Abstract
Brain tauopathies are characterized by abnormal processing of tau protein. While somatodendritic tau mislocalization has attracted considerable attention in tauopathies, the role of tau pathology in axonal transport, connectivity and related dysfunctions remains obscure. We have previously shown using the squid giant synapse that presynaptic microinjection of recombinant human tau protein (htau42) results in failure of synaptic transmission. Here, we evaluated molecular mechanisms mediating this effect. Thus, the initial event, observed after htau42 presynaptic injection, was an increase in transmitter release. This event was mediated by calcium release from intracellular stores and was followed by a reduction in evoked transmitter release. The effect of htau42 on synaptic transmission was recapitulated by a peptide comprising the phosphatase-activating domain of tau, suggesting activation of phosphotransferases. Accordingly, findings indicated that htau42-mediated toxicity involves the activities of both GSK3 and Cdk5 kinases.
1. Introduction
Present knowledge indicates that all brain tauopathies involve the generation of aberrantly phosphorylated, truncated, and misfolded tau neurotoxic species (Rao et al., 2014, Kovacs, 2015). Synaptic dysfunction and abnormalities in axonal transport are early pathogenic events in tauopathies that precede the formation of neurofibrillary tangles (NFTs) and neuronal cell death (Majid et al., 2014, Polydoro et al., 2014, Jadhav et al., 2015). Normally, a substantial amount of cellular tau is sorted into axons (Rao et al., 2014, Jadhav et al., 2015), and there is compelling evidence to suggest that the missorting of tau into the somatodendritic compartment plays a pathological role in tauopathies (Zempel and Mandelkow, 2014). Nevertheless, pathological axonal tau localizations are also prominent (Rao et al., 2014, Tai et al., 2014, Jadhav et al., 2015). Furthermore, it has been recently proposed that pathological-tau spreading may occur trans-synaptically from pre- to the post-synaptic sites (de Calignon et al., 2012). In addition, misfolded tau species may be internalized at the axon terminals and be transported retrogradely (Wu et al., 2013). It is therefore evident that the presynaptic issues represent a prominent parameter in the tauopathies. Presently, the mechanisms linking axonal tau pathology to synaptic dysfunction remain elusive; in part because of the synaptic size limitations that are characteristic of mammalian forms preventing direct access to the synaptic machinery.
To address the possibility that tau accumulation and/or mislocalization at the presynapse triggers synaptic dysfunction we evaluated acute effects of “human wild type” tau protein using the squid synapse preparation. Our previous results demonstrated that recombinant human tau isoform (full length h-tau42) induces a short-lasting increase in spontaneous transmitter release, followed by a rapid decrease and failure of synaptic transmission (Moreno et al., 2011). Microinjected htau42 became phosphorylated at the pathological AT8 antibody epitope. Intriguingly, endogenous tau levels are within 1-2μM ranges in vivo and perfusion of 25μM of wild type htau42 in squid axoplasm did not affect axonal transport (Morfini et al., 2007). These observations suggest that the loss of synaptic function which is characteristic of Alzheimer's disease and other tauopathies involve an abnormal presynaptic distribution of tau, rather than an overall increase in cellular tau levels (Yuan et al., 2008).
In the present study, we found evidence indicating that microinjection of htau42 in synaptic terminals abnormally increases levels of cytosolic calcium, presumably from intracellular stores. Additional experiments indicate that the phosphatase-activating domain (PAD (Kanaan et al., 2011)) comprising aminoacids 2-18 of htau42 is necessary and sufficient to produce disruption of synaptic transmission. Pharmacological experiments indicate that the toxic effect of htau42 on synaptic function involves the activities of cyclin-dependent protein kinase 5 (Cdk5) and glycogen synthase kinase 3 (GSK3) (LaPointe et al., 2009). Taken together, these results identify multiple pathogenic events associated with tau-mediated synapto-toxicity at the molecular level, therefore providing novel therapeutic targets to address synaptic dysfunction in tauopathies.
2. Material and Methods
2.1. Recombinant tau proteins
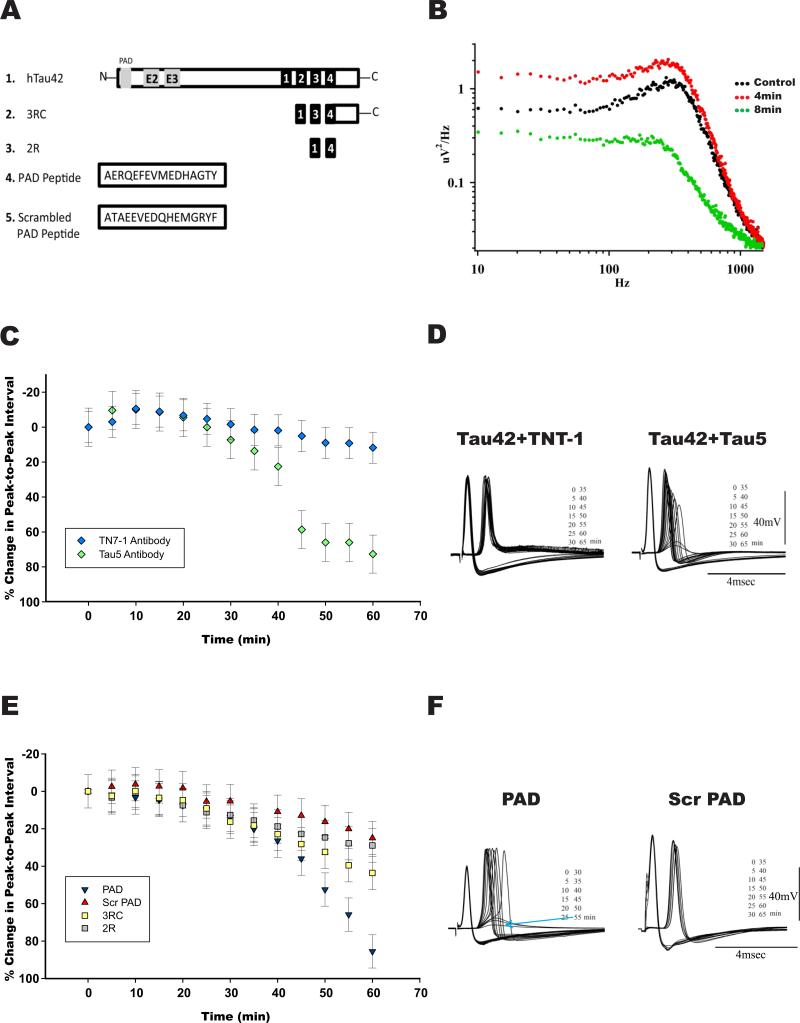
Wild type human tau htau42 (isoform with four tubulin binding motifs and two extra exons in the N-terminal domain which contains 441 a.a.), its variant htau 3RC (a protein which contains three tubulin binding motifs and the carboxyl terminal region) and the 2R fragment which has 62 amino acids were isolated as previously described (Perez et al., 2001) (see figure 2). PAD peptide and Scrambled PAD peptide from (GenScript). Figure 2A shows a schematic representation of the different tau constructs.
Figure 2. The PAD domain of htau42 is necessary and sufficient to block synaptic transmission.
A) Schematic diagram of the tau constructs used 1) Full length wild type human tau42 (htau42), the largest isoform of tau found in the mature brain, contains the PAD region (in gray), exons 2 and 3 (E2 and E3) and four tubulin binding motifs (black boxes) 2) 3RC, a protein construct which contains three tubulin binding motifs (black boxes) and the carboxyl terminal region [C], 3) 2R fragment which has 62 amino acids with two tubulin binding motifs (black boxes) 4) PAD peptide, 5) Scrambled PAD peptide. B) Power spectra of spontaneous post-synaptic noise. Noise recording at the post-synaptic terminal were taken at 1-min intervals, before PAD injection [Control, black dots] following 4 min [red dots] and 8 min after PAD injection [green dots] as indicated). Spontaneous release is determined by synaptic noise power spectrum. Note the rapid increase in noise 4 min after microinjection, indicating higher spontaneous release followed by drastic reduction within a 4 min interval (reading taken at a 1/min rate). C) Time course of synaptic transmission changes following presynaptic microinjection of: i) htau 42 plus anti-PAD antibody TNT-1, which blocks the toxic effect of htau42 (blue rhombi), ii) htau 42 plus Tau5 antibody, which does not interfere with htau42 effect (green rhombi). D) Representative example of evoked responses to direct presynaptic stimulation in synapses coinjected with htau42 and TNT-1 left, or with Tau5 antibodies right. E) Time course of synaptic transmission changes following presynaptic microinjection of: i) PAD peptide (blue triangles)—note a similar effect to that of full length tau [htau42], ii) scrambled PAD peptide producing no significant changes (red triangles) and iii) Fragments 3RC (yellow squares) or 2R (gray squares) also producing no significant synaptic block at the 60 min time point. F) Representative recordings of a synapse microinjected with PAD peptide, showing complete block at 55 min (indicated by blue arrow) and an example of a synapse injected with scrambled PAD peptide, showing no significant change in the pre or postsynaptic spikes (right).
Note: In figures C and E: Y axis identifies the percentage of change (interspike time between pre and postsynaptic) from the baseline (0%).
2.2. Other Reagents
SB216763 (Tocris), 2-Aminoethoxydiphenylborane (2-APB), Xestospongin C, and dantrolene (Sigma-Aldrich), ING-135 was a generous gift from Dr Kozikowski, TFP5 was described before in (Shukla et al., 2013), TNT-1 and tau5 antibodies were also previously described (LaPointe et al., 2009). The drug concentration for each of the reagents were chosen base on the IC50/EC50, and the effective doses reported in the literature in different preparations, as specified in Table 1. Dose related target specificity for the different reagents is also presented in table 1.
Table 1.
| Target(s) of Interest | EC50 | IC50 | Other Targets | IC50 | |
|---|---|---|---|---|---|
| 2-APB | IP3Rs | 10*μM[1] 42#μM[2] |
SERCA SOC Channels TRP Channels |
91μM[3] 15-20*μM[1] 10-15+μM[1] |
|
| Xestospongin C | IP3Rs | 358nM[4] | SERCA RyRs |
67&μM[5] >20μM[4] |
|
| Dantrolene | RyRs | 30.2μM[6] | Bcl-2[7] Phospholipase A2[7] IP3Rs ? [8] |
||
| ING-135 | GSK-3 | 7nM[9] | Other 22 kinases panel |
>10μM[9] | |
| SB216763 | GSK-3 | 34.3nM[10] | AMPK GSK-3β MAPK PKA PKBα PKCα SGK And others |
>10μM[10] | |
| TFP-5 | Cdk5 | Blocks 90% of Cdk5-p35 activity at 0.05 μM[11] | |||
| TNT-1 | Tau Phosphatase-activating domain (PAD) | 5.5ng/ml[12] |
= DT40 cells
= Microsomal fraction of rat cerebellum
=HEK293 cells
=A7r5 smooth-muscle cells
2.3. Electrophysiology and Microinjections
The squid stellate ganglia isolation from the mantle and the electrophysiological techniques used have been described previously (Llinas et al., 1985). In short two glass micropipette electrodes impaled the largest (most distal) presynaptic terminal digit at the synaptic junction site while the postsynaptic axon was impaled by one microelectrode at the junctional site. One of the pre-electrodes was used for pressure microinjection of the different protein/peptides or other compounds (as described in each experiment) and also supported voltage clamp current feedback, while the second monitored membrane potential. The exact location of injection, the diffusion and steady-state distribution of the different treatments/fluorescent dye mix (0.001% dextran fluorescein) were monitored using a fluorescence microscope attached to a Hamamatsu camera system (ARGUS 100, Middlesex, NJ). Microinjections were normalized by determining that the amount of fluorescence that reached the presynaptic terminals was comparable. Recordings were done when fluorescence values (measured as arbitrary units) were 20- 25X over background (using the same power gain). In all experiments a good correlation was observed between the localization of the fluorescence and the electrophysiological findings.
2.5. Statistical Methods
Linear mixed models were used to evaluate the outcome ‘Peak to Peak Interval’ over 7 times [between 0-60 min, for all treatments]. The p values presented were adjusted for multiple testing [by FDR method] at the 60 min. The analysis was performed in log-scale, due to the skeweness of the outcome data. We used fixed effects for treatment, time, time square and their interactions with treatment. Both interactions [time and time square by treatment were significant- p<0.0001, for both]. We used random effects for intercept and slope for time, for each experiment. Fixed effects were used to estimate the treatments effects over time. Random effects were used to account for the repeated measures for each animal over time, and natural variability between animals. In this model, absence of postsynaptic spike, due to amplitude reduction of postsynaptic potential to sub-threshold level was also included. When spikes were generated, their amplitude was evaluated using the same linear mixed model. No significant amplitude differences were observed between any of the groups, therefore this data is not reported throughout the manuscript.
3. Results
The initial set of experiments (Fig. 1A-D) addressed mechanisms triggered by injection of wild type recombinant htau42 (80nM) in the synaptic compartment. Evoked pre- and post-synaptic action potentials were recorded in current-clamp mode following our standard protocol (Llinas et al., 1985). The synapse was activated by direct depolarization of the presynaptic terminal every 5 min (low-frequency protocol). Microinjected htau42 was monitored by fluorescence microscopy using a fluorescent dye/protein mix and correlated with its effect on synaptic release (see materials and methods). Once the fluorescent material injected into the axon reached the presynaptic terminal htau42 produced a recordable inhibition of synaptic transmission within 45+/−15 min post-injection (Fig. 1A&B), as reported before (Moreno et al., 2011).
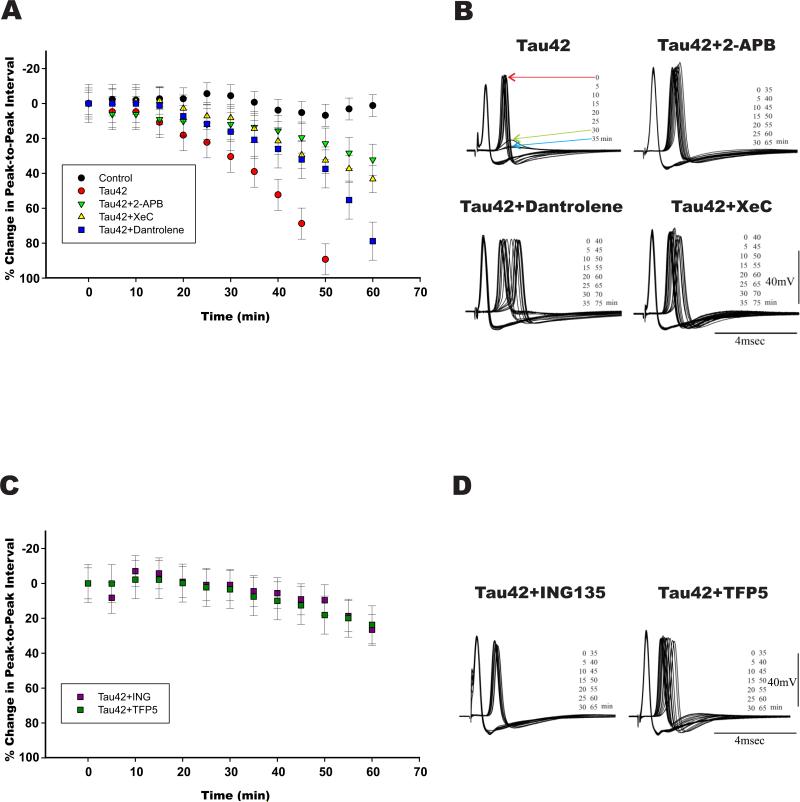
Figure 1. Exogenously injected htau42 requires IP3 receptors, GSK3 and Cdk5 activities to induce blockade of synaptic transmission.
A) Synaptic transmission time courses: i) vehicle injected axons (black dots). Note that interspike time is maintained relatively constant during the recording period, ii) htau42 injected synapses (red dots), iii) synapses coinjected with: htau42 and 2-APB (green triangles), htau42 and Xestospongin C (yellow triangles) and htau42 and dantrolene (blue squares). Note that synaptic transmission block observed with htau42 was prevented by the IP3Rs inhibitors and delayed by dantrolene. B) Representative pre- and post-synaptic potentials following direct presynaptic axon stimulation every five min, first evoked spike identified by red arrow. Synaptic transmission starts to fail at 30 min, in this example (green arrow), and is completely blocked at 35 min (blue arrow) following h-tau42 preinjection (Tau42; left upper panel). Coinjection of recombinant htau42 protein with the IP3Rs blockers (Tau42+2-APB or Tau42+XeC) prevented htau42 synapto-toxicity for the time of the experiment (65 or 75 min respectively). Dantrolene had a variable response on htau42 toxicity and in this case delayed synaptic block. C) Synaptic transmission time courses for synapses coinjected with htau42 and the GSK3 inhibitor ING-135 (ING, purple squares dots), or with the Cdk5/p35 modulator TFP5 peptide (TFP5, green squares). D) Representative pre- and post-synaptic potentials following direct presynaptic axon stimulation every five min, shown are synapses coinjected with htau42 and ING135, left panel and htau42 plus TFP5, right panel. Note that synaptic transmission block induced by htau42 (A) was prevented by ING-135 and TFP5.
Note: Y axis identifies the percentage of change (interspike time between pre and postsynaptic) from the baseline (0%).
3.1. IP3 receptors are involved in hTau mediated synapto-toxicity
We have previously shown that shortly after (5 +/−1.5 min) microinjected recombinant htau42 protein reached the presynaptic terminal a transient increment in post-synaptic noise was observed, indicative of an increment in spontaneous neurotransmitter release (Lin et al., 1990). This effect was followed by a rapid noise level reduction, characteristic of the dysfunction that follows htau42 injection (Moreno et al., 2011). A plausible mechanism underlying the transient post-synaptic noise increase is that htau42 modulates presynaptic calcium stores, which are thought to be involved in transmitter release in other preparations (Collin et al., 2005). Based on these prior results, in addition to our own, the transient htau42 dependent increase of spontaneous release was assigned to an increase in cytoplasmic calcium concentration, presumably via endoplasmic reticulum (ER) IP3 or Ryanodine receptor (IP3Rs, RYRs) activation. This hypothesis was tested by presynaptic coinjection of either htau42 with IP3R or RyRS inhibitors, a) Xestospongin C (XeC, 20 μM; n=4), b) 2-Aminoethoxydiphenylborane (2-APB; 100 μM; n=5) or c) with the RyR blocker dantrolene (300 μM; n=4) (Maruyama et al., 1997, Collin et al., 2005).
For the statistical analysis of these data and related findings described below, the pre to postsynaptic spike interval was determined at baseline (time=0) and then again after microinjection in 5 min intervals. Following this procedure, interspike time was used as an indicator of changes in synaptic delay (see i.e. Fig. 1A). Linear mixed models were used to evaluate the outcome of interspike delay and in selected cases spike amplitude (See methods for details).
Comparison of the interspike time of synapses injected with 1) htau42, 2) htau42+APB and 3) htau42+XeC demonstrated significant interspike time differences for both APB and XeC groups (p= 0.0007 and p=0.0055 respectively). Significance was also observed when synapses injected with htau42 alone or htau42+Dantrolene were compared p=0.047 (unadjusted p=0.02; Fig. 1A and B). Additionally 50-70 min after htau42 was microinjected a complete absence of post-synaptic response was observed in 100% of the injected synapses, while only one complete block at this time point was observed in htau42+dantrolene injected synapses and none in htau42+APB, htau42+XeC or controls (not shown).
These findings indicate that both IP3Rs blockers (APB and XeC) prevented htau42 mediated synaptic block (Fig. 1 A and B). The effect of RyR blocker dantrolene was also significant but had a slower onset (Fig. 1A and B). Microinjection of either compound alone did not produce changes in synaptic transmission at the pre- or postsynaptic sites, whereas IP3Rs block abolished the initial phase of htau42 mediated increased post-synaptic noise (not shown). These data indicate that the transient increase in intracellular calcium associated with htau42 injection likely results from activation of the ER-calcium IP3Rs, but both RyR and IP3Rs are involved in the toxic effect mediated by htau42.
3.2. GSK3B and Cdk5 activities are required for htau42 toxicity
The actual presence of a squid tau epitope phosphorylated by GSK3 and Cdk5/p35 kinases was demonstrated by recognition via AT8 antibodies in squid axoplasm (Morfini et al., 2002, Morfini et al., 2004). Previous experiments have shown that microinjected htau42 becomes rapidly phosphorylated at the AT8 epitope (Moreno et al., 2011), suggesting that either GSK3, Cdk5/p35 or both may phosphorylate exogenously injected htau 42 at the presynaptic terminal. To address this possibility, synapses were co-microinjected with htau42 and either the GSK3 inhibitor ING-135 (100 nM; n=4) (Kozikowski et al., 2007, LaPointe et al., 2009) or with TFP5, a truncated p35-derived peptide that modulates Cdk5/p35 activity (200 nM; n=5) (Shukla et al., 2013).
As shown in Figs 1C-D, synapses co-injected with htau42+ING or with htau42+TFP5 demonstrated a significant lack in neurotransmitter release reduction, compared with those injected with htau42 alone (p= 0.007 and p=0.0009, respectively). Moreover, an unrelated pharmacological inhibitor of GSK3, SB216763 (Acevedo et al., 2014), also prevented the toxic effects of htau42 in coinjection experiments at 15 μM (not shown). Synapses injected with only ING or with TFP5, demonstrated no significant effect on post-synaptic response compared to control (vehicle-injected synapses; p=0.7 and p=0.55 respectively). These findings strongly indicate that the toxic effect of microinjected htau42 on synaptic function involves the activities of endogenous GSK3 and CdK5/p35.
3.3. The PAD domain of tau is necessary and sufficient to trigger synaptic transmission failure
Phosphorylation of htau42 at the AT8 epitope induces abnormal exposure of the amino terminal region of tau, a domain referred to as phosphatase-activating domain (PAD, aa 1-18) (Kanaan et al., 2011). Since AT8 immunoreactivity was observed in htau42-injected synapses ((Moreno et al., 2011)), we addressed the issue of whether the PAD motif was necessary to observe the toxic effect on synaptic function induced by htau42. This was implemented by co-injecting, htau42 with either TNT-1 (10 μM; n=6), an antibody that binds to PAD and prevents PAD-mediated activation of the PP1-GSK-3 cascade (Kanaan et al., 2011), or with Tau5 antibody (10 μM; n=6), which recognizes a.a. 210-230 of tau (LaPointe et al., 2009). Synapses co-injected with htau42 and TNT-1 showed no inhibition of synaptic transmission, compared to those injected with htau42 alone (p=0.0018). In contrast, synapses co-injected with htau42 and Tau5 displayed block of neurotransmitter release within 55+/− 10 min, showing no statistical differences from synapses injected with htau42 alone (p=0.056; Figs. 2 C&D).
Based on the above observations, we next questioned whether the PAD domain mediated the toxic effect of tau42 on synaptic transmission. Presynaptic microinjection of PAD peptide (2.5 μM; n=8) and of scrambled peptide at same concentration (n=5) [see methods and (Kanaan et al., 2011) for details] demonstrated that microinjected PAD peptide blocked synaptic transmission with a similar time course as htau42 (complete block within 55+/− 10 min, compared to htau42; p=0.071). In contrast, no significant changes in post-synaptic response were observed in scrambled PAD peptide-injected synapses after 90 min of recording, compared to vehicle-injected control synapses (p=0.93) (Figs. 2E&F). Noise analysis experiments further showed that PAD peptide transiently increased post-synaptic noise (Fig. 2B). Together, these results indicate that the PAD domain mediates the synaptic inhibitory effect of htau42. In order to evaluate whether other htau42 regions promoted synaptic toxicity as well, we microinjected recombinant tau constructs 3RC (containing the three tubulin binding motifs and the carboxyl terminal region) and 2R (containing two tubulin binding motifs) (Perez et al., 2001) (Fig. 2A). Microinjection of tau fragments 3RC or 2R at either low (80 nM; n=4 per group) or high concentrations (2 μM; n=5 per group) produced no significant inhibition of synaptic transmission during the first hour of recording compared to vehicle-injected synapses p=0.7 and p=0.60 respectively (high concentration experiments). After one hour, a slow blocking effect of postsynaptic response was observed with slightly faster effect seen in 3RC-injected synapses (Fig. 2E). Note that during this protocol (slow presynaptic activation), the post-synaptic response remained stable for over 180 min in control synapses (Llinas et al., 1985).
Collectively, results from these experiments indicate that the PAD region of tau is necessary and sufficient to produce synaptic toxicity. Our results also indicate that other tau regions might marginally contribute to htau42-induced synapto-toxicity, as indicated by the minor effect of 3R and 2R tau constructs lacking the PAD region on synaptic function. Of note, The PAD sequence (100% residues) is only present in human or monkey tau, and no obvious PAD domains in other microtubule-associated proteins, including MAP2 and MAP1B were observed in a sequence comparison analysis (not shown).
4. Discussion
4.1. Modulation of IP3 receptors by htau42
In this study, the IP3 inhibitors 2-APB and Xestospongin C effectively blocked the synaptic failure induced by htau42, identifying calcium disregulation as a key element of tau-related pathology. The experimental results presented here indicate that htau42 itself induces an initial transient calcium increment necessary for its toxic effect on synaptic dysfunction. Based on these findings, we propose that calcium release from the ER is an early pathogenic event, triggered by preterminal-mislocalized htau42, which triggers a set of downstream events leading to synaptic vesicle exocytosis block, resulting in synaptic transmission failure.
Presently, there is clear evidence for ER calcium stores (IP3Rs and RyRs) dysfunction in AD (Pierrot et al., 2006, Supnet et al., 2006, Cheung et al., 2008). These abnormalities have been related to beta amyloid pathology but to our knowledge, the modulation of IP3 receptor, by tau protein related pathology had not been reported before. There is evidence, however, that in mice overexpressing mutant tau P301L and in human AD brains- abnormally phosphorylated tau tends to associate with ER membranes (Perreault et al., 2009). While the pharmacological experiments presented here do not provide direct evidence that htau42 modifies directly ER calcium release, the results strongly suggest that calcium release from ER (directly or indirectly) is required for htau42 to induce synaptic toxicity. Although Xestospongin C and 2-APB have multiple targets (see table 1) these two IP3 inhibitors with different modes of action had similar effect. This finding suggests a common target, i.e. that they are both acting on IP3Rs. Although ryanodine is a highly specific modulator of RyRs, a different blocker was used, because the effect seen by htau42—(increased synaptic noise) was short lasting and it may overlap with the initial opening of RyRs observed with ryanodine, which is followed by the stored calcium depletion. The effect of hatu42 in RYRs, may also be relevant in tauopathies and deserve further evaluation. A significant finding reported here relates to the fact that ER calcium release can modulate spontaneous transmitter release, as demonstrated by the IP3R mediated increase in post-synaptic noise induced by htau42 injection (Fig 2B). Up to now, the mechanism by which htau42 modulates IP3Rs remains unknown. It is established that calcium levels are tightly controlled in synaptic terminals (Rizzuto and Pozzan, 2006) requiring, therefore, that the calcium rise mediated by htau42 be highly compartmentalized, and close to the release site to have an impact on synaptic vesicles dynamics.
4.2. Identification of the toxic regions of htau42 and its relation to phosphorylated tau
In the squid axon, perfusion of aggregated forms of wild type human tau inhibited fast axonal transport (FAT). The toxic effect of pathogenic tau species on FAT was mapped to the PAD domain (Kanaan et al., 2011). Interestingly, htau42 inhibited synaptic transmission at 80nM concentration, whereas perfusion of htau42 in squid axoplasm did not affect FAT at concentrations up to 25μM (Morfini et al., 2007). In contrast to non-phosphorylated htau42, htau42 phosphorylated at the AT8 site did affect FAT when perfused in axoplasm (Kanaan et al., 2011). In agreement with such findings we had previously showed that htau42 in the synapse becomes phosphorylated (Moreno et al., 2011). Thus, present experiments demonstrate that the PAD region of htau42 was necessary and sufficient parameter determining the toxic effect of tau on synaptic transmission as indicated by: 1) the ability of PAD peptide to recapitulate the toxic effect of full-length htau42 at the synapse and 2) the prevention of htau42 synapto-toxicity by monoclonal antibody TNT-1, which specifically recognizes PAD with high affinity (Kanaan et al., 2011)). Unlike TNT-1, an anti-tau antibody recognizing a. a. 210-230 (Tau5) did not prevent htau42-induced synpato-toxicity. Other tau domains (3RC and 2R) minimally affected synaptic transmission, suggesting that the effect of htau42 may implicate more than one mechanism and protein regions, notwithstanding the necessary and sufficient role of PAD in synaptic block. In addition, our results also indicate that GSK3 and Cdk5/p35 activities are required in triggering htau42 toxicity. Specifically, the highly specific GSK3 inhibitors ING-135 and SB216763 (the latter not illustrated here) blocked the inhibitory effect of htau42 on synaptic transmission when co-injected with htau42. These findings are in agreement with previous experiments in the isolated squid axon, demonstrating that AT8-tau, and PAD domain activate the PP1-GS3K cascade (LaPointe et al., 2009, Kanaan et al., 2011).
Our findings suggest that htau42 might undergo a conformational change within the presynaptic terminal, such that PAD is abnormally exposed. This effect would trigger aberrant activation of the PP1-GSK3 cascade (Wang et al., 1994, Morfini et al., 2004, Kanaan et al., 2011), which ultimately would lead to abnormal phosphorylation of synaptic proteins and affect synaptic transmission. The role of Cdk5/p35 in this mechanism remains unclear; it is probable that htau42 becomes “primed” by this kinase, which would then become available for phosphorylation by GSK3. Regardless of the above, the prevention of htau42 toxicity by TFP5 is consistent with recent data demonstrating that modulation of Cdk5/p35 activity by TFP5 ameliorated behavioral deficits in AD mouse models (Shukla et al., 2013, Sundaram et al., 2013).
4.3 Axonal dysfunction in tauopathies
Early neuropathological studies of frontotemporal dementia (FTD) reported evidence of abnormal enlargement of presynaptic terminal, which contained spheroids rich in tau protein (Zhou et al., 1998). Recent observations in a mouse model of FTD identified the active role of axonal endocytosis in the internalization of pathological tau within synapses (Wu et al., 2013). On the other hand it has been suggested that axonal transport may fail during the progression of AD (Cash et al., 2003). Results from studies in the squid axoplasm and giant synapse preparation support and extends those findings as follows: 1) microtubule dependent axonal transport is indeed impaired by pathological forms of phosphorylated tau in an acute fashion (Kanaan et al., 2011). 2) AT8-phosphorylated tau acutely inhibits glutamatergic synaptic transmission in the presynaptic terminal (Moreno et al., 2011). 3) The present work identified molecular events initiated by excess htau42 in the presynaptic terminal. These events would be expected to promote a synergistic mechanism leading to decreased neuronal connectivity. Future experiments are needed to elucidate more rigorously the effect of hTau42 on ER calcium release.
Based on these results above, a model is proposed where the presence of htau42 at the presynaptic terminal induces abnormal calcium release from intracellular stores. This event is proposed to trigger a pathogenic cascade involving, activation of a PP1-GSK3 pathway, aberrant phosphorylation of synaptic proteins, including tau, and synaptic release failure (Fig 3).
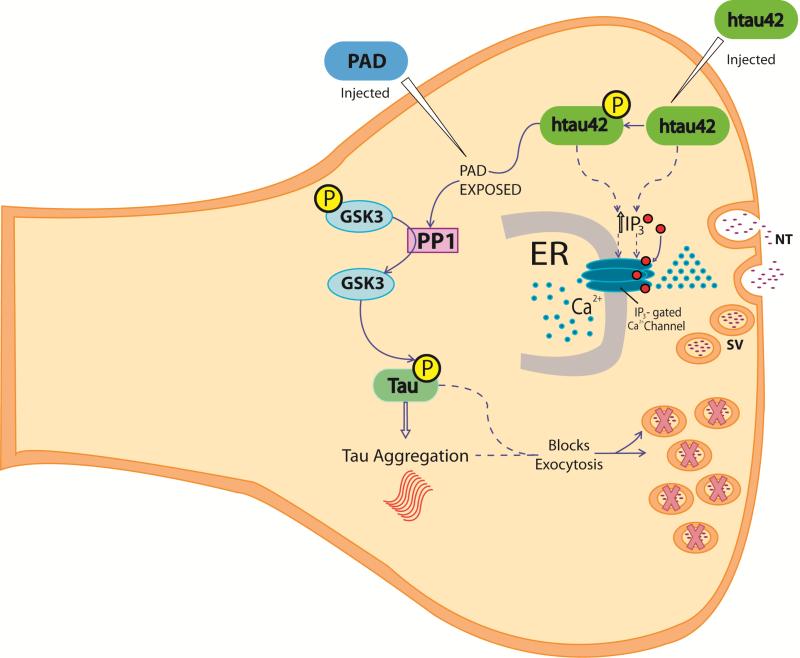
Figure 3. Pathogenic events triggered by wild type htau42 at the presynaptic site.
In disease state, wild type htau42 and/or phosphorylated tau at the synapse induce IP3 receptor activity directly or indirectly (discontinuous arrows and red dots [IP3]) resulting in increased intracellular calcium release (blue dots) in close contact with presynaptic vesicles (SV). Phosphorylation of microinjected htau42 by Cdk5/p35 and/or GSK3 at the AT8 epitope results in increased PAD domain exposure, which induces PP1 and consequently increased GSK3 activation. These events would then promote increased htau42 phosphorylation and aggregation, which in turn would promote abnormalities in synaptic vesicle and exocytosis failure (indicated by X marked SV). Microinjected PAD (PAD injected) recreated htau 42 effects (htau42 injected). Note that the exact temporal relationship of these events, in particular after tau induced increased calcium, remains to be elucidated.
P= Phosphate group
ER: Endoplasmic Reticulum
PAD: Phosphatase-Activating Domain
SV: Synaptic vesicle
NT= Neurotransmitter
Novel findings.
-
1)
htau42 in the presynaptic terminal induces calcium release from the ER
-
2)
the acute-modulation of IP3R and RyR by tau protein (directly or indirectly)
-
3)
identification of a synaptotoxic-tau region (PAD)
-
4)
the modulation of downstream kinases by PAD
Acknowledgment
To Dr D. Stefanov from SUNY Downstate for his help with statistical methods. Nicholas Kanaan of Michigan State Univ for providing stocks of the TNT-1 antibody and PAD peptide, Alan Kozikowski for the ING-135 inhibitor,
Supported by NS/NINDS/NIH HHS to R L; NIH AG027476 to H. Moreno; and a donation from ToyamaPharmaceuticals to RL and MS. NIH NS066942 (to GM), NS23868, NS23320, NS41170, NS082730 (to STB) and a Zenith Award from the Alzheimer's Association grants to STB and grant from FAPESP (Science Foundation for the São Paulo State, Brasil; 19011-0) to JEM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo KM, Opazo CM, Norrish D, Challis LM, Li QX, White AR, Bush AI, Camakaris J. Phosphorylation of amyloid precursor protein at threonine 668 is essential for its copper-responsive trafficking in SH-SY5Y neuroblastoma cells. J Biol Chem. 2014;289:11007–11019. doi: 10.1074/jbc.M113.538710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash AD, Aliev G, Siedlak SL, Nunomura A, Fujioka H, Zhu X, Raina AK, Vinters HV, Tabaton M, Johnson AB, Paula-Barbosa M, Avila J, Jones PK, Castellani RJ, Smith MA, Perry G. Microtubule reduction in Alzheimer's disease and aging is independent of tau filament formation. Am J Pathol. 2003;162:1623–1627. doi: 10.1016/s0002-9440(10)64296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin T, Marty A, Llano I. Presynaptic calcium stores and synaptic transmission. Curr Opin Neurobiol. 2005;15:275–281. doi: 10.1016/j.conb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, Pitstick R, Sahara N, Ashe KH, Carlson GA, Spires-Jones TL, Hyman BT. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav S, Katina S, Kovac A, Kazmerova Z, Novak M, Zilka N. Truncated tau deregulates synaptic markers in rat model for human tauopathy. Front Cell Neurosci. 2015;9:24. doi: 10.3389/fncel.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan NM, Morfini GA, LaPointe NE, Pigino GF, Patterson KR, Song Y, Andreadis A, Fu Y, Brady ST, Binder LI. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J Neurosci. 2011;31:9858–9868. doi: 10.1523/JNEUROSCI.0560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs GG. Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol. 2015;41:3–23. doi: 10.1111/nan.12208. [DOI] [PubMed] [Google Scholar]
- Kozikowski AP, Gaisina IN, Yuan H, Petukhov PA, Blond SY, Fedolak A, Caldarone B, McGonigle P. Structure-based design leads to the identification of lithium mimetics that block mania-like effects in rodents. possible new GSK-3beta therapies for bipolar disorders. J Am Chem Soc. 2007;129:8328–8332. doi: 10.1021/ja068969w. [DOI] [PubMed] [Google Scholar]
- LaPointe NE, Morfini G, Pigino G, Gaisina IN, Kozikowski AP, Binder LI, Brady ST. The amino terminus of tau inhibits kinesin-dependent axonal transport: implications for filament toxicity. J Neurosci Res. 2009;87:440–451. doi: 10.1002/jnr.21850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JW, Sugimori M, Llinas RR, McGuinness TL, Greengard P. Effects of synapsin I and calcium/calmodulin-dependent protein kinase II on spontaneous neurotransmitter release in the squid giant synapse. Proc Natl Acad Sci U S A. 1990;87:8257–8261. doi: 10.1073/pnas.87.21.8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, McGuinness TL, Leonard CS, Sugimori M, Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci U S A. 1985;82:3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid T, Ali YO, Venkitaramani DV, Jang MK, Lu HC, Pautler RG. In vivo axonal transport deficits in a mouse model of fronto-temporal dementia. Neuroimage Clin. 2014;4:711–717. doi: 10.1016/j.nicl.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Moreno H, Choi S, Yu E, Brusco J, Avila J, Moreira JE, Sugimori M, Llinas RR. Blocking Effects of Human Tau on Squid Giant Synapse Transmission and Its Prevention by T-817 MA. Front Synaptic Neurosci. 2011;3:3. doi: 10.3389/fnsyn.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Mizuno N, Kikkawa M, Brady ST. Tau binding to microtubules does not directly affect microtubule-based vesicle motility. J Neurosci Res. 2007;85:2620–2630. doi: 10.1002/jnr.21154. [DOI] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Brown H, Pant HC, Pigino G, DeBoer S, Beffert U, Brady ST. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 2004;23:2235–2245. doi: 10.1038/sj.emboj.7600237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 2002;21:281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M, Arrasate M, Montejo De Garcini E, Munoz V, Avila J. In vitro assembly of tau protein: mapping the regions involved in filament formation. Biochemistry. 2001;40:5983–5991. doi: 10.1021/bi002961w. [DOI] [PubMed] [Google Scholar]
- Perreault S, Bousquet O, Lauzon M, Paiement J, Leclerc N. Increased association between rough endoplasmic reticulum membranes and mitochondria in transgenic mice that express P301L tau. J Neuropathol Exp Neurol. 2009;68:503–514. doi: 10.1097/NEN.0b013e3181a1fc49. [DOI] [PubMed] [Google Scholar]
- Pierrot N, Santos SF, Feyt C, Morel M, Brion JP, Octave JN. Calcium-mediated transient phosphorylation of tau and amyloid precursor protein followed by intraneuronal amyloid-beta accumulation. J Biol Chem. 2006;281:39907–39914. doi: 10.1074/jbc.M606015200. [DOI] [PubMed] [Google Scholar]
- Polydoro M, Dzhala VI, Pooler AM, Nicholls SB, McKinney AP, Sanchez L, Pitstick R, Carlson GA, Staley KJ, Spires-Jones TL, Hyman BT. Soluble pathological tau in the entorhinal cortex leads to presynaptic deficits in an early Alzheimer's disease model. Acta Neuropathol. 2014;127:257–270. doi: 10.1007/s00401-013-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, McBrayer MK, Campbell J, Kumar A, Hashim A, Sershen H, Stavrides PH, Ohno M, Hutton M, Nixon RA. Specific calpain inhibition by calpastatin prevents tauopathy and neurodegeneration and restores normal lifespan in tau P301L mice. J Neurosci. 2014;34:9222–9234. doi: 10.1523/JNEUROSCI.1132-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- Shukla V, Zheng YL, Mishra SK, Amin ND, Steiner J, Grant P, Kesavapany S, Pant HC. A truncated peptide from p35, a Cdk5 activator, prevents Alzheimer's disease phenotypes in model mice. FASEB J. 2013;27:174–186. doi: 10.1096/fj.12-217497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram JR, Poore CP, Sulaimee NH, Pareek T, Asad AB, Rajkumar R, Cheong WF, Wenk MR, Dawe GS, Chuang KH, Pant HC, Kesavapany S. Specific inhibition of p25/Cdk5 activity by the Cdk5 inhibitory peptide reduces neurodegeneration in vivo. J Neurosci. 2013;33:334–343. doi: 10.1523/JNEUROSCI.3593-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supnet C, Grant J, Kong H, Westaway D, Mayne M. Amyloid-beta-(1-42) increases ryanodine receptor-3 expression and function in neurons of TgCRND8 mice. J Biol Chem. 2006;281:38440–38447. doi: 10.1074/jbc.M606736200. [DOI] [PubMed] [Google Scholar]
- Tai HC, Wang BY, Serrano-Pozo A, Frosch MP, Spires-Jones TL, Hyman BT. Frequent and symmetric deposition of misfolded tau oligomers within presynaptic and postsynaptic terminals in Alzheimer's disease. Acta Neuropathol Commun. 2014;2:146. doi: 10.1186/s40478-014-0146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QM, Fiol CJ, DePaoli-Roach AA, Roach PJ. Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J Biol Chem. 1994;269:14566–14574. [PubMed] [Google Scholar]
- Wu JW, Herman M, Liu L, Simoes S, Acker CM, Figueroa H, Steinberg JI, Margittai M, Kayed R, Zurzolo C, Di Paolo G, Duff KE. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem. 2013;288:1856–1870. doi: 10.1074/jbc.M112.394528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A, Kumar A, Peterhoff C, Duff K, Nixon RA. Axonal transport rates in vivo are unaffected by tau deletion or overexpression in mice. J Neurosci. 2008;28:1682–1687. doi: 10.1523/JNEUROSCI.5242-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempel H, Mandelkow E. Lost after translation: missorting of Tau protein and consequences for Alzheimer disease. Trends Neurosci. 2014;37:721–732. doi: 10.1016/j.tins.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Zhou L, Miller BL, McDaniel CH, Kelly L, Kim OJ, Miller CA. Frontotemporal dementia: neuropil spheroids and presynaptic terminal degeneration. Ann Neurol. 1998;44:99–109. doi: 10.1002/ana.410440116. [DOI] [PubMed] [Google Scholar]
References for Table 1
- 1.Ma HT, et al. Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry channels. J Biol Chem. 2001;276(22):18888–96. doi: 10.1074/jbc.M100944200. [DOI] [PubMed] [Google Scholar]
- 2.Maruyama T, et al. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122(3):498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 3.Missiaen L, et al. 2-Aminoethoxydiphenyl borate affects the inositol 1,4,5-trisphosphate receptor, the intracellular Ca2+ pump and the non-specific Ca2+ leak from the non-mitochondrial Ca2+ stores in permeabilized A7r5 cells. Cell Calcium. 2001;29(2):111–6. doi: 10.1054/ceca.2000.0163. [DOI] [PubMed] [Google Scholar]
- 4.Gafni J, et al. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19(3):723–33. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 5.De Smet P, et al. Xestospongin C is an equally potent inhibitor of the inositol 1,4,5-trisphosphate receptor and the endoplasmic-reticulum Ca(2+) pumps. Cell Calcium. 1999;26(1-2):9–13. doi: 10.1054/ceca.1999.0047. [DOI] [PubMed] [Google Scholar]
- 6.Korolkiewicz R, et al. Pharmacological characterization of the contractile effects of galanin (1-29)-NH2, galantide and galanin (1-14)-(alpha-aminobutyric acid8)scyliorhinin-I in the rat gastric fundus. Fundam Clin Pharmacol. 1997;11(6):576–83. doi: 10.1111/j.1472-8206.1997.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 7.Muehlschlegel S, Sims JR. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit Care. 2009;10(1):103–15. doi: 10.1007/s12028-008-9133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei H, Perry DC. Dantrolene is cytoprotective in two models of neuronal cell death. J Neurochem. 1996;67(6):2390–8. doi: 10.1046/j.1471-4159.1996.67062390.x. [DOI] [PubMed] [Google Scholar]
- 9.Kozikowski AP, et al. Structure-based design leads to the identification of lithium mimetics that block mania-like effects in rodents. possible new GSK-3beta therapies for bipolar disorders. J Am Chem Soc. 2007;129(26):8328–32. doi: 10.1021/ja068969w. [DOI] [PubMed] [Google Scholar]
- 10.Coghlan MP, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000;7(10):793–803. doi: 10.1016/s1074-5521(00)00025-9. [DOI] [PubMed] [Google Scholar]
- 11.Zheng YL, et al. A 24-residue peptide (p5), derived from p35, the Cdk5 neuronal activator, specifically inhibits Cdk5-p25 hyperactivity and tau hyperphosphorylation. J Biol Chem. 2010;285(44):34202–12. doi: 10.1074/jbc.M110.134643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanaan NM, et al. Pathogenic forms of tau inhibit kinesin-dependent axonal transport through a mechanism involving activation of axonal phosphotransferases. J Neurosci. 2011;31(27):9858–68. doi: 10.1523/JNEUROSCI.0560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]