Abstract
Chronic, massive, irreparable rotator cuff tears remain one of the most challenging pathologies in shoulder surgery to treat. Because of this, many treatment options exist for the management of chronic retracted rotator cuff tears. Superior capsule reconstruction is one option that provides the potential to restore and rebalance the force couples necessary for dynamic shoulder function. We describe an all-arthroscopic technique using an acellular dermal allograft for superior capsule reconstruction indicated for patients with a deficient superior rotator cuff. This technique provides an option for patients with an irreparable rotator cuff tear without compromising future treatment options. Although this is a relatively new and unproven method for treating chronic irreparable rotator cuff tears, our short-term results are promising. Nevertheless, larger studies with long-term follow-up are required to fully evaluate the success of this technique.
Chronic massive rotator cuff tears remain one of the most challenging pathologies in shoulder surgery to treat. Because of the chronic nature of these rotator cuff tears, limited excursion and fatty atrophy develop, which decrease the healing and functional potential after primary repair.1, 2, 3, 4, 5, 6 Many treatment options have been proposed for the treatment of patients with chronic, massive, irreparable rotator cuff tears.1, 7, 8, 9, 10, 11, 12, 13, 14 Depending on patient demographic characteristics and expectations, options include patch augmentation, partial repair, and reverse total shoulder arthroplasty. These options work well in specific populations but are not a panacea for all patients with this debilitating condition. Patches have been used successfully to augment primary repairs but fail to provide the structural integrity needed to span a gap in the setting of an irreparable tear.15 Partial repair is another option, but the risk of a recurrent tear can be as high as 52%.16 Reverse total shoulder arthroplasty is a good option for the elderly population but has failed to provide similar clinical results in a younger population.17
Ideally, the treatment for patients with a chronic massive cuff tear should include restoration of balanced force couples with a biologically viable scaffold with adequate structural integrity that can span a gap in the setting of tendon loss. One recent approach to chronic tendon loss has been proposed by Mihata et al.18 They noted that patients with a chronic irreparable rotator cuff tear have a defect in the superior capsule and cuff, and they suggested that a reconstruction of the superior capsule with use of a fascia lata autograft could address the gap and restore glenohumeral kinematics, resulting in improved functionality of the joint. Their original technique is uniquely different from previous descriptions in that, by securing the graft to the superior glenoid neck, the superior capsule is reconstructed, as opposed to bridging a gap between the remnant rotator cuff and its lateral insertion on the greater tuberosity. Although their early results are promising, the fascia lata graft is thin, requiring doubling of the construct and a relatively large donor incision for harvesting. As an alternative, we describe an all-arthroscopic superior capsule reconstruction with an acellular dermal allograft (2-mm ArthroFlex Patch; Arthrex, Naples, FL). Snyder et al.19 have previously shown success with this graft with minimal immunologic risk. This graft has also been shown to integrate well and provide a scaffold for neovascularization while maintaining structural integrity.19 We have adapted this graft and incorporated it into the superior capsular reconstruction described by Mihata et al. The advantages of this graft include the following: no donor-site morbidity, ease of preparation, thickness and strength of construct, and biologic incorporation. The purpose of this report is to describe, in detail, an arthroscopic technique for superior capsule reconstruction with an acellular dermal allograft (Video 1). A list of surgical pearls is provided in Table 1.
Table 1.
Surgical Pearls
| 1. The surgeon should be sure to perform a thorough bursectomy to view the entire cuff defect to the glenoid surface. |
| 2. A spinal needle should be used to localize the anterior and posterior glenoid anchor trajectory. The posterior glenoid anchor trajectory can be variable and may be best established from the Neviaser portal, the portal of Wilmington, or a mid-lateral approach, which is established off the lateral edge of the acromion. |
| 3. The surgeon should pass the glenoid anchors and medial row of tuberosity anchors before graft passage. Suture passage should be performed outside of the shoulder. |
| 4. For graft passage, a large cannula such as the Arthrex 10-mm Passport can ensure ease of passage without soft-tissue interposition. |
| 5. A double-pulley technique should be used to advance the medial side of the graft, and the surgeon should be sure that the sutures stay on tension during graft passage to ensure that they do not become entangled. Passing all 4 limbs of the medial suture through the graft allows it to sit more flush on the glenoid surface. |
| 6. The medial-row sutures should be secured to a second lateral row of anchors to maximize healing surface area. |
| 7. The surgeon should ensure that the peripheral edges of the graft (anteriorly and posteriorly) are secured to the native remnant tissue to relink the construct. Posteriorly, this should be a complete reapproximation to the intact cuff, whereas anteriorly, the lateral-most aspect of the rotator interval should be closed. |
| 8. The graft should be fixed with the arm in neutral rotation with approximately 20° to 30° of abduction. |
Surgical Technique
Step 1: Preoperative Workup
Patients who present to our clinic with shoulder pain undergo a routine history and physical examination. If impingement signs are seen along with pseudoparalysis, patients undergo a diagnostic and therapeutic subacromial injection. If this fails to provide adequate relief of pain or improvement of function, magnetic resonance imaging of the shoulder is obtained. If a large rotator cuff tear is found, it is assessed as to its chronicity, fatty infiltration, and ability to heal if repaired. The patient is then counseled concerning both nonoperative and operative options. If the patient is physiologically young and the tear is deemed irreparable, superior capsule reconstruction can be considered among other surgical options. As with any surgical decision, the patient is counseled on the risks, benefits, and expectations of surgery. We consider this option in young patients who have massive contracted cuff tears. We obtain consent from all patients for repair versus reconstruction because the decision on reparability is generally made intraoperatively after releases and attempts at cuff mobilization have been exhausted.
Step 2: Surgical Positioning and Diagnostic Arthroscopy
The patient is brought to the operating room, and after the induction of general anesthesia, he or she is examined under anesthesia, which is standard for all arthroscopic shoulder procedures. The patient is positioned in the lateral decubitus position on a beanbag, with the use of a padded arm sleeve (STAR sleeve; Arthrex), in balanced lateral suspension. A posterior portal is established approximately 1 cm medial and 2 cm distal to the posterolateral acromial border. The arthroscope is introduced, and additional portals are established by an outside-in technique under direct visualization with the use of a switching stick. The anterosuperior portal is established first, approximately 1 cm inferior to the clavicle and lateral to the coracoid. A standard diagnostic arthroscopy is performed within the glenohumeral joint, and any pathology is treated appropriately. If the long head of the biceps tendon has not previously undergone tenotomy, either a tenotomy or tenodesis is performed according to surgeon preference. In patients with a debilitating, chronic, retracted rotator cuff tear, our preference is a simple tenotomy, which can be performed with an ablation device (ArthroCare; Smith & Nephew, Andover, MA) or with curved Mayo scissors inserted through the anterior incision. To allow efficient switching of the camera and instruments throughout the case, 8.25-mm cannulas (Arthrex) are liberally used. The subacromial space is then entered through the posterior portal, and a midlateral portal is established. If indicated, subacromial decompression and acromioplasty are performed. Capsular and subacromial releases, as well as an anterior rotator interval slide, are performed to attempt primary repair. If the cuff can be mobilized with these techniques, a primary or revision repair is accomplished. If the cuff tear is too retracted and cannot be mobilized without undue tension, we proceed with superior capsular reconstruction.
Step 3: Superior Glenoid Neck Preparation
To secure the medial edge of the graft to the glenoid, the superior glenoid neck is addressed and prepared (Fig 1). The medial edge of the graft should be secured to the glenoid neck inferior to any residual rotator cuff tissue. The superior glenoid neck immediately medial to the superior labrum is biologically prepared to create a vascular bed for securing the medial aspect of the graft. This is achieved by removing all soft tissue on the superior glenoid neck with an ablation device (ArthroCare) or arthroscopic shaver and creating bleeding bone with a rasp (Glenoid Rasp; Arthrex) or burr. The prepared area corresponds to the medial attachment of the graft and should extend anteriorly to the edge of the rotator interval (or edge of the supraspinatus remnant) and posteriorly to the edge of the posterior cuff remnant. Specifically, the location of the 2 medial anchors (2.4-mm Bio-SutureTak; Arthrex) is usually around the 2-o'clock position anteriorly at the base of the coracoid and between the 9- and 10-o'clock position posteriorly.
Fig 1.
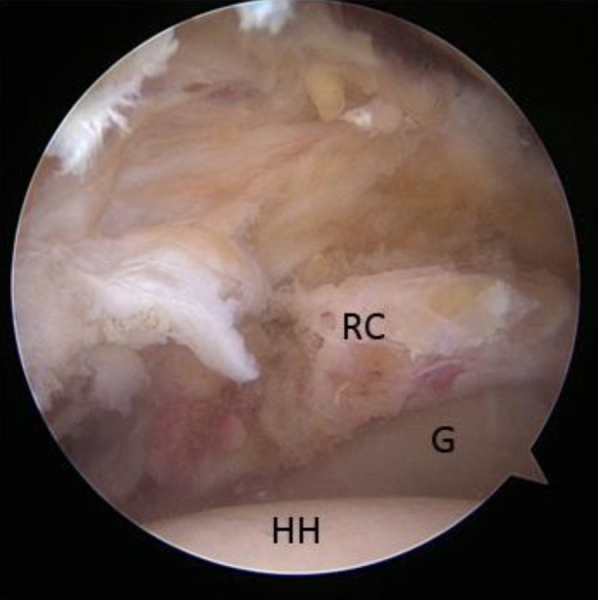
View from lateral portal of glenoid (G) and rotator cuff remnant with prepared superior glenoid neck. (HH, humeral head; RC, rotator cuff remnant tissue.)
Step 4: Placement of Medial Anchors
A small incision is made to create either a Neviaser portal or posterolateral portal—depending on the previously determined location of the posterior cuff remnant. A spinal needle can be used to assess which portal provides the best trajectory. This is created to place the posterosuperior anchor (SutureTak BioComposite; Arthrex) on the glenoid neck (Fig 2). Once this anchor is drilled and placed, the suture tails are grasped and brought out of the midlateral portal. The anterior portal is then used to place a second medial anchor even with the base of the coracoid process, which usually corresponds to just anterior to the biceps tendon root. Once placed, the suture ends are also brought out of the midlateral portal.
Fig 2.
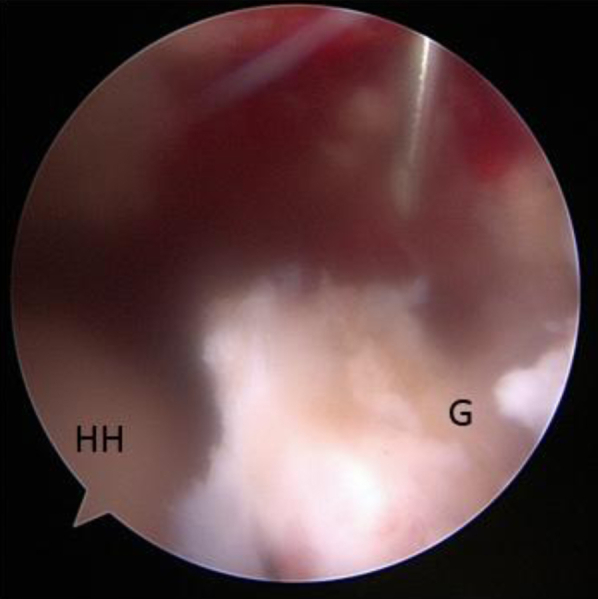
View from posterior portal using a spinal needle to locate the correct portal placement through the Neviaser portal for the posterosuperior anchor into the superior glenoid neck (G). (HH, humeral head.)
Step 5: Graft Sizing and Preparation
The dimensions of the rotator cuff defect are measured using a calibrated arthroscopic probe. The anterior-posterior distance is measured at the neck of the glenoid and at the lateral rotator cuff footprint. This can be performed through either the anterosuperior or posterior portal. In addition, the surgeon measures the medial-lateral distance through the midlateral portal, keeping in mind to add 12 mm to this distance to accommodate double-row fixation over the greater tuberosity. These measurements are then used to size and prepare the acellular dermal graft (ArthroFlex). The graft is prepared on the back table, sized, and trimmed accordingly. A marking pen is used to mark the position of suture insertion of the graft for later arthroscopic identification (Fig 3). It is important to place all sutures at least 5 mm from the edge of the graft to avoid losing fixation by the sutures pulling through the graft. Because of the graft thickness and durability, it is difficult to consistently and efficiently penetrate the graft with a free needle. Therefore we use an arthroscopic suture passer (Scorpion; Arthrex) to pass suture both arthroscopically and during graft preparation. In addition, using the Scorpion suture passer during graft preparation helps confirm that the graft can be easily penetrated before attempting it arthroscopically.
Fig 3.
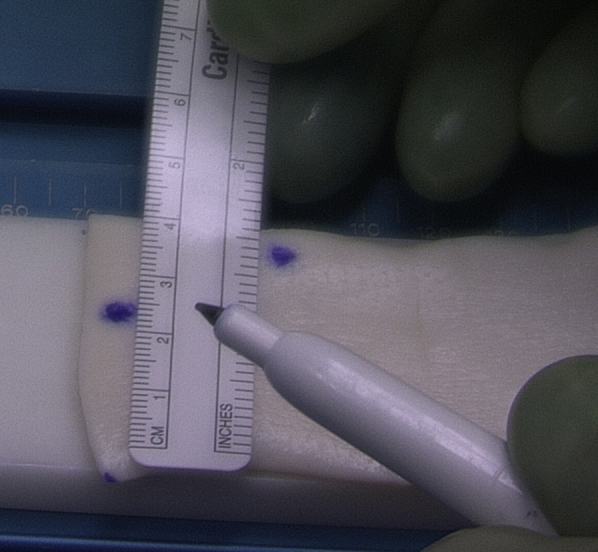
The acellular dermal allograft is sized according to subacromial measurements. The final graft shape is usually trapezoidal depending on the superior capsule defect.
Step 6: Graft Insertion and Medial Fixation
The graft is brought just outside the shoulder near the midlateral portal, and the sutures are passed through their respective holes in the graft (Fig 4). A double-pulley technique, as described by Koo et al.,20 is used to insert the graft into the shoulder. This is performed by tying 1 strand from each anchor to each other over a switching stick and then advancing down into the shoulder by pulling on the 2 untied suture ends. Because of the size of the graft, it may be necessary to pass the graft into the shoulder without a cannula. Once the graft is in its desired position medially, the 2 untied suture ends are tied arthroscopically over the graft, securing its medial attachment (Fig 5).
Fig 4.
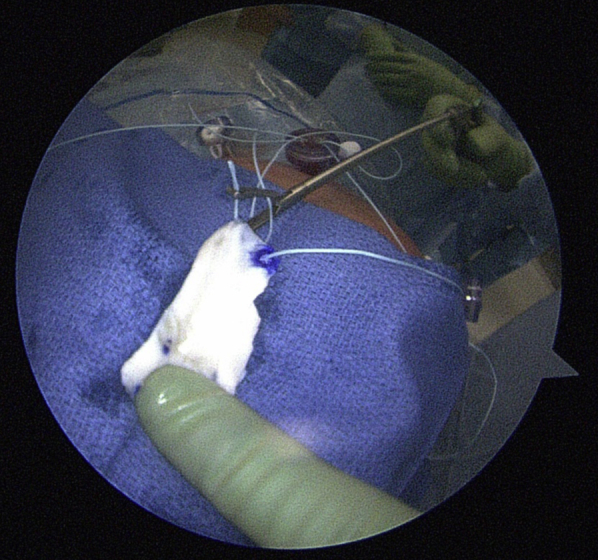
After graft sizing and preparation, by use of a double-pulley technique, the acellular dermal allograft is placed near the shoulder and a single suture end from each medial anchor is passed through the medial edge of the graft.
Fig 5.
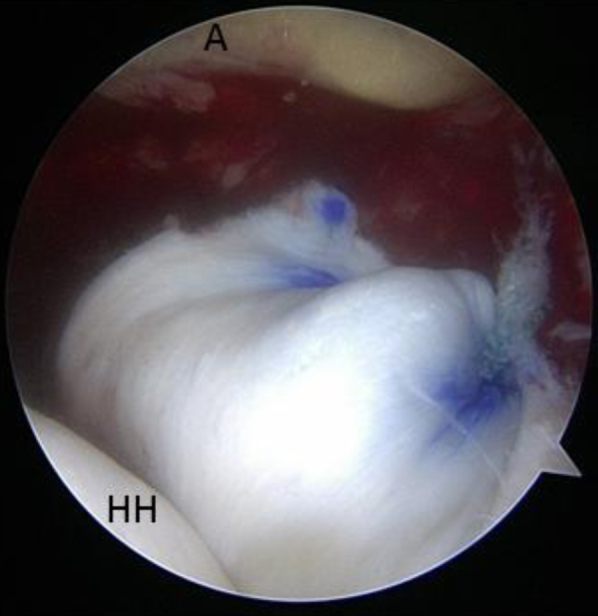
View from posterolateral portal. By use of a double-pulley technique, the medial edge of the graft is secured to the superior glenoid neck. In this view the graft is tucked into the glenohumeral articulation for visualization purposes. (A, acromion; HH, humeral head.)
Step 7: Lateral Graft Fixation to Humerus
Securing the lateral graft is accomplished using a double-row transosseous-equivalent technique with the arm at 45° of abduction. Two single-loaded anchors (Corkscrew FT; Arthrex) are placed just lateral to the articular margin of the humeral head. A spinal needle can be used to determine the best trajectory for placement of the medial-row anchors. Typically, this trajectory exits the shoulder just lateral to the acromion. Internal and external rotation of the arm can assist in anchor placement. One anchor should be placed anteriorly near the superior aspect of the biceps groove and another anchor secured posteriorly near the posterior-medial edge of the greater tuberosity. The sutures from each anchor are passed through their respective area of the graft with an arthroscopic suture-passing device (Scorpion) and tied arthroscopically. Two lateral-row anchors (SwiveLock; Arthrex) are then placed both anteriorly and posteriorly along the lateral rotator cuff footprint on the greater tuberosity. The midlateral portal can be used to place these 2 anchors. As with the medial anchors, internal and external rotation of the humerus can assist in creating the optimal trajectory for the lateral-row anchors. The suture ends are then secured to the lateral row in a transosseous-equivalent double-row fashion using knotless lateral-row anchors (SwiveLock) (Fig 6).
Fig 6.
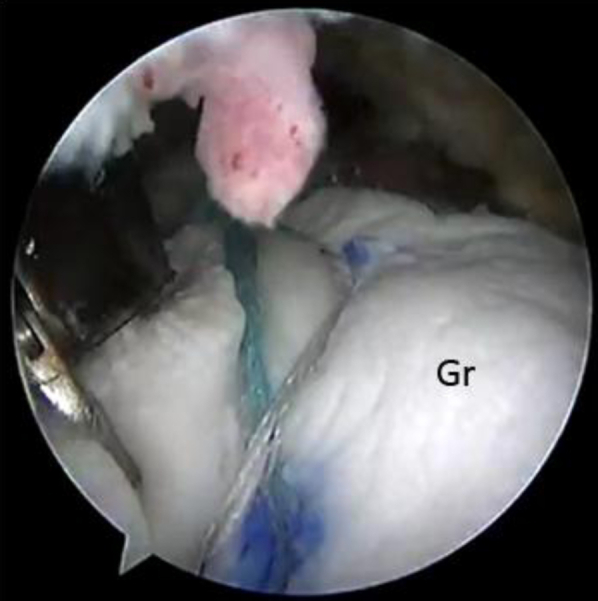
View from posterior portal after double-row fixation of the graft laterally over the greater tuberosity. (Gr, acellular dermal allograft.)
Step 8: Anterior and Posterior Edge Side-to-Side Fixation
Once the medial and lateral rows are secured, side-to-side simple sutures are placed using the arthroscopic suture passer, securing the posterior edge of the graft to the free edge of the teres minor or, if partially intact, the infraspinatus tendon (Fig 7). While the surgeon is viewing from the anterior portal, the midlateral portal can be used to pass a free suture through the anterior edge of the intact posterior cuff near the musculotendinous junction, which is usually located just lateral to the glenoid. Once passed, the sutures can be brought out of the posterior portal and an arthroscopic suture passer can be used to pass the inferior strand of the suture through the posterior edge of the graft. This suture is then tied arthroscopically. The technique can then be repeated to pass additional simple sutures, securing the posterior graft to the posterior rotator cuff tendon as it extends laterally. To maintain access to the inferior graft for passage of sutures, it is important to start securing the posterior graft first medially and then work laterally.
Fig 7.
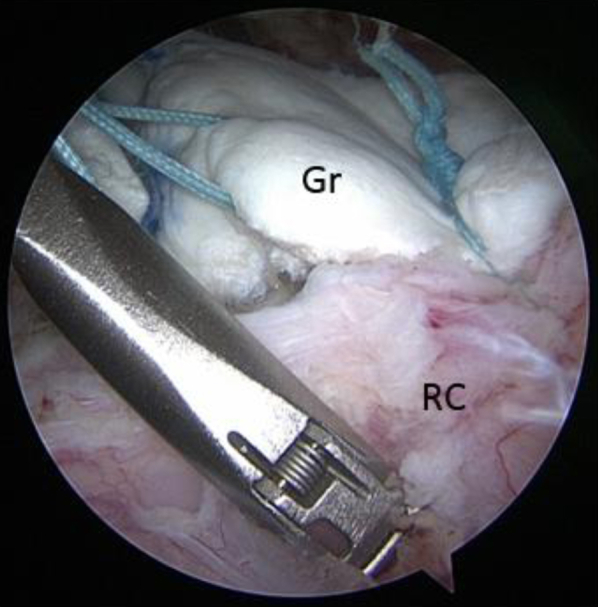
View from posterior portal showing side-to-side simple suturing of the posterior edge of the graft to the intact posterior cuff muscle and tendon with a FastPass Scorpion device (Arthrex). (Gr, acellular dermal allograft; RC, posterior intact rotator cuff.)
Anteriorly, side-to-side sutures are used to secure the anterolateral edge of the graft to the superolateral subscapularis tendon. This is accomplished by passing a free suture through the superolateral subscapularis tendon using the arthroscopic suture passer with access through the midlateral portal. The suture ends are brought out of the anterior portal, and the inferior suture strand is passed through the anterolateral graft using the arthroscopic suture passer. The suture is then tied arthroscopically. Alternatively, as in the case of a small partial upper subscapularis tear, an anchor can be used to secure the anterior edge of the lateral part of the graft to the anterolateral humeral head near the biceps groove. This can be accomplished by passing a free suture through the graft and securing it with a knotless anchor near the superior edge of the subscapularis tendon. The rotator interval can then be left open (which is our preference) or, if desired, can be closed with side-to-side sutures securing the anterior edge of the graft to the superior subscapularis tendon. Alternatively, if desired, the anterior edge of the graft can be secured to the rotator interval tissue to help provide a seal of the glenohumeral joint from the subacromial space (Fig 8). These simple sutures may also be passed with a suture-shuttling device and tied arthroscopically.
Fig 8.
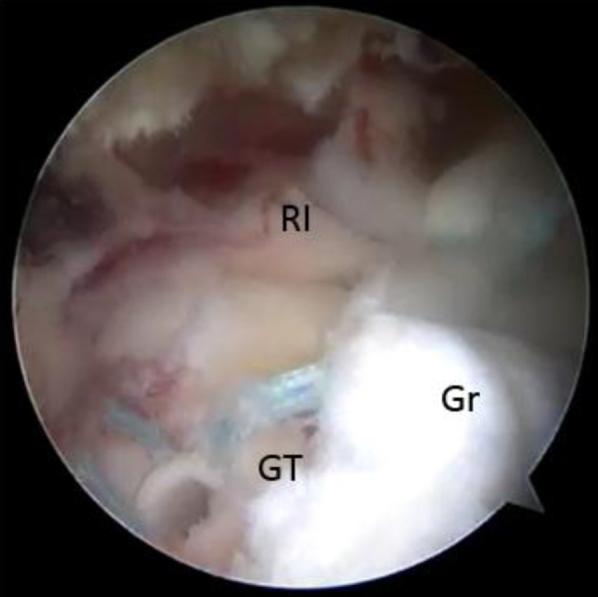
View from lateral portal of side-to-side fixation of the graft anterior edge secured laterally to an anchor near the biceps groove and medially to available rotator interval tissue (RI). Care should be taken not to close the entire rotator interval because doing so could result in increased postoperative stiffness. (Gr, acellular dermal allograft; GT, greater tuberosity.)
Step 9: Final Inspection, Closure, and Postoperative Rehabilitation
Once the graft is secured (Fig 9), the humerus can be gently moved through its range of motion to test the tension on the graft and its response to motion while being arthroscopically visualized. All portals are then closed in a standard manner. Postoperatively, the patient is treated by a massive repair protocol. This includes placement in a shoulder immobilizer with an abduction pillow. The patient is allowed to perform elbow, wrist, and hand exercises, along with gentle passive glenohumeral motion, for 6 weeks. Progressive motion is begun at 6 weeks postoperatively, and strengthening is begun at 12 weeks. The patient gradually returns to activity when motion, strength, and confidence return over a 6-month postoperative period.
Fig 9.
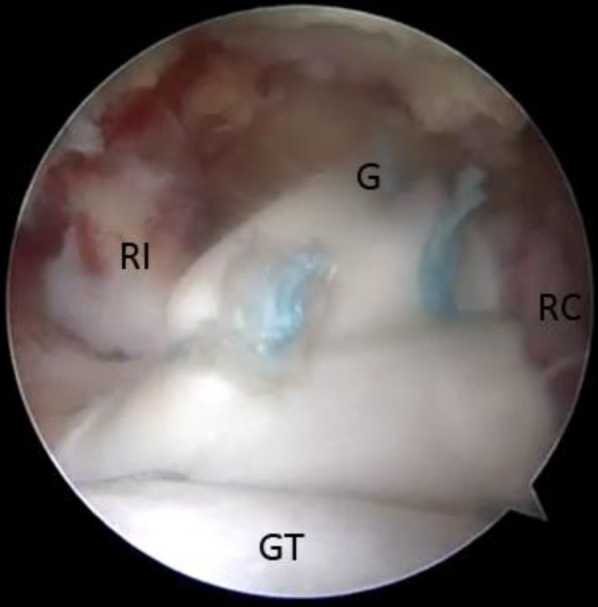
View from lateral portal of final graft construct. The graft is secured to the superior glenoid neck (G) medially (top), to the rotator interval (RI) anteriorly (left), and to the intact posterior rotator cuff (RC) (right). (GT, greater tuberosity with attached graft.)
Discussion
Treatment of chronic, massive, irreparable rotator cuff tears in a young active population presents a significant challenge to shoulder surgeons. In addition to desiring pain relief, patients with this pathology usually have a desire to regain functionality. In an older population, reverse total shoulder arthroplasty has been shown to help restore function and improve pain with short- to medium-term follow-up.21 Even in this population, however, the rate of postoperative complications can be as high as 20%.22 In patients younger than 65 years, the complication rate for reverse total shoulder arthroplasty in the setting of an irreparable rotator cuff tear has been reported to be as high as 38%.17
An ideal solution for the young active patient with a chronic massive rotator cuff tear has not been established. Both allograft and synthetic substitutes continue to be evaluated as scaffolds in the setting of chronic, massive, irreparable rotator cuff tears.23 Using a patch graft attached to the lateral edge of the remnant rotator cuff tendon has yielded disappointing results thus far. Moore et al.15 performed an allograft reconstruction in 32 patients with irreparable rotator cuff tears and obtained magnetic resonance imaging scans in 15 of them at an average follow-up of 31 months. All 15 shoulders showed a recurrent tear of the rotator cuff–graft construct. They concluded, on the basis of University of California, Los Angeles scores, that allograft repair of the rotator cuff was equivalent to simple debridement and subacromial decompression and recommended against this type of repair given the added risk of complications associated with allograft tissue.
An advantage of superior capsule reconstruction is that it provides an option to restore and rebalance the force couples necessary for dynamic shoulder function and does not sacrifice any future treatment options. By securing the graft to the glenoid neck, integration of the graft into the native rotator cuff is not required for function. The graft functions to add superior stability to the glenohumeral joint, thus restoring more anatomic force couples and improving function. The graft also links the posterior and anterior rotator cuff and may also function as a soft-tissue spacer and checkrein against superior migration of the humeral head. With the graft secured to the glenoid and humerus, one could reason that there may be increased medial-lateral tension on the graft from simple adduction of the extremity—resulting in potential failure of the construct. Mihata et al.18 studied this concept in a cadaveric model and noted that if the graft was secured while the extremity was abducted 45°, the incidence of failure of fascia lata graft by medial-lateral tension during adduction was zero. Overall, the risks of this technique are similar to those that accompany a standard rotator cuff repair procedure, with the addition of the minimal added risk of a possible host reaction to the graft.
Arthroscopic superior capsule reconstruction is, however, a relatively new and unproven method of treating chronic irreparable rotator cuff tears, and long-term outcomes have not been reported. Mihata et al.18 performed arthroscopic superior capsule reconstruction with autograft fascia lata and showed significant improvements in pain, acromiohumeral distance, and motion at an average follow-up of 34 months. They also noted significant improvements in clinical outcomes. The American Shoulder and Elbow Surgeons, Japanese Orthopaedic Association, and University of California, Los Angeles scores all improved significantly, from 23.5, 48.3, and 9.9 preoperatively to postoperative scores of 92.9, 92.6, and 32.4, respectively. Acellular dermal allograft tissue is a convenient substantial substitute for normal superior capsular tissue and eliminates donor-site morbidity, as well as time required for graft harvest. The clinical outcomes at our institution are relatively short-term but have shown early promising results. Larger studies with longer-term follow-up are required to evaluate the efficacy of this technique fully.
Footnotes
The authors report the following potential conflict of interest or source of funding: J.M.T. receives support from Arthroscopy Association of North America, Journal of Shoulder and Elbow Surgery, Orthopedics Today Hawkins Foundation, Arthrex, and Depuy Mitek.
Supplementary Data
Technique for superior capsule reconstruction with acellular dermal allograft. The patient is positioned in the lateral decubitus position with the extremity in balanced traction. An acellular dermal allograft patch is arthroscopically secured medially to the superior glenoid neck, posteriorly to the intact rotator cuff, laterally to the greater tuberosity, and anteriorly to the rotator interval tissue.
References
- 1.Bedi A., Dines J., Warren R., Dines D. Massive tears of the rotator cuff. J Bone Joint Surg Am. 2010;92:1894–1908. doi: 10.2106/JBJS.I.01531. [DOI] [PubMed] [Google Scholar]
- 2.Melis B., Nemoz C., Walch G. Muscle fatty infiltration in rotator cuff tears: Descriptive analysis of 1688 cases. Orthop Traumatol Surg Res. 2009;95:319–324. doi: 10.1016/j.otsr.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Melis B., Wall B., Walch G. Natural history of infraspinatus fatty infiltration in rotator cuff tears. J Shoulder Elbow Surg. 2010;19:757–763. doi: 10.1016/j.jse.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Oh J., Kim S., Choi J., Kim Y., Oh C. Reliability of the grading system for fatty degeneration of rotator cuff muscles. Clin Orthop Relat Res. 2009;468:1558–1564. doi: 10.1007/s11999-009-0818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oh J., Kim S., Kang J., Oh C., Gong H. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med. 2010;38:672–678. doi: 10.1177/0363546509352460. [DOI] [PubMed] [Google Scholar]
- 6.Goutallier D., Postel J., Bernageau J., Lavau L., Voisin M. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994:78–83. [PubMed] [Google Scholar]
- 7.Gerber C., Maquieira G., Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg Am. 2006;88:113–120. doi: 10.2106/JBJS.E.00282. [DOI] [PubMed] [Google Scholar]
- 8.Neviaser J., Neviaser R., Neviaser T. The repair of chronic massive ruptures of the rotator cuff of the shoulder by use of a freeze-dried rotator cuff. J Bone Joint Surg Am. 1978;60:681–684. [PubMed] [Google Scholar]
- 9.Savarese E., Romeo R. New solution for massive, irreparable rotator cuff tears: The subacromial “biodegradable spacer.”. Arthrosc Tech. 2012;1:e69–e74. doi: 10.1016/j.eats.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warner J., Parsons I. Latissimus dorsi tendon transfer: A comparative analysis of primary and salvage reconstruction of massive, irreparable rotator cuff tears. J Shoulder Elbow Surg. 2001;10:514–521. doi: 10.1067/mse.2001.118629. [DOI] [PubMed] [Google Scholar]
- 11.Bigliani L., Cordasco F., McIlveen S., Musso E. Operative repair of massive rotator cuff tears: Long-term results. J Shoulder Elbow Surg. 1992;1:120–130. doi: 10.1016/1058-2746(92)90089-L. [DOI] [PubMed] [Google Scholar]
- 12.Gerber C., Fuchs B., Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505–515. doi: 10.2106/00004623-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Rockwood C.A., Jr., Williams G.R., Jr., Burkhead W.Z., Jr. Debridement of degenerative, irreparable lesions of the rotator cuff. J Bone Joint Surg Am. 1995;77:857–866. doi: 10.2106/00004623-199506000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Rokito A., Cuomo F., Gallagher M., Zuckerman J. Long-term functional outcome of repair of large and massive chronic tears of the rotator cuff. J Bone Joint Surg Am. 1999;81:991–997. doi: 10.2106/00004623-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Moore D., Cain E., Schwartz M., Clancy W.J. Allograft reconstruction for massive, irreparable rotator cuff tears. Am J Sports Med. 2006;34:392–396. doi: 10.1177/0363546505281237. [DOI] [PubMed] [Google Scholar]
- 16.Berth A., Neumann W., Awiszus F., Pap G. Massive rotator cuff tears: Functional outcome after debridement or arthroscopic partial repair. J Orthop Traumatol. 2010;11:13–20. doi: 10.1007/s10195-010-0084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ek E., Neukom L., Catanzaro S., Gerber C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: Results after 5 to fifteen years. J Shoulder Elbow Surg. 2013;22:1199–1208. doi: 10.1016/j.jse.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Mihata T., Lee T., Watanabe C. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy. 2013;29:459–470. doi: 10.1016/j.arthro.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Snyder S., Arnoczky S., Bond J., Dopirak R. Histologic evaluation of a biopsy specimen obtained 3 months after rotator cuff augmentation with GraftJacket Matrix. Arthroscopy. 2009;25:329–333. doi: 10.1016/j.arthro.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Koo S., Burkhart S., Ochoa E. Arthroscopic double-pulley remplissage technique for engaging Hill-Sachs lesions in anterior shoulder instability repairs. Arthroscopy. 2009;25:1343–1348. doi: 10.1016/j.arthro.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Boileau P., Gonzalez J., Chuinard C., Bicknell R., Walch G. Reverse total shoulder arthroplasty after failed rotator cuff surgery. J Shoulder Elbow Surg. 2009;18:600–606. doi: 10.1016/j.jse.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Mulieri P., Dunning P., Klein S., Pupello D., Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92:2544–2556. doi: 10.2106/JBJS.I.00912. [DOI] [PubMed] [Google Scholar]
- 23.Ricchetti E., Aurora A., Iannotti J., Derwin K. Scaffold devices for rotator cuff repair. J Shoulder Elbow Surg. 2012;21:251–265. doi: 10.1016/j.jse.2011.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technique for superior capsule reconstruction with acellular dermal allograft. The patient is positioned in the lateral decubitus position with the extremity in balanced traction. An acellular dermal allograft patch is arthroscopically secured medially to the superior glenoid neck, posteriorly to the intact rotator cuff, laterally to the greater tuberosity, and anteriorly to the rotator interval tissue.


