Abstract
For over three decades now, the T cell receptor (TCR) for antigen has not ceased to challenge the imaginations of cellular and molecular immunologists alike. T cell antigen recognition transcends every aspect of adaptive immunity: it shapes the T cell repertoire in the thymus and directs T cell-mediated effector functions in the periphery, where it is also central to the induction of peripheral tolerance. Yet, despite its central position, there remain many questions unresolved: how can one TCR be specific for one particular peptide-major histocompatibility complex (pMHC) ligand while also binding other pMHC ligands with an immunologically relevant affinity? And how can a T cell’s extreme specificity (alterations of single methyl groups in their ligand can abrogate a response) and sensitivity (single agonist ligands on a cell surface are sufficient to trigger a measurable response) emerge from TCR–ligand interactions that are so low in affinity? Solving these questions is intimately tied to a fundamental understanding of molecular recognition dynamics within the many different contexts of various T cell–antigen presenting cell (APC) contacts: from the thymic APCs that shape the TCR repertoire and guide functional differentiation of developing T cells to the peripheral APCs that support homeostasis and provoke antigen responses in naïve, effector, memory, and regulatory T cells. Here, we discuss our recent findings relating to T cell antigen recognition and how this leads to the thymic development of foreign-antigen-responsive αβT cells.
I. Introduction
With the ongoing identification and characterization of new T cell lineages with distinctive immunological functions, we increasingly appreciate how diverse and dynamic the T cell compartment really is. Such attributes are not surprising in view of the daunting task T cells are entrusted with: to distinguish “self” from “foreign” based on protein composition and then neutralize “foreign” with little time left before pathogens breach the first lines of innate defense. To manage this job, education, memory, networking, and fine-tuning capabilities are of the essence. As we now know, most of these predicates of adaptive immunity depend per se on T cell antigen recognition. Yet, though considerable strides have been made, many of the parameters by which antigen recognition operates are not fully understood.
Here, we principally discuss the mechanisms by which the peripheral T cell receptor (TCR) repertoire arises, so we must first set the scene. The T cell’s schoolhouse is the thymus, an organ whose bulk is comprised of the thymocytes that it educates, but whose structure is imparted by a spider’s web of stromal cells that includes the epithelial cells that act as teachers. The thymus is separated anatomically into an outer cortex and an inner medulla, both of which are composed of distinct epithelial cells1 and further populated with bone-marrow-derived macrophages and dendritic cells (DCs; although these are relatively more abundant in the medulla).2 T cell precursors are delivered to the junction between these layers and then migrate outward into the cortex, eventually reaching the outermost subcapsular zone (SCZ).3 These are double-negative (DN) thymocytes, so called because they are devoid of the coreceptors CD4 and CD8, and at this stage, they also lack a TCR.
The molecular genetic construction of the TCR is intimately and causally linked with T cell development. DNs in the outer cortex3 initiate recombination of the TCRβ locus, and are allowed to develop further only upon successful generation of TCRβ protein,4 as read out by TCRβ’s capacity to generate TCR signals in tandem with an invariant pre-TCRα partner.5 Once past this checkpoint, immature T cells form a TCRα gene and upregulate CD4 and CD8 to become CD4+CD8+ double-positive (DP) thymocytes. This represents the first T cell population that can receive signals from pMHC,6 and these cells spend the next several days navigating the web of cortical and then medullary antigen presenting cells (APCs), testing their newly minted TCRs against their host’s self-determinants.
II. TCR Structure and Diversity
TCRs are expressed on the surfaces of thymic and peripheral T cells as a disulfide-linked heterodimer composed of two glycosylated type I membrane proteins: TCRα (40–45 kDa) and TCRβ (40–50 kDa). Quite similar in structure to a Fab antibody fragment, the extracellular portion contains two variable (Vα, Vβ) immunoglobulin (Ig)-like domains and then two constant Ig domains (Cα, Cβ), which are followed by a transmembrane region and a short cytoplasmic tail. Relatively random VJ (TCRα) and VDJ (TCRβ) gene rearrangement at the DN stage of thymic development allows for a potential diversity on the order of ~104 TCR combinations,7 while N-nucleotide and P-nucleotide additions within the junctional CDR3 regions of TCRα and TCRβ increase the potential diversity enormously. In theory, roughly 1015 different αβTCR heterodimers could be produced,8 exceeding the number of T cells in a mouse (~2 × 108) or human (~2 × 1011).
The concentration of diversity in the CDR3s and the relative lack in the germline V-region-encoded CDR1s and CDR2s seems to be tailor-made for recognizing diverse peptides in comparatively monomorphic MHC molecules.8 Studies with mutated peptides9,10 and experiments with transplanted CDR3s11 showed a close relationship between CDR3s and peptide specificity. Structural studies of TCR–pMHC complexes have also generally borne this out by showing that the CDR3 loops form a large share of contacts with the peptide, while CDRs1 and 2 more often contact predominantly the helices that comprise the MHC’s peptide binding cleft12,13 (though exceptions do exist to this generality).14 This is not to say that the mode of binding for a particular pair of V regions to a particular MHC is the same across all TCR–pMHC pairs. Rather, the CDRs1 and 2 appear to adopt one of a handful of discrete docking angles over the MHC.15 As another line of evidence, a study of the binding between the 2B4 TCR and its pMHC ligand showed that mutations in the contact area between TCR and MHC led primarily to defects in the initial association of the two, while mutations in the contact area between TCR and peptide led primarily to defects in the stability of the bound complex. We suggested that this might be by design: that the CDRs1 and 2 might allow the 2B4 TCR to associate readily with pMHCs bearing many different peptides, but that the more stable association that leads to TCR triggering would be dictated more by the peptide and CDR3s16 (Fig. 1). As no preselection TCR has been examined in this way, it is impossible to say whether this property is a result of evolutionary pressure on the germline V regions or is the result of thymic selection.17
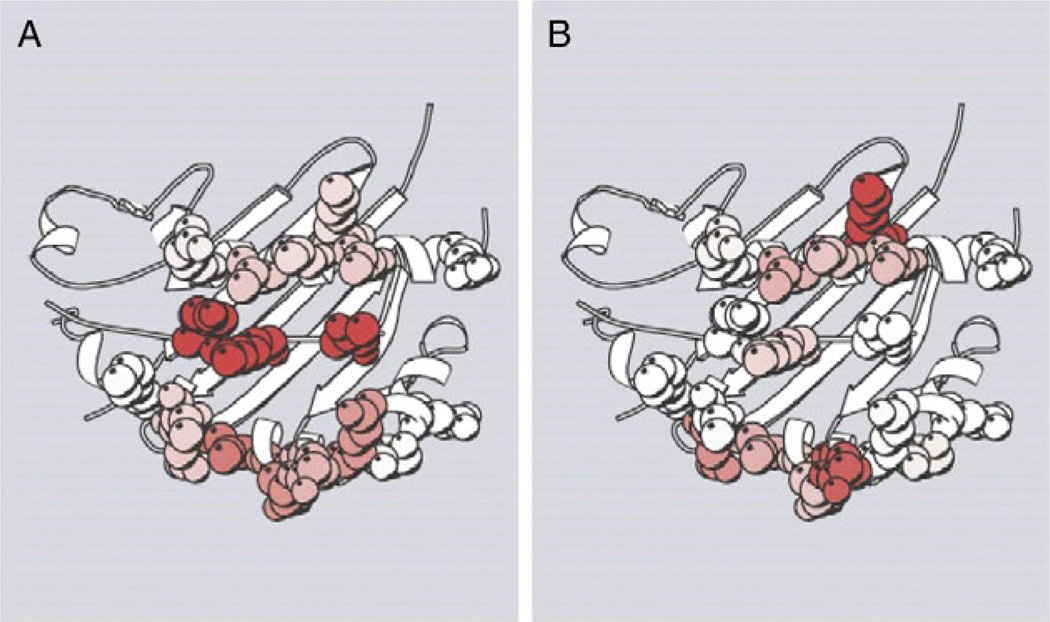
Fig. 1.
ϕ analysis of the contribution of MCC–I-Ek surface residues to 2B4 TCR–pMHC association versus stabilization. The peptide-binding pocket of I-Ek and the MCC peptide are shown as white ribbons. Residues studied are shown as space-filling representations and are color-coded with respect to their effect on stability and association. (A) Contributions of residues to complex stability (ΔΔG). Residues are shaded white (no effect, ΔΔG = 0 kcal mol−1) to gray (maximum effect in this study, ΔΔG > 3.14 kcal mol−1). (B) Contributions of residues to complex association (ϕ). Residues are shaded white (no interaction, ϕ = 0) to gray (maximum interaction in this study, ϕ = 0.73). Figure originally from Ref. 16.
In reality, many fewer than 1015 distinct functional specificities are apparent in the peripheral T cell repertoire. Studies in mice have shown that the frequency of naïve CD8+ or CD8+ T cells that are specific for a particular foreign pMHC tetramer lies in the range of dozens to a few hundred per 108.18,19 Our own studies of naïve human CD8+ T cells yield a very similar answer (Yu and Davis, unpublished data), giving an estimate of functional ligand diversity that can be distinguished by naïve αβT cells of 106–107. The relationship of TCRs with ligands is not remotely one-to-one, as TCRs can exhibit substantial polyspecificity for pMHCs,20 and distinct TCRs can recognize the same pMHC.21 Rather, this relationship appears to be many-to-many22 and, as there has not been any estimation of the clonality of these tetramer-positive populations, it is difficult to estimate the repertoire’s actual diversity in terms of TCR protein sequences based on this method. Earlier estimates of the diversity based on TCR gene sequencing arrived at an answer of 107–108 distinct TCRs among human peripheral blood T cells.23 Taken together, this might lead us to guess, very roughly, that each T cell in mice has on average ~10 identical twins. This would accord with still earlier results that found that it was common to observe identical TCRs arising in clones raised against a particular antigen from immunization of different mice.24 That is, there is typically a significant TCR overlap between individual mice, so each antigen reactivity is likely represented by a handful of distinct TCRs in mice, and each of these TCRs is likely to be represented by multiple naïve T cells.
Even though these estimates are imprecise, they leave a substantial gap between the number of theoretically distinct TCRs and the number that occupy the peripheral lymphatics of mice and humans. While some of this loss in diversity can be explained by the mechanics of TCR recombination4 or the inability of particular TCRαs to pair with particular TCRβs, more than 90% of successfully rearranged TCRs are lost in the thymus along with the T cells that bore them.25,26 In the following sections we discuss the knowledge we have gained in recent years regarding which self-pMHCs guide the selection of these TCRs, how immature T cells access this self-antigenic information, and how a microRNA helps guarantee proper self-tolerization. And finally, we speculate on which TCRs are selected and why they are chosen.
III. Thymic Self-Peptides Involved in T Cell Selection
Cognate antigen, that is, the pMHC that acts as an agonist for a particular TCR and activates mature T cells bearing that TCR, also generally induces apoptosis in immature DP thymocytes in vitro and in vivo.27 These agonist interactions often occur with affinities in the range of 1–40 µM in terms of their dissociation constants (KD’s).28,29 For mature T cells, whether the dissociation constant better predicts the biological potency of the pMHC, as opposed to the half-life of the TCR–pMHC interaction or a combination of thermodynamic parameters, is a topic of contemporary debate and is described elsewhere.29 However, for immature T cells, across three distinct class I-restricted TCR systems, the threshold separating positively from negatively selecting pMHC was found to be consistent and very narrow in terms of the KD of TCR–pMHC interaction (T1, S14, and OT-I TCRs).30 This analysis was performed using soluble photo-crosslinkable pMHCs on the surfaces of T cells and so describes the threshold in terms of apparent KD (which includes the small contribution of CD8 to binding),31 but the stronger pMHC ligands examined bound tightly enough to be measureable in terms of monomeric affinity and could be used for comparison. The positive/negative selection threshold thus appears to lie very close to the range of affinities found for agonists. Furthermore, earlier studies had measured the monomeric affinities of two positively selecting altered peptide ligands, E1 and R4, in the OT-I system (i.e., variants of the agonist Ova peptide). At 20 µM28 and 40–57 µM28,32 at 25 °C, respectively, these measurements also support the idea of a narrow affinity gap between positive and negative selection.
There is a snare, however. When E1 was coexpressed alongside the OT-I TCR transgene, in otherwise peptide-deficient mice, OT-I T cells were positively selected and exported to the periphery. But these cells were functionally compromised and hyporesponsive,33 similar to the way in which the P14 T cells34 selected by very low doses of agonist peptide in fetal thymic organ cultures (FTOCs) had earlier been found to be compromised.35 Whether E1-selected cells bearing the OT-I TCR would contribute to the peripheral repertoire in normal mice, then, is in question.
Two naturally occurring H-2Kb-binding peptides that promote positive selection of OT-I T cells have also been known for some time.36,37 Both these peptides bore structural similarities to the nominal Ova antigen, prompting the authors to suggest that structural similarity might underlie the potential of self-peptides to promote selection. Until recently, however, we did not know the affinity of the OT-I receptor for these peptide-H-2Kb. We have now determined their affinities and, somewhat surprisingly, they are much weaker than the a fore measured E1 and R4.38 Indeed, they bound so poorly at 25 °C that they could be measured only at 10 °C, where their dissociation was sufficiently slow to calculate an accurate KD. In order to more accurately compare them, we therefore also measured the KD of Ova-H-2Kb at this temperature. At a KD of 136 mM for Catnb-H-2Kb (15.6-fold lower than Ova-H-2Kb at 10 °C) and 211 mM for Cappa1-H-2Kb (24.3-fold lower than Ova-H-2Kb at 10 °C), these endogenous selecting ligands bind OT-I two- to sixfold more weakly than R4 (8.8-fold lower than Ova-H-2Kb at 25 °C) and E1 (4.4-fold lower than Ova-H-2Kb at 25 °C). These results significantly broaden the spectrum of self-peptides, in terms of affinity, that are relevant for thymic selection. Also, while the threshold between positive and negative selection appears quite sharp in vitro,30 for the admittedly few naturally occurring selecting peptides, it appears much broader. This in turn might also help us understand the TCR specificity fostered by the thymus: it seems quite a task to accurately distinguish pMHCs that differ by only twofold in affinity, but if the thymus effectively removes this “middle ground” by negative selection or attenuation,33 or by some other mechanism, then the task becomes much easier.
With respect to the identification of selecting peptides, the study of class II-restricted systems has lagged behind, as no selecting peptides have been described until recently. But we have now begun to close that gap. Recently, our group39 as well as that of Paul Allen and colleagues40 reported the identification of endogenous I-Ek-bound peptides that can drive the in vitro selection of functionally mature 5C.C7 or AND TCR transgenic T cells on the B10.BR background, respectively (Fig. 2). Among the 95 tested, 6 different peptides were found with this property for 5C.C7, the most potent among them being an endogenous retroviral Gag-Pol-polyprotein-derived peptide (which we refer to as Gag-Pol or GP), whereas for AND, 1 peptide, a gp250-derived peptide, was found among the same pool. In its putative I-Ek-binding register, GP harbors four residues that are identical to those in the agonist Moth Cytochrome C (MCC) and a fifth residue that is a chemically conservative substitution (the P1 leucine, which is an isoleucine in MCC). These identical and similar residues occupy the I-Ek-contacting positions,42 while the TCR-contacting side chains are very different. This strengthens the argument that positively selecting ligands for a given TCR are structurally similar to that of TCR’s nominal antigen37,45 and/or that the selecting ligand might help to enforce an MHC conformation that is similar to that of the nominal antigen-MHC.46 The ability of antibodies raised against the complex of nominal antigen-MHC to block positive selection in two different TCR systems further supported this case.47,48 Again, there is a snare, though: the third data point does not follow this rule. The gp250 peptide that positively selects AND T cells is structurally very distinct from the agonist MCC: only the P1 and P9 anchor residues are conserved, while the P4 and P6 anchors are chemically quite distinct (L → T and Q → S, respectively, from MCC to gp250), and the TCR contacts are likewise quite distinct. Nor does this represent degenerate recognition of gp250 by AND because single mutations of the gp250 peptide destroy its ability to select AND T cells. Even a substitution at P2 that restores the “MCC-like” alanine residue abrogates rather than strengthens AND reactivity. To levy a parting defense of the role for structural similarity, however, we found that an ATPase 11c-derived peptide could weakly promote positive selection of 5C.C7 thymocytes and that this peptide could also negatively select 5C.C7 thymocytes at high doses. The ATPase 11c peptide is highly similar to the agonist MCC and, unlike GP, it is chemically identical to MCC at known TCR-contacting residues (P2, P5, and P7).42
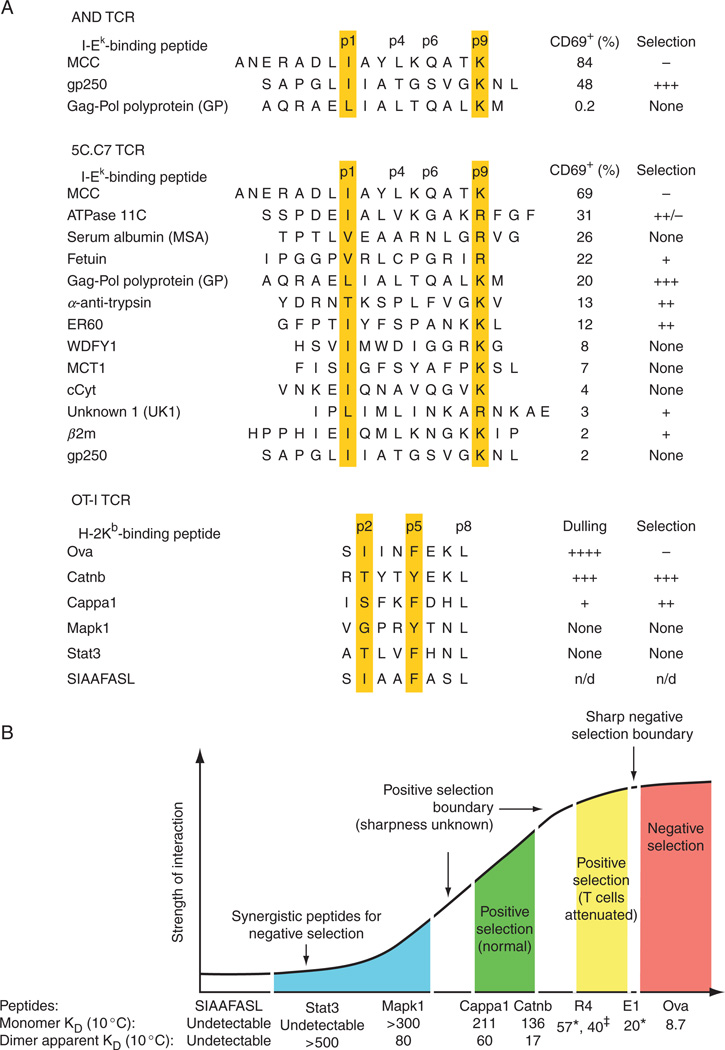
Fig. 2.
Summary of sequence and thermodynamic data for the self-peptides with reactivity toward the AND, 5C.C7, and OT-I TCRs. (A) Amino acid sequences of a subset of the known I-Ek-bound self-peptides41 and H-2Kb-bound self-peptides (Ref. 37; the Cappa1 peptide was originally described in Ref. 36). I-Ek-binding peptides are shown in their predicted I-Ek-binding register; MHC pocket residues are highlighted.42,43 The peptides’ reactivity toward the indicated TCR was measured by CD69 upregulation of DP thymocytes bearing that TCR toward the indicated peptide presented by APCs or platebound I-Ek.39,40 For OT-I, reactivity was measured in terms of CD4 CD8 coreceptor downregulation, or dulling.37 The ability of these peptides to positively select T cells bearing the indicated TCR was assessed: for AND, in reaggregate thymus cultures with the ANV41.2 cTEC cell line as APCs; for 5C.C7, in 5C.C7 Ii−/− fetal thymus organ cultures (FTOCs); and for OT-I, in OT-I TAP−/− FTOCs. (B) A summary of affinities of the OT-I TCR for different peptide–H-2Kb and a schematic of the relation between affinity and selection outcome. Positive selection data for E1 (EIINFEKL) and R4 (SIIRFEKL) are from Ref. 44, positive selection data for the self-peptides are from Ref. 37. Affinity measurements are from Ref. 38 except for ☼ (Ref. 28), at 25 °C, and ‡ (Ref. 32), also at 25 °C.
Speaking more generally in terms of the thymic peptide repertoire, prior work has shown that the bulk of the quantity of self-peptides presented in the thymus represents a limited number of distinct species and these are roughly the same species as those found on splenic APCs in the periphery.49 It must also be noted, however, that multiple genetic programs that are unique to thymic APCs and result in alteration of the peptide repertoire presented by those APCs have been identified, presumably to better select maturing T cells. For example, the autoimmune regulator, or AIRE, directs the expression of tissue-restricted proteins (e.g., pancreatic-, salivary gland-, ovary-specific proteins) in a set of medullary epithelial cells,50 which are then presented directly51 and/or cross-presented by nearby phagocytic APCs52 to achieve tolerance against those tissues. Lymphotoxin-β receptor regulates a similar expression of tissue-restricted antigens (TRAs) in a distinct subset of medullary thymic epithelial cells (mTECs).53 While many thymic DCs, both cortical and medullary, are autochthonous, DCs also migrate into the thymus from the skin and blood, and potentially from other sites. These DCs reside primarily at the corticomedullary junction and their role in promoting central tolerance has also been confirmed.54 With respect to positive selection, cortical thymic epithelial cells (cTECs) are unique among epithelial cells in that they express class II, which is obviously an advantage for educating CD4+ T cells. They also have adaptations in their MHC loading pathways that are thought to facilitate selection. For example, cTECs express cathepsin L, which diversifies and/or alters the repertoire of self-peptides presented by class II molecules.55 In the class I loading pathway, cTECs constitutively express a thymus-restricted β5t proteasome subunit along with a β5i subunit that is IFNγ-inducible in somatic cells, which similarly results in a more diverse array of class I-presented peptides.56 Both these adaptations are necessary for a fully diverse T cell repertoire, although whether their necessity stems simply from the increased peptide diversity (as suggested by the classic experiments of Hogquist)57 or from the presentation of uniquely cTEC-restricted peptides remains to be seen.58 In either case, these mechanisms all illustrate the pains taken by the thymus to broaden the scope of the immunological “self” presented to developing T cells.
IV. Synaptic and Nonsynaptic Interactions in the Thymus
As discussed above, the thymus contains a wealth of information regarding the host’s antigenic self. To optimize its education, a thymocyte would be best served by referencing all of it against its TCRs. But how to accomplish this? The thymus is vast, on a cellular scale, and the thymocyte’s task is great. Since the APCs cannot go to the thymocytes, the thymocytes must go to the APCs. And this is what they do, in a manner well suited to their purpose.
The first clues pointing to a potential mechanism came from the earliest studies of TCR transgenic mice. Here, it was found that a relatively linear increase in the total number of selected T cells resulted from an increase in the surface density of MHC on the selecting epithelium59 (but no increase was observed with increased TCR density on the developing T cells).60 This contrasted with negative selection, which could be induced by minute amounts of agonist ligand and which titrated precipitously at these very low doses.35 And, although this positive selection “niche” can be saturated in mice expressing TCR transgenes, who therefore have abnormally large numbers of “selectable” immature T cells, it does not appear to be saturated in normal mice.59,61 The idea of a niche for positive selection was expanded by Merkenschlager and colleagues,62 who generated reaggregate thymus cultures in which only some cTECs expressed the selecting ligand. In this case, a dose-dependent and fairly linear increase in T cell selection could be observed as the proportion of selecting to nonselecting cTECs increased. Again, negative selection differed, in that only very few negatively selecting APCs were required to obliterate nearly all autoresponsive DP thymocytes. A physical niche characterized by a static interaction of thymocytes with positively selecting cTECs was suggested as the explanation for these data.63
With the benefit of new studies exploring the migration of maturing T cells within the thymus, we can reexamine this interpretation. While DN thymocytes arrived at the SCZ by virtue of directed migration in response to CXCR4 ligands,64 DPs do not appear initially to follow a directed pattern of migration on their journey back through the cortex. Rather, they adopt a pattern of motility best described as a random walk,65 where nonresponsiveness to cues for directed migration is actively maintained by a repressor of chemokine receptor signaling, the G protein-coupled receptor kinase-interactor 2 (GIT2).66 Upon encountering a positively selecting cTEC, signaling ensues and the DP arrests its motility.67,68 However, this arrest is short-lived, on the order of 5–10 min, and the DP does not form a stable contact or “synapse” with the stimulating cTEC,69 as a mature T cell would in response to a stimulatory APC in most70,71 but not all cases.72 After this brief interlude, motility resumes. But this transient contact alone is insufficient for commitment to positive selection: the DP must find new selecting cTECs, or return to an old one, in order to maintain active Nuclear Factor of Activated T cells (NFAT) and other chromosome remodeling machinery long enough73,74 to continue its maturation.69 Thus, it seems more likely that the selecting niche is actually scattered along the DP’s path and is measured not in terms of cellular space available to swaddle DPs but in terms of the probability of finding sufficiently many distinct sources of selecting pMHCs as the DP meanders its way through the cortex. This hypothesis, which we have described as a “gauntlet” model, can explain how selection could be linear with both the number of selecting APCs and with the total density of selecting MHC, as both affect the probability of encountering a selecting cTEC. In further support of this, positive selection is attenuated in the absence of GIT2 and the random walk behavior that it promotes.66 The majority of thymocytes likely do not have sufficiently self-peptide-self-MHC-compatible TCRs, and these fail to survive for want of promaturation TCR signals.
The cortex holds additional perils for developing thymocytes as well.75 We know that negative selection is brutally efficient both in terms of the number of APCs required to achieve maximal clonal deletion in thymus cultures (Ref. 62 and our unpublished data) and the number of agonist pMHC ligands required to achieve deletion in vitro.69,76,77 Cortical DCs are potent negative selectors2 and, although cTECs generally lack costimulatory molecules that are required for direct induction of negative selection,78–80 at least a subset of cTECs also can contribute to deletional tolerance.81 It seems likely that, for a thymocyte burdened with an autoreactive TCR, these APCs will be very hard to avoid. After all, the thymocyte is already compelled to bounce around the cortex to seek out new brief cTEC contacts from which to glean positively selecting signals. Upon encounter with a thymic DC or TEC that presents negatively selecting ligands in reaggregate thymus cultures, rather than arrest and resume its motility, the thymocyte stops and forms a stable contact, typified by TCR accumulation at the interface with the APC as in a mature T cell synapse.82,83 In thymic slices, negatively selecting thymocyte–DC interactions appear less stable, with the thymocyte restricted in its movements to ~30 µM, but whether synapses form in this situation could not be addressed.84 In vitro, this negatively selecting synapse can be formed in response to as few as two agonist pMHCs, and apoptosis ensues within 90 minutes.69 In the case of cTEC-mediated negative selection, simultaneous or subsequent interaction with distinct APCs may also be important,85 but this has not yet been addressed by the microscopy studies. Finally, we can show that this synapse formation is necessary for efficient negative selection in vitro and in thymus cultures, as shown by antibody blockade of LFA-1 and the ensuing inability of thymocytes to form stable contacts with agonist-presenting APCs (Ref. 69 and our unpublished data). This is similar to mature T cells, which require the prolonged contact with APCs that is afforded by synapse formation to commit to expansion and effector function.86
We have likened this process to a gauntlet because to have any chance of survival and to be initiated into the peripheral repertoire, the developing thymocyte must “run the gauntlet” of the thymic cortex (Fig. 3). It must continually find suitable low-affinity self-pMHCs to maintain active NFAT in the nucleus, yet at any moment it may encounter an APC bearing a number of negatively selecting peptides that is sufficient to induce synapse formation and apoptosis.
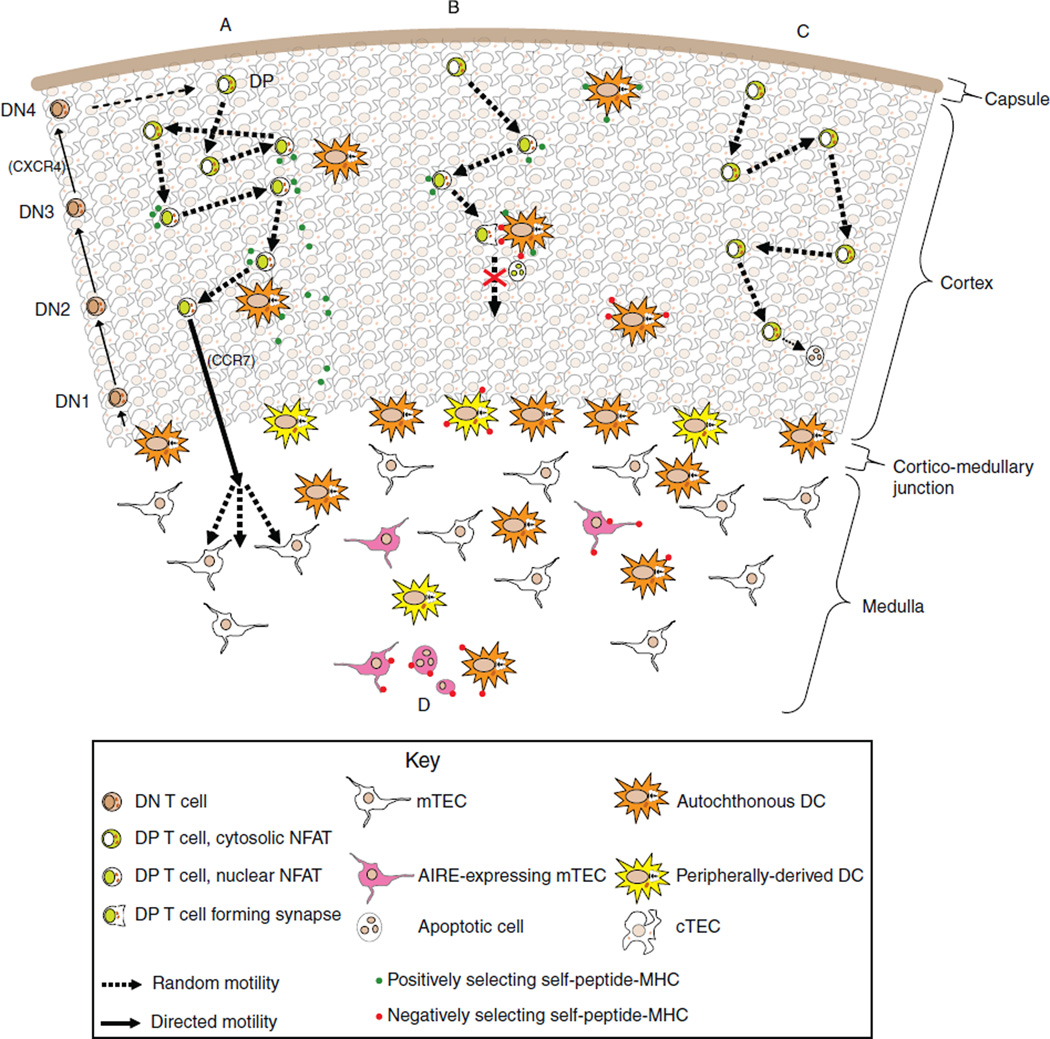
Fig. 3.
The gauntlet model of thymic T cell maturation. A Schematic of thymocyte migration in response to developmental and TCR-triggered cues. (A) A thymocyte whose eventual fate is positive selection and maturation. Common lymphoid progenitors (CLPs) initially seed the thymus87 and migrate outward in a directed fashion in response to chemokine signaling,3 while discrete anatomic locations within the cortex support TCR recombination and maturation to the DP stage. Once these DN cells become DPs at the SCZ, they instead adopt a random-walk pattern of migration. When a DP encounters positively selecting pMHCs, it pauses briefly and initiates signaling, which includes NFAT nuclear translocation and activation, but does not include synapse formation with the stimulating cTEC. Individually, these contacts are too brief to support the sustained signaling that is required for maturation to proceed,73,74 and so reencounter with additional positively selecting cTECs presumably is required. When sufficient signaling has occurred, the DP transitions to the late DP/semi-mature SP stage,88 which includes upregulation of CCR789 and downregulation of GIT2,66 allowing directed migration toward the medulla. Additional signals within the medulla support further maturation,90 while encounters with medullary APCs (which can present antigens not found in the cortex) run the risk of negative selection.84 (B) A thymocyte whose eventual fate is negative selection in response to a cortically presented antigen. DPs’ random walk motility increases the probability that they will encounter fairly sparse cortical DCs that can present negatively selecting self-antigens. Once encountered, the DP forms an immunological synapse that prevents it from leaving the APC,82 providing sufficient time to complete the apoptotic response.69 (C) A thymocyte whose eventual fate is neglect. (D) Distinct medullary APCs present antigens derived from disparate sources. DCs can migrate from the blood and skin, or develop intrathymically. Subsets of mTECs possess genetic programs that lead to expression of tissue-restricted antigens. These TRAs might be presented directly by mTECs,51 or cross-presented by other medullary APCs such as DCs.52
A thymocyte that escapes deletion and receives sufficiently many positively selecting signals continues its maturation program. This includes upregulation of the chemokine receptor CCR7, which prompts migration toward the medulla89,91 in a directed fashion.65 Entry into the medulla is then further gated by a pertussis-toxin-sensitive mechanism.92 Once migrated there, the thymocyte is exposed to new APCs with new self-antigens that can delete autoreactive thymocytes whose cognate self-antigens are not present in the cortex88,93: AIRE-expressing medullary TECs, for example,50,94 and peripherally derived DCs.54 The necessity of this step is evidenced by the failure of central tolerance in CCR7-deficient mice.95,96 Alternatively, if the thymocyte’s TCR passes this final test, it is fostered and eventually exported to the periphery as a naïve T cell.90
V. miR-181a, A MicroRNA That Enhances T Cell Sensitivity and Promotes Tolerance
Another feature of developing thymocytes is their heightened sensitivity toward pMHC ligands, over and above the already remarkable sensitivity of mature T cells, both in terms of the quality97 and number76,77 of ligands they can recognize. For example, while mature CD4+98 and CD8+99 T cells require contact with ~3–10 agonist pMHCs to mount a full effector response, DP thymocytes require only 2 to trigger apoptosis.69 Alternative sialylation of cell surface molecules could partially explain this heightened sensitivity,100 as could lower CD5 expression in an independent study101 and more recently high CD45 expression,102 but other factors clearly are at play.
One such factor is miR-181a, a microRNA previously identified as playing a role in influencing the B/T lineage decision.103 microRNAs are ~20–22 nucleotide RNAs in their mature form and exert their influence in combination with proteins of the RNA-induced silencing complex (RISC) by targeting mRNAs for degradation or translational repression.104 We previously showed105 that miR-181a suppresses multiple phosphatases that negatively regulate the TCR signaling cascade: Ptpn22, which targets the activating phosphorylation sites of lck and ZAP70106,107; Shp2, an effector for inhibitory transmembrane adapter proteins (TRAPs) such as SIT and TRIM, which have also been shown to influence thymocyte sensitivity and tolerance108; DUSP5, which dephosphorylates activated Erk in the nucleus; and DUSP6, which targets cytosolic Erk.109 While each of these four targets was repressed only moderately, from two- to fivefold, overexpression of miR-181a in mature T cells resulted in a dramatic shift in their responsiveness, in the form of both higher absolute calcium signals, an ability to respond to fewer agonist pMHCs than are normally required, and the ability to respond to pMHC ligands that normally are too weak to stimulate a response.105 This resulted from increases in both steady-state and TCR-signaling-induced levels of activated lck and Erk. This mirrors the similar and elegantly delineated mechanism by which the balance of the lck-activatory phosphatase CD45 and the lck-inhibitory kinase Csk modulates TCR sensitivity in developing T cells.102
With regard to thymic selection, we also found that miR-181a was expressed at about four- to eightfold higher levels in DP thymocytes than in mature T cells. When miR-181a was inhibited in 5C.C7 transgenic thymus cultures by use of an anti-miR-181a antagomir,110 a reduction in the number of positively selected T cells could be observed. A similar diminution of the TCR response could be seen in negatively selecting thymus cultures, where a relatively high number of 5C.C7 thymocytes survived in the miR-181a-inhibited thymi. These results led us to suggest that miR-181a was at least in part responsible for thymocyte hyperreactivity toward pMHC and that the developmentally regulated expression of miR-181a at the DP stage might represent a mechanism favoring central tolerance.
This presumably would operate by raising the sensitivity of DP cells and allowing the deletion of thymocytes bearing TCRs with moderate affinity for self-pMHC. As the remaining T cells mature, they would reduce their miR-181a expression and lose the ability to respond to these moderate-affinity pMHCs. This might create a buffer zone in terms of affinity for self, presumably representing KD’s in the range of ~50–100 µM (Fig. 1). In order to test whether such a buffer exists and whether it is important for self-tolerance, we subsequently made use of the various I-Ek-bound self-peptides that we had characterized in the 5C.C7 system.39 First, to test whether miR-181a is important for efficient induction of central tolerance, we allowed wild type T cells to mature in miR-181a-inhibited thymus cultures and then challenged these T cells with syngeneic APCs. Compared to the T cells from unmanipulated thymi, which remained quiescent, miR-181a inhibition resulted in a small but appreciable population of autoreactive T cells as measured by CD69 upregulation and the production of inflammatory cytokines. To test whether these autoresponsive T cells were responding toward particular self-peptides of especially high affinity, we made use of the two self-peptides that we had previously found to have the highest capacity to stimulate the 5C.C7 TCR, GP, and ATPase 11c (Fig. 4). By fixing the 5C.C7β chain but allowing the TCRα chain to vary as normal, we hoped to generate a preselection repertoire that would be biased toward recognizing these two peptides. We then allowed the 5C.C7β-transgenic thymocytes to mature in thymi in which miR-181a was inhibited. Using GP-I-Ek and ATPase-I-Ek tetramers, we could then see that the miR-181a-inhibited thymi had allowed the maturation of many more GP- and ATPase-reactive T cells, as compared to otherwise unmanipulated 5C. C7β-transgenic thymi. Taken together, these results strongly support a role for developmentally regulated miR-181a-dependent hypersensitivity in enforcing central tolerance. This in turn supports the notion that, in addition to the spectrum of self-determinants presented by disparate APCs and the affinity of a thymocyte’s TCR for those self-pMHCs, the sensitivity of a thymocyte’s TCR signaling network is also a critical parameter determining the outcome of selection.
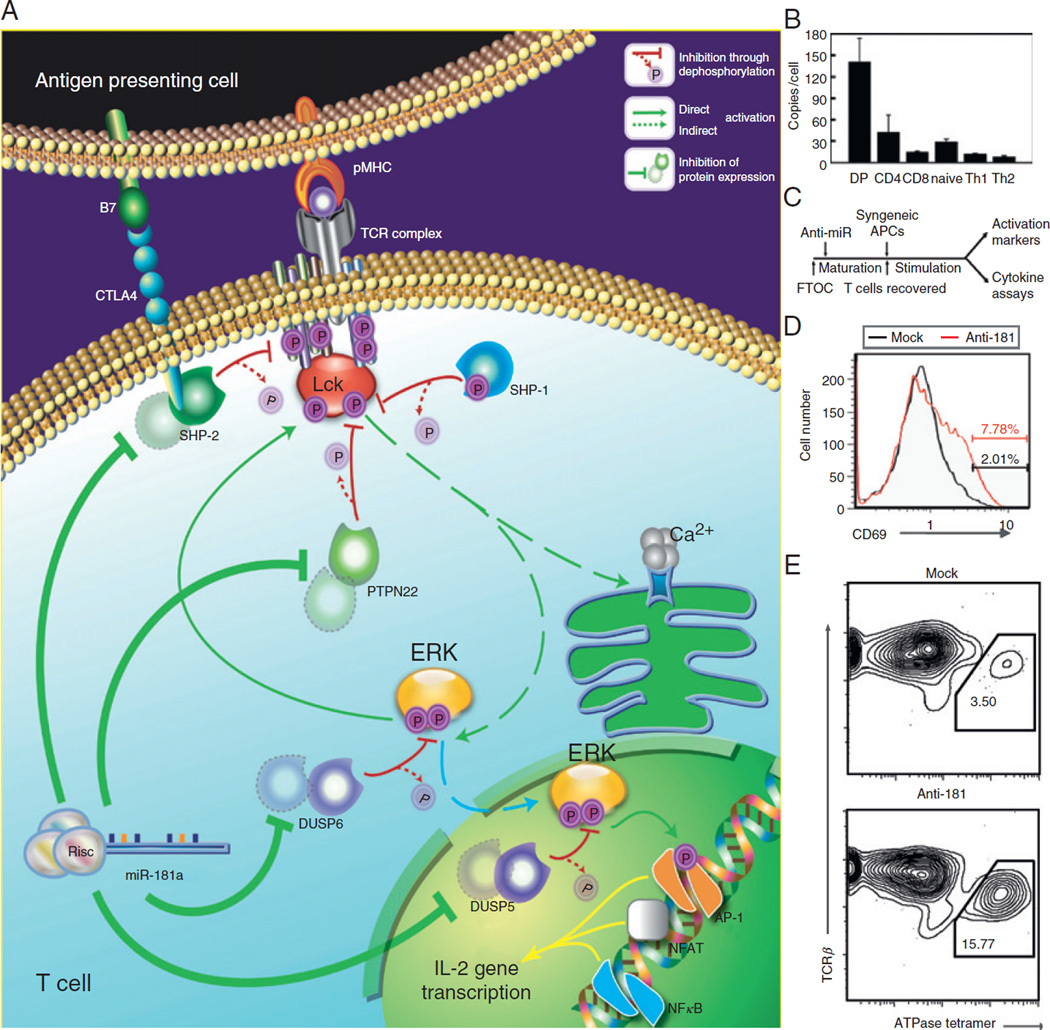
Fig. 4.
miR-181a-mediated hypersensitivity in thymic selection. (A) A schematic of miR-181a’s targets and their positions in a mature T cell’s TCR signaling cascade. (B) Expression level of miR-181a in developing T cell populations, as measured by quantitative real-time PCR. (C) Schematic of the protocol used to test the effect of miR-181a inhibition on T cell development. E16 FTOC were established and treated with antagomir specific for miR-181a, or with seed-region-scrambled antagomir which does not reduce miR-181a levels. After 5–6 days, mature T cells were harvested from these FTOCs, washed to remove any residual antagomirs, and then cocultured with syngeneic (B10.BR) splenic APCs. (D) B10.BR FTOC (in which T cells possess a normally diverse TCR repertoire) were treated in this way, and the resulting mature T cell populations were assayed for expression of the activation marker CD69 by FACS. Gated CD4+ T cells are shown: miR-181a inhibition in the FTOC resulted in a small population of autoreactive T cells. (E) 5C.C7β-transgenic B10.BR FTOC were treated as in part (C), and the resulting T cells were assayed using an ATPase 11c-I-Ek tetramer by FACS. Gated CD4+ T cells are shown: miR-181a inhibition in the FTOC resulted in an increased proportion of T cells that recognize the ATPase 11c self-peptide. Parts (A, B) are originally from Ref. 105 parts (C–E) are originally from Ref. 39.
How, then, is miR-181a regulated during T cell maturation? miR-181a downregulation might simply be part and parcel of one of the many known genetic programs that support T cell maturation.111 A more intriguing possibility is that TCR signaling directly regulates miR-181a expression. Stochastic variation in the expression level of positive and negative regulators has been shown to lead to differences in the strength of signal transduction among individual cells,112 and it has been suggested that epigenetic regulation of other negative regulators might accomplish similar modulation of signal strength in response to thymic TCR signaling.113,114 As preliminary evidence for this possibility, we found that TCR signaling in response to both agonists and positively selecting ligands could dramatically downregulate the level of mature miR-181a within 1 hour.39 We do not yet know the mechanism underlying this unusually rapid miRNA downregulation, however, and we do not know whether it is this direct mechanism or another entirely separate mechanism that results in the low level of miR-181a in mature T cells. It is tempting to speculate, however, that this might also explain why T cells selected by high-affinity positively selecting ligands appear to be attenuated in their responsiveness in the periphery.33 If especially strong TCR signals in the thymus induce higher than normal miR-181a downregulation and if this low miR-181a expression is maintained during the remainder of the T cell’s maturation, then this low miR-181a might render the resulting T cell population hyporesponsive. Also, interestingly, miR-181a is even more highly expressed in DN T cells than in DPs, and miR-181a enhances progression of T cells from the DN to the DP stage in OP9-DL1 cocultures.105,115 This even higher level of miR-181a expression might in turn cause an even higher degree of TCR hypersensitivity, which might allow DNs to receive maturation cues from the transient dimerization of pre-TCR.5,116 Whether the ensuing miR-181a downregulation then results directly from these pre-TCR signals, or as the indirect result of the DN-to-DP developmental program, is also unknown.
VI. The Selecting Ligand and the Purpose of Selection
The critical role for self-MHC in the positive selection of developing T cells has been well accepted,117–119 and when mice bearing monoclonal T cell populations became available, this requirement was also found in most cases to be specific to the particular MHC allele to which that TCR was restricted, in terms of its ability to respond to foreign-peptide-self-MHC.21,120 This dovetailed beautifully with the established phenomenon of self-restriction of polyclonal T cell populations to thymic MHC, and self-MHC has been credited with self-restriction ever since.121 More recently, evidence for the importance of self-MHC in selection has also come from the TCR side of the equation. In the context of a polyclonal repertoire, mutations of residues within CDR2β that contact invariant portions of I-Ab reduced the number of selected T cells in I-Ab+ mice.122 While we have been forced to admit that there is also a requirement for specific self-peptides,37,48,57 this requirement has been generally characterized as permissive, in that the peptide might allow the invariant portions of the MHC to adopt a particular conformation that is reminiscent of the agonist-MHC46 or might itself act as a structural mimic of the agonist.37
Self-peptides have taken a back seat to self-MHC in terms of their influence on the peripheral TCR repertoire, and it is not immediately clear why this should be. A strong case can be made for the role of self-peptides, based on evidence that is very parallel to the case for self-MHC. Absence of class I or class II molecules results in the near absence of CD8+ or CD4+ T cells, respectively. Mutations in the peptide-binding cleft of I-Ek were found to have a similar effect in mice transgenic for an I-Ek-restricted TCR.123 Reducing the repertoire of self-peptides from its usual diversity to a handful of distinct species also results in a >95% deficit in T cell numbers in a polyclonal setting: whether by β2m or TAP mutation in class I systems33,57 or combined invariant chain/HLA-DM mutation in class II systems.124,125 In a beautiful demonstration of the peptide’s importance, the size of the selected CD8+ T cell repertoire in b2m-deficient thymi could be reconstituted in a dose-dependent fashion by the addition of increasingly complex mixtures of exogenous peptide.57 Upon later examination of thymi with monoclonal expression of the OT-I TCR, only 2 out of 27 tested endogenously derived H-2Kb-binding peptides were capable of supporting positive selection.37 And, in fact, this fraction overestimates the actual proportion of selecting peptides among the self-peptide repertoire by roughly twofold, as the peptides were split into two fractions prior to mass spectrometric identification and only the active half was assayed further. More recently, we39 and Lo et al.40 have mirrored this result using the class II-restricted 5C.C7 and AND TCRs, respectively: 6 of 95 naturally occurring I-Ek-bound peptides could reconstitute positive selection of 5C.C7 (5 of them only very weakly) and 1 of 95 peptides could drive selection of AND T cells. As an additional demonstration of the critical role for specific peptides in selection, it should be noted that, although 5C.C7 and AND recognize the same nominal antigen (a pigeon or moth cytochrome c-derived peptide presented by I-Ek), the self-peptides that select these two TCRs are distinct. So, while self-MHC is necessary for positive selection, it is also clearly not sufficient. These data, both old and new, are substantial hurdles for the idea that positive selection confers self-MHC-restriction.
It is certainly a compelling idea that T cells should be self-referential on the level of an individual animal. But how necessary is it that TCRs reference the invariant portions of self-MHC in each successive generation? Recently, the possibility that MHC restriction is encoded at least partially in the germline elements of the TCR’s V regions has received new attention. If TCRs are predisposed to bind MHC by virtue of their natural history, then the critical question for self-restriction is whether thymic selection can improve the likelihood that a selected T cell will be able to recognize a foreign-peptide-self-MHC. Some 90% of thymocytes are thought to be discarded because they fail positive selection.25,26 The present model of self-restriction posits that they fail because they could not bind to foreign-peptide-self-MHC or at least that they are less able to bind foreign-peptide-self-MHC complexes than those thymocytes that survive. This has been difficult to address directly, as it requires an extensive evaluation of the preselection TCR repertoire not only in terms of TCR sequence but also in terms of pMHC binding. But the indirect evidence that is available points to a different answer. Earlier work had shown that T cells selected by a TCR-nonspecific stimulus (either anti-TCR antibody126 or Ly6A.2127) were as reactive toward a third-party MHC as T cells from a normal mouse. But as this third-party MHC was not “self” (as indeed these T cells had no “self” to speak of), it remained possible that positive selection would in fact focus the repertoire on the particular MHC allele(s) present in the thymus. This possibility became less likely, however, in light of more recent results showing that T cells that arise in negative-selection-deficient mice are cross-reactive toward multiple MHC alleles.128 While negative selection presumably reigns in this cross-reactivity in a normal animal,20 positive selection appears to generate a repertoire that is not particularly focused on the selecting MHC allele. Also compelling in terms of the TCR side of the relationship was the finding that this cross-reactivity depended on conserved residues within the CDRs1 and 2.129 As another test of the germline compatibility of TCRs with MHC, the class II homolog HLA-DM was expressed on the cell surface in MHC-deficient mice, and this could not restore T cell selection.130 The question remains open, however, whether this failure resulted from the lack of positive evolutionary pressure on the invariant MHC-like features of HLA-DM or the inability of HLA-DM to present a diverse peptide repertoire (just as the monolithic class II pMHC of Ii/H2M-deficient mice failed to select T cells).125
To build a parallel TCR-centric argument, one might consider the impact on positive selection of fixing CDR1 and 2 and allowing only CDR3 to vary. If T cells are selected for their compatibility with the invariant portions of MHC, then CDR1 and 2 should be sufficient to drive selection irrespective of the CDR3 sequence. If, on the other hand, they are selected for their ability to bind specific pMHC combinations, then the CDR3 should prove critical. We can find an answer in the classic study of Sant’Angelo.131 Here, the authors utilized a transgenic mouse bearing the beta chain of a class II-restricted TCR and examined the sequences of TCRα chains from T cells at various stages of development. By further restricting their analysis to TCRαs using Vα2.3, they effectively considered a set of T cells where five of six CDRs were fixed and only CDR3α could be diverse. In this case, only 6% of DP cells possessed TCRα chains that could be found among peripheral CD4+ T cells. So fixing favorable CDRs1 and 2 certainly did not give these TCRs a free pass in terms of selection; in fact, they conferred a selection efficiency that was barely distinguishable from the selection efficiency we estimate from the proportion of DPs to CD4+ SPs in a normal mouse. By generating mice that are limited to Vα2.3/Vβ8.2 but whose CDR3s are subject to normal diversification, we find a similar inefficiency of positive selection on an H-2b background, even though both Vα2.3 and Vβ8.2 are utilized by large numbers of T cells in b-haplotype mice (and also accounting for the more limited opportunity for receptor editing due to the Vα transgene; Campbell and Davis, unpublished data). Similarly, several different transgenic mice bearing TCRs with identical Vα11/Vβ3 usage displayed markedly different efficiencies of positive selection,61 as did TCRs distinguished by a single mutation in the V–J junction.132 But, remarkably, the Sant’Angelo study also showed that the TCRs that they found among CD4+ SP T cells still were sufficient to very accurately confer appropriate CD4+ lineage choice in the thymus. This further underscores the point that, rather than being driven by degenerate binding of CDRs1 and 2 with MHC, positive selection relies upon specific interactions of CDR3s with particular self-pMHC(s).
Rather than foretelling the reactivity with an as-yet-unknown peptide, previous work has told us that the same self-pMHC that selected a T cell is operative in the periphery, in that it signals the TCR-dependent homeostasis of naïve CD4+133 and CD8+ T cells.134,135 A later investigation also demonstrated a role for the selecting self-pMHC in potentiating T cell triggering, in this case by maintaining naïve T cells’ CD3 chains in a state of semi-phosphorylation and thus suspending them in a state of near-activation.136 Lately, we have presented data that could provide a more causal link between positive selection and peripheral activation. It has been known that soluble monomeric agonist pMHC ligands are generally incapable of initiating signaling events in T cells and that dimeric agonist pMHC was sufficient.137–140 In the case of activated 5C.C7 CD4+ T cells, we could show that heterodimeric engagement of TCRs with a strong agonist pMHC paired with particular self-pMHC (in the form of a covalently linked heterodimer of pMHCs) could trigger calcium and cytokine signaling.141 We further hypothesized that those particular self-pMHCs that could partner with antigenic peptides to support mature 5C.C7 T cell activation would be operative in positive selection.142,143
There are now two studies that support this hypothesis, one from our own work with the 5C.C7 TCR39 and another from the work of Lo et al.40 with the AND TCR. In the case of 5C.C7, an endogenous retrovirus-derived GP polyprotein peptide was the strongest of 6 positively selecting peptides among the 95 self-peptides tested, and this GP peptide could enhance naïve 5C.C7 T cell activation in response to minute amounts of exogenously added agonist MCC peptide while other self-peptides could not. In the case of AND, one positively selecting peptide was found among the same test set of 95 self-peptides, and this peptide could enhance naïve AND T cell activation in the context of platebound agonist MCC–self-peptide heterodimers, while again other self-peptides could not. Additional studies will certainly be needed to properly evaluate the model of selection-for-coagonist. Nonetheless, we feel it better explains the results of recent years. As one particularly striking example, Chu et al.144 examined the populations of naïve T cells that bound to a particular antigen-I-Ab tetramer, in b-haplotype and in d-haplotype mice. Contrary to what one would expect based on the selection-for-self-restriction model, the two mice harbored very similar numbers of these cells. However, the tetramer-binding cells from the d-haplotype mouse had a fivefold reduced capacity for activation. Thus positive selection had seemingly improved the responsiveness of those T cells without improving their ability to bind agonist peptide-self-MHC. We would suggest that these cells have instead been educated with respect to which self-peptide-I-Ab could support TCR triggering when paired with the agonist-I-Ab. In this case, the critical feature imparted by thymic selection would not be the TCRs’ affinity for the agonist pMHC but their fine specificity for much lower affinity self-peptide-MHCs.
There are also hurdles to overcome. One wrinkle in this story is that one of the 5C.C7 “coactivating” self-peptides found in the initial heterodimer study, ER60, was not found to coactivate naïve 5C.C7 T cells when stimulation was done using APCs. This could possibly be due to the different activation states of the T cells used, as the prior study had utilized preactivated T cells which have been shown to be more sensitive to restimulation.145 Alternatively, this might have been due to a difference in the agonist peptides used, as the 2005 study used a stronger K5 variant of the MCC agonist peptide, which might relax the affinity requirement for the partnering self-peptide. This is plausible, as the later study showed ER60 to be among the five weakly positively selecting peptides for 5C.C7, and so its affinity for the 5C.C7 TCR is likely only slightly weaker than GP’s. Finally, this discrepancy might have resulted from a difference between the covalently linked heterodimeric pMHCs and cell-surface-bound pMHCs, in terms of how they trigger TCRs. This possibility will be discussed further in the following section.
VII. On the Role of Self-Peptides in the Activation of CD4+ Versus CD8+ T Cells
Another wrinkle is the disparate role of self-pMHC so far observed in the activation of CD4+ and CD8+ T cells. One report suggested that self-pMHC did not influence OT-I T cell activation, based on the comparison of the ability of RMA versus RMA-S cells to act as APCs.146 As RMA-S cells lack TAP, they lack the capacity to process their own self-peptides for presentation by class I molecules. However, they do retain a small but significant amount of surface class I molecules147 and so this data could be interpreted as suggesting only that self-pMHC are not required in large numbers to observe an enhancement of agonist signaling. A second series of reports148,149 also used RMA-S cells, but in those studies added the various self-peptides known to bind H-2Kb exogenously37 in order to stabilize and increase H-2Kb surface expression. In this case, an enhancing effect on agonist signaling was observed when H-2Kb expression was rescued, but the identity of the self-peptide made no difference in the magnitude of signaling, even though a subset of the self-peptides tested are known to positively select the OT-I TCR.37 This strongly suggested that the invariant portions of the self-H-2Kb were responsible for the enhancement of TCR signaling. This could occur through simultaneous binding of TCR to the agonist pMHC and local enrichment of CD8 by binding to the self-pMHC. And, because we know that CD8 contributes to the stabilization of TCR–pMHC31,150 while CD4 does not,151 this result seemed to represent a very plausible explanation for the differential role of specific self-peptides in activating the two types of T cell. However, it represents a serious blow to the idea that positive selection selects T cells for their ability to utilize specific self-peptides in the periphery. This is because regardless of their eventual lineage decision, positive selection initially operates on the same population of DP T cells, is triggered by the same TCR signals,152 and depends on very particular and comparably scarce self-pMHCs in the case of both class I37 and class II.40,153 How, then, could their mechanisms of signaling be so different? And if the identity of the selecting peptide is so important for both types of T cell, why do CD4+ T cells remember those peptides in the periphery, while CD8+ T cells forsake them?
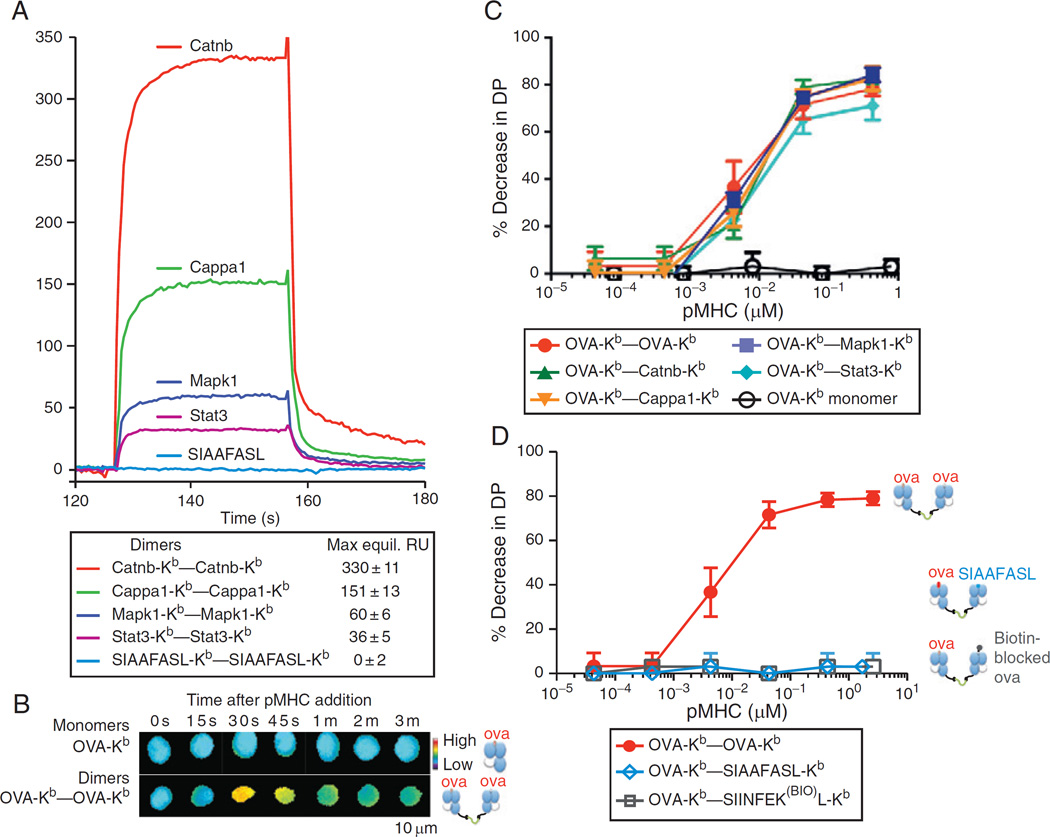
To answer these questions, we examined signaling by the OT-I TCR using the soluble heterodimer technique that had initially illuminated the role of self-peptides in triggering CD4+ T cells.154 Our results using Ova-Kb–self-peptide-Kb heterodimers essentially recapitulated the results of Yachi et al.149: the agonist Ova-Kb could partner with any of the self-peptide-Kb tested to provoke a calcium response in OT-IDP thymocytes or negative selection in OT-I H-2K−/− FTOCs. This was true irrespective of whether the self-peptide-Kb was a positively selecting peptide or a nonselecting peptide such as Mapk1 or Stat3. However, a different picture began to emerge in our examination of positive selection. Here, a calcium response or a positive selection response was observed only when the positively selecting Catnb-Kb was partnered with another Catnb-Kb or with the other positively selecting ligand, Cappa1-Kb. Catnb-Kb could not support signaling when partnered with the nonselecting Mapk1-Kb or Stat3-Kb. This suggested that both partners in the pMHC heterodimer needed to bind TCR in order to cause a signal. And, in fact, we could show thatMapk1-Kb and Stat3-Kb do bind to the OT-I TCR, albeit very weakly, by measuring the interaction of dimeric Mapk1-Kb or Stat3-Kb with OT-I. Finally, we could abrogate this OT-I binding by generating a TCR-contact-mutated version of the Ova antigen, SIAAFASL, or by sterically blocking Ova’s central TCR-binding residue with a biotin. Heterodimers of these peptide-Kbs paired with Ova-Kb were unable to stimulate calcium flux or selection of OT-I thymocytes even when used at a concentration 100-fold higher than was sufficient to observe signaling with the Ova-Kb–Mapk1-Kb or Ova-Kb–Stat3-Kb. Thus we could show that TCR engagement by both partners of the heterodimeric ligand was necessary for thymocyte signaling in this class I-restricted system, as had previously been observed for class II-restricted T cells.
These data also allow us to comment on how faithfully soluble heterodimers recapitulate the natural situation of membrane-bound pMHCs. From our studies of the self-peptide–H-2Kbs, it is clear that the covalently linked heterodimers do increase the operational avidity, or apparent affinity, of the ligands involved. This might explain why we can observe an enhancement of 5C.C7 T cell activation by ER60 in the context of the heterodimers,154 but not in the context of APC-presented ligands.39 But, critically, the dimers do preserve the rank order of affinities of the individual component pMHCs38 (Fig. 5). And, further, the increased avidity is not sufficient to explain why the heterodimeric ligand signals while the monomeric ligand does not. This is because we now have a direct measurement of the dimer’s apparent KD, and so we can directly compare the dimers to monomers in terms of how many receptors they are binding. For example, we have measured the calcium response of OT-I DP T cells when their TCRs are engaged by monomeric Ova/H-2Kb at a concentration above the KD of this interaction (at concentrations of 17 µM; KD = 6–10 µM).28,38 Remarkably, when more than half of the T cell’s TCRs are bound by the monomeric agonist pMHC, no signaling whatsoever results. We can then compare this to the result of engaging TCRs with a soluble homodimeric Cappa1-H-2Kb–Cappa1-H-2Kb. OT-I’s apparent affinity for this dimeric ligand is ~60 µM, yet at concentrations well below this (e.g., 8.7 µM), the homodimeric Cappa1-H-2Kb induces robust calcium responses in OT-I DP T cells. The same picture emerges when the OT-I DP cells are exposed to the monomeric or dimeric pMHCs in the context of the whole thymus: agonist monomers are completely nonstimulatory even at very high concentrations (in terms of positive selection, negative selection, or upregulation of activation markers such as CD69), while the Cappa1-H-2Kb homodimers and Catnb-H-2Kb homodimers which have lower apparent affinities, can mediate positive selection at lower concentrations.
Fig. 5.
Self-peptide-MHC contributes to class I-restricted thymocyte signaling in a peptide-specific manner. (A) Surface Plasmon Resonance (SPR) measurement of binding of soluble dimeric complexes of the indicated self-peptide-Kbs to immobilized OT-I TCR. The Mapk1 and Stat3 self-peptide-Kbs bound OT-I with a measurable affinity, while the engineered “null” SIAAFASL-Kb did not. (B) Dimeric but not monomeric Ova-Kb drives calcium signaling by OT-I DP thymocytes. Thymocytes were taken from OT-I H-2K−/− H-2D−/− mice, loaded with the ratiometric calcium dye Fura2am, and exposed to high concentrations of soluble monomeric or dimeric Ova-Kb. Fura2 ratios (340/380 nm) are shown in a false-color scale. (C) Homodimeric or heterodimeric Ova-Kb, but not monomeric Ova-Kb, drives negative selection in whole thymi. E16 FTOC were established from OT-I H-2K−/− H-2D−/− mice, and the indicated soluble monomeric or dimeric H-2Kbs were added at varying concentrations. After 3–4 days, FTOC were disrupted, and the resulting thymocyte populations were assayed by FACS: the percentage of surviving DP thymocytes, relative to untreated control FTOCs, are shown. Ova-Kb monomers had no effect on thymocyte selection, even when added at concentrations above the KD of the Ova-Kb/OT-I interaction, where more than 50% of the thymocytes’ TCRs are expected to be bound by the Ova-Kb. Ova-Kb homodimers, or Ova-Kb–self-peptide-Kb heterodimers, could drive negative selection of the OT-I thymocytes to a similar extent, and at an equally low dose. (D) The ability of Ova-Kb–self-peptide-Kb to negatively select depends on the self-peptide-Kb’s affinity for the OT-I TCR. FTOC were established and assayed as in part (C). Negative selection did not result from even very high doses of the Ova-Kb–SIAAFASL-Kb (in which the peptide’s OT-I-contacting residues are mutated), or the Ova-Kb–SIINFEK(BIO)L-Kb (in which a primary predicted OT-I contacting residue in the Ova peptide, the p7 K, is modified with a biotin at the lysine’s free amine group). Data are originally from Ref. 38.
Finally, although we believe that we have provided evidence for a more unified mechanism of self-peptide-supported TCR triggering in CD4+ and CD8+ T cells, it is harder to make the connection between positively selecting ligands and peripheral activation for the CD8+ T cells. Whereas signaling by the mature 5C.C7 and AND CD4+ T cells could only be enhanced by the selecting peptide, we now have multiple independent sets of data that suggest that CD8+ T cells overshoot this mark. That is, in the OT-I system, positive selection is driven only by a high-affinity subset of the self-peptides that can enhance mature T cell responses. On the one hand, this observation could still be consistent with our hypothesis that positive selection produces a repertoire of T cells that can respond to agonist pMHC paired with self-pMHC. By forcing the OT-ITCR to recognize a ligand with a KD of 100–200 µM affinity in order to mature, this would guarantee that the OT-ITCR has coactivating peptides available to it in the periphery, even if weaker pMHCs (~300 µM) are also sufficient. In this case, it seems that thymic selection would waste potentially useful TCRs. And, as every additional naïve CD8+ T cell appears to be useful in combating infection,19 this would seem to be a less than optimal mechanism. On the other hand, we know that naïve CD8+ T cells require self-pMHC for their homeostatic survival, and that this peripheral maintenance is dependent on the same self-pMHCs that selected those T cells in the thymus.135 In this case, perhaps the weaker ~300 µM self-pMHCs are insufficient for OT-I’s peripheral maintenance. None of these speculations is particularly satisfying, and it is clear that further investigation of other class I-restricted TCRs will be needed to resolve this.
VIII. Conclusions
The T cell repertoire is molded by a wealth of information about the self. Here we have briefly summarized recent findings relating to how this information is packaged in the thymus for developing T cells’ consideration, how thymocytes go about accessing it, and the nature of the information that gives a developing T cell its imprimatur to join the peripheral repertoire.
On the first point, we have accumulated an excellent description of the movement of thymocytes within and between the different anatomic compartments of the thymus,65,155 and we have begun to understand the molecular queues that underlie this movement, both developmentally regulated66,92,156 and TCR-triggered.68,69,82 We also have excellent evidence suggesting that the APCs in these distinct physical locations present nonoverlapping sets of self-peptides, in addition to being phenotypically different in terms of their ability to promote positive versus negative selection.58 This evidence is mostly indirect, coming from studies of mutations in the MHC loading pathway and their influence on the efficiency of T cell selection.56,124,157,158 For example, we know that positive selection depends on thymocytes’ ability to “scan” the cortex in a wide-ranging and randomly directed fashion,66,69 and we know that positive selection depends on self-peptides that are quite rare.37,39,40 While the indirect evidence is compelling, we can only infer the TCR–pMHC interaction that connects these. We also know that negative selection depends on APC-specific mechanisms of TRA presentation,50 and that limiting thymocytes’ access to the medulla results in autoimmunity.95 But did this occur specifically because thymocytes were denied access to self-peptides, as opposed to another tolerizing property of the medulla?
Clearly these questions would benefit from a more complete identification of the peptides that actually occupy the MHCs on the surfaces of thymic APCs.58 But making the connection between these peptides and selection events will be a daunting task. Positive selection relies on the diversity of the self-peptide repertoire, such that a very small but especially diverse subset of the total mass of self-pMHC can support positive selection of a large diversity of T cells.125 We do not know how many peptides this subset might represent: antibodies that block positive selection bound ~3–5% of self-pMHCs,47,48 and this can serve as a reasonable upper limit, but we have no good sense of the lower limit. In the extreme case, it is quite possible that two phenotypically indistinguishable APCs might present nonoverlapping sets of peptides due only to stochastic variation, combined perhaps with epigenetic variegation.159 This might offer an explanation for why thymocytes’ cortical “scanning” motility is beneficial for their selection: perhaps multiple cTECs must be examined in order to find a selecting interaction.66,69 But it would also make it incredibly difficult to isolate the particular peptides that are relevant for any given TCR. Negatively selecting self-peptides present a similar conundrum. Thymocytes are capable of responding toward two high-affinity pMHCs,69 and can seek out such peptides presented by one in 20 APCs.62 If we conservatively estimate that the APCs involved express ~25,000–50,000 MHC molecules each, then we are talking about a detection limit of between two and four parts per million. Only radical improvements in mass spectrometry, or entirely new technology, will allow us to directly see the peptides that T cells can see.
T cells require only a week to develop a profound understanding of the self, and so we can continue to learn about the nature of self from the T cells themselves. A renewed interest and ability to detect very rare pMHC tetramer-binding T cell populations has yielded new information regarding the diversity of the naïve TCR repertoire and by extension has placed constraints on the number of distinct TCRs that emerge from the thymus.17,160 Comparing these results with a similar survey of the preselection repertoire would be very valuable in assessing how positive and negative selection shape the mature T cell pool in a normal individual. There are obstacles to overcome here as well, as thymocytes’ low surface expression of TCR makes tetramer analysis difficult,161 and multiple genetic programs conspire to make TCR ligation a lethal proposition.111,162,163 The tetramer approach also a priori forces us to select peptide targets prior to data collection, and so such an analysis could not be unbiased without a very large sample size. This is a critical difficulty, as the affinity model of selection predicts that tetramers made from positively selecting pMHCs (e.g., KD > 50 µM) should not detect the T cells they select,38 whereas the altered self model predicts the opposite.58 Thus a more global analysis may be useful. Advances in high-throughput DNA sequencing technology could be leveraged here, as they have been in the study of antibody diversity.164 On a sufficient scale, such studies have the potential not only to precisely gauge the diversity of pre- and postselection TCR repertoires but also on a population level to assess the degree of coevolution of TCR and MHC genes.165 But, as valuable as they are, such techniques will have difficulty connecting what they discover about TCRs to the self-peptides that are relevant for those TCRs’ selection and survival.135
If we are to understand the mechanics of positive selection without a complete understanding of the self-peptides involved, then we must answer this critical question: what is the selecting parameter, and what is the selected property? Does a TCR’s weak affinity for self-peptide-self-MHC predict high affinity for an as-yet-unknown peptide-self-MHC? We do not favor this model, because in this case the selecting parameter must be the affinity afforded by the TCR’s contact with genetically encoded MHC residues. Several lines of evidence indicate that this property is satisfied prior to selection,20,126 and that peptide-specific contacts are crucial for selection.57,131 Perhaps the positive selection parameter is the breadth of the TCR’s reactivity toward self-pMHC, and the selected property is a high degree of polyspecificity.20,22 This might in turn confer the potential for each selected T cell to recognize multiple distinct antigens and thereby improve the chance that any one T cell will be useful in combating infection. We know, however, that positive selection can proceed when a particular TCR is afforded an abundance of a single pMHC species.37,39,40 Still, we can only remark this property in the case of three distinct TCRs among many thousands or millions, and so this may represent the exception rather than the rule. If these do represent an exception, then we might also discard the coinciding observation that selection can be driven by low-affinity peptides presented ubiquitously throughout the thymus. This would allow us to consider the possibility that thymocytes are selected by high-affinity interactions with a unique set of “altered self” peptides on cTECs, and that autoimmunity can be averted because these peptides will never be seen again by mature T cells.58,166 The selecting parameter might then be a “training set” of agonist cortical peptides, with a completely distinct “test set” due to peptide-selective differences in the MHC loading pathway among peripheral and somatic cells. In this case, the selected property is agonist reactivity with ostensibly “nonself”-peptide-self-MHC and therefore could perfectly predict self-restriction of the peripheral repertoire. But, as noted earlier, the uniqueness of cTECs’ peptide repertoire is inferred from mutations of the MHC loading pathway and the ensuing impact on T cell selection. The extent to which peripheral APCs can successfully hide this very dangerous “training set” of agonist self-peptides is far from clear. The fidelity required for this separation of self to be a successful strategy is enormous, because we know that allowing T cells even the tiniest sniff of those cortical agonist peptides could spell disaster.98,142
Finally, we will discuss the possibility that we currently favor. Perhaps the TCR’s weak affinity for self-peptide-self-MHC predicts improved responsiveness toward as-yet-unknown peptide-self-MHCs specifically because the self-peptide-self-MHCs participate in the latter process. We have suggested a mechanism for TCR triggering based on heterodimeric engagement of TCR with agonist pMHC together with self-pMHC, which could explain the basis for the selected parameter in this case.143 More recently, we have also shown that this dimer-based mechanism of TCR triggering can be extended to explain how self-peptide-MHCs trigger selection in the thymus.38 The triggering mechanism can be conserved, despite the weaker ligands involved, at least in part because developmental regulation of miR-181a lowers the signaling threshold in DP thymocytes.105 Lastly, there are now two peptides that can demonstrate that the property of self-pMHC contributing to peripheral responses is a correlate of the selection process.39,40 Further studies in additional TCR systems will certainly be needed to support the universality of this observation, however, and additional evidence is still required to settle the precise molecular mechanism governing TCR signaling in the context of cell–cell interactions.167 More advanced molecular imaging techniques will be invaluable in addressing this last point,151 while cell and tissue imaging will continue to give us clues as to which APCs are relevant to developing T cells and how the twain meet.66 And, while significant advances in the detection of macromolecules will be required to directly assess the self-peptide repertoire, we are hopeful that high-throughput and systems-level analyses will allow us to test the predictions of the above models against the actual polyclonal TCR repertoire in healthy individuals.
References
- 1.Rodewald HR. Thymus organogenesis. Annu Rev Immunol. 2008;26:355–388. doi: 10.1146/annurev.immunol.26.021607.090408. [DOI] [PubMed] [Google Scholar]
- 2.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205:2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrie HT. Cell migration and the control of post-natal T-cell lymphopoiesis in the thymus. Nat Rev Immunol. 2003;3:859–866. doi: 10.1038/nri1223. [DOI] [PubMed] [Google Scholar]
- 4.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6:728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki S, Saito T. Molecular basis for pre-TCR-mediated autonomous signaling. Trends Immunol. 2007;28:39–43. doi: 10.1016/j.it.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Singer A, Bosselut R. CD4/CD8 coreceptors in thymocyte development, selection, and lineage commitment: analysis of the CD4/CD8 lineage decision. Adv Immunol. 2004;83:91–131. doi: 10.1016/S0065-2776(04)83003-7. [DOI] [PubMed] [Google Scholar]
- 7.Gascoigne NR, Chien Y, Becker DM, Kavaler J, Davis MM. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984;310:387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- 8.Davis MM, Bjorkman PJ. T-cell antigen receptor genes and T-cell recognition. Nature. 1988;334:395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- 9.Sorger SB, Paterson Y, Fink PJ, Hedrick SM. T cell receptor junctional regions and the MHC molecule affect the recognition of antigenic peptides by T cell clones. J Immunol. 1990;144:1127–1135. [PubMed] [Google Scholar]
- 10.Ehrich EW, Devaux B, Rock EP, Jorgensen JL, Davis MM, Chien YH. T cell receptor interaction with peptide/major histocompatibility complex (MHC) and super antigen/MHC ligands is dominated by antigen. J Exp Med. 1993;178:713–722. doi: 10.1084/jem.178.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katayama CD, Eidelman FJ, Duncan A, Hooshmand F, Hedrick SM. Predicted complementarity determining regions of the T cell antigen receptor determine antigen specificity. EMBO J. 1995;14:927–938. doi: 10.1002/j.1460-2075.1995.tb07074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia KC, Degano M, Stanfield RL, Brunmark A, Jackson MR, Peterson PA, et al. An alphabeta T cell receptor structure at 2.5 A and its orientation in the TCR–MHC complex. Science. 1996;274:209–219. doi: 10.1126/science.274.5285.209. [DOI] [PubMed] [Google Scholar]
- 13.Garboczi DN, Ghosh P, Utz U, Fan QR, Biddison WE, Wiley DC. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 14.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 15.Garcia KC, Adams JJ, Feng D, Ely LK. The molecular basis of TCR germline bias for MHC is surprisingly simple. Nat Immunol. 2009;10:143–147. doi: 10.1038/ni.f.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu LC, Tuot DS, Lyons DS, Garcia KC, Davis MM. Two-step binding mechanism for T-cell receptor recognition of peptide MHC. Nature. 2002;418:552–556. doi: 10.1038/nature00920. [DOI] [PubMed] [Google Scholar]
- 17.Jenkins MK, Chu HH, McLachlan JB, Moon JJ. On the composition of the preimmune repertoire of T cells specific for peptide-major histocompatibility complex ligands. Annu Rev Immunol. 2010;28:275–294. doi: 10.1146/annurev-immunol-030409-101253. [DOI] [PubMed] [Google Scholar]
- 18.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huseby ES, White J, Crawford F, Vass T, Becker D, Pinilla C, et al. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Berg LJ, Pullen AM, de St Fazeka, Groth B, Mathis D, Benoist C, et al. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- 22.Wucherpfennig KW, Allen PM, Celada F, Cohen IR, De Boer R, Garcia KC, et al. Polyspecificity of T cell and B cell receptor recognition. Semin Immunol. 2007;19:216–224. doi: 10.1016/j.smim.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–961. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 24.Sant’Angelo DB, Waterbury PG, Cohen BE, Martin WD, Van Kaer L, Hayday AC, et al. The imprint of intrathymic self-peptides on the mature T cell receptor repertoire. Immunity. 1997;7:517–524. doi: 10.1016/s1074-7613(00)80373-8. [DOI] [PubMed] [Google Scholar]
- 25.Scollay RG, Butcher EC, Weissman IL. Thymus cell migration. Quantitative aspects of cellular traffic from the thymus to the periphery in mice. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 26.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 27.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 28.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, et al. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong KM, Piepenbrink KH, Baker BM. Conformational changes and flexibility in T-cell receptor recognition of peptide-MHC complexes. Biochem J. 2008;415:183–196. doi: 10.1042/BJ20080850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naeher D, Daniels MA, Hausmann B, Guillaume P, Luescher I, Palmer E. A constant affinity threshold for T cell tolerance. J Exp Med. 2007;204:2553–2559. doi: 10.1084/jem.20070254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia KC, Scott CA, Brunmark A, Carbone FR, Peterson PA, Wilson IA, et al. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature. 1996;384:577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 32.Alam SM, Davies GM, Lin CM, Zal T, Nasholds W, Jameson SC, et al. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–237. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 33.Stefanski HE, Mayerova D, Jameson SC, Hogquist KA. A low affinity TCR ligand restores positive selection of CD8+ T cells in vivo. J Immunol. 2001;166:6602–6607. doi: 10.4049/jimmunol.166.11.6602. [DOI] [PubMed] [Google Scholar]
- 34.Ashton-Rickardt PG, Van Kaer L, Schumacher TN, Ploegh HL, Tonegawa S. Peptide contributes to the specificity of positive selection of CD8+ T cells in the thymus. Cell. 1993;73:1041–1049. doi: 10.1016/0092-8674(93)90281-t. [DOI] [PubMed] [Google Scholar]
- 35.Hogquist KA, Jameson SC, Bevan MJ. Strong agonist ligands for the T cell receptor do not mediate positive selection of functional CD8+ T cells. Immunity. 1995;3:79–86. doi: 10.1016/1074-7613(95)90160-4. [DOI] [PubMed] [Google Scholar]
- 36.Hogquist KA, Tomlinson AJ, Kieper WC, McGargill MA, Hart MC, Naylor S, et al. Identification of a naturally occurring ligand for thymic positive selection. Immunity. 1997;6:389–399. doi: 10.1016/s1074-7613(00)80282-4. [DOI] [PubMed] [Google Scholar]
- 37.Santori FR, Kieper WC, Brown SM, Lu Y, Neubert TA, Johnson KL, et al. Rare, structurally homologous self-peptides promote thymocyte positive selection. Immunity. 2002;17:131–142. doi: 10.1016/s1074-7613(02)00361-8. [DOI] [PubMed] [Google Scholar]
- 38.Juang J, Ebert PJ, Feng D, Garcia KC, Krogsgaard M, Davis MM. Peptide-MHC heterodimers show that thymic positive selection requires a more restricted set of self-peptides than negative selection. J Exp Med. 2010;207:1223–1234. doi: 10.1084/jem.20092170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009;10:1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lo W-L, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide-MHC ligand positively selects and augments peripheral CD4+ T cell activation and survival. Nat Immunol. 2009;10:1155–1161. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felix NJ, Donermeyer DL, Horvath S, Walters JJ, Gross ML, Suri A, et al. Alloreactive T cells respond specifically to multiple distinct peptide-MHC complexes. Nat Immunol. 2007;8:388–397. doi: 10.1038/ni1446. [DOI] [PubMed] [Google Scholar]
- 42.Reay PA, Kantor RM, Davis MM. Use of global amino acid replacements to define the requirements for MHC binding and T cell recognition of moth cytochrome c (93–103) J Immunol. 1994;152:3946–3957. [PubMed] [Google Scholar]
- 43.Fremont DH, Stura EA, Matsumura M, Peterson PA, Wilson IA. Crystal structure of an H-2Kb-ovalbumin peptide complex reveals the interplay of primary and secondary anchor positions in the major histocompatibility complex binding groove. Proc Natl Acad Sci USA. 1995;92:2479–2483. doi: 10.1073/pnas.92.7.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 45.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 46.Stefanski HE, Jameson SC, Hogquist KA. Positive selection is limited by available peptide-dependent MHC conformations. J Immunol. 2000;164:3519–3526. doi: 10.4049/jimmunol.164.7.3519. [DOI] [PubMed] [Google Scholar]
- 47.Baldwin KK, Reay PA, Wu L, Farr A, Davis MM. A T cell receptor-specific blockade of positive selection. J Exp Med. 1999;189:13–24. doi: 10.1084/jem.189.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viret C, He X, Janeway CA., Jr On the self-referential nature of naive MHC class II-restricted T cells. J Immunol. 2000;165:6183–6192. doi: 10.4049/jimmunol.165.11.6183. [DOI] [PubMed] [Google Scholar]
- 49.Marrack P, Ignatowicz L, Kappler JW, Boymel J, Freed JH. Comparison of peptides bound to spleen and thymus class II. J Exp Med. 1993;178:2173–2183. doi: 10.1084/jem.178.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 51.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 52.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204:2521–2528. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Seach N, Ueno T, Fletcher AL, Lowen T, Mattesich M, Engwerda CR, et al. The lymphotoxin pathway regulates Aire-independent expression of ectopic genes and chemokines in thymic stromal cells. J Immunol. 2008;180:5384–5392. doi: 10.4049/jimmunol.180.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 55.Honey K, Nakagawa T, Peters C, Rudensky A. Cathepsin L regulates CD4+ T cell selection independently of its effect on invariant chain: a role in the generation of positively selecting peptide ligands. J Exp Med. 2002;195:1349–1358. doi: 10.1084/jem.20011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nitta T, Murata S, Sasaki K, Fujii H, Ripen AM, Ishimaru N, et al. Thymoproteasome shapes immunocompetent repertoire of CD8+ T cells. Immunity. 2010;32:29–40. doi: 10.1016/j.immuni.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 57.Hogquist KA, Gavin MA, Bevan MJ. Positive selection of CD8+ T cells induced by major histocompatibility complex binding peptides in fetal thymic organ culture. J Exp Med. 1993;177:1469–1473. doi: 10.1084/jem.177.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 59.Berg LJ, Frank GD, Davis MM. The effects of MHC gene dosage and allelic variation on T cell receptor selection. Cell. 1990;60:1043–1053. doi: 10.1016/0092-8674(90)90352-f. [DOI] [PubMed] [Google Scholar]
- 60.Labrecque N, Whitfield LS, Obst R, Waltzinger C, Benoist C, Mathis D. How much TCR does a T cell need? Immunity. 2001;15:71–82. doi: 10.1016/s1074-7613(01)00170-4. [DOI] [PubMed] [Google Scholar]
- 61.Yelon D, Berg LJ. Structurally similar TCRs differ in their efficiency of positive selection. J Immunol. 1997;158:5219–5228. [PubMed] [Google Scholar]
- 62.Merkenschlager M, Benoist C, Mathis D. Evidence for a single-niche model of positive selection. Proc Natl Acad Sci USA. 1994;91:11694–11698. doi: 10.1073/pnas.91.24.11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merkenschlager M. Tracing interactions of thymocytes with individual stromal cell partners. Eur J Immunol. 1996;26:892–896. doi: 10.1002/eji.1830260426. [DOI] [PubMed] [Google Scholar]
- 64.Plotkin J, Prockop SE, Lepique A, Petrie HT. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J Immunol. 2003;171:4521–4527. doi: 10.4049/jimmunol.171.9.4521. [DOI] [PubMed] [Google Scholar]
- 65.Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 2005;3:e160. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phee H, Dzhagalov I, Mollenauer M, Wang Y, Irvine DJ, Robey E, et al. Regulation of thymocyte positive selection and motility by GIT2. Nat Immunol. 2010;11:503–511. doi: 10.1038/ni.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bousso P, Bhakta NR, Lewis RS, Robey E. Dynamics of thymocyte–stromal cell interactions visualized by two-photon microscopy. Science. 2002;296:1876–1880. doi: 10.1126/science.1070945. [DOI] [PubMed] [Google Scholar]
- 68.Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat Immunol. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- 69.Ebert PJ, Ehrlich LI, Davis MM. Low ligand requirement for deletion and lack of synapses in positive selection enforce the gauntlet of thymic T cell maturation. Immunity. 2008;29:734–745. doi: 10.1016/j.immuni.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 71.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 72.Gunzer M, Schafer A, Borgmann S, Grabbe S, Zanker KS, Brocker EB, et al. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–332. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- 73.Kisielow P, Miazek A. Positive selection of T cells: rescue from programmed cell death and differentiation require continual engagement of the T cell receptor. J Exp Med. 1995;181:1975–1984. doi: 10.1084/jem.181.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu X, Bosselut R. Duration of TCR signaling controls CD4−CD8 lineage differentiation in vivo. Nat Immunol. 2004;5:280–288. doi: 10.1038/ni1040. [DOI] [PubMed] [Google Scholar]
- 75.Viret C, Sant’Angelo DB, He X, Ramaswamy H, Janeway CA., Jr A role for accessibility to self-peptide-self-MHC complexes in intrathymic negative selection. J Immunol. 2001;166:4429–4437. doi: 10.4049/jimmunol.166.7.4429. [DOI] [PubMed] [Google Scholar]
- 76.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER. Cutting edge: negative selection of immature thymocytes by a few peptide-MHC complexes: differential sensitivity of immature and mature T cells. J Immunol. 1999;162:3117–3120. [PubMed] [Google Scholar]
- 78.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 80.Laufer TM, Fan L, Glimcher LH. Self-reactive T cells selected on thymic cortical epithelium are polyclonal and are pathogenic in vivo. J Immunol. 1999;162:5078–5084. [PubMed] [Google Scholar]
- 81.Ahn S, Lee G, Yang SJ, Lee D, Lee S, Shin HS, et al. TSCOT+ thymic epithelial cell-mediated sensitive CD4 tolerance by direct presentation. PLoS Biol. 2008;6:e191. doi: 10.1371/journal.pbio.0060191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Richie LI, Ebert PJ, Wu LC, Krummel MF, Owen JJ, Davis MM. Imaging synapse formation during thymocyte selection: inability of CD3zeta to form a stable central accumulation during negative selection. Immunity. 2002;16:595–606. doi: 10.1016/s1074-7613(02)00299-6. [DOI] [PubMed] [Google Scholar]
- 83.Hailman E, Burack WR, Shaw AS, Dustin ML, Allen PM. Immature CD4(+)CD8(+) thymocytes form a multifocal immunological synapse with sustained tyrosine phosphorylation. Immunity. 2002;16:839–848. doi: 10.1016/s1074-7613(02)00326-6. [DOI] [PubMed] [Google Scholar]
- 84.Le Borgne M, Ladi E, Dzhagalov I, Herzmark P, Liao YF, Chakraborty AK, et al. The impact of negative selection on thymocyte migration in the medulla. Nat Immunol. 2009;10:823–830. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Punt JA, Havran W, Abe R, Sarin A, Singer A. T cell receptor (TCR)-induced death of immature CD4+CD8+ thymocytes by two distinct mechanisms differing in their requirement for CD28 costimulation: implications for negative selection in the thymus. J Exp Med. 1997;186:1911–1922. doi: 10.1084/jem.186.11.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 87.Serwold T, Ehrlich LI, Weissman IL. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood. 2008;113:807–815. doi: 10.1182/blood-2008-08-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kishimoto H, Sprent J. Negative selection in the thymus includes semimature T cells. J Exp Med. 1997;185:263–271. doi: 10.1084/jem.185.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, et al. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200:493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park JH, Adoro S, Guinter T, Erman B, Alag AS, Catalfamo M, et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol. 2010;11:257–264. doi: 10.1038/ni.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Misslitz A, Pabst O, Hintzen G, Ohl L, Kremmer E, Petrie HT, et al. Thymic T cell development and progenitor localization depend on CCR7. J Exp Med. 2004;200:481–491. doi: 10.1084/jem.20040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ehrlich LI, Oh DY, Weissman IL, Lewis RS. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity. 2009;31:986–998. doi: 10.1016/j.immuni.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cho HJ, Edmondson SG, Miller AD, Sellars M, Alexander ST, Somersan S, et al. Cutting edge: identification of the targets of clonal deletion in an unmanipulated thymus. J Immunol. 2003;170:10–13. doi: 10.4049/jimmunol.170.1.10. [DOI] [PubMed] [Google Scholar]
- 94.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 95.Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24:165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 96.Nitta T, Nitta S, Lei Y, Lipp M, Takahama Y. CCR7-mediated migration of developing thymocytes to the medulla is essential for negative selection to tissue-restricted antigens. Proc Natl Acad Sci USA. 2009;106:17129–17133. doi: 10.1073/pnas.0906956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pircher H, Rohrer UH, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 98.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 99.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 100.Starr TK, Daniels MA, Lucido MM, Jameson SC, Hogquist KA. Thymocyte sensitivity and supramolecular activation cluster formation are developmentally regulated: a partial role for sialylation. J Immunol. 2003;171:4512–4520. doi: 10.4049/jimmunol.171.9.4512. [DOI] [PubMed] [Google Scholar]
- 101.Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Muller W, Killeen N, et al. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 102.Zikherman J, Jenne C, Watson S, Doan K, Raschke W, Goodnow CC, et al. CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development. Immunity. 2010;32:342–354. doi: 10.1016/j.immuni.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 104.Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev. 2004;5:396–400. doi: 10.1038/nrg1328. [DOI] [PubMed] [Google Scholar]
- 105.Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT, Tang J, et al. Identification of substrates of human protein-tyrosine phosphatase PTPN22. J Biol Chem. 2006;281:11002–11010. doi: 10.1074/jbc.M600498200. [DOI] [PubMed] [Google Scholar]
- 107.Zikherman J, Hermiston M, Steiner D, Hasegawa K, Chan A, Weiss A. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. J Immunol. 2009;182:4093–4106. doi: 10.4049/jimmunol.0803317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koelsch U, Schraven B, Simeoni L. SIT and TRIM determine T cell fate in the thymus. J Immunol. 2008;181:5930–5939. doi: 10.4049/jimmunol.181.9.5930. [DOI] [PubMed] [Google Scholar]
- 109.Theodosiou A, Ashworth A. MAP kinase phosphatases. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-reviews3009. (REVIEWS3009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 111.Jones ME, Zhuang Y. Acquisition of a functional T cell receptor during T lymphocyte development is enforced by HEB and E2A transcription factors. Immunity. 2007;27:860–870. doi: 10.1016/j.immuni.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feinerman O, Veiga J, Dorfman JR, Germain RN, Altan-Bonnet G. Variability and robustness in T cell activation from regulated heterogeneity in protein levels. Science. 2008;321:1081–1084. doi: 10.1126/science.1158013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schmedt C, Saijo K, Niidome T, Kuhn R, Aizawa S, Tarakhovsky A. Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature. 1998;394:901–904. doi: 10.1038/29802. [DOI] [PubMed] [Google Scholar]
- 114.Wong P, Barton GM, Forbush KA, Rudensky AY. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J Exp Med. 2001;193:1179–1187. doi: 10.1084/jem.193.10.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mao TK, Chen CZ. Dissecting microRNA-mediated gene regulation and function in T-cell development. Methods Enzymol. 2007;427:171–189. doi: 10.1016/S0076-6879(07)27010-7. [DOI] [PubMed] [Google Scholar]
- 116.Yamasaki S, Ishikawa E, Sakuma M, Ogata K, Sakata-Sogawa K, Hiroshima M, et al. Mechanistic basis of pre-T cell receptor-mediated autonomous signaling critical for thymocyte development. Nat Immunol. 2006;7:67–75. doi: 10.1038/ni1290. [DOI] [PubMed] [Google Scholar]
- 117.Bevan MJ, Fink PJ. The influence of thymus H-2 antigens on the specificity of maturing killer and helper cells. Immunol Rev. 1978;42:3–19. doi: 10.1111/j.1600-065x.1978.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 118.Zinkernagel RM, Callahan GN, Althage A, Cooper S, Klein PA, Klein J. On the thymus in the differentiation of "H-2 self-recognition" by T cells: evidence for dual recognition? J Exp Med. 1978;147:882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zuniga-Pflucker JC, Longo DL, Kruisbeek AM. Positive selection of CD4−CD8+ T cells in the thymus of normal mice. Nature. 1989;338:76–78. doi: 10.1038/338076a0. [DOI] [PubMed] [Google Scholar]
- 120.Kisielow P, Teh HS, Bluthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 121.Hogquist KA, Jameson SC, Bevan MJ. The ligand for positive selection of T lymphocytes in the thymus. Curr Opin Immunol. 1994;6:273–278. doi: 10.1016/0952-7915(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 122.Scott-Browne JP, White J, Kappler JW, Gapin L, Marrack P. Germline-encoded amino acids in the alphabeta T-cell receptor control thymic selection. Nature. 2009;458:1043–1046. doi: 10.1038/nature07812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bhayani HR, Hedrick SM. The role of polymorphic amino acids of the MHC molecule in the selection of the T cell repertoire. J Immunol. 1991;146:1093–1098. [PubMed] [Google Scholar]
- 124.Barton GM, Rudensky AY. Evaluating peptide repertoires within the context of thymocyte development. Semin Immunol. 1999;11:417–422. doi: 10.1006/smim.1999.0199. [DOI] [PubMed] [Google Scholar]
- 125.Barton GM, Rudensky AY. Requirement for diverse, low-abundance peptides in positive selection of T cells. Science. 1999;283:67–70. doi: 10.1126/science.283.5398.67. [DOI] [PubMed] [Google Scholar]
- 126.Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 127.Henderson SC, Berezovskaya A, English A, Palliser D, Rock KL, Bamezai A. CD4+ T cells mature in the absence of MHC class I and class II expression in Ly-6A.2 transgenic mice. J Immunol. 1998;161:175–182. [PubMed] [Google Scholar]
- 128.Huseby ES, Crawford F, White J, Kappler J, Marrack P. Negative selection imparts peptide specificity to the mature T cell repertoire. Proc Natl Acad Sci USA. 2003;100:11565–11570. doi: 10.1073/pnas.1934636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rubtsova K, Scott-Browne JP, Crawford F, Dai S, Marrack P, Kappler JW. Many different Vbeta CDR3s can reveal the inherent MHC reactivity of germline-encoded TCR V regions. Proc Natl Acad Sci USA. 2009;106:7951–7956. doi: 10.1073/pnas.0902728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim HJ, Guo D, Sant’Angelo DB. Coevolution of TCR–MHC interactions: conserved MHC tertiary structure is not sufficient for interactions with the TCR. Proc Natl Acad Sci USA. 2005;102:7263–7267. doi: 10.1073/pnas.0502751102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sant’Angelo DB, Lucas B, Waterbury PG, Cohen B, Brabb T, Goverman J, et al. A molecular map of T cell development. Immunity. 1998;9:179–186. doi: 10.1016/s1074-7613(00)80600-7. [DOI] [PubMed] [Google Scholar]
- 132.Kaye J, Vasquez NJ, Hedrick SM. Involvement of the same region of the T cell antigen receptor in thymic selection and foreign peptide recognition. J Immunol. 1992;148:3342–3353. [PubMed] [Google Scholar]
- 133.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 134.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8 + T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 136.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 137.Boniface JJ, Rabinowitz JD, Wulfing C, Hampl J, Reich Z, Altman JD, et al. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands [corrected] Immunity. 1998;9:459–466. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- 138.Stone JD, Stern LJ. CD8 T cells, like CD4 T cells, are triggered by multivalent engagement of TCRs by MHC-peptide ligands but not by monovalent engagement. J Immunol. 2006;176:1498–14505. doi: 10.4049/jimmunol.176.3.1498. [DOI] [PubMed] [Google Scholar]
- 139.Cochran JR, Cameron TO, Stern LJ. The relationship of MHC-peptide binding and T cell activation probed using chemically defined MHC class II oligomers. Immunity. 2000;12:241–250. doi: 10.1016/s1074-7613(00)80177-6. [DOI] [PubMed] [Google Scholar]
- 140.Cochran JR, Cameron TO, Stone JD, Lubetsky JB, Stern LJ. Receptor proximity, not intermolecular orientation, is critical for triggering T-cell activation. J Biol Chem. 2001;276:28068–28074. doi: 10.1074/jbc.M103280200. [DOI] [PubMed] [Google Scholar]
- 141.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 142.Davis MM, Krogsgaard M, Huse M, Huppa JB, Lillemeier BF, Li QJ. T cells as a self-referential, sensory organ. Annu Rev Immunol. 2007;25:681–695. doi: 10.1146/annurev.immunol.24.021605.090600. [DOI] [PubMed] [Google Scholar]
- 143.Krogsgaard M, Juang J, Davis MM. A role for "self" in T-cell activation. Semin Immunol. 2007;19:236–244. doi: 10.1016/j.smim.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chu HH, Moon JJ, Takada K, Pepper M, Molitor JA, Schacker TW, et al. Positive selection optimizes the number and function of MHCII-restricted CD4+ T cell clones in the naive polyclonal repertoire. Proc Natl Acad Sci USA. 2009;106:11241–11245. doi: 10.1073/pnas.0902015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stefanova I, Dorfman JR, Tsukamoto M, Germain RN. On the role of self-recognition in T cell responses to foreign antigen. Immunol Rev. 2003;191:97–106. doi: 10.1034/j.1600-065x.2003.00006.x. [DOI] [PubMed] [Google Scholar]
- 146.Sporri R, Reis e Sousa C. Self peptide/MHC class I complexes have a negligible effect on the response of some CD8+ T cells to foreign antigen. Eur J Immunol. 2002;32:3161–3317. doi: 10.1002/1521-4141(200211)32:11<3161::AID-IMMU3161>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 147.Ljunggren HG, Stam NJ, Ohlen C, Neefjes JJ, Hoglund P, Heemels MT, et al. Empty MHC class I molecules come out in the cold. Nature. 1990;346:476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- 148.Yachi PP, Ampudia J, Gascoigne NR, Zal T. Nonstimulatory peptides contribute to antigen-induced CD8− T cell receptor interaction at the immunological synapse. Nat Immunol. 2005;6:785–792. doi: 10.1038/ni1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yachi PP, Lotz C, Ampudia J, Gascoigne NR. T cell activation enhancement by endogenous pMHC acts for both weak and strong agonists but varies with differentiation state. J Exp Med. 2007;204:2747–2757. doi: 10.1084/jem.20062610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wooldridge L, van den Berg HA, Glick M, Gostick E, Laugel B, Hutchinson SL, et al. Interaction between the CD8 coreceptor and major histocompatibility complex class I stabilizes T cell receptor-antigen complexes at the cell surface. J Biol Chem. 2005;280:27491–27501. doi: 10.1074/jbc.M500555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huppa JB, Axmann M, Mortelmaier MA, Lillemeier BF, Newell EW, Brameshuber M, et al. TCR–peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463:963–967. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bosselut R, Feigenbaum L, Sharrow SO, Singer A. Strength of signaling by CD4 and CD8 coreceptor tails determines the number but not the lineage direction of positively selected thymocytes. Immunity. 2001;14:483–494. doi: 10.1016/s1074-7613(01)00128-5. [DOI] [PubMed] [Google Scholar]
- 153.Ebert PJ, Baker JF, Punt JA. Immature CD4+CD8+ thymocytes do not polarize lipid rafts in response to TCR-mediated signals. J Immunol. 2000;165:5435–5442. doi: 10.4049/jimmunol.165.10.5435. [DOI] [PubMed] [Google Scholar]
- 154.Krogsgaard M, Davis MM. How T cells ’see’ antigen. Nat Immunol. 2005;6:239–245. doi: 10.1038/ni1173. [DOI] [PubMed] [Google Scholar]
- 155.Robey EA, Bousso P. Visualizing thymocyte motility using 2-photon microscopy. Immunol Rev. 2003;195:51–57. doi: 10.1034/j.1600-065x.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- 156.Griffith AV, Fallahi M, Nakase H, Gosink M, Young B, Petrie HT. Spatial mapping of thymic stromal microenvironments reveals unique features influencing T lymphoid differentiation. Immunity. 2009;31:999–1009. doi: 10.1016/j.immuni.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tourne S, Nakano N, Viville S, Benoist C, Mathis D. The influence of invariant chain on the positive selection of single T cell receptor specificities. Eur J Immunol. 1995;25:1851–1856. doi: 10.1002/eji.1830250709. [DOI] [PubMed] [Google Scholar]
- 158.Ignatowicz L, Kappler J, Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84:521–529. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- 159.Venanzi ES, Melamed R, Mathis D, Benoist C. The variable immunological self: genetic variation and nongenetic noise in Aire-regulated transcription. Proc Natl Acad Sci USA. 2008;105:15860–15865. doi: 10.1073/pnas.0808070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Day CL, Seth NP, Lucas M, Appel H, Gauthier L, Lauer GM, et al. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J Clin Invest. 2003;112:831–842. doi: 10.1172/JCI18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Savage PA, Davis MM. A kinetic window constricts the T cell receptor repertoire in the thymus. Immunity. 2001;14:243–252. doi: 10.1016/s1074-7613(01)00106-6. [DOI] [PubMed] [Google Scholar]
- 162.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 163.Kovalovsky D, Pezzano M, Ortiz BD, Sant’Angelo DB. A novel TCR transgenic model reveals that negative selection involves an immediate, Bim-dependent pathway and a delayed, Bim-independent pathway. PLoS ONE. 2010;5:e8675. doi: 10.1371/journal.pone.0008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Weinstein JA, Jiang N, White RA, 3rd, Fisher DS, Quake SR. High-throughput sequencing of the zebrafish antibody repertoire. Science. 2009;324:807–810. doi: 10.1126/science.1170020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jiang X, Fares MA. Identifying coevolutionary patterns in human leukocyte antigen (Hla) molecules. Evolution; Int J Org Evol. 2009;64:1429–1445. doi: 10.1111/j.1558-5646.2009.00903.x. [DOI] [PubMed] [Google Scholar]
- 166.Marrack P, Kappler J. The T cell receptor. Science. 1987;238:1073–1079. doi: 10.1126/science.3317824. [DOI] [PubMed] [Google Scholar]
- 167.Ma Z, Sharp KA, Janmey PA, Finkel TH. Surface-anchored monomeric agonist pMHCs alone trigger TCR with high sensitivity. PLoS Biol. 2008;6:e43. doi: 10.1371/journal.pbio.0060043. [DOI] [PMC free article] [PubMed] [Google Scholar]