SUMMARY
The molecular chaperone Hsp90 protects deregulated signaling proteins that are vital for tumor growth and survival. Tumors generally display sensitivity and selectivity toward Hsp90 inhibitors; however, the molecular mechanism underlying this phenotype remains undefined. We report that the mitotic checkpoint kinase Mps1 phosphorylates a conserved threonine residue in the amino-domain of Hsp90. This, in turn, regulates chaperone function by reducing Hsp90 ATPase activity while fostering Hsp90 association with kinase clients, including Mps1. Phosphorylation of Hsp90 is also essential for the mitotic checkpoint because it confers Mps1 stability and activity. We identified Cdc14 as the phosphatase that dephosphorylates Hsp90 and disrupts its interaction with Mps1. This causes Mps1 degradation, thus providing a mechanism for its inactivation. Finally, Hsp90 phosphorylation sensitizes cells to its inhibitors, and elevated Mps1 levels confer renal cell carcinoma selectivity to Hsp90 drugs. Mps1 expression level can potentially serve as a predictive indicator of tumor response to Hsp90 inhibitors.
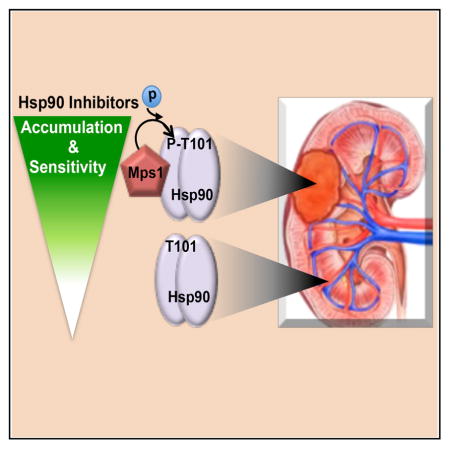
Graphical abstract
INTRODUCTION
Heat shock protein-90 (Hsp90) is an essential molecular chaperone in eukaryotes, and it is involved in the maturation, protection, and activation of a group of proteins referred to as “clients,” (see the website maintained by D. Picard; https://www.picard.ch/downloads/Hsp90interactors.pdf) (Picard, 2002; Röhl et al., 2013; Taipale et al., 2010). Hsp90 clients are enriched in signal transducers, including protein kinases and transcription factors. Hsp90 and a distinct set of co-chaperone proteins such as Cdc37 “hold” these clients in a stabilized state in which they can respond to activating signals (Röhl et al., 2013; Taipale et al., 2010). Hsp90 chaperone activity is coupled to its ATPase activity (Panaretou et al., 1998), which is tightly regulated by co-chaperone proteins and post-translational modifications (PTMs) such as phosphorylation, acetylation, ubiquitination, and SUMOylation (covered in detail in a recent review; Walton-Diaz et al., 2013).
Cancer cells rely on the Hsp90 chaperone machinery to protect an array of mutated and overexpressed oncoproteins from misfolding and degradation. Thus, Hsp90 is a critical facilitator of “oncogene addiction” and cancer cell survival. Emerging clinical data identify Hsp90 inhibition as a promising therapeutic strategy to treat cancer (Neckers and Trepel, 2014). Cancer cells appear to be particularly sensitive to Hsp90 inhibitors compared to non-transformed cells (Chiosis and Neckers, 2006), and Hsp90 inhibitors are retained by tumors in vivo far longer than in normal tissues (Kamal et al., 2003). However, the molecular basis of these phenomena remains undefined.
The mitotic checkpoint, or mitotic spindle assembly checkpoint prevents missegregation of chromosomes by arresting cells in metaphase until all chromosomes are properly aligned. The evolutionarily conserved dual specificity protein kinase, Mps1, is required for this process, as was recently reviewed in detail (Liu and Winey, 2012). High expression and PTM of Mps1 are involved in its activation, whereas the major route of Mps1 inactivation is degradation (Liu and Winey, 2012). Overexpression of Mps1 also causes abnormal chromosome segregation during mitosis, i.e., aneuploidy, a hallmark of cancers associated with high risk for tumorigenesis. (Kops et al., 2005; Musacchio and Salmon, 2007). High levels of Mps1 kinase are found in colon cancer tissues and several tumor cell lines such as U937, HeLa, HEY, OCC1, Bewo, T987, and SW480 (Ling et al., 2014; Yen and Kao, 2005).
In the present study, we found that Mps1 is highly expressed in tumors from patients with renal cell carcinoma (RCC), including clear cell RCC (ccRCC), papillary type I and type II RCC, oncocytoma, and angiomyolipoma (AML), compared to adjacent normal tissue. Accumulation of the Hsp90 inhibitor ganetespib (GB) in tumors from RCC patients prompted us to ask whether Mps1-mediated phosphorylation and regulation of Hsp90 chaperone function is the molecular basis of tumor sensitivity and selectivity to Hsp90 inhibitors.
RESULTS
Mps1 Phosphorylates a Conserved Threonine Residue of Yeast and Human Hsp90
Hsp90 is a post-translationally modified protein, (reviewed in Walton-Diaz et al., 2013). Here, we show the phosphorylation of T101 in the N-domain of yeast Hsp90 (yHsp90) by using an assay that we have reported previously (Mollapour et al., 2011; see Experimental Procedures) (Figures 1A and 1B). T101 was mutated to a non-phosphorylatable alanine in yHsp90 that contained a PreScission protease cleavage site between the N-domain and the adjacent charged linker (Figure 1A). The wild-type (WT) yHsp90 and T101A mutant were also hexahistidine-tagged at their N-domains and were expressed as the sole copies of yHsp90 in yeast. The yHsp90-His6 proteins were isolated from cell lysates using nickel-nitrilotriacetic acid (Ni-NTA) agarose and then treated with PreScission protease to isolate yHsp90 N-domains. This was confirmed by western blot analysis using anti-hexahistidine antibody. Using our previously established pan-anti-phospho-threonine antibody (Mollapour et al., 2011), we were able to observe the threonine phosphorylation of yHsp90 N-domain, and this signal was significantly reduced in T101A-yHsp90 mutant (Figure 1B). We have also previously shown the phosphorylation of T22 in the N-domain of yHsp90 (Mollapour et al., 2011). Mutation of both T22 and T101 to non-phosphorylatable alanine completely abolished the threonine phosphorylation of N-domain yHsp90 (Figure S1A). These data suggest that T22 and T101 are the only phospho-threonine sites in the yHsp90 N-domain.
Figure 1. Phosphorylation of a Conserved Threonine Residue in the N-Domain of Yeast and Human Hsp90.
(A) Pymol cartoon of the Hsp90 monomer without Sba1 (PDB: 2CG9). The cyan region represents the N-terminal domain (NTD), yellow represents the middle domain (MD), and orange represents the C-terminal domain (CTD). The PreScission protease region is highlighted in pink. Alignment of yeast and human Hsp90 showing the conservation of T101 and T115 amino acid residues.
(B and C) N-domain phosphorylation of (B) WT yHsp90-His6 (WT) and T101A-yHsp90-His6 (T101A) or (C) WT hHsp90α-FLAG (WT) and T115A-hHsp90α-FLAG (T115A) detected by immunoblotting with anti-hexahistidine for yHsp90 and anti-FLAG for hHsp90α and pan-anti-phosphothreonine antibodies after PreScission protease digestion. IP, immunoprecipitation; Phos-Thr, phospho-threonine.
(D) Yeast cells expressing WT yHsp90-His6 (WT) or T101A-yHsp90-His6 (T101A) and carrying yMPS1-MYC under GAL1-promoter were grown on raffinose (−) or galactose (+). N-domain threonine phosphorylation was assessed after pull-downs and after PreScission protease digestion and immunoblotting.
(E) yMps1 mediates threonine phosphorylation of WT yHsp90 and T101A-yHsp90 (T101A) mutant in vitro. Bacterially expressed and purified yHsp90-His6 and T101A mutant were used as substrates for yMps1. Threonine phosphorylation was detected by immunoblotting with pan-anti-phospho-threonine antibody. SE, short exposure; LE, long exposure.
(F) Bacterially expressed and purified hHsp90α-His6 and T115A mutant were used as substrates for hMps1. Threonine phosphorylation was detected by immunoblotting with pan-anti-phospho-threonine antibody.
See also Figures S1 and S2.
Next, the corresponding T101 (i.e., T115) was mutated to alanine in hHsp90α-FLAG. This mutant also contained a PreScission site between the N-domain and the charged linker. This allowed us to transiently express and isolate the N-domain of hHsp90α from HEK293 cells and detect phosphorylation of the T115 site (Figure 1C). Previous work has shown that protein kinase C (PKC)γ targets T115 in hHsp90α (Lu et al., 2014). We showed that PKCγ phosphorylates hHsp90α or yHsp90 in vitro; however, we were unable to observe PKCγ-dependent phosphorylation of T115-hHsp90α or T101-yHsp90 (Figure S1B). Therefore, in an attempt to identify the kinase(s) targeting T101/T115, we used the yeast deletion library for the non-essential kinases (110 strains) obtained from EUROSCARF (http://www.euroscarf.de/search.php?name=Order) (Figures S2A and S2B; Table S1). The yeast essential kinases library (19 kinases) under the control of the GAL1-promoter was obtained from Dharmacon (http://dharmacon.gelifesciences.com/). These libraries were transformed with either WT yHsp90 or T101A mutant (containing the PreScission site), and then the levels of N-domain phosphorylation were assessed by western blot analysis (Figures S2C and S2D). We found that only overexpression of the essential protein kinase Mps1 causes an increase in the phosphorylation of T101-yHsp90 (Figure 1D). Next, we used purified recombinant yeast Mps1 (yMps1) in an in vitro kinase assay with bacterially expressed and purified WT yHsp90 and T101A mutant. Our data showed that yMps1 phosphorylates only the T101 in yHsp90 in vitro (Figure 1E). Since Mps1 is an evolutionarily conserved protein kinase (Abrieu et al., 2001), we used the purified human Mps1 (hMps1), also known as PYT/TTK in an in vitro kinase assay. hMps1 phosphorylates only the T115 residue in hHsp90α in vitro (Figure 1F).
T101/T115 Phosphomutants Affect Hsp90 ATPase Activity and Co-chaperone Binding
The chaperone activity of Hsp90 is coupled to its ability to bind and hydrolyze ATP (Panaretou et al., 1998). Therefore, we expressed and isolated yHsp90 and the phosphomutants from yeast and determined their ATPase activity, using a small-scale PiPer Phosphate Assay (Thermo Fisher Scientific) as previously described (Kamal et al., 2003) (see Experimental Procedures). T101A had a similar (80%) ATPase activity compared to the WT yHsp90, whereas the T101E-yHsp90 ATPase was reduced by 62% (Figure S3A). Next, we expressed and purified yHsp90 and hHsp90α from bacteria and phosphorylated them in vitro using recombinant yMps1 and hMps1, respectively (Figures S3B and S3C). The ATPase activity of the phosphorylated and non-phosphorylated Hsp90 proteins were determined using the aforementioned method. Consistent with our earlier result, Mps1-mediated phosphorylation of Hsp90 reduced the ATPase activity of yHsp90 by 69% and of hHsp90α by 59% (Figures 2A and 2B; Figures S3D–S3F).
Figure 2. Impact of T101-yHsp90 and T115-hHsp90α Phosphorylation on ATPase Activity and Co-chaperones Binding.
(A) Relative ATPase activity of WT yHsp90-His6 and yMps1 mediated phospho-T101-yHsp90-His6. ATPase activity was inhibited by 10 μM GB. Error bars represent SD of three independent experiments. ****p < 0.0001.
(B) Relative ATPase activity of WT hHsp90α-His6 and hMps1 mediated phospho-T115-hHsp90α-His6. ATPase activity was inhibited by 10 μM GB. Error bars represent SD of three independent experiments. ****p < 0.0001.
(C) yHsp90 was isolated from yeast with empty plasmid (C), WT yHsp90-His6 (WT), T101A-yHsp90-His6 (T101A), and T101E-yHsp90-His6 (T101E). Interaction of the co-chaperones yAha1, Cdc37p50, Sba1p23, and Sti1Hop were detected by immunoblotting. Equivalent precipitation of yHsp90 was confirmed by blotting for hexahistidine.
(D) HEK293 cells were transfected with empty plasmid (C), hHsp90α-FLAG (WT), T115A-hHsp90α-FLAG (T115A), or T115E-hHsp90α-FLAG (T115E). Hsp90-FLAG was immunoprecipitated (IP) with anti-FLAG M2 affinity gel and interaction with hAha1 or p50cdc37, p23Sba1, or p60Hop was examined by immunoblotting.
Next, we examined the interaction of several important regulatory Hsp90 co-chaperones with T101-yHsp90 and T115-hHsp90α phosphomutants. Lysates of yeast expressing WT yHsp90, T101A, or T101E were subjected to affinity pull-down with Ni-NTA agarose, and interacting co-chaperones were examined by western blot analysis. The interaction of both mutants with yAha1 was abolished, interaction with Cdc37p50 was significantly enhanced, and interaction with Sti1HOP was unchanged (Figure 2C). In contrast, association of Sba1p23 with T101A was lost, but its interaction with T101E was slightly enhanced compared to the WT yHsp90 (Figure 2D). We also obtained similar results with mammalian co-chaperones. HEK293 cells were transiently transfected with FLAG-hHsp90α or its T115A or T115E mutants. WT hHsp90α and its mutants interacted with p60HOP with similar affinity, while the mutants bound slightly stronger to p50Cdc37 as compared to WT (Figure 2D). However, association of both mutants with human Aha1 (hAha1) was abolished, and T115A interaction with p23 was significantly reduced (Figure 2D). Taken together, our data suggest that phosphorylation of T101/T115 is a dynamic process that stabilizes N-domain dimerization and Sba1p23 association, which itself is known to slow the rate of ATP hydrolysis (Siligardi et al., 2004). It follows that dephosphorylation of T101/T115 is necessary to permit ATP hydrolysis.
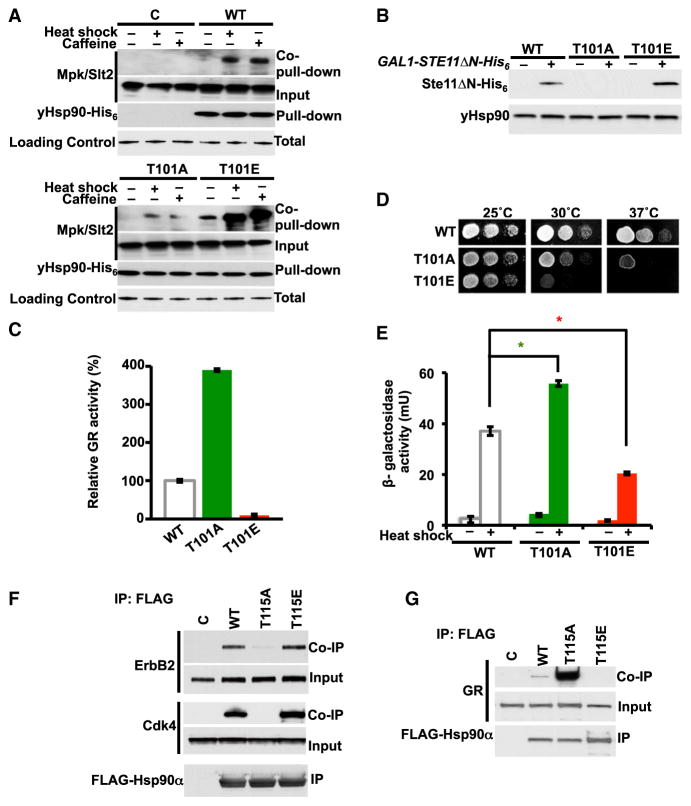
Phosphorylation of T101/T115 Selectively Impacts the Chaperoning of Clients
The importance of the T101 residue on chaperone activity was first reported 20 years ago (Nathan and Lindquist, 1995). Here, we tested the ability of T101-yHsp90 phosphomutants to chaperone known endogenous clients in yeast. The mitogen-activated protein kinase (MAPK) kinase Mpk1/Slt2 (an Erk5 ortholog) is an Hsp90 client. Activation of this kinase with heat shock or caffeine stress strengthens its interaction with yHsp90 (Truman et al., 2006). Mpk1/Slt2 from heat-shocked yeast cells (39°C for 40 min) or yeast stressed with 10 mM caffeine interacts with yHsp90. These stresses activate the cell wall integrity pathway in yeast. However, interaction of Mpk1/Slt2 with T101A-yHsp90 was significantly reduced (Figure 3A). Conversely T101E-yHsp90 associated with Mpk1/Slt2, even in the absence of stress (Figure 3A). We observed similar data with another endogenous yeast Hsp90 client, active Ste11 kinase (Ste11ΔN), the ortholog of mammalian Raf-1 (Flom et al., 2008; Louvion et al., 1998). The non-phosphorylatable T101A was unable to interact with the active Ste11 kinase, while association of the phosphomimic T101E mutant with active Ste11 was slightly enhanced compared to wild-type Hsp90 (Figure 3B).
Figure 3. Threonine Phosphorylation of the Hsp90 N-Domain Impacts the Chaperoning of Clients.
(A) Interaction of WT yHsp90-His6 (WT), T101A-yHsp90-His6 (T101A), and T101E-yHsp90-His6 (T101E) mutants with Mpk1/Slt2 was examined after yeast cells were stressed with 10 mM caffeine or heat shocked at 39°C for 60 min (activator of Mpk1/Slt2 MAP kinase). Empty plasmid was used as a control (C). yHsp90-His6 isolated by Ni-NTA and interaction with Mpk1/Slt2 were examined by immunoblotting.
(B) WT yHsp90-His6 (WT), T101A, and T101E yeast strains transformed with Ste11ΔN-His6 under GAL1-promoter were grown on raffinose (−) or galactose (+) media, and Ste11ΔN-His6 protein expression was examined by immunoblotting. α-tubulin was used as loading control.
(C) GR-lacZ activity was assessed in the same yeast strains as described above. Data are expressed as a percentage of the activity in WT cells, and results from three independent experiments are depicted as the mean ± SD. **p < 0.005.
(D) A 1:10 dilution series of 107 cells per ml of WT yHsp90-His6 (WT), T101A, and T101E yeast strains were spotted on YPDA agar. Plates were incubated at 25°C, 30°C and 37°C for 3 days.
(E) WT yHsp90-His6 (WT), T101A, and T101E yeast strains expressing heat shock element (HSE)-lacZ were heat shocked (40 min at 39°C). Heat shock response was measured in three independent experiments. All data represent mean ± SD. *p < 0.05.
(F) SkBr3 cells were transfected with empty plasmid (C), hHsp90α-FLAG (WT), T115A-hHsp90α-FLAG (T115A), or T115E-hHsp90α-FLAG (T115E). Hsp90-FLAG was immunoprecipitated (IP) with anti-FLAG M2 affinity gel, and co-immunoprecipitated ErB2 and Cdk4 were detected by immunoblotting. Phos-Thr, phospho-threonine.
(G) HEK293 cells were transfected with indicated Hsp90 constructs. Hsp90-FLAG was immunoprecipitated with anti-FLAG M2 affinity gel, and co-immunoprecipitated GR was detected by immunoblotting.
Next, we examined the impact of T101 phosphomutants on the chaperoning of two non-kinase clients. The mammalian glucocorticoid receptor (GR) is a well-characterized Hsp90 client that provides an Hsp90 functional assay in yeast (Pratt et al., 2004). WT yeast cells, as well as the T101A and T101E mutants, were transformed with plasmids constitutively expressing GR and a glucocorticoid-regulated lacZ reporter gene (Garabedian and Yamamoto, 1992). In this case, the GR activity was almost four times higher in the T101A mutant than in the WT cells; conversely, the phosphomimetic T101E mutant showed impaired GR activity (Figure 3C). Next, we tested the effect of T101 phosphorylation on Heat shock factor-1 (Hsf1) transcriptional activity. Hsf1 is an Hsp90 client, and its interaction with this chaperone suppresses Hsf1 activity (Zou et al., 1998). Hsp90 inhibition, due to mutation or pharmacologic inhibition, leads to induction of Hsf1 activity in the absence of heat shock stress (Hjorth-Sørensen et al., 2001). Temperature sensitivity of the T101I mutant has been reported previously (Nathan and Lind-quist, 1995). Here, we showed the phosphomutant T101E to be temperature sensitive (ts) at 30°C and T101A at 37°C (Figure 3D). The heat shock response in the T101A mutant upon heat stress was 1.5-fold higher than in WT yeast cells but was 2-fold lower than in WT for the T101E mutant (Figure 3E). We observed similar results with T115-hHsp90α phosphomutants. HEK293 cells were transiently co-transfected with the Hsp90 kinase client, ErbB2, and one of the following constructs: WT hHsp90α-FLAG, T115A, or T115E mutants. While we were able to co-immunoprecipitate ErbB2 with the WT hHsp90α-FLAG, ErbB2 interaction with T115A was significantly reduced (Figure 3F). We obtained similar data with endogenous Cdk4 (another Hsp90 kinase client) (Figure 3F). Interaction of the phosphomimetic mutant T115E with both ErbB2 and Cdk4 was slightly increased (Figure 3F). Consistent with our observation in yeast, T115A-hHsp90α interaction with GR was significantly increased, whereas T115E-hHsp90α association with GR was completely abolished (Figure 3G). These data show that phosphorylation of T101/T115 increases the interaction with, and activity of, several kinase clients but inhibits the interaction/activity of the steroid hormone receptor GR.
Phosphorylation of T101/T115 Is Essential for Mps1 Activity and Mitotic Checkpoint
Previous work has suggested that co-chaperones Cdc37 and Sti1 are involved in the chaperoning of yMps1 (Schutz et al., 1997). We evaluated whether Mps1 is an Hsp90 client and also examined the impact of T101/T115 phosphorylation on the chaperoning of Mps1 itself. Treating the yeast cells with 40 μM GB, an Hsp90 inhibitor, led to degradation of the endogenously Myc-epitope-tagged yMps1 protein (Figure 4A). This phenomenon was reversed by pre-treating the yeast cells with the proteasome inhibitor MG132 (Figure 4B). We next evaluated yMps1 protein stability in Hsp90-T101 phosphomutants by cycloheximide (CHX) chase analysis and found that yMps1-Myc was markedly stable in WT and T101E yeast cells. Conversely, yMps1-Myc expressed in the non-phosphorylatable T101A mutant background was undetectable after 60-min treatment with cycloheximide (Figure 4C).
Figure 4. Mps1-Mediated Phosphorylation of Hsp90 Is Essential for Mitotic Arrest.
(A) WT yHsp90-His6 yeast cells with endogenously Myc-epitope-tagged yMps1 were treated with 40 μM GB for indicated time points in liquid YPDA media. The stability of yMps1-Myc was assessed by immunoblotting.
(B) WT yHsp90-His6 yeast cells with endogenous yMps1-Myc were treated with either 40 μM GB for 24 hr or 50 mM proteasome inhibitor MG132 for 24 hr. Cells were also pretreated with MG132 1 hr prior to treatment with GB.
(C) Yeast cells expressing yHsp90-His6 (WT), T101A, and T101E with endogenous yMps1-Myc were treated with 100 μg/ml CHX. yHsp90-His6 and yMps1-Myc proteins were detected by immunoblotting at the indicated times. Data were also quantified and presented as bar charts. Error bars represent SD. OD, optical density.
(D) Yeast with yHsp90-His6 (WT), T101A-yHsp90-His6 (T101A), and T101E-yHsp90-His6 (T101E) mutants with endogenous yMps1-Myc were used to isolate yHsp90-His6 by Ni-NTA, and interaction of yMps1 was examined by immunoblotting. Empty plasmid was used as a control (C).
(E) HEK293 cells were transfected with empty plasmid (C), hHsp90α-FLAG (WT), T115A-hHsp90α-FLAG (T115A), or T115E-hHsp90α-FLAG (T115E). Hsp90-FLAG was immunoprecipitated (IP) with anti-FLAG M2 affinity gel, and interaction of human Mps1 was examined by immunoblotting.
(F) yMps1-Myc was isolated from yeast cells expressing yHsp90-His6 (WT), T101A-yHsp90-His6 (T101A), and T101E-yHsp90-His6 (T101E). yMps1 activity was examined by immunoblotting using pan-phospho-threonine antibody.
(G) Flow cytometry analysis of yHsp90-His6 (WT), T101A-yHsp90-His6 (T101A), and T101E-yHsp90-His6 (T101E) mutants grown in liquid YPDA over-night at 25°C. 57% of WT, 54% of T101A, and 77% of T101E cells were arrested in G2.
(H) yHsp90-His6 (WT) and T101A-yHsp90-His6 (T101A) cells containing GAL-yMPS1-GST were grown in liquid YP-raffinose overnight at 25°C and then transferred to YP-galactose for 2 hr at 25°C. Flow cytometry analysis revealed only WT cell accumulation in G2 (75%).
(I) Budding morphology of distributions in WT, T101, and mps1-1 yeast mutants. Cells were arrested in G1 with an α-factor at 25°C and then released in medium containing 20 μg/ml nocodazole for 4 hr at either 25°C or 37°C. 100 cells were scored for large buds (L), multiply budded (M), and unbudded (U). Cells were scored from three independent experiments.
All data represent mean ± SD. **p < 0.005.
Next, we examined the interaction of yMps1 with the T101 phosphomutants. The interaction of the non-phosphorylatable T101A mutant with yMps1-Myc was completely abrogated. In contrast, the interaction between T101E and yMps1-Myc was stronger than the interaction between WT yHsp90 and yMps1 (Figure 4D). We obtained similar results with the WT hHsp90α and the T115 phosphomutants. The T115A mutant was unable to bind to hMps1, whereas the phosphomimetic T115E associated more strongly than WT hHsp90α with hMps1 (Figure 4E). Our data suggest a model in which Mps1-mediated phosphorylation of T101/T115 enhances its interaction with Hsp90, possibly increasing Mps1 kinase activity.
To test these hypotheses, we isolated Mps1-Myc from yeast expressing WT yHsp90 and T101 phosphomutants and used pan-phospho-threonine antibody to examine the autophosphorylation/activity of yMps1. Threonine phosphorylation of yMps1 was detected in WT yeast cells and was increased in the T101E mutant. However yMps1 phosphorylation was significantly reduced in the T101A mutant (Figure 4F). Our data suggest that yMps1 activation and phosphorylation of T101-yHsp90 facilitates the interaction of yMps1 with yHsp90, which, in turn, is important for maintaining yMps1 kinase activity.
We decided to test this model further by determining whether the T101E yeast cells experience mitotic arrest. Previous work has shown that overexpression of yMps1 causes cell cycle arrest at G2 but with morphologically normal spindles (Hardwick et al., 1996). Here, we examined yeast cells expressing WT yHsp90, T101A, or T101E mutants for any cell cycle defect and discovered that T101E mutant yeast were arrested in G2 (Figure 4G). Next, we overexpressed yMps1-GST under the GAL1-promoter in WT or T101A mutant yeast. Cells were grown on media containing raffinose in order to suppress the GAL1-promoter and then shifted to galactose-containing media to induce the GAL1-promoter. In agreement with previous reports (Hardwick et al., 1996), overexpression of yMps1 arrested WT cells in G2, but it had no effect in T101A mutant yeast (Figure 4H). Mps1 kinase is essential for the mitotic checkpoint (also known as the spindle checkpoint); therefore, Mps1 deletion is lethal in yeast (Abrieu et al., 2001). The ts mps1-1 mutant is viable at the permissive temperature (25°C). We synchronized the WT, mps1-1, and T101A-yHsp90 yeast cells in G1, with an α-factor at 25°C. The cells were then released in medium containing the microtubule poison nocodazole, a known activator of the mitotic checkpoint, for a further incubation of 4 hr at either 25°C or 37°C. WT, mps1-1, and T101A cells were arrested with large buds in nocodazole-containing medium at 25°C (Figure 4I). In contrast, after 4 hr in medium containing nocodazole at 37°C, T101A mutants, like mps1-1 yeast, had multiple-budded cells (Figure 4I). Taken together, our data suggest that Mps1 phosphorylation of T101-yHsp90 is essential for its kinase activity and also for mitotic arrest.
Quantitative Proteomic Analysis of Phosphorylated Hsp90
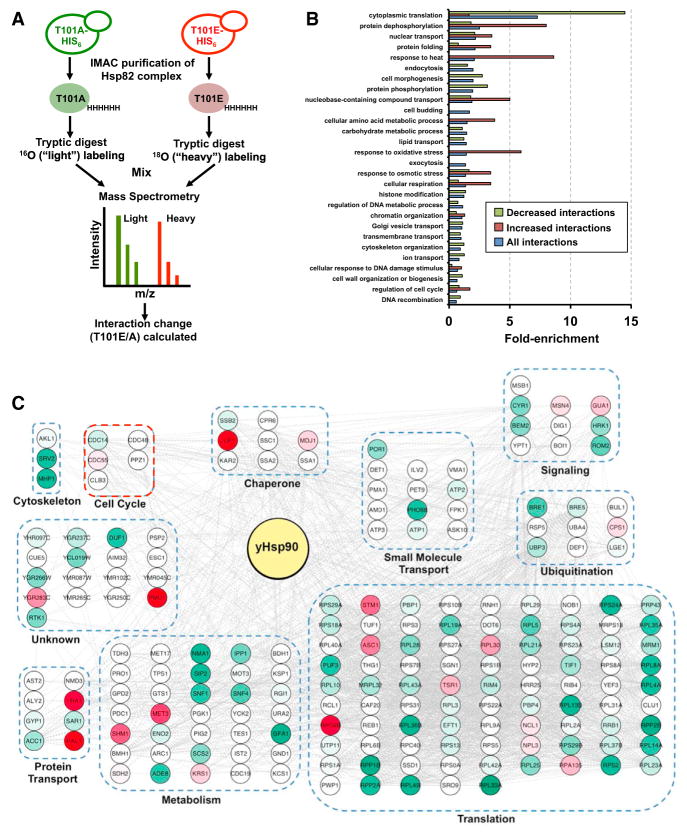
Our data have shown that phosphorylation of T101 promotes Hsp90 interaction with certain client proteins. In order to quantitatively identify the global interactome of Hsp90 as the result of T101 phosphorylation, we purified the T101A and T101E-yHsp90-His6 interactomes and compared them by isotope-coded liquid chromatography-tandem mass spectrometry (LC-MS/MS; Figure 5A). Based on high-confidence peptide matches, 474 yHsp90 partners were identified (Table S2). Gene Ontology (GO) analysis of these interactors revealed “response to stress” and “protein dephosphorylation” as the most enriched categories for increased association and “cytoplasmic translation” as the most enriched category for dissociation (Figure 5B). Next, we analyzed individual proteins that were associated or dissociated with yHsp90 as the result of T101 phosphorylation. A large number of ribosomal proteins involved in “transcription/translation” were dissociated from yHsp90; surprisingly, there was no change in protein interactions involved in “chromatin regulation” (Figure 5C). We also discovered that T101 phosphorylation enhanced yHsp90 interaction with Cdc55, but it enhanced dissociation from Cdc14, grouped under the “cell cycle” node (Figure 5C). Cdc55 phosphatase promotes mitotic entry (Healy et al., 1991; Wang and Burke, 1997), whereas Cdc14 phosphatase is required for mitotic exit (Bremmer et al., 2012; Mocciaro and Schiebel, 2010).
Figure 5. T101 Phosphorylation Alters the yHsp90 Interactome.
(A) A schematic illustration of the methodology used to identify the yHsp90 interactome. Cells expressing either T101A- or T101E-yHap90-His6 were grown to mid log. yHsp90 complexes purified via immobilized metal ion affinity chromatography (IMAC) and trypsinized. T101E-yHsp90-His6 interacting peptides were isotopically labeled with 18 O, mixed 1:1 with T101A-yHsp90-His6 interacting peptides, and then analyzed by quantitative LC-MS/MS.
(B) GO term analysis of yHsp90 interactors. They were classified by cellular process using GO Slim. Enrichment for each process is shown relative to occurrence in non-essential genome.
(C) Effect of T101 phosphorylation on yHsp90 interactome. yHsp90 interactors were grouped into non-redundant functional categories. Interactions are colored based on change; white nodes represent unchanged interactions, red nodes are significantly increased interactions, and green nodes are significantly decreased interactions.
Cdc14 Dephosphorylates T101 and Disrupts yMps1-yHsp90 Complex
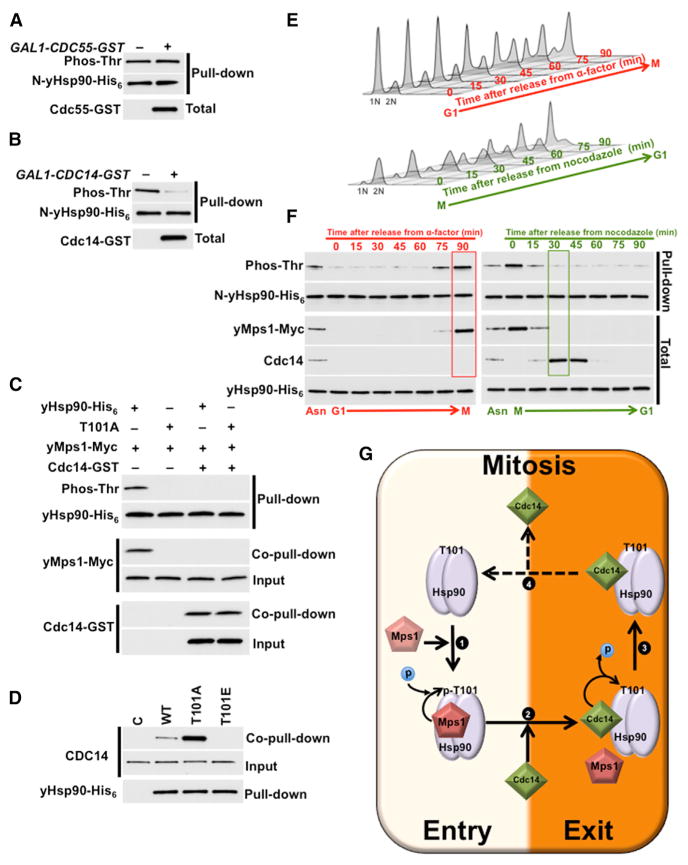
To determine whether either Cdc55 or Cdc14 is involved in the dephosphorylation of T101, we overexpressed glutathione S-transferase (GST)-epitope-tagged Cdc55 and Cdc14 under a galactose-inducible promoter (GAL1-promoter) in yeast with either WT yHsp90-His6 or T101A mutant. After inducing CDC55-GST or CDC14-GST expression by growing the cells on medium containing galactose for 2 hr, we detected both proteins by western blot analysis using anti-GST antibody (Figures 6A and 6B). Next, we examined the phosphorylation status of T101 in the N-domain of yHsp90 using the PreScission cleavage procedure (Mollapour et al., 2011). Over-expression of Cdc55 did not change the threonine phosphorylation of N-domain yHsp90, whereas overexpression of Cdc14 reduced the threonine phosphorylation of the N-domain (Figures 6A and 6B). To investigate whether Cdc14 directly targets T101, we used bacterially expressed and purified WT yHsp90-His6 and T101A mutant bound to Ni-NTA agarose. We also overexpressed and purified GAL1-yMPS1-MYC from yeast by immunoprecipitation. Purified yMps1-Myc was salt-stripped with 0.5 M NaCl to disrupt co-immunoprecipitated proteins. We found that yMps1 phosphorylates only T101 in yHsp90 (Figure 6C). Our in vitro data also showed that T101A-yHsp90 mutant did not form a complex with yMps1. This result suggests that yMps1 only targets T101, and this is essential for yHsp90-yMps1 complex formation (Figure 6C). These data are in agreement with our in vivo findings (Figure 4D). Next, we incubated the phosphorylated T101-yHsp90 with immunoprecipitated and salt-stripped Cdc14-GST from yeast. This phosphatase had the ability to dephosphorylate T101 and then form a strong complex with de-phosphorylated yHsp90. Interestingly, yMps1 was dissociated from yHsp90 under these conditions (Figure 6C). We confirmed these data by isolating hexahistidine-epitope-tagged yHsp90 proteins from cell lysates using Ni-NTA agarose beads. The endogenous Cdc14 interaction with yHsp90 was assessed by immunoblotting using anti-Cdc14 antibody (Figure 6D). Cdc14 interacted with the WT yHsp90, and this association was significantly enhanced with the non-phosphorylatable T101A mutant (Figure 6D). Conversely, the phosphomimetic T101E mutant did not associate with the Cdc14-GST. Our data suggest that phosphorylation/dephosphorylation of T101-Hsp90 may be part of the regulation of the cell cycle. We validated this point by synchronizing yeast cells in G1 using an α-factor and then releasing in media containing nocodazole in order to arrest the cells in mitosis (early anaphase) (Figure 6E). Conversely, yeast cells were also arrested in mitosis and then released in media with an α-factor. Cell cycle status was examined every 15 min. Next, we isolated the N-domain yHsp90-His6 by using the PreScission protease cleavage and monitored the phosphorylation of T101 by western blot analysis. Our earlier data have shown that T22 and T101 are the only phospho-threonine sites in the yHsp90 N-domain (Figure S1A). Since T22 phosphorylation does not change at different stages of the cell cycle (Mollapour et al., 2011), we concluded that the change in the threonine phosphorylation of N-domain yHsp90 is because of the modification of T101. Our data show that T101 is not phosphorylated in G1, and as cells undergo mitosis, the levels of T101 phosphorylation increases significantly (Figure 6F). This is consistent with yMps1 increase in mitosis and absence of the phosphatase Cdc14 from G1 to mitosis (Figure 6F). Next, we examined T101 phosphorylation in cells exiting mitosis and entering G1. As yeast cells exit mitosis, the yMps1 level decreases, coinciding with an increase in the Cdc14 (Figure 6F).
Figure 6. Cdc14 Phosphatase Targets yHsp90 and Dephosphorylates T101.
(A and B) Yeast expressing WT yHsp90-His6 (WT) and carrying (A) CDC55-GST or (B) CDC14-GST under GAL1-promoter were grown on YP-raffinose (−) overnight at 25°C and then shifted to YP-galactose (+) for 2 hr at 25°C. N-domain threonine phosphorylation of yHsp90 was assessed after pull-downs, PreScission protease digestion, and immunoblotting. Phos-Thr, phospho-threonine.
(C) yMps1-Myc was isolated from yeast, salt-stripped (0.5 M NaCl), and used to phosphorylate bacterially expressed and purified yHsp90-His6 (WT) and T101A-yHsp90-His6 (T101A). Cdc14-GST isolated from yeast, salt-stripped (0.5 M NaCl), and used to dephosphorylate T101. Total and phosphorylated yHsp90 was detected by immunoblotting.
(D) Yeast cells with yHsp90-His6 (WT), T101A-yHsp90-His6 (T101A), and T101E-yHsp90-His6 (T101E) mutants were isolated by Ni-NTA, and interaction of Cdc14 was examined by immunoblotting. Empty plasmid was used as a control (C).
(E) Yeast cells were released from G1 (2.5 μg/ml α-factor) to mitosis (20 μg/ml nocodazole) or from mitosis to G1 and then analyzed by flow cytometry every 15 min.
(F) Phosphorylation of the N-yHsp90-His6 (WT) from cells in (E) was analyzed by immunoblotting with pan-anti-phospho-threonine antibody. Cdc14, yMps1-Myc and full-length yHsp90-His6 protein levels were also detected by immunoblotting.
(G) Schematic representation of yMps1-mediated phosphorylation and Cdc14-facilitated dephosphorylation of T101-yHsp90. (1) yMps1 binds to yHsp90 and phosphorylates T101, and this promotes formation of yHsp90:yMps1 complex. (2) Cdc14 mediates dephosphorylation of T101, (3) disrupts yHsp90:yMps1 complex, and promotes yHsp90:Cdc14 association. (4) Possible dissociation of Cdc14 allows yMps1 binding and restarting the phosphorylation cycle.
Taken together, our findings suggest that yMps1 phosphorylates T101-yHsp90, and this is essential for the mitotic checkpoint (Figure 6G). The Cdc14 phosphatase, in turn, de-phosphorylates T101 and dissociates yHsp90-yMps1 complex, leading to its inactivation.
Elevated hMps1 Levels in RCC Confer Selectivity to Hsp90 Inhibitors
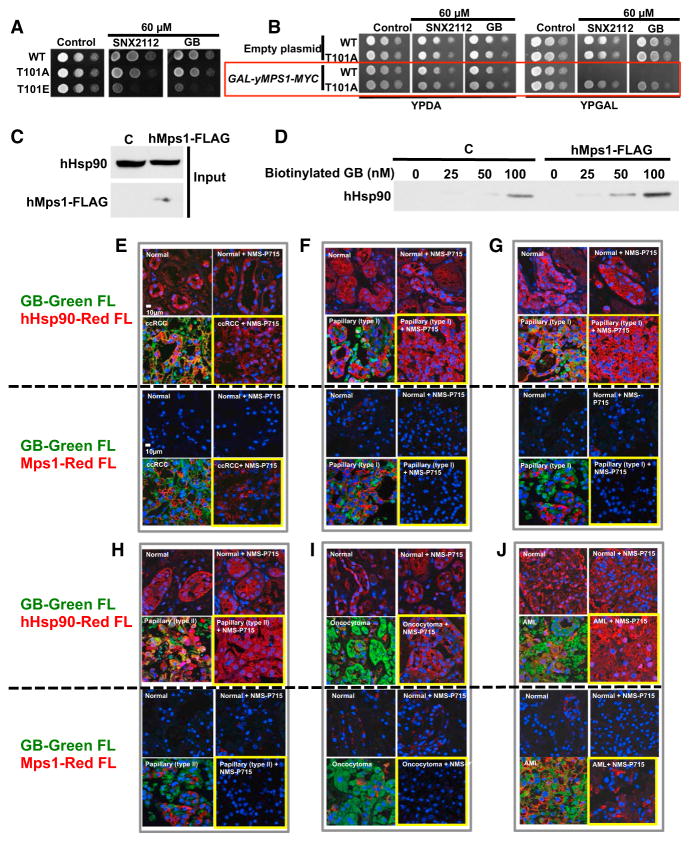
Although Hsp90 is highly expressed in most cells, Hsp90 inhibitors selectively kill cancer cells compared to normal cells. We observed that the T101E phosphomimetic yeast mutant is sensitive to the pharmacologic Hsp90 inhibitors geldanamycin and radicicol, as well as to the clinically evaluated Hsp90 inhibitors, SNX2112 and GB (Figure 7A; Figure S4A). Overexpression of yMps1 in yeast also conferred sensitivity to both SNX2112 and GB (Figure 7B). This sensitivity was not observed in the T101A mutant, suggesting that yMps1-mediated phosphorylation of T101 is essential for the drug sensitivity. We further validated this finding by transiently overexpressing hMps1-FLAG in HEK293 cells (Figure 7C), and consequently enhancing WT hHsp90 (both α and β isoforms) binding to biotinylated-GB (Figure 7D). These data suggest that hyperphosphorylation of T101 or T115, as the result of overactive Mps1, sensitizes yeast and mammalian cells to Hsp90 inhibitors.
Figure 7. Mps1-Mediated Phosphorylation of Hsp90 Sensitizes Cells to Hsp90 Drugs and Confers Tumor Selectivity to Hsp90 Inhibitors.
(A) Yeast expressing yHsp90-His6 (WT), and indicated phospho-T101 mutants were spotted at a 1:10 dilution series of 107 cells per milliliter on YPDA agar containing 60 μM Hsp90 inhibitor SNX2112 or GB. Plates were incubated at 28°C for 4 days.
(B) Strains in (A) containing either empty plasmid (pYES2) or GAL-yMps1-MYC were grown on raffinose media overnight at 25°C. A 1:10 dilution series of 107 cells per ml were spotted on YPDA or YPGAL agar containing 60 μM SNX2112 or GB. Plates were incubated at 28°C for 4 days.
(C) HEK293 cells were transiently transfected with empty plasmid (C) or hMps1-FLAG. Protein expression was assessed by immunoblotting.
(D) Lysates from (C) were incubated with indicated amounts of biotinylated-GB, followed by streptavidin agarose beads, and Hsp90 was detected by immunoblotting.
(E–J) Adjacent normal tissues and (E) ccRCC , (F and G) papillary type I, (H) papillary type II, (I) on-cocytoma, and (J) AML were dissected into 3-mm3 pieces that were cultured in medium containing 10 μM NMS-P715 (Mps1 inhibitor) for 24 hr. Tissues were then incubated with 0.5 μM BODIPY fluorophore-conjugated GB (STA-12-9455; GB-Green-FL) for an additional 6 hr. Tissues were fixed and immunostained for either Hsp90 (hHsp90-Red FL), or hMps1 (hMps1-Red FL). DNA was stained with DAPI for immunofluorescence analysis.
See also Figure S4.
Hsp90 inhibitors selectively accumulate in cancer cells compared to normal cells. Therefore, we tested for possible overexpression of Mps1 in tumors that could account for this observation. We used tumors and adjacent normal tissues from patients with RCC. Based on histopathology, RCCs are classified into multiple types. We used tumors from patients with ccRCC, papillary type I and type II RCCs, oncocytoma, and AML (Figure S4B). Within 15 min of removal of tumors, by radical or partial nephrectomy, human RCC tumors were dissected into 3-mm3 pieces and cultured in medium containing 10 μM NMS-P715 (a potent Mps1 inhibitor) (Colombo et al., 2010) for 24 hr. The tumors were incubated with 0.5 μM BODIPY fluorophore-conjugated GB (STA-12-9455; FL-GB) for an additional 6 hr.
Tissues were fixed, and accumulation of FL-GB was examined by confocal immunofluorescence microscopy. The tissues were co-stained with hHsp90 and hMps1. As expected, the FL-GB (green fluorescent) was primarily accumulated in the tumors but not in the normal tissues (Figures 7E–7J). hMps1 level (red fluorescent) was also elevated in tumors compared to the level in the normal tissues (Figures 7E–7J). However, treating the tumors with the hMps1 inhibitor NMS-P715 abolished the accumulation of FL-GB (Figures 7E–7J). It is noteworthy that hHsp90 levels (red fluorescent) were unaffected in the normal and tumor tissues, even after treatment with NMS-P715 (Figures 7E–7J); however, hMps1 levels were significantly reduced in the NMS-P715-treated tumors (Figures 7E–7J).
We further validated these data by examining the affinity of hHsp90 from the normal and tumor tissues for binding to bio-tinylated-GB. We discovered that the hHsp90 from tumors had a higher affinity for biotinylated-GB compared to the adjacent normal tissues (Figures 8A–8F). Tumors treated with the hMps1 inhibitor NMS-P715 reduced the binding of hHsp90 to biotinylated-GB (Figures 8A–8G). Taken together, our data suggest that overexpression of Mps1 can account for tumor sensitivity and tumor selectivity of Hsp90 inhibitors (Figure 8H).
Figure 8. Overexpression of Mps1 in RCC Increases Hsp90 Binding to GB.
(A–F) ccRCC (A), papillary type I (B and C), papillary type II (D), oncocytoma (E), and AML (F) tissues were cultured in medium with 10 μM NMS-P715 (Mps1 inhibitor) or without drug for 24 hr. After protein lysate preparation, Hsp90 binding to GB was assessed by biotinylated-GB and immunoblotting.
(G) Hsp90 in samples (A)–(F) was detected by immunoblotting using anti-Hsp90-antibody.
(H) Overexpression and hyperactivity of Mps1 in kidney tumor compared to the adjacent normal tissue confer cancer cell selectivity and sensitivity to Hsp90 inhibitor GB.
DISCUSSION
In this study, we documented the phosphorylation of a single conserved threonine residue in the N-domain of both yeast (T101) and mammalian (T115) Hsp90. Previous work has shown that T101I has an ATP affinity similar to that of the WT yHsp90 but reduced ATPase activity and AMP-PNP-dependent N-domain dimerization (Prodromou et al., 2000). T101 is located within the lid segment of yHsp90, and in the “open” conformation, the side chain of T101 packs into a hydrophobic cavity formed by the side chains of I12 and L15 and by the Cα and Cβ of E11. Upon N-domain dimerization, this interaction is disrupted and exposes the side chain of T101 on the top surface of the lid. Mutations to alanine or glutamic acid stabilize the hydrophobic interaction in the “open” conformation and destabilize the exposure of the side chain in the “closed” conformation. Our data show that phosphorylation of T101 significantly reduces the yHsp90 ATPase activity, whereas lack of phosphorylation has minimum impact on ATP hydrolysis in yeast. In terms of interaction with co-chaperones, neither non-phosphorylatable nor phosphomimic mutants in yeast or human Hsp90 bound to Aha1; however, both mutants had a strong interaction with Cdc37. Phosphorylation of Hsp90 also enhanced binding and chaperoning of several kinase clients while reducing the activity of the non-kinase client GR.
Recent work has shown that PKCγ phoshorylates T115-hHsp90α (Lu et al., 2014). We were unable to verify these results using both yeast and human Hsp90 proteins in an in vitro kinase assay. We were also unable to show yeast PKC1-mediated phosphorylation of T101 in yeast overexpressing PKC1.
We screened the yeast non-essential kinase deletion as well as essential kinase overexpression libraries and identified yMps1 as the kinase targeting T101-yHsp90. yMps1 is an evolutionarily conserved dual-specificity protein kinase required for (1) duplication of centrosomes in metazoans or spindle pole body (SPB) in yeast and (2) the mitotic spindle assembly checkpoint (also known as the mitotic checkpoint) (Liu and Winey, 2012). However, its primary cellular substrate has remained elusive. Previous work has demonstrated that yMps1 activity requires the co-chaperone Cdc37 (Schutz et al., 1997). yMps1 is essential in eukaryotic cells, and it was shown that both conditional and non-conditional alleles of cdc37 are lethal in combination with the mps1-1 ts mutant in yeast (Schutz et al., 1997). Winey’s lab has also reported that mutations in yHSP90 and in the co-chaperones YDJ1 and STI1 display synthetic lethality with the mps1-1 mutation (Jones et al., 1999). Our data show that Mps1 is a bona fide Hsp90 client, and its phosphorylation of T101-yHsp90 and T115-hHsp90α is required for mitotic arrest. Our data also show that yMps1 does not stably interact with the non-phos-phorylatable T101A-yHsp90 mutant, suggesting that yMps1-mediated phosphorylation stabilizes interaction of this kinase with Hsp90. It is noteworthy that our proteomic data did not identify yMps1 interaction with T101E-yHsp90. This might be because of the low abundance of yMps1 kinase in cells (Liu and Winey, 2012). Our quantitative proteomic analysis, however, identified the Cdc14 phosphatase responsible for dephosphorylation of T101. Our data suggest a regulatory paradigm in which Mps1 phosphorylation of Hsp90 leads to formation of a strong complex between these two proteins and, consequently, causes mitotic arrest. The Cdc14 phosphatase dephosphorylates T101 and disrupts the Mps1-Hsp90 complex. This leads to the degradation of Mps1. We posit that this process is vital for mitotic exit, since Cdc14 phosphatase activity is essential for this step.
Mps1 kinase activity is essential for spindle checkpoint signaling (Hardwick et al., 1996). Overexpression of Mps1 is coupled to an increase in its kinase activity and it causes abnormal chromosome segregation during mitosis, i.e., aneuploidy, a hallmark of cancers associated with high risk for tumorigenesis. (Kops et al., 2005; Musacchio and Salmon, 2007). We found that overexpression of yMps1 made yeast cells sensitive to Hsp90 inhibitors, and this phenotype depends on the phosphorylation of T101. Also, overexpression of hMps1 in mammalian cells increased their binding to GB.
High levels of hMps1 kinase are found in colon cancer tissues and several tumor cell lines such as U937, HeLa, HEY, OCC1, Bewo, T987, and SW480 (Ling et al., 2014; Yen and Kao, 2005). Here, we observed high levels of hMps1 in different kidney tumors (ccRCC, AML, oncocytoma, papillary type 1 and type 2 RCCs) compared to the adjacent normal tissue. We also found that the Hsp90 inhibitor GB selectively accumulates in these tumor specimens. However, pharmacologic inhibition of hMps1 prevented the accumulation of GB in the tumor tissue, suggesting that high expression/activity of hMps1 may be characteristic of some cancers and can potentially serve as a predictive indicator of tumor response to Hsp90 inhibitors.
EXPERIMENTAL PROCEDURES
Yeast Strains, Plasmids, and Growth Media
Yeast strain pp30 (MAT a, trp1-289, leu2-3, -112, his3-200, ura3-52, ade2-101, lys2-801, hsc82KANMX4, hsp82KANMX4) was used in this study (Panaretou et al., 1998). Plasmids were constructed as described previously (Mollapour et al., 2011). yMps1 was C-terminally Myc tagged using primers listed in Table S3 and pFA6a-13Myc-His3MX6 plasmid as the template. The EUROSCARF collection of haploid non-essential kinase deletions in BY4741 (MAT a; Δhis3-Δ1; leu2-Δ0; met15-Δ0; ura3-Δ0) background (http://www.euroscarf.de/search.php?name=Order) was used to screen for T101 phosphorylation (Table S1). The yeast essential kinases library under the control of GAL1-promoter was obtained from Dharmacon (http://dharmacon.gelifesciences.com/). Detailed procedures, a list of primers (Table S3), and media conditions for both yeast and mammalian cells are provided in the Supplemental Information.
Protein Extraction, Isolation, PreScission Protease Cleavage, and Immunoblotting
Preparation and analysis of total protein lysate by western blot was described previously (Mollapour et al., 2010). A detailed method for protein precipitations, cleavage by PreScission protease, and detection by western blotting and assays for Hsp90 client binding and activity for both yeast and mammalian systems are presented in the Supplemental Information.
Interactome Analysis of Phosphorylation of T101-yHsp90 by Mass Spectrometry
Yeast cells expressing T101A-yHsp90-His6 (T101A) or T101E-yHsp90-His6 (T101E) as the sole yHsp90 protein were grown to mid-log phase. T101A and T101E interactomes were purified using Ni-NTA agarose, separated by SDS-PAGE, and subjected to proteomic analysis. Briefly, gel slices were subjected to in-gel proteolysis; tryptic peptides were purified, differentially labeled by carboxyl-terminal 18O exchange, and analyzed via LC-MS/MS as described in the Supplemental Information.
In Vitro Kinase Assay
Yeast Hsp90 and the mutant T101A were N-terminally His6-tagged using the pRSETA plasmid. They were then expressed in bacteria, and 2 mg of protein extracts were incubated with 50 μl of Ni-NTA agarose. yMps1-Myc and Cdc14-GST were expressed and purified from yeast. These proteins were also salt-stripped with 0.5 M NaCl prior to addition to the in vitro reaction as previously described (Mollapour et al., 2010).
Flow Cytometric Analysis
Flow cytometric analysis was performed as described previously (Mollapour et al., 2010). A detailed procedure is found in the Supplemental Information.
Isothermal Titration Calorimetry and Kd Determinations
Isothermal titration calorimetry (ITC) and diffusion constant (Kd) determinations were performed as described previously (Prodromou et al., 2000). Additional information can be found in the Supplemental Information.
Hsp90 ATPase Activity In Vivo
Yeast Hsp90-His6 and the T101A and T101E mutants were isolated, and their ATPase activity was measured as previously described (Kamal et al., 2003), with exceptions detailed in the Supplemental Information.
Ex Vivo Culture and Analysis of Human RCC Tumors
Tumor tissues of the patients with conventional RCC were obtained with written informed consent from the Department of Urology at SUNY (State University of New York) Upstate Medical University. Patients had no history of hereditary Von Hippel-Lindau (VHL) disease.
At the time of radical or partial nephrectomy, which was done with <15 min of renal ischemia, RCC tumors were dissected into 3- to 5-mm3 pieces and cultured on a presoaked gelatin sponge (Johnson & Johnson) in 24-well plates containing 2 ml RPMI-1640 with 10% FBS, antibiotic/antimycotic solution, with or without 10 μM NMS-P715. Tissues were cultured at 37°C for 24 hr, followed by the addition of 100 nM fluorescently labeled GB (STA-12-9455; FL-GB) and further incubation at 37°C for 6 hr.
Using an ex vivo method as previously described (Kedar et al., 1982), approximately 107 cells were isolated from the RCC solid tumor analysis by flow cytometry and western blot.
Statistical Analysis
Data were analyzed with Student’s t test. Asterisks in figures indicate significant differences (p < 0.05). Error bars represent the SD for three independent experiments.
Supplementary Material
Highlights.
Mps1 phosphorylates a conserved threonine in Hsp90 and regulates its function
Hsp90 phosphorylation by Mps1 is essential for the mitotic checkpoint
Cdc14 phosphatase dephosphorylates Hsp90 and disrupts the Mps1-Hsp90 complex
Mps1 phosphorylation of Hsp90 confers tumor selectivity to its inhibitors
Acknowledgments
The authors thank Dr. W. Feng and Mr. A. McCulley for insightful scientific discussion and helpful comments. We are grateful to Dr. J. Johnson (University of Idaho) for STE11ΔN plasmid, Dr. T. Haystead (Duke University) for the Hsp90 inhibitor SNX2112, Dr. W. Ying (Synta Pharmaceuticals) for the Hsp90 inhibitor ganetespib, Dr. D. Masison (National Institute of Diabetes and Digestive and Kidney Diseases) for Sti1 antibody, Dr. M. Snyder (Stanford University) for Cdc55-GST and Cdc14-GST constructs, and Dr. C. Boone (University of Toronto) for the mps1-1 strain. This work was supported with funds from NCI R01 CA164492 (S.J.K.), Wellcome Trust 095605/Z11/Z (C.P.), SUNY Upstate Medical University, Foundation for Upstate Medical University, One Square Mile of Hope Foundation, and Carol M. Baldwin Breast Cancer Fund (M.M., D.B., M.R.W, D.D).
Footnotes
ACCESSION NUMBERS
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (Vizcaíno et al., 2014) and are available under accession number PRIDE: PXD001969.
Supplemental Information includes Supplemental Experimental Procedures, four figures, and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2015.12.084.
AUTHOR CONTRIBUTIONS
Conceptualization: M.R.W. and M.M.; methodology: M.R.W., A.W.T., S.M.J., M.A., B.P., S.L., C.P., S.J.K., O.S., J.B.T., W.G.S.-S., D.B., L.N., G.B., and M.M.; investigation: M.R.W., A.W.T., D.M.D., S.M.J., R.C., R.B., K.B., D.W., S.W., D.E.P., T.C., S.T., D.B., G.B., and M.M.; writing—original draft: M.R.W., D.M.D., D.B.; writing—review and editing, project administration, visualization, supervision, and funding acquisition, M.M.
References
- Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbé JC. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 2001;106:83–93. doi: 10.1016/s0092-8674(01)00410-x. [DOI] [PubMed] [Google Scholar]
- Bremmer SC, Hall H, Martinez JS, Eissler CL, Hinrichsen TH, Rossie S, Parker LL, Hall MC, Charbonneau H. Cdc14 phosphatases preferentially dephosphorylate a subset of cyclin-dependent kinase (Cdk) sites containing phosphoserine. J Biol Chem. 2012;287:1662–1669. doi: 10.1074/jbc.M111.281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiosis G, Neckers L. Tumor selectivity of Hsp90 inhibitors: the explanation remains elusive. ACS Chem Biol. 2006;1:279–284. doi: 10.1021/cb600224w. [DOI] [PubMed] [Google Scholar]
- Colombo R, Caldarelli M, Mennecozzi M, Giorgini ML, Sola F, Cappella P, Perrera C, Depaolini SR, Rusconi L, Cucchi U, et al. Targeting the mitotic checkpoint for cancer therapy with NMS-P715, an inhibitor of MPS1 kinase. Cancer Res. 2010;70:10255–10264. doi: 10.1158/0008-5472.CAN-10-2101. [DOI] [PubMed] [Google Scholar]
- Flom GA, Lemieszek M, Fortunato EA, Johnson JL. Farnesylation of Ydj1 is required for in vivo interaction with Hsp90 client proteins. Mol Biol Cell. 2008;19:5249–5258. doi: 10.1091/mbc.E08-04-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garabedian MJ, Yamamoto KR. Genetic dissection of the signaling domain of a mammalian steroid receptor in yeast. Mol Biol Cell. 1992;3:1245–1257. doi: 10.1091/mbc.3.11.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick KG, Weiss E, Luca FC, Winey M, Murray AW. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science. 1996;273:953–956. doi: 10.1126/science.273.5277.953. [DOI] [PubMed] [Google Scholar]
- Healy AM, Zolnierowicz S, Stapleton AE, Goebl M, DePaoli-Roach AA, Pringle JR. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol Cell Biol. 1991;11:5767–5780. doi: 10.1128/mcb.11.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth-Sørensen B, Hoffmann ER, Lissin NM, Sewell AK, Jakobsen BK. Activation of heat shock transcription factor in yeast is not influenced by the levels of expression of heat shock proteins. Mol Microbiol. 2001;39:914–923. doi: 10.1046/j.1365-2958.2001.02279.x. [DOI] [PubMed] [Google Scholar]
- Jones MH, Bachant JB, Castillo AR, Giddings TH, Jr, Winey M. Yeast Dam1p is required to maintain spindle integrity during mitosis and interacts with the Mps1p kinase. Mol Biol Cell. 1999;10:2377–2391. doi: 10.1091/mbc.10.7.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- Kedar E, Ikejiri BL, Bonnard GD, Herberman RB. A rapid technique for isolation of viable tumor cells from solid tumors: use of the tumor cells for induction and measurement of cell-mediated cytotoxic responses. Eur J Cancer Clin Oncol. 1982;18:991–1000. doi: 10.1016/0277-5379(82)90248-6. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- Ling Y, Zhang X, Bai Y, Li P, Wei C, Song T, Zheng Z, Guan K, Zhang Y, Zhang B, et al. Overexpression of Mps1 in colon cancer cells attenuates the spindle assembly checkpoint and increases aneuploidy. Biochem Biophys Res Commun. 2014;450:1690–1695. doi: 10.1016/j.bbrc.2014.07.071. [DOI] [PubMed] [Google Scholar]
- Liu X, Winey M. The MPS1 family of protein kinases. Annu Rev Biochem. 2012;81:561–585. doi: 10.1146/annurev-biochem-061611-090435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvion JF, Abbas-Terki T, Picard D. Hsp90 is required for pheromone signaling in yeast. Mol Biol Cell. 1998;9:3071–3083. doi: 10.1091/mbc.9.11.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XA, Wang X, Zhuo W, Jia L, Jiang Y, Fu Y, Luo Y. The regulatory mechanism of a client kinase controlling its own release from Hsp90 chaperone machinery through phosphorylation. Biochem J. 2014;457:171–183. doi: 10.1042/BJ20130963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocciaro A, Schiebel E. Cdc14: a highly conserved family of phosphatases with non-conserved functions? J Cell Sci. 2010;123:2867–2876. doi: 10.1242/jcs.074815. [DOI] [PubMed] [Google Scholar]
- Mollapour M, Tsutsumi S, Donnelly AC, Beebe K, Tokita MJ, Lee MJ, Lee S, Morra G, Bourboulia D, Scroggins BT, et al. Swe1Wee1-dependent tyrosine phosphorylation of Hsp90 regulates distinct facets of chaperone function. Mol Cell. 2010;37:333–343. doi: 10.1016/j.molcel.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollapour M, Tsutsumi S, Truman AW, Xu W, Vaughan CK, Beebe K, Konstantinova A, Vourganti S, Panaretou B, Piper PW, et al. Threonine 22 phosphorylation attenuates Hsp90 interaction with cochaperones and affects its chaperone activity. Mol Cell. 2011;41:672–681. doi: 10.1016/j.molcel.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Nathan DF, Lindquist S. Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol Cell Biol. 1995;15:3917–3925. doi: 10.1128/mcb.15.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L, Trepel JB. Stressing the development of small molecules targeting HSP90. Clin Cancer Res. 2014;20:275–277. doi: 10.1158/1078-0432.CCR-13-2571. [DOI] [PubMed] [Google Scholar]
- Panaretou B, Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998;17:4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci. 2002;59:1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, Galigniana MD, Morishima Y, Murphy PJ. Role of molecular chaperones in steroid receptor action. Essays Biochem. 2004;40:41–58. doi: 10.1042/bse0400041. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Panaretou B, Chohan S, Siligardi G, O’Brien R, Ladbury JE, Roe SM, Piper PW, Pearl LH. The ATPase cycle of Hsp90 drives a molecular ‘clamp’ via transient dimerization of the N-terminal domains. EMBO J. 2000;19:4383–4392. doi: 10.1093/emboj/19.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhl A, Rohrberg J, Buchner J. The chaperone Hsp90: changing partners for demanding clients. Trends Biochem Sci. 2013;38:253–262. doi: 10.1016/j.tibs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Schutz AR, Giddings TH, Jr, Steiner E, Winey M. The yeast CDC37 gene interacts with MPS1 and is required for proper execution of spindle pole body duplication. J Cell Biol. 1997;136:969–982. doi: 10.1083/jcb.136.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siligardi G, Hu B, Panaretou B, Piper PW, Pearl LH, Prodromou C. Co-chaperone regulation of conformational switching in the Hsp90 ATPase cycle. J Biol Chem. 2004;279:51989–51998. doi: 10.1074/jbc.M410562200. [DOI] [PubMed] [Google Scholar]
- Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- Truman AW, Millson SH, Nuttall JM, King V, Mollapour M, Prodromou C, Pearl LH, Piper PW. Expressed in the yeast Saccharomyces cerevisiae, human ERK5 is a client of the Hsp90 chaperone that complements loss of the Slt2p (Mpk1p) cell integrity stress-activated protein kinase. Eukaryot Cell. 2006;5:1914–1924. doi: 10.1128/EC.00263-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaíno JA, Deutsch EW, Wang R, Csordas A, Reisinger F, Ríos D, Dianes JA, Sun Z, Farrah T, Bandeira N, et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat Biotechnol. 2014;32:223–226. doi: 10.1038/nbt.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton-Diaz A, Khan S, Bourboulia D, Trepel JB, Neckers L, Mollapour M. Contributions of co-chaperones and post-translational modifications towards Hsp90 drug sensitivity. Future Med Chem. 2013;5:1059–1071. doi: 10.4155/fmc.13.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Burke DJ. Cdc55p, the B-type regulatory subunit of protein phosphatase 2A, has multiple functions in mitosis and is required for the kinetochore/spindle checkpoint in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17:620–626. doi: 10.1128/mcb.17.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TJ, Kao GD. Mitotic checkpoint, aneuploidy and cancer. Adv Exp Med Biol. 2005;570:477–499. doi: 10.1007/1-4020-3764-3_17. [DOI] [PubMed] [Google Scholar]
- Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.