1980 | 18 | |
| 1981–1990 | 10 | |
| 1991–2000 | 7 | |
| 2001–2010 | 9 | |
| 2011-Present | 170 | |
| ZIKV topic category | ||
| Epidemiology | 132 | |
| Pathogenesis | 89 | |
| Virus study | 43 | |
| Surveillance | 13 | |
| Diagnostic tests | 12 | |
| Vector study | 12 | |
| Transmission | 10 | |
| Mitigation | 3 | |
| Qualitative | 1 | |
| Other model | 1 | |
| Study design | ||
| Observational study | 209 | |
| Outbreak investigation | 67 | |
| Case study or case-series | 62 | |
| Prevalence survey | 48 | |
| Surveillance or monitoring program | 14 | |
| Cross-sectional | 7 | |
| diagnostic test evaluation | 7 | |
| Molecular epidemiology | 6 | |
| Longitudinal study | 1 | |
| Case control | 1 | |
| Experimental study | 24 | |
| Challenge trial | 22 | |
| Controlled trial | 2 | |
| Vector mapping model | 2 | |
In the 233 included articles, human populations were the most frequently reported (191/233) with 15 studies focusing solely on pediatric populations (<16 years old), Table 2. Blood was the most common sample (179/191) used to evaluate ZIKV infection or seropositivity in humans, Table 2. Monkeys were the most frequently studied non-human hosts (7/13) followed by three recent studies (4 citations) on bats from which none report ZIKV isolation, Table 2. Mice were used for the majority of animal model experiments (13/15) while monkeys were used in 4/15 studies, Table 2.
Table 2. Human and non-human host populations studied in 233 included articles.
| Category | Count | ||
|---|---|---|---|
| Humans | 191 | ||
| adults/ all ages | 176 | ||
| pediatric only | 15 | ||
| human samples | |||
| blood | 179 | ||
| urine | 16 | ||
| saliva | 10 | ||
| fetus / placenta | 8 | ||
| amniotic fluid | 5 | ||
| head circumference | 4 | ||
| cerebrospinal fluid | 4 | ||
| semen | 2 | ||
| breast milk | 2 | ||
| nasopharynx swab | 2 | ||
| conjunctivae swab | 1 | ||
| Host | 13 | ||
| bats | 4 | ||
| chicken/ duck (domestic) | 1 | ||
| domestic ruminants | 1 | ||
| horse | 1 | ||
| monkey | 7 | ||
| rodents | 2 | ||
| small mammals | 1 | ||
| wild birds | 1 | ||
| Animal model | experimental animals | 15 | |
| Mice | 13 | ||
| Non-human primates | 4 | Rhesus monkey | |
| Other animal species | 2 | cotton rats, guinea pigs, rabbits | |
| Vectors | 45 mosquito species | 26 | See Table 4 |
ZIKV virus studies
A small number of studies examined the attributes of ZIKV, Table 3. Early reports describe a 40 mμ, spherical shaped virus [37] that infects the nucleus of cells [27]. Others examined the antigenic relatedness of various Flaviviruses [26,38]. The virus growth, replication and survival in human cell lines [39] and in vivo (mice) was investigated [40] and the pathogenesis in mice has also been described [38,41,42]. The evaluation of a mouse model for virus propagation is described in three studies [42–44]; the mouse model was also used in 13 studies to identify and propagate ZIKV. In one report, researchers describe the use of chick embryos and inoculation of the yolk sac for virus propagation as being comparable to intracerebral inoculation of mice [45]. Earlier studies were identified that examined the cross neutralization tests and cross complement fixation tests on a number of new and known viruses to examine their relatedness [45] and used a monkey model to show there was no cross-immunity with yellow-fever and ZIKV [46].
Table 3. 45 studies examined the ZIKV characteristics: genotype, molecular characterisation, pathogenic attributes and virus propagation in mice.
| Number of studies | |
|---|---|
| Genotype reported: | |
| Asian lineage | 33 |
| East/Central/South African lineage (Uganda) | 9 |
| West African lineage (Senegal/Nigerian) | 5 |
| Molecular characterisation: | |
| Phylogeny was reported (e.g. dentogram, tree) | 26 |
| Virus was reported to be partially or fully sequenced | 25 |
| Evolution of the virus discussed | 12 |
| Examined proportion of nucleotide identities among strains and/or codon adaptation and fitness | 5 |
| Pathogenic attributes: | |
| Virus propagation in mice via intracerebral inoculation | 4 |
| Antigenic relatedness of Flaviviruses | 2 |
| Viral entry into cells | 2 |
| Virus morphology (ZIKV: 40 mµ, spherical in shape) | 1 |
| Presence of virus-specific antigen in the nuclei of Zika virus-infected cells (vertebrate & invertebrate) | 1 |
| Virus growth in vitro | 1 |
| Virus spread within mice | 1 |
| Virus propagation in chick embryos | 1 |
ZIKV genotype was provided in 17.6% (41/233) of studies, 36 observational studies and 5 experimental studies, Table 3 [21,32,39,46–81]. One study failed to report the genotype even though sequencing was done [82]. Due to the outbreaks in Australasia since 2007 and more recently in the Americas, many of the studies reporting genotype reported the Asian type (33/41), Table 3. With respect to studies that lend evidence to the molecular characterisation of the virus, authors frequently reported the virus had been partially sequenced, provided a gene bank number or provided a phylogenic tree in the paper [21,47,50–55,57–63,66–81,83]. A number of studies discussed ZIKV genetic relatedness other Flaviviruses and the evolution of ZIKV [47,50–54,57–59,63,68,70,83]. In a few studies the evolution of the virus (12/41) was discussed or proportions of nucleotide identities across strains (5/41) were provided in the paper. A recent paper examined codon adaptation and fitness [51] and concluded that across studies no particular mutations or evidence of adaptation to a new vector would explain the recent spread through the Pacific Islands to the Americas.
Zika Vectors
Known ZIKV vectors are all mosquitos; no evidence was identified for any other type of vector. Table 4 reports on the 26 mosquito vector studies that tested 45 different mosquito species for either a natural infection with ZIKV or evaluated the mosquito species for vector competence to transmit ZIKV. Eighteen species of mosquitos were found to be positive for ZIKV during epidemiological sampling in Africa and Asia from 1956 to 2015 and eight were evaluated experimentally for vector competence, Table 4. Ae. aegypti 15/26, and Ae. africanus 10/26 were investigated most often. Ae. albopictus, a species of particular interest as a possible vector for ZIKV in North America, was evaluated for vector competence in one study and as a naturally infected vector of ZIKV in two studies.
Table 4. Vector species evaluated in 26 studies for carriage of ZIKV or vector competence to transmit ZIKV.
| Ref | Mosquito species | Number of Studies | Challenge trials | Naturally infected with ZIKV studies |
|---|---|---|---|---|
| Total Studies: | 26 | 10 | 12 | |
| [31,35,64,83–95] | Aedes aegypti | 16 | 6 | 5 |
| [31] | Aedes aegypti formosus | 1 | 1 | |
| [1,35,38,44,70,91,95–98] | Aedes africanus | 10 | 2 | 4 |
| [65,83,87] | Aedes albopictus | 3 | 1 | 1 |
| [97] | Aedes apicoargenteus | 1 | 1 | |
| [93] | Aedes argentepunctatus | 1 | ||
| [93] | Aedes cozi | 1 | ||
| [93,94] | Aedes cumminsi | 2 | ||
| [35,86,91,93,99] | Aedes dalzielii | 5 | 4 | |
| [86] | Aedes fowleri | 1 | 1 | |
| [35,86,91–94,96,99] | Aedes furcifer | 8 | 4 | |
| [85,100] | Aedes hensilii | 2 | 1 | |
| [91] | Aedes hirsutus | 1 | 1 | |
| [98] | Aedes ingrami | 1 | ||
| [35,84,86,91,93–96,99] | Aedes luteocephalus | 9 | 1 | 5 |
| [35,91,94] | Aedes metallicus | 3 | 1 | |
| [35,86] | Aedes minutus | 2 | 1 | |
| [35,86,93] | Aedes neoafricanus | 3 | 1 | |
| [70,93,94] | Aedes opok | 3 | 1 | |
| [35] | Aedes patas | 1 | ||
| [83,95] | Aedes simpsoni | 2 | ||
| [35,86,91,93,99] | Aedes taylori | 5 | 3 | |
| [84,91,93] | Aedes unilineatus | 3 | 1 | 1 |
| [85] | Aedes vexans | 1 | ||
| [35,84,86,91–93,95,99] | Aedes vittatus | 8 | 1 | 2 |
| [35] | Aedes coustani | 1 | 1 | |
| [91] | Anopheles coustani | 1 | ||
| [35,83,94] | Anopheles gambiae | 3 | ||
| [94] | Anopheles nili | 1 | ||
| [35] | Cercopithecus aethiops | 1 | ||
| [98] | Chrysops centurionis | 1 | ||
| [98] | Chrysops funebris | 1 | ||
| [85] | Coquillettidia crassipes | 1 | ||
| [98] | Culex annulioris | 1 | ||
| [85] | Culex gossi | 1 | ||
| [85] | Culex nigropunctatus | 1 | ||
| [91] | Culex perfuscus | 1 | 1 | |
| [94] | Culex poicilipes | 1 | ||
| [83,85] | Culex quinquefasciatus | 2 | ||
| [85] | Culex sitiens | 1 | ||
| [83] | Eretmapodites quinquevittatus | 1 | ||
| [85] | Lutzia fuscana | 1 | ||
| [83] | Mansonia africana | 1 | ||
| [98] | Mansonia aurites | 1 | ||
| [35,83,91,99] | Mansonia uniformis | 4 | 2 |
Experimental studies examining vector competence and feeding activity under laboratory conditions (24–29 C and 75–95% relative humidity) mainly focused on Ae. aegypti and provided data on infection rates, dissemination rates, transmission rates, minimum infection rate, entomologic inoculation rate or mean biting rate, Table 5. Studies on Ae. aegypti demonstrated that individual mosquitos held under laboratory conditions transmitted ZIKV 33–100% of the time and transmission of the virus occurred irrespective of whether the mosquito was engorged with a blood meal [90]. In one experiment infected Ae. aegypti transmitted ZIKV to a rhesus monkey 72 days post mosquito infection [89]. Mitigation by biological control was investigated in one experiment where the researcher examined the transferability of mosquito resistance to ZIKV between species (Ae. aegypti and Ae. formosus), however they concluded the resistance gene is complex and not easily manipulated [31].
Table 5. Summary of eight studies on vector competence and behaviour.
| Ref | Country | Year study conducted | Mosquito Species | Infection rate | Dissemination rate | Transmission rate | Proportion infected | Proportion disseminated ZIKV | MIR | EIR | MBR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Africa | 3dpi | 5 dpi | 8 dpi | 10 dpi | 15 dpi | 20–60 dpi | 4 dpi | 7 dpi | 10 dpi | 15 dpi | |||||||||
| [84] | Senegal | 2015 | Ae. aegypti | highest 10dpi | 0% | 0–10% | 0–50% | 55% | 5.80% | ||||||||||
| Ae. unilineatus | highest 10dpi | 0–10% | 18.70% | 5.30% | |||||||||||||||
| Ae. vittatus | highest 10dpi | 0% | 0–100% | 0–100% | 20–50% | 14.40% | 27% | ||||||||||||
| Ae. luteocephalus | highest 15dpi | 0% | 0–100% | 20–50% | 75% | 42.20% | |||||||||||||
| [91] | Senegal | 2011/04–2011/12 | Ae. aegypti, Ae. africanus, Ae. dalzielii, Ae. furcifer, Ae. vittatus, Cx. perfuscus, Ae. taylori, Ae. hirsutus and Ae. metallicus, Ae. unilineatus, Ma. Unif, An. coustani, An. andoustani | 3–10 | 0.005–0.052 | 0.48 to 12.78 | |||||||||||||
| [90] | Senegal | 1976 | Ae. aegypti | 88% | 79–100% (7–30 dpi)/ titer 2–6 | ||||||||||||||
| [89] | Nigeria | 1956 | Ae. aegypti | 10 3.4 | 104.7 to 5.6 | ||||||||||||||
| Asia | |||||||||||||||||||
| [65] | Singapore | 2013 | Ae. albopictus | 100% at 3dpi | 25%+ | 50%+ | 100% + | 100%+ | 100%+ | first isolate+ | 100%+ | ||||||||
| [64] | Singapore | 2012 | Ae. aegypti | 87.5% at 3 dpi, 100% at 6 dpi | first obs 4 dpi + | 62%+ | 100%+ | 100%+ | |||||||||||
| [85,100] | Yap island | 2007/04–2007/07 | Ae. (Stegomyia) hensilii | 7.1% (4.9 titre*) | 0% (4.9% titer*) | ||||||||||||||
| 80% (5.7 titer*) | 12.5% (5.7 titer*) | ||||||||||||||||||
| 86.1% (5.9 titre*) | 22.5% (5.9 titer*) | ||||||||||||||||||
* log10 pfu/ml, environment "humid" and 28C, dpi = days post infection,
+ dissemination rate = divide the number of infected salivary glands by the total number of mosquitoes with infected midgets. Transmission rate = divide the number of positive saliva by the number of infected salivary glands. MIR = Minimum infection rate, estimated number of positive mosquitoes per 1000 mosquitoes tested. EIR = Entomologic inoculation rate, number of infected mosquito bites per person, per evening. MBR = mean biting rate, number of female mosquitoes captured per person per evening
Active mosquito surveillance programs were reported in three studies in Uganda, Senegal and Burkina Faso between 1946 and 1986, where routine samples were collected [35,94,97]. Studies that conducted surveillance activities or prevalence surveys of mosquitos for ZIKV were identified from Africa and Asia and several species were implicated as possible vectors including Ae. aegypti (5 studies) and Ae. albopictus (1 study), Table 6. The results of the studies in Table 6 were not necessarily conducted when ZIKV was actively circulating.
Table 6. Twelve studies from Africa and Asia between 1956 and 2011 that sampled mosquitos for ZIKV.
Studies were conducted as prevalence surveys or part of entomological surveillance for arboviruses.
| Ref | Country | Date / place of sampling | ZIKV positive mosquito species | Positive/N | Other epidemiological information | Other mosquito species tested |
|---|---|---|---|---|---|---|
| Africa | ||||||
| [94] | Burkina Faso | 1984/09–1984/11: area not specified | Ae. furcifer, Ae. luteocephalus, Ae. opok | 9/ 1853 pools | Based on entomological arbovirus surveillance 1983–1986. | Ae. aegypti, Cx. poicilipes, Ae.metallicus, Ae. cumminsi, Anopheles gambiae, An. nili |
| [92] | Côte d’Ivoire | 1999: area not specified | Ae. vittatus, Ae. furcifer, Ae. aegypti. | 3/159 pools | ||
| [83] | Gabon | 2007: Libreville suburbs | Ae. albopictus | 2/247 pools | First isolation of ZIKV in Gabon in 2007 during a simultaneous outbreak of Chikungunya and Dengue. | Ae. aegypti, Cx quinquefasciatus, Ae. simpsoni complex, An. gambiae, M. africana, M. uniformis, Cx. quinquefasciatus, Eretmapodites quinquevittatus |
| [95] | Nigeria | 1969: Jos Plateau | Ae. luteocephalus | 2/205 pools | Ae. aegypti, Ae. africanus, Ae. vittatus, Ae. simpsoni | |
| [91] | Senegal | 2011/06–2011/12: Kedougou area | Ae. luteocephalus (5 pools), Ae. africanus (5), Ae. vittatus (3), Ae. taylori (2), Ae. dalzielii (2), Ae. hirsutus (2) and Ae. metallicus (2), Ae. aegypti (1), Ae. unilineatus (1), M. uniformis (1), Cx. perfuscus (1), An. coustani (1) | 31/1700 pools | Thirty-one ZIKV infected pools: June (9.7%), September (32.2%), October (35.5%), November (19.3%) and December (3.2%) | An. coustani, Ae. andoustani, Ae. furcifer |
| [93] | Senegal | 1990: Senegal | Ae. aegypti, Ae. dalzielii, Ae. luteocephalus | 3/151 pools | Ae. taylor, Ae. unilineatus, Ae. cozi, Ae. neoafricanus, Ae. opok, Ae. cumminsii, Ae. argentepunctatus, Ae. furcifer, Ae. vittatus | |
| [86] | Senegal | 1988: Southern Senegal | Ae. furcifer (25/62 pools), Ae. aegypti, Ae. dalzielii, Ae. taylori, Ae. neoafricanus, Ae. fowleri, Ae. minutus | 27/435 pools | ZIKV seemed to have an epizootic annual cycle. In 16 ZIKV positive pools, dengue-2 was also isolated. | |
| 1989: Southern Senegal | 29/654 pools | |||||
| 1990: Southern Senegal | 3/497 pools | |||||
| 1991: Southern Senegal | 3/1264 pools | |||||
| [99] | Senegal | 1972: area not specified | Ae. dalzielii, Ae. furcifer, Ae. luteocephalus, Ae. vittatus, Ae. taylori, M. uniformis | 2/204 | 130,000 mosquitos from 69 different species were examined 1972–77. | |
| 1973: area not specified | 16/798 | |||||
| 1976: area not specified | 19/599 pools | |||||
| [38] | Uganda | 1956/05–1956/08: Lunya forest | Ae. africanus | 2/11 pools | 11 pools = 1355 mosquitos | |
| [98] | Uganda | 1961/11–1963/06: Zika forest | Ae. africanus | 13/688 pools | Positive pools occurred 1962/05-1962/09 and 1962/11. | M. aurites, C. centurionis, C. funebris, C. annulioris, A. ingrami |
| [97] | Uganda | 1969–1970: Zika Forest | Ae. africanus (14 isolates), Ae. apicoargenteus (1) | 15/105 pools | No ZIKV isolation for 6 years and 73 days (since 1962/11): 8 isolations 1969/04–1969/06 and a 192 day lapse before 7 isolations 1970/04–1970/08. | |
| Asia | ||||||
| [87] | Malaysia | 1966: Bentong | Ae. aegypti | 1/296 pools | (pools- mosquitos) Ae. aegypti (58–1,277), Ae. albopictus (59–4492), 23 other Aedes species (179–27636) |
Several epidemiological studies examining risk factors for mosquito abundance, human exposure to mosquitos and conditions for ZIKV infected mosquitos were identified by the scoping review. Observations include some mosquito species such as Ae. aegypti are well suited to urban environments, are often trapped within homes [87] and are able to reproduce in water containers which are frequently found in homes. For example, on Yap Island during the 2007 outbreak 87% of the homes were found to have mosquito infested water containers [85,100]. Biting behaviour of mosquitos was shown to increase in the two months following rainfall and decreased at temperatures less than 13°C [97]. A study conducted in Senegal found the forest canopy and forest ground areas were more likely to yield ZIKV positive mosquitoes [91]. From 13 species of mosquitos examined, Ae. furcifer showed significant temporal variation and Ae. luteocephalus and Ae. taylori showed a significant correlation between biting and infection rates with a one month lag time [91].
A vector mapping model based on mosquito data collected in Senegal between 1972 and 2008 demonstrated ZIKV was not correlated with the incidence of other viruses, the abundance of mosquitos, mean temperature or humidity [35]. However, ZIKV was shown to increase 12% (95% CI = 5%-21%) for each additional inch of rain above the baseline, was positively correlated with Chikungunya virus at a 6 year lag time, and was correlated with Ae. fuciferi and Ae. taylori at a 13 year lag time [35]. The minimum infection rate for Ae. furcifer and Ae. taylori was 80% (95% CI = 41%-94%) lower than for Ae. luteocephalus [35].
Non-human hosts of ZIKV
Zika virus was first isolated in 1947 in a rhesus sentinel monkey used for yellow fever surveillance [1]. Subsequent studies in Africa to evaluate potential hosts of ZIKV examined a number of small mammals in the Zika forest and bats in Uganda, but failed to identify ZIKV or seroreactivity to ZIKV in any sample, Table 7 [98,101]. Surveys of various monkeys in Uganda, Gabon and Burkina Faso showed a range of seroprevalence for ZIKV from 0% to >50% in some species, Table 7 [97,102–104]. Surveys from Indonesia (1978) and Pakistan (1983) sampled a number of rodents, bats, wild birds and domestic livestock; they reported some ZIKV seropositivity in rodents, bats, ducks, horses and large domestic livestock [28,105]. One study, published in two papers, on the orangutans in the Sabah region of Malaysia reported a higher seroprevalence among free-ranging orangutans compared to the semi-captive group and higher seroprevalence among humans than orangutans [22,23]. They hypothesize that ZIKV among orangutans may be incidental infections from mosquitos that had contact with viremic humans or another sylvatic cycle [22,23]. At this time it has been experimentally demonstrated that infected mosquitos can transmit ZIKV to mice and monkeys under controlled laboratory conditions [89]. However, no studies were identified that examined the relative importance of non-human primates or other potential hosts compared to humans in the transmission cycle of ZIKV.
Table 7. Potential host populations sampled for ZIKV in nine studies conducted between 1952 and 2016.
| Ref | Country | Date / place of sampling | Population sampled (positive/N) |
|---|---|---|---|
| Africa | |||
| [104] | Burkina Faso | 1983–1984 | Monkey, not specified (0/9) |
| [103] | Gabon | 1979–1980: | Monkey, Simian (9/34) |
| [101] | Uganda | 2009–2013: area not described | Bats (0/1067) |
| [106] | Uganda, Kenya, Democratic Republic of Congo | 2011–2012 | Bats (conference proceeding, no data) |
| [107] | Uganda | after 2011 | Bats (conference proceeding, no data) |
| [102] | Uganda | 1972: Entebbe area | Monkey (9/21): grey vervet and red tail |
| [97] | Uganda | 1969–1970: Around Kisubi forest and Bwame forest | Monkeys: vervet monkey (23/52), redtail monkey (4/21), mona monkey (0/1), black mangabey (2/4), lowland colobus (5/11), others (7/16) |
| [98] | Uganda | 1962–1963: Zika forest | (0/25) "small mammals": 1 potto, 3 palm civets, 4 squirrels, 2 tree rats, 7 giant pouched rats, 8 field rats |
| [1] | Uganda | 1947/04: Zika forest | (1/1) Rhesus sentinel monkey: first identification of ZIKV |
| Asia | |||
| [28] | Indonesia | 1978: Lombok | Horse (3/15), cow (4/41), carabao (1/13), goat (7/35), chicken (0/78), duck (2/52), bat (6/71), rat (0/25), wild bird (0/17) |
| [22] [23] | Malaysia | 1996–1998: Sabah region | Orangutans semi- captive (1/31) and free-ranging (5/40) |
| [105] | Pakistan | 1983: Pakistan | Rodents: Tatera indicia (3/47), Meriones hurrianae (2/33), Bandicota bengalensis (1/2) cow (0/45), buffalo (0/33), sheep (1/46), goat (1/48) |
Pathogenesis of ZIKV infection in rhesus monkeys has been described for two animals; the initial sentinel monkey developed fever and was viremic on day 3 of the fever. The second monkey who was subcutaneously challenged with ZIKV showed no signs of disease, but was viremic on days 4–5 and tested seropositive 30 days after exposure [1,44]. Mice were primarily used across 13 laboratory studies published between 1952 and 1976 to study pathogenesis, transmission and to propagate ZIKV, Table 8 [1,38,42,44]. Pathogenesis of ZIKV infection in mice mainly consists of degeneration of nerve cells, wide-spread softening of the brain and often porencephaly [38,42], with little effect on other major organs [38]. In one experiment challenged rats, guinea pigs and rabbits did not show signs of illness, however, the rabbits became seropositive while the guinea pigs died [38]. No ZIKV could be identified in the guinea pigs to indicate the virus had a role in the deaths [38].
Table 8. List of studies that used an animal model to propagate ZIKV or investigate the pathology and transmission of ZIKV in mice, monkeys or other laboratory animals.
| Ref | Country | Year | Study objective | Animal model |
|---|---|---|---|---|
| [40] | England | 1976 | Pathogenesis | mice |
| [42] | England | 1971 | Pathogenesis | mice |
| [46] | Uganda | 1970 | ZIKV cross-immunity with yellow fever | rhesus and vervet monkeys |
| [87] | Malaysia | 1969 | ZIKV propagation | mice |
| [98] | Uganda | 1964 | ZIKV propagation | mice |
| [108] | Malaysia, Thailand and N. Vietnam. | 1963 | ZIKV propagation | mice |
| [38] | Uganda | 1958 | Histopathogenesis | mice |
| [89] | Nigeria | 1956 | ZIKV propagation | mice |
| [88] | Nigeria | 1956 | Transmission | mice |
| [41] | United States | 1955 | Histopathogenesis | mice |
| [43] | Nigeria | 1954 | ZIKV propagation | mice |
| [37] | United States | 1953 | ZIKV propagation | mice |
| [1] | Uganda | 1952 | ZIKV propagation | mice |
| Transmission | Rhesus monkey | |||
| [44] | Uganda | 1952 | ZIKV propagation | mice |
| Isolation | rhesus monkey | |||
| Pathogenesis | cotton rats, guinea pigs, rabbits | |||
| [45] | Uganda and Tanzania | 1951 | ZIKV propagation | chick embryo |
ZIKV in humans
Epidemiology
Studies investigating the epidemiology of ZIKV in humans (120/233) provided prevalence estimates (46) or summarized an outbreak situation (74) from 1952–2016 and included the following healthy populations: general and pediatric populations, blood donors and military personnel and acute viral fever patients as indicated in Table 9. The majority of the prevalence surveys and outbreak reports were from the Americas (44), Asia/Australasia (42) and Africa (30); some reported for more than one region. Risk factors for testing ZIKV positive were examined in three studies. On Yap Island, a study found men 77% [95% CI, 72% to 83%] were more likely to have IgM antibodies against ZIKV virus compared to women 68% [95% CI, 62% to 74%], which was a relative risk of 1.1 [95% CI, 1.0 to 1.2] [100]. Higher density housing was significantly associated with increased risk of arbovirus infections in Kenya (1970) [109]. A recent study from Zambia found that indoor residual spraying had an adjusted odds ratio of 0.81, 95% CI [0.66, 0.99] and having an iron sheet roof on the home was protective against being ZIKV seropositive. However, increasing age, farming and having a grass roof significantly increased the risk of being ZIKV seropositive [110].
Table 9. One hundred and twenty prevalence studies and outbreak reports that report ZIKV infection in human populations published between 1952 and 2016.
| Ref | Country | Date / place of sampling | Population sampled | Measure-ment | Prevalence/ Rate | Positive / N | Comment |
|---|---|---|---|---|---|---|---|
| Africa | |||||||
| [111] | Angola | 1960: several regions | General population- adults | Prevalence | 27.0% | 133/492 | (20/42 Neutralization test positive) |
| [112] | Burundi | 1980–1982: many areas | General population- all ages | Prevalence | 1.4% | 9/623 | |
| [113] | Cameroon | 2010: Buea and Tiko in the Fako division (80% of the population) | All ages- hospital submissions | Prevalence | 38.0% | 30/79 | |
| [114] | Cameroon | 1972: several areas | General population- all ages | Prevalence | 7.0% | 83/1186 | |
| [115] | Cape Verde | 2015/09/27–2015/12/06 | General population- all ages | Outbreak | 4744 cases | 165 (sept 27-Oct14) and 4744 (Sept 27- Dec 6) suspected cases. (17 confirmed) | |
| [116] | Cape Verde | 2015/10–2016/01/17 | General population- all ages | Outbreak | 7081 cases | ||
| [117] | Cape Verde | 2015/10–2016/01/31 | General population- all ages | Outbreak | 7258 cases | ||
| [118] | Central African Republic | 1979: M'Bomou region (population of 30 000) | General population- adults | Prevalence | 59.0% | 271/459 | Prevalence: Bangassou 47%/111, M'Ballazime 62.4%/125, Iongofongo 68.5%/162, Ouango 47.5%/61 |
| [92] | Côte d’Ivoire | 1999/08: Kakpin | Adult | Prevalence | 54.2% | 13/24 | |
| [119] | Ethiopia | 2013: Dire Dawa town | All ages- hospital submissions | Prevalence | 10.0% | 5/50 | |
| [83] | Gabon | 2007–2010: entire country | All ages- hospital submissions | Prevalence | 0.1% | 4/4312 | |
| [103] | Gabon | 1979/10–1980/03: Franceville | General population- adults | Prevalence | 14.7% | 29/197 | |
| [120] | Gabon | 1975: South East Gabon | General population- adults | Prevalence | ?/1276 | ZIKV positive number not specified | |
| [121] | Guinea | 2010: N'Zerekore and Faranah | All ages- hospital submissions | Prevalence | ?/151, number ZIKV positive not reported. | ||
| [122,123] | Kenya | 2013: Kenya | General population- all ages | Prevalence | 0.0% | 0/351 | |
| [124] | Kenya | 1969: Ahero | Paediatric- school age children | Prevalence | 7.2% | 40/559 | |
| [109] | Kenya | 1966–1968: Central Nyanza, Kitui district, Malindi district | General population- all ages | Prevalence | 17.6% | 475/2698 | Prevalence: Central Nyanza 3.3%, Kitui district 1.3%, Malindi district 52% |
| [125] | Nigeria | 1980: near Kainji Dam | General population- all ages | Prevalence | 56.2% | 150/267 | |
| [126] | Nigeria | 1975: Igbo-Ora | General population- all ages | Prevalence | 20.0% | 4/20 | 1 virus isolation |
| [127] | Nigeria | 1971–1974: Oshogbo, Igbo-Ora, Ibadan and Oyo. | General population- pediatric | Prevalence | 27.0% | 51/189 | 2 ZIKV isolations /10778 samples |
| [128] | Nigeria | 1965: Benue Plateau State | General population- all ages | Prevalence | 21.4% | 15/70 | |
| [128] | Nigeria | 1970–1971: Benue Plateau State | General population- all ages | Prevalence | 12.2% | 18/147 | Samples taken during a yellow fever outbreak 1970 |
| [129] | Nigeria | 1964–1970: whole country | Paediatric- hospital submissions | Prevalence | 0.0% | 3/12613 | 3 ZIKV isolates were from 1968/ from 171 isolated arboviruses |
| [43] | Nigeria | 1952: Uburu | General population- adults | Prevalence | 59.5% | 50/84 | |
| [130] | Nigeria | 1955: Ilobi | all ages | Prevalence | 55.1% | 114/207 | |
| [130] | Nigeria | 1951: Ilaro | all ages | Prevalence | 44.3% | 43/97 | |
| [131] | Senegal | 2009/07–2013/03: eastern Senegal (population:141 226) | Adults- clinic submissions | prevalence | 0.1% | 9/13845 | All 9 IgM ZIKV positive cases were in 2011 only. |
| [86] | Senegal | 1990: Southern Senegal | General population- adults | Prevalence | 2.8% | 11/396 | |
| [86] | Senegal | 1988: Southern Senegal | General population- adults | Prevalence | 10.1% | 46/456 | |
| [132] | Senegal | 1972–5: several areas | General population- all ages | Prevalence | 58.3% | 1432/2457 | |
| [133] | Sierra Leone | 1972: several areas | Paediatric | Prevalence | 6.9% | 62/899 | |
| [44] | Uganda | 1952 Zika forest, Kampala, Bwamba, West Nile areas | General population- all ages | Prevalence | 6.1% | 6/99 | Prevalence: Zika forest 0%, Kampala 0%, Bwamba 20%, West Nile 9.5%. |
| [45] | Uganda and Tanzania | 1951: not reported | General population- all ages | Prevalence | ?/127 | Order of prevalence: Bwamba > Ntaya > ZIKV > Uganda S > West Nile > Bunyamwera > Semliki Forest viruses | |
| [110] | Zambia | 2010: Western and North-Western province | General population- all ages | Prevalence | 6.0% | 217/3625 | |
| Asia / Australasia | |||||||
| [134–136] | American Samoa | up to 2016-02-07 | General population- all ages | Outbreak | 551 cases (2 confirmed) | ||
| [137–142] | Cook Islands | 2014 | General population- all ages | Outbreak | 932 cases (18 confirmed and 50/80 in Tahiti confirmed.) | ||
| [79,138] | Easter Island (Chile) | 2014/01–2014/06: Easter Island | General population- all ages | Outbreak prevalence | 2.3% | 89/3860 | Easter Island population = 3860 |
| [143] | Fiji | 2014/08/ | General population- all ages | Outbreak | . | 2/6 suspect cases were ZIKV confirmed | |
| [72,116, 139,144–148] | French Polynesia | 2013/10/30–2014/04 | General population- all ages | Outbreak Attack rate | 12% (10–40 depending on the island) | 32000/268207 | 8750 suspected cases. (383–460 confirmed) Estimate: 32000 cases, 11% of the population 268, 207 on 67 islands |
| [144] | French Polynesia | 2014/02/04–2014/03/13 | General population- all ages | Prevalence | 41.3% | Estimated that 50% of people were asymptomatic | |
| [137] | French Polynesia | 2013–2014: French Polynesia | General population- all ages | Outbreak | 8746 cases (>30000 medical consultations) | ||
| [71,147] | French Polynesia | 2013/11–2014/02: French Polynesia | Adult- blood donors | Outbreak prevalence | 2.8% | 42/1505 | 11/42 reported ZIKV like illness 3 to 10 days after donation |
| [149] | French Polynesia | 2011/07–2013/10: French Polynesia | Adults- blood donors | Prevalence | 0.8% | 5/593 | |
| [53] | Indonesia | 2014/12-2015/04 | All ages- hospital submissions dengue negative | Prevalence | 1% | 1/103 | |
| [150] | Indonesia | 2004–2005: Bandung, West Java | All ages- hospital submissions dengue negative | Prevalence | ?/95, number ZIKV positive not reported. | ||
| [28] | Indonesia | 1978: Lombok | General population- all ages | Prevalence | 2.0% | 9/446 | |
| [151] | Indonesia | 1973: Tamampu, Malili, Balikpapan (South Sulawesi and East Kalimantan) | General population- all ages | Prevalence | 9.5% | 21/222 | Prevalence: 27/160 Timampu, 24/40 Malili, 20/22 Balikpapan |
| [23] | Malaysia | 1996–1997: Borneo | General population- all ages | Prevalence | 44.1% | 9/30 native & 40/81 immigrant | |
| [108] | Malaysia | 1953–4: Kuala Lumpur | General population- all ages | Prevalence | 75.0% | 75/100 | |
| [135] | Marshall Islands | 2016/02/14 | General population- all ages | Outbreak | First confirmed case. 1/6 suspect cases positive ZIKV | ||
| [137–139,142, 152,153] | New Caledonia | 2013/11–2014/07 | General population- all ages | Outbreak | 1400 cases | ||
| [105] | Pakistan | 1983 | Adult | Prevalence | 2.3% | 1/43 | |
| [154–156] | Samoa | 2015/09–2015/12/06 | Gen pop- all ages | Outbreak | 3/40 suspect cases were confirmed. | ||
| [140,157–165] | Solomon Islands | 2015/02–2015/05/24 | General population- all ages | Outbreak | 310 cases (5 confirmed) | ||
| [166] | Thailand | 2015: Northern Thailand | Adult | Prevalence | 80% | 16/21 | 16/21 showed sero-reactivity to ZIKV, only 2 exclusively to ZIKV |
| [108] | Thailand | 1954: Thailand | Adult | Prevalence | 16.0% | 8/25 south & 0/25 north | |
| [135, 167,168] | Tonga | 2016/01–2016/02/14 | gen pop- all ages | outbreak | . | > 800 suspect cases and 2 confirmed | |
| [157,162] | Vanuatu | 2015/04/26 | General population- all ages | Outbreak | 1st confirmed case | ||
| [108] | Vietnam | 1954: North Vietnam, River Delta of Tomkin area | General population- all ages | Prevalence | 4.0% | 2/50 | |
| [100, 147] | Yap Island, Micronesia | 2007/04/01–2007/08/09 | General population- all ages | Outbreak prevalence | 74.3% | 414/557 sero-prevalence | Yap Island had a population of 7391 at time of outbreak RT-PCR positive |
| Outbreak prevalence | 14.5% | 185/1276 | |||||
| attack rate | 14.6/1000 persons | Ranged 3.6–21.5/1000 by community | |||||
| Infection rate | 73% (95% CI, 68 to 77) | ||||||
| Clinical symptoms | 18% (95% CI, 10 to 27) | 919 (95% CI, 480 to 1357)/6892 | |||||
| South and Central America | |||||||
| [169] | Barbados | 2016/01/14-15 | General population- all ages | Outbreak | 3 cases: autochthonous transmission 2016/01 | ||
| [117,170] | Brazil | 2015/05–2015/02 | General population- all ages | Outbreak | 497593–1 482 701 cases estimated (they stopped counting) | ||
| [4,171, 172] | Brazil | 2015/05-2015/12/01 | General population- all ages | Outbreak | 3 fatalities: One man with co-morbidities, one healthy 20 year old woman and an infant. | ||
| [173] | Brazil | 2015/02–2015/06: Salvador Brazil | General population- all ages | Outbreak attack rate | 5.5 cases/1000 persons | 14835 cases (not confirmed) | |
| [174] | Bolivia | 2016-01-08 | General population- all ages | Outbreak | 1 case: autochthonous transmission 2016/01 | ||
| [117,170,175] | Colombia | 2015/10/31–2016/02/06 | General population- all ages | Outbreak | 31 555 cases (1504 confirmed) | ||
| [116] | Colombia | 2015/10/31–2016/01/23 | General population- all ages | Outbreak | 20297 cases | ||
| [176] | Colombia | 2015/09/22–2016/01/02 | General population- all ages | Outbreak | 11712 cases (746 confirmed) and 1 fatality: sickle cell disease (previously associated with severe dengue symptoms) | ||
| [4,54, 171] | Colombia | 2015/10/31–2015/12/01 | All ages | Outbreak | autochthonous transmission 9/22 confirmed cases Sincelejo area | ||
| [177] | Dominican Republic | 2016/01/23 | General population- all ages | Outbreak | 10 cases; autochthonous transmission 2016/01 | ||
| [169] | Ecuador | 2016/01/14-15 | General population- all ages | Outbreak | 2 cases; autochthonous transmission 2016/01 | ||
| [116,178] | El Salvador | 2015/11–2015/12/31 | General population- all ages | Outbreak | 3836 suspect cases, 46 hospitalizations and 1 death; patient had many co-morbidities | ||
| [4] | El Salvador | 2015/11–2015/12/01 | General population- all ages | Outbreak | autochthonous transmission 2015/11 | ||
| [179] | French Guiana | 2015-12-21 | General population- all ages | 1 case; autochthonous transmission 2015/12 | |||
| [4,180] | Guatemala | 2015/11–2015/12/01 | General population- all ages | Outbreak | autochthonous transmission 2015/11 | ||
| [181,182] | Guadeloupe | 1015/11/23–2016/01/21 | General population- all ages | Outbreak | 1 case confirmed | ||
| [169,181] | Guyana | 1015/11/23–2016/01/21 | General population- all ages | Outbreak | 164 cases (45 confirmed) autochthonous transmission 2016/01 | ||
| [183] | Haiti | 2016/01/18 | General population- all ages | Outbreak | 5 cases, autochthonous transmission 2016/01 | ||
| [184] | Honduras | 2015/12/21 | General population- all ages | Outbreak | 1 case, autochthonous transmission 2015/12 | ||
| [179,181] | Martinique | 1015/11/23–2016/01/21 | General population- all ages | Outbreak | 1255 cases (102 confirmed), autochthonous transmission 2015/12 | ||
| [4,185] | Mexico | 2015/11–2015/12/01 | General population- all ages | Outbreak | autochthonous transmission 2015/11 | ||
| [186,187] | Panama | 2015/11–2015/12/14 | General population- all ages | Outbreak | 95 cases, (4 confirmed) autochthonous transmission 2015/12 | ||
| [4,188] | Paraguay | 2015/11–2015/12/01 | General population- all ages | Outbreak | 6 cases, autochthonous transmission 2015/11 on Brazilian border | ||
| [189,190] | Puerto Rico | 2015/11/23-2016/01/28 | General population- all ages | Outbreak | 155 cases (30/73 confirmed), 3 hospitalizations. autochthonous transmission 2015/11 | ||
| [181] | St. Barthelemy | 2015/11/23–2016/01/21 | General population- all ages | Outbreak | |||
| [181,182] | St. Martin | 2015/11/23–2016/01/21 | General population- all ages | Outbreak | 1 case confirmed | ||
| [4,191, 192] | Suriname | 2015/11 | General population- all ages | Outbreak | 5/6 cases confirmed. autochthonous transmission reported November 2015 | ||
| [193] | Trinidad | 1953–1954: Trinidad | General population- all ages | Prevalence | 0.0% | 0/15 | |
| [4,194] | Venezuela | 2015/11–2015/12/01 | General population- all ages | Outbreak | 7 cases (4 confirmed) autochthonous transmission 2015/11 on Brazilian border | ||
| [195] | Virgin Islands (US) | 2016/01/25 | General population- all ages | Outbreak | 1 case, autochthonous transmission 2016/01 | ||
| Travel related- notifiable disease report | |||||||
| [196] | New Zealand | 2015: New Zealand | General population- all ages | Incidence rate | 0.1 per 100,000 | 6 cases | |
| [197] | New Zealand | 2014: New Zealand | General population- all ages | Incidence rate | 1.3 per 100,000 | 57 cases total | |
| [198] | Europe | up to 2016/02/25 | General population- all ages | 177 cases from 15 countries. Austria (1), Czech Rep (2), Denmark (1), Finland (2), France (66), Germany (20), Ireland (3), Italy (6), Malta (1), Netherlands (30), Portugal (7), Spain (27), Sweden (2), Slovenia (1), UK (8). | |||
Clinical Symptoms, Complications and Pathogenesis
Clinical symptoms associated with ZIKV illness in humans is reported in 72 studies and described in Table 10, including the first experimentally challenged human case recorded in Nigeria (1956) [88] to present cases in South America and the Caribbean region. Thirty four studies report travel related cases in individuals returning from various affected countries. Thirty five studies report on complications with ZIKV, Table 11. Publications that described the clinical features of ZIKV infection in humans were mainly case reports/case series (57/78), epidemiological surveys (12/78), outbreak investigations (11/78), and one human challenge experiment (some studies fell into multiple categories). For most studies (58/78) the total number of cases was <6 while other results summarized the clinical features of up to 13,786 cases. The majority of studies have been published in the past decade and are based on ZIKV acquired infections in South-Central America and Caribbean region (54/75) and Asia/Australasia (48/75) with a number of publications reporting on both regions. A wide range of clinical symptoms are reported for ZIKV infection, most common are mild fever and a maculopapular rash followed by joint pain, headache and more recently bilateral conjunctivitis; see the summary at the bottom of Table 10 for averages across studies. In a recent study, researchers found an association with more severe symptoms in patients that were co-infected with malaria [131]. Six studies reported co-infections with dengue and/or chikungunya [74,116,117,144,146,199], one study reported co-infection with Influenza B and another with Human Immunodeficiency Virus [81,189]. Some of these studies provide evidence for the viremic period, suggested which samples and diagnostic tests to use at various stages of infection and the possible range of incubation periods, however, only one study investigated the immune response in humans [200]. Tappe (2015), investigated the role of cytokines and chemokines in the pathogenesis of ZIKV infected patients from the acute stage (≤ 10 days after symptom onset) to convalescent stage (> 10 days after disease onset) [200].
Table 10. Pathogenesis: Studies (n = 72) reporting on clinical symptoms in humans from the first experimentally challenged human in Nigeria (1956) to present (March 1, 2016) cases in the South America and Caribbean region divided by studies with n>6 (25.4%) and n<6 (74.6%).
| Ref | Study Year | Country of ZIKV Exposure | N | Fever | Joint pain | Rash Rash | Con-junc-tivitis | Muscle pain | Head-ache | Retro-orbit-al pain | Edema | Lymphadenopathy | Malaise | Asthenia | Sore throat/ cough | Nausea/ vomiting/ diarrhea | Hemato-spermia | COMMENT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outbreak Investigations, Cohort or cross-sectional studies | ||||||||||||||||||
| South- Central America and Caribbean | ||||||||||||||||||
| [173] | 2015 | Brazil | 13786 | 35% | 27% | 100% | 22% | 26% | Summary of the clinical picture from indeterminate acute exanthematous illness in Salvador, Brazil. Many cases not confirmed. | |||||||||
| [73] | 2015 | Brazil | 24 | 38% | 86% | 54% | 58% | |||||||||||
| [201] | 2015 | Brazil | 29 | 45% | 38% | 72% | 79% | 17% | 14% itchy | |||||||||
| [202] | 2015 | Brazil | 10 | 70% | 70% | 70% | 100% microcephaly in infant. | |||||||||||
| [76] | 2015 | Brazil | 8 | 75% | 88% | 100% | 75% | 75% | 50% | 75% | 38% | 8/8/ Pain scale applied to six cases -pain levels high in most patients, with levels of zero (1/7), seven (2/7), nine (1/7) or 10 (2/7). Duration 2–15 days. | ||||||
| [79] | 2014 | Easter Island (Chile) | 89 | 100% | 100% | 100% | 100% | 100% | Clinical picture of the 89 positive cases on Easter Island (total population 3860) | |||||||||
| [177] | 2016 | Dominican Republic | 10 | 100% | 60% | 100% | 80% | 50% | 60% | 60% | ||||||||
| [188] | 2015 | Paraguay | 6 | 100% | 100% | 100% | 100% | 100% | 100% | |||||||||
| [189] | 2015–2016 | Puerto Rico | 30 | 73% | 73% | 77% | 77% | 3% | 3% chills and abdominal pain | |||||||||
| [61] | 2015/10 | Suriname | 5 | 100% | 100% | |||||||||||||
| Asia and Australasia | ||||||||||||||||||
| [144–146] | 2013–14 | French Polynesia | 297 | 72% | 65% | 93% | 63% | 44% | 46% | 16% | 47% | 78% | 23% | 28% | Adenopathies 15%, mouth ulcers 4% | |||
| [203] | 2014 | New Caledonia | 6 | 83% | 67% | 100% | 50% | 67% | 17% | 17% | 50% | 17% | 6/6 itching | |||||
| [77] | 2012 | Thailand | 7 | 100% | 29% | 100% | 29% | 29% | 14% | 29% | 1/7 Rhinorrhea | |||||||
| [100,147] | 2007 | Yap Island, Micronesia | 31 | 65% | 65% | 90% | 55% | 48% | 45% | 39% | 19% | 10% | Clinical picture of 31 confirmed cases identified during a survey of 173 randomly selected households on Yap Island during the outbreak in 2007. | |||||
| [204] | 1977 | Indonesia | 7 | 100% | 29% | 14% | 14% | 14% | 86% | 43% | 1/7 haematuria, 4/7 anorexia, 2/7 chills, 5/7 stomach ache, 3/7 dizziness, 3/7 constipations, 2/7 hypotension, | |||||||
| Case Studies < 5 people | ||||||||||||||||||
| South- Central America and Caribbean | ||||||||||||||||||
| [205] | 2016 | Brazil | 1 | 100% | 100% | 100% | bilateral ocular discomfort, blurry vision, and mild redness on day 7 | |||||||||||
| [206] | 2015 | Brazil | 3 | 33% | 33% | Pregnant mothers—no laboratory confirmation | ||||||||||||
| [81] | 2015 | Brazil | 1 | 100% | 100% | 100% | 100% | 100% | Case had HIV. | |||||||||
| [207] | 2015 | Brazil | 1 | 100% | 100% | 100% | 100% | 100% | ||||||||||
| [52] | 2015 | Brazil | 1 | 100% | 100% | 100% | 100% itchy | |||||||||||
| [55] | 2015 | Brazil | 1 | 100% | 100% | 100% | 100% | 100% | 100% itchy | |||||||||
| [208] | 2015 | Brazil | 1 | 100% | 100% | 100% | 100% | 100% | 100% | 100% chills | ||||||||
| [176] | 2015 | Colombia | 1 | 100% | 100% | 100% | 100% | 100% Abdominal pain and jandice. Comorbidity: Sickle cell disease (>5 yrs). Patient died. | ||||||||||
| [209] | 2015 | Colombia | 1 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | ||||||||
| [199] | 2015 | Colombia | 1 | 100% | 100% | 100% | 100% | 100% | Case had co-infection with dengue and chikungunya. | |||||||||
| [210] | 2016 | Costa Rica | 1 | 100% | 100% | 100% | 100% | 100% | ||||||||||
| [211] | 2016 | Costa Rica | 1 | 100% | 100% | 100% | 100% | 100% | ||||||||||
| [210] | 2016 | Curacao | 1 | 100% | 100% | 100% | 100% diarrhea | |||||||||||
| [210] | 2016 | Jamaica | 1 | 100% | 100% | 100% | 100% | 100% | 100% abdominal pain, retro orbital pain and vomiting | |||||||||
| [212] | 2016 | Martinique, Brazil and Colombia | 3 | 67% | 33% | 100% | 100% | 100% | 67% | 33% | 33% | |||||||
| [210] | 2016 | Nicaragua | 2 | 100% | 100% | 100% | ||||||||||||
| [213] | 2015 | Suriname | 1 | 100% | 100% | 100% | 100% | 100% | 100% itchy, painful skin | |||||||||
| [214] | 2016 | Suriname | 1 | 100% | 100% | 100% | 100% | 100% | 100% | 100% itchy and subcutaneous haematomas on arms and legs (day 10) day 29 diagnosed: immune-mediated thrombocytopenia | ||||||||
| [215] | 2016 | Venezuela | 2 | 100% | 50% | 100% | 100% | 100% | ||||||||||
| [195] | 2016 | Virgin Islands (US) | 1 | 100% | 100% | 100% | 100% | |||||||||||
| Asia and Australasia | ||||||||||||||||||
| [82] | 2010 | Cambodia | 1 | 100% | 100% | 100% | ||||||||||||
| [216] | 2015 | Cook Islands | 2 | 100% | 100% | 50% | 100% | 100% | ||||||||||
| [57] | 2014 | Cook Islands | 1 | 100% | 100% | 100% | 100% | 100% | ||||||||||
| [217] | 2014 | Cook Islands | 2 | 100% | 100% | 100% | 100% | 100% | ||||||||||
| [218] | 2014 | French Polynesia | 2 | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 1/2 slight gingival bleeding for a few days | |||||
| [74] | 2014 | French Polynesia | 2 | 100% | 100% | 50% | 50% | 100% | 50% | 50% | 100% | 50% | 2 cases had co-infection with two different dengue strains. | |||||
| [219] | 2014 | French Polynesia | 1 | 100% | 100% | 100% | 100% | |||||||||||
| [59] | 2013 | French Polynesia | 1 | 100% | 100% | 100% | 100% | 100% | ||||||||||
| [220] | 2013 | French Polynesia | 1 | 100% | 100% | 100% | 100% | 100% | 100% | |||||||||
| [221] | 2013 | French Polynesia | 1 | 100% | 100% | 100% | 100% | 100% | ||||||||||
| [72] | 2013 | French Polynesia | 3 | 100% | 100% | 100% | 67% | 100% | 33% | 100% | 1/3 aphthous ulcer | |||||||
| [222] | 2013 | French Polynesia | 2 | 50% | 100% | 50% | Pregnant cases had ZIKV infection immediately before or after birth; newborns developed ZIKV (2/2 RT-PCR positive, 1/2 rash) with no complications (one had gestational diabetes) | |||||||||||
| [223] | 2013 | French Polynesia | 1 | 100% | 100% | 100% | 100% | Developed Guillain-Barré syndrome at day 7 (see complications table) | ||||||||||
| [69] | 2013 | French Polynesia | 2 | 100% | 50% | 100% | 50% | 50% | 50% | |||||||||
| [67] | 2013 | French Polynesia | 1 | 100% | 100% | 100% | 100% | 100% | 100% | |||||||||
| [224] | 2013 | French Polynesia | 1 | 100% | 100% | 100% | 100% | 100% | 100% | |||||||||
| [53] | 2014–15 | Indonesia | 1 | 100% | 100% | 100% | 100% | 100% | ||||||||||
| [78] | 2015 | Indonesia | 1 | 100% | 100% | 100% | 100% | |||||||||||
| [66] | 2012 | Indonesia | 1 | 100% | 100% | 100% | 100% | 100% | 100% | |||||||||
| [225] | 2014 | Malaysia | 1 | 100% | 100% | 100% | 100% | 100% | 100% | 1/1 burning sensation of palms of hands and soles of feet. 1/1 sudden development of bilateral dull and metallic hearing—left ear a very short delay between a sound and perception of sound, duration 10 days with gradual resolution. | ||||||||
| [58] | 2015 | Maldives | 1 | 100% | 100% | 100% | 100% | |||||||||||
| [226] | 2015 | New Caledonia | 1 | 100% | 100% | Gave birth, 37 wk, at ZIKV onset. | ||||||||||||
| [75] | 2012 | Philippines | 1 | 100% | 100% | 100% | 100% | 100% | 100% | 1/1 stomach pain, anorexia | ||||||||
| [227] | 2014 | Thailand | 2 | 50% | 100% | 50% | 100% | 50% | 50% | 50% diffuse pain | ||||||||
| [228] | 2016 | Thailand | 1 | 100% | 100% | |||||||||||||
| [229] | 2014 | Thailand | 1 | 100% | 100% | 100% | 100% | |||||||||||
| [60] | 2013 | Thailand | 1 | 100% | 100% | 100% | 100% | 100% | 100% | 1/1 chills, 1/1 mouth blisters (appeared after 2 days); symptomatic for 16 days | ||||||||
| [230] | 2013 | Thailand | 1 | 100% | 100% | 100% | 100% | 100% | ||||||||||
| Africa | ||||||||||||||||||
| [127] | 1971 | Nigeria | 2 | 100% | 50% | 50% | ||||||||||||
| [88] | 1956 | Nigeria | 1 | 100% | 100% | 100% | Laboratory challenge of human volunteer | |||||||||||
| [43] | 1952 | Nigeria | 3 | 100% | 67% | 67% | 33% | 33% | 1/3 jaundice (but concurrent epidemic of jaundice), 1/3 albumin in the urine on day 3–5. | |||||||||
| [231] | 2008 | Senegal | 3 | 100% | 100% | 33% | 67% | 100% | 66% | 33% | 66% | 50% | 2/3 chills, 1/3 prostatitis, 2/3 aphthous ulcers on lip (day 4), 1/3 photophobia, 2/3 reported arthralgia reoccurring for several months. | |||||
| [232] | 1962 | Uganda | 1 | 100% | 100% | 100% | 100% | 100% | 100% | |||||||||
| Europe | ||||||||||||||||||
| [233] | 1973 | Portugal | 1 | 100% | 100% | 100% | Laboratory acquired ZIKV: 1/1/ chills, 1/1 sweating | |||||||||||
| Symptom Frequency across case studies | 84% | 57% | 79% | 54% | 43% | 41% | 13% | 16% | 10% | 22% | 28% | 11% | 7% | 3% | ||||
| Symptom Frequency across Observational studies | 66% | 54% | 85% | 41% | 45% | 31% | 15% | 13% | 5% | 14% | 5% | 3% | 12% | 1% | ||||
Fever (typically reported as low grade 38–40 C lasting usually 2–4 days, range 1–8 days).
Joint pain (usually wrist, fingers, ankles or knees affected, some reports of low back pain. Pain lasts typically less than a week, but there are reports of intermittent arthralgia for 1–2 months).
Rash (common description: diffuse pink maculopapular rash which covered the face, neck, trunk and upper arms, occasionally this is reported as "itchy", typically appeared around day 2 of illness and faded around day 5, although there are cases where the rash lasted up to 2 weeks.).
Conjunctivitis (red eyes)—mostly reported to occur in both eyes.
Muscle pain (reported to range from mild to severe and last 2–7 days).
Headache (mild headache often the first symptom and is reported to last 2–4 days).
Retro-orbital pain. Edema (usually in hands/fingers or ankles/feet) duration approximately 7 days.).
Lymphadenopathy—duration up to 2 weeks. Often reported in cervical area.
Malaise 2–14 days.
Asthenia—2 to 14 days and often preceded rash by up to 48 hours.
Sore throat/cough (duration 4 days).
Nausea/vomiting/ diarrhea.
Hematospermia (occurred at day 5 to 14 after symptoms appeared)
Table 11. Complications reported to be associated with ZIKV infection in 35 case reports/ case series and outbreak reports.
20 reports on birth defects and microcephaly in pregnant women (2013–2016) and 24 on Guillain-Barré syndrome following ZIKV infection (probable and confirmed) during outbreaks in the Pacific Islands and the Americas (2011–2016).
| Ref | Country | Study year | Complication | N | ZIKV confirmed? | Description of clinical findings: |
|---|---|---|---|---|---|---|
| Microcephaly and other birth defects potentially associated with ZIKV infection | ||||||
| [148] | French Polynesia | 2014 | Birth Defects: fetal cerebral anomaly | 18 | 4/6 amniotic fluid samples were RT-PCR positive | French Polynesia has ~4000 births/year. Retrospective analysis of birth defects involving the central nervous system indicated 18 cases in 2014 (vs. 4 in 2013 and 3 in 2012) where the mothers may have been infected with ZIKV early in pregnancy. Amniotic fluid samples from standard testing procedures showed 4/6 samples were ZIKV positive. Since our search, an additional paper on this set of cases has been published. |
| [206] | Brazil | 2015 | Birth defects: microcephaly | 3 | 33% mothers had clinical symptoms. No testing done. | CT scan and ocular examination showed all infants had unilateral ocular findings: gross macular pigment mottling and foveal reflex loss. Well-defined macular neuroretinal atrophy was detected in one child. |
| [80] | Brazil | 2015 | Birth defects: microcephaly | 8 | 25% positive amniotic fluid, 75% mothers had clinical symptoms during pregnancy | Amniotic fluid positive fetuses (n = 2), note mother’s serum RT-PCR1 was negative: both eyes had cataracts and intraocular calcifications, and one eye was smaller than the other. Fetuses from mothers with clinical symptoms (n = 6): fetal neurosonograms showed 33% cases with cerebellar involvement and 16% with severe arthrogryposis. |
| [201] | Brazil | 2015 | Birth defects: Microcephaly and ocular defects | 29 | 29 cases | 29 infants age 1–6 months. 23/29 mothers had clinical ZIKV infections: 18/29 first trimester, 4/29 second trimester, 1/29 third trimester. 6 had no symptoms of ZIKV; 10 patients had ocular findings and were presumed to have been exposed to ZIKV. |
| [52] | Brazil | 2015 | Birth defects: Microcephaly | 2 | 2 cases: first and second trimester ZIKV infections | Case 1: normal ultrasound at 16wk, ZIKV at 18wk, ultrasound at 21 weeks detected microcephaly, confirmed at 27wk. Baby born at 40wks with head circumference of 30cm. Case 2: ZIKV at 10wk, 22wk ultrasound indicated fetal head <10th percentile, 25wk indicated microcephaly, term delivery neonate presented with severe ventriculomegaly, microphthalmia, cataract, and severe arthrogryposis in the legs and arms. Amniotic fluid positive at 28wks. |
| [55] | Brazil | 2015/10 | Microcephaly | 1 | RT-PCR positive on fetal brain sample | Mother had ZIKV at 13 weeks gestation. Ultrasound at 14 and 20 weeks were normal. Ultrasound at 29 and 32 weeks showed retardation of growth with normal amniotic fluid and placenta, a head circumference below the second percentile for gestation (microcephaly), moderate ventriculomegaly. Brain structures were blurred, calcifications and no other fetal structural abnormalities. Fetal, umbilical, and uterine blood flows were normal. RT-PCR1 ZIKV positive in the fetal brain sample (6.5×107 viral RNA copies per milligram of tissue). |
| [202] | Brazil | 2015/12 | Birth defects: Microcephaly and ocular findings | 10 | 10 cases | 10 cases had clinical diagnosis of ZIKV vertical infection (mothers 7/10 rash, 6 in 1st trimester) and diagnosed with ophthalmological abnormalities |
| [234] | Brazil | 2015/08–2015/10 | Birth defects: Microcephaly | 35 | 35 cases. 25/35 severe microcephaly, 17/35 at least one neurologic abnormality. | 26/35 mothers recalled a rash during pregnancy: first trimester 21/26 and 5/26 in the second trimester. Pathology: Computed tomography scans and transfontanellar cranial ultrasounds showed a consistent pattern of widespread brain calcifications, ventricular enlargement secondary to cortical/subcortical atrophy, excessive and redundant scalp skin in 11 (31%) cases, also suggests acute intrauterine brain injury, indicating an arrest in cerebral growth. |
| [235] | Brazil | up to 2015/11/21 | Birth defects: Microcephaly | 739 | 739 cases (1 death) | Nov 7, 2015: case definition revised from <33cm to <32cm. |
| [4,172] | Brazil | up to 2015/11/30 | Birth defects: Microcephaly | 1248 | 1,248 cases (7 deaths) | 1,248 cases equates to 99.7/100,000 live births have microcephaly. Brazil noted many affected women appear to have been infected with ZIKV in first trimester (no data) |
| [236] | Brazil | up to 2015/12/05 | Birth defects: Microcephaly | 1761 | 1761 cases (19 deaths) | |
| [170, 237] | Brazil | up to 2016/01/31 | Birth defects: Microcephaly or CNS malformation | 4783 | 4783 cases (76 deaths) | 5/76 deaths were ZIKV positive. Historic average 163/year. |
| [116,117,238] | Brazil | 2015/11–2016/02/13 | Birth defects: Microcephaly or other CNS involvement | 5280 | 5280 cases (108 deaths) | Brazil 2001 to 2014 had an average of 163 microcephaly cases/year. Validation of 1345 cases of microcephaly is complete: 837 discarded, 508 confirmed by 421/462 cases radiological findings and 41/462 ZIKV confirmed infection. |
| [48] | Brazil | 2016 | Birth defects: Microcephaly | 1 | 1 case | 20 year old mother: microcephaly detection at 18 weeks, pregnancy terminated at 32 weeks. Fetal tissues: cerebral cortex, medulla oblongata and cerebrospinal and amniotic fluid ZIKV RT-PCR positive. |
| [116, 239] | Hawaii | 2016/01/08 | Birth defects: Microcephaly | 1 | Case of microcephaly, confirmed ZIKV | The mother likely acquired ZIKV in Brazil (May 2015) and her newborn acquired the infection in utero. |
| [240] | USA | 2015/08/01–2016/02/07 | Birth defects: Microcephaly, miscarriage | 9 | 3/9 fetuses ZIKV positive.9/9 mothers ZIKV confirmed. | 1st trimester (6/9): 2 miscarriages, 2 terminations, 1 microcephaly case, one not born yet. Trimester 3 (3/9): 3 apparently healthy infants. Travel to American Samoa, Brazil, El Salvador, Guatemala, Haiti, Honduras, Mexico, Puerto Rico. In USA 151/257 pregnancies tested for ZIKV, 8 IgM positive. |
| Guillain-Barré syndrome (GBS) or other neurological complications potentially associated with ZIKV infection | ||||||
| [116, 117, 170, 172,241,242] | Brazil | 2015/11 | GBS | 7 | 7/10 GBS patients were ZIKV positive | 1708 GBS cases (2015) vs. 1439 cases (2014). |
| [4,116, 117,170,172,237,242] | Brazil | 2015/05/01–2015/07/13 | GBS | 42 | 62% (26/42) were ZIKV positive | |
| [173] | Brazil | 2015/02/15-2015/06/25 | GBS | 24 | 24 cases of suspect GBS were reported during an outbreak of 14,835 cases of “indeterminate acute exanthematous illness” in Salvador, Brazil (3rd largest city). Confirmation tests not done for ZIKV, chikungunya or dengue. | |
| [116,117, 175,237] | Colombia | 2015/12/27–2016/01/31 | GBS | 86 | 86 cases in 2 months | Historic average is 242 GBS /year (20 cases /month). |
| [117,178,237,242, 243] | El Salvador | 2015/12–2016/01/09 | GBS | 46 | 46–118 GBS cases in 5 weeks (2 deaths) | 2014 average was 169 cases/year. Case series 12/22 GBS patients had ZIKV within the 15 days prior to GBS. |
| [116,117,137,138, 144–147, 172,242,244,245] | French Polynesia | Nov 2013-Feb 2014 | GBS | 42 | 42 cases of GBS and increased incidence of neurological complications were reported associated with the ZIKV outbreak in the Pacific Islands. 37/42 GBS cases reported having a viral syndrome 6 (4–10) days before the onset of GBS. GBS symptoms peaked at 6 (4–9) days and by 3 months after discharge, 24 (57%) patients were able to walk without assistance. All GBS patients were hospitalized, median 11days (7–20) (N = 42) and 51 days (16–70) for ICU patients (n = 10).Symptoms: Clinical presentation at hospital admission included generalised muscle weakness (74%), inability to walk (44%), facial palsy (64%), 39 (93%) patients had increased (>0·52 g/L) protein concentration in their CSF, 16 (38%) patients were admitted to ICU and 12 (29%) required respiratory assistance. All cases (100%) received immunoglobulin treatment and one (2%) had plasmapheresis. | |
| [245] | French Polynesia | Nov 2013-Feb 2014 | GBS | 42 | Case control study: | If the ZIKV attack rate in French Polynesia was 66%, the risk of GBS was 0·24/1000 Zika virus infections. Patient and control samples drawn at several time points were examined by RT-PCR, IgM / IgG and PRNT2 reaction. 98% of GBS patients were IgM or IgG positive, 19% cross-reacted with dengue, but 100% were confirmed ZIKV positive by PRNT. Compared to a control group of hospitalized patients, the odds of a GBS patient being ZIKV positive was 59·7 (95% CI 10·4 –+∞); p<0·0001. And for PRNT the odds was 34·1 (95% CI 5·8 –+∞) p<0·0001. No association was detected for dengue test results and GBS. |
| [223] | French Polynesia | 2013 | GBS | 1 | Case report, sero-positive | Polynesian woman, early 40s had ZIKV symptoms 7 days before neurological symptoms. No past medical history except acute articular rheumatism. Day 0: evaluated for paraesthesia of the four limb extremities. Day 1: admitted to hospital, paraesthesia had evolved into ascendant muscular weakness suggestive of GBS. Day 3: developed tetraparesis predominant in the lower limbs, with paraesthesia of the extremities, diffuse myalgia, and a bilateral, but asymmetric peripheral facial palsy. Deep tendon reflexes absent. No respiratory or deglutition disorders. Chest pain developed related to a sustained ventricular tachycardia, and orthostatic hypotension, both suggestive of dysautonomia. Electromyogram confirmed a diffuse demyelinating disorder, with elevated distal motor latency, elongated F-wave, conduction block and acute denervation, without axonal abnormalities. Day 13: discharged with paraparesis requiring the use of a walking frame, and the facial palsy slowly disappeared. Day 40, able to walk without help and muscular strength score was 85/100. |
| [181, 237] | Martinique | 2016/01/21 | GBS | 6 | 6/6 GBS cases were ZIKV positive | |
| [246] | New Caledonia | 2011/01/01–2014/12/31 | GBS | 42 | 42 cases of GBS between 2011–2014 investigated. | 42 cases of GBS: incidence 2011 = 2.6 (0.66–4.54)/100000 vs. 2014 = 5.09 (2/49-7.56)/100000 = NOT a significant difference. 13 (30%) cases occurred between March and July 2014 (during ZIKV outbreak), 6 in April 2014. These patients indicated 2 confirmed and 2 suspect ZIKV cases and 4 dengue cases preceded GBS. |
| [189] | Puerto Rico | 2015/11/23-2016/01/28 | GBS | 1 | 1 ZIKV positive case developed GBS | |
| [116, 117,170] | Suriname | 2015/12–2016/01/21 | GBS | 13 | 10 GBS (2015) and 3 GBS (2016) | Historic average is 4/year. 2/10 2015 cases were ZIKV confirmed. |
| [214] | Suriname | 2016/01 | immune-mediated thrombocytopenia | 1 | ZIKV positive patient | Developed normal clinical symptoms of ZIKV, and on day 29 diagnosed immune-mediated thrombocytopenia. |
| [117, 170,175,237] | Venezuela | 2016/01/01–2016/02/10 | GBS and other neurological symptoms | 252 | 252 GBS (3 confirmed) | Up to 76% of patients reported clinical symptoms of ZIKV. 65% had comorbidities. 3 cases with other neurological symptoms were ZIKV positive. |
1 RT-PCR = reverse transcription-polymerase chain reaction,
2 PRNT = plaque reduction neutralization test
Complications associated with ZIKV infection in humans are reported in Table 11. The research and attention concentrated on all potential complications of ZIKV has increased significantly in the last few months; we identified that many official reports and papers published the same information multiple times. A number of small case series or case studies investigating complications with ZIKV have demonstrated a chronological association between ZIKV infection in pregnant women and development of microcephaly in the fetus, ZIKV infection in fetuses and newborns from mothers exposed to ZIKV at various points during pregnancy, and ZIKV preceding neurological conditions, mainly Guillain-Barre syndrome in adults, Table 11. A significant increase in microcephaly has been reported in Brazil and a good deal of research and scrutiny of the existing data is on-going to determine the degree that ZIKV is contributing to microcephaly cases. Two studies discuss the criteria used to categorize infants as positive for microcephaly and the potential impact on the numbers reported in Brazil [247,248]; one study shows evidence of an increase in microcephaly in Brazil starting in 2012 prior to ZIKV arriving in the Americas [248]. Other birth defects have also been noted including a number of ocular abnormalities and hydrops fetalis [48,201]. Hopefully on-going cohort studies will provide better evidence for the role ZIKV plays in birth defects and fetal death. Outside of Brazil, no other country has noted the increase in microcephaly at the time of writing (March 1, 2016), however several countries in the Americas and Australasia have noted an increase in Guillain-Barré Syndrome coinciding with a ZIKV outbreak, Table 11 [146,242,246].
Transmission
ZIKV is known to be transmitted by mosquitos; however there is also increasing evidence that intrauterine transmission and sexual transmission via semen may occur, Table 12. It has also been suggested that transmission by blood transfusion is possible, although no cases have been reported [71]. Two studies examined the efficacy of amotosalen combined with UVA light treatment of plasma and recommended that it was effective to deactivate ZIKV [30,32].
Table 12. Studies reporting various modes of human to human transmission and one human to mosquito transmission study.
| Refid | Year of study | Country | Study design | Mode of transmission | Comment |
|---|---|---|---|---|---|
| [80] | 2015 | Brazil | Case study | human- intrauterine | 2 mothers with clinical symptoms of ZIKV were serum negative, but amniotic fluid positive. |
| [116] | 2016 | Brazil | Outbreak investigation | human- intrauterine | 17 infants with microcephaly and 5 fetuses were ZIKV positive |
| [52] | 2016 | Brazil | Case study | human- intrauterine | Mothers presented with ZIKV at 10 and 18 weeks. Fetuses developed microcephaly after ZIKV infection and were positive for ZIKV in fetal tissue |
| [226] | 2015/07 | New Caledonia | Case study | human- breast milk | No transmission was noted, but virus was cultured from breast milk. |
| [71] | 2013/11/21-2014/02/17 | French Polynesia | Cross-sectional | human—blood transfusion | 42/1505 blood donations were ZIKV positive, 11/42 donors reported having ZIKV like symptoms within 10 days of the donation. |
| [222,242] | 2013/12–2014/02 | French Polynesia | Case study | human- intrauterine | 2 mothers had active ZIKV infections at delivery. Transmission was thought to have occurred either intrauterine or during delivery. |
| human- direct contact | |||||
| human- breast milk | |||||
| [103] | 1979/10–1980/03 | Gabon | Prevalence | human- intrauterine | 1/28 mother-newborn sera pairs positive for ZIKV. |
| [215] | 2016 | United States | Case study | human—sexual transmission | 7 days after first patient developed symptoms, patient 2 developed symptoms. |
| [227] | 2016 | Italy | Case study | human—sexual transmission | 19 days after patient one developed symptoms, patient 2 developed symptoms. |
| [231] | 2008 | United States | Case study | human—sexual transmission | Hematospermia reported in case 1 and is thought to have transmitted ZIKV to case 3 by sexual transmission. Another possibility is that direct contact and exchange of other bodily fluids, such as saliva, could have resulted in ZIKV transmission, but illness did not develop in their 4 children. |
| [88] | 1956 | Nigeria | Challenge trial | human- mosquito | Single experimental case was unsuccessful at transmission of ZIKV to Ae. aegypti, possibly because the case had a very low titre. |
Testing for ZIKV infection
Serum samples were most commonly used to diagnose ZIKV infection, but other samples including saliva, nasopharyngeal swabs, urine, semen, amniotic fluid and breast milk have also been used to detect ZIKV infection with RT-PCR, Table 13. There were very few studies (9) that evaluated a diagnostic test for ZIKV and of those only 33% reported data on test performance and none reported on a commercial test, Table 14. Of note, several studies captured in this review discuss potential cross-reactivity of some dengue serology tests during the acute phases of ZIKV infection, therefore laboratories and physicians need to follow readily available guidelines [249] so appropriate samples are taken for accurate diagnosis [208,211,212,227].
Table 13. Non-serum samples for ZIKV rtPCR testing reported in 29 studies 2013–2015.
| Ref | Year of study | Country | Sample (N) | Test | Days since symptoms appeared | Comment |
|---|---|---|---|---|---|---|
| [78] | 2015 | Australia | Nasopharyngeal | RT-PCR1 | 2 | |
| [80] | 2015 | Brazil | Amniotic Fluid | RT-PCR | NR | 2 mothers with clinical symptoms of ZIKV were serum negative, but amniotic fluid positive. |
| [238] | 2016 | Brazil | Urine | RT-PCR | NR | ZIKV detected in urine and saliva samples. Transmission potential unknown |
| [52] | 2015 | Brazil | Urine | RT-PCR | 70–126 | Negative Urine |
| Amniotic Fluid | RT-PCR | 70–126 | Positive amniotic fluid | |||
| [49] | 2016 | Brazil | Urine | RT-PCR | 4–14 | Urine positive 4–14 days (7 days longer than serum) |
| [4] | 2015 | Brazil | Fetal organs | RT-PCR | NR | Positive: blood and organ samples (brain, liver, spleen, kidney, lung, and heart). |
| [56] | 2015 | Brazil | Fetal brain and placenta | RT-PCR | NR | Only fetal brain tissue was positive |
| [168,242] | 2016 | Brazil | Fetal tissue | RT-PCR | NR | Tissue samples were positive |
| [172] | 2015 | Brazil | Fetal tissue | RT-PCR | NR | Only central nervous system samples were positive. Organs (heart, lung, liver or placenta) were negative. |
| Amniotic Fluid | RT-PCR | NR | Amniotic fluid was positive | |||
| [60] | 2013 | Canada | Urine | RT-PCR | 6 | Patient was RT-PCR serum positive on day 6 and 9, IgM positive from day 77 onward. |
| Nasopharyngeal | RT-PCR | 6 | ||||
| [143] | 2015 | Fiji | Saliva | RT-PCR | NR | ZIKV positive, sample taken for diagnosis |
| [58] | 2015 | Finland | Urine | RT-PCR | 7 | RT-PCR of serum was negative |
| [212] | 2015–2016 | France | Urine | RT-PCR | 3 | Blood, urine and saliva positive on day 3 and sero-conversion occurred day 8. |
| Saliva | RT-PCR | 3 | ||||
| [145,146,220,250] | 2013–2014 | French Polynesia | Saliva | RT-PCR | 3 (1–8) | 19.2% tested positive by saliva while negative in blood; 8.8% tested positive in blood while negative by saliva (n = 319), McNemar test, p = 0.0117. Oral swabs are non-invasive; sensitivity of testing is increased by collecting both oral swabs and blood samples. |
| [222] | 2013–2014 | French Polynesia | Breast Milk (2) | RT-PCR | 3–8 | Breast milk in two cases positive on day 3–8. Sequential samples not done. |
| Urine | RT-PCR | 8 | Mother and infant urine positive on day 8, negative day11 | |||
| Saliva | RT-PCR | 3 | Infant and mother positive on day 3 of illness. | |||
| [221] | 2013 | French Polynesia | Urine | RT-PCR | 17 | RT-PCR of serum negative |
| Semen | RT-PCR | 14–17 | ||||
| [148] | 2013/09-2014/03 | French Polynesia | Amniotic Fluid | RT-PCR | NR | Retrospective evaluation of amniotic fluid samples from cases of fetal cerebral dysfunction |
| [229] | 2014 | Japan | Urine | RT-PCR | 7 | RT-PCR of serum negative |
| [69] | 2013–2014 | Japan | Urine | RT-PCR | NR | RT-PCR of serum negative, first time ZIKV virus particles reported in urine. |
| [214] | 2016 | Netherlands | Urine | RT-PCR | 17–18 | Urine positive and serum negative |
| [203] | 2014 | New Caledonia | Urine (6) | RT-PCR | 10 to >20 | Urine estimated viral load: 0.7–220.106 copies/ml. Detectable in urine about 7 days longer than serum. Infectious particles not isolated. |
| [226] | 2015/07 | New Caledonia | Breast Milk | RT-PCR | 4 | ZIKV RT-PCR positive in serum on day 3, in breast milk on day 4 and was confirmed by the presence of a cytopathic effect and by RT-qPCR 39 million RNA copies per mL |
| [116,237,251] | 2016 | Martinique | Urine | RT-PCR | NR | Urine sample part of routine sample collection. |
| [55,238] | 2015 | Slovenia | Fetal organs and tissue | RT-PCR | 133 | Only fetal brain tissue positive. Tested: placenta, lungs, heart, skin, spleen, thymus, liver, kidneys, and cerebral cortex. |
| [217] | 2014 | United Kingdom | Semen | RT-PCR | 28 | May be a good sample if the initial viremic blood samples are not available. |
| [239] | 2016 | United States | Cerebrospinal fluid | IgM & PRNT | NR | Mother and fetus were IgM and PRNT2 positive. |
1 RT-PCR = reverse transcription-polymerase chain reaction
2 PRNT = plaque reduction neutralization test
Table 14. Nine studies evaluated the performance of diagnostic tests for ZIKV—none were reported to be commercially available and in most studies evaluation was not on clinical samples.
| Ref | Country | Year published | Diagnostic tests compared/evaluated | Data available? | Comment |
|---|---|---|---|---|---|
| [212] | France | 2011-Present | Dengue false positive | insufficient data | Protocol for ZIKV testing given dengue IgM false positive during acute ZIKV infection. |
| [96] | Senegal | 2011-Present | RT-PCR1: one step / quantitative | Yes: Sn4, raw data, detection limits | Developed a rapid, sensitive and specific real time PCR for the detection of ZIKV circulating in Africa and Asia |
| [252] | Singapore | 2011-Present | RT-PCR | Yes: Sp4, detection limits | Laboratory Sn/Sp good, but not tested on clinical samples |
| [253] | Senegal | 2001–2010 | RT-PCR: one step | Yes: Sn, detection limits | Lab evaluation only, not tested on clinical isolates. |
| [62] | Yap Island, Micronesia | 2001–2010 | Clinical diagnosis (by signs and symptoms), serological tests (IgM and IgG), PRNT2, RT-PCR | insufficient data | Describes that IgM cross-reactivity with dengue occurs when ZIKV infection occurs in individuals seropositive for dengue. (This has since been reported in a number of case studies where dengue IgM initially climbs, but then decreases and the patient never sero converts to IgG dengue, but will develop IgG to ZIKV) |
| [24] | France | 1991–2000 | Sequencing the virus, RT-PCR | insufficient data | Single set of primers for universal amplification of Flaviviruses: based on conserved elements in the 3' untranslated region of mosquito-borne Flavivirus (YF, DEN, JE, WN and ZIKV)3 RNA and in the protein NS5. Recombinant plasmids can then be used to identify the specific virus in one round of rtPCR with hybridization in 2 days. |
| [104] | Senegal | 1981–1990 | Serological tests (IgM), complement fixation test | Yes: Sp | IgM combined with virus isolation is recommended for surveillance as specific diagnosis from a single serum sample is possible. |
| [254] | England | 1971–1980 | Cross-neutralization | insufficient data | Older study, technique obsolete |
| [255] | England | 1960–1970 | Micro-culture method | insufficient data | Older study, technique obsolete |
1 RT-PCR = reverse transcription-polymerase chain reaction
2 PRNT = plaque reduction neutralization test
3 YF = yellow fever; DEN = dengue fever; JE = Japanese encephalitis; WN = West Nile virus
4 Sn = sensitivity, Sp = specificity
From the studies (n = 76) where details of cases and diagnosis were reported, RT-PCR was most frequently used (68.4%) followed by serology (40.8%) and virus isolation (3.9%); one study conducted a serum cytokine analysis [200]. Among the studies that used serology (n = 31), IgM was used in 80.6% of studies, IgG in 41.9% and plaque reduction neutralization tests (PRNT) in 67.7%. Four studies published before 1982 used hemagglutination-inhibition tests, complement fixation tests and neutralization tests [127,204,232,233].
Discussion
In this scoping study we identified 233 primary research papers, reports, theses and conference proceedings on ZIKV published between 1952 and March 1, 2016. There is evidence that the Asian lineage of ZIKV, which had been historically shown to be circulating in Malaysia, Philippines, Pakistan, Thailand and Cambodia, caused an outbreak on Yap Island in 2007 and continued to spread, affecting a number of the Polynesian islands from 2012 to present. In 2014 the virus traveled to South America where autochthonous circulation was recognised in Brazil in early 2015. At this time (March 2016), 31 countries in South-Central America and the Caribbean are reporting local transmission [3].
Historically ZIKV was not known to cause severe illness in humans and for the first 60 years since its initial identification in Uganda (1947) there were only a few accounts of illness caused by ZIKV. These were mainly in laboratory workers who inadvertently became exposed to the virus. Epidemiological studies in this time period were mainly conducted in Africa and evaluated populations for seropositivity to ZIKV, but not active infection. In most countries where the population was tested for ZIKV, researchers found a proportion of the population had been exposed. This proportion varied widely and was likely affected by the types of samples and tests used, a variety of climatic factors, vectors present and their abundance, and other competing viruses circulating in the area. ZIKV can circulate at the same time as other viruses e.g. dengue and Chikungunya, sharing the same vector, however there is no evidence that exposure, infection or immunity to one virus impacts the outcome of a ZIKV infection or provides protection.
During the recent outbreaks from 2007 to present, reporting of data concerning clinical characteristics of ZIKV has significantly increased. Based on the Yap Island outbreak, 18% of exposed individuals are likely to experience symptoms, thus there is likely substantial under-reporting of ZIKV infections as 80% of infected individuals may be asymptomatic [100]. Symptoms are usually mild and very similar to other co-circulating viruses (dengue and chikungunya), which makes diagnosis based on clinical symptoms challenging [173]. The World Health Organization released its interim case definition for ZIKV as a person presenting with clinical symptoms: rash or fever and one or more of arthralgia, arthritis or conjunctivitis and a positive IgM with epidemiological link (probable case) or a sample positive for ZIKV RNA or IgM with a positive PRNT for ZIKV vs. other flaviviruses [256]. However clinical identification of ZIKV is difficult due to similarities with other co-circulating viruses (e.g. dengue and chikungunya) and testing is currently the responsibility of specialized laboratories [257].
Potential complications reported following ZIKV infection are of great concern to the public health community. Associations with birth defects and neurological complications have been reported relatively recently causing the World Health Organization to issue an alert for precautionary measures and intense investigation to close the knowledge gaps associated with ZIKV potential to cause birth defects; mainly microcephaly [4,258–260]. Reports of increased incidence of GBS and other neurological complications were noted in the French Polynesian and New Caledonia outbreaks [137,223,246] and have since been noted in several countries in South and Central America [117,173]. Currently Brazil, El Salvador, Venezuela, Colombia and Suriname have reported an increased incidence and Puerto Rico and Martinique have reported ZIKV associated cases of GBS [117,237,261]. A number of studies are underway to try to understand who is at high risk of developing GBS and other neurological symptoms following ZIKV infection [245]. Although birth defects such as microcephaly linked to ZIKV have been reported in Brazil and possibly from French Polynesia [148], it is not known why microcephaly has not been reported by other affected countries. This may be a matter of population size, a lag time between ZIKV spread and the birth of affected infants, and a lack of awareness that ZIKV may impact fetal development. Hopefully the retrospective and prospective studies underway in Brazil and other ZIKV affected countries will improve our understanding of the role of ZIKV in causing microcephaly, other birth defects and negative pregnancy outcomes as this is a current knowledge gap.
Guidelines have been developed for the diagnosis of ZIKV infection in humans and based on the studies captured in this review RT-PCR is most common on serum samples and is recommended for samples collected within the first 7 days of symptoms. Many studies were identified that used other samples (saliva, nasopharyngeal swabs, urine, cerebral spinal fluid, semen, amniotic fluid and breast milk) and RT-PCR to identify ZIKV RNA which would be indicative of ZIKV infection. Studies on some of these other sample types may offer a longer or later window of sampling for identification of the virus RNA with RT-PCR, but none have been widely used within primary research studies to date. The identification of ZIKV RNA in various types of bodily fluids raises questions of human to human transmission via saliva, semen and breast milk. At this time only sexual transmission via semen has been reported. ZIKV has been identified in semen up to 72 days post symptoms; however, this is based on a small number of observations and further research is needed to confirm and better understand this finding. Given the potential for ZIKV survival in semen for a long period of time, current guidelines suggest precautions are taken for up to 6 months following possible exposure to ZIKV to try to prevent some of the growing number of sexually transmitted cases reported from Europe and North America [215,221,227,262–264]. Intrauterine transmission has also been shown to be highly plausible. The first case study was from French Polynesia in women who were already infected at time of delivery and infection was confirmed in both mothers and newborns; the newborns recovered from ZIKV infection without complications [222]. Studies from Brazil and other countries have now provided further evidence for the impact of ZIKV on fetuses in utero as several studies have reported ZIKV in amniotic fluid and deceased fetuses and infants with severe birth defects [55,80,172,214,222,240,251]. The implications of ZIKV infection during gestation and variability in the severity of birth defects depending on when maternal ZIKV exposure occurs during pregnancy are current knowledge gaps that are the focus of research progressing in the Americas [48,52,55,234,240,259]. Based on the first few case series of women exposed to ZIKV during pregnancy with documented fetal/infant outcomes, the data suggest ZIKV infection up to 20 weeks of gestation may be the highest risk for negative outcomes such as fetal death or birth defects [48,52,55,234,240,259]. Transmission by blood transfusion is also possible, although there are currently no documented cases of this type of transmission. There are studies from French Polynesia indicating the possible risk of transfusion due to subclinical viremic blood donors. Two studies demonstrated the efficacy of photochemical treatment of plasma to prevent plasma transfusion-transmitted ZIKV and potentially other arboviruses [30,32,71].
Only 56 publications on ZIKV preceded the 2007 Yap Island outbreak, which was the first time that wide spread illness due to ZIKV was documented. This research was primarily published in Africa and focused on vectors, hosts, and understanding the virus rather than on the burden of illness in humans. These articles provide evidence for the vector competence of several mosquito species to be infected with ZIKV and their ability to transmit ZIKV. They also suggest that non-human primates can be infected with ZIKV and may have a role in its transmission cycle. However, there is not enough evidence to conclude whether non-human primates are a reservoir or incidental host of ZIKV [23]. Very few surveys have been conducted on other animal species and the literature doesn’t suggest other potential reservoirs including bats. Thus, identification of the reservoirs or potential spectrum of hosts for ZIKV remains a knowledge gap.
Molecular evaluation of ZIKV through time has been carried out in a few studies and suggest that ZIKV emerged in Uganda around the 1920s and demonstrated little preference for host or vector during its spread through west and central Africa and Asia [68,83]. They demonstrated that the ZIKV cycle may be 1–2 years compared to 5–8 year cycles seen by other viruses [68]. To date a significant change in the ZIKV genome that lead to increased pathogenesis in humans, increased virulence or spread to new competent vectors has not been described in the literature. Although given the range of vectors shown to be competent for ZIKV transmission, it is possible that ZIKV recently started cycling in a vector species it historically had not. Further research on the evolution of ZIKV and competent vectors may explain this knowledge gap.
Mitigation strategies to prevent or control ZIKV were the topic of one experimental study examining the complexity of a resistance gene in Ae. aegypti formosus and concluded that the resistance to ZIKV in this mosquito was complex and not easily transferred to other mosquito species [31]. Although general mosquito mitigation is outside the scope of this review, it is relevant to the prevention of ZIKV and other mosquito transmitted diseases.
This scoping review identified and characterized the global literature on ZIKV (up to March 1st, 2016) and identified several knowledge gaps with respect to its epidemiology, the burden of disease in humans and complications related to ZIKV infection. Historically there has been little research on this virus, however, given the current spread of ZIKV through Australasia and the Americas, significant research resources have been allocated to closing many of the knowledge gaps identified in this scoping review. Future updates of this review will likely demonstrate enhanced evidence and understanding of ZIKV and its impact on public health.
Supporting Information
(DOCX)
(XLSX)
Acknowledgments
We would like to thank Janet Harris and the NML library staff for their help with article procurement.
Data Availability
All relevant data are within the paper and its Supporting Information files. All relevant studies are listed in the reference list; references are publicly available.
Funding Statement
This study was funded through the Public Health Agency of Canada (http://www.phac-aspc.gc.ca). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dick GWA, Kitchen SF, Haddow AJ. Zika Virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46: 509–520. [DOI] [PubMed] [Google Scholar]
- 2.Marano G, Pupella S, Vaglio S, Liumbruno GM, Grazzini G. Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus. 2015: 1–6. 10.2450/2015.0066-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center for Disease Control and Prevention. Zika Webpage. 2016.
- 4.World Health Organization. Epidemiological alert: neurological syndrome, congenital malformations, and Zika virus infection Implications for public health in the Americas. WHO; 2015, December 1. [Google Scholar]
- 5.Pham MT, Rajic A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. 2014: n/a-n/a. 10.1002/jrsm.1123. [DOI] [PMC free article] [PubMed]
- 6.Colquhoun HL, Levac D, O'Brien KK, Straus S, Tricco AC, Perrier L, et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol. 2014;67: 1291–1294. 10.1016/j.jclinepi.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 7.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005;8: 19–32. [Google Scholar]
- 8.Levac D, Colquhoun H, O'Brien KK. Scoping studies: Advancing the methodology. Implementation Science. 2010;5 10.1186/1748-5908-5-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young I, Waddell L, Sanchez J, Wilhelm B, McEwen SA, Rajic A. The application of knowledge synthesis methods in agri-food public health: recent advancements, challenges and opportunities. Prev Vet Med. 2014;113: 339–355. 10.1016/j.prevetmed.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 10.Tusevljak N, Rajic A, Waddell L, Dutil L, Cernicchiaro N, Greig J, et al. Prevalence of zoonotic bacteria in wild and farmed aquatic species and seafood: a scoping study, systematic review, and meta-analysis of published research. Foodborne Pathog Dis. 2012;9: 487–497. 10.1089/fpd.2011.1063 [DOI] [PubMed] [Google Scholar]
- 11.European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus infection outbreak, French Polynesia. ECDC; 2014, February 14. [Google Scholar]
- 12.European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus infection outbreak, Brazil and the Pacific region– 25 May 2015. ECDC; 2015, May 25. [Google Scholar]
- 13.European Centre for Disease Prevention and Control. Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barré syndrome (first update) 21 January 2016. ECDC; 2016, January 21. [Google Scholar]
- 14.Nhan T, Musso D. Emergence of the Zika virus. Virologie. 2015;19: 225–235. [DOI] [PubMed] [Google Scholar]
- 15.Yasri S, Wiwanitkit V. New human pathogenic dengue like virus infections (Zika, Alkhumraand Mayaro viruses): A short review. Asian Pac J Trop Dis. 2015;5: S31–S32. 10.1016/S2222-1808(15)60851-9 [DOI] [Google Scholar]
- 16.Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: Following the path of dengue and chikungunya? Lancet. 2015;386: 243–244. 10.1016/S0140-6736(15)61273-9 [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Morales AJ. Zika: The new arbovirus threat for latin america. J Infect Dev Ctries. 2015;9: 684–685. 10.3855/jidc.7230 [DOI] [PubMed] [Google Scholar]
- 18.Salim Mattar V, Marco Gonzalez T. Now is the time for the Zika virus. Rev MVZ Cordoba. 2015;20: 4511–4512. [Google Scholar]
- 19.Joob B, Wiwanitkit V. Zika virus infection and dengue: A new problem in diagnosis in a dengue-endemic area. Ann Trop Med Public Health. 2015;8: 145–146. 10.4103/1755-6783.162402 [DOI] [Google Scholar]
- 20.Derraik JG, Slaney D. Notes on Zika virus—An emerging pathogen now present in the South Pacific. Aust New Zealand J Public Health. 2015;39: 5–7. 10.1111/1753-6405.12302 [DOI] [PubMed] [Google Scholar]
- 21.Kuno G, Chang G-J. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch Virol. 2007;152: 687–696. 10.1007/s00705-006-0903-z [DOI] [PubMed] [Google Scholar]
- 22.Kilbourn AM, Karesh WB, Wolfe ND, Bosi EJ, Cook RA, Andau M. Health evaluation of free-ranging and semi-captive orangutans (Pongo pygmaeus pygmaeus) in Sabah, Malaysia. J Wildl Dis. 2003;39: 73–83. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe ND, Kilbourn AM, Karesh WB, Rahman HA, Bosi EJ, Cropp BC, et al. Sylvatic transmission of arboviruses among Bornean orangutans. Am J Trop Med Hyg. 2001;64: 310–316. [DOI] [PubMed] [Google Scholar]
- 24.Pierre V, Drouet M, Deubel V. Identification of mosquito-borne flavivirus sequences using universal primers and reverse transcription/polymerase chain reaction. Res Virol. 1994;145: 93–104. 10.1016/S0923-2516(07)80011-2 [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Pulgarin DF, Acevedo-Mendoza WF, Cardona-Ospina JA, Rodríguez-Morales AJ, Paniz-Mondolfi AE. A bibliometric analysis of global Zika research. Travel Med Infect Dis. 2015. 10.1016/j.tmaid.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 26.Baba SS, Fagbami AH, Olaleye OD. Antigenic relatedness of selected flaviviruses: Study with homologous and heterologous immune mouse ascitic fluids. Rev Inst Med Trop Sao Paulo. 1998;40: 343–349. [DOI] [PubMed] [Google Scholar]
- 27.Buckley A, Gould EA. Detection of virus-specific antigen in the nuclei or nucleoli of cells infected with Zika or Langat virus. J Gen Virol. 1988;69: 1913–1920. [DOI] [PubMed] [Google Scholar]
- 28.Olson JG, Ksiazek TG, Gubler DJ, Lubis SI, Simanjuntak G, Lee VH, et al. A survey for arboviral antibodies in sera of humans and animals in Lombok, Republic of Indonesia. Ann Trop Med Parasitol. 1983;77: 131–137. [DOI] [PubMed] [Google Scholar]
- 29.Pan American Health Organization. Epidemiological Alert Zika virus infection 7 May 2015. PAHO; 2015. [Google Scholar]
- 30.Aubry M, Richard V, Musso D. Amotosalen and ultraviolet a light inactivate zika virus in plasma. Vox Sang. 2015;109: 189–190. [Google Scholar]
- 31.Miller BR, Mitchell CJ. Genetic selection of a flavivirus-refractory strain of the yellow fever mosquito Aedes aegypti. Am J Trop Med Hyg. 1991;45: 399–407. [DOI] [PubMed] [Google Scholar]
- 32.Aubry M, Richard V, Green J, Broult J, Musso D. Inactivation of Zika virus in plasma with amotosalen and ultraviolet A illumination. Transfusion. 2016;56: 33–40. 10.1111/trf.13271 [DOI] [PubMed] [Google Scholar]
- 33.Sabogal-Roman JA, Murillo-García DR, Yepes-Echeverri MC, Restrepo-Mejia JD, Granados-Álvarez S, Paniz-Mondolfi AE, et al. Healthcare students and workers' knowledge about transmission, epidemiology and symptoms of Zika fever in four cities of Colombia. Travel Med Infect Dis. 2015. 10.1016/j.tmaid.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 34.Bogoch II, Brady OJ, Kraemer MUG, German M, Creatore MI, Kulkarni MA, et al. Anticipating the international spread of Zika virus from Brazil. The Lancet. 2016;387: 335–336. 10.1016/S0140-6736(16)00080-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Althouse BM, Hanley KA, Diallo M, Sall AA, Ba Y, Faye O, et al. Impact of climate and mosquito vector abundance on sylvatic arbovirus circulation dynamics in senegal. Am J Trop Med Hyg. 2015;92: 88–97. 10.4269/ajtmh.13-0617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kucharski AJ, Funk S, Eggo RM, Mallet HP, Edmunds WJ, Nilles EJ. Transmission dynamics of Zika virus in island populations: a modelling analysis of the 2013{14 French Polynesia outbreak. bioRxiv. EPUB: February 7, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reagan RL, Brueckner AL. Comparison by electron microscopy of the Ntaya and Zika viruses. Tex Rep Biol Med. 1953;11: 347–351. [PubMed] [Google Scholar]
- 38.Weinbren MP, Williams MC. Zika virus: Further isolations in the zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg. 1958;52: 263–268. [DOI] [PubMed] [Google Scholar]
- 39.Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, et al. Biology of Zika virus infection in human skin cells. J Virol. 2015;89: 8880–8896. 10.1128/JVI.00354-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Way HJ, Bowen ETW, Platt GS. Comparative studies of some African arboviruses in cell culture and in mice. J Gen Virol. 1976;30: 123–130. [DOI] [PubMed] [Google Scholar]
- 41.Reagan RL, Chang SC, Brueckner AL. Electron micrographs of erythrocytes from Swiss albino mice infected with Zika virus. Tex Rep Biol Med. 1955;13: 934–938. [PubMed] [Google Scholar]
- 42.Bell TM, Field EJ, Narang HK. Zika virus infection of the central nervous system of mice. Archiv f Virusforschung. 1971;35: 183–193. 10.1007/BF01249709 [DOI] [PubMed] [Google Scholar]
- 43.MacNamara FN. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48: 139–145. [DOI] [PubMed] [Google Scholar]
- 44.Dick GWA. Zika virus (II). Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46: 521–534. [DOI] [PubMed] [Google Scholar]
- 45.Rockefeller Foundation. Rockefeller Foundation Annual Report. Zika pages 131–140. Rockefeller Foundation, NY, USA: 1951. [Google Scholar]
- 46.Henerson B, Cheshire P, Kirya G, Lule M. Immunologic Studies with Yellow Fever and Selected African Group B Arboviruses in Rhesus and Vervet Monkeys. The American Journal of Tropical Medicine and Hygiene. 1970;19: 110 [DOI] [PubMed] [Google Scholar]
- 47.Lanciotti RS, Lambert AJ, Holodniy M, Saavedra S, del Carmen Castillo Signor L. Phylogeny of Zika virus inWestern Hemisphere,2015 [letter]. Emerg Infect Dis. 2016. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarno M, Sacramento GA, Khouri R, do Rosario MS, Costa F, Archanjo G, et al. Zika Virus Infection and Stillbirths: A Case of Hydrops Fetalis, Hydranencephaly and Fetal Demise. PLoS Negl Trop Dis. 2016;10 10.1371/journal.pntd.0004517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de M Campos R, Cirne-Santos C, Meira GL, Santos LL, de Meneses MD, Friedrich J, et al. Prolonged detection of Zika virus RNA in urine samples during the ongoing Zika virus epidemic in Brazil. J Clin Virol. 2016;77: 69–70. S1386-6532(16)30001-4 [pii]. 10.1016/j.jcv.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 50.Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol. 1998;72: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freire CCM, lamarino A, Neto DF, Sall AA, Zanotto PMA. Spread of the pandemic Zika virus lineage is associated with NS1 codon usage adaptation in humans. bioRxiv. 2015. [Google Scholar]
- 52.Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016. S1473-3099(16)00095-5 [pii]. [DOI] [PubMed] [Google Scholar]
- 53.Perkasa A, Yudhaputri F, Haryanto S, Hayati RF, Chairin NM, Antonjaya U, et al. Isolation of Zika Virus from Febrile Patient, Indonesia. Emerg Infect Dis. 2016;22 10.3201/eid2205.151915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camacho E, Paternina-Gomez M, Blanco PJ, Osorio JE, Aliota MT. Detection of Autochthonous Zika Virus Transmission in Sincelejo, Colombia. Emerging Infectious Disease journal. 2016;22 10.3201/eid2205.160023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016. 10.1056/NEJMoa1600651 [DOI] [PubMed] [Google Scholar]
- 56.Martines RB, Bhatnagar J, Keating MK, Silva-Flannery L, Muehlenbachs A, Gary J, et al. Notes from the Field: Evidence of Zika Virus Infection in Brain and Placental Tissues from Two Congenitally Infected Newborns and Two Fetal Losses—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65: 159–160. 10.15585/mmwr.mm6506e1 [DOI] [PubMed] [Google Scholar]
- 57.Pyke AT, Daly MT, Cameron JN, Moore PR, Taylor CT, Hewitson GR, et al. Imported zika virus infection from the cook islands into australia, 2014. PLoS Curr. 2014;6 10.1371/currents.outbreaks.4635a54dbffba2156fb2fd76dc49f65e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korhonen EM, Huhtamo E, Smura T, Kallio-Kokko H, Raassina M, Vapalahti O. Zika virus infection in a traveller returning from the Maldives, June 2015. Euro Surveill. 2016;21 10.2807/1560-7917.ES.2016.21.2.30107 [DOI] [PubMed] [Google Scholar]
- 59.Baronti C, Piorkowski G, Charrel RN, Boubis L, LeparcGoffart I, Lamballerie X. Complete coding sequence of Zika virus from a French Polynesia outbreak in 2013. Genome Announcements. 2014;2: e00500–14. 10.1128/genomeA.00500-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fonseca K, Meatherall B, Zarra D, Drebot M, MacDonald J, Pabbaraju K, et al. First case of Zika virus infection in a returning Canadian traveler. Am J Trop Med Hyg. 2014;91: 1035–1038. 10.4269/ajtmh.14-0151. 10.4269/ajtmh.14-0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enfissi A, Codrington J, Roosblad J, Kazanji M, Rousset D. Zika virus genome from the Americas. Lancet. 2016;387: 227–228. 10.1016/S0140-6736(16)00003-9. 10.1016/S0140-6736(16)00003-9 [DOI] [PubMed] [Google Scholar]
- 62.Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14: 1232–1239. 10.3201/eid1408.080287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, et al. Genetic characterization of zika virus strains: Geographic expansion of the asian lineage. PLoS Negl Trop Dis. 2012;6 10.1371/journal.pntd.0001477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li MI, Wong PSJ, Ng LC, Tan CH. Oral Susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika Virus. PLoS Negl Trop Dis. 2012;6 10.1371/journal.pntd.0001792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong P-J, Li M-I, Chong C, Ng L, Tan C. Aedes (Stegomyia) albopictus (Skuse): A Potential Vector of Zika Virus in Singapore. PLoS Negl Trop Dis. 2013;7 10.1371/journal.pntd.0002348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwong JC, Druce JD, Leder K. Case report: Zika virus infection acquired during brief travel to indonesia. Am J Trop Med Hyg. 2013;89: 516–517. 10.4269/ajtmh.13-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wæhre T, Maagard A, Tappe D, Cadar D, Schmidt-Chanasit J. Zika virus infection after travel to Tahiti, December 2013. Emerg Infect Dis. 2014;20: 1412–1414. 10.3201/eid2008.140302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faye O, Freire CCM, Iamarino A, Faye O, de Oliveira JVC, Diallo M, et al. Molecular Evolution of Zika Virus during Its Emergence in the 20th Century. PLoS Negl Trop Dis. 2014;8: 36 10.1371/journal.pntd.0002636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kutsuna S, Kato Y, Takasaki T, Moi ML, Kotaki A, Uemura H, et al. Two cases of zika fever imported from french polynesia to Japan, December to January 2013. Eurosurveillance. 2014;19. [DOI] [PubMed] [Google Scholar]
- 70.Berthet N, Nakouné E, Kamgang B, Selekon B, Descorps-Declère S, Gessain A, et al. Molecular characterization of three zika flaviviruses obtained from sylvatic mosquitoes in the Central African Republic. Vector Borne Zoonotic Dis. 2014;14: 862–865. 10.1089/vbz.2014.1607 [DOI] [PubMed] [Google Scholar]
- 71.Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Eurosurveillance. 2014;19. [DOI] [PubMed] [Google Scholar]
- 72.Cao-Lormeau V. RE: Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis. 2014;20: 1960 10.3201/eid2006.140138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21: 1885–1886. 10.32301/eid2110.150847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dupont-Rouzeyrol M, O’Connor O, Calvez E, Daures M, John M, Grangeon J, et al. Co-infection with zika and dengue viruses in 2 patients, New Caledonia, 2014. Emerg Infect Dis. 2015;21: 381–382. 10.3201/eid2102.141553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alera MT, Hermann L, Tac-An IA, Klungthong C, Rutvisuttinunt W, Manasatienkij W, et al. Zika virus infection, philippines, 2012. Emerg Infect Dis. 2015;21: 722–724. 10.3201/eid2104.141707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zanluca C, De Melo VCA, Mosimann ALP, Dos Santos GIV, dos Santos CND, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110: 569–572. 10.1590/0074-02760150192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buathong R, Hermann L, Thaisomboonsuk B, Rutvisuttinunt W, Klungthong C, Chinnawirotpisan P, et al. Detection of zika virus infection in Thailand, 2012–2014. Am J Trop Med Hyg. 2015;93: 380–383. 10.4269/ajtmh.15-0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leung GH, Baird RW, Druce J, Anstey NM. Zika Virus Infection in Australia Following a Monkey Bite in Indonesia. Southeast Asian J Trop Med Public Health. 2015;46: 460–464. [PubMed] [Google Scholar]
- 79.Tognarelli J, Ulloa S, Villagra E, Lagos J, Aguayo C, Fasce R, et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol. 2015: 1–4. 10.1007/s00705-015-2695-5 [DOI] [PubMed] [Google Scholar]
- 80.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo De Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: Tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47: 6–7. 10.1002/uog.15831 [DOI] [PubMed] [Google Scholar]
- 81.Calvet GA, Filippis AMB, Mendonça MCL, Sequeira PC, Siqueira AM, Veloso VG, et al. First detection of autochthonous Zika virus transmission in a HIV-infected patient in Rio de Janeiro, Brazil. J Clin Virol. 2016;74: 1–3. 10.1016/j.jcv.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 82.Heang V, Yasuda CY, Sovann L, Haddow AD, da Rosa APT, Tesh RB, et al. Zika virus infection, Cambodia, 2010. Emerg Infect Dis. 2012;18: 349–351. 10.3201/eid1802.111224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, et al. Zika Virus in Gabon (Central Africa) - 2007: A New Threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8 10.1371/journal.pntd.0002681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Diagne CT, Diallo D, Faye O, Ba Y, Faye O, Gaye A, et al. Potential of selected Senegalese Aedes spp. mosquitoes (Diptera: Culicidae) to transmit Zika virus. BMC Infect Dis. 2015;15 10.1186/s12879-015-1231-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ledermann JP, Guillaumot L, Yug L, Saweyog SC, Tided M, Machieng P, et al. Aedes hensilli as a Potential Vector of Chikungunya and Zika Viruses. PLoS Negl Trop Dis. 2014;8 10.1371/journal.pntd.0003188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Monlun E, Zeller H, Le Guenno B, Traoré-Lamizana M, Hervy JP, Adam F, et al. Surveillance of the circulation of arbovirus of medical interest in the region of eastern Senegal. Bull Soc Pathol Exot. 1993;86: 21–28. [PubMed] [Google Scholar]
- 87.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18: 411–415. [DOI] [PubMed] [Google Scholar]
- 88.Bearcroft WGC. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg. 1956;50: 438–441. [PubMed] [Google Scholar]
- 89.Boorman JPT, Porterfield JS. A simple technique for infection of mosquitoes with viruses transmission of zika virus. Trans R Soc Trop Med Hyg. 1956;50: 238–242. [DOI] [PubMed] [Google Scholar]
- 90.Cornet M, Robin Y, Adam C, Valade M, Calvo MA. Comparison between experimental transmission of yellow fever and Zika viruses in Aedes aegypti. Cahiers O R S T O M Serie entomologie medicale et parasitologie. 1979;17: 47–53. [Google Scholar]
- 91.Diallo D, Sall AA, Diagne CT, Faye O, Faye O, Ba Y, et al. Zika virus emergence in mosquitoes in Southeastern Senegal, 2011. 2014;9: e10; 44 ref. 10.1371/journal.pone.0109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.AkouaKoffi C, Diarrassouba S, Benie VB, Ngbichi JM, Bozoua T, Bosson A, et al. Inquiry into a fatal case of yellow fever in Cote d'Ivoire in 1999. Bulletin de la Societe de Pathologie Exotique. 2001;94: 227–230. [PubMed] [Google Scholar]
- 93.Traore Lamizana M, Zeller H, Monlun E, Mondo M, Hervy JP, Adam F, et al. Dengue 2 outbreak in southeastern Senegal during 1990: virus isolations from mosquitoes (Diptera: Culicidae). J Med Entomol. 1994;31: 623–627. [DOI] [PubMed] [Google Scholar]
- 94.Robert V, Lhuillier M, Meunier D. Yellow fever virus, dengue 2 virus and other arboviruses isolated from mosquitoes in Burkina Faso during 1983–86. Entomological and epidemiological considerations. Bull Soc Pathol Exot. 1993;86: 90–100. [PubMed] [Google Scholar]
- 95.Lee VH, Moore DL. Vectors of the 1969 yellow fever epidemic on the Jos Plateau, Nigeria. Bull World Health Organ. 1972;46: 669–673. [PMC free article] [PubMed] [Google Scholar]
- 96.Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught Mosquitoes. Virol J. 2013;10 10.1186/1743-422X-10-311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McCrae AWR, Kirya BG. Yellow fever and Zika virus epizootics and enzootics in Uganda. Trans R Soc Trop Med Hyg. 1982;76: 552–562. 10.1016/0035-9203(82)90161-4 [DOI] [PubMed] [Google Scholar]
- 98.Haddow AJ, Williams MC, Woodall JP, Simpson DI, Goma LK. Twelve Isolations of Zika Virus from Aedes (Stegomyia) Africanus (Theobald) Taken in and Above a Uganda Forest. Bull World Health Organ. 1964;31: 57–69. [PMC free article] [PubMed] [Google Scholar]
- 99.Cornet M, Robin Y, Chateau R, Heme G, Adam C, Valade M, et al. Isolations of arboviruses in eastern Senegal from mosquitoes (1972–1977) and notes on the epidemiology of viruses transmitted by Aedes spp., especially yellow fever. Cahiers ORSTOM, Entomologie Medicale et Parasitologie. 1979;17: 149–163. [Google Scholar]
- 100.Duffy MR, Chen T, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. New Engl J Med. 2009;360: 2536–2543. 10.1056/NEJMoa0805715 [DOI] [PubMed] [Google Scholar]
- 101.Kading Mossel, Borland Ledermann, Panella Crabtree, et al. Arbovirus surveillance in bats in Uganda. American Journal of Tropical Medicine and Hygiene Conference. 2014;91: 260. [Google Scholar]
- 102.Kirya BG, Okia NO. A yellow fever epizootic in Zika Forest, Uganda, during 1972: Part 2: Monkey serology. Trans R Soc Trop Med Hyg. 1977;71: 300–303. 10.1016/0035-9203(77)90104-3 [DOI] [PubMed] [Google Scholar]
- 103.Saluzzo JF, Ivanoff B, Languillat G, Georges AJ. Serological survey for arbovirus antibodies in the human and simian populations of the South East of Gabon. Bull Soc Pathol Exot Fil. 1982;75: 262–266. [PubMed] [Google Scholar]
- 104.Saluzzo JF, Sarthou JL, Cornet M. Specific ELISA-IgM antibodies for diagnosis and epidemiological surveillance of flaviviruses in Africa. Ann Inst Pasteur Virol. 1986;137: 155–170. 10.1016/S0769-2617(86)80201-5 [DOI] [Google Scholar]
- 105.Darwish MA, Hoogstraal H, Roberts TJ, Ahmed IP, Omar F. A sero-epidemiological survey for certain arboviruses (Togaviridae) in Pakistan. Trans R Soc Trop Med Hyg. 1983;77: 442–445. 10.1016/0035-9203(83)90106-2 [DOI] [PubMed] [Google Scholar]
- 106.Crockett Ledermann, Borland Powers, Panella Crabtree, Miller. Arbovirus surveillance and virus isolations from bats in Uganda, Kenya, and the democratic republic of the Congo. American Journal of Tropical Medicine and Hygiene Conference. 2012;87: 170. [Google Scholar]
- 107.Crockett Crabtree, Ledermann Borland, Powers Mutebi, et al. Investigations into mosquito blood feeding patterns on wildlife and a potential role for bats in arbovirus transmission cycles in Uganda. American Journal of Tropical Medicine and Hygiene Conference. 2011;85: 374–375. [Google Scholar]
- 108.Pond WL. Arthropod-borne virus antibodies in sera from residents of South-East Asia. Trans R Soc Trop Med Hyg. 1963;57: 364–371. [DOI] [PubMed] [Google Scholar]
- 109.Geser A, Henderson BE, Christensen S. A multipurpose serological survey in Kenya. 2. Results of arbovirus serological tests. Bull World Health Organ. 1970;43: 539–552. [PMC free article] [PubMed] [Google Scholar]
- 110.Babaniyi OA, Mwaba P, Songolo P, Mazaba-Liwewe ML, Mweene-Ndumba I, Masaninga F, et al. Seroprevalence of Zika virus infection specific IgG in Western and North-Western Provinces of Zambia. International Journal of Public Health and Epidemiology. 2015, January;4: 110–114. [Google Scholar]
- 111.Kokernot RH, Casaca VMR, Weinbren MP, McIntosh BM. Survey for antibodies against arthropod-borne viruses in the sera of indigenous residents of Angola. Trans R Soc Trop Med Hyg. 1965;59: 563–570. [DOI] [PubMed] [Google Scholar]
- 112.Rodhain F, Carterton B, Laroche R, Hannoun C. Human arbovirus infections in Burundi: results of a seroepidemiological survey, 1980–1982. Bull Soc Pathol Exot Filiales. 1987;80: 155–161. [PubMed] [Google Scholar]
- 113.Fokam EB, Levai LD, Guzman H, Amelia PA, Titanji VP, Tesh RB, et al. Silent circulation of arboviruses in Cameroon. East Afr Med J. 2010;87: 262–268. [DOI] [PubMed] [Google Scholar]
- 114.Boche R, Jan C, Le Noc P, Ravisse P. Immunologic survey on the incidence of arboviruses in the Pygmy population in Eastern Cameroon (Djoum area). Bull Soc Pathol Exot Fil. 1974;67: 126–140. [PubMed] [Google Scholar]
- 115.World Health Organization. Zika virus infection–Cape Verde Disease Outbreak News. 21 December 2015. WHO. 2015. [Google Scholar]
- 116.World Health Organization. Neurological Syndrome and Congenital Anomalies Situration Report. 5 February 2016. WHO. 2016. [Google Scholar]
- 117.World Health Organization. Zika Virus Microcephaly and Gullian-Barre Syndrome: Situation Report. 19 February 2016. WHO. 2016. [Google Scholar]
- 118.Saluzzo JF, Gonzalez JP, Herve JP, Georges AJ. Serological survey for the prevalence of certain arboviruses in the human population of the south-east area of Central African Republic. Bull Soc Pathol Exot Fil. 1981;74: 490–499. [PubMed] [Google Scholar]
- 119.Tsegaye. Virological and serological investigation of the first dengue fever outbreak in Ethiopia. American Journal of Tropical Medicine and Hygiene Conference. 2014;91: 53. [Google Scholar]
- 120.Jan Ch., Languillat G, Renaudet J, Robin Y. A serologic survey on arboviruses in Gabon. Bull Soc Pathol Exot Fil. 1978;71: 140–146. [PubMed] [Google Scholar]
- 121.De Araujo LJ, Bausch D.G., Mores C.N. Serological evidence of co-circulating dengue virus serotypes, an underreported arboviral disease in Guinea. Am J Trop Med Hyg. 2010;83: 314.20682874 [Google Scholar]
- 122.Ofula Wurapa. Evidence of circulation of selected arboviruses in ijara and marigat districts, Kenya. American Journal of Tropical Medicine and Hygiene Conference. 2013;89: 278. [Google Scholar]
- 123.Ofula Wurapa. Seroprevalence of selected arboviruses in ijara and marigat districts,Kenya. American Journal of Tropical Medicine and Hygiene Conference. 2012;87: 126. [Google Scholar]
- 124.Bowen ETW, Simpson DIH, Platt GS, Way H, Bright WF, Day J, et al. Large scale irrigation and arbovirus epidemiology, Kano plain, Kenya II. Preliminary serological survey. Trans R Soc Trop Med Hyg. 1973;67: 702–709. 10.1016/0035-9203(73)90041-2 [DOI] [PubMed] [Google Scholar]
- 125.Adekolu-John EO, Fagbami AH. Arthropod-borne virus antibodies in sera of residents of Kainji Lake Basin, Nigeria 1980. Trans R Soc Trop Med Hyg. 1983;77: 149–151. 10.1016/0035-9203(83)90053-6 [DOI] [PubMed] [Google Scholar]
- 126.Fagbami A. Epidemiological investigations on arbovirus infections at Igbo-Ora, Nigeria. Trop Geogr Med. 1977;29: 187–191. [PubMed] [Google Scholar]
- 127.Fagbami AH. Zika virus infections in Nigeria: Virological and seroepidemiological investigations in Oyo State. J Hyg. 1979;83: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Monath TP, Wilson DC, Casals J. The 1970 yellow fever epidemic in Okwoga District, Benue Plateau State, Nigeria. 3. Serological responses in persons with and without pre-existing heterologous group B immunity. BULL WHO. 1973;49: 235–244. [PMC free article] [PubMed] [Google Scholar]
- 129.Moore DL, Causey OR, Carey DE, Reddy S, Cooke AR, Akinkugbe FM, et al. Arthropod borne viral infections of man in Nigeria, 1964–1970. Ann Trop Med Parasitol. 1975;69: 49–64. [DOI] [PubMed] [Google Scholar]
- 130.Macnamara FN, Horn DW, Porterfield JS. Yellow fever and other arthropod-borne viruses. A consideration of two serological surveys made in South Western Nigeria. Trans R Soc Trop Med Hyg. 1959;53: 202–212. [DOI] [PubMed] [Google Scholar]
- 131.Sow A, Loucoubar C, Diallo D, Faye O, Ndiaye Y, Senghor CS, et al. Concurrent malaria and arbovirus infections in Kedougou, southeastern Senegal. Malar J. 2016;15: 47-016-1100-5. 10.1186/s12936-016-1100-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Renaudet J, Jan C, Ridet J, Adam C, Robin Y. A serological survey of arboviruses in the human population of Senegal. Bull Soc Pathol Exot Filiales. 1978;71: 131–140. [PubMed] [Google Scholar]
- 133.Robin Y, Mouchet J. Serological and entomological survey of yellow fever in Sierra Leone. Bull Soc Pathol Exot. 1975;68: 249–258. [PubMed] [Google Scholar]
- 134.Direction de la santé Bureau de veille sanitaire. Surveillance et veille sanitaire en Polynésie française. Données du 01 au 07 Février 2016 (Semaine 05). Direction de la santé Bureau de veille sanitaire. 2016.
- 135.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 6, ending 14 Feb, 2016. WHO; 2016. [Google Scholar]
- 136.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 7, ending 21 Feb, 2016. WHO; 2016. [Google Scholar]
- 137.Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, et al. Concurrent outbreaks of dengue, chikungunya and zika virus infections–An unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012–2014. Eurosurveillance. 2014;19. [DOI] [PubMed] [Google Scholar]
- 138.Institut de Veille Sanitaire. Bulletin Hebdomadaire International N°442, 05 au 11 mars 2014. Institut de Veille Sanitaire; 2014. [Google Scholar]
- 139.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 17, ending 27 Apr, 2014. WHO; 2014. [Google Scholar]
- 140.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 20, ending 17 May, 2015. WHO; 2015. [Google Scholar]
- 141.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 20, ending 18 May, 2014. WHO; 2014. [Google Scholar]
- 142.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 21, ending 25 May, 2014. WHO; 2014. [Google Scholar]
- 143.World Health Organization. Pacific syndromic surveillance report. Week 33, ending 16 August, 2015. WHO; 2015. [Google Scholar]
- 144.Mallet HP. Emergence du virus Zika en Polynésie française [presentation]. Conference: 15es JNI, Bordeaux du 11 au 13 juin 2014. 2014.
- 145.Agence Regionale de Sante. Emergence des maladies infectieuses. Bulletin de veille sanitaire. no2 Jun-Aug 2014. Agence Regionale de Sante; 2014. [Google Scholar]
- 146.Mallet HP, Vial AL, Musso D. Bilan de l'Epidemie a Virus Zika en Polynesie Francaise, 2013–2014. Bulletin d'Information Sanitaires, Epidemiologiques et Statistiques. 2015. [Google Scholar]
- 147.Institut de Veille Sanitaire. Virus Zika Polynesie 2013–2014, Isle de yap, Micronesia 2007 (janvier 2014). Institut de Veille Sanitaire; 2014. [Google Scholar]
- 148.Jouannic JM, Friszer S, Leparc-Goffart I, Garel C, Eyrolle-Guignot D. Zika virus infection in French Polynesia. Lancet. 2016. S0140-6736(16)00625-5 [pii]. [DOI] [PubMed] [Google Scholar]
- 149.Aubry M, Finke J, Teissier A, Roche C, Broult J, Paulous S, et al. Seroprevalence of arboviruses among blood donors in French Polynesia, 2011–2013. Int J Infect Dis. 2015;41: 11–12. 10.1016/j.ijid.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 150.Antonjaya. Study of arboviruses in archived specimens from acute febrile illness studies in bandung,Indonesia. American Journal of Tropical Medicine and Hygiene Conference. 2012;87: 122. [Google Scholar]
- 151.Van Peenen PFD, Joseph SW, Casals J. Arbovirus antibodies in Indonesians at Malili, South Sulawesi (Celebes) and Balikpapan, E. Kalimantan (Borneo). Ann Trop Med Parasitol. 1975;69: 475–482. [Google Scholar]
- 152.World Health Organization, Western Pacific Region, Division of Pacific Technical Support; Pacific Syndromic Surveillance: Week 24, ending 15 Jun, 2014. WHO; 2014. [Google Scholar]
- 153.World Health Organization, Western Pacific Region, Division of Pacific Technical Support; Pacific Syndromic Surveillance: Week 30, ending 27 Jul, 2014. WHO; 2014. [Google Scholar]
- 154.World Health Organization, Western Pacific Region, Division of Pacific Technical Support; Pacific Syndromic Surveillance: Week 49, ending 6 Dec, 2015. WHO; 2015. [Google Scholar]
- 155.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 37, ending 13 Sep, 2015. WHO; 2015. [Google Scholar]
- 156.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 46, ending 15 Nov, 2015. WHO; 2015. [Google Scholar]
- 157.World Health Organization. Pacific syndromic surveillance report. Week 17, ending 26 April, 2015. WHO; 2015. [Google Scholar]
- 158.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 22, ending 31 May, 2015. WHO; 2015. [Google Scholar]
- 159.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 12, ending 22 Mar, 2015. WHO; 2015. [Google Scholar]
- 160.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 14, ending 5 Apr, 2015. WHO; 2015. [Google Scholar]
- 161.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 16, ending 19 Apr, 2015. WHO; 2015. [Google Scholar]
- 162.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 17, ending 22 Apr, 2015. WHO; 2015. [Google Scholar]
- 163.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 18, ending 3 May, 2015. WHO; 2015. [Google Scholar]
- 164.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 19, ending 10 May, 2015. WHO; 2015. [Google Scholar]
- 165.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 21, ending 24 May, 2015. WHO; 2015. [Google Scholar]
- 166.Wikan N, Suputtamongkol Y, Yoksan S, Smith DR, Auewarakul P. Immunological evidence of Zika virus transmission in Thailand. Asian Pac J Trop Med. 2016;9: 141–144. 10.1016/j.apjtm.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 167.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 4, ending 31 Jan, 2016. WHO; 2016. [Google Scholar]
- 168.World Health Organization, Western Pacific Region, Division of Pacific Technical Support. Pacific Syndromic Surveillance: Week 5, ending 7 Feb, 2016. WHO; 2016. [Google Scholar]
- 169.World Health Organization. Zika virus infection–Guyana, Barbados and Ecuador Disease Outbreak News. 20 January 2016. WHO; 2016. [Google Scholar]
- 170.World Health Organization. Zika and Potential Complications. Situation Report. 12 February 2016. WHO; 2016. [Google Scholar]
- 171.World Health Organization. Zika virus infection–Brazil and Colombia Disease Outbreak News. 21 October 2015. WHO; 2015. [Google Scholar]
- 172.Pan American Health Organization. Epidemiological Alert Neurological syndrome, congenital malformations, and Zika virus infection Implications for public health in the Americas 1 December 2015. PAHO; 2015. [Google Scholar]
- 173.Cardoso CW, Paploski IA, Kikuti M, Rodrigues MS, Silva MM, Campos GS, et al. Outbreak of Exanthematous Illness associated with Zika, Chikungunya, and Dengue viruses, Salvador, Brazil. Emerg Infect Dis. 2015;21: 2274–2276. 10.3201/eid2112.151167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.World Health Organization. Zika virus infection–Bolivia Disease Outbreak News. 20 January 2016. WHO; 2016. [Google Scholar]
- 175.World Health Organization. Guillain-Barré syndrome–Colombia and Venezuela Disease Outbreak News. 12 February 2016. WHO; 2016. [Google Scholar]
- 176.Arzuza-Ortega L, Polo A, Pérez-Tatis G, López-García H, Parra E, Pardo-Herrera LC. Fatal Zika virus infection in girl with sickle cell disease, Colombia [letter]. Emerg Infect Dis. 2016. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.World Health Organization. Zika virus infection–Dominican Republic Disease Outbreak News. 27 January 2016. WHO; 2016. [Google Scholar]
- 178.World Health Organization. Guillain-Barré syndrome–El Salvador Disease Outbreak News. 21 January 2016. WHO; 2016. [Google Scholar]
- 179.World Health Organization. Zika virus infection–France—French Guiana and Martinique Disease Outbreak News. 8 January 2016. WHO; 2016. [Google Scholar]
- 180.World Health Organization. Zika virus infection–Guatemala Disease Outbreak News. 27 November 2015. WHO; 2015. [Google Scholar]
- 181.Agence Regionale de Sante. Emergence du virus Zika aux Antilles Guyane Situation épidémiologique Point épidémiologique du 21 janvier—N°3 / 2016. Agence Regionale de Sante; 2016. [Google Scholar]
- 182.World Health Organization. Zika virus infection–France—Saint Martin and Guadeloupe Disease Outbreak News, 21 January 2016. WHO; 2016. [Google Scholar]
- 183.World Health Organization. Zika virus infection–Haiti Disease Outbreak News. 21 January 2016. WHO; 2016. [Google Scholar]
- 184.World Health Organization. Zika virus infection–Honduras Disease Outbreak News. 21 December 2015. WHO; 2015. [Google Scholar]
- 185.World Health Organization. Zika virus infection–Mexico Disease Outbreak News. 3 December 2015. WHO; 2015. [Google Scholar]
- 186.World Health Organization. Zika virus infection–Panama Disease Outbreak News. 22 December 2015. WHO. 2015. [Google Scholar]
- 187.World Health Organization. Zika virus infection–Panama Disease Outbreak News. 5 December 2015. WHO; 2015. [Google Scholar]
- 188.World Health Organization. Zika virus infection–Paraguay Disease Outbreak News. 3 December 2015. WHO. 2015. [Google Scholar]
- 189.Thomas DL, Sharp TM, Torres J, Armstrong PA, Munoz-Jordan J, Ryff KR, et al. Local Transmission of Zika Virus—Puerto Rico, November 23, 2015-January 28, 2016. MMWR Morb Mortal Wkly Rep. 2016;65: 154–158. 10.15585/mmwr.mm6506e2 [DOI] [PubMed] [Google Scholar]
- 190.World Health Organization. Zika virus infection–United States of America—Puerto Rico Disease Outbreak News. 8 January 2016. WHO; 2016. [Google Scholar]
- 191.World Health Organization. Zika virus infection–Suriname Disease Outbreak News. 13 November 2015. WHO; 2015. [Google Scholar]
- 192.World Health Organization. Zika virus infection–Suriname Disease Outbreak News. 11 November 2015. WHO; 2015. [Google Scholar]
- 193.Downs WG, Anderson CR, Theiler M. Neutralizing antibodies against certain viruses in the sera of residents of Trinidad, B.W.I. Carib Med J. 1965;27: 39–53. [DOI] [PubMed] [Google Scholar]
- 194.World Health Organization. Zika virus infection–Venezuela Disease Outbreak News. 3 December 2015. WHO; 2015. [Google Scholar]
- 195.World Health Organization. Zika virus infection–United States of America. — United States Virgin Islands Disease Outbreak News. 29 January 2016. WHO; 2016. [Google Scholar]
- 196.New Zealand Public Health. An outbreak of mumps-like illness. New Zealand Public Health Surveill Rep. 2015;13. [Google Scholar]
- 197.New Zealand Public Health. A summary of the key trends in notifiable diseases for 2014. New Zealand Public Health Surveill Rep. 2015;13. [Google Scholar]
- 198.UK government. Health Protection Report: volume 10, issue 8, News February 26th. Zika epidemiological update. Public Health England; 2016. [Google Scholar]
- 199.Villamil-Gomez WE, Gonzalez-Camargo O, Rodriguez-Ayubi J, Zapata-Serpa D, Rodriguez-Morales AJ. Dengue, chikungunya and Zika co-infection in a patient from Colombia. J Infect Public Health. 2016. S1876-0341(15)00221-X [pii]. [DOI] [PubMed] [Google Scholar]
- 200.Tappe D, Pérez-Girón JV, Zammarchi L, Rissland J, Ferreira DF, Jaenisch T, et al. Cytokine kinetics of Zika virus-infected patients from acute to reconvalescent phase. Med Microbiol Immunol. 2015: 1–5. 10.1007/s00430-015-0445-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Freitas B, Oliveira Dias J, Prazeres J. Ocular Findings in Infants With Microcephaly Associated With Presumed Zika Virus Congenital Infection in Salvador, Brazil. JAMA Ophthalmol. 2016;Published online February 9, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ventura CV, Maia M, Ventura BV, Linden VV, Araujo EB, Ramos RC, et al. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol. 2016;79: 1–3. 10.5935/0004-2749.20160002 [DOI] [PubMed] [Google Scholar]
- 203.Gourinat A, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of zika virus in urine. Emerg Infect Dis. 2015;21: 84–86. 10.3201/eid2101.140894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Olson JG, Ksiazek TG, Suhandiman, Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75: 389–393. 10.1016/0035-9203(81)90100-0 [DOI] [PubMed] [Google Scholar]
- 205.Fontes BM. Zika virus-related hypertensive iridocyclitis. Arq Bras Oftalmol. 2016;79: 63–2749.20160020. 10.5935/0004-2749.20160020 [DOI] [PubMed] [Google Scholar]
- 206.Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. 2016;387: 228 10.1016/S0140-6736(16)00006-4 [DOI] [PubMed] [Google Scholar]
- 207.Zammarchi L, Tappe D, Fortuna C, Remoli ME, Günther S, Venturi G, et al. Zika virus infection in a traveller returning to europe from Brazil, march 2015. Eurosurveillance. 2015;20. [DOI] [PubMed] [Google Scholar]
- 208.Gyurech D, Schilling J, Schmidt-Chanasit J, Cassinotti P, Kaeppeli F, Dobec M. False positive dengue NS1 antigen test in a traveller with an acute Zika virus infection imported into Switzerland. Swiss Med Wkly. 2016;146: w14296 10.4414/smw.2016.14296 [DOI] [PubMed] [Google Scholar]
- 209.Weitzel T, Cortes CP. Zika Virus Infection Presenting with Postauricular Lymphadenopathy. Am J Trop Med Hyg. 2016. 16–0096 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.World Health Organization. Zika virus infection–Region of the Americas Disease Outbreak News. 8 February 2016. WHO; 2016. [Google Scholar]
- 211.Chen LH. Zika Virus Infection in a Massachusetts Resident After Travel to Costa Rica: A Case Report. Ann Intern Med. 2016. 10.7326/L16-0075 [DOI] [PubMed] [Google Scholar]
- 212.Maria AT, Maquart M, Makinson A, Flusin O, Segondy M, Leparc-Goffart I, et al. Zika virus infections in three travellers returning from South America and the Caribbean respectively, to Montpellier, France, December 2015 to January 2016. Euro Surveill. 2016; 21 10.2807/1560-7917.ES.2016.21.6.30131 [DOI] [PubMed] [Google Scholar]
- 213.Goorhuis A. Zika Virus- Netherlands ex Suriname. ProMED-mail. 2015.
- 214.Karimi O, Goorhuis A, Schinkel J, Codrington J, Vreden SG, Vermaat JS, et al. Thrombocytopenia and subcutaneous bleedings in a patient with Zika virus infection. Lancet. 2016. S0140-6736(16)00502-X [pii]. [DOI] [PubMed] [Google Scholar]
- 215.World Health Organization. Zika virus infection–United States of America Disease Outbreak News. 12 February 2016. WHO; 2016. [Google Scholar]
- 216.Macesic N, Abbott IJ, Johnson DF. Fever and rash in a husband and wife returning from the Cook Islands. Clin Infect Dis. 2015;61: 1445 and 1485–1486. 10.1093/cid/civ408 [DOI] [PubMed] [Google Scholar]
- 217.Hearn Brooks. Identification of the first case of imported Zika Fever to the UK: A novel sample type for diagnostic purposes and support for a potential non-vectorborne route of transmission. American Journal of Tropical Medicine and Hygiene Conference. 2014;91: 62–63. [Google Scholar]
- 218.Zammarchi L, Stella G, Mantella A, Bartolozzi D, Tappe D, Günther S, et al. Zika virus infections imported to Italy: Clinical, immunological and virological findings, and public health implications. J Clin Virol. 2015;63: 32–35. 10.1016/j.jcv.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 219.Brust KB, Prince WS, Fader RC. Trouble in paradise. IDCases. 2014;1: 95–96. 10.1016/j.idcr.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Musso D, Roche C, Nhan T, Robin E, Teissier A, Cao-Lormeau V. Detection of Zika virus in saliva. J Clin Virol. 2015;68: 53–55. 10.1016/j.jcv.2015.04.021 [DOI] [PubMed] [Google Scholar]
- 221.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau V. Potential sexual transmission of zika virus. Emerg Infect Dis. 2015;21: 359–361. 10.3201/eid2102.141363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Besnard M, Lastère S, Teissier A, Cao-Lormeau VM, Musso D. Evidence of perinatal transmission of zika virus, French Polynesia, December 2013 and February 2014. Eurosurveillance. 2014;19. [PubMed] [Google Scholar]
- 223.Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastãre S, Valour F, et al. Zika virus infection complicated by guillain-barré syndrome a case report, French Polynesia, December 2013. Eurosurveillance. 2014;19. [DOI] [PubMed] [Google Scholar]
- 224.Summers DJ, Acosta RW, Acosta AM. Zika Virus in an American Recreational Traveler. J Travel Med. 2015;22: 338–340. 10.1111/jtm.12208 [DOI] [PubMed] [Google Scholar]
- 225.Tappe D, Nachtigall S, Kapaun A, Schnitzler P, Günther S, Schmidt-Chanasit J. Acute Zika virus infection after travel to Malaysian Borneo, September 2014. Emerg Infect Dis. 2015;21: 911–913. 10.3201/eid2105.141960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dupont-Rouzeyrol M, Biron A, O'Connor O, Huguon E, Descloux E. Infectious Zika viral particles in breastmilk. Lancet. 2016. S0140-6736(16)00624-3 [pii]. [DOI] [PubMed] [Google Scholar]
- 227.Venturi G, Zammarchi L, Fortuna C, Remoli ME, benedetti E, Florentini C, et al. An Autochthonous case of zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill. 2016;21. [DOI] [PubMed] [Google Scholar]
- 228.Center for Disease Control, ROC (Taiwan). As first imported case of Zika virus infection identified in Taiwan, Taiwan CDC to list Zika virus infection as Category II Notifiable Infectious Disease and raises travel notice level for Central and South America and six countries in Southeast Asia: 2016-01-19. Center for Disease Control, ROC (Taiwan);. 2016. [Google Scholar]
- 229.Shinohara K, Kutsuna S, Takasaki T, Moi ML, Ikeda M, Kotaki A, et al. Zika fever imported from Thailand to Japan, and diagnosed by PCR in the urines. J Travel Med. 2016;23: 10.1093/jtm/tav011 Print 2016 Jan. 10.1093/jtm/tav011. [DOI] [PubMed] [Google Scholar]
- 230.Tappe D, Rissland J, Gabriel M, Emmerich P, Günther S, Held G, et al. First case of laboratory-confirmed zika virus infection imported into Europe, November 2013. Eurosurveillance. 2014;19. [DOI] [PubMed] [Google Scholar]
- 231.Foy BD, Kobylinski KC, Foy JLC, Blitvich BJ, da Rosa AT, Haddow AD, et al. Probable Non-Vector-borne Transmission of Zika Virus, Colorado, USA. Emerg Infect Dis. 2011;17: 880–882. 10.3201/eid1705.101939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Simpson DI. Zika Virus Infection in Man. Trans R Soc Trop Med Hyg. 1964;58: 335–338. [PubMed] [Google Scholar]
- 233.Filipe AR, Martins CMV, Rocha H. Laboratory infection with Zika virus after vaccination against yellow fever. Arch Ges Virusforsch. 1973;43: 315–319. [DOI] [PubMed] [Google Scholar]
- 234.Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, et al. Possible Association Between Zika Virus Infection and Microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65: 59–62. 10.15585/mmwr.mm6503e2 [DOI] [PubMed] [Google Scholar]
- 235.World Health Organization. Microcephaly–Brazil Disease Outbreak News. 27 November 2015. WHO; 2015. [Google Scholar]
- 236.World Health Organization. Microcephaly–Brazil Disease Outbreak News. 15 December 2015. WHO; 2015. [Google Scholar]
- 237.Pan American Health Organization. Reported increase of congenital microcephaly and other central nervous system symptoms—10 February 2016. PAHO; 2016. [Google Scholar]
- 238.Pan American Health Organization. Zika Epidemiological Update—17 February 2016. PAHO; 2016. [Google Scholar]
- 239.World Health Organization. Microcephaly–United States of America Disease Outbreak News. 12 February 2016. WHO; 2016. [Google Scholar]
- 240.Meaney-Delman D, Hills Sl, Williams C, Galang RR, Iyengar P, Hennenfent AK, et al. Zika Virus Infection Among U.S. Pregnant Travelers: August 2015 –February 2016. MMWR Morb Mortal Wkly Rep. 2016;65. [DOI] [PubMed] [Google Scholar]
- 241.World Health Organization. Guillain-Barré syndrome–Brazil Disease Outbreak News. 8 February 2016. WHO; 2016. [Google Scholar]
- 242.Pan American Health Organization. Epidemiological Update Neurological syndrome, congenital anomalies, and Zika virus infection 17 January 2016. PAHO; 2016. [Google Scholar]
- 243.World Health Organization. Zika virus infection–El Salvador Disease Outbreak News. 27 November 2015. WHO; 2015. [Google Scholar]
- 244.Direction de la Santé - Bureau de Veille sanitaire. Surveillance de la dengue et du zika en Polynésie française. Données actualisées au 14 février 2014. Direction de la Santé - Bureau de Veille sanitaire. 2014.
- 245.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016. S0140-6736(16)00562-6 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Millon P. Epidemiologie des syndromes de Gullain-Barre en Nouvelle-Caledonie entre 2011 et 2014: influence des arboviroses. Medecine humaine et pathologie. 2015;1153577. [Google Scholar]
- 247.Victora CG, Schuler-Faccini L, Matijasevich A, Ribeiro E, Pessoa A, Barros FC. Microcephaly in Brazil: how to interpret reported numbers? 2016;387: 621–624. 10.1016/S0140-6736(16)00273-7 [DOI] [PubMed] [Google Scholar]
- 248.Araújo J, Regis CT, Gomes RG, Tavares TR, Santos CR, Assunção PM, et al. Microcephaly in northeastern Brazil: a review of 16 208 births between 2012 and 2015. Bull World Health Organ. E-pub: 4 February 2016. [Google Scholar]
- 249.Center for Disease Control and Prevention. Subject: Revised diagnostic testing for Zika, chikungunya, and dengue viruses in US Public Health Laboratories. CDC; 2016. [Google Scholar]
- 250.Cao-Lormeau, Roche, Teissier, Musso. Epidemiological and molecular features of dengue, Zika and Chikungunya concurrent outbreaks in the pacific, 2014. American Journal of Tropical Medicine and Hygiene Conference. 2014;91: 588. [Google Scholar]
- 251.World Health Organization. Guillain-Barré syndrome–France—Martinique Disease Outbreak News. 8 February 2016. WHO; 2016. [Google Scholar]
- 252.Balm MN, Lee CK, Lee HK, Chiu L, Koay ES, Tang JW. A diagnostic polymerase chain reaction assay for Zika virus. J Med Virol. 2012;84: 1501–1505. 10.1002/jmv.23241 [DOI] [PubMed] [Google Scholar]
- 253.Faye O, Faye O, Dupressoir A, Weidmann M, Ndiaye M, Alpha Sall A. One-step RT-PCR for detection of Zika virus. J Clin Virol. 2008;43: 96–101. 10.1016/j.jcv.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 254.De Madrid AT, Porterfield JS. The flaviviruses (group B arboviruses): a cross-neutralization study. J Gen Virol. 1974;23: 91–96. 10.1099/0022-1317-23-1-91 [DOI] [PubMed] [Google Scholar]
- 255.De Madrid AT, Porterfield JS. A simple micro-culture method for the study of group B arboviruses. Bull World Health Organ. 1969;40: 113–121. [PMC free article] [PubMed] [Google Scholar]
- 256.World Health Organization. Zika virus disease: Interim case definitions. 12 February 2016. WHO; 2016. [Google Scholar]
- 257.Center for Disease Control and Prevention. New CDC Laboratory Test for Zika Virus Authorized for Emergency Use by FDA. Media Release. Friday, March 18, 2016. Available: http://www.cdc.gov/media/releases/2016/s0318-zika-lab-test.html. Accessed March 22, 2016. [Google Scholar]
- 258.World Health Organization. WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. WHO; 2016, February 1. [Google Scholar]
- 259.Vouga M, Musso D, Van Mieghem T, Baud D. CDC guidelines for pregnant women during the Zika virus outbreak. Lancet. 2016. S0140-6736(16)00383-4 [pii]. [DOI] [PubMed] [Google Scholar]
- 260.Heymann DL, Hodgson A, Sall AA, Freedman DO, Staples JE, Althabe F, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet. 2016;387: 719–721. 10.1016/S0140-6736(16)00320-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.World Health Organization. Zika situation report (February 16, 2016): Zika virus, Microcephaly and Guillain-Barré syndrome. WHO; 2016. [Google Scholar]
- 262.Oster AM, Brooks JT, Stryker JE, Kachur RE, Mead P, Pesik NT, et al. Interim Guidelines for Prevention of Sexual Transmission of Zika Virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65: 120–121 2p. 10.15585/mmwr.mm6505e1 [DOI] [PubMed] [Google Scholar]
- 263.[Anonymous]. CDC investigating 14 new reports of possible sexual transmission of Zika virus. American Health Line. First Look. 2016: n/a. [Google Scholar]
- 264.World Health Organization. Prevention of potential sexual transmission of Zika virus. Interim guidance. 18 February 2016. WHO; 2016;WHO/ZIKV/MOC/16.1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. All relevant studies are listed in the reference list; references are publicly available.