Abstract
Rationale
While dementia affects 6–10% of persons aged 65 years or older, the industrialized world has witnessed an alarming rise in obesity. However, obesity’s influence on dementia remains poorly understood.
Methods
We conducted a systematic review and meta-analysis. Pubmed search between 1995 and 2007 resulted in 10 relevant prospective cohort studies with endpoints being dementia or its sub-types and main predictors including adiposity measures, particularly body mass index (BMI). Seven of them were included in our meta-analysis.
Results
Findings from previous cohort studies are mixed. The effect of obesity on Alzheimer’s Disease (AD) and vascular dementia (VaD) was stronger in studies with long follow-up time (>10 years) and young baseline age (<60 years). Weight gain and having a high waist circumference (WC) or skinfold thicknesses increased the risk of dementia. Our meta-analysis indicates a U-shaped association between BMI and dementia, as dementia risk was increased for obesity and underweight, though obesity’s effect on AD but not VaD was statistically significant (RR for AD: 1.80, 95% CI: 1.00, 3.29).
Conclusions
Findings from previous cohort studies are mixed and our meta-analysis shows a moderate association between obesity and the risks for dementia. Future studies are needed to understand the biological mechanisms.
Keywords: Obesity, dementia, Alzheimer’s disease, Vascular dementia, Aging
Introduction
As populations age, all cognitive disorders, including dementia, become more common. Dementia affects 6–10% of people aged 65 years or older, two-thirds of which are accounted by Alzheimer’s Disease (AD) 1. In fact, Alzheimer’s disease currently ranks as the eighth leading cause of death among the elderly in the United States 2 and a pooled analysis for mortality risk by dementia status yielded a risk ratio of 2.63 (2.17, 3.21), independently of age, sex and education 3. Despite its strong association with age 1, 4, dementia is still considered as a preventable condition and a large number of modifiable risk factors have been studied in relation to its occurrence. Vascular disease and its risk factors (such as smoking, alcohol, physical inactivity, high intake of saturated fats and low intake of antioxidants and n-3 fatty acids) are receiving increasing attention as potentially modifiable factors associated with cognitive decline and dementia in older adults 5–18. Stroke, cardiovascular disease, peripheral vascular disease, hypertension and diabetes have each been associated with cognitive deficits or dementia in various elderly population cohorts 19–28 and in cross-sectional studies 29–32. Dementia, particularly of the vascular type (VaD), is conceptually linked with cardiovascular complications. Recent research efforts have included AD as a potential outcome for poor cardiovascular profile 33. One group of risk factors were measures of adiposity and weight status, particularly body mass index (BMI in kg/m2), obesity (BMI≥30) and central obesity as measured by waist circumference (WC) among others. However, there is still no consensus as to their direct impact on dementia, independently of other cardiovascular factors, such as type 2 diabetes, hypertension and dyslipidemia.
The industrialized world has witnessed an alarming rise in the prevalence of obesity. Currently, almost two-thirds of American adults (66.3%) are overweight or obese, 32.4% are obese. In Americans aged 60 and over, around 72% are overweight or obese, while over 35% are obese 34. Among this age group, the prevalence of central obesity is around 50% among men and around 70% among women 35. Consequently, even a small impact of these adiposity measures on risk of dementia and its sub-types carries great public health implications.
The main objective of this paper is to systematically evaluate recent studies that examined the association between BMI and other measures of adiposity on incident dementia, VaD and AD in later life. Further, we conducted a meta-analysis to pool our findings and compare results across exposure-outcome dyads. Although two recent systematic reviews examined the association between obesity and dementia 33, 36, they share several main limitations, such as not including two more recent important cohort studies36, 37, and not reaching consistent conclusions. More importantly, they did not provide a formal meta-analysis to quantify the strength of the association based on different studies.
Materials and Methods
Data Extraction
Our study inclusion criteria for our meta-analysis are: (a) prospective cohort studies; (b) baseline age should be at least 40 years or older; (c) sample size at baseline >100; (d) follow-up time ≥ two years; (e) outcomes of are either incident dementia, or incident subtypes of dementia (AD or VaD) or a combination of these; (f) exposure recorded as either BMI, obesity/overweight or a measure of central obesity or a combination; (g) A measure of association was provided as relative risk (RR), odds ratio (OR) or hazard ratio (HR), or can be estimated from the published results.
A PUBMED search (January, 1995 to June, 2007) was conducted to identify English-language human studies which included keywords (MESH) of “dementia” and “obesity”. Of the 61 references that were retrieved, only seven studies met our selection criteria. Using the ‘related article’ capabilities of PUBMED, we found three additional relevant studies. Therefore, ten studies were included in our systematic review, and seven 36–42 had adequate data (e.g. OR or RR or HR) for our meta-analysis, while the other three studies did not provide measures of association that could be used to obtain pooled estimates. A database was built accordingly using Endnote ver. X 43. The characteristics of these ten studies are summarized in Table 1.
Table 1.
Main characteristics and findings of review and meta-analysis
| Authors | Year | Study name | Country | Baseline age, sex | Sample size | # of dementia cases | Dementia assessment | Follow-up time | Adiposity measures/Criteria | Main findings | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Luchsinger et al., 36 | 2007 | __ | USA | 77.0±5.7, both | 980 | Dementia: 181 | DSM-IV 109 | 5 years | BMI, WC, Weight change:
measured BMI and WC quartiles Ref: BMI:<23.4 kg/m2 |
No significant association between BMI and
dementia or its sub-types. After controlling for age, sex, education, ethnic group and ApoE ε4 status: |
|
| AD: 112 | NINCDS-ADRDA 110 | WC: ≤83 cm. |
WC>97 cm. for
VaD: HR: 2.3 (1.0, 5.1) |
||||||||
| VaD: 53 | ICD10: 111 | Weight change: Loss Stable (ref) Gain |
Weight gain (vs. stable weight) for
VaD: HR: 2.8 (1.0, 7.9) |
||||||||
|
| |||||||||||
| Whitmer et al.,37 | 2007 | Kaiser Permanente | USA | 40–45, both | 10,136 | AD: 477 | ICD9: 3331.0 112 | 36 years | BMI: measured WHO categories Ref: 18.5–24.9 kg/m2 |
After controlling for age, sex, education,
race, marital status, smoking, hyperlipidemia, hypertension, diabetes,
ischemic heart disease and stroke: BMI≥30 for AD: HR:3.10 (2.19, 4.38) BMI≥30 for VaD: HR: 5.01 (2.98, 8.43) |
|
| VaD: 132 | ICD9: 290.4 112 |
25≤BMI≤29.9 for
AD: HR: 2.09 (1.69, 2.60) 25≤ BMI≤29.9 for VaD: HR: 1.95 (1.29, 2.96) BMI<18.5 for AD: HR: 1.07 (0.50, 2.27) BMI<18.5 for VaD: HR: 0.65 (0.09, 4.72) |
|||||||||
|
| |||||||||||
| Hayden et al., 38 | 2006 | Cache County study | USA | 65+, both | 3,123 | Dementia: 141 | DSMIII-R 113 | 3.2 years | BMI: reported (self and proxy) | After controlling for age, sex, education, ApoEε4 status, hypertension, high cholesterol, diabetes, stroke, CABG and MI: | |
| AD: 104 | NINCDS-ADRDA 110 | ||||||||||
| VaD: 37 | NINDS-AIREN114 | Obesity: BMI≥30
kg/m2 Ref: <30 kg/m2 |
Obesity for
dementia: HR: 1.76 (1.03, 2.88) Obesity for AD: HR: 1.93 (1.05, 3.36) Obesity for VaD: HR: 1.16 (0.37, 3.12) |
||||||||
|
| |||||||||||
| Kivipelto et al., 39 | 2005 | Cardiovascular Risk Factors, Aging and Dementia (CAIDE) | Finland | 65–79, both | 1,449 | Dementia: 61 | DSM-IV109 | 21 years | BMI: measured Normal:≤25 kg/m2 Overweight: 25–30 Obese:>30 Ref: normal |
After controlling for age, sex, education,
follow-up time, SBP, DBP, total cholesterol, smoking, ApoE status, and
history of diabetes, MI and stroke: Obese for dementia: OR: 1.88 (0.76, 4.63) |
|
| AD: 48 | NINCDS-ADRDA 110 |
Overwt. for
dementia: OR: 0.99 (0.47, 2.15) Obese for AD: OR: 1.76 (0.67, 4.61) |
|||||||||
|
| |||||||||||
| Whitmer et al., 42 | 2005 | Kaiser Permanente | USA | 40–45, both | 10,276 | Dementia: 713 | ICD-9: 2900.0,7809.3, 3310.0,2904.1, 2900.1 112 | 27 years | BMI: measured Obese: ≥30 kg/m2 Overweight: 25–29.9 Normal:18.6–24.9 Underweight: ≤18.5 Ref: Normal SST and TST: quintiles Ref: Lowest quintile |
After controlling for age, sex, education,
race, marital status and comorbidity (hypertension, diabetes,
hyperlipidemia, stroke and ischemic heart
disease): obese for dementia: HR: 1.74 (1.34, 2.26) overwt. for dementia: HR: 1.35 (1.14, 1.60) underwt.for.dementia: HR: 1.24 (0.70, 2.21) Women >Men Highest SST quintile for dementia: HR: 1.72 (1.36, 2.18) Highest TST quintile for dementia: HR: 1.59 (1.24,2.04) |
|
|
| |||||||||||
| Rosengren et al., 41 | 2005 | Primary Prevention Study | Sweden | 47–55y, men | 7,402 | AD: 22 | ICD-8,9,10: 290.10, 290B or 331A, F00.0-F00.1 or F00.9 111, 112 | 25 years | BMI: measured Six categories: <20.0 to ≥30 kg/m2 Ref: 20–22.5 kg/m2 |
After controlling for age, smoking, social
class, SBP, diabetes mellitus, and
cholesterol: Obese for all dementia: HR: 1.84 (1.01, 3.34) |
|
| Dementia (primary): 154 | ICD-8,9,10 111, 112 |
Obese for. dementia as primary
diag.: HR: 2.54 (1.20, 5.36) |
|||||||||
| Dementia (secondary): 78 | ICD-8,9,10 111, 112 | ||||||||||
|
| |||||||||||
| Gustafson et al., 50 | 2003 | __ | Sweden | 70y, both | 382 | At ages 70, 75, 79y: Dementia: 34, 34, 33 |
DSM-IIIR 113 | 18 years | BMI: measured | After controlling for DBP, cardiovascular
disease, cigarette smoking, socioeconomic status, and treatment for
hypertension: BMI (1-unit increase) vs. total dementia*: HR: 1.13 (1.04, 1.24) |
|
| AD: 17,17,17 | NINCDS-ADRDA 110 | Continuous var. | HR: 1.13 (1.04, 1.24) HR: 1.15 (1.05, 1.26) |
||||||||
| VaD: 16,15,14 | NINDS-AIREN114 |
BMI (1-unit increase) vs.
AD*: HR: 1.36 (1.16, 1.59) HR: 1.35 (1.19, 1.53) HR: 1.23 (1.10, 1.37) BMI (1-unit increase) vs. VaD*: HR: 1.01 (0.88, 1.15) HR: 1.07 (1.02, 1.12) HR: 1.00 (0.89, 1.13) *BMI measured at 70, 75 and 79y, respectively. |
|||||||||
|
| |||||||||||
| Nourhashemi et al., 40 | 2003 | PAQUID | France | 65+, both | 3,646 | Dementia: 221 | __ | DSM-IIIR 113 | 8 years | BMI: reported | After controlling for sex, age,
age*sex, education, alcohol, and tobacco
consumption: BMI≥27vs.dementia: RR: 0.83 (0.59, 1.18) BMI<21vs.dementia: RR: 1.48 (1.08, 2.04) |
| Ref: 23–26 kg/m2 | (dementia at any follow-up time) | ||||||||||
|
| |||||||||||
| Kalmijn et al. 41 | 2000 | Honolulu- Asia Aging Study (HAAS) | USA, Japanese-American | 45–66y, men | 3,734 | Dementia: 215 | DSM-IIIR 113 | 25 years | BMI: measured Continuous: 1 SD=2.9 kg/m2 |
After controlling for age and
education: BMI (1 SD increase) vs. dementia: RR: 1.21 (1.05, 1.40) |
|
| AD: 82 | NINCDS-ADRDA 110 | SST: measured 1 SD=6.5 mm (central obesity) |
SST (1 SD increase) vs.
dementia: RR: 1.21 (1.06, 1.40) |
||||||||
| VaD: 73 | CADTS 115 | ||||||||||
|
| |||||||||||
| Yoshitake et al., 51 | 1995 | __ | Japan | 65+, both | 828 | Dementia: 103 | DSM-IIIR 113 | 7 years | BMI: measured SST/TST ratio | After controlling for age, smoking and other comorbidities: | |
| AD:42 | NINCDS-ADRDA 110 | Continuous var. |
BMI (1 unit increase) vs.
AD: RR: 0.75 (0.54, 1.03) |
||||||||
| VaD:50 | NINDS-AIREN114 |
BMI (1 unit increase) vs.
VaD: RR: 1.31 (0.98, 1.74) There was no association between SST/TST ratio and dementia. |
|||||||||
Abbreviations: AD: Alzheimer’s Disease; BMI: Body Mass Index (in kg/m2); WC: Waist circumference (in centimeters); VaD: Vascular dementia; DSM: diagnostic and statistical manual; WHO: World Health Organization; ICD: International Classification of Disease; HAAS: Honolulu-Asia Aging Study; CAIDE: Cardiovascular Risk Factors, Aging and Dementia; OR: Odds Ratio; HR: Hazard Ratio; RR: Risk Ratio; SST: Subscapular Skinfold Thickness; TST: Triceps Skinfold Thickness; NINCDS-ADRDA: National Institute of Neurological and Communicative Disorders and Stroke -- the Alzheimer’s Disease and Related Disorders Association; NINDS-AIREN: National Institute of Neurological and Communicative Disorders and Stroke--Association Internationale pour la Recherche et l’Enseignement en Neurosciences.
Statistical Analysis
We conducted a meta-analysis to assess the strength of the association between selected risk factors and our main outcomes. While outcomes included incident AD, VaD or dementia, risk factors considered were obesity, overweight, central obesity and BMI or weight changes. In this analysis, the original reported effect measures such as HR were pooled using random effects models when test for homogeneity between studies was significant or fixed effect models when otherwise. The pooled RR was obtained by averaging the natural logarithm of the relative measure in question, weighted by the inverse of their respective variances44. DerSimonian and Laird’s method was used in the random-effects model to further incorporate between-study variability45.
Next, in stratified meta-analyses, we examined potential sources of heterogeneity, including sex, baseline age group (<60 years vs. ≥60 years), follow-up time (<10 years vs. ≥10 years). In all our meta-analyses, we only considered fully adjusted models in each study which most often adjusted for measures of association for socio-demographic factors (age, sex, education, marital status, race) in addition to either lifestyle factors (e.g. smoking, physical inactivity), several co-morbid conditions (e.g. diabetes, hypertension, dyslipidemia, cardiovascular or cerebrovascular disease), a genetic factor (usually ApoEε4 carrier status), or a combination of all these factors.
Moreover, after estimating pooled measures of effect (RR), we computed the population attributable risk (PAR) whenever the analysis was based on at least two datapoints, given the current estimates of adult obesity prevalence (Pr) in the United States 34. PAR was computed as follows:
| Eq. 1 |
with point estimates and confidence limits of RR being applied to this formula and Prexp being the prevalence of the exposure (e.g. obesity).
In addition, we assessed publication bias using the Begg’s funnel plots. The measures of association (RR) were plotted on a logarithmic scale against their corresponding SEs for each study 46, 47. We also assessed publication bias by two formal tests, the Begg-adjusted rank correlation test, and the Egger’s regression asymmetry test. Our meta-analysis was conducted using STATA 9.0 (StataCorp, College Station, TX)48. Statistical significance of regression coefficients associated with risk or hazard ratios was set at P<0.05.
RESULTS
Systematic Review
Table 1 describes the ten studies included in our systematic review. Four of the ten retrieved studies were conducted in the United States and all ten were recently published except for one in the 1990s. Most studies (8 out of 10) involved a balanced number of men and women while two included only men. Baseline ages in four studies included subjects in their 40s, while the other six studies started out with the elderly group (≥65 years). Follow-up time ranged from 3.2 to 36 years. Sample size ranged from 382 to 10,136 subjects. In total, the average person-time of follow-up from all the studies was 1,007,911. Many studies adopted a three-step process in diagnosing dementia and its subtypes, starting with a screening phase (using tools such as the Mini-Mental State Exam or MMSE), then a diagnostic phase (using DSM-IV or DSM-IIIR or ICD8,9 or 10) and finally a differential diagnosis phase which detects sub-types of dementia (mainly using tools such as NINCDS-ADRDA for AD and NINDS-AIREN or WHO or CADTS for VaD) (see Table 1 for references).
Eight out of the ten studies assessed the effect of adiposity measures on incident AD, while six assessed their influence on incident VaD and eight examined overall incident dementia as the endpoint. In terms of exposures, obesity was of interest in eight studies, and continuous BMI in four, central obesity in two and weight change in one.
BMI and dementia
BMI was studied as a continuous variable in four of the selected cohort studies. In one study, mean BMI±SD was significantly larger among subjects with dementia as primary diagnosis for hospitalization compared to those who did not have dementia (26.0±3.1 vs. 25.5±3.3; p<0.05). This study cohort consisted of 7,402 men aged 47–55 years who were followed for an average of 25 years up till hospitalization 41. Another study conducted among 3,734 middle-aged Japanese-American men who were followed up for an average of 25 years found that 1 SD (2.9 kg/m2) higher BMI was associated with an RR of 1.21 (95% CI: 1.05–1.40) for dementia, after adjusting for age and education 49. In a third study 50, a representative cohort of 392 non-demented Swedish adults was followed up from age 70 to 88 years. It was found that among women, for each unit increase in baseline BMI at age 70, AD risk increased by 36% (RR=1.36 (1.16, 1.59)). The RR tended to be weaker but statistically significant when BMIs at ages 75 and 79y were considered. No significant association was found among men or for VaD outcome, which may be due to the smaller sample size. However, an earlier study conducted among 828 subjects aged 65 years or more at baseline found after 7 years of follow-up no significant association between baseline and incident AD (RR=0.75 (0.54, 1.03)) or with VaD (RR=1.31 (0.98–1.74)) 51.
Obesity, overweight and dementia: a dose-response relationship?
Using variable cutoffs for BMI, eight studies assessed the effect of obesity and/or overweight at baseline on incidence of dementia, AD and/or VaD. For overall dementia, obesity was found to be associated with this incident outcome in two 41, 42 out of five studies with available data 36, 39–42. The first study that found a positive association followed up a sample of 10,276 middle-aged US men and women for 27 years and is to date the largest cohort study to test our hypothesis of interest. This study indicated that the risk of incident dementia was increased by 74% among obese subjects at baseline (HR=1.74 (1.34 to 2.26)), while overweight people (BMI=25.0–29.9) had a 35% greater risk of dementia (1.35, 1.14 to 1.60) compared with those of normal weight (BMI=18.6–24.9), indicating a dose-response relationship 42. In the second study which was conducted among 7,402 middle-aged Swedish men who were followed-up for 25 years, results were even stronger, particularly among the obese when compared to the normal weight (HR: 1.98, 95% confidence interval 1.10–3.56) 41. However, obesity, whether defined by the 30 or 27 kg/m2 cutoff point was not a significant risk factor of dementia according to three large cohort studies. Compared to the two studies cited above, those three cohorts had smaller sample sizes, baseline age was higher on average including the 65 years and over group in two studies of the three, and the number of dementia cases was less than 200 36, 39, 40. Thus, limited statistical power might have prevented significant associations.
In contrast, for incident AD, a positive association with obesity was found in two 37, 41 out of five studies 36–39, 41. The Cache County Study showed that obesity increased the risk of AD in women (adjusted HR=2.23 (1.09–4.30)), but not among men (1.48 (0.41–4.18)). It is worth noting that in this analysis, adjustment was made for sodio-demographics (age, education) as well as genetic (ApoE genotype) and health-related factors (hypertension, high cholesterol, diabetes, stroke, CABG and MI)38. Using a similar methodology for assessing incident AD, the Kaiser Permanente study found that when the model was adjusted for age and education only, HRs were weaker than when additional adjustment was made on race, sex, marital status, smoking and chronic conditions (hyperlipidemia, hypertension, diabetes, ischemic heart disease and stroke). In the fully adjusted models, the HR for obesity and incident AD among both sexes was 3.10, 95% CI: 2.19, 4.38. Among men and women, the HRs were 2.60, 95%CI: 1.44, 4.69 and 3.38, 95% CI: 2.20, 5.19, respectively, indicating a stronger effect among women. In the case of overweight, the age and education adjusted model yielded a HR of 1.27, 95% CI: 1.04, 1.54. Adjusting further for all other covariates, gave an HR of 2.09, 95% CI: 1.69, 2.60. Similar to obesity, overweight had a stronger impact on the incidence of AD among women compared to men (HRs 2.45 vs. 1.74, respectively)37.
Finally for incident VaD, the association was significant in one 37 out of three studies36–38. In that study and in terms of obesity (BMI≥30), age, sex, and education adjusted models yielded an HR for incident VaD of 3.01 (1.86, 4.85), while the fully adjusted model increased the HR [5.01 (2.98, 8.43)]. There were no significant gender differences in the association. But regarding overweight, women showed a stronger association than men, the HRs being 2.45 and 2.09 respectively 37.
In summary, findings from the available studies are mixed, and only a few indicate a dose-response relationship.
Central obesity, weight change, body fat and dementia
Aside from BMI, other measures were used in various studies to assess the effect of central obesity on incident dementia, including WC, as well as the effect of body fat measured with triceps skinfold thickness (TST) and subscapular skinfold thickness (SST). The earliest study conducted among 828 non-demented Japanese subjects aged 65 years or more and followed up for 7 years suggested that SST/TST ratio was not associated with incidence of AD or VaD 51. In contrast, another study followed 3,734 Japanese-American middle-aged men for 25 years and found that an increase in SST by 6.5 mm increased the risk of dementia by 21% (RR: 1.21, 95% CI: 1.06–1.40). This effect was almost identical to an increase in BMI by 2.9 kg/m2 49. Moreover, another shows that the upper quintiles of TST and SST increased the risk of dementia by around 50% after adjustment for age, education, race, marital status and sex (RR for SST: 1.54, 95% CI: 1.23, 1.94; RR for TST: 1.49, 95% CI: 1.04, 1.97). Additional control for co-morbid conditions yielded higher average effects (RR for SST: 1.72; RR for TST: 1.59)42.
Another recent study explored the impact of central obesity and weight gain on risk of dementia and followed 980 elderly subjects for 5 years and suggested that WC as a continuous variable was not associated with dementia (HR, 1.0; 95% CI, 0.9–1.0), nor was it associated with incident AD or VaD. However, the upper quartile of WC (>97 cm.) increased the risk of VaD (RR: 2.3, 95% CI:1.0, 5.1). Their analysis controlled for age, sex, education, ethnicity and ApoEε4 status. The study also shows a positive association between weight gain and the risk of VaD (RR: 2.8, 95% CI: 1.0, 7.9) 36.
Meta-analysis
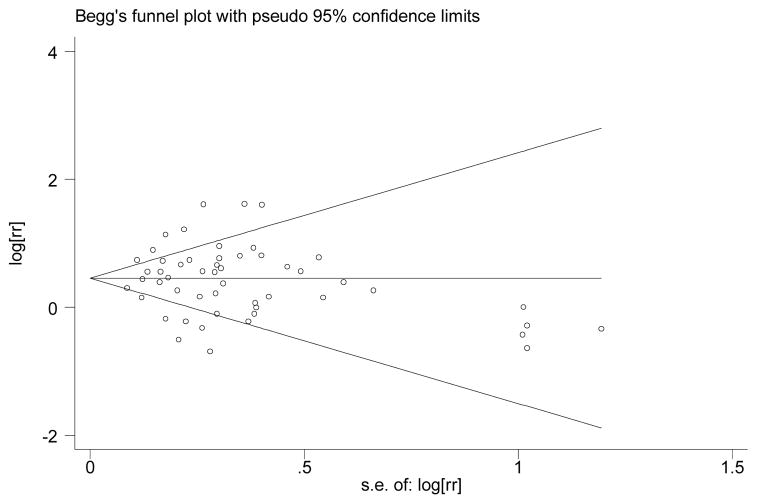
Our meta-analysis was conducted on seven studies which had adequate measures of association that could be pooled together, with 138 data points consisting of HRs, ORs or RRs with their confidence intervals and with focus on categorical adiposity measures, mainly BMI, WC, skinfolds and weight change in relation to total incident dementia, AD or VaD. For each study we entered between 4 and 45 data points for our meta-analysis. However, for our main analyses we selected those data points in which the model within each study was fully adjusted for the potential confounders considered. Taking all those data points together (n=70), we assessed publication bias using Begg’s funnel plot which is presented in Appendix A. While there seems to be some clustering at the low SE end, two statistical tests (Begg-adjusted rank correlation test and the Egger’s regression asymmetry test) indicated no significant publication bias.
Appendix A.
Funnel plot for all RR, HR and OR measures used for meta-analysis: assessment of publication bias for fully adjusted models with BMI (categories) as main exposure (n=52 datapoints)*
* Egger’s regression asymmetry test: −0.14±0.54, P=0.791;
Begg-adjusted rank correlation test: z=0.62; P=0.533.
† RR: Relative Risk; HR: Hazard Ratio; OR: Odds Ratio; s.e.: standard error.
Weight status and dementia
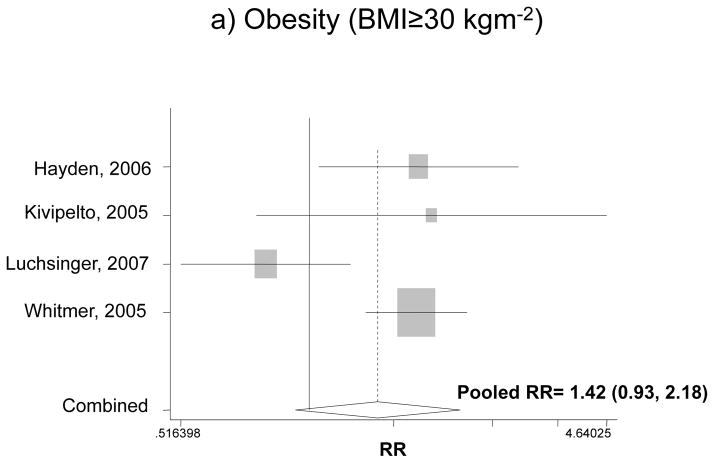
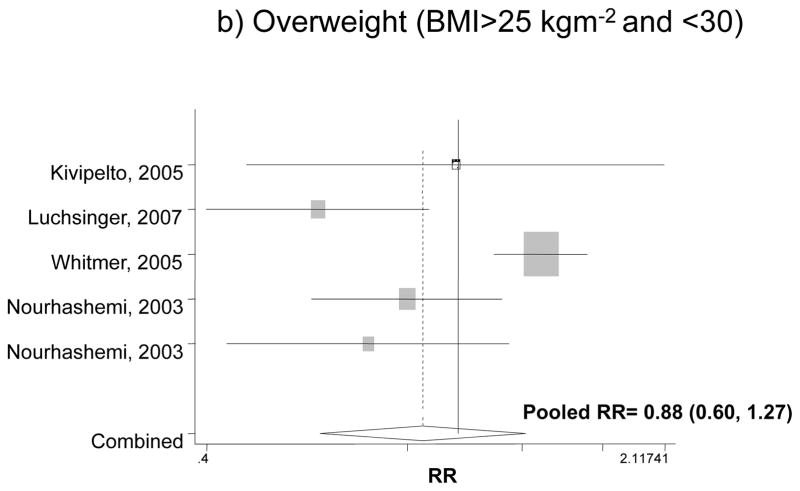
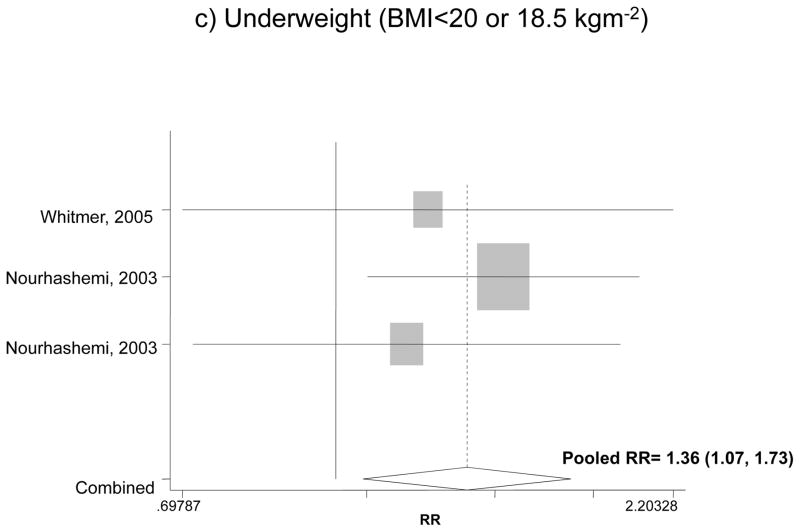
Figure 1 shows the association between BMI categories and incident dementia for the fully adjusted models in each study and findings of our pooled analysis. Our pooled RR for obesity and overall dementia for both men and women indicated an increased risk by 42% compared to normal weight, though the effect was not significant (RR: 1.42, 95%CI: 0.93, 2.18). The pooled RR for overweight in relation to dementia did not indicate a significant association (RR=0.88; 95% CI: 0.60, 1.27). Comparing underweight with normal weight, however, there was a 42% increase in the risk of dementia based on a pooled RR (RR: 1.42, 95% CI: 1.07, 1.87).
Figure 1.
The association between BMI categories and incident dementia*
* Based on fully adjusted models (sociodemographic+lifestyle and/or co-morbid conditions and/or genetic factors), for both genders together, and normal BMI range being the reference category.
Obesity and subtype of dementia
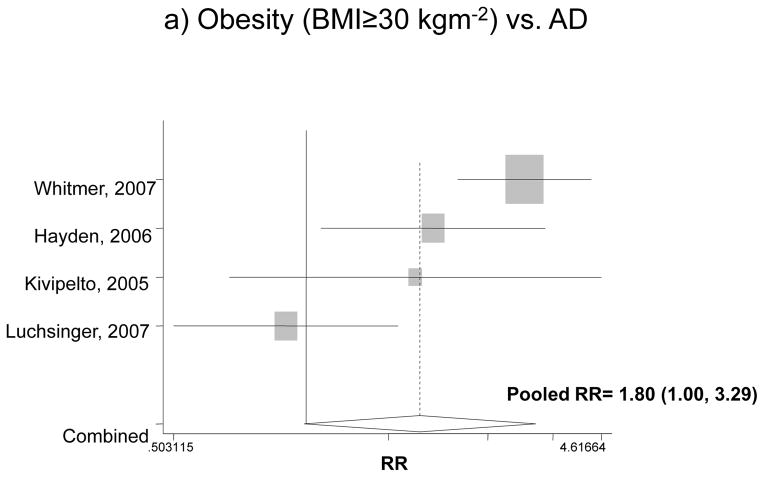
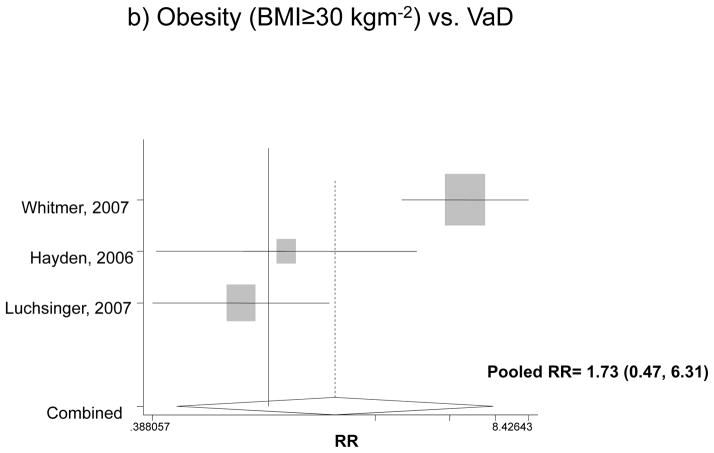
Looking specifically at each type of diagnosis, Figure 2 attempts to pool data on the association between obesity and incident AD or VaD, using the fully adjusted models. For both genders, the pooled RR for AD was estimated at 1.80 with a 95% CI of 1.00 to 3.29, indicating that compared to normal weight subjects, obese people have an 80% higher risk for incident AD. However, in the case of VaD when both sexes were pooled together, obesity did not have a significant effect. This was not the case when each gender was considered separately. Among men, the pooled RR for obesity and dementia was 2.32 with a 95% CI of 1.37 to 3.9, while among women, the effect was even stronger: pooled RR=3.01 with a 95% CI of 2.09 to 4.33. Based on the same studies 37, 38, though imprecise, the effect of obesity on incident VaD indicated an appreciable and statistically significant increased risk among both men and women (RR>3).
Figure 2.
The association between obesity and incident AD and VaD*
* Based on fully adjusted models (sociodemographic+lifestyle and/or co-morbid conditions and/or genetic factors), for both genders together, and normal BMI range being the reference category.
AD: Alzheimer’s Disease; VaD: Vascular Dementia.
Subgroup analyses
Table 2 presents further subgroup analyses for the pooled RR of obesity and incident dementia and sub-types. The stratifying variables included baseline age, sex and follow-up time, and only data points for fully adjusted models were considered. RRs of obesity and dementia did not differ significantly across strata. In the case of AD, a positive association was found with obesity which had a stronger effect when baseline age was <60 years and follow-up time ≥10 years (Significant Q-test, p<0.05). The same pattern was observed for VaD. For models adjusted only for socio-demographic variables such as age, sex, race/ethnicity and marital status, the overall RR pooled estimates for all outcomes were greater in magnitude compared to those in the fully adjusted models, even though the Q-test comparing those two models (fully vs. partially adjusted) was not statistically significant.
Table 2.
Subgroup analyses: pooled RR or HR and 95% confidence interval (CI) of obesity and incident dementia and sub-types in prospective cohort studies in the general adult population: fully adjusted models‡
| Dementia | AD | VaD | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| RR | 95% CI | RR | 95% CI | RR | 95% CI | |
|
|
||||||
| All | 1.42 | 0.93, 2.18 | 1.80 | 1.00, 3.29* | 1.73 | 0.47, 6.31 |
| (n=4) | (n=4) | (n=3) | ||||
| Baseline age (y) | ||||||
| <60 | 1.74 | 1.34, 2.26* | 3.10 | 2.19, 4.38*,† | 5.01 | 2.97, 8.43*,† |
| 60+ | 1.31 | 0.71, 2.39 | 1.38 | 0.94, 1.66* | 0.90 | 0.49, 1.64 |
| (n= 1 and 3) | (n= 1 and 3) | (n= 1 and 2) | ||||
| Sex | ||||||
| Men | 1.59 | 1.17, 2.15* | 2.32 | 1.37, 3.92* | 4.07 | 1.93, 8.58* |
| Women | 2.07 | 1.49, 2.88* | 3.00 | 2.09, 4.33* | 3.70) | 1.99, 6.88* |
| (n=3 and 1) | (n=2 and 2) | (n= 2 and 2 | ||||
| Follow-up time (y) | ||||||
| <10 | 1.17 | 0.54, 2.54 | 1.32 | 0.87, 1.99† | 0.90 | 0.49, 1.64† |
| ≥10 | 1.75 | 1.36, 2.25* | 2.91 | 2.10, 4.03* | 5.01 | 2.98, 8.43* |
| (n=2 and 2) | (n=2 and 2) | (n=2 and 1) | ||||
P<0.05 for the null hypothesis that lnRR=0.
Significant test for heterogeneity (Q-test) with 1 degrees of freedom, comparing RRs between sex, baseline age and follow-up strata: P-value<0.05.
Models were adjusted for socio-demographic factors in addition to some lifestyle factors, co-morbid conditions and/or genetic factors (mainly ApoE ε4 carrier status).
AD: Alzheimer’s Disease; VaD: Vascular Dementia; RR: Relative Risk; n: study datapoint used in fully adjusted models.
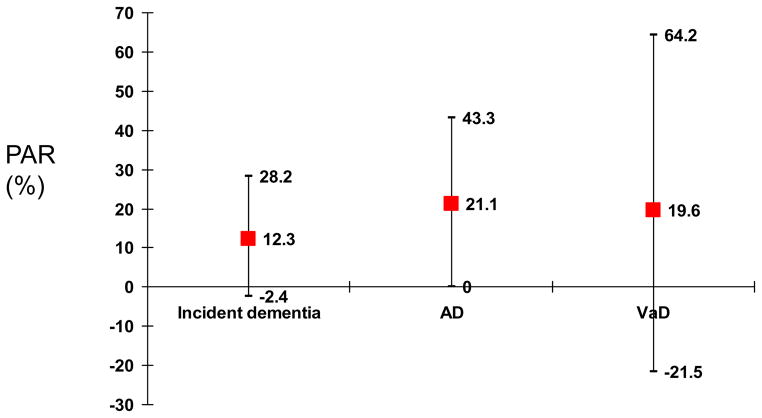
Population Attributable Risk
Figure 3. shows PAR which estimates the proportion of disease (dementia and its sub-types) in the study population that is attributable to the exposure (measures of obesity). Overall, while around 12% of dementia risk was attributed to obesity. PAR was even higher in the case of AD (21.1%).
Figure 3.
Population Attributable Risk with 95% CI for obesity and dementia and its subtypes using current estimates of adult obesity prevalence (NHANES, 1999–2002)*
Source: 34
*Estimates of obesity prevalence were applied to the PAR formula as the average for the age groups (40–59 and 60+), which was 33.35%.
DISCUSSION
The influence of obesity on dementia has been a subject of controversy for many years. Our systematic review of large prospective cohort studies with at least five years of follow-up time shows that previous findings are mixed and inconclusive. Our meta-analysis of these findings suggest that obesity and central obesity seem to play an independent role in the etiology of AD and in some cases of VaD, even when control is made on socio-demographic, lifestyle and health-related co-morbid factors. Overall, there seems to be a U-shaped relation between BMI status and dementia, both obesity and underweight increasing the risk of dementia. Our pooled analyses show that obesity’s effect on AD but not VaD was statistically significant (RR for AD: 1.80 (1.00, 3.29)). The effect of obesity on AD and VaD was particularly strong and significant in studies with follow-up time greater than 10 years and baseline ages lower than 60 years. RRs for obesity and AD were slightly stronger among women and the opposite was true for obesity and VaD. Moreover, research indicated that adiposity measured using skinfold thicknesses increased the risk of dementia in a few studies 42, 49, while central obesity (high WC) may increase the risk of VaD but not AD 36. In the US population, about 21% of AD incidence was attributed to obesity. It is worth noting that obesity and abdominal obesity (defined using waist-hip-ratio cutoffs) in later life have been strongly associated with prevalent AD based on at least one recent case-control study. In this study, adding metabolic disorders to the logistic regression models further increased the strength of the association as was found in our meta-analysis52.
It is well-established that obesity in general and central obesity in particular is only one component of an etiologic cluster known as the metabolic syndrome. Up to this point, it was still unclear whether having high adiposity is an independent risk factor for dementia and its sub-types when we take into account the other components of this syndrome. Our meta-analysis suggests that obesity is an independent risk factor for AD in analyses adjusted for other components such as hypertension, type 2 diabetes and dyslipidemia among others. Some of the independent biologic mechanisms for the effect of adiposity on cognitive functioning include the fat-brain axis 53 and the hypothalamic-pituitary-adipose axis 54. Recent experimental data proved that compounds secreted by adipose tissues such as leptin and adiponectin may regulate energy expenditure and hyperphagic responses by interacting with the hypothalamus. Leptin, when administered directly into the hippocampus of mice, improves memory processing and may also shape the hypothalamus during the earliest stages55.
It is possible that part of the effect of obesity on dementia risk is mediated by the other components of the metabolic syndrome. First of all, obesity increases the risk of hypertension 56, and some previous studies suggest an association between hypertension and cognitive decline in middle age and later 57–59, although these studies provided inconsistent results. These differences seem to arise mostly from methodological and sampling variations between studies. However, in general, they have indicated that cognitive function tends to be poorer and decline to be faster with increased blood pressure (BP). In addition, this positive association was seen both among the elderly and middle-aged adults. For instance, a longitudinal study over a period of 25 to 30 years on older adults and concluded that people who maintain elevated systolic BP throughout their adult lives are at increased risk for reduced verbal learning and memory function20. Another recent study 21 conducted among the Established Populations for Epidemiologic Studies of the Elderly (EPESE) cohort of East Boston aged 65 to 102 years at baseline suggested a U-shaped association between BP and cognitive decline.
Second, several prospective studies have assessed type 2 diabetes mellitus as a risk factor for incident Alzheimer disease (AD) and decline in cognitive function. A recent study 25 examined 824 older Catholic nuns, priests and brothers annually for up to 9 years to assess their cognitive function. After adjusting for age, sex and educational level, the HR was 1.65 (1.10–2.47). Moreover, the presence of type 2 diabetes increased the risk of cognitive decline in perceptual speed by 44% (P=0.02) but not in other cognitive systems. Similar findings were replicated in another recent study 26, which found that risk of developing cognitive impairment -- based on five standardized tests -- among women with Impaired Fasting Glucose (IFG) or diabetes was increased by almost twofold (age and treatment-adjusted OR = 1.64; 95% CI 1.03 to 2.61 for IFG; OR = 1.79; 95% CI 1.14 to 2.81 for diabetics). Finally, in a longitudinal study of 258 elderly subjects, a significant interaction was found between hypertension and diabetes and the presence of both diseases tended to produce pronounced Mini-Mental State Examination (MMSE) cognitive decline28.
Third, the relation between plasma lipid levels and the risks for AD and VaD remains unclear. Cholesterol alters the degradation of the amyloid precursor protein, which plays a major role in the pathogenesis of AD 60. Moreover, cerebrovascular disease which is associated with dyslipidemia, may be related to the risk of AD 61. Previous literature showed that reduced HDL-C 62, 63 and apolipoprotein A-I levels 62 as well as increased levels of lipoprotein A 64 were observed among VaD patients. However, this association was not found in other studies 65, 66. Conflicting results were also noted in studies relating total cholesterol 67, 68, HDL-C 7, 64, 69 and LDL-C 7, 67 levels with AD. A recent cohort study 70 among 4,316 Medicare recipients, 65 years and older, residing in Northern Manhattan, NY, showed a weak association between lipid levels and the risk of VaD. Similarly, the risk of AD was independent of both lipid levels and use of agents to lower them.
The metabolic syndrome was recently studied as a risk factor for cognitive decline among 1,616 elders71, and found that among high-functioning elders, those with metabolic syndrome exhibit an increased risk of developing cognitive impairment and decline over 4 years. This association remained after adjusting for demographic and lifestyle variables as well as chronic health conditions. The increased rate of cognitive impairment was primarily observed in those elders who had high levels of serum markers of inflammation, suggesting that at least some of the increased risk associated with the metabolic syndrome is modified by inflammation. Other recent studies linking metabolic syndrome to incident or prevalent dementia, AD and VaD came to similar conclusions, though various measurements of the metabolic syndrome were used 49, 72–74. In fact, in one study only hyperinsulinemia and diabetes were associated with increased risk of incident AD and VaD rather than the metabolic syndrome as a whole73. However, in another study which used the NCEP ATP III definition for metabolic syndrome, the whole syndrome was significantly associated with prevalent AD, particularly among women74.
While disentangling the effect of adiposity from that of the metabolic syndrome as a whole has been a major obstacle in fully understanding the etiology of dementia, other complexities should be addressed as well. In fact, BMI is also associated with physical inactivity 31, 75–83 and depressive symptoms 15, 25, 84, 85, both of which are linked to cognitive impairment as well. First, in terms of physical inactivity, a recent cohort study 75 conducted on 4,615 men and women aged 65 years or more (the 1991–92 Canadian Study of Health and Aging) who were cognitively healthy at baseline found that high levels of physical activity were associated with reduced risk of cognitive impairment (OR= 0.59 (0.41–0.83)), AD (OR=0.50 (0.28–0.90)) and dementia of any type (OR=0.63 (0.40–0.98)). Similar findings were obtained by other recent prospective studies 31, 76–83 and one cross-sectional study86. Several mechanisms may underlie the potentially protective effects of physical activity on cognitive function, including sustained cerebral blood flow 87, improved aerobic capacity and cerebral nutrient supply 88, 89 as well as growth factors, specifically the brain-derived neurotropic factor, which is a molecule that increases neuronal survival, enhances learning, and protects against cognitive decline 90, 91.
Second, in terms of depressive symptoms’ effect on cognition, a recent study conducted on 4,392 older people in Chicago suggested that for each depressive symptom, the rate of cognitive decline increased by about 5%. Hence, it was suggested that depressive symptoms predict cognitive decline in old age 92. Several other studies came to similar conclusions in the overall population of older adults 84, 85, 93, while other studies did not find an association 94, 95 and still others found the association only in those with baseline cognitive impairment 96 or relatively more education 97. Thus, lack of control for such variables constitute a major limitation in assessing the independent effect of BMI on the risks for dementia.
It is worth noting that in addition to the moderately strong association between obesity and incident AD (RR=1.80), we found a weak to moderate association between underweight and incident dementia (pooled RR=1.36) based on two cohort studies 40, 42, which was consistent with several cross-sectional and case-control studies52. In addition, a number of other cohort studies suggested that weight loss and BMI loss were associated with incident AD or dementia 98–100 as well as cognitive decline101. The possible mechanisms include change in eating habits and access to adequate nutrition due to memory impairment 102–105, and increase in anorectic adipokines or inflammatory cytokines with frailty contributing to loss in BMI among subjects with AD 106–108.
In conclusion, findings from recent cohort studies from diverse populations are mixed. Our meta-analysis suggests an independent effect of obesity on dementia in general and incident AD in particular, but the associations are relatively weak. While the effect of BMI on dementia is a U-shaped one, reducing the prevalence of obesity is a promising strategy in preventing progression from normal aging into AD. Future cohort studies should attempt to disentangle the effect of BMI from other components of the metabolic syndrome, address residual confounding by other covariates such as physical activity and depressive symptoms and attempt to fully understand the related biological mechanisms.
References
- 1.Hendrie HC. Epidemiology of dementia and Alzheimer’s disease. Am J Geriatr Psychiatry. 1998;6:S3–18. doi: 10.1097/00019442-199821001-00002. [DOI] [PubMed] [Google Scholar]
- 2.Centers of Disease Control and Prevention. Death: preliminary data 2003. Natl Vital Stat Rep. 2005:53. [PubMed] [Google Scholar]
- 3.Dewey ME, Saz P. Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: a systematic review of the literature. Int J Geriatr Psychiatry. 2001;16:751–61. doi: 10.1002/gps.397. [DOI] [PubMed] [Google Scholar]
- 4.Abate C, Ferrari-Ramondo V, Di Iorio A. Risk factors for cognitive disorders in the elderly: A review. Archives of Gerontology and Geriatrics. 1998;(suppl 6):7–15. [Google Scholar]
- 5.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–55. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 6.Geerlings MI, Ruitenberg A, Witteman JC, van Swieten JC, Hofman A, van Duijn CM, et al. Reproductive period and risk of dementia in postmenopausal women. Jama. 2001;285:1475–81. doi: 10.1001/jama.285.11.1475. [DOI] [PubMed] [Google Scholar]
- 7.Anttila T, Helkala EL, Viitanen M, Kareholt I, Fratiglioni L, Winblad B, et al. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. Bmj. 2004;329:539. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngandu T, Helkala EL, Soininen H, Winblad B, Tuomilehto J, Nissinen A, et al. Alcohol Drinking and Cognitive Functions: Findings from the Cardiovascular Risk Factors Aging and Dementia (CAIDE) Study. Dement Geriatr Cogn Disord. 2006;23:140–49. doi: 10.1159/000097995. [DOI] [PubMed] [Google Scholar]
- 9.Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med. 2005;352:245–53. doi: 10.1056/NEJMoa041152. [DOI] [PubMed] [Google Scholar]
- 10.Galanis DJ, Joseph C, Masaki KH, Petrovitch H, Ross GW, White L. A longitudinal study of drinking and cognitive performance in elderly Japanese American men: the Honolulu-Asia Aging Study. Am J Public Health. 2000;90:1254–9. doi: 10.2105/ajph.90.8.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganguli M, Vander Bilt J, Saxton JA, Shen C, Dodge HH. Alcohol consumption and cognitive function in late life: a longitudinal community study. Neurology. 2005;65:1210–7. doi: 10.1212/01.wnl.0000180520.35181.24. [DOI] [PubMed] [Google Scholar]
- 12.Logan AC, Katzman M. Major depressive disorder: probiotics may be an adjuvant therapy. Med Hypotheses. 2005;64:533–8. doi: 10.1016/j.mehy.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Espeland MA, Coker LH, Wallace R, Rapp SR, Resnick SM, Limacher M, et al. Association between alcohol intake and domain-specific cognitive function in older women. Neuroepidemiology. 2006;27:1–12. doi: 10.1159/000093532. [DOI] [PubMed] [Google Scholar]
- 14.Wright CB, Elkind MS, Luo X, Paik MC, Sacco RL. Reported alcohol consumption and cognitive decline: the northern Manhattan study. Neuroepidemiology. 2006;27:201–7. doi: 10.1159/000096300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. Jama. 2002;287:3230–7. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 16.Morris MC. Diet and Alzheimer’s disease: what the evidence shows. MedGenMed. 2004;6:48. [PMC free article] [PubMed] [Google Scholar]
- 17.Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Wilson RS, et al. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch Neurol. 2003;60:940–6. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 18.Beydoun MA, Kaufman JS, Satia JA, Rosamond W, Folsom AR. Plasma n-3 fatty acids and risk of cognitive decline among older adults: The Atherosclerosis Risk in Communities (ARIC) study. Am J Clin Nutr. 2007 doi: 10.1093/ajcn/85.4.1103. In press. [DOI] [PubMed] [Google Scholar]
- 19.Kalmijn S, Feskens EJ, Launer LJ, Stijnen T, Kromhout D. Glucose intolerance, hyperinsulinaemia and cognitive function in a general population of elderly men. Diabetologia. 1995;38:1096–102. doi: 10.1007/BF00402181. [DOI] [PubMed] [Google Scholar]
- 20.Swan GE, Carmelli D, Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke. 1998;29:2334–40. doi: 10.1161/01.str.29.11.2334. [DOI] [PubMed] [Google Scholar]
- 21.Glynn RJ, Beckett LA, Hebert LE, Morris MC, Scherr PA, Evans DA. Current and remote blood pressure and cognitive decline. Jama. 1999;281:438–45. doi: 10.1001/jama.281.5.438. [DOI] [PubMed] [Google Scholar]
- 22.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–8. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 23.Alves de Moraes SA, Szklo M, Knopman D, Sato R. The relationship between temporal changes in blood pressure and changes in cognitive function: Atherosclerosis Risk in Communities (ARIC) Study. Preventive Medicine. 2002;33:258–63. doi: 10.1006/pmed.2002.1077. [DOI] [PubMed] [Google Scholar]
- 24.Stewart R, Prince M, Mann A. Age, vascular risk, and cognitive decline in an older, British, African-Caribbean population. J Am Geriatr Soc. 2003;51:1547–53. doi: 10.1046/j.1532-5415.2003.51504.x. [DOI] [PubMed] [Google Scholar]
- 25.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–6. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 26.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–63. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 27.Fontbonne A, Berr C, Ducimetiere P, Alperovitch A. Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care. 2001;24:366–70. doi: 10.2337/diacare.24.2.366. [DOI] [PubMed] [Google Scholar]
- 28.Hassing LB, Hofer SM, Nilsson SE, Berg S, Pedersen NL, McClearn G, et al. Comorbid type 2 diabetes mellitus and hypertension exacerbates cognitive decline: evidence from a longitudinal study. Age Ageing. 2004;33:355–61. doi: 10.1093/ageing/afh100. [DOI] [PubMed] [Google Scholar]
- 29.Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the Rotterdam Study. Bmj. 1994;308:1604–8. doi: 10.1136/bmj.308.6944.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips NA, Mate-Kole CC. Cognitive deficits in peripheral vascular disease. A comparison of mild stroke patients and normal control subjects. Stroke. 1997;28:777–84. doi: 10.1161/01.str.28.4.777. [DOI] [PubMed] [Google Scholar]
- 31.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. Jama. 2004;292:1447–53. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 32.Harrington F, Saxby BK, McKeith IG, Wesnes K, Ford GA. Cognitive performance in hypertensive and normotensive older subjects. Hypertension. 2000;36:1079–82. doi: 10.1161/01.hyp.36.6.1079. [DOI] [PubMed] [Google Scholar]
- 33.Gorospe EC, Dave JK. The risk of dementia with increased body mass index. Age Ageing. 2007;36:23–9. doi: 10.1093/ageing/afl123. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Beydoun MA. The Obesity Epidemic in the United States--Gender, Age, Socioeconomic, Racial/Ethnic, and Geographic Characteristics: A Systematic Review and Meta-Regression Analysis. Epidemiol Rev. 2007 doi: 10.1093/epirev/mxm007. [DOI] [PubMed] [Google Scholar]
- 35.Okosun IS, Choi ST, Boltri JM, Parish DC, Chandra KM, Dever GE, et al. Trends of abdominal adiposity in white, black, and Mexican-American adults, 1988 to 2000. Obes Res. 2003;11:1010–7. doi: 10.1038/oby.2003.139. [DOI] [PubMed] [Google Scholar]
- 36.Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64:392–8. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–9. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 38.Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20:93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 39.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–60. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 40.Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60:117–9. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 41.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005;165:321–6. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 42.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Bmj. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Endnote Thomson T. M.: Philadelphia, PA 2006
- 44.Petitti DB. Statistical methods in meat-analysis. In: Petitti DB, editor. Meta-analysis. Decision Analysis, and cost-effectiveness analysis. 2. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 45.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 46.Egger M, Smith GD, Altman DG. Systematic Reviews in heatlh care: Meta-analysis in context. 2. London. UK: the BMJ Publishing Group; 2001. [Google Scholar]
- 47.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stata Statistical Software: Release 9.0. Stata Corporation; College Station, TX: 2005. [Google Scholar]
- 49.Kalmijn S, Foley D, White L, Burchfiel CM, Curb JD, Petrovitch H, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol. 2000;20:2255–60. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 50.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003;163:1524–8. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 51.Yoshitake T, Kiyohara Y, Kato I, Ohmura T, Iwamoto H, Nakayama K, et al. Incidence and risk factors of vascular dementia and Alzheimer’s disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45:1161–8. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 52.Razay G, Vreugdenhil A, Wilcock G. Obesity, abdominal obesity and Alzheimer disease. Dement Geriatr Cogn Disord. 2006;22:173–6. doi: 10.1159/000094586. [DOI] [PubMed] [Google Scholar]
- 53.Elmquist JK, Flier JS. Neuroscience. The fat-brain axis enters a new dimension. Science. 2004;304:63–4. doi: 10.1126/science.1096746. [DOI] [PubMed] [Google Scholar]
- 54.Schaffler A, Binart N, Scholmerich J, Buchler C. Hypothesis paper Brain talks with fat--evidence for a hypothalamic-pituitary-adipose axis? Neuropeptides. 2005;39:363–7. doi: 10.1016/j.npep.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 55.Harvey J, Shanley LJ, O’Malley D, Irving AJ. Leptin: a potential cognitive enhancer? Biochem Soc Trans. 2005;33:1029–32. doi: 10.1042/BST20051029. [DOI] [PubMed] [Google Scholar]
- 56.Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr. 1998;68:1157–73. doi: 10.1093/ajcn/68.6.1157. [DOI] [PubMed] [Google Scholar]
- 57.El-Atat F, Aneja A, McFarlane S, Sowers J. Obesity and hypertension. Endocrinol Metab Clin North Am. 2003;32:823–54. doi: 10.1016/s0889-8529(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 58.Hall JE, Jones DW, Kuo JJ, da Silva A, Tallam LS, Liu J. Impact of the obesity epidemic on hypertension and renal disease. Curr Hypertens Rep. 2003;5:386–92. doi: 10.1007/s11906-003-0084-z. [DOI] [PubMed] [Google Scholar]
- 59.Najman DM, Kapoor P, Serrano A, Tckachenko D. Hypertension and obesity. Arch Intern Med. 2003;163:1114–5. doi: 10.1001/archinte.163.9.1114-b. author reply 15-6. [DOI] [PubMed] [Google Scholar]
- 60.Burns M, Duff K. Cholesterol in Alzheimer’s disease and tauopathy. Ann N Y Acad Sci. 2002;977:367–75. doi: 10.1111/j.1749-6632.2002.tb04839.x. [DOI] [PubMed] [Google Scholar]
- 61.Geula C, Farlow M, Cummings J, Morris J, Scheltens PRA. Alzheimer’s disease: Translating neurochemical insights into chemical benefits. Journal of Clinical Psychiatry. 2000;61:791–802. doi: 10.4088/jcp.v61n1012. [DOI] [PubMed] [Google Scholar]
- 62.Kuriyama M, Takahashi K, Yamano T, Hokezu Y, Togo S, Osame M, et al. Low levels of serum apolipoprotein A I and A II in senile dementia. Jpn J Psychiatry Neurol. 1994;48:589–93. doi: 10.1111/j.1440-1819.1994.tb03019.x. [DOI] [PubMed] [Google Scholar]
- 63.Muckle TJ, Roy JR. High-density lipoprotein cholesterol in differential diagnosis of senile dementia. Lancet. 1985;1:1191–3. doi: 10.1016/s0140-6736(85)92866-1. [DOI] [PubMed] [Google Scholar]
- 64.Kuriyama M, Hokezu Y, Togo S, Nagata K, Takahashi K, Igakura T, et al. Serum lipids, lipoproteins and apolipoproteins in patients with senile dementia. Nippon Ronen Igakkai Zasshi. 1992;29:559–64. doi: 10.3143/geriatrics.29.559. [DOI] [PubMed] [Google Scholar]
- 65.Klich-Raczka A, Necki M, Wizner B, Baron T, Adamkiewicz-Piejko A, Gryglewska B, et al. Vascular dementia and systemic changes. Przegl Lek. 2002;59:269–71. [PubMed] [Google Scholar]
- 66.Wieringa GE, Burlinson S, Rafferty JA, Gowland E, Burns A. Apolipoprotein E genotypes and serum lipid levels in Alzheimer’s disease and multi-infarct dementia. Int J Geriatr Psychiatry. 1997;12:359–62. [PubMed] [Google Scholar]
- 67.Scacchi R, De Bernardini L, Mantuano E, Vilardo T, Donini LM, Ruggeri M, et al. DNA polymorphisms of apolipoprotein B and angiotensin I-converting enzyme genes and relationships with lipid levels in Italian patients with vascular dementia or Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9:186–90. doi: 10.1159/000017045. [DOI] [PubMed] [Google Scholar]
- 68.Lesser G, Kandiah K, Libow LS, Likourezos A, Breuer B, Marin D, et al. Elevated serum total and LDL cholesterol in very old patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2001;12:138–45. doi: 10.1159/000051248. [DOI] [PubMed] [Google Scholar]
- 69.Michikawa M. Cholesterol paradox: is high total or low HDL cholesterol level a risk for Alzheimer’s disease? J Neurosci Res. 2003;72:141–6. doi: 10.1002/jnr.10585. [DOI] [PubMed] [Google Scholar]
- 70.Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61:705–14. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama. 2004;292:2237–42. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 72.Martins IJ, Hone E, Foster JK, Sunram-Lea SI, Gnjec A, Fuller SJ, et al. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol Psychiatry. 2006;11:721–36. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 73.Muller M, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dement Geriatr Cogn Disord. 2007;24:185–92. doi: 10.1159/000105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vanhanen M, Koivisto K, Moilanen L, Helkala EL, Hanninen T, Soininen H, et al. Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology. 2006;67:843–7. doi: 10.1212/01.wnl.0000234037.91185.99. [DOI] [PubMed] [Google Scholar]
- 75.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 76.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–8. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 77.van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: the FINE Study. Neurology. 2004;63:2316–21. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- 78.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. Jama. 2004;292:1454–61. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 79.Lytle ME, Vander Bilt J, Pandav RS, Dodge HH, Ganguli M. Exercise level and cognitive decline: the MoVIES project. Alzheimer Dis Assoc Disord. 2004;18:57–64. doi: 10.1097/01.wad.0000126614.87955.79. [DOI] [PubMed] [Google Scholar]
- 80.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–51. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 81.Schuit AJ, Feskens EJ, Launer LJ, Kromhout D. Physical activity and cognitive decline, the role of the apolipoprotein e4 allele. Med Sci Sports Exerc. 2001;33:772–7. doi: 10.1097/00005768-200105000-00015. [DOI] [PubMed] [Google Scholar]
- 82.Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–16. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 83.Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 84.Prince M, Lewis G, Bird A, Blizard R, Mann A. A longitudinal study of factors predicting change in cognitive test scores over time, in an older hypertensive population. Psychol Med. 1996;26:555–68. doi: 10.1017/s0033291700035637. [DOI] [PubMed] [Google Scholar]
- 85.Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56:425–30. doi: 10.1001/archpsyc.56.5.425. [DOI] [PubMed] [Google Scholar]
- 86.Cerhan JR, Folsom AR, Mortimer JA, Shahar E, Knopman DS, McGovern PG, et al. Correlates of cognitive function in middle-aged adults. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Gerontology. 1998;44:95–105. doi: 10.1159/000021991. [DOI] [PubMed] [Google Scholar]
- 87.Rogers RL, Meyer JS, Mortel KF. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc. 1990;38:123–8. doi: 10.1111/j.1532-5415.1990.tb03472.x. [DOI] [PubMed] [Google Scholar]
- 88.Dustman RE, Ruhling RO, Russell EM, Shearer DE, Bonekat HW, Shigeoka JW, et al. Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiol Aging. 1984;5:35–42. doi: 10.1016/0197-4580(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 89.Spirduso WW. Physical fitness, aging, and psychomotor speed: a review. J Gerontol. 1980;35:850–65. doi: 10.1093/geronj/35.6.850. [DOI] [PubMed] [Google Scholar]
- 90.Gomez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764:1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- 91.Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exerc Sport Sci Rev. 2002;30:75–9. doi: 10.1097/00003677-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 92.Wilson RS, Mendes De Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry. 2004;75:126–9. [PMC free article] [PubMed] [Google Scholar]
- 93.Wilson RS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JS, Bach J, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59:364–70. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 94.Dufouil C, Fuhrer R, Dartigues JF, Alperovitch A. Longitudinal analysis of the association between depressive symptomatology and cognitive deterioration. Am J Epidemiol. 1996;144:634–41. doi: 10.1093/oxfordjournals.aje.a008974. [DOI] [PubMed] [Google Scholar]
- 95.Henderson AS, Korten AE, Jacomb PA, Mackinnon AJ, Jorm AF, Christensen H, et al. The course of depression in the elderly: a longitudinal community-based study in Australia. Psychol Med. 1997;27:119–29. doi: 10.1017/s0033291796004199. [DOI] [PubMed] [Google Scholar]
- 96.Bassuk SS, Berkman LF, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Arch Gen Psychiatry. 1998;55:1073–81. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- 97.Geerlings MI, Schoevers RA, Beekman AT, Jonker C, Deeg DJ, Schmand B, et al. Depression and risk of cognitive decline and Alzheimer’s disease. Results of two prospective community-based studies in The Netherlands. Br J Psychiatry. 2000;176:568–75. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- 98.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–7. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 99.Buchman AS, Schneider JA, Wilson RS, Bienias JL, Bennett DA. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67:1949–54. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- 100.Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. J Am Geriatr Soc. 1996;44:1147–52. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 101.Kretsch MJ, Green MW, Fong AK, Elliman NA, Johnson HL. Cognitive effects of a long-term weight reducing diet. Int J Obes Relat Metab Disord. 1997;21:14–21. doi: 10.1038/sj.ijo.0800353. [DOI] [PubMed] [Google Scholar]
- 102.Morris CH, Hope RA, Fairburn CG. Eating habits in dementia. A descriptive study. Br J Psychiatry. 1989;154:801–6. doi: 10.1192/bjp.154.6.801. [DOI] [PubMed] [Google Scholar]
- 103.Cooper JK, Mungas D. Serotonin response in sweet-food craving Alzheimer’s disease subjects. Aging (Milano) 1992;4:165–9. doi: 10.1007/BF03324086. [DOI] [PubMed] [Google Scholar]
- 104.Mungas D, Cooper JK, Weiler PG, Gietzen D, Franzi C, Bernick C. Dietary preference for sweet foods in patients with dementia. J Am Geriatr Soc. 1990;38:999–1007. doi: 10.1111/j.1532-5415.1990.tb04423.x. [DOI] [PubMed] [Google Scholar]
- 105.Wang PN, Yang CL, Lin KN, Chen WT, Chwang LC, Liu HC. Weight loss, nutritional status and physical activity in patients with Alzheimer’s disease. A controlled study. J Neurol. 2004;251:314–20. doi: 10.1007/s00415-004-0316-4. [DOI] [PubMed] [Google Scholar]
- 106.Nourhashemi F, Andrieu S, Gillette-Guyonnet S, Reynish E, Albarede JL, Grandjean H, et al. Is there a relationship between fat-free soft tissue mass and low cognitive function? Results from a study of 7,105 women. J Am Geriatr Soc. 2002;50:1796–801. doi: 10.1046/j.1532-5415.2002.50507.x. [DOI] [PubMed] [Google Scholar]
- 107.Strawbridge WJ, Shema SJ, Balfour JL, Higby HR, Kaplan GA. Antecedents of frailty over three decades in an older cohort. J Gerontol B Psychol Sci Soc Sci. 1998;53:S9–16. doi: 10.1093/geronb/53b.1.s9. [DOI] [PubMed] [Google Scholar]
- 108.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–8. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 109.APA. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 110.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 111.World Health Organization. The ICD-10 Classification of Mental and Behavioral Disorders: Diagnostic Criteria for Research. World Health Organization; Geneva, Switzerland: 1993. [Google Scholar]
- 112.Wetterling T, Kanitz RD, Borgis KJ. Comparison of different diagnostic criteria for vascular dementia (ADDTC, DSM-IV, ICD-10, NINDS-AIREN) Stroke. 1996;27:30–6. doi: 10.1161/01.str.27.1.30. [DOI] [PubMed] [Google Scholar]
- 113.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. American Psychiatric Association; Washington, DC: 1987. Revised. [Google Scholar]
- 114.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–60. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 115.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–80. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]