Abstract
Background
A meta-analysis was conducted to assess the impact of radiation-induced ovarian ablation (RT-OA) on amenorrhea cessation rates, progression-free survival, and overall survival in pre/perimenopausal women with breast cancer.
Materials and methods
The Medline, CANCERLIT, and Cochrane Library databases and search engines were searched to identify randomized controlled studies comparing RT-OA with control for early or metastatic breast cancer. Further, radiotherapy doses, techniques, and associated side effects were evaluated.
Results
Six controlled trials with a total patient population of 3,317 were identified. Pooled results from these trials showed significant amenorrhea rates (P<0.00001) and increase in progression-free survival in patients treated with RT-OA (P<0.00001). However, there was no difference in overall survival (P=0.37). The majority of patients were treated with larger field sizes with parallel-opposed anteroposterior and posteroanterior pelvic fields. RT-OA was generally well tolerated. Radiotherapy doses of 1,500 cGy in five fractions, 1,500 cGy in four fractions, 1,600 cGy in four fractions, and 2,000 cGy in ten fractions were associated with excellent amenorrhea rates. The resultant funnel plot showed no publication bias (Egger test P=0.16).
Conclusion
RT-OA is cost-effective and can safely be used in pre/perimenopausal women with metastatic breast cancer, or if luteinizing hormone-releasing hormone analogs are contraindicated, or in patients in whom fertility preservation is not an issue. Radiation dose of 1,500 cGy in five fractions, 1,500 cGy in four fractions, 1,600 cGy in four fractions, and 2,000 cGy in ten fractions showed more efficacies. However, further studies incorporating three-dimensional conformal radiotherapy and intensity-modulated radiotherapy are warranted.
Keywords: radiation-induced ovarian ablation, pre/perimenopausal, breast cancer, meta-analysis
Introduction
In premenopausal patients with breast cancer, up to 90% of estrogen is produced by the ovaries; thus, ovarian ablation (OA) has been widely accepted for the treatment of breast cancer since 1896.1 However, luteinizing hormone-releasing hormone (LHRH) analogs, which act on the hypothalamic–pituitary–ovarian axis to suppress circulating estrogens to postmenopausal levels, have largely replaced the irreversible methods of OA, especially radiation-induced ovarian ablation (RT-OA), because of their ease of administration, tolerability, less morbidity, and a lower likelihood of permanent amenorrhea, with the potential for restoration of fertility.2 The relative efficacies of RT-OA and medical ovarian suppression have never been studied in large randomized clinical trials. However, data from two trials performed in patients with metastatic breast cancer comparing LHRH analogs to surgical or RT-OA suggest that LHRH analogs have similar efficacy to these other treatment modalities.3,4 Extrapolation of these results to women with early-stage breast cancer is problematic because the optimal duration of ovarian suppression in the adjuvant setting has not been established.5 The Early Breast Cancer Trialists’ Collaborative Group meta-analysis of 12 randomized controlled trials (RCTs) enrolling a total of 2,102 patients has reported ~25% relative reduction in the risks of recurrence and mortality at 15 years of follow-up in premenopausal women with early invasive breast cancer who underwent oophorectomy or RT-OA compared with those receiving no adjuvant therapy.6 RT-OA is still a reasonable alternate option for premenopausal patients with breast cancer who are not candidates for LHRH analogs and is also a cost-effective procedure of OA in many countries.7
We undertook the present meta-analysis with the aim of determining the efficacy, amenorrhea response rates, progression-free survival (PFS), overall survival (OS), and optimal dose of radiation therapy for OA in premenopausal women with breast cancer.
Materials and methods
The search criteria included the studies that were either complete articles of RCTs or retrospective if they were well controlled. The abstracts with full details were also included. The Medline, CANCERLIT, and Cochrane Library databases were searched using the terms “ovarian ablation”, “ovarian suppression”, “breast cancer”, “radiotherapy”, and “radiation”. These terms were then combined to search for randomized controlled reviews and meta-analyses. The relevant articles were retrieved by two methodologists, and only studies that met the following criteria were included:
Patients had histologically confirmed breast carcinoma.
Patients had received RT-OA, with or without hormonal therapy. Control groups were tamoxifen alone, chemotherapy plus tamoxifen, or chemotherapy alone.
The studies that included only surgical oophorectomy and medical ovarian suppression were excluded.
Outcome measures
The outcome measures were PFS, OS, cessation of menstrual cycles, efficacy of radiotherapy according to different doses, and late toxicity.
Quality assessment
Internal validity of included RCTs was assessed using the Cochrane Risk of Bias tool, which consists of six domains: 1) selection bias, 2) performance bias, 3) detection bias, 4) attrition bias (incomplete outcome data), 5) reporting bias, and 6) other sources of bias. Each separate domain was labeled according to a “low”, “unclear”, or “high” risk of bias.8 A trial was finally rated as “low risk of bias” (all six domains rated as low risk), “high risk of bias” (one or more domains rated as high risk), and “unclear risk of bias”.
Review analysis
All analyses were carried out on an intention-to-treat analysis basis. For the categorical variables, weighted risk ratios and their 95% CIs were calculated using the Review Manager (RevMan, version 5.0) software application, provided by the Cochrane Collaboration (part of the meta-analytic software program MetaView version 9.1.0: Update Software, Oxford, UK). The results were tested for heterogeneity at the significant level of P<0.05. If there was evidence of heterogeneity, a random-effects model was used for meta-analysis; otherwise, fixed-effects model was used. The odds ratio (OR) and 95% CI were calculated for each trial and presented in forest plot.
We determined the PFS, OS, and cessation of menses on the basis of the follow-up period mentioned in each trial. We also determined the efficacy of various fractionations of radiotherapy in terms of cessation menses.
Publication bias was evaluated using the funnel graph, the Begg–Mazumdar’s adjusted rank correlation test,9 and the Egger’s test.10 For heterogeneity, we carried out the Cochran’s Q-test to determine whether the studies are homogenous.
Results
Yield of search strategy and characteristics of eligible studies
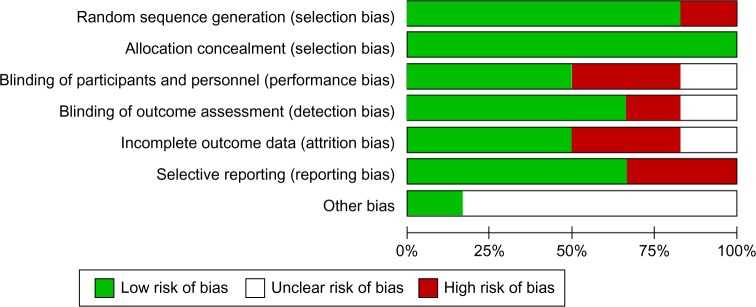
The electronic search revealed 712 relevant citations. After screening, 32 full-text articles were retrieved for further assessment. Finally, six studies were identified that met the criteria (Figure 1); the total population of these studies was 3,317 patients. Details are shown in Table 1.4,11–15 The studies were conducted in several countries. All studies were multicenter trials. All studies included peri- or premenopausal women with early, locally advanced, or metastatic breast cancer. All studies reported on cessation of menses, PFS, and OS outcomes of RT-OA. Two RCTs (33.3%) were rated to be “high risk” of bias, three trials (50%) were considered “low risk”, and one trial (16.7%) was classified as unclear risk of bias (Figure 2).
Figure 1.
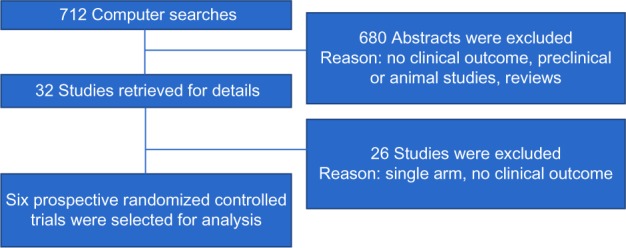
Schema diagram according to the quality of reporting of meta-analysis criteria.
Table 1.
Characteristics of studies of RT-OA in breast cancer
| Study | Period | Country | Inclusion criteria | Study design | Ovarian ablation type | Radiotherapy dose and fraction size | End points | Follow-up |
|---|---|---|---|---|---|---|---|---|
| Adjuvant Breast Cancer Trials Collaborative Group11 | 1993–2000 | UK, India, Sri Lanka, Egypt, Malta, Saudi Arabia, New Zealand, Pakistan, Singapore | Pre/peri menopausal women; early breast cancer; T1-3aN0-1M0 | Arm A: ovarian ablation/suppression (982 patients); Arm B: control (997 patients) | Radiation, surgical oophorectomy, LHRH analogs | 1,600 cGy in four fractions | Cessation of menses, PFS, OS | 10 years |
| Boccardo et al4 | 1988–1992 | Italy | Pre/peri menopausal women; metastatic breast cancer | Arm A: ovarian ablation (15 patients); Arm B: LHRH analogs (22 patients); Arm C: ovarian ablation + tamoxifen (18 patients); Arm D: LHRH analogs + tamoxifen (20 patients) | Radiation, surgical oophorectomy, LHRH analogs | 1,500 cGy in five fractions | Cessation of menses, PFS, OS | 4 years |
| Ejlertsen et al12 | 1990–1998 | Denmark | Pre/peri menopausal women; early breast cancer; T1-3aN0-1M0 | Arm A: ovarian ablation (317 patients); Arm B: chemotherapy CMF (445 patients) | Radiation | 1,500 cGy in five fractions | Cessation of menses, PFS, OS | 5 years |
| International Breast Cancer Study Group Trial 11-9313 | 1993–1998 | Switzerland | Pre/peri menopausal women; early breast cancer; T1-3aN0-1M0 | Arm A: ovarian ablation (37 patients); Arm B: chemotherapy AC (83 patients) | Radiation, surgical oophorectomy, LHRH analogs | 1,600 cGy in four fractions, 1,500 cGy in four fractions | Cessation of menses, PFS, OS | 8 years |
| North Central Cancer Treatment Group; Cancer and Leukemia Group B; South West Oncology Group, and Eastern Cooperative Oncology Group14 | 1994–1997 | USA | Pre/peri menopausal women; early breast cancer; T1-3aN0-1M0 | Arm A: ovarian ablation (19 patients); Arm B: tamoxifen (20 patients) | Radiation | 2,000 cGy in ten fractions | Cessation of menses, PFS, OS | 3 years |
| Scottish Cancer Trial Breast Group15 | 1993–1998 | UK | Pre/peri menopausal women; early breast cancer; T1-3aN0-1M0 | Arm A: ovarian ablation (180 patients); Arm B: tamoxifen (172 patients) | Radiation, surgical oophorectomy | 4.5–5 Gy single fraction, 1,500 cGy in five fractions | Cessation of menses, PFS, OS | 10 years |
Abbreviations: RT-OA, radiation-induced ovarian ablation; LHRH, luteinizing hormone-releasing hormone; PFS, progression-free survival; OS, overall survival; CMF, cyclophosphamide, methotrexate, 5 Flour-uracil; AC, adriamycin, cyclophosphamide.
Figure 2.
Summary of risk bias assessment.
Cessation of menstrual cycles
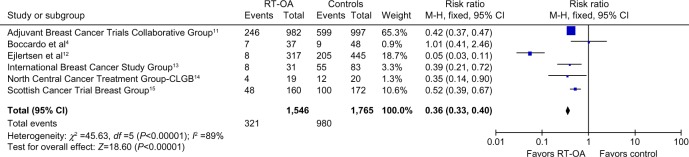
All six studies with a population of 1,546 patients analyzed the cessation of menstrual cycles as one of the outcomes. The cessation of menstrual cycles was significantly high in patients with RT-OA (P<0.00001). The pooled OR was 0.36 (95% CI: 0.33, 0.40). The result of the test for heterogeneity was not statistically significant. The overall benefit from OA and control to suppress the menstrual cycles is shown in Figure 3.
Figure 3.
Forest plot showing amenorrhea rates for all subgroups.
Abbreviations: CLGB, Cancer and Leukemia Group B; df, degrees of freedom; RT-OA, radiation-induced ovarian ablation; M-H, Mantel-Haenszel.
Progression-free survival
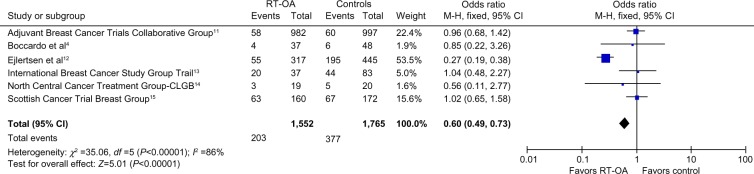
All six studies with 1,552 patients examined the PFS as one of the outcomes. Among the six studies, only one study12 showed decrease in all-site recurrences (OR: 0.27, 95% CI: 0.19, 0.38), thus improving PFS in RT-OA arm (P<0.00001). Overall ORs showed a significant difference between RT-OA and control arms (Figure 4).
Figure 4.
Forest plot showing progression-free survival rates for all subgroups.
Abbreviations: CLGB, Cancer and Leukemia Group B; df, degrees of freedom; RT-OA, radiation-induced ovarian ablation; M-H, Mantel-Haenszel.
Overall survival
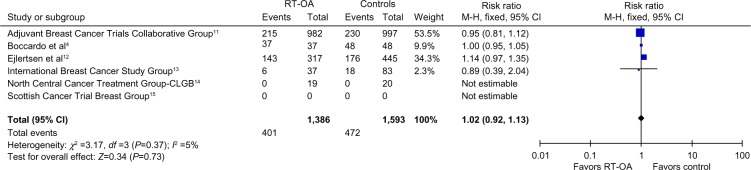
All the mentioned studies, with 1,386 patients, addressed the OS as one of the outcomes. One study showed minimal improvement in OS, four studies showed no difference, and one study showed better survival in control arm. The pooled ORs were not statistically different between RT-OA and control arms (P=0.37), without any heterogeneity (Figure 5).
Figure 5.
Forest plot showing progression-free survival rates for all subgroups.
Abbreviations: CLGB, Cancer and Leukemia Group B; df, degrees of freedom; RT-OA, radiation-induced ovarian ablation; M-H, Mantel-Haenszel.
Radiotherapy techniques, dose, efficacy, and toxicity
In all studies, patients were treated with parallel-opposed pelvic fields, that is, anteroposterior/posteroanterior, and the dose was prescribed to midplane. Mean field sizes used were 10×15 cm2 to include both ovaries. In four studies, the upper border was kept at the inferior sacroiliac joint and lower border was kept at mid-obturator foramen. Lateral borders were 1 cm laterally to the pelvic sidewall. However, in two studies, the upper border was kept at sacral promontory and lower border was kept at the bottom of obturator foramen (Figure 6). All studies used conventional radiotherapy, without computed tomography (CT) data. The majority of patients were treated on cobalt 60 and non-multileaf collimators linear accelerators. No uniform pattern of radiotherapy dose and fraction size was used in analyzed studies. However, radiotherapy doses of 1,500 cGy in five fractions followed by 1,500 cGy in four fractions, 1,600 cGy in four fractions, 2,000 cGy in ten fractions, and 36 Gy in 18 fractions were found more related with complete cessation of menstrual cycles. RT-OA was generally well tolerated, with minimal and manageable side effects. No grade 3 or 4 toxicities were seen in any prophylaxis or treatment group. No treatment-related deaths or second malignancy in irradiated site were reported.
Figure 6.
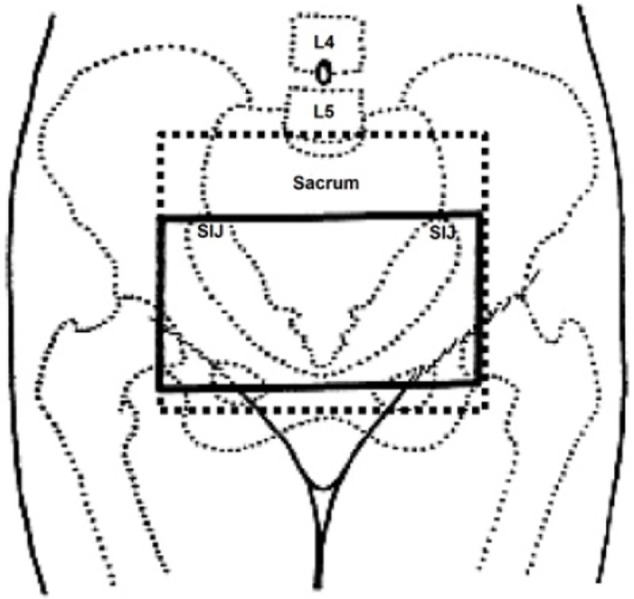
Treatment fields for ovarian ablation used in included studies.
Abbreviations: L4, lumbar 4; L5, lumbar 5; SIJ, sacroiliac joint.
Publication bias
The funnel plot revealed the narrow funnel (Figure 7), showing no significant publication bias (P-values from Begg–Mazumdar’s test and Egger’s test were 0.25 and 0.16, respectively). The narrow shape may be attributed to the large number of pooled patients.
Figure 7.
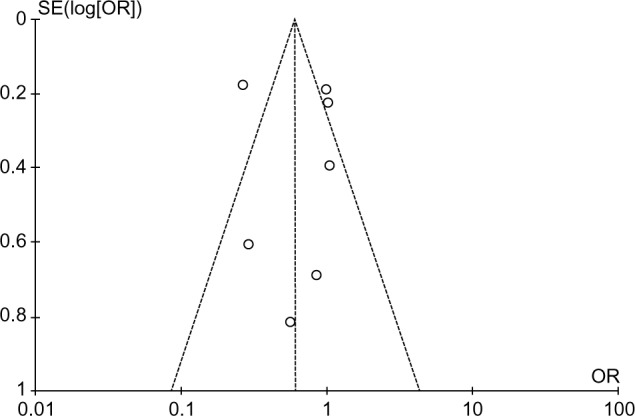
Funnel plot to evaluate publication bias.
Abbreviations: SE, standard error; OR, odds ratio.
Discussion
Over the past 2 decades, with the advent of the LHRH analogs, the use of RT-OA in premenopausal women with breast cancer has been declined. Recent data from currently published clinical trials of LHRH analogs in the adjuvant setting for premenopausal women with ER+ breast cancer are supportive of clinical benefit.16 Similarly, Zoladex in Premenopausal Patients trial has shown that the medical OA with goserelin for 2 years in premenopausal patients with ER-positive (ER+) disease produced a statistically significant benefit in terms of disease-free survival, PFS, and a trend toward improvement in OS, irrespective of concurrent adjuvant tamoxifen or chemotherapy.17,18 However, RT-OA is still being used in many countries for its low cost and easy administration and can be offered in premenopausal women with relative contraindications to hormonal therapy, especially tamoxifen and LHRH analogs. Evidence supporting an increase in the risk of venous thrombo-embolism (VTE) with tamoxifen treatment comes from the “National Surgical Adjuvant Breast and Bowel Project-1 trial findings, which showed that 35 women on tamoxifen developed VTE compared with just 22 women in the placebo group. Among all women aged 49 years or younger in the trial, there were eight cases recorded in the placebo group versus eleven in the tamoxifen group with a relative risk of 1.39 (95% CI: 0.51, 3.99).19 Further, a recent meta-analysis of randomized breast carcinoma prevention trials that compared tamoxifen with placebo concluded that the risk of VTE was increased nearly twofold in tamoxifen users (relative risk: 1.9; 95% CI: 1.4, 2.6; P<0.0001).20
The results of our meta-analysis of RT-OA studies suggest that RT-OA is associated with better amenorrhea rates and minimal improvement in PFS rates in pre/perimenopausal women with early and metastatic breast cancer. RT-OA or ovarian suppression-related improved PFS is debatable, as five trials showed no improvement in PFS in present meta-analysis; however, recent review by The Cochrane Breast Cancer Group has shown a trend toward improved PFS and OS in premenopausal patients with breast cancer who received an LHRH analog plus tamoxifen with chemotherapy combination in comparison to chemotherapy alone.17
Further pooled adjusted estimates from the prospective studies showed that RT-OA with radiotherapy doses of 1,500 cGy in five fractions, 1,500 cGy in four fractions, 1,600 cGy in four fractions, and 2,000 cGy in ten fractions were the most beneficial among all available dose-fractionation protocols. The results of this meta-analysis support the use of RT-OA as an appropriate option for the pre/perimenopausal patients who are not candidates for LHRH analog-induced ovarian suppression. The advantages of RT-OA are the short treatment time (1 week or 2 weeks), low costs, and manageable adverse events. However, potential risk of second malignancy is of great concern, especially in pre/perimenopausal patients with early breast cancer. The included studies did not address this issue after RT-OA, but the explanation could be the short follow-up in the included studies.
However, no formal method to localize the ovarian position was sorted out in the included studies. In all studies, the patients were treated with conventional radiotherapy methods, that is, the larger parallel-opposed pelvic fields (anteroposterior/posteroanterior), without any pretreatment localization of ovaries. Featherstone et al21 used ultrasound localization of the ovaries prior to RT-OA in 70 patients. They found that the craniocaudal ovarian position varied from 2.5 cm above the lower aspect of the sacroiliac joint to 2.0 cm above the symphysis pubis, and the field size used was larger to include both ovaries. Counsell et al22 assessed the variation in ovarian position on CT pelvis images in 81 pre/perimenopausal women. They found that, in 83% of cases, the ovaries were located within the upper two-thirds of a ring defined by the sacroiliac joints, the bony side wall of the pelvis. Due to ovarian movements probably in relation to the degree of bladder filling or rectal distension, they recommended the treatment volumes for conventional RT-OA to extend from the inferior border of the fifth lumbar vertebra down to a level traversing the middle of the femoral heads and 1 cm lateral to the pelvic side walls as described in Figure 6.
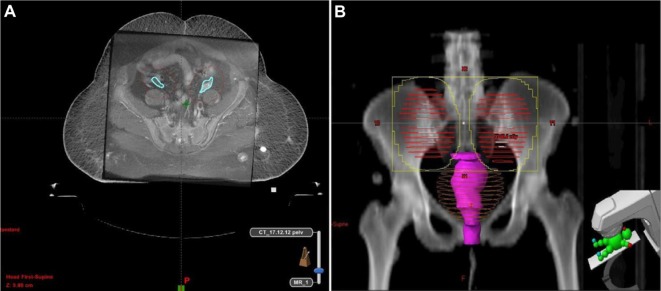
However, with the advent of novel radiotherapy techniques, the position of the ovaries can be delineated on diagnostic CT or magnetic resonance imaging fused with CT simulation data.23 Peters et al24 assessed the extent of ovarian movement on magnetic resonance imaging images in patients with gynecological malignancies. They have reported that left ovary movement was 17.0±9.67 mm and right ovary movement was 19.7±11.95 mm (P=0.31). Based on this information, planning target volume of 1.5–2.0 cm should be added around the ovaries during three-dimensional conformal radiotherapy or intensity-modulated radiotherapy-based OA to ensure that the ovaries are within the high-radiation-dose area (Figure 8A and B).
Figure 8.
(A) MR imaging fusion with CT simulation data to delineate ovaries and planning target volume and (B) Beam’s eye view.
Abbreviations: CT, computed tomography; MR, magnetic resonance.
There were two main limitations of our meta-analysis: 1) no comparison was performed between RT-OA and medical ovarian suppression to compare efficacy and 2) potential selection bias as some patients in the included studies were treated using surgical oophorectomy, although the number was very small.
Conclusion
Even with similar efficacy, the use of RT-OA in pre/perimenopausal women with early or metastatic breast cancer is decreasing in comparison to LHRH analogs; however, RT-OA is cost-effective and can safely be used in patients with metastatic breast cancer, or if LHRH analogs are contraindicated, or in patients in whom fertility preservation is not an issue. Radiation doses of 1,500 cGy in five fractions, 1,500 cGy in four fractions, 1,600 cGy in four fractions, and 2,000 cGy in ten fractions are associated with more amenor-rhea rates. However, adverse effects can be minimized by RT-OA using three-dimensional conformal radiotherapy and intensity-modulated radiotherapy, for which further studies are warranted.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Prowell TM, Davidson NE. What is the role of ovarian ablation in the management of primary and metastatic breast cancer today? Oncologist. 2004;9(5):507–517. doi: 10.1634/theoncologist.9-5-507. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Wu SG, Wang JJ, et al. Ovarian ablation using goserelin improves survival of premenopausal patients with stage II/III hormone receptor-positive breast cancer without chemotherapy-induced amenorrhea. Cancer Res Treat. 2015;47(1):55–63. doi: 10.4143/crt.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor CW, Green S, Dalton WS, et al. Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: an intergroup study. J Clin Oncol. 1998;16(3):994–999. doi: 10.1200/JCO.1998.16.3.994. [DOI] [PubMed] [Google Scholar]
- 4.Boccardo F, Rubagotti A, Perrotta A, et al. Ovarian ablation versus goserelin with or without tamoxifen in pre-perimenopausal patients with advanced breast cancer: results of a multicentric Italian study. Ann Oncol. 1994;5(4):337–342. doi: 10.1093/oxfordjournals.annonc.a058837. [DOI] [PubMed] [Google Scholar]
- 5.Jonat W, Kaufmann M, Sauerbrei W, et al. Zoladex Early Breast Cancer Research Association Study Goserelin versus cyclophosphamide, metho trexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: the Zoladex Early Breast Cancer Research Association Study. J Clin Oncol. 2002;20(24):4628–4635. doi: 10.1200/JCO.2002.05.042. [DOI] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group Ovarian ablation in early breast cancer: overview of the randomised trials. Lancet. 1996;348(9036):1189–1196. [PubMed] [Google Scholar]
- 7.Bese NS, Iribas A, Dirican A, Oksuz D, Atkovar G, Ober A. Ovarian ablation by radiation therapy: is it still an option for the ablation of ovarian function in endocrine responsive premenopausal breast cancer patients? Breast. 2009;18(5):304–308. doi: 10.1016/j.breast.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Altman DG, Gotzsche PC, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begg CB, Mazumdar M. Operating characteristics of rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 10.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adjuvant Breast Cancer Trials Collaborative Group Ovarian ablation or suppression in premenopausal early breast cancer: results from the international adjuvant breast cancer ovarian ablation or suppression randomized trial. J Natl Cancer Inst. 2007;99(7):516–525. doi: 10.1093/jnci/djk109. [DOI] [PubMed] [Google Scholar]
- 12.Ejlertsen B, Mouridsen HT, Jensen MB, et al. Similar efficacy for ovarian ablation compared with cyclophosphamide, methotrexate, and fluorouracil: from a randomized comparison of premenopausal patients with node-positive, hormone receptor-positive breast cancer. J Clin Oncol. 2006;24(31):4956–4962. doi: 10.1200/JCO.2005.05.1235. [DOI] [PubMed] [Google Scholar]
- 13.Thurlimann B, Price KN, Gelber RD, et al. Is chemotherapy necessary for premenopausal women with lower-risk node-positive, endocrine responsive breast cancer? 10-year update of International Breast Cancer Study Group Trial 11-93. Breast Cancer Res Treat. 2009;113(1):137–144. doi: 10.1007/s10549-008-9912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes LL, Gray RJ, Solin LJ, et al. Eastern Cooperative Oncology Group. Southwest Oncology Group. Cancer and Leukemia Group B. North Central Cancer Treatment Group Efficacy of radiotherapy for ovarian ablation: results of a breast intergroup study. Cancer. 2004;101(5):969–972. doi: 10.1002/cncr.20481. [DOI] [PubMed] [Google Scholar]
- 15.Thomson CS, Twelves CJ, Mallon EA, Leake RE, Scottish Cancer Trials Breast Group. Scottish Cancer Therapy Network Adjuvant ovarian ablation vs. CMF chemotherapy in premenopausal breast cancer patients: trial update and impact of immunohistochemical assessment of ER status. Breast. 2002;11(5):419–429. doi: 10.1054/brst.2002.0451. [DOI] [PubMed] [Google Scholar]
- 16.Leung SF, Tsao SY, Teo PM, Choi PH, Shiu WC. Ovarian ablation failures by radiation: a comparison of two dose schedules. Br J Radiol. 1991;64(762):537–538. doi: 10.1259/0007-1285-64-762-537. [DOI] [PubMed] [Google Scholar]
- 17.Goel S, Sharma R, Hamilton A, Beith J. LHRH agonists for adjuvant therapy of early breast cancer in premenopausal women. Cochrane Database Syst Rev. 2009;4:CD004562. doi: 10.1002/14651858.CD004562.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum M, Hackshaw A, Houghton J, et al. ZIPP International Collaborators Group Adjuvant goserelin in premenopausal patients with early breast cancer: results from the ZIPP study. Eur J Cancer. 2006;42(7):895–904. doi: 10.1016/j.ejca.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 20.Cuzick JH, Powles T, Veronesi U, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361(9354):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 21.Featherstone C, Harnett AN, Brunt AM. Ultrasound localization of the ovaries for radiation-induced ovarian ablation. Clin Oncol (R Coll Radiol) 1999;11(6):393–397. doi: 10.1053/clon.1999.9090. [DOI] [PubMed] [Google Scholar]
- 22.Counsell R, Bain G, Williams MV, Dixon AK. Artificial radiation menopause: where are the ovaries? Clin Oncol (R Coll Radiol) 1996;8(4):250–253. doi: 10.1016/s0936-6555(05)80664-8. [DOI] [PubMed] [Google Scholar]
- 23.Schlemmer HP. Imaging for new radiotherapy techniques. Cancer Imaging. 2010;10:S73. [Google Scholar]
- 24.Peters NH, Patterson AJ, Horan G, Gregory D, Sala E. Assessment of ovarian movement on consecutive pelvic MRI examinations in patients with gynaecological malignancies: what is the planning organ-at-risk volume for radiotherapy? Breast Cancer Res Treat. 2009;113(1018):137–144. doi: 10.1259/bjr/22205448. [DOI] [PMC free article] [PubMed] [Google Scholar]