Abstract
Objective:
The brain reserve hypothesis links larger maximal lifetime brain growth (MLBG, estimated with intracranial volume [ICV]) with lower risk for cognitive decline/dementia. We examined whether larger MLBG is also linked to less physical disability progression over 5 years in a prospective sample of treatment-naive patients with multiple sclerosis (MS).
Methods:
Physical disability was measured with the Expanded Disability Status Scale (EDSS) at baseline and 5-year follow-up in 52 treatment-naive Serbian patients with MS. MRI measured disease burden (cerebral atrophy, T2 lesion volume) and MLBG: a genetically determined, premorbid (established during adolescence, stable thereafter) patient characteristic estimated with ICV (adjusted for sex). Logistic regression tested whether MLBG (smaller vs larger) predicts disability progression (stable vs worsened) independently of disease burden.
Results:
Disability progression was observed in 29 (55.8%) patients. Larger MLBG predicted lower risk for progression (odds ratio 0.13, 95% confidence interval 0.02–0.78), independently of disease burden. We also calculated absolute change in EDSS scores, and observed that patients with smaller MLBG showed worse EDSS change (0.91 ± 0.71) than patients with larger MLBG (0.42 ± 0.87).
Conclusions:
Larger MLBG was linked to lower risk for disability progression in patients with MS over 5 years, which is the first extension of the brain reserve hypothesis to physical disability. MLBG (ICV) represents a clinically available metric that may help gauge risk for future disability in patients with MS, which may advance the science and practice of early intervention. Potential avenues for future research are discussed.
Physical disability progression varies widely across persons with multiple sclerosis (MS), making it difficult to identify patients at greatest risk for disability. This is an obstacle for early intervention research and clinical practice (e.g., choice of disease-modifying drugs [DMDs]).
The brain reserve hypothesis1 posits that developmental differences in maximal lifetime brain growth (MLBG, estimated with intracranial volume [ICV]) afford differential reserve against cognitive impairment/dementia among elders2 and patients with MS,3 with larger MLBG linked to lower risk. Larger MLBG is linearly related to larger neuronal count4 (and, by extension, synaptic count), which may (1) support the development of robust neural networks resistant to disease-related disruption or (2) provide additional degrees of freedom for plastic reorganization in response to disease, thereby protecting against cognitive dysfunction.
It is unknown whether the brain reserve hypothesis also applies to physical disability in any neurologic population, including MS. In this initial study, we investigate whether larger MLBG is linked to lower risk for physical disability progression over 5 years in a sample of treatment-naive Serbian patients with MS, independently of disease-related brain changes (T2 lesion volume, cerebral atrophy). If so, MLBG (ICV) may represent a stable,5 genetically determined,6 easily measured, and clinically available marker of risk for future disability in patients with MS.
METHODS
Patients.
Fifty-two patients with MS (39 women) from Belgrade, Serbia, were evaluated at baseline and after median follow-up of 5 years (interquartile range 1.0). Patients had adult-onset MS to avoid differences in brain development. Baseline age was ≤60 years to avoid age-related frailty (e.g., muscle weakness, slowed walking),7 which could affect Expanded Disability Status Scale (EDSS) scores in a way unrelated to MS. Patients were free of exacerbations for at least 4 weeks prior to baseline and follow-up. Patients received no DMD treatment from baseline through follow-up. Baseline characteristics were as follows: age: 42.8 ± 10.3 years; time since diagnosis: 10.3 ± 9.7 years; phenotypes: 7 clinically isolated syndrome (CIS), 25 relapsing-remitting MS (RRMS), 7 secondary progressive MS, 13 primary progressive MS; EDSS: 3.5 ± 2.0, median 3.5, range 0–7.5. Patients with CIS were reclassified as RRMS by follow-up.
Standard protocol approvals, registrations, and patient consents.
Approval was received from the local ethical standards committee on human research. Patients provided written informed consent.
Physical disability progression.
Physical disability was evaluated with the EDSS at baseline and follow-up. Clinically meaningful change in disability was classified as stable or progressed using standard criteria8 (increase of ≥1.0 if baseline EDSS ≤5.0, or ≥0.5 if baseline EDSS ≥5.5).
Normalized brain volume and T2 lesion volume (T2LV).
Using a 1.5T Siemens Avanto scanner, the following brain images were acquired at baseline and follow-up: 3D T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) and axial dual-echo (DE) turbo spin echo (see reference 3 for sequence details). Absolute brain volume (ABV) was obtained from MPRAGE scans using SIENAX. Consistent with standard procedures, ABV was adjusted for developmental differences in MLBG, estimated with ICV. Specifically, SIENAX volume scaling factor (VSF, a proxy of ICV) was derived from the transformation that matches the extracted brain and skull to standard space brain and skull images (derived from Montreal Neurological Institute [MNI] 152 standard image): VSF >1 and <1 derived for ICVs smaller and larger than the MNI atlas, respectively. ABV was multiplied by VSF to calculate normalized brain volume (NBV). T2LV was measured from DE scans using a thresholding segmentation technique (Jim 5.0, www.xinapse.com) by coauthors expert in the identification and quantification of MS lesions (P.P. and A.M., 6 and 7 years of experience, respectively). To measure change in disease burden over time, SIENA quantified percentage brain volume change (PBVC) from baseline to follow-up, and T2LV change was calculated as follow-up minus baseline.
Maximal lifetime brain growth.
Consistent with previous work,3,5 MLBG was estimated with ICV. More specifically, the reciprocal of the SIENAX VSF was taken so that larger values correspond to larger ICV, and regression-adjusted for sex (because men have larger ICV). ICV is an established estimate of MLBG in neuroimaging research (for background, see reference 9) and in general.6 Indeed, NBV is calculated as ABV adjusted for ICV, which controls for developmental differences in MLBG. This procedure regards developmental differences in MLBG as error variance to be controlled; however, the brain reserve hypothesis posits that such differences are important. MLBG is genetically determined,6 established during adolescence,5 and independent of age- or disease-related variables (e.g., cerebral atrophy).6 In our sample, ICV was unrelated to NBV (r = −0.142, p = 0.315) or T2LV (r = 0.085, p = 0.548). Unlike NBV, which decreases with age and disease, ICV remains stable throughout adulthood,5 and therefore remains a proxy of MLBG. NBV was negatively associated with age in our sample (r = −0.535, p < 0.001), and decreased over time (t[51] = 3.36, p = 0.001). MLBG was unrelated to age (r = −0.032, p = 0.821) and did not change (t[51] = 0.38, p = 0.704). Baseline and follow-up ICV were nearly perfectly correlated (r = 0.976). Also, VSF (our measure of ICV) was very highly correlated with a volumetric measure of ICV (r = 0.94). Our sample was divided into smaller and larger MLBG based on a median split of ICV (median 1,331.4 cm3 for women, 1,490.2 cm3 for men). There were no differences in demographic or disease burden variables between smaller and larger MLBG groups (table e-1 on the Neurology® Web site at Neurology.org).
Statistical analyses.
After assessing for outliers and winsorizing as appropriate, we investigated differences in demographic and disease burden variables between patients with disability progression vs stable patients (independent t tests and χ2 tests as appropriate). Variables differing between groups were entered in block one of a logistic regression predicting disability progression (stable, progressed). The contribution of MLBG (ICV) was evaluated in block 2. We predicted that larger MLBG would be independently linked to lower risk for progression. Next, we calculated absolute change in EDSS (follow-up minus baseline) for each patient, and regression-adjusted change for covariates in block one (above). An independent t test investigated differences in EDSS change between patients with larger and smaller MLBG. We predicted that EDSS change would be greater in patients with smaller MLBG.
RESULTS
Disability progression was observed in 29 patients (55.8%). Patients who progressed had lower baseline NBV, higher baseline T2LV, and worse brain volume loss over time (PBVC), and were more likely to have a progressive course (table e-2). The logistic regression model predicting disability progression controlling for these covariates was significant (χ2[6] = 27.43, p < 0.001), and patients with larger MLBG were at lower risk for disability progression (Wald[1] = 4.99, p = 0.026, odds ratio 0.13 [95% confidence interval 0.02–0.78]).
Disability (EDSS) increased from baseline to follow-up (EDSS change: mean = 0.67 ± 1.14, median = 0.5, range = −1.5 to 3.0; one-sample t[51] = 4.25, p < 0.001). Adjusted for covariates, patients with smaller MLBG showed worse disability progression (EDSS change = 0.91 ± 0.71) than patients with larger MLBG (0.42 ± 0.87; t[50] = 2.25, p = 0.029, see figure 1 and figure e-1).
Figure 1. Disability progression across patients with multiple sclerosis with lower and higher maximal lifetime brain growth (intracranial volume).
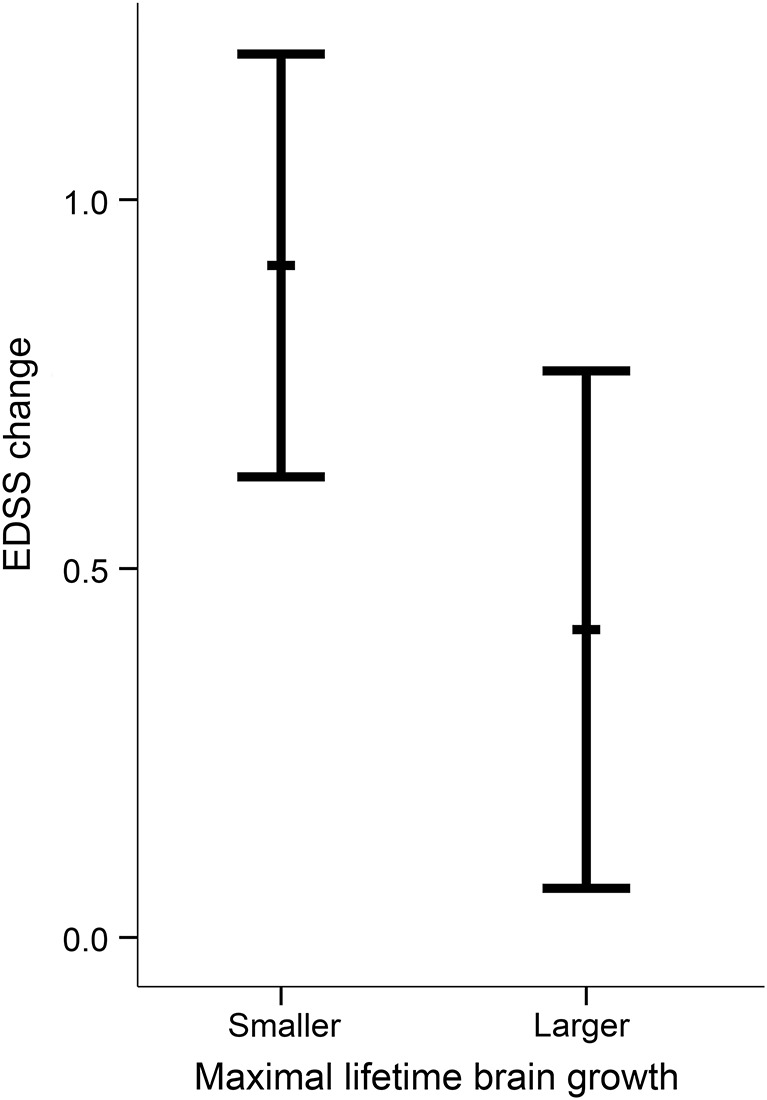
Change in Expanded Disability Status Scale (EDSS) scores from baseline to follow-up (adjusted for covariates) was larger for patients with multiple sclerosis with smaller maximal lifetime brain growth (MLBG) (mean ± SD: 0.91 ± 0.71) than larger MLBG (0.42 ± 0.87). Error bars represent 95% confidence intervals.
DISCUSSION
In this initial extension of the brain reserve hypothesis to physical disability, patients with MS with larger MLBG were at lower risk for disability progression over 5 years. Consideration of MLBG (estimated with ICV) may help identify patients with MS at greatest risk for future disability, which would advance early intervention/preventative medicine.
We measured physical disability with the EDSS: the most widely used measure of physical disability in MS research, including clinical trials. Our sample mimics clinical trial control groups, as patients were not treated with DMDs. Recruitment of patients with smaller MLBG may increase risk for disability progression within clinical trial samples, which may increase statistical power (appendix e-1).
Our sample was relatively small; however, 5-year longitudinal follow-up in treatment-naive patients with MS is a unique strength. Indeed, our sample may be regarded as a natural history study. That said, our findings require replication in larger longitudinal samples, which may utilize more comprehensive assessments of disability progression. Although the EDSS is the most widely used measure of disability in patients with MS, there are some limitations of this tool. For instance, the EDSS is a nonlinear ordinal scale, and the short range of scores makes it insensitive to more subtle changes in disability (for commentary, see reference 10). Future research with larger samples is needed to precisely quantify the relative contribution of MLBG to disability progression independently of disease burden, but the current work represents a necessary proof of concept. Consistent with research on brain reserve against cognitive disability, we estimated brain reserve with MLBG. Future research may work to identify reserve within specific functional networks, which will likely increase the size (and clinical usefulness) of relationships. MLBG (ICV) may be correlated with other variables, such as height. Although ICV has a unique genetic basis and is only modestly correlated with height,6 future research should investigate whether the link between MLBG and disability is mediated through other such variables. Finally, research should also investigate reserve against physical disability in aging and other neurologic diseases.
Supplementary Material
GLOSSARY
- ABV
absolute brain volume
- CIS
clinically isolated syndrome
- DE
dual-echo
- DMD
disease-modifying drug
- EDSS
Expanded Disability Status Scale
- ICV
intracranial volume
- MLBG
maximal lifetime brain growth
- MNI
Montreal Neurological Institute
- MPRAGE
magnetization-prepared rapid gradient echo
- MS
multiple sclerosis
- NBV
normalized brain volume
- PBVC
percentage brain volume change
- RRMS
relapsing-remitting multiple sclerosis
- T2LV
T2 lesion volume
- VSF
volume scaling factor
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
J.F. Sumowski drafted the manuscript for content, contributed to the study concept and design and analysis/interpretation of the data, and performed statistical analyses. M.A. Rocca assisted in drafting the manuscript for content and analysis/interpretation of data. V.M. Leavitt assisted in drafting the manuscript for content and analysis/interpretation of data. A. Meani assisted in the analysis of data. S. Mesaros contributed to the study concept and design and acquisition of the data, and performed clinical evaluations. J. Drulovic contributed to the study concept and design and acquisition of the data, and performed clinical evaluations. P. Preziosa assisted in the analysis of data. C.G. Habeck assisted in drafting the manuscript for content and analysis/interpretation of data. M. Filippi assisted in drafting the manuscript for content and analysis/interpretation of data.
STUDY FUNDING
Ministry of Science, Republic of Serbia (175031); NIH (HD060765).
DISCLOSURE
J. Sumowski received a speaker's honorarium from Biogen Idec. M. Rocca received speaker's honoraria from Biogen Idec, Excemed, and Novartis and receives research support from the Italian Ministry of Health and Fondazione Italiana Sclerosi Multipla. V. Leavitt and A. Meani report no disclosures relevant to the manuscript. S. Mesaros has received speaker grants form Merck Serono SA and travel grants from Bayer Schering Pharma. J. Drulovic has received research grant support from Bayer Schering Pharma and speaker honoraria from Merck Serono SA and Bayer Schering Pharma. P. Preziosa received speaker's honoraria from Novartis. C. Habeck reports no disclosures relevant to the manuscript. M. Filippi is Editor-in-Chief of the Journal of Neurology; serves on scientific advisory boards for Teva Pharmaceutical Industries Ltd.; has received funding for travel from Bayer Schering Pharma, Biogen Idec, Merck Serono, and Teva Pharmaceutical Industries Ltd.; serves as a consultant to Bayer Schering Pharma, Biogen Idec, Merck Serono, Novartis, Pepgen Corporation, and Teva Pharmaceutical Industries Ltd.; serves on speakers' bureaus for Bayer Schering Pharma, Biogen Idec, Merck Serono, and Teva Pharmaceutical Industries Ltd.; and receives research support from Bayer Schering Pharma, Biogen Idec, Novartis, Merck Serono, Teva Pharmaceutical Industries Ltd., Fondazione Italiana Sclerosi Multipla, the Italian Ministry of Health, CurePSP, and the Jacques and Gloria Gossweiler Foundation (Switzerland). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Satz P. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology 1993;7:273–295. [Google Scholar]
- 2.Schofield PW, Logroscino G, Andrews HF, Albert S, Stern Y. An association between head circumference and Alzheimer's disease in a population-based study of aging and dementia. Neurology 1997;49:30–37. [DOI] [PubMed] [Google Scholar]
- 3.Sumowski JF, Rocca MA, Leavitt VM, et al. Brain reserve and cognitive reserve protect against cognitive decline over 4.5 years in MS. Neurology 2014;82:1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haug H. Brain sizes, surfaces, and neuronal sizes of the cortex cerebri: a stereological investigation of man and his variability and a comparison with some mammals (primates, whales, marsupials, insectivores, and one elephant). Am J Anat 1987;180:126–142. [DOI] [PubMed] [Google Scholar]
- 5.Courchesne E, Chisum HJ, Townsend J, et al. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 2000;216:672–682. [DOI] [PubMed] [Google Scholar]
- 6.Ikram MA, Fornage M, Smith AV, et al. Common variants at 6q22 and 17q21 are associated with intracranial volume. Nat Genet 2012;44:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 8.Comi G, Jeffery D, Kappos L, et al. Placebo-controlled trial of oral laquinimod for multiple sclerosis. N Engl J Med 2012;366:1000–1009. [DOI] [PubMed] [Google Scholar]
- 9.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage 2004;23:724–738. [DOI] [PubMed] [Google Scholar]
- 10.Cohen JA, Reingold SC, Polman CH, Wolinsky JS; International Advisory Committee on Clinical Trials in Multiple S. Disability outcome measures in multiple sclerosis clinical trials: current status and future prospects. Lancet Neurol 2012;11:467–476. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


