Abstract
Objective:
We pooled the results of studies on natural history of cavernous malformations (CM) to calculate point estimates and investigate main sources of heterogeneity.
Methods:
We searched MEDLINE, EMBASE, and ISI Web of Science for relevant studies published before May 2015. We used fixed or random effects models and meta-regression to pool the data.
Results:
Twenty-five studies were entered into the meta-analysis (90–1,295 patients depending on the analysis). Bleeding was defined as symptomatic hemorrhage plus radiologic evidence of hemorrhage. Sources of heterogeneity were identified as mixture of hemorrhage and rehemorrhage, mixture of rehemorrhage before and after 2 years of first bleeding, brainstem vs other locations, and calculation method. The rehemorrhage rate was higher than the hemorrhage rate (incidence rate ratio 16.5, p < 0.001, 95% confidence interval [CI] 9.7–28.0). Rehemorrhage within 2 years of the first hemorrhage was higher than after that (incidence rate ratio 1.8, p = 0.042, 95% CI 1.5–2.0). In two metaregression models, rough estimate of the annual incidence rate of hemorrhage was 0.3% (95% CI 0.1%–0.5%) and 2.8% (2.5%–3.3%) per person year in nonbrainstem and brainstem lesions and rough estimate of annual rehemorrhage rate per person year was 6.3% (3%–13.2%) and 32.3% (19.8%–52.7%) in nonbrainstem and brainstem lesions. Median time to rehemorrhage was 10.5 months. Posthemorrhage full recovery was 38.8%/person-year (28.7%–48.8%). Posthemorrhage full recovery or minimal disability was 79.5%/person-year (74.3%–84.8%). Mortality after bleeding was 2.2%.
Conclusions:
The incidence of symptomatic hemorrhage or rehemorrhage is higher in brainstem lesions. First symptomatic hemorrhage increases the chance of symptomatic rehemorrhage, which decreases after 2 years.
Cavernous malformations (CMs) are closely packed abnormal blood vessels lined with endothelial cells that do not exhibit intervening tight junctions. These vessels lack muscular and elastic layers and are filled with blood at various stages of thrombosis and organization. In the brain and spinal cord, CMs are usually surrounded by hemosiderin and gliosis. There is usually no neural tissue inside the lesion. They are the second most common type of vascular lesion in CNS, comprising 10%–15% of all vascular malformations.1
CMs can present incidentally, with seizure or with focal neurologic symptoms. CMs can produce acute, subacute, waxing and waning, or chronic symptoms. Symptoms can be attributed to hemorrhage inside or outside the boundary of lesion, mass effect, or other processes. Familial cases tend to have multiple lesions, in comparison with sporadic cases, which most often have one identified lesion.2
Despite numerous studies about the natural history of CMs, results of studies so far have been heterogeneous. This may be due to different study designs or inclusion of subgroups of patients with different incidence rates (IRs) of new events. Therefore, providing overall IR of the events, or evaluating the risk factors based on the overall IR, might be misleading.
In the present study, we performed a systematic literature review of studies that evaluated natural history of brain and spinal cord CMs. We initially investigated sources of heterogeneity and subsequently estimated the risk of bleeding in CMs by pooling the data in more homogeneous groups of patients.
METHODS
This study followed the PRISMA statement. The protocol and the planned analysis were not published in advance.
Search strategy and selection criteria.
We searched 3 databases—MEDLINE (1950–May 2015), EMBASE (1980–May 2015), and Web of Science (1976–May 2015)—with all possible combinations of CM key words, MESH terms, and EMTREE terms up to May 2015 (supplemental material on the Neurology® Web site at Neurology.org). We also included published search methodology to extract natural history studies.3,4 Moreover, we inspected references of all included studies and previously published review articles for further relevant studies.
We included all prospective and retrospective studies that evaluated at least 20 untreated patients, to decrease chance of error, with brain or spinal cord CM. We did not exclude any article on the basis of language. In all included studies, a specified follow-up period including the inception point and definition of hemorrhage (if hemorrhage has been evaluated) had to be specified. Diagnosis had to be established using MRI, CT, or histology. Presurgical follow-up was also included. We defined hemorrhage as acute or subacute onset of symptoms accompanied by radiologic, pathologic, surgical, or CSF evidence of recent extralesional or intralesional hemorrhage.
Data extraction and management.
The first and second authors independently inspected the citations identified in the search process. We discussed any disagreement with other authors. We identified duplicate citations by bibliometric data of each article. After selection of relevant titles and abstracts, we obtained full reports for compliance of studies with eligibility criteria. We contacted the study author if further information was needed. In each study, several parameters were evaluated for assessing risk of bias (supplemental material).
Our predefined outcome measures were annual hemorrhage and rehemorrhage rate (person-year or lesion-year), annual rehemorrhage rate before and after 2 years (person-year or lesion-year), annual event or recurrent event rate (person-year or lesion-year), hemorrhage fatality rate, and morbidity (determined by any measure).
Time of birth was considered as an inception point of follow-up for calculating hemorrhage rate in retrospective studies because of lack of date of diagnosis. In prospective studies, time of inclusion in the study was the inception point. Therefore, patients were asymptomatic at the inception point for calculating hemorrhage rate. For calculating rehemorrhage rate, the inception point was the time of first symptomatic hemorrhage in all retrospective studies and prospective studies. Rehemorrhage after 2 years was defined as any rehemorrhage after 2 years (regardless of whether or not there was rehemorrhage within 2 years). We chose a 2-year cutoff based on a previous report that suggested there was a relative decrease in the hemorrhage rate about 2 years after a hemorrhage.5
If possible, we calculated pure hemorrhage or rehemorrhage rates in studies in which hemorrhage and rehemorrhage were mixed together; otherwise we excluded that measure from calculating pure hemorrhage or rehemorrhage rates. We also excluded suspected mixture of hemorrhage and rehemorrhage from the meta-analysis. In some studies, for calculating the rehemorrhage rate, all symptomatic patients at the time of diagnosis were included in calculations instead of those confirmed to have hemorrhage at the time of diagnosis.6–9 We excluded the point estimate (PE) from meta-analysis when there was any concern regarding this.6 In 2 studies, there were some patients who had bleeding prior to inclusion into the study. In these cases, the time from bleeding until inclusion into the study was ignored.10,11 We did not extract data from one group of patients twice.11–20
Assessment of heterogeneity.
We assessed heterogeneity by inspecting the graphs and use of χ2, p value, and I2 statistics. We considered a p value of less than 0.10 as significant. We interpreted I2 value of 50% or greater as high heterogeneity. In case of heterogeneity, we investigated the reason. The main predefined reasons for investigating heterogeneity were (1) hemorrhage vs rehemorrhage, (2) before and after 2 years of rehemorrhage, (3) location of CMs, and (4) type of calculation. See the supplemental material for heterogeneity and bias assessment.
Statistical analysis.
If we did not interpret the data as being heterogeneous, a fixed effect model (FEM) was used (with the Hedges g method of weighting). Whenever data were heterogeneous, we investigated reasons for heterogeneity in the included studies with particular attention to the study method. If we could find a reason for heterogeneous results, we separated the study or a group of studies responsible for the heterogeneity from the rest of the studies. If we could not find a reason for heterogeneity, we tried to find outlier studies responsible for overall heterogeneity with applying random effect variance shift model (RVSM) using R software (supplemental material).21 When we could not use the 2 latter mentioned solutions for dealing with a heterogeneous study, a random effect model using moment-based method was carried out for analysis. When we could attribute heterogeneity among studies to predefined subgroups for assessment (supplemental material), we presented the data in subgroups and also compared relevant subgroups with each other. For comparing subgroups, we preferably extracted or calculated IR ratio in each study (this is a ratio of IR in each group) and then combined them. We used this for comparing hemorrhage vs rehemorrhage and rehemorrhage before and after 2 years (supplemental material). We used logarithmic transformation of IR, or IR ratio and their confidence intervals, for deriving the PE and then converted them back to normal scale. We also used a meta-regression model to assess the source of heterogeneity as well as deriving PEs. Meta-regression, by allowing for controlling the study level variables (in the present article, proportion of brainstem lesions), makes linear prediction of outcome possible based on different values of covariates. We extracted proportion of brainstem cases from the articles for inclusion into the meta-regression model. If proportion of brainstem cases were not given for patients at risk of hemorrhage and rehemorrhage separately, we used the proportion of brainstem cases in the whole number of patients. We conducted sensitivity analysis to assess how choosing our method of synthetizing the data (including type of included studies) affected the results. All analyses except RVSM were carried out using STATA version 11 (College Station, TX).
RESULTS
The supplemental material shows the study flowchart (figure e-1) and final included studies.5–13,15,16,18,22–38 We had access to individual patient data in 2 studies and calculated relevant information from these articles.5,35 See the supplemental material for characteristics of included studies including definition of hemorrhage, type of diagnosis, and quality assessment (tables e-1 and e-2).
Identifying source of heterogeneity.
Person-year vs lesion-year.
For calculating hemorrhage rate, all studies censored the patients after first bleeding. In case of multiple lesions, if calculations are based on lesion-year of follow-up, this results in right side censoring and underestimation of hemorrhage rate because of ignoring other lesions while including their lesion-year of follow-up for calculating the whole incidence rate of hemorrhage.
Most of the studies mixed lesion-year of follow-up in lesions that bled with lesion-year of asymptomatic lesions for calculating the rehemorrhage rates. This underestimates the rehemorrhage rates.
Therefore, we decided to use person-year of follow-up for our calculations.
Hemorrhage vs rehemorrhage.
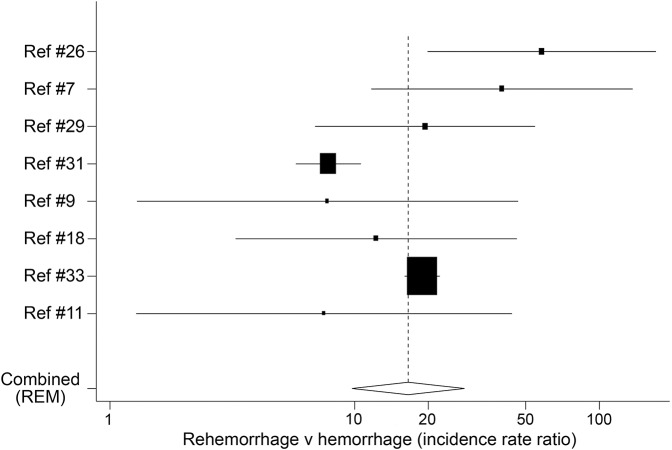
Result of a random effect model meta-analysis showed that rehemorrhage rates were significantly higher than hemorrhage rates (table 1, figure 1)7,9,11,18,26,29,31,33 (supplemental material).
Table 1.
Predictors of hemorrhage

Figure 1. Rehemorrhage rate vs hemorrhage rate.
Dashed vertical line represents pooled value. Analysis was done on logarithmic scale and the x-axis is shown with transformation to normal scale. REM = random effect model. On the x-axis, 1 represents null hypothesis.
Rehemorrhage before and after 2 years.
Rehemorrhage before 2 years of first bleeding was higher than after that (table 1, figure e-2). One study29 had an exceptionally high estimate compared with the other studies.5,18,29,34,35 This was the only study that followed patients for more than 5 years. Downweighting the mentioned study using RVSM + variance shift (p = 0.042) or omission of the study (p = 0.043) all yielded significant results (supplemental material) indicating rehemorrhage rate before 2 years is higher than after that.
Brainstem vs other locations.
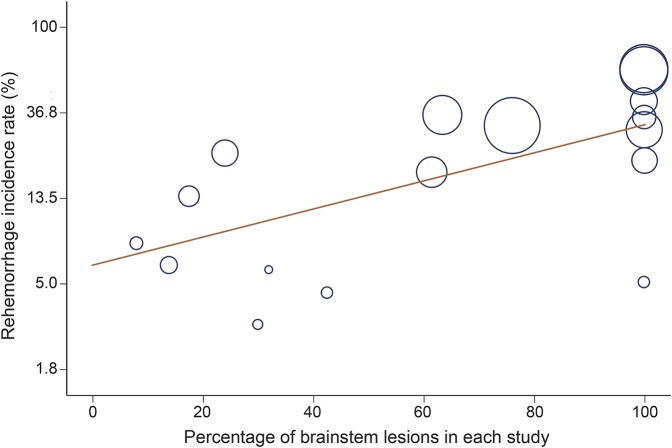
Based on the meta-regression model, risk of rehemorrhage in brainstem lesions was significantly more than in nonbrainstem lesions (table 2, figure 2).5,7–11,18,26,29,31–38 This identified brainstem location as a source of heterogeneity. One study censored patients after second hemorrhage.8 In 2 studies, type of censoring after second hemorrhage could not be ascertained.18,29 The rest of the included studies did not censor the cases after second hemorrhage.
Table 2.
Point estimate of incidence rates in natural history of cavernous malformation

Figure 2. Percentage of brainstem cavernous malformation lesions in each study and the overall reported incidence rate of rehemorrhage.
Radius of each circle represents the precision of each study.
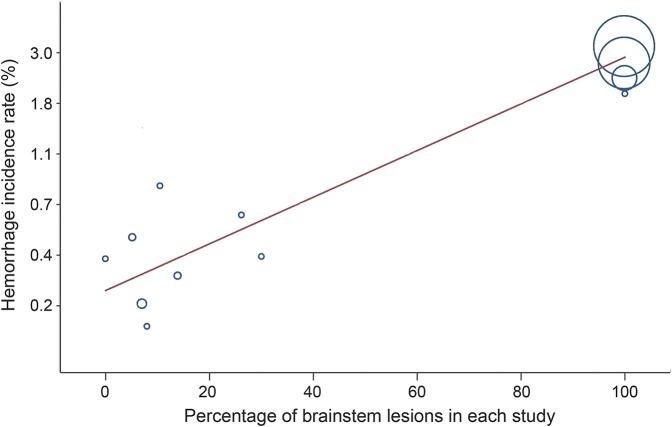
In another meta-regression model, hemorrhage rate was higher in brainstem lesions compared with other locations (table 2, figure 3).6,7,9,11,13,18,22,26,27,29,31,33 This model identified brainstem as a source of heterogeneity as well. In 4 included studies, at baseline not all patients were asymptomatic and some of them had seizure or minor symptoms not attributable to hemorrhage.9,18,26,29 Four studies calculated hemorrhage rates assuming lesions were present from birth.13,22,31,33 Excluding these 4 studies, there was still strong correlation between percentage of brainstem lesions in each study and hemorrhage rate (r = 0.85, p = 0.0003). Residual I2 of the model became 0 with adjusted R2 of 99.5%. This means most of the heterogeneity among studies was accounted for by controlling for the percentage of brainstem lesions in each study.
Figure 3. Percentage of brainstem cavernous malformation lesions in each study and the overall reported incidence rate of hemorrhage.
Radius of each circle represents the precision of each study.
Other locations.
We could not assess risk of hemorrhage in other locations mainly due to mixture of hemorrhage and rehemorrhage in studies (supplemental material).
Median time of rehemorrhage.
In 12 included studies,5,7–9,11,15,18,29,32,33,35,36 50% of patients who rehemorrhaged did so within 10.5 months of the first hemorrhage (table 2). See the supplemental material for detailed analysis.
Predictors of bleeding.
We could not evaluate heterogeneity related to sex, age, size of lesion, associated developmental venous malformation, multiple vs single lesions, or pregnancy. Subgroup analysis was not possible because of different definitions of hemorrhage, mixture of subgroups of patients as identified in this study, and not appropriate reporting the incidence rate (supplemental material). Meta-regression with inclusion of more than one variable was not possible because of lack of enough included studies.
Familial vs nonfamilial.
In familial series, asymptomatic hemorrhage was also included in the calculation of hemorrhage and rehemorrhage rate and rates were mostly mixed.24,25,28 We recalculated pure hemorrhage rate in 1 study, which was slightly higher (1.43%) than IR of nonfamilial cases.28 Therefore, we decided not to use this value in meta-regression model to prevent unnecessary mixture of different groups of patients.
Morbidity.
Based on FEM meta-analysis, complete recovery after hemorrhage was about 40% (table 2).9,31 Using FEM, no or minimal disability (defined as no need for assistance and minimal symptoms) after hemorrhage was about 80% (table 2).13,26,31
Case fatality rate.
With inclusion of 7 studies,9,11,18,23,26,30,31 overall case fatality rate after acute hemorrhagic event based on FEM meta-analysis was 2.2%. Duration of follow-up after bleeding was not mentioned in most of the articles (it was assumed early after bleeding).
DISCUSSION
This is a well-designed meta-analysis of the natural history of CMs that formally assessed source of heterogeneity among studies and identified a more homogeneous subset of patients. This makes our estimations more robust. Mixtures of subgroups of patients with different IR of hemorrhage was the main source of heterogeneity (hemorrhage vs rehemorrhage, location, rehemorrhage before and after 2 years). This mixture of patients can confound evaluation of other potential risk factors such as age, sex, size, pregnancy, and associated developmental venous anomaly. We did not perform meta-analysis in aforementioned potential risk factors since we believed mixture of different subsets of patients can confound the results. Measures such as odds ratio or relative risk without considering the time of follow-up in 2 groups of comparison can result in biased conclusion and be a potential source of heterogeneity. Not calculating the lesion-year or person-year of follow-up appropriately was identified as another source of bias and subsequently a source of heterogeneity. Part of heterogeneity among studies regarding different risk factors of hemorrhage (e.g., age, sex, pregnancy, location, developmental venous anomaly) can be attributed to not considering the aforementioned factors in analysis.
Recently a meta-analysis on the natural history of CMs was published.39 Our study has several merits over the previously published article. First, with our comprehensive search strategy and complex statistical method, we were able to include more studies and identified more subgroups of patients with homogeneous risk of hemorrhage and reported on different aspects of hemorrhage including morbidity and mortality. Second, we differentiated between hemorrhage and rehemorrhage. Third, we showed there is a clustering of rehemorrhage and, therefore, we reported annual incidence rate as opposed to 5-year incidence rate. Fourth, we clearly identified sources of heterogeneity among studies and explained appropriate methodology of calculations to prevent bias.
The point estimates for comparison of hemorrhage vs rehemorrhage and brainstem vs other locations gives a rough estimate of comparison and is not intended to calculate the exact magnitude of difference as for each of these comparisons all heterogeneity sources could not be controlled. Hemorrhage and rehemorrhage rates obtained from meta-regression are also just an estimate for brainstem and nonbrainstem lesions for the same reason. An important finding was complete resolution of heterogeneity among studies in hemorrhage rate by using brainstem percentage in each study as a covariate. Even by excluding studies that calculated the hemorrhage rate assuming lesions were present from birth, the correlation between hemorrhage rate and brainstem lesions stayed high.
Since only symptomatic hemorrhage was evaluated, our data show that brainstem CMs are more symptomatic. The higher risk in brainstem CM may be because small, low-pressure hemorrhages that might be asymptomatic from a supratentorial CM are more likely to cause symptoms in the brainstem. Further study is needed to evaluate symptomatic hemorrhage and rehemorrhage rate in highly eloquent cortical locations.
Particular attention should be made in calculating incidence rate based on lesion-year or person-year of follow-up. In patients with multiple lesions, if hemorrhage incidence rate based on lesion-year of follow-up is reported, individuals should be followed for hemorrhage in other lesions to prevent right side censoring. In similar situations, for calculating hemorrhage or rehemorrhage incidence rates based on person-year of follow-up, location of the lesions should be considered since differently seated lesions might exhibit different chance of bleeding (brainstem vs other locations).
In patients with multiple lesions, only subsequent hemorrhage in the same lesion that bled before should be considered as rehemorrhage and bleeding in other lesions that never bled before should not be considered as rehemorrhage (This can underestimate the lesion-year of rehemorrhage).
As our study showed, since the rate of rehemorrhage is different before and after 2 years, hazard ratio, which allows for the change in incidence rate during the follow-up period, is a more suitable measure than IR to evaluate potential risk factors of rehemorrhage.
Natural history studies of familial cases of CMs have suggested that familial lesions might have a higher risk of bleeding.24,25,28 We suggest part of this difference is attributable to inclusion of asymptomatic hemorrhage in familial studies whereas nonfamilial studies defined hemorrhage as a symptomatic event with evidence of bleeding on radiologic imaging or pathology at the time of surgery (familial series had imaging surveillance as opposed to other studies that performed imaging once the patient was symptomatic). Other method differences may contribute to differences in familial and nonfamilial series such as use of screening in familial cases. Available data, however, do not permit any definitive conclusions.
Case-fatality rate and morbidity of patients after hemorrhage might have been underestimated in this study because of exclusion of patients who underwent surgery. Inclusion of well-designed retrospective studies made our study more comprehensive but might have affected our results. However, we investigated risk of bias (supplemental material), and performed sensitivity analysis to ensure our results are robust. The nature of our outcome selection (symptomatic hemorrhage) also makes retrospective studies more reliable.
Regarding hemorrhage and rehemorrhage rates, exclusion of surgical cases had minor effect on point estimates since all included studies tried to add follow-up time into their calculations prior to any intervention.
Bleeding in cavernomas confers low risk of death and morbidity. Location of the lesions is an important risk factor for symptomatic hemorrhage (brainstem location). Prior hemorrhage increases the risk of rehemorrhage. Rehemorrhage is considerably more common during the first 2 years after the first bleeding. Lesion-year or person-year of follow-up should be appropriately calculated. IR of hemorrhage or rehemorrhage should not be mixed in future studies.
Supplementary Material
GLOSSARY
- CM
cavernous malformation
- FEM
fixed effect model
- IR
incidence rate
- PE
point estimate
- REML + VS
restricted maximal likelihood + variance shift
- RVSM
random effect variance shift model
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
S.T. and R.L.M. were responsible for study design. S.T. and A.M. collected data. S.T. performed data analysis and data interpretation. S.T. drafted the paper. S.A. and F.G.B. provided individual patient data and collaborated in data synthesis. All authors critically revised the manuscript.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
S. Taslimi, A. Modabbernia, S. Amin-Hanjani, and F. Barker report no disclosures relevant to the manuscript. L. Macdonald: Physicians Services Incorporated Foundation, Brain Aneurysm Foundation, Canadian Institutes for Health Research, and the Heart and Stroke Foundation of Canada. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Washington CW, McCoy KE, Zipfel GJ. Update on the natural history of cavernous malformations and factors predicting aggressive clinical presentation. Neurosurg Focus 2010;29:E7. [DOI] [PubMed] [Google Scholar]
- 2.Dziedzic T, Kunert P, Matyja E, Ziora-Jakutowicz K, Sidoti A, Marchel A. Familial cerebral cavernous malformation. Folia Neuropathol 2012;50:152–158. [PubMed] [Google Scholar]
- 3.Wilczynski NL, Haynes RB. Optimal search strategies for detecting clinically sound prognostic studies in EMBASE: an analytic survey. J Am Med Inform Assoc 2005;12:481–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound prognostic studies in MEDLINE: an analytic survey. BMC Med 2004;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker FG, II, Amin-Hanjani S, Butler WE, et al. Temporal clustering of hemorrhages from untreated cavernous malformations of the central nervous system. Neurosurgery 2001;49:15–24; discussion 24–25. [DOI] [PubMed] [Google Scholar]
- 6.Mathiesen T, Edner G, Kihlstrom L. Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg 2003;99:31–37. [DOI] [PubMed] [Google Scholar]
- 7.Al-Holou WN, O'Lynnger TM, Pandey AS, et al. Natural history and imaging prevalence of cavernous malformations in children and young adults [clinical article]. J Neurosurg Pediatr 2012;9:198–205. [DOI] [PubMed] [Google Scholar]
- 8.Hauck EF, Barnett SL, White JA, Samson D. Symptomatic brainstem cavernomas. Neurosurgery 2009;64:61–70; discussion 70–71. [DOI] [PubMed] [Google Scholar]
- 9.Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg 1997;87:190–197. [DOI] [PubMed] [Google Scholar]
- 10.Kupersmith MJ, Kalish H, Epstein F, et al. Natural history of brainstem cavernous malformations. Neurosurgery 2001;48:47–53. [DOI] [PubMed] [Google Scholar]
- 11.Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg 1995;83:820–824. [DOI] [PubMed] [Google Scholar]
- 12.Murillo-Bonilla LM, Cantu-Brito C, Arauz-Gongora A, Higuera-Calleja J, Padilla-Rubio J, Barinagarrementeria-Aldatz F. Cavernous angiomas: clinical observations and prognosis in 133 patients. Rev Invest Clin 2003;55:387–393. [PubMed] [Google Scholar]
- 13.Cantu C, Murillo-Bonilla L, Arauz A, Higuera J, Padilla J, Barinagarrementeria F. Predictive factors for intracerebral hemorrhage in patients with cavernous angiomas. Neurol Res 2005;27:314–318. [DOI] [PubMed] [Google Scholar]
- 14.Kondziolka D, Monaco EA, III, Lunsford LD. Cavernous malformations and hemorrhage risk. Prog Neurol Surg 2013;27:141–146. [DOI] [PubMed] [Google Scholar]
- 15.Moriarity JL, Wetzel M, Clatterbuck RE, et al. The natural history of cavernous malformations: a prospective study of 68 patients. Neurosurgery 1999;44:1166–1173. [PubMed] [Google Scholar]
- 16.Clatterbuck RE, Moriarity JL, Elmaci I, Lee RR, Breiter SN, Rigamonti D. Dynamic nature of cavernous malformations: a prospective magnetic resonance imaging study with volumetric analysis. J Neurosurg 2000;93:981–986. [DOI] [PubMed] [Google Scholar]
- 17.Josephson CB, Leach JP, Duncan R, et al. Seizure risk from cavernous or arteriovenous malformations: prospective population-based study. Neurology 2011;76:1548–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Shahi Salman R, Hall JM, Horne MA, et al. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol 2012;11:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, Hao SY, Tang J, et al. Clinical course of untreated pediatric brainstem cavernous malformations: hemorrhage risk and functional recovery. J Neurosurg Pediatr 2014;13:471–483. [DOI] [PubMed] [Google Scholar]
- 20.Li DA, Hao SYU, Jia GJ, Wu Z, Zhang LIW, Zhang JT. Hemorrhage risks and functional outcomes of untreated brainstem cavernous malformations. J Neurosurg 2014;121:32–41. [DOI] [PubMed] [Google Scholar]
- 21.Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry 2013;74:15–25. [DOI] [PubMed] [Google Scholar]
- 22.Del Curling O, Jr, Kelly DL, Jr, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg 1991;75:702–708. [DOI] [PubMed] [Google Scholar]
- 23.Kim DS, Park YG, Choi JU, Chung SS, Lee KC. An analysis of the natural history of cavernous malformations. Surg Neurol 1997;48:9–18. [DOI] [PubMed] [Google Scholar]
- 24.Zabramski JM, Wascher TM, Spetzler RF, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg 1994;80:422–432. [DOI] [PubMed] [Google Scholar]
- 25.Labauge P, Brunereau L, Levy C, Laberge S, Houtteville JP. The natural history of familial cerebral cavernomas: a retrospective MRI study of 40 patients. Neuroradiology 2000;42:327–332. [DOI] [PubMed] [Google Scholar]
- 26.Aiba T, Tanaka R, Koike T, Kameyama S, Takeda N, Komata T. Natural history of intracranial cavernous malformations. J Neurosurg 1995;83:56–59. [DOI] [PubMed] [Google Scholar]
- 27.Robinson JR, Awad IA, Little JR. Natural-history of the cavernous angioma. J Neurosurg 1991;75:709–714. [DOI] [PubMed] [Google Scholar]
- 28.Labauge P, Brunereau L, Laberge S, Houtteville JP. Prospective follow-up of 33 asymptomatic patients with familial cerebral cavernous malformations. Neurology 2001;57:1825–1828. [DOI] [PubMed] [Google Scholar]
- 29.Flemming KD, Link MJ, Christianson TJH, Brown RD., Jr Prospective hemorrhage risk of intracerebral cavernous malformations. Neurology 2012;78:632–636. [DOI] [PubMed] [Google Scholar]
- 30.Menon G, Gopalakrishnan CV, Rao BR, Nair S, Sudhir J, Sharma M. A single institution series of cavernomas of the brainstem. J Clin Neurosci 2011;18:1210–1214. [DOI] [PubMed] [Google Scholar]
- 31.Fritschi JA, Reulen HJ, Spetzler RF, Zabramski JM. Cavernous malformations of the brain stem: a review of 139 cases. Acta Neurochir 1994;130:35–46. [DOI] [PubMed] [Google Scholar]
- 32.Porter RW, Detwiler PW, Spetzler RF, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg 1999;90:50–58. [DOI] [PubMed] [Google Scholar]
- 33.Wang CC, Liu A, Zhang JT, Sun B, Zhao YL. Surgical management of brain-stem cavernous malformations: report of 137 cases. Surg Neurol 2003;59:444–454; discussion 454. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa T, McInerney J, Kondziolka D, Lee JY, Flickinger JC, Lunsford LD. Long-term results after stereotactic radiosurgery for patients with cavernous malformations. Neurosurgery 2002;50:1190–1197; discussion 1197–1198. [DOI] [PubMed] [Google Scholar]
- 35.Amin-Hanjani S, Ogilvy CS, Candia GJ, Lyons S, Chapman PH. Stereotactic radiosurgery for cavernous malformations: Kjellberg's experience with proton beam therapy in 98 cases at the Harvard Cyclotron. Neurosurgery 1998;42:1229–1236; discussion 1236–1238. [DOI] [PubMed] [Google Scholar]
- 36.Li D, Yang Y, Hao SY, et al. Hemorrhage risk, surgical management, and functional outcome of brainstem cavernous malformations. J Neurosurg 2013;119:996–1008. [DOI] [PubMed] [Google Scholar]
- 37.Pandey P, Westbroek EM, Gooderham PA, Steinberg GK. Cavernous malformation of brainstem, thalamus, and basal ganglia: a series of 176 patients. Neurosurgery 2013;72:573–589; discussion 588–589. [DOI] [PubMed] [Google Scholar]
- 38.Ferroli P, Sinisi M, Franzini A, Giombini S, Solero CL, Broggi G. Brainstem cavernomas: long-term results of microsurgical resection in 52 patients. Neurosurgery 2005;56:1203–1212; discussion 1212–1214. [DOI] [PubMed] [Google Scholar]
- 39.Horne MA, Flemming KD, Su IC, et al. Clinical course of untreated cerebral cavernous malformations: a meta-analysis of individual patient data. Lancet Neurol Epub 2015 Dec 1. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.