Abstract
An efficient palladium-catalyzed α-alkenylation of pyridylmethyl ethers with vinyl bromides is presented. A Pd/NIXANTPHOS-based catalyst system enables a mild and chemoselective coupling between a variety of pyridylmethyl ethers and vinyl bromides in good to excellent yields. Under the mild conditions, β,γ-unsaturated products are obtained without isomerization or Heck byproducts observed.
Graphical Abstract

Transition metal catalyzed vinylations of organic compounds with alkenyl halides is an attractive transformation as well as a long-standing challenge to synthetic chemists.1 Direct alkenylation of carbanion nucleophiles with vinyl halides is one of the most efficient and economic approaches for installing β,γ-unsaturated units. Despite the large number of transition metal catalyzed methods to mediate C(sp3)–H functionalization reactions with aryl halides electrophiles, use of vinyl halides has been limited to enolate2 and nitronate3 nucleophiles (Scheme 1a–b). Methods employing less acidic nucleophiles have met with very limited success.
Scheme 1.
Transition-Metal-Catalyzed Alkenylation Chemistry
We have reported a series of catalytic functionalizations of weakly acidic sp3-hybridized C–H bonds via a deprotonative–cross-coupling process (DCCP).4 These reactions involve reversible in situ deprotonation of substrates (pronucleophiles) to generate nucleophiles for transmetalation with the catalyst. Recently, we disclosed a versatile and chemoselective arylation of 2-pyridylmethyl ethers and tandem arylation/[1,2]-Wittig rearrangement.5 Despite these efforts, the direct intermolecular C–H alkenylation of pyridylmethyl ethers with vinyl bromides remains unknown. Herein, we report a novel monoalkenylation of pyridylmethyl ethers with vinyl bromides. The pKa values of the benzylic C–H’s of 2- and 4-pyridylmethyl ethers are unknown, but likely similar to those of 2- and 4-methylpyridine (pKa’s 32–34 in THF).6 These high pKa values make the identification of base/solvent and catalyst combinations that will promote deprotonation of the pyridylmethyl ether, but not the more acidic product, challenging. Furthermore, because the product contains an olefin, byproduct formation derived from the Heck reaction must be suppressed.
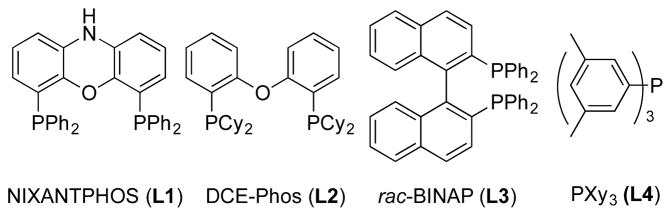
We initiated studies of the coupling between 4-pyridylmethyl ethyl ether 1a and 2-bromoprop-1-ene 2a with ligand screening on a microscale (0.01 mmol). A total of 41 electronically diverse mono- and bidentate phosphines were examined using Pd(OAc)2 as the metal source, LiN(SiMe3)2 as the base, CPME as the solvent, 12 h of reaction time, and 65 °C (see the Supporting Information for details). The four most promising ligands from this screen were NIXANTPHOS (L1), DCE-Phos (L2), rac-BINAP (L3), and PXy3 (L4) (Figure 1). Laboratory scale-up (0.1 mmol) led to the alkenylation product in 78% (NIXANTPHOS), 60% (DCE-Phos), 59% (rac-BINAP), and 49% (PXy3) assay yields (AY) after 1 h (AY determined by 1H NMR analysis, Table 1, entries 1–4). Interestingly, reducing the catalyst loading from 10 to 2.5 mol % led to a similar assay yield (75%, entry 5). The moderate yield inspired us to conducted a second microscale screen (0.01 mmol) focusing on palladium sources {Pd(OAc)2, Pd(dba)2, [PdCl(allyl)]2, PdCl2(COD), Pd(NCPh)2Cl2, [Pd(2-methylallyl)Cl]2} and four solvents [THF, CPME, DME, and toluene] with LiN(SiMe3)2 at 65 °C for 1 h. The two top Pd/solvent combinations from this screen were [PdCl(allyl)]2/CPME and Pd(dba)2/CPME. Scaling to 0.1 mmol resulted in 74% ([PdCl(allyl)]2) and 88% (Pd(dba)2) assay yields (entries 6 and 7, respectively).
Figure 1.
Structures of leading ligands in preliminary catalyst identification with Pd(OAc)2 (Table 1).
Table 1.

| |||||
|---|---|---|---|---|---|
| Pd source | L | Pd/L (mol %) | base | assay yield (%) | |
| 1 | Pd(OAc)2 | L1 | 10/15 | LiN(SiMe3)2 | 78 |
| 2 | Pd(OAc)2 | L2 | 10/15 | LiN(SiMe3)2 | 60 |
| 3 | Pd(OAc)2 | L3 | 10/15 | LiN(SiMe3)2 | 59 |
| 4 | Pd(OAc)2 | L4 | 10/15 | LiN(SiMe3)2 | 49 |
| 5 | Pd(OAc)2 | L1 | 2.5/3.75 | LiN(SiMe3)2 | 75 |
| 6 | [PdCl(allyl)]2 | L1 | 2.5/3.75 | LiN(SiMe3)2 | 74 |
| 7 | Pd(dba)2 | L1 | 2.5/3.75 | LiN(SiMe3)2 | 88 |
| 8 | Pd(dba)2 | L1 | 2.5/5.0 | LiN(SiMe3)2 | 88 |
| 9 | Pd(dba)2 | L1 | 2.5/2.5 | LiN(SiMe3)2 | 96 |
| 10 | Pd(dba)2 | L1 | 2.5/2.5 | NaN(SiMe3)2 | 60 |
| 11 | Pd(dba)2 | L1 | 1.0/1.0 | LiN(SiMe3)2 | 95(94)c |
| 12 | Pd(dba)2 | L1 | 0.5/0.5 | LiN(SiMe3)2 | 77 |
Reactions conducted on a 0.1 mmol scale using 1 equiv of 1a, and 1.5 equiv of 2a.
Assay yields determined by 1H NMR spectroscopy of the crude reaction mixtures.
Isolated yield after chromatographic purification.
The Pd:NIXANTPHOS ratio was next examined. Changing the Pd/ligand ratio from 1:1.5 to 1:2 resulted in no change in AY (entry 8). When a 1:1 Pd/L ratio was employed, however, the AY increased to 96% (entry 9). This increase in activity is likely due to inhibition by excess ligand.7 Under identical reaction conditions, with the exception of substitution of NaN(SiMe3)2 for LiN(SiMe3)2, only a 60% AY was obtained (entry 10), highlighting the important role of the counterion in DCCPs with NIXANTPHOS. Further reducing the catalyst loading to 1 mol % provided a 95% AY and 94% isolated yield (entry 11). Attempts to further reduce the amount of the catalyst to 0.5 mol % resulted in a drop in the AY (77%, entry 12).
Starting from the optimized conditions in Table 1 (entry 11), we explored the alkenylation of 4-pyridylmethyl ethyl ether 1a with a variety of vinyl bromides (Table 2). In general, at a 1 mol % catalyst loading, alkenylated products were formed with very good yields with no product isomerization observed by 1H NMR of the crude reaction mixtures. As noted, 2-bromoprop-1-ene 2a rendered product 3aa in 94% yield. Trisubstituted 2-bromo-3-methylbut-2-ene 2b provided product in 91% yield under standard conditions. Coupling of 1a with trans- and cis-2-bromobut-2-ene (2c and 2d) delivered 3ac and 3ad in 92% and 80% yields using a 2.5 and 1 mol % catalyst loading, respectively. We observed that the geometries of products were retained in each case. 1-Bromo-2-methylprop-1-ene 2e and (bromomethylene) cyclohexane 2f were successfully coupled with 1a to afford 3ae and 3af in 71% and 70% yields, respectively. Vinyl bromides with aryl substituents (1-bromovinyl)benzene (2g) and hindered bromotriphenyl-ethylene (2h) afforded 3ag and 3ah in 80% and 53% yields at 1 and 10 mol % Pd loadings, respectively. Finally, (1-bromovinyl)trimethylsilane successfully coupled with 1a furnishing the product 3ai in 65% yield (1 mol % catalyst).
Table 2.
Scope of Vinyl Bromides in α-Alkenylation of 4-Pyridylmethyl Ethyl Ether 1aa
Reactions conducted on 0.2 mmol scale using 1 equiv of 1a and 1.5 equiv of vinyl bromide 2. Isolated yields after chromatographic purification.
2.5 mol % Pd loading.
10 mol % Pd loading, 3 h reaction time.
0.5 h reaction time.
3 h reaction time.
We next examined the alkenylation of 4- and 2-pyridylmethyl ethers bearing different ether O–R substituents (Table 3). In general, all the pyridylmethyl alkyl or aryl ethers exhibited a good to excellent yield with no isomerization products observed (1H NMR). The 4-pyridylmethyl methyl ether (1b) coupled with 2-bromoprop-1-ene (2a) to give product 3ba in 93% yield with a 1 mol % catalyst loading. 4-Pyridylmethyl tert-butyl ether (1c) underwent alkenylation with 2-bromoprop-1-ene (2a) providing a 72% yield. Alkenylation of 4-pyridylmethyl cyclohexyl ether (1d) with a 5 mol % catalyst loading gave a 50% yield after 10 h. 4-Pyridylmethyl alkyl ethers, including methyl ether (1b) and tert-butyl ether (1c), underwent coupling with 2-bromo-3-methylbut-2-ene (2b) to give products 3bb and 3cb in 97% and 75% yields under the standard conditions. Furthermore, 4-pyridylmethyl aryl ethers, including phenyl (1f), 4-isopropylphenyl (1g), 4-methoxy-phenyl (1h), and 4-fluorophenyl (1i), were coupled with 2-bromo-3-methylbut-2-ene (2b) (10 mol % catalyst), leading to products 3fb, 3gb, 3hb, and 3ib in 65%, 71%, 62%, and 74% yields, respectively.
Table 3.
Scope of Pyridylmethyl Ethers in Alkenylation with Vinyl Bromidesa
Reactions conducted on a 0.2 mmol scale using 1 equiv of pyridylmethyl ethers 1 and 1.5 equiv of vinyl bromides 2. Isolated yield after chromatographic purification.
1.0 mol % Pd loading.
10 h reaction time.
10 mol % Pd loading, 5 h reaction time.
As mentioned earlier, 2-picoline derivatives are less acidic than 4-picolines by a factor of about 100. This difference in acidity is reflected in the reduced suitability of 2-pyridylmethyl ethers in this chemistry. The 2-pyridylmethyl tert-butyl ether (1e) and tert-butyldimethylsilyl ether (1j) underwent coupling with 2-bromoprop-1-ene (2a) and 2-bromo-3-methylbut-2-ene (2b) to provide the corresponding products 3ea, 3ja, and 3jb in 65%, 51%, and 50% yields, respectively. Other 2-pyridylmethyl ethers exhibited poor yields.
Finally, we evaluated the scalability of our method. Conducting the alkenylation of 1b with 2-bromoprop-1-ene 2a on a 10 mmol scale with a 1.0 mol % Pd loading (Scheme 2) provided product 3ba in 83% yield (1.35 g).
Scheme 2.
Gram Scale Preparation of 4-(1-Methoxy-2-methylallyl)pyridine (3ba)
In summary, we have developed an efficient and versatile palladium-catalyzed alkenylation of pyridylmethyl ethers with vinyl bromides. The screening of ligands indicated that a (NIXANTPHOS)Pd-based catalyst exhibited the highest reactivity. Under our reaction conditions, a range of pyridylmethyl ethers underwent coupling with vinyl bromides in good to excellent yields. Importantly, no product isomerization or sequential Heck reactions were observed. These observations suggest that an enantioselective version may be possible. Efforts to develop enantioselective catalysts for this reaction are currently being pursued.
Supplementary Material
Acknowledgments
P.J.W. thanks the National Science Foundation (CHE-1464744) and National Institutes of Health (NIGMS 104349) for financial support. X.Y. thanks the Program for China Scholarship Council (201408535034), NSFC (21462049), and Excellent Young Talents of Yunnan University.
Footnotes
Notes
The authors declare no competing financial interest.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.or-glett.6b00815.
Procedures, characterization data for all new compounds (PDF)
References
- 1.Denmark SE, Butler CR. Chem Commun. 2009;1:20. doi: 10.1039/b809676g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For a review on Pd- and Ni-catalyzed alkenylation of enolates, see: Ankner T, Cosner CC, Helquist P. Chem - Eur J. 2013;19:1858. doi: 10.1002/chem.201202798.For selected examples on the development of the Pd-catalyzed α-alkenylation of enolates, see: Grigalunas M, Ankner T, Norrby PO, Wiest O, Helquist P. Org Lett. 2014;16:3970. doi: 10.1021/ol5017965.Grigalunas M, Ankner T, Norrby PO, Wiest O, Helquist P. J Am Chem Soc. 2015;137:7019. doi: 10.1021/jacs.5b02945.Grigalunas M, Norrby PO, Wiest O, Helquist P. Angew Chem, Int Ed. 2015;54:11822. doi: 10.1002/anie.201505895.Hamada T, Buchwald SL. Org Lett. 2002;4:999. doi: 10.1021/ol025563p.Huang J, Bunel E, Faul MM. Org Lett. 2007;9:4343. doi: 10.1021/ol7019839.Chieffi A, Kamikawa K, Åhman J, Fox JM, Buchwald SL. Org Lett. 2001;3:1897. doi: 10.1021/ol0159470.Hardegger LA, Habegger J, Donohoe TJ. Org Lett. 2015;17:3222. doi: 10.1021/acs.orglett.5b01312.Piers E, Marais PC. J Org Chem. 1990;55:3454.Piers E, Oballa RM. Tetrahedron Lett. 1995;36:5857.Solé D, Peidró E, Bonjoch J. Org Lett. 2000;2:2225. doi: 10.1021/ol005973i.For recent examples of the use of Pd-catalyzed α-alkenylation in synthesis, see: Liu F, Li C. Org Biomol Chem. 2014;12:637. doi: 10.1039/c3ob41883a.Edwankar CR, Edwankar RV, Deschamps JR, Cook JM. Angew Chem, Int Ed. 2012;51:11762. doi: 10.1002/anie.201206015.Cosner CC, Bhaskara Reddy Iska V, Chatterjee A, Markiewicz JT, Corden SJ, Löfstedt J, Ankner T, Richer J, Hulett T, Schauer DJ, Wiest O, Helquist P. Eur J Org Chem. 2013;2013:162.Yao Y, Liang G. Org Lett. 2012;14:5499. doi: 10.1021/ol3026395.
- 3.Padilla-Salinas R, Walvoord RR, Tcyrulnikov S, Kozlowski MC. Org Lett. 2013;15:3966. doi: 10.1021/ol401747u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Li M, Gonzalez-Esguevillas M, Berritt S, Yang X, Bellomo A, Walsh PJ. Angew Chem, Int Ed. 2016;55:2825. doi: 10.1002/anie.201509757. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) McGrew GI, Stanciu C, Zhang J, Carroll PJ, Dreher SD, Walsh PJ. Angew Chem, Int Ed. 2012;51:11510. doi: 10.1002/anie.201201874. [DOI] [PubMed] [Google Scholar]; (c) Gao F, Kim BS, Walsh PJ. Chem Commun. 2014;50:10661. doi: 10.1039/c4cc05307a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Bellomo A, Zhang J, Trongsiriwat N, Walsh PJ. Chem Sci. 2013;4:849. [Google Scholar]; (e) Cao X, Sha SC, Li M, Kim BS, Morgan C, Huang R, Yang X, Walsh PJ. Chem Sci. 2016;7:611. doi: 10.1039/c5sc03704b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Li M, Yucel B, Adrio J, Bellomo A, Walsh PJ. Chem Sci. 2014;5:2383. doi: 10.1039/C3SC53526F. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Zhang J, Bellomo A, Creamer AD, Dreher SD, Walsh PJ. J Am Chem Soc. 2012;134:13765. doi: 10.1021/ja3047816. [DOI] [PubMed] [Google Scholar]; (h) Li M, Yucel B, Jimenez-Hernandez J, Rotella M, Fu Y, Walsh PJ. Adv Synth Catal. doi: 10.1002/adsc.201600075. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Zheng B, Li M, Gao G, He Y, Walsh PJ. Adv Synth Catal. doi: 10.1002/adsc.201600090. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Kim BS, Jimenez J, Gao F, Walsh PJ. Org Lett. 2015;17:5788. doi: 10.1021/acs.orglett.5b02898. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Li M, Berritt S, Walsh PJ. Org Lett. 2014;16:4312. doi: 10.1021/ol502043j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Gao F, Kim BS, Walsh PJ. Chem Sci. 2016;7:976. doi: 10.1039/c5sc02739j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rivero AR, Kim BS, Walsh PJ. Org Lett. 2016;18:1590. doi: 10.1021/acs.orglett.6b00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Bordwell FG. Acc Chem Res. 1988;21:456. [Google Scholar]; (b) Fraser RR, Mansour TS, Savard S. J Org Chem. 1985;50:3232. [Google Scholar]
- 7.(a) Barrios-Landeros F, Carrow BP, Hartwig JF. J Am Chem Soc. 2009;131:8141. doi: 10.1021/ja900798s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Barrios-Landeros F, Hartwig JF. J Am Chem Soc. 2005;127:6944. doi: 10.1021/ja042959i. [DOI] [PubMed] [Google Scholar]; (c) Klingensmith LM, Strieter ER, Barder TE, Buchwald SL. Organometallics. 2006;25:82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.