Abstract
Fabrication of cellular constructs with spatial control of cell location (±5 μm) is essential to the advancement of a wide range of applications including tissue engineering, stem cell and cancer research. Precise cell placement, especially of multiple cell types in co- or multi-cultures and in three dimensions, can enable research possibilities otherwise impossible, such as the cell-by-cell assembly of complex cellular constructs. Laser-based direct writing, a printing technique first utilized in electronics applications, has been adapted to transfer living cells and other biological materials (e.g., enzymes, proteins and bioceramics). Many different cell types have been printed using laser-based direct writing, and this technique offers significant improvements when compared to conventional cell patterning techniques. The predominance of work to date has not been in application of the technique, but rather focused on demonstrating the ability of direct writing to pattern living cells, in a spatially precise manner, while maintaining cellular viability. This paper reviews laser-based additive direct-write techniques for cell printing, and the various cell types successfully laser direct-written that have applications in tissue engineering, stem cell and cancer research are highlighted. A particular focus is paid to process dynamics modeling and process-induced cell injury during laser-based cell direct writing.
1. Introduction
The ability to pattern cells is fundamental to the precise control of cellular microenvironments and to create tissue constructs, which are needed to understand cellular interactions in normal or diseased tissues, direct stem cell differentiation and to build functional tissue replacements. By controlling cell location in relation to neighboring cells, an in vitro culture or construct can be created to replicate the in vivo cellular environment. Such a construct can yield physiologically relevant cellular responses and functions, and thus enables more rapid and accurate biomaterial and drug discovery. In particular, the ability to control a cell’s location and spatial proximity in relation to neighboring homogeneous and heterogeneous cell types is highly desired to investigate cell–cell and cell–extracellular matrix (ECM) interactions, signaling pathways and gene expression. By dictating cell location and cellular proximity, the modes of cellular signaling (direct cell contact, paracrine signals or endocrine signals) are also affected. Controlling the types of cells, as well as their mode(s) of communication, will directly influence cellular behavior, gene expression and subsequent intercellular signaling and cell function. For these reasons, precise cell patterning in two- and three dimensions provides profound opportunities for basic science investigations, as well as applications in tissue engineering and regenerative medicine.
1.1. Importance of cellular patterning for tissue engineering
The current state of the art in tissue engineering involves homogeneously seeding cells into preformed scaffolds, and then manipulating that scaffold through chemical and mechanical stimulation to achieve desired structure and mechanical properties. Numerous, wide-ranging materials are used to create these scaffolds. For example, some current scaffold materials for engineered tendon replacements have been collagen sponges [1], porcine small intestine submucosa (SIS) [2], poly-L-lactic acid (PLLA) [3] and silk [4]. Despite their biocompatibility and ability to be seeded with the desired cells, many scaffolds are restricted in size and shape due to diffusion limitations on receiving nutrients and transporting wastes. Such limits highlight the necessity of vasculature, which to date very few engineered tissue constructs have been able to incorporate [5]. Therefore, the spatial patterning of diverse cell types would be highly desired and advantageous to tissue engineering; having control over individual cell location in addition to biological and synthetic scaffolding would allow the building of a cellular construct on a cell-by-cell basis, from the bottom-up. When coupled with the ability to use multiple cell types, this precise control makes the incorporation of secondary structures, such as vasculature, feasible to achieve the desired structure and cellular make-up.
1.2. Importance of cell patterning for in vitro culture
1.2.1. Stem cells
Stem cells represent an unrestricted cell source for basic science, cell therapy and tissue regeneration, due to their self-renewal capacity and differentiation potential [6]. The renewal, differentiation and assembly of stem cells are governed by the stem cell niche, or microenvironment, which is composed of ECM, soluble factors and neighboring cells [7–9]. Proteins of the ECM help to regulate cell signaling in a spatially-patterned fashion by providing structural support to cells, integrating complex cellular signals and controlling the distribution and activation of growth factors [10]. A number of approaches have been employed in attempts to recapitulate the stem cell niche or desired aspects of the microenvironment, to understand and/or influence the cell’s fate decision. Engineered biomaterials have been created to mimic the three dimensionality, nanofibrous structure, molecular factors and mechanical properties of ECM to regulate stem cell differentiation [11–19]. In addition, micro- and nanofabrication, such as electrospinning, nanopatterning and microfluidics, offer new ways for biomaterials to mimic stem cell microenvironments for the spatial control of stem cell fate [20–23]. For example, nanopatterned surfaces could affect stem cell adhesion [24, 25]; nanofibers (made of PLLA, polyamide or poly-caprolacton) could promote self-renewal or lineage-specific differentiation [26–30] as well as nanotubes (made of TiO2 or carbon) [31–33]. The cell patterning of microchip and microfluidic systems has also been employed to study the stem cell microenvironment, or to form uniform embryoid bodies for in vivo-like stem cell differentiation [34–38]. These works focused mainly on the influence of the patterned surface on embryonic stem cell adhesion and differentiation. Very recently, it has been realized how important in vivo cellular spatial positioning is in influencing differentiation and function, as well as the physiology of health and disease [39, 40]. The ability to control the lineage-specific differentiation of stem cells in the appropriate location is vital to tissue morphogenesis and regeneration [23, 41]. Furthermore, the cellular composition of engineered stem cell microenvironments also displays a profound influence on regulating stem cell differentiation, with different cell fates achieved by varying cellular spacing or proximity as well as different types of neighboring cells in co-cultures [42, 43]. Therefore, in order to realize directed, lineage-specific stem cell differentiation, an innovative approach is needed to organize all of these factors into a complex, interactive, structural stem cell microenvironment to facilitate cell-fate decision in a proper spatiotemporal manner.
1.2.2. Cancer cells
Similar to applications in stem cell research, the study of cancer induction, proliferation, migration, metastasis, apoptosis and treatments would benefit greatly from cellular patterning. In particular, the patterning of multiple cell types for co- or multi-cultures would allow for the replication or mimicry of the in vivo environment, necessary to gain insight into cellular communications within the microenvironment of developing tumors. Although the interaction between cancer cells and neighboring cells plays a crucial role in cancer metastasis and anticancer drug resistance, much of the current in vitro cancer research is conducted by uniformly co-culturing normal tissue cells and carcinomas or monolayers of the two different cell types [44]. Such studies do not allow for spatial influences on cells in culture, and therefore cannot replicate the distribution of carcinomas and non-cancerous cells (e.g., fibroblasts) as observed physiologically. As a result, the types of cells in communication as well as the modes by which they communicate do not represent those in a tumor. Through cell patterning, an engineered tumor construct can be created by patterning the different cell types to mimic the cellular distribution of a histologic section. Such a construct would replicate in vivo cellular communication in an in vitro model, to provide unique insight into cancer metastasis and more specific cellular interactions. This type of engineered tumor would be very useful in fundamental studies of tumor development and applications in cancer drug screening.
1.2.3. High-throughput analysis of novel biomaterials
Rapidly fabricated and customizable idealized cellular patterns and constructs can greatly increase the scope of high-throughput testing. High-throughput and combinatorial processing has been used for biomaterial development and allows for more efficient assessment of cellular and biomaterial interactions, due to the ability to quickly run multiple tests [45–47]. Patterned arrays of both homogeneous and heterogeneous idealized cellular constructs can be used for the combinatorial analysis of cell-to-cell, cell-to-drug and cell-to-biomaterial interactions.
Laser-based direct writing provides the platform for a bottom-up approach to build cellular constructs for tissue engineering applications and precise cellular environments for stem cell and cancer research, and for constructing three-dimensional in vitro tissue models for biomaterial discovery, drug screening and toxicity testing. This paper reviews laser-based additive direct-write techniques that have been used for printing cells with these applications in mind. A review of process dynamics during laser-based cell direct writing is also emphasized.
1.3. Overview of laser-based direct-write techniques for cell patterning
Laser direct writing was first used to write patterns of metals for processing mesoscopic conformal passive electronic devices [48–52]. Thick films of Ag, BaTiO3 and NiCr have been printed to construct conductors, capacitors and resistors, respectively, with a spatial resolution between ~1 and 3 μm using laser-based additive direct writing [50]. It was this resolution and reproducibility that made laser direct-write techniques desirable for adaptation to biomedical applications, such as cell printing. Additionally, many of the laser-based techniques are now based on rapid prototyping technology and methodologies, allowing cells and other biological materials to be printed using computer-aided design and computer-aided manufacturing (CAD/CAM). The more traditional, and widely used, cell patterning techniques include micro-contact printing [53, 54], photolithography [55], dip pen nanolithography [56] and ink-jet printing [57]. However, in this review we will focus only on laser-based cell patterning. The most prolific laser-based direct-write techniques for cellular applications are laser-induced forward transfer (LIFT), absorbing film-assisted laser-induced forward transfer (AFA-LIFT), biological laser processing (BioLP), matrix-assisted pulsed laser evaporation direct writing (MAPLE DW) and laser-guided direct writing (LG DW). These methods will be reviewed with a special focus on the specific techniques employed for cell printing, and the various cell types that have been written will be discussed. A comprehensive review of early work in this area, that also includes non-laser-based printing techniques for living cells, is given elsewhere [58].
LIFT, AFA-LIFT, BioLP and MAPLE DW have some distinct similarities in methodology for the direct writing of cells (figure 1). These direct-write techniques utilize laser transparent print ribbons on which one side is coated with cells that are either adhered to a biological polymer through initial cellular attachment or uniformly suspended in a thin layer of liquid (usually cell culture medium mixed with glycerol) or a hydrogel. A receiving substrate is coated with a biopolymer or cell culture medium to maintain cellular adhesion and sustained growth, mounted on motorized stages and positioned facing the cell-coated side of the ribbon. A pulsed laser beam is transmitted through the ribbon and is used to propel cells from the ribbon to the receiving substrate. The rapid volatilization of the cellular support layer on the ribbon creates the force necessary to allow the cells to cross the small (700–2000 μm [77]) gap between the ribbon and receiving substrate. Rather than employing a print ribbon and pulsed laser, LG DW relies on a weakly focused continuous laser to target an individual cell from a liquid cell suspension and propel it to a growth surface using optical forces.
Figure 1.

Representative schematic of a LIFT, AFA-LIFT, BioLP or MAPLE DW system used for cell printing.
LIFT [52, 59–64] traditionally employs a high-powered pulsed laser. In this process, the quartz or glass print ribbon is coated with a thin layer of metal or other laser-absorbing biocompatible materials, to protect the cells from the high-power laser pulses. The cells of interest are suspended in either a culture medium or a hydrogel and uniformly spread onto the bottom side of the ribbon (figure 2). The suspension is then vaporized with a laser pulse focused onto the metal layer, which acts to propel the cell suspension from the print ribbon to a receiving substrate.
Figure 2.

Schematic (side view) of a LIFT print ribbon (not to scale).
AFA-LIFT [65–68] is similar in technique to LIFT, but uses a thick (~100 nm) sacrificial layer of a metal on the ribbon to interact with the laser. A version of AFA-LIFT called BioLP [69–76] uses motorized receiving stages and a CCD camera to help in focusing the laser. BioLP uses a sacrificial metal layer (75–100 nm thick) to have a rapid thermal expansion, which propels small volumes of cell suspensions from the ribbon to the receiving substrate with little heating of the cell suspension as demonstrated by high speed imaging showing a stream of fluid, instead of vapor leaving the ribbon [69]. Various thin metal layers, such as Au, Ag, Ti and TiO2, have been used with success. Cells have been suspended in both culture medium and various hydrogels (figure 3).
Figure 3.

Schematic (side view) of an AFA-LIFT or BioLP print ribbon (not to scale).
MAPLE DW [77–80] is a technique similar to AFA-LIFT, but utilizes a low-powered, pulsed laser operating in the UV or near-UV region. The underside of the print ribbon is first coated with a sacrificial biological attachment layer such as a basement membrane matrix (i.e. Matrigel® (BD Bioscience, Bedford, MA)) to allow for initial cell attachment (figure 4). The low power and UV wavelength help to ensure that the laser does not penetrate to the cell attachment layer. Localized volatilization of the attachment layer at the ribbon/biopolymer interface allows for the transfer spot to be propelled and fall onto a receiving substrate. Initial cell attachment to the transparent biopolymer layer is required on the ribbon; the coupling with a CCD camera and computer-controlled X–Y–Z translation stages allows for the visualization and subsequent targeting of specific cells on the ribbon. MAPLE DW provides CAD/CAM control and processing along with selective cell patterning.
Figure 4.

Schematic (side view) of a MAPLE DW print ribbon (not to scale).
LG DW [81–84] and laser guidance [85–87] typically use a laser operating at an ~800 nm wavelength. The laser beam is weakly focused into a liquid suspension of cells and the force of the light moves the cells from the suspension onto a translating receiving substrate (figure 5(a)), but the working distance is usually limited to less than 300 μm. To increase the working distance, the laser beam can also be coupled with hollow optical fibers to carry cells over millimeter to centimeter distances to the growth surface (figure 5(b)). The addition of computer control and a CCD camera for visualization allows for selecting specific cells and creating precise patterns.
Figure 5.
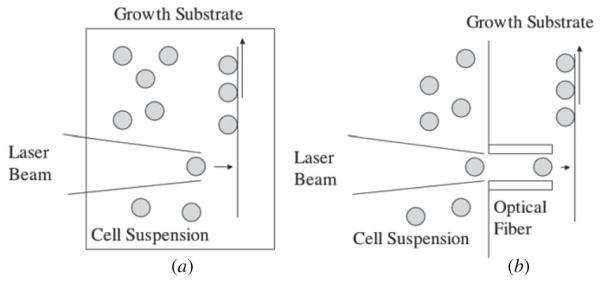
(a) Laser-guided direct writing. (b) LG DW with an optical fiber (adapted from [82]) (not to scale).
2. Applications of laser-based cell printing for tissue engineering, stem cell, and cancer research
Laser-based additive writing has the ability to create precise user-generated patterns for a variety of cell types and biomaterials. The control of cellular positioning can be utilized to create spatially precise cultures of cells, including co- and multi-cultures, as well as to build biological constructs in a bottom-up, cell-by-cell fashion. Such a technique is of great interest in research involving cells whose response is highly sensitive to their microenvironment, such as carcinomas and stem cells. The application of cellular laser direct writing can therefore have a significant influence on applications in cancer research, as well as tissue engineering and regenerative medicine. However, the predominance of work to date has not been in application of the technique, but rather focused on demonstrating the ability of direct writing to pattern living cells, in a spatially precise manner, while maintaining cellular viability. These demonstrations are summarized below, with particular focus on cancer cells, stem cells and cells with tissue engineering applications; they represent an important first step in realizing cellular applications of laser-based direct writing.
2.1. Laser direct writing of cancer- and tumor-derived cells
The ability to create precise in vitro cultures of cancer cells is essential for replicating the in vivo tumor microenvironment to better understand cellular communication in culture. In-depth cellular studies can help to answer fundamental questions regarding the influence of spatial and geometric locations on cancer induction, proliferation, metastasis and cell-to-cell communication between carcinomas and healthy normal cells. The studies reviewed below describe the various types of cancer- and tumor-derived cells that have been laser direct written.
2.1.1. MAPLE DW
Pluripotent embryonal mouse carcinoma cells (P19) were written using MAPLE DW with CAD/CAM control and Matrigel®-coated ribbons and receiving dishes [88]. A spin coater was used to apply the Matrigel® layer to the ribbon to increase uniformity and repeatability. The transfer process resulted in greater than 95% viability when patterned onto a thick layer of Matrigel® (up to 100 μm) while using an ArF pulsed laser (wavelength of 193 nm) and fluences around 400 mJ cm−2. Additionally, DNA damage, possibly induced by the UV irradiation, was evaluated with both neutral and alkaline comet assays to assess for double-strand and single-strand DNA breaks, respectively, demonstrating little to no evidence of DNA damage. In order to evaluate the pluripotency of the P19 cells post-transfer, the cells were exposed to retinoic acid and DMSO. The transferred cells differentiated into both the neuronal and muscle lineage as expected, verifying that laser direct writing did not negatively affect the cell’s pluripotency. This study showed that laser transfer can create viable patterns of pluripotent cells in a manner that does not cause DNA damage from the UV laser pulses while still retaining the cell’s natural pluripotent ability to differentiate.
MAPLE DW was also used to direct write human osteosarcoma (MG-63) and rat cardiac cells [78]. A pulsed laser (193 nm wavelength) with fluences ranging from 157 to 315 μJ cm−2 printed the cells from a suspension of culture medium and 5% glycerol onto Matrigel®-coated receiving dishes. Repeatedly (3 times) depositing MG-63 cells from a Matrigel®-coated ribbon onto the same location on a Matrigel®-coated receiving substrate resulted in stacks of viable cells of 50–100 μm height, demonstrating the ability of MAPLE DW to create three-dimensional cellular constructs.
To investigate the co-deposition of bioceramics with viable cells, osteosarcoma (MG-63) and hydroxyapatite (HA) constructs were patterned using MAPLE DW [80]. HA was introduced to the ribbon in a glycerol/water solution and then transferred using an optimized fluence of 0.22 J cm−2. MG-63 cells were written from a commercially available ECM (ATCC, Manassas, VA)-coated ribbon onto an ECM-coated receiving substrate using a fluence of 0.15 J cm−2. Co-deposition of HA and MG-63 involved suspending HA in a liquid ECM prior to polymerization on the ribbon and then allowing MG-63 cell attachment. The HA-MG-63 combination was then patterned onto an ECM-coated receiving substrate. The study succeeded in patterning the HA, MG-63 and the combination HA-MG-63. The novelty of writing inorganic scaffold materials in addition to viable cells has many applications and could be beneficial for building three-dimensional constructs.
B35 neuronal cells, which are derived from neonatal rat nervous system tumors (neuroblastoma), were patterned into lines or rectangular arrays using MAPLE DW [77]. The B35 cells were printed from a Matrigel®-coated ribbon onto a Matrigel®-coated receiving substrate. Along with verifying cell viability and three-dimensional axonal growth into the Matrigel® , a TUNEL assay was completed and no increase in cell apoptosis from the MAPLE DW process was found. It was found that fluences over 0.08 J cm−2 increased the number of dead cells in the live/dead assay, but by adjusting the laser fluence from 0.02 J cm−2 to 0.08 J cm−2, it was possible to control the penetration depth of the cells into the Matrigel® receiving substrate. A similar study was completed using B35 cells, but the ribbon had an additional coating with a triazene polymer dynamic release layer prior to the Matrigel® coating and subsequent seeding with cells [89]. The triazene was shown to be non-cytotoxic and allowed for a slight reduction in the laser fluences required for cellular transfer, demonstrating the potential utility of dynamic release layers for printing cells. In the most recent example of MAPLE DW, an ArF pulsed excimer laser (193 nm wavelength) with CAD/CAM control and Matrigel®-coated ribbons and receiving surfaces were used to create viable arrays of MCF-7 breast cancer cells [90].
2.1.2. BioLP
BioLP has also been used to direct write osteosarcoma (MG-63) into three-dimensional structures [70]. The earliest BioLP technique coated the ribbon in 75–85 nm of Ti or TiO2, with the cells suspended in culture medium and glycerol, and transferred to a Matrigel®-coated receiving substrate using a 266 nm wavelength laser. A three-dimensional structure was constructed with two layers of MG-63 cells, by manually spreading Matrigel® on top of the first transfer and then writing a second group of cells on top. Feasibility of patterning MG-63 in heterogeneous cultures of patterned arrays on Matrigel® with mouse endothelial cells (EOMA GFP) was also shown [73]. An additional study showed that BioLP was capable of printing MG-63 with single cell resolution onto a Matrigel®-coated receiving substrate [72]. Cell concentration and probability dictate the number of spots targeted that will have single cells. Based on a cell analog (10 μm diameter beads), a concentration of 1 × 107 beads ml−1 on the ribbon has a probability of 0.37 of achieving a single cell per laser shot. Growth curves of laser-written single cells did not differ from growth curve of controls. Heat shock protein (HSP) expression (a protein which may be expressed when a cell is exposed to heat stress) was also found to be minimal for the laser-transferred MG-63 compared to controls.
MAPLE DW has been successful in printing osteosarcoma (MG-63), tumor-derived neuronal cells (B35), pluripotent embryonal carcinoma (P19) and breast cancer (MCF-7) cells. The efficacy of the technique was demonstrated with high cell viability (live/dead assay), little to no DNA damage (comet assay) and no increase in cell apoptosis (TUNEL assay) after MAPLE DW. BioLP had also successfully printed MG-63 into three-dimensional structures, heterogeneously with mouse endothelial cells (ECs) and with single-cell resolution while showing minimal HSP expression.
2.2. Laser direct writing of stem cells and cells with tissue engineering applications
Cellular constructs for tissue engineering and regenerative medicine would have greater engineering control of structure and function than that seen in scaffold-based constructs, if built from the bottom-up on a cell-by-cell basis. However, to build a cellular construct, many different cell types need to be involved. To demonstrate the ability of laser-based direct writing for building cellular constructs, the studies reviewed below describe the numerous types of cells that have been laser direct written and have tissue engineering applications.
2.2.1. MAPLE DW
Wu et al [91] transferred viable Chinese hamster ovary cells (CHOs) to a receiving substrate from a hydrogel-coated ribbon, which was one of the first demonstrations of using a pulsed laser direct-write technique to print viable mammalian cells. In the most recent example of MAPLE DW, an ArF pulsed excimer laser operating at 193 nm with CAD/CAM control was used. Human dermal fibroblasts, mouse C2C12 myoblasts, bovine pulmonary artery endothelial (BPAEC), breast cancer (MCF-7) and rat neural stem cells were patterned from Matrigel®-coated ribbons and receiving surfaces into arrays. All cell transfers used the same protocol to demonstrate the versatility of the technique for multiple applications and cell types [90].
2.2.2. AFA-LIFT
AFA-LIFT was used to laser direct-write rat Schwann and astroglial and pig lens epithelial cells [68]. A quartz ribbon was first coated with a silver layer (~100 nm thick) by vacuum evaporation and then the cell suspension was spread on top with a thickness of ~140–160 μm. Using a laser beam area of 0.07 mm2 and a fluence of 360 mJ cm−2, the cells were transferred to a gelatin-coated receiving substrate which was situated above the ribbon. A time-resolved study of the transfer showed that the cells were ejected from the ribbon at a velocity of 122 m s−1 with an acceleration over 107 g. Although the cells experienced large forces, the few nanosecond exposure minimized the effect on cell viability. This study determined that viable cell transfer required the cells to be deposited in a wet environment and onto a substrate that supports sustained growth and cell adhesion.
2.2.3. LIFT
A modified LIFT technique was used to direct write mouse embryonic stem cells [92]. A thick film of polyimide (4 μm) was spin coated onto glass slides and then oven cured, representing a shift from the traditionally used thin laser absorbing metal layers. Mouse embryonic stem cells were suspended in a 1:1 mixture of glycerol and cell culture medium and spread onto the thick polyimide layer. Using a laser with a 30 μm beam diameter focused to the polyimide/glass interface and a 355 nm wavelength, viable cells were transferred to a Matrigel®-coated (thickness ~500 μm) Petri dish.
NIH3T3 fibroblasts, HaCaT keratinocytes and human mesenchymal stem cells (hMSC) were laser direct written into patterns using LIFT and a 1064 nm wavelength laser with fluences ranging from 3 to 6 J cm−2 [63]. The ribbon surface was first coated with 55–60 nm of gold and the cells were suspended in a mixture of blood plasma and alginate hydrogel and then laser written on a Matrigel®-coated substrate for their long-term growth. Survivability after LIFT for the fibroblasts and keratinocytes was ~98% and hMSC was ~90%. Apoptosis of transferred cells was unaltered from controls, and single- and double-strand DNA damage as measured by a comet assay showed no significant increase. Additionally, it was found that LIFT did not alter the phenotype of the hMSCs or initiate differentiation. Koch et al also demonstrated a two-dimensional checkerboard-pattern co-culture of fibroblasts and keratinocytes, which shows significant tissue engineering potential.
The ability of LIFT to selectively seed a three-dimensional scaffold with two different cell types was demonstrated by Ovsianikov et al [64]. A hexagonal porous tubular scaffold, constructed out of poly(ethylene glycol) diacrylate, was fabricated using two-photon polymerization (2PP) and then placed in an aqueous solution, maintaining a moist environment to support cell transfer. Ovine ECs and ovine vascular smooth muscle-like cells (vSMCs) were suspended onto a gold-coated ribbon in a mixture of blood plasma, alginate hydrogel and cell culture medium and then printed into the scaffold using a 1064 nm wavelength laser. To mimic the cellular distribution of a blood vessel, ECs were printed into the intimal layer and the vSMCs into the medial layer of the scaffold. The controlled placement of the two different cell types in a three-dimensional scaffold by LIFT allowed for a radial distribution of cell types, presenting a method for seeding scaffolds with greater spatial precision and selectivity than homogeneous cell seeding.
2.2.4. BioLP
BioLP was used to print bovine aortic endothelial cells (BAEC) to investigate intercellular communication post-transfer [75]. The cells were suspended in growth medium and 5% glycerol, spread evenly into a 10–100 μm thick layer, on a metal or metal oxide backed ribbon, and then printed onto Matrigel® using a pulsed laser (248 nm wavelength). Transferred cells were analyzed for the expression of HSP, which may be expressed when exposed to heat stress. No change in the expression of HSP was observed between controls and printed cells using fluences ranging from 0.15 J cm−2 up to 1.5 J cm−2. Viability was also found to be near 100%, but dropped to 90% at the highest fluence. BAEC were seen growing together and beginning to form tubular structures after 24 h of growth. Othon et al [76] used BioLP and a laser with a wavelength of 266 nm to print three-dimensional lines of olfactory ensheathing cells. The cells were suspended in culture media and 0.35% methylcellulose, spread evenly onto a 40 nm thick TiO2 film-coated ribbon and patterned onto Matrigel® to form lines of single cells. Three-dimensional lines with larger cell numbers were constructed by layering Matrigel® on previously printed cells.
A high-throughput variation on BioLP (HT-BioLP) used a near-IR laser operating at a wavelength of 1064 nm and a fluence of 1.2 J cm−2 to write ECs (EA.hy926) [93]. The ribbon was first sputter coated with a 20–30 nm thick layer of gold, and transferred cells were embedded in sodium alginate (1% w/v in either water or PBS) and 10% w/v glycerol, and then written on a sodium alginate-coated receiving substrate. This demonstrated the ability of HT-BioLP to rapidly write customized patterns (~1 s for a complex pattern) of viable ECs with CAD/CAM control.
Complex structures such as branch and stem structures were printed with human umbilical vein endothelial cells (HUVECs) and human umbilical vascular smooth muscle cells (HUVSMC) [94] using BioLP and the methods developed previously by Barron et al [73]. The large-scale stem structures were 15 mm in length and complete with multiple branches (3 mm in length). To maintain moisture during cell transfer a moat filled with water was constructed to surround the printing stage, but printing time was limited to ~10 min due to substrate drying. Each cell type, HUVEC and HUVSMC, was printed separately onto Matrigel® with success. More interestingly, a co-culture was created by printing HUVSMCs into the stem/branch structure, on top of a stem/branch structure of HUVECs that had been printed 1 day earlier. The construction of larger scale structures that incorporate two different cell types is an important step in laser direct writing.
2.2.5. LG DW
LG DW was used to pattern embryonic chick spinal cord cells by suspending the cells at a low density in culture medium and then using a 800 nm wavelength laser to propel them to an untreated glass growth surface [83]. To increase the distance from the cell suspension to the glass growth surface from ~300 μm up to 7 mm, the cells were guided through hollow optical fibers. Even with the long exposures to laser irradiation that is required for cell guidance to the growth surface, the cells remained viable. Using a similar method, multipotent adult progenitor cells (MAPCs) were patterned into two-dimensional lines on a substrate, using a laser operating at a wavelength of 830 nm, with power of 200 mW, and a 5 μm focused beam. It was estimated that the cells experienced an axial force of 10 pN in this process and moved with an average velocity of 88 μm s−1 using Stokes law for a rigid sphere [87].
Nahmais et al [81] developed a protocol to apply LG DW for the construction of hepatic endothelial sinusoid-like structures. HUVECs were patterned from a culture medium and 40% Percoll solution into horizontal lines onto Matrigel® . Hepatocytes were then seeded on top of the pattern to create the co-cultures that began to resemble liver-sinusoid like structures [84]. However, due to the low refractive index of the HUVECs resulting in a small optical force, polystyrene micro-beads (4.5 μm diameter) that were coated with either VEGF, collagen or laminin had to be added in order to adhere to the cells so that they could be laser guided and propelled to the growth surface. While the beads end up in the patterned culture, they were released from the cells at a rate of 10% per day, and were found to have no negative effects on the cell culture. A three-dimensional structure was constructed by patterning HUVECs onto collagen, a collagen gel was layered on top of the first pattern and then a second pattern of HUVEC was added onto the final collagen gel layer.
Embryonic chick forebrain neurons were patterned with laser guidance (800 nm wavelength) using a semi-automatic computer-guided system and incubated chamber [85]. The cells were suspended in culture medium and the user then centers the laser on a floating cell, pushing it in the Z direction to the correct X and Y coordinates. A visual feedback loop was used to ensure proper guidance. Cell guidance times ranged from 10–30 s/cell up to 120 s/cell; however, cell viability and morphology (as determined by neurite outgrowth) were unaltered. The chick forebrain neurons were also analyzed for 800 nm laser exposure at 100 mW and 300 mW for 10 s and 60 s, respectively, and the viability and neurite outgrowth were not significantly different from controls [86].
A wide range of cell types has been laser direct written into precise locations displaying the potential for a variety of biomedical applications (table 1). Laser direct writing has unique advantages over the traditional cell patterning techniques. It is a rapid transfer technique that is customizable in terms of patterns (figure 6), cell types and application. Furthermore, the optical process allows for real-time verification of cellular transfer and specific cell targeting. Many of the direct writing techniques are able to employ CAD/CAM and have single cell or near-single cell resolution. The cell transfer can be performed to a homogeneous growth surface to ensure cellular proliferation is controlled by normal cell–cell interactions. Laser-based direct writing also enables the creation of precise patterns of cells to form co- and multi-cultures. Patterns can be created in three dimensions, layer-by-layer, either by repeated printing of cells on a single spot or by adding layers of matrix. Despite the fact that either UV or IR lasers are used in direct writing, and the printed cell is physically transferred from a ribbon, across a gap, to a receiving substrate, the cell viability post-transfer is near 100% depending on laser fluences. Various assays have shown little to no DNA damage present following cell transfer, as well as no significant elevation of HSP expression (indicative of a cell’s exposure to stress), following cell transfer.
Table 1.
Laser-based direct-write techniques and the various cell types that have been successfully printed with each technique, displaying the potential for many biomedical applications.
| Laser-based direct-write technique |
Cell types |
|---|---|
| LIFT | Fibroblasts (NIH3T3) [63] |
| HaCaT keratinocytes [63] | |
| Human mesenchymal stem cells [63] |
|
| Ovine endothelial cells [64] | |
| Ovine vascular smooth muscle-like cells [64] |
|
| Mouse embryonic stem cells [92] | |
| AFA-LIFT | Rat Schwann cells [68] |
| Astroglial cells [68] | |
| Pig lens epithelial cells [68] | |
| MAPLE DW | Human osteosarcoma (MG-63) [78, 80] |
| Pluripotent embroyonal carcinoma (P19) [88] |
|
| Mouse myoblast (C2C12) [90] | |
| Human dermal fibroblasts [90] | |
| Bovine pulmonary artery endothelial cells (BPAEC) [90] |
|
| Breast carcinoma (MCF-7) [90] | |
| Rat neural stem cells [90] | |
| Tumor-derived neuronal cells (B35) [77, 89] |
|
| Rat cardiac cells [78] | |
| Human colon cancer cells (HT-29) [100] |
|
| Chinese hamster ovary cells (CHOs) [91] | |
| BioLP | Human osteosarcoma (MG-63) [70, 72, 73] |
| Olfactory ensheathing cells [76] | |
| Mouse endothelial cells (EOMA GFP) [73] |
|
| Endothelial (EA.hy926) [93] | |
| Human umbilical-vein endothelial cells (HUVEC) [94] |
|
| Human umbilical-vein smooth muscle cells (HUVSMC) [94] |
|
| Bovine pulmonary aorta endothelial (BAEC) [75] |
|
| LG DW | Embryonic chick forebrain neurons [85, 86] |
| Embryonic chick spinal cord cells [83] | |
| Human umbilical-vein endothelial cells (HUVEC) [81, 84] |
|
| Multipotent adult progenitor cells (MAPC) [87] |
Figure 6.

A2 × 3 array of human dermal fibroblast cells (a) immediately following laser direct writing, and (b) at 30 min after transfer, displaying maintained pattern registry and viability as cells begin to attach and spread.
However, the current state of laser direct writing for cell printing appears to be still in the development and verification stage. These techniques are extremely successful at patterning a wide variety of cell types, and show great promise for applications that require cellular spatial control, such as creating precise co-culture and multi-cultures, and building cellular biological constructs. Great efforts have been made thus far in the development of the direct-writing technology, and the demonstration of these various techniques to transfer living cells. As these techniques begin to transition from demonstration to application, we envision that the spatial control offered will have a great influence on a number of areas of research, particularly in cancer research, tissue engineering and regenerative medicine.
3. Similarity in methods of laser direct writing
The aforementioned laser-based direct-write methodologies share some procedural similarities that help in their reproducibility and repeatability. Many have applied thin film processing techniques, such as spin coating and sputter coating, to achieve uniform surfaces on both the ribbon and receiving substrate. Additionally, most of the direct-write methods have incorporated motorized and computer-controlled stages into the process, either to position the print ribbon, the receiving substrate or both. Not only does this lead to improved speed, such positional control makes the direct-write process more precise and repeatable. Furthermore, independent computer control of both the ribbon and substrate stages allows for greater efficiency in ribbon usage, the specification of both target cell and print location, and also provides the framework for automated image-based cell depositions.
All these direct-write techniques, LIFT, AFA-LIFT, BioLP, MAPLE DW and LG DW, have utilized the commercially available biopolymer, Matrigel® , either on the ribbon, on the receiving substrate or both. On the print ribbon, Matrigel® is used to facilitate cell adhesion to the ribbon by providing a layer on which cells can spread and attach, whereas on the receiving substrate it provides a viscoelastic matrix which dissipates the energy of transfer and acts as a long-term growth surface for printed cells. Matrigel® is also a valuable tool for the creation of three-dimensional constructs.
Although proven to be a widely used and effective growth surface, Matrigel® possesses some disadvantages that may limit its usefulness for precise cell cultures and other applications of laser direct writing. Matrigel® contains a number of growth factor constituents that may interfere with many of the cellular processes under investigation. Furthermore, it also contains a variety of proteins and ECM components; the presence and concentrations of which may not be prescribed for a particular cellular direct-write application or function. For these reasons, the use of Matrigel® may preclude or greatly limit the utility of laser direct writing for precise cell cultures [95]. Therefore, the ability to pattern cells and have precise control over growth substrate constituents, without additional or extraneous growth factors or proteins, is essential for more in-depth studies of cell proliferation, differentiation, cellular growth factor secretion and protein production. Any material added to the ribbon or the receiving substrate in the direct writing process must be evaluated for efficacy, not only to ensure effective transfer and cell viability, but also to confirm that it does not impart any unforeseen affect on the cells.
Laser-based approaches have many advantages that have been outlined above, but when compared to other approaches to direct-write biomaterials they also present challenges which need to be addressed and some inherent disadvantages. Unlike conventional printing approaches like inkjet or photolithography, laser-based approaches (LIFT, AFA-LIFT, BioLP and MAPLE DW) require ribbon fabrication. This presents a challenge in terms of the laser absorption and transfer dynamics as well as the proximity and manipulation of the ribbon with respect to the receiving substrate. Moreover, for conformal coatings on non-planar substrates, especially concave surfaces, the manipulation of a ribbon and receiving substrate can become onerous, if not impossible. While past work has demonstrated no damage to single- and double-stranded DNA [62, 86], the use of intense laser radiation will always be challenged in terms of photonic cell damage. In an effort to further limit potential UV laser radiation, LIFT, AFA-LIFT and BioLP all apply an intermediate thin film layer (Au, Ag, Ti, TiO2) on the print ribbon. However, this may have other unintended consequences. Both metallic nanoparticles (100–700 nm) and even microparticles (up to 15 μm) were found on the growth surface following a simulated cell transfer from a ribbon using a UV laser (248 nm wavelength) that was focused onto a silver intermediate thin film [66]. Nanoparticles may be cytotoxic at certain concentrations and have cellular affects that are not yet fully characterized [96]. This cytotoxic potential may necessitate careful rinsing of the growth surface and monitoring of the construct post-laser transfer to ensure the removal of all nanoparticles. Moreover, non-transparent metal films will preclude imaging of cells on the ribbon before deposition, thus making targeting specific cells impossible.
Unlike the aforementioned conventional approaches, the cell transfer process presents an extreme kinematic profile, though over a short time [66]. The dynamic range of deposition does extend to single cell transport, but the other extreme (depositing macroscopic amounts of material) may limit long-range applications in large constructive fabrication of tissues and organs, complex wound repair and regenerative medicine. Electronic materials deposition was demonstrated at speeds of 1 m s−1 with high repetition rate lasers (300 000 Hz using a tripled Nd:YVO4 laser) [97], but even before reaching this extreme, the inertia of receiving substrate motion prevents complex material designs. Lastly, there is one challenge that cellular direct-writing approaches, in general, face in their application to building scaffold-free cellular constructs, namely the ability to provide accelerated tissue maturation [98, 99]. While direct-write approaches assemble tissue constructs using cells as the building blocks, as opposed to growing them in scaffolds, the maturation of a cellular construct into a bioactive tissue must be rapid (hours to days depending on the complexity of the extracellular environment required) without the structural support of a scaffold. The lack of an initial support structure in a cell-based construct makes the application of mechanical stimulation (e.g., pressure, compression, tension, fluid flow), used as a cue for tissue development and maturation, a unique challenge and opportunity.
4. Process dynamics in laser cell direct writing
Biofabrication process-induced injury to cells, especially fragile mammalian cells, still poses a significant challenge to achieve satisfactory post-transfer cell viability [100, 101]. For example, cell injury may occur during the cell droplet ejection from supporting media (such as the printer orifice in inkjet printing and the quartz support in MAPLE DW), the travel through an air gap and the subsequent impact/collision with the receiving substrate during cell droplet landing. It was found that the post-transfer cell viability depends on the cell droplet ejection speed and the coating thickness of the receiving substrate in MAPLE DW [88]. High-speed imaging showed that the velocities of MAPLE DW-ejected material could range from 50 to 1000 m s−1 [67, 102]. The transferred cells may not be viable if the impact between the cell and the receiving culture coating/substrate, during the cell droplet landing, leads to cell impact-induced stress resulting in membrane rupture. For laser-based cell transfer to be a viable technology, process dynamics and process-induced cell injury must be understood and prevented. We can employ modeling techniques to gain insight into the process and mechanical concerns of laser direct writing. The whole laser-based cell transfer process can be modeled as three sequential events: cell droplet formation, cell droplet travel in air and cell droplet landing. While the modeling of these events can be pursued for all the aforementioned laser direct-write techniques, in this review we focus our modeling discussion on MAPLE DW. The two main events, cell droplet formation and landing, are discussed in detail in the following sections, and the process-induced cell injury is also commented.
4.1. Modeling of bubble expansion dynamics during cell droplet formation
A cell droplet is formed as the result of bubble expansion on the print ribbon. A schematic of the laser-induced bubble formation and expansion process in a typical laser-assisted cell direct-write setup (MAPLE DW herein) can be seen in figure 7. While the MAPLE DW schematic is shown here, the proposed modeling approach is still applicable to the other aforementioned sacrificial energy absorbing layer-based processes by assuming that the energy conversion thickness (usually less than 100 nm) is negligible. During the bubble expansion process, after the bubble is formed, a high-pressure shock wave is generated, which interacts with the cells inside the cell suspension coating.
Figure 7.
(a) Laser direct writing schematic and (b) modeling domain for the bubble expansion-induced cell deformation [103] (not to scale).
Bubble expansion and formation can be modeled using a computational domain (figure 7(b)). There are four materials: the vaporized bubble gas, air, the hydrogel (coating material herein) and the cells. Typically, the cell is modeled as a solid-type material using a Lagrangian mesh for its straightforward and fast implementation, while the bubble, coating and air are modeled using Eulerian meshes to avoid any extreme element distortion of these materials during ejection. The cell/hydrogel interaction is modeled using the appropriate Euler/Lagrange coupling option to capture the viscosity effect within the cell boundary layer, and the interactions among the hydrogel, bubble gas and air are modeled by defining these materials in multi-material groupings.
The ejection velocity of the cell droplet is important to determine the cell viability during the subsequent cell droplet landing process. The ejection velocity plays an integral role in determining the initial velocity at which the cell droplet impacts the receiving substrate. The cell droplet ejection velocity should be well controlled to minimize cell injury during cell droplet landing. The cell center velocity evolution during the ejection process is seen in figure 8. The cell velocity oscillates initially, then smoothes out gradually and plateaus to a constant ejection velocity (107 m s−1 herein). The initial velocity oscillation is attributed to the elasticity of cell, implying a negative acceleration. Due to the compressibility of the hydrogel, there is a delay in the velocity response to the bubble expansion (figure 8). After about 2 μs, the cell droplet has a very weak connection with the coating and starts to disassociate from the hydrogel coating with a constant velocity.
Figure 8.

Evolution of cell center velocity [103].
Wang et al [103] found that (1) the cell can first accelerate to as high as 109 m s−2 at the beginning period of bubble expansion and then quickly approaches zero in an oscillatory manner; fortunately, this high acceleration period only lasts for a very short period (about 0.1 μs) and (2) the pressure that cells experience can be very high at the beginning period of bubble expansion and quickly decreases to zero in an oscillatory fashion, as seen from the cell acceleration evolution. The cell top surface region usually experiences the highest pressure level, followed by the bottom surface and the middle regions.
It should be pointed out that in addition to the bubble expansion-induced stress wave, the thermoelastic stress wave may also injure the transferred cells. Generally speaking, the pressure generated by the phase explosion-induced bubble expansion is usually one order of magnitude higher than that due to the thermoelastic effect [104, 105], and the effect of thermoelastic stress wave is usually negligible in predicting the droplet formation and ejection-induced mechanical profile.
4.2. Modeling of impact dynamics during cell droplet landing
During landing, cell droplets undergo significant deceleration and impact(s). However, the cells survive a much higher external force than they are able to withstand under steady state conditions (e.g. cell lysis typically occurs at 10 g). This landing process, and its impact-induced stress, can be modeled using the mass, momentum and energy conservation equations, respectively [106, 107]. These equations hold true for cells and both hydrogels of the droplet and the substrate coating. Besides boundary and initial conditions, proper material models, which include the equation of state, constitutive model and failure criteria, are also indispensable in solving these equations. The equation of state is used to define the corresponding functional relationship between pressure, density and internal energy. The constitutive model defines the stress dependence on related strain, the stain rate and temperature. In addition, a material model also generally includes a failure criterion to determine whether the material fails and loses its ability to support stress/strain.
Some representative simulation results of landing are presented in figure 9 when a 50 m s−1 cell droplet hits a rigid substrate coated with a 30 μm thick hydrogel. A cell droplet with a cell in the center is modeled using a mesh-free smooth particle hydrodynamic (SPH) method. It can be seen that there are two different impacts during the process under the specified conditions. The first impact is between the cell droplet and the hydrogel coating, and the second impact is between the cell and the rigid substrate after the cell passes through the coating following the first impact. As the landing process continues, the hydrogel-enclosed cell droplet gradually merges into the substrate coating. Before the cell immerses into the coating (figure 9(a)), it is the outside hydrogel enclosure that is subject to the majority of the impact-induced stress. This shows that the outside hydrogel enclosure of the cell plays an important role in alleviating the impact-induced stress to the cell by absorbing the strain energy. Around 0.16 μs later, the impact between the cell and the hydrogel coating occurs. After the cell is immersed in the coating (figure 9(b)), the outside hydrogel enclosure and the coating bear relatively lower stresses, although the cell experiences higher stresses.
Figure 9.
Simulated cell droplet landing process at (a) 5.9322 ns and (b) 2.4865 μs [107].
Through modeling studies [106, 107], it was found that: (1) the cell peripheral regions, especially the bottom peripheral region, usually experience a higher stress level than that of the inner regions. It indicates that the cell membrane is easily affected by the impact-induced mechanical injury during cell direct writing; (2) the cell mechanical loading profile and the post-transfer cell viability depend on the cell droplet initial velocity and the substrate coating thickness. Generally, a larger initial velocity poses a higher probability of cell injury, and a substrate coating can significantly relieve the cell mechanical injury severity; and (3) two important impact processes may occur during the cell droplet landing process after ejection: the first impact between the cell droplet and the substrate coating and the second impact between the cell and the substrate. It is assumed that the impact-induced cell injury depends on not only the magnitudes of stress, acceleration and/or shear strain but also the cell loading history. In fact, it is the collective momentum change of the cell over the whole impact duration, rather than peak values of stress, acceleration and/or strain, that is critical in determining the cell viability during laser direct writing.
4.3. Discussion on process-induced cell injury
Cell injury during MAPLE DW might be associated with both mechanical cell injury and biochemical injury (thermal injury and UV injury). The injury should be modeled through the intrinsic and extrinsic pathways, given process-induced stress and/or thermal information. The process-induced mechanical stress during MAPLE DW may come from two different processes: the cell droplet formation (acceleration) and the cell droplet landing on the receiving substrate (deceleration). During the droplet formation process, the rapid expansion of the high-pressure bubble accelerates the forming cell droplet. Such an acceleration can be as high as 105–109 g: the higher the laser fluence, the higher the pressure, resulting in a higher acceleration/velocity. The impact during the cell droplet landing process also brings a significantly higher deceleration to cells as simulated [106, 107], and the cell viability was found to be closely related to the thickness of the receiving substrate coating [88]. For most cases, the dependence of cell viability on the laser fluence has indicated the existence of mechanical injury, since higher laser fluence led to higher cell acceleration (or deceleration) and velocity, resulting in lower cell viability. It should be pointed out that it is due to acceleration (or deceleration)-induced normal/shear stress, strain and/or strain rate that injures cells being transferred over in less than 5 μs [107].
The process-induced thermal injury to cells or tissues might be of concern as the laser energy absorbed may cause the deactivation of enzymes, denaturation of proteins and vaporization/carbonization of cells. During the short laser pulse duration (such as 12 ns), the thermal penetration due to the Fourier heat conduction is only a few micrometers, as the whole cell droplet formation usually happens within a few microseconds. Compared to the typical coating thickness (around 100 μm), the possible heat-affected zone and the resulting thermal injury are considered negligible.
UV radiation from laser pulses can injure living cells and induce DNA damage. Fortunately, the cellular support material on the ribbon (a water-based biological solution in this study) absorbs a majority of the UV energy [100]. In addition, the UV light in MAPLE DW is constrained inside a confined volume at the quartz ribbon and the coating interface. For a 100 μm thick coating, approximately 5% of coating might be affected by UV radiation (193 nm), resulting in very limited UV radiation-related injury to cells [100]. Furthermore, comet assays of DNA damage showed that UV injury was not detected within the limits of the assay for MAPLE DW [88], and laser micro-dissection and laser pressure catapulting [108]. Therefore, UV radiation-induced injury is also considered to be negligible.
During typical laser-assisted cell transfer, it is concluded: (1) the mechanical injury, which can be observed immediately, is mainly controlled by the laser fluence; and (2) the biochemical injury (e.g. DNA damage) might be controlled by thermal injury, UV injury and/or mechanical injury-induced secondary injury. In addition, the post-transfer cell proliferation process may also involve the recovery of some quiescent cells. Reversible cell injury is generally quantified as functional and morphologic changes that are reversible if the damaging stress is removed. More specific analyses of cellular changes before and after the process, as well as tracking of quiescent cells, should be conducted to identify these quiescent cells.
5. Conclusions and future directions
Laser-based cellular printing techniques have displayed their accuracy, robustness and efficiency for patterning multiple cell types, with wide-ranging applications. Modeling and in vitro studies of post-transfer cells have shown that laser-based direct-write techniques do not hinder the cells’ ability to proliferate, migrate and differentiate and show almost no signs of trauma post-transfer on the molecular, genetic or cellular level.
With these most recent advances in the methodologies and years of development, laser-based direct-write techniques can now be used for investigating spatially regulated cellular interactions, cancer development and progression, tissue morphogenesis and regeneration, stem cell maintenance and differentiation, biomaterial discovery, drug screening and toxicity testing. Laser direct writing is an unrivaled platform for creating precise cellular co-cultures, multi-cultures and biological or synthetic cellular constructs. By utilizing different ribbons, numerous heterogeneous cell types can be written in relation to each other, with great precision. The optical nature of laser direct-write techniques affords the opportunity to incorporate machine vision, and to employ optically selective cell targeting and transfer. Additionally, application of rapid prototype technology, such as CAD/CAM processing and automation, can enable high-throughput fabrication and make laser direct writing a more prolific method for precisely patterning cells.
Laser direct-write techniques are extremely successful at printing viable cells and we look forward to applying this technology, which has been long in development, to investigate fundamental cellular questions regarding spatial and geometric locations and the bottom-up building of cellular constructs.
Acknowledgments
DTC, YX and DBC would like to thank the National Institutes of Health for support (1R56DK088217-01). YH thanks the support from the National Textile Center and the National Science Foundation (CMMI-CAREER 0747959) and the assistance of Yafu Lin and Wei Wang of Clemson University.
References
- [1].Nirmalanandhan VS, et al. Combined effects of scaffold stiffening and mechanical preconditioning cycles on construct biomechanics, gene expression, and tendon repair biomechanics. Tissue Eng. 2009;15:2103–11. doi: 10.1089/ten.tea.2008.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nguyen TD, et al. Effects of cell seeding and cyclic stretch on the fiber remodeling in an extracellular matrix-derived bioscaffold. Tissue Eng. 2009;15:957–63. doi: 10.1089/ten.tea.2007.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Inui A, et al. Potency of double-layered poly L-lactic acid scaffold in tissue engineering of tendon tissue. Int. Orthop. 2009 doi: 10.1007/s00264-009-0917-8. doi:10.1007/s00264-009-0917-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sahoo S, Toh SL, Goh JC. A bFGF-releasing silk/PLGA-based biohybrid scaffold for ligament/tendon tissue engineering using mesenchymal progenitor cells. Biomaterials. 2010;31:2990–8. doi: 10.1016/j.biomaterials.2010.01.004. [DOI] [PubMed] [Google Scholar]
- [5].Ko HC, Milthorpe BK, McFarland CD. Engineering thick tissues—the vascularisation problem. Eur. Cell Mater. 2007;14:1–18. doi: 10.22203/ecm.v014a01. [DOI] [PubMed] [Google Scholar]
- [6].Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–55. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- [7].Watt FM, Hogan BLM. Out of Eden: stem cells and their niches. Science. 2000;287:1427–30. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- [8].Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- [9].Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–9. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [12].Engler AJ, Sweeney HL, Discher DE, Schwarzbauer JE. Extracellular matrix elasticity directs stem cell differentiation. J. Musculoskelet. Neuronal. Interact. 2007;7:335. [PubMed] [Google Scholar]
- [13].Even-Ram S, Artym V, Yamada KM. Matrix control of stem cell fate. Cell. 2006;126:645–7. doi: 10.1016/j.cell.2006.08.008. [DOI] [PubMed] [Google Scholar]
- [14].Hui EE, Bhatia SN. Micromechanical control of cell–cell interactions. Proc. Natl Acad. Sci. USA. 2007;104:5722–6. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dawson E, Mapili G, Erickson K, Taqvi S, Roy K. Biomaterials for stem cell differentiation. Adv. Drug Deliv. Rev. 2008;60:215–28. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- [16].Chai C, Leong KW. Biomaterials approach to expand and direct differentiation of stem cells. Mol. Ther. 2007;15:467–80. doi: 10.1038/sj.mt.6300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv. Drug Deliv. Rev. 2008;60:199–214. doi: 10.1016/j.addr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ferreira L, Squier T, Park H, Choe H, Kohane DS, Langer R. Human embryoid bodies containing nano- and microparticulate delivery vehicles. Adv. Mater. 2008;20:2285–91. [Google Scholar]
- [19].Lutolf MP, Blau HM. Artificial stem cell niches. Adv. Mater. 2009;21:3255–68. doi: 10.1002/adma.200802582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Park J, et al. Microfabrication-based modulation of embryonic stem cell differentiation. Lab Chip. 2007;7:1018–28. doi: 10.1039/b704739h. [DOI] [PubMed] [Google Scholar]
- [21].Metallo CM, Mohr JC, Detzel CJ, de Pablo JJ, Van Wie BJ, Palecek SP. Engineering the stem cell microenvironment. Biotechnol. Prog. 2007;23:18–23. doi: 10.1021/bp060350a. [DOI] [PubMed] [Google Scholar]
- [22].Dellatore SM, Garcia AS, Miller WM. Mimicking stem cell niches to increase stem cell expansion. Curr. Opin. Biotechnol. 2008;19:534–40. doi: 10.1016/j.copbio.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ruiz SA, Chen CS. Emergence of patterned stem cell differentiation within multicellular structures. Stem Cells. 2008;26:2921–7. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yim EK, Darling EM, Kulangara K, Guilak F, Leong KW. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials. 2009;31:1299–306. doi: 10.1016/j.biomaterials.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gerecht S, Bettinger CJ, Zhang Z, Borenstein JT, Vuniak-Novakovic G, Langer R. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials. 2007;28:4068–77. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–10. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- [27].Li WJ, et al. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599–609. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- [28].Nur EKA, Ahmed I, Kamal J, Schindler M, Meiners S. Three-dimensional nanofibrillar surfaces promote self-renewal in mouse embryonic stem cells. Stem Cells. 2006;24:426–33. doi: 10.1634/stemcells.2005-0170. [DOI] [PubMed] [Google Scholar]
- [29].Kang XH, et al. Adipogenesis of murine embryonic stem cells in a three-dimensional culture system using electrospun polymer scaffolds. Biomaterials. 2007;28:450–8. doi: 10.1016/j.biomaterials.2006.08.052. [DOI] [PubMed] [Google Scholar]
- [30].Xie JW, et al. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials. 2009;30:354–62. doi: 10.1016/j.biomaterials.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Oh S, et al. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl Acad. Sci. USA. 2009;106:2130–5. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sridharan I, Kim T, Wang R. Adapting collagen/CNT matrix in directing hESC differentiation. Biochem. Biophys. Res. Commun. 2009;381:508–12. doi: 10.1016/j.bbrc.2009.02.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chao TI, et al. Carbon nanotubes promote neuron differentiation from human embryonic stem cells. Biochem. Biophys. Res. Commun. 2009;384:426–30. doi: 10.1016/j.bbrc.2009.04.157. [DOI] [PubMed] [Google Scholar]
- [34].Rosenthal A, Macdonald A, Voldman J. Cell patterning chip for controlling the stem cell microenvironment. Biomaterials. 2007;28:3208–16. doi: 10.1016/j.biomaterials.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lii J, Hsu WJ, Parsa H, Das A, Rouse R, Sia SK. Real-time microfluidic system for studying mammalian cells in 3D microenvironments. Anal. Chem. 2008;80:3640–7. doi: 10.1021/ac8000034. [DOI] [PubMed] [Google Scholar]
- [36].Torisawa YS, et al. Efficient formation of uniform-sized embryoid bodies using a compartmentalized microchannel device. Lab Chip. 2007;7:770–6. doi: 10.1039/b618439a. [DOI] [PubMed] [Google Scholar]
- [37].Karp JM, et al. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip. 2007;7:786–94. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- [38].Torisawa YS, Mosadegh B, Luker GD, Morell M, O’Shea KS, Takayama S. Microfluidic hydrodynamic cellular patterning for systematic formation of co-culture spheroids. Integr. Biol. 2009;1:649–54. doi: 10.1039/b915965g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hurtley S. Location, location, location introduction. Science. 2009;326:1205–5. doi: 10.1126/science.326.5957.1205. [DOI] [PubMed] [Google Scholar]
- [40].Chang HY. Anatomic demarcation of cells: genes to patterns. Science. 2009;326:1206–7. doi: 10.1126/science.1175686. [DOI] [PubMed] [Google Scholar]
- [41].Vunjak-Novakovic G. Patterning stem cell differentiation. Cell Stem Cell. 2008;3:362–3. doi: 10.1016/j.stem.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–41. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cheng LZ, Hammond H, Ye ZH, Zhan XC, Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131–42. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- [44].Heneweer M, Muusse M, Dingemans M, de Jong PC, Van Den Berg M, Sanderson JT. Co-culture of primary human mammary fibroblasts and MCF-7 cells as an in vitro breast cancer model. Toxicol. Sci. 2005;83:257–63. doi: 10.1093/toxsci/kfi025. [DOI] [PubMed] [Google Scholar]
- [45].Kohn J, Welsh WJ, Knight D. A new approach to the rationale discovery of polymeric biomaterials. Biomaterials. 2007;28:4171–7. doi: 10.1016/j.biomaterials.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Simon CG, Jr, Stephens JS, Dorsey SM, Becker ML. Fabrication of combinatorial polymer scaffold libraries. Rev. Sci. Instrum. 2007;78:072207. doi: 10.1063/1.2755761. [DOI] [PubMed] [Google Scholar]
- [47].Peters A, Brey DM, Burdick JA. High-throughput and combinatorial technologies for tissue engineering applications. Tissue Eng. 2009;15:225–39. doi: 10.1089/ten.TEB.2009.0049. [DOI] [PubMed] [Google Scholar]
- [48].Pique A, Chrisey DB, Auyeung RCY, Fitz-Gerald J, Wu HD, McGill RA, Lakeou S, Wu PK, Nguyen V, Duignan M. A novel laser transfer process for direct writing of electronic and sensor materials. Appl. Phys. 1999;69:5279–84. [Google Scholar]
- [49].Fitz-Gerald JM, et al. Matrix assisted pulsed laser evaporation direct write (MAPLE DW): a new method to rapidly prototype active and passive electronic circuit elements. Mat. Res. Soc. Proc. 2000;625:99–110. [Google Scholar]
- [50].Chrisey DB, et al. New approach to laser direct writing active and passive mesoscopic circuit elements. Appl. Surf. Sci. 2000;154:593–600. [Google Scholar]
- [51].Zergioti I, Mailis S, Vainos NA, Fotakis C, Chen S, Grigoropoulos CP. Microdeposition of metals by femtosecond excimer laser. Appl. Surf. Sci. 1998;127:601–5. [Google Scholar]
- [52].Fogarassy E, Fuchs C, Kerherve F, Hauchecorne G, Perriere J. Laser-induced forward transfer—a new approach for the deposition of high-Tc superconducting thin-films. J. Mater. Res. 1989;4:1082–86. [Google Scholar]
- [53].Zinchenko YS, Coger RN. Engineering micropatterned surfaces for the coculture of hepatocytes and Kupffer cells. J. Biomed. Mater. Res. 2005;75:242–48. doi: 10.1002/jbm.a.30399. [DOI] [PubMed] [Google Scholar]
- [54].Folch A, Jo BH, Hurtado O, Beebe DJ, Toner M. Microfabricated elastomeric stencils for micropatterning cell cultures. J. Biomed. Mater. Res. 2000;52:346–53. doi: 10.1002/1097-4636(200011)52:2<346::aid-jbm14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [55].Rohr S, Fluckiger-Labrada R, Kucera JP. Photolithographically defined deposition of attachment factors as a versatile method for patterning the growth of different cell types in culture. Pflugers Arch.—Eur. J. Physiol. 2003;446:125–32. doi: 10.1007/s00424-002-1000-0. [DOI] [PubMed] [Google Scholar]
- [56].Salaita K, Wang Y, Mirkin CA. Applications of dip-pen nanolithography. Nat. Nanotechnol. 2007;2:145–55. doi: 10.1038/nnano.2007.39. [DOI] [PubMed] [Google Scholar]
- [57].Roth EA, Xu T, Das M, Gregory C, Hickman JJ, Boland T. Inkjet printing for high-throughput cell patterning. Biomaterials. 2004;25:3707–15. doi: 10.1016/j.biomaterials.2003.10.052. [DOI] [PubMed] [Google Scholar]
- [58].Ringeisen BR, Othon CM, Barron JA, Young D, Spargo BJ. Jet-based methods to print living cells. Biotechnol. J. 2006;1:930–48. doi: 10.1002/biot.200600058. [DOI] [PubMed] [Google Scholar]
- [59].Fernandez-Pradas JM, Colina M, Serra P, Dominguez J, Morenza JL. Laser-induced forward transfer of biomolecules. Thin Solid Films. 2004; – :27–30. [Google Scholar]
- [60].Dinca V, Farsari M, Kafetzopoulos D, Popescu A, Dinescu M, Fotakis C. Patterning parameters for biomolecules microarrays constructed with nanosecond and femtosecond UV lasers. Thin Solid Films. 2008;516:6504–11. [Google Scholar]
- [61].Duocastella M, Fernandez-Pradas JM, Serra P, Morenza JL. Laser-induced forward transfer of liquids for miniaturized biosensors preparation. J. Laser Micro Nanoeng. 2008;3:1–4. [Google Scholar]
- [62].Duocastella M, Colina M, Fernandez-Pradas JM, Serra P, Morenza JL. Study of the laser-induced forward transfer of liquids for laser bioprinting. Appl. Surf. Sci. 2007;253:7855–9. [Google Scholar]
- [63].Koch L, et al. Laser printing of skin cells and human stem cells. Tissue Eng. C Methods. 2009 doi: 10.1089/ten.TEC.2009.0397. [DOI] [PubMed] [Google Scholar]
- [64].Ovsianikov A, et al. Laser printing of cells into 3D scaffolds. Biofabrication. 2010;2:014104. doi: 10.1088/1758-5082/2/1/014104. [DOI] [PubMed] [Google Scholar]
- [65].Hopp B, Smausz T, Antal Z, Kresz N, Bor Z, Chrisey D. Absorbing film assisted laser induced forward transfer of fungi (Trichoderma conidia) J. Appl. Phys. 2004;96:3478–81. [Google Scholar]
- [66].Smausz T, Hopp B, Kecskemeti G, Bor Z. Study on metal microparticle content of the material transferred with absorbing film assisted laser induced forward transfer when using silver absorbing layer. Appl. Surf. Sci. 2006;252:4738–42. [Google Scholar]
- [67].Hopp B, et al. Time-resolved study of absorbing film assisted laser induced forward transfer of. Trichoderma longibrachiatum. J. Phys. D: Appl. Phys. 2005;38:833–7. [Google Scholar]
- [68].Hopp B, et al. Survival and proliferative ability of various living cell types after laser-induced forward transfer. Tissue Eng. 2005;11:1817–23. doi: 10.1089/ten.2005.11.1817. [DOI] [PubMed] [Google Scholar]
- [69].Barron JA, Young HD, Dlott DD, Darfler MM, Krizman DB, Ringeisen BR. Printing of protein microarrays via a capillary-free fluid jetting mechanism. Proteomics. 2005;5:4138–44. doi: 10.1002/pmic.200401294. [DOI] [PubMed] [Google Scholar]
- [70].Barron JA, Spargo BJ, Ringeisen BR. Biological laser printing of three dimensional cellular structures. Appl. Phys. 2004;79:1027–30. [Google Scholar]
- [71].Barron JA, Rosen R, Jones-Meehan J, Spargo BJ, Belkin S, Ringeisen BR. 2004 Biological laser printing of genetically modified Escherichia coli for biosensor applications. Biosens. Bioelectron. 20:246–52. doi: 10.1016/j.bios.2004.01.011. [DOI] [PubMed] [Google Scholar]
- [72].Barron JA, Krizman DB, Ringeisen BR. Laser printing of single cells: statistical analysis, cell viability, and stress. Ann. Biomed. Eng. 2005;33:121–30. doi: 10.1007/s10439-005-8971-x. [DOI] [PubMed] [Google Scholar]
- [73].Barron JA, Wu P, Ladouceur HD, Ringeisen BR. Biological laser printing: a novel technique for creating heterogeneous 3-dimensional cell patterns. Biomed. Microdevices. 2004;6:139–47. doi: 10.1023/b:bmmd.0000031751.67267.9f. [DOI] [PubMed] [Google Scholar]
- [74].Barron JA, Young HD, Ringeisen BR, Dlott DD, Krizman DB. Biological laser printing as an alternative to traditional protein arrayers. Proc. SPIE. 2005;5699:517–25. [Google Scholar]
- [75].Chen CY, Barron JA, Ringeisen BR. Cell patterning without chemical surface modification: cell-cell interactions between printed bovine aortic endothelial cells (BAEC) on a homogeneous cell-adherent hydrogel. Appl. Surf. Sci. 2006;252:8641–5. [Google Scholar]
- [76].Othon CM, Wu XJ, Anders JJ, Ringeisen BR. Single-cell printing to form three-dimensional lines of olfactory ensheathing cells. Biomed. Mater. 2008;3:034101. doi: 10.1088/1748-6041/3/3/034101. [DOI] [PubMed] [Google Scholar]
- [77].Patz TM, et al. Three-dimensional direct writing of B35 neuronal cells. J. Biomed. Mater. Res. 2006;78:124–30. doi: 10.1002/jbm.b.30473. [DOI] [PubMed] [Google Scholar]
- [78].Barron JA, Ringeisen BR, Kim HS, Spargo BJ, Chrisey DB. Application of laser printing to mammalian cells. Thin Solid Films. 2004; – :383–7. [Google Scholar]
- [79].Chrisey DB, et al. Laser deposition of polymer and biomaterial films. Chem. Rev. 2003;103:553–76. doi: 10.1021/cr010428w. [DOI] [PubMed] [Google Scholar]
- [80].Doraiswamy A, Narayan RJ, Harris ML, Qadri SB, Modi R, Chrisey DB. Laser microfabrication of hydroxyapatite-osteoblast-like cell composites. J. Biomed. Mater. Res. 2007;80:635–43. doi: 10.1002/jbm.a.30969. [DOI] [PubMed] [Google Scholar]
- [81].Nahmias Y, Odde DJ. Micropatterning of living cells by laser-guided direct writing: application to fabrication of hepatic endothelial sinusoid-like structures. Nat. Protoc. 2006;1:2288–96. doi: 10.1038/nprot.2006.386. [DOI] [PubMed] [Google Scholar]
- [82].Odde DJ, Renn MJ. Laser-guided direct writing for applications in biotechnology. Trends Biotechnol. 1999;17:385–9. doi: 10.1016/s0167-7799(99)01355-4. [DOI] [PubMed] [Google Scholar]
- [83].Odde DJ, Renn MJ. Laser-guided direct writing of living cells. Biotechnol. Bioeng. 2000;67:312–8. doi: 10.1002/(sici)1097-0290(20000205)67:3<312::aid-bit7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- [84].Nahmias Y, Schwartz RE, Verfaillie CM, Odde DJ. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnol. Bioeng. 2005;92:129–36. doi: 10.1002/bit.20585. [DOI] [PubMed] [Google Scholar]
- [85].Pirlo RK, Dean DM, Knapp DR, Gao BZ. Cell deposition system based on laser guidance. Biotechnol. J. 2006;1:1007–13. doi: 10.1002/biot.200600127. [DOI] [PubMed] [Google Scholar]
- [86].Rosenbalm TN, Owens S, Bakken D, Gao BZ. Cell viability test after laser guidance. Proc. SPIE. 2006;6084:8418–8. (article no 608418) [Google Scholar]
- [87].Nahmias YK, Gao BZ, Odde DJ. Dimensionless parameters for the design of optical traps and laser guidance systems. Appl. Opt. 2004;43:3999–4006. doi: 10.1364/ao.43.003999. [DOI] [PubMed] [Google Scholar]
- [88].Ringeisen BR, et al. Laser printing of pluripotent embryonal carcinoma cells. Tissue Eng. 2004;10:483–91. doi: 10.1089/107632704323061843. [DOI] [PubMed] [Google Scholar]
- [89].Doraiswamy A, et al. Excimer laser forward transfer of mammalian cells using a novel triazene absorbing layer. Appl. Surf. Sci. 2006;252:4743–7. [Google Scholar]
- [90].Schiele NR, et al. Laser direct writing of combinatorial libraries of idealized cellular constructs: biomedical applications. Appl. Surf. Sci. 2009;255:5444–7. [Google Scholar]
- [91].Wu PK, et al. The deposition, structure, pattern deposition, and activity of biomaterial thin-films by matrix-assisted pulsed-laser evaporation (MAPLE) and MAPLE direct write. Thin Solid Films. 2001;398:607–14. [Google Scholar]
- [92].Kattamis NT, Purnick PE, Weiss R, Arnold CB. Thick film laser induced forward transfer for deposition of thermally and mechanically sensitive materials. Appl. Phys. Lett. 2007;91:171120. [Google Scholar]
- [93].Guillemot F, et al. High-throughput laser printing of cells and biomaterials for tissue engineering. Acta Biomater. 2009;6:2494–500. doi: 10.1016/j.actbio.2009.09.029. [DOI] [PubMed] [Google Scholar]
- [94].Wu PK, Ringeisen BR. Development of human umbilical vein endothelial cell (HUVEC) and human umbilical vein smooth muscle cell (HUVSMC) branch/stem structures on hydrogel layers via biological laser printing (BioLP) Biofabrication. 2010;2:014111. doi: 10.1088/1758-5082/2/1/014111. [DOI] [PubMed] [Google Scholar]
- [95].Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res. 1992;202:1–8. doi: 10.1016/0014-4827(92)90397-q. [DOI] [PubMed] [Google Scholar]
- [96].Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- [97].Zhang CP, et al. The realization of a multi-layer band pass filter using laser direct-write techniques. Proc. SPIE. 2002;4828:23–8. [Google Scholar]
- [98].Mironov V, Prestwich G, Forgacs G. Bioprinting living structures. J. Mater. Chem. 2007;17:2054–60. [Google Scholar]
- [99].Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164–74. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lin YF, Huang G, Huang Y, Tzeng TRJ, Chrisey DB. Effect of laser fluence in laser-assisted direct writing of human colon cell. Rapid Protoyping J. 2010;16:202–8. [Google Scholar]
- [101].Lin YF, Huang Y, Wang GY, Tzeng TRJ, Chrisey DB. Effect of laser fluence on yeast cell viability in laser-assisted cell transfer. J. Appl. Phys. 2009;106:043106. [Google Scholar]
- [102].Young D, Auyeung RCY, Pique A, Chrisey DB, Dlott DD. Time-resolved optical microscopy of a laser-based forward transfer process. Appl. Phys. Lett. 2001;78:3169–71. [Google Scholar]
- [103].Wang W, Li G, Huang Y. Modeling of bubble expansion-induced cell mechanical profile in laser-assisted cell direct writing. Trans. ASME, J. Manuf. Sci. Eng. 2009;131:051013. [Google Scholar]
- [104].Sigrist MW, Kneubuhl FK. Laser-generated stress waves in liquids. J. Acoust. Soc. Am. 1978;64:1652–63. [Google Scholar]
- [105].Park HK, Kim D, Grigoropoulos CP, Tam AC. Pressure generation and measurement in the rapid vaporization of water on a pulsed-laser-heated surface. J. Appl. Phys. 1996;80:4072–81. [Google Scholar]
- [106].Wang W, Huang Y, Chrisey DB. Numerical study of cell droplet and hydrogel coating impact process in cell direct writing. Trans. NAMRI/SME. 2007;35:217–24. [Google Scholar]
- [107].Wang W, Huang Y, Grujicic M, Chrisey DB. Study of impact-induced mechanical effects in cell direct writing using smooth particle hydrodynamic method. Trans. ASME, J. Manuf. Sci. Eng. 2008;130:021012. [Google Scholar]
- [108].Vogel A, Lorenz K, Horneffer V, Huttmann G, von Smolinski SD, Gebert A. Mechanisms of laser-induced dissection and transport of histologic specimens. Biophys. J. 2007;93:4481–500. doi: 10.1529/biophysj.106.102277. [DOI] [PMC free article] [PubMed] [Google Scholar]




