Abstract
Objectives
Among patients with atrial fibrillation (AF), female gender has been associated with an increased risk of stroke and a decreased likelihood of receiving oral anticoagulant therapy (OAT). The elderly are less likely to receive OAT due to concerns about falling and frailty. This study assessed the appropriateness of OAT among women and the elderly, looking for patterns of under-treatment or unnecessary treatment.
Design
Retrospective cohort study.
Setting
Primary care practices of an academic healthcare system.
Participants
1,585 adults with non-valvular AF seen between March 2013 and February 2014.
Intervention
Treatment recommendations were made by an Atrial Fibrillation Decision Support Tool (AFDST) based on projections for quality-adjusted life expectancy calculated by a decision analytic model that integrates patient-specific risk factors for stroke and hemorrhage.
Results
Treatment was discordant from AFDST-recommended treatment in 45% (326/725) of women and in 39% (338/860) of men (p = 0.02). While current treatment was discordant from recommended in 35% (89/258) of the elderly (age ≥ 85), and in 43% (575/1328) of patients < 85 years of age (p = 0.01) a large proportion of under-treated elderly patients were receiving aspirin as the sole antithrombotic agent.
Conclusion
Physicians should understand that female gender is a significant risk factor for AF-related stroke and incorporate this into decision-making about thromboprophylaxis. By treating older patients with aspirin instead of oral anticoagulant therapy we are exposing them to a significant risk of bleeding with little to no benefit relative to reduction of AF-related stroke risk.
Keywords: atrial fibrillation, anticoagulation, gender differences, elderly, warfarin, novel oral anticoagulants, aspirin
INTRODUCTION
With the aging of the U.S. population, the number of individuals with atrial fibrillation (AF) will increase substantially from over 2.2 million to more than 3 million by the year 2020.1 The contribution of AF to the burden of stroke also increases with age. In the Framingham study, 23.5% of strokes in individuals ≥ 80 years of age were due to AF.2 It is also worth noting that due to the higher risk of AF-related stroke in the elderly, the impact of anticoagulation on the absolute risk reduction of stroke is greater in the elderly than in younger patients.
Surveys exploring barriers to optimal anticoagulation have identified numerous issues. Beyth and colleagues noted that physicians were less likely to prescribe warfarin for older patients.3 Others have shown that advanced age, female gender, and rural residency were associated with underuse of oral anticoagulant therapy.4 The therapeutic options for oral anticoagulant therapy (OAT) have evolved over time from vitamin K oral anticoagulants such as warfarin to a number of novel agents only recently approved for use in the United States by the FDA (dabigatran, rivaroxaban, apixaban, and edoxaban). The reasons for under-treatment of women are unclear, but likely relate to an incorrect perception that women are at lower risk of stroke.5 The pivotal physician-related factor seems to be an insufficiently balanced evaluation of the risk versus benefit of oral anticoagulant therapy. A recent systematic review comparing current treatment practices for stroke prevention in AF with a variety of published guidelines, including those of the American College of Chest Physicians (ACCP), and the American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS), showed underuse of OAT in high risk patients in the majority of 54 studies reviewed.6 Among patients in 29 studies with a history of prior stroke or transient ischemic attack (TIA) who should all be receiving anticoagulant therapy, treatment levels averaged less than 60% (range 19% - 81.3%). Among high risk patients with a CHADS2 score ≥ 2 treatment levels averaged less than 70% (range 39% - 92.3%). While there has been a trend towards improvement in utilization of anticoagulant therapy over the past decade, a recently published study of community-based practices in the Christiana Care Health System in northern Delaware continued to show substantial underutilization with almost one-third of high risk patients (CHADS2 score ≥ 2) never receiving anticoagulant therapy despite the absence of identified barriers to such treatment.7 In an analysis of predictors of warfarin use among the 1,141 patients studied there was a trend for men to be more likely to receive treatment than women. In an analysis of predictors of warfarin interruptions, there was a trend towards an increased risk in women.
Middle-aged women have been found to be at significantly increased risk of death from AF.8 The addition of female gender to AF-related stroke risk scoring systems such as the CHA2DS2VASc underscores the observation that female gender is a risk modifier for AF-related stroke in the presence of additional stroke risk factors. That being said, the risk of stroke in women with AF is underappreciated by clinicians.9 Furthermore, studies have shown that populations of women at equivalent risk of stroke are less likely to receive anticoagulant therapy than men.10
There also is evidence that women, particularly older women, are less likely to receive OAT for AF.5, 11, 12 Fang and colleagues found that women not taking oral anticoagulants were at higher risk for stroke than men at both younger and older ages, with an adjusted relative risk of 1.6 (95% CI – 1.0 – 2.3) and 1.8 (95% CI – 1.4 – 2.3) respectively among patients ≤ 75 years of age and > 75 years of age.13
Others have found that older patients are less likely to receive anticoagulant therapy.14 Despite improvements over the past decade, still fewer than half of eligible elderly patients are receiving warfarin.15, 16 Surveys of physicians cite fall risk, dementia, and non-adherence as factors in elderly patients that deter them from prescribing warfarin in this population of patients.17-19
With the recent approval and availability of 4 non-vitamin K antagonist oral anticoagulants (NOACs), concerns have been raised regarding the safety of these agents in the elderly. Reasons for such concerns include the high frequency of renal impairment, low body mass index in the frail and most-elderly, and altered body composition of muscle and fat.20 There have been a number of reports of increased bleeding risk among the elderly receiving treatment with dabigatran.21-23 While no randomized trials have specifically examined the efficacy and safety of the NOACs in the elderly, a meta-analysis of 10 randomized trials (RCTs) of more than 25,000 patients ≥75 years, the risk of major bleeding was not significantly different between the NOACs and conventional therapy, usually warfarin. In the 4 trials involving patients with AF, NOACs were more effective than warfarin in the prevention of stroke in the elderly.24
This study is aimed at producing a better understanding of the appropriateness of antithrombotic therapy* for thromboprophylaxis among women and the elderly, looking for patterns of either under-treatment or unnecessary treatment. It also provides some insight into the process of generating patient-specific reports regarding current and optimal anticoagulation therapy in an academic healthcare system. Our hypothesis was that in our cohort of AF patients we would find that women and older patients are under-treated with OAT compared with the most recent recommendations of the AHA/ACC/HRS.25 We selected this guideline since it was both the most recent AF guideline released in the United States (March 2014), and it utilizes the more recently developed CHA2DS2VASc for determining AF-related stroke risk. We also hypothesized the same would be true even after accounting for AF-related stroke risk and bleeding risk on anticoagulant therapy, using an Atrial Fibrillation Decision Support Tool (AFDST) developed at our institution.
METHODS
We conducted a retrospective cohort study of 1,585 adults, ages 28-93, with non-valvular AF or flutter seen in ambulatory primary care practices of an academic healthcare system serving the Cincinnati tristate region. We used our health system’s clinical data store to identify 9,270 patients with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), diagnosis of atrial fibrillation (427.31) or atrial flutter (427.32) who did not have diagnoses of mitral valve disease (394.x), aortic valve disease (395.x), heart valve transplant (V42.2) or heart valve replacement (V42.3). We abstracted one year of data at the beginning of March 2014 (March 1, 2013-February 28, 2014). During this one-year period of time, 4,021 AF patients were seen in any of the outpatient practices in our health system, and 1,585 were seen in the primary care network (PCN). The institutional review board at the University of Cincinnati approved this study.
Information needed to calculate stroke risk using CHA2DS2VASc26, major hemorrhage using HAS-BLED27, and intracerebral hemorrhage (ICH)27, was extracted from the clinical data store using the active problem list and a combination of laboratory values and clinical measurements. Current antithrombotic therapy was retrieved from the active medication list. Data were stored on a secure server at our Center for Health Informatics as discrete elements hosted on MYSQL™. SAS data files were created as necessary for statistical analyses using unique coded patient identifiers. Further details are described separately.28
Treatment recommendations were made by an Atrial Fibrillation Decision Support Tool (AFDST) which we developed. The computational engine of the AFDST is a patient-specific decision analytic model comprised of a 29-state Markov simulation which projects quality-adjusted life expectancy (QALE) for each of 3 strategies - 1) no antithrombotic therapy, 2) aspirin, and 3) oral anticoagulant therapy (warfarin in the base case) for each individual patient.29 The decision model uses information from the EHR to integrate patient-specific risk factors for stroke and hemorrhage in its calculations.28, 29 Decision model construction and analysis was done using a standard computer program (Decision Maker, Boston, Massachusetts). Further details of the patient-specific decision analytic model are described separately.28 While the decision analytic model used efficacy and bleeding risk data from warfarin for the oral anticoagulation strategy, the AFDST recommendation was for any oral anticoagulant if the warfarin strategy was better than either no antithrombotic therapy or aspirin. Thus, the AFDST was slightly conservative in its recommendations for OAT, given more recent data regarding the efficacy and bleeding risks associated with recently approved novel oral anticoagulants (NOACs). Finally, while recommendations of guidelines promulgated by the ACCP and the AHA/ACC/HRS are based solely upon stroke risk, the AFDST quantitatively incorporates both AF-related stroke and bleeding risk. SAS was used to perform simple descriptive statistical analyses and to develop multivariable regression models. All reported p-values are derived from models in which the provider is a random factor and denominator degrees of freedom are based on numbers of patients. The study alpha was a two-tailed p = .05, unadjusted for multiple tests.
RESULTS
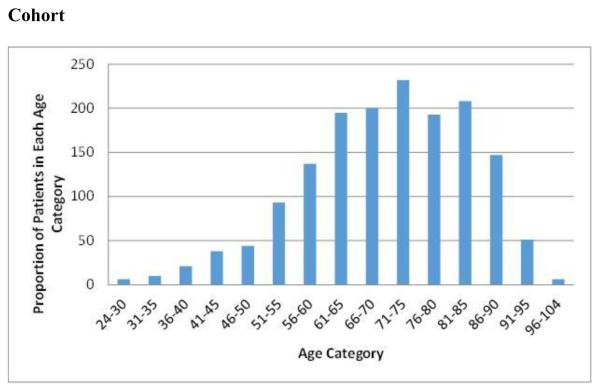
Characteristics for the 1,585 patients in the study are described in Table 1. Patients’ ages ranged between 28 years and 93 years, with a mean age of 70.5 years. Figure 1 shows the distribution of age across 5-year increments. Forty-six percent of patients were women. The mean CHA2DS2VASc and HAS-BLED scores were 3.7 and 2.1, respectively. Fifty percent of the cohort was receiving some form of OAT, most commonly warfarin (see Table 1). Only a small proportion of patients (11%) were receiving any of the non-vitamin K oral anticoagulants. There was a trend towards lower levels of OAT in women than in men but the difference was not statistically significant, 48% (347/726) and 52% (449/860) (p = 0.06), respectively.
Table 1.
Patient Characteristics
| Number | 1,585 |
| Age [mean, (SD)] | 70.5 (13.4) |
| Female [n, (%)] | 726 (46) |
| CHA2DS2VASc [mean, (SD)] | 3.7 (1.9) |
| HAS-BLED [mean, (SD)] | 2.1 (1.2) |
| Receiving oral anticoagulant therapy [n, (%)] † |
797 (50) |
| Current treatment discordant from AFDST-recommended‡ treatment (n, %) |
664 (42) |
| Current Treatment | n, (%) |
| No antithrombotic therapy | 214 (14) |
| Aspirin only | 575 (36) |
| Warfarin | 611 (39) |
| Aspirin/Clopidogrel | 1 |
| Aspirin/Prasugrel | 1 |
| Dabigatran | 66 (4) |
| Rivaroxaban | 83 (5) |
| Apixaban | 27 (2) |
Warfarin, dabigatran, rivaroxaban, apixaban, edoxaban or aspirin/clopidogrel or prasugrel combination.
AFDST – Atrial Fibrillation Decision Support Tool.
Figure 1. Distribution of Patient Ages in Atrial Fibrillation Cohort.
Five-year age categories are displayed on the x-axis, while the y-axis reports the proportion of patients in each age category in our atrial fibrillation cohort. The mean age is 70.5.
The AFDST recommended OAT for 1,374 (87%) patients, aspirin for 65 (4%), and no antithrombotic therapy for 146 (9%) patients. Overall, antithrombotic therapy was concordant with AFDST recommendations in 58% of patients. Fifty-seven percent of patients for whom OAT or aspirin was recommended were receiving such treatment (788 and 37, respectively), while 64% of those for whom no antithrombotic therapy was recommended (94) were receiving no antithrombotic therapy. Antithrombotic therapy was discordant from that recommended by the AFDST in 42% of patients. Thirty-seven percent of patients for whom the tool recommended OAT were receiving only aspirin or no antithrombotic therapy; 2% of patients for whom the tool recommended aspirin were receiving either no antithrombotic therapy or OAT; and 3% of patients for whom the tool recommend no antithrombotic therapy were receiving either aspirin or OAT.
As shown at the bottom of Table 2a, current treatment was discordant from recommended treatment in 45% (326/725) of women and in 39% (338/860) of men (p = 0.02). In addition, 44% (321/725) of women were “under-treated,” receiving aspirin or no OAT when OAT was recommended, while only 31% (263/860) of men were “under-treated” (p < 0.0001). Thus, women were 1.8 times more likely to be under-treated than men. The most recent update of the American Heart Association/American College of Cardiology/Heart Rhythm Society (AHA/ACC/HRS) guideline for antithrombotic therapy in patients with AF recommends OAT for CHA2DS2VASc scores ≥2, no antithrombotic therapy for a CHA2DS2VASc of 0, and states that any therapy is reasonable for a CHA2DS2VASc of 125. However, women with a CHA2DS2VASc of 1 (i.e., whose only risk factor is female gender) are at very low risk for stroke and no antithrombotic treatment is an appropriate choice, as recommended in European guidelines.30 Comparing current treatment to the most recent AHA/ACC/HRS guideline-based therapy†, 51% (370/725) of women and 37% (317/860) of men had treatment that was discordant with the guideline (p < 0.0001), resulting in an even greater disparity between the sexes (see Table 2b).
Table 2a.
Type of discordance among men and women with treatment discordant from AFDST‡.
| AFDST Recommended Treatment |
Current Treatment | Women | Men | ||
|---|---|---|---|---|---|
| Number of Patients (n) |
% | Number of Patients (n) |
% | ||
| Oral Anticoagulant Therapy |
None | 78 | 24% | 53 | 16% |
| Aspirin | 243 | 75% | 210 | 62% | |
| Aspirin | None | 1 | 0% | 11 | 3% |
| Oral Anticoagulant Therapy |
2 | 1% | 14 | 4% | |
| None | Aspirin | 2 | 1% | 24 | 7% |
| Oral Anticoagulant Therapy |
0 | 0% | 26 | 8% | |
| Total | 326 | 338 | |||
AFDST – Atrial Fibrillation Decision Support Tool.
Table 2b.
Type of discordance among men and women with treatment discordant from guidelines.
| Treatment Recommended by AHA/ACC/HRS Guideline |
Current Treatment |
Women | Men | ||
|---|---|---|---|---|---|
| Number of Patients (n) |
% | Number of Patients (n) |
% | ||
| Oral Anticoagulant Therapy† |
None | 83 | 22% | 57 | 18% |
| Aspirin | 258 | 70% | 231 | 73% | |
| None‡ | Aspirin | 21 | 6% | 22 | 7% |
| Oral Anticoagulant Therapy |
8 | 2% | 7 | 2% | |
| Total | 370 | 317 | |||
AHA/ACC/HRS indicates American Heart Association/American College of Cardiology/Heart Rhythm Society.
Oral anticoagulant therapy recommended for CHA2DS2VASc score ≥2.
No antithrombotic therapy recommended for CHA2DS2VASc score=0; or 1 in women without additional risk factors.
In non-stratified analyses, there was no significant difference in the use of OAT in older and younger patients; 55% (141/258) of patients 85 years of age or older were receiving OAT compared with 49% (654/1328) of those < 85 years of age (p = 0.13). As shown at the bottom of Table 3, among the very elderly (age ≥ 85) current treatment was discordant from recommended treatment in 35% (89/258), while treatment was discordant among 43% (575/1328) of patients < 85 years of age (p < 0.01). Similar proportions of patients under 85 years of age, 37% (497/1328) and ≥ 85 years of age,” 34% (87/258) were “under-treated (p = 0.30). Aspirin use was no different between patients ≥ 85 and < 85 years of age (36%).
Table 3.
Type of discordance among patients < 85 or ≥ 85 years of age with treatment discordant from AFDST‡.
| AFDST Recommended Treatment |
Current Treatment | Age < 85 | Age ≥ 85 | ||
|---|---|---|---|---|---|
| Number of Patients (n) |
% | Number of Patients (n) |
% | ||
| Oral Anticoagulant Therapy |
None | 113 | 20% | 18 | 20% |
| Aspirin | 384 | 67% | 69 | 78% | |
| Aspirin | None | 12 | 2% | 0 | 0% |
| Oral Anticoagulant Therapy |
16 | 3% | 0 | 0% | |
| None | Aspirin | 25 | 4% | 1 | 1% |
| Oral Anticoagulant Therapy |
25 | 4% | 1 | 1% | |
| Total | 575 | 89 | |||
AFDST – Atrial Fibrillation Decision Support Tool.
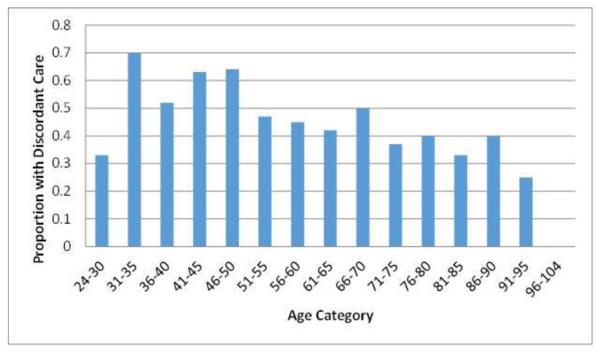
We further examined age categories in 5-year increments and found that discordant therapy was as high as 60-70% in those between the ages of 31 and 50 (see Figure 2).
Figure 2. Proportion of Patients with Discordant Care by Age Category.
Five-year age categories are displayed on the x-axis, while the y-axis reports the proportion of patients in each age category who had treatment that was discordant from the recommendations of the Atrial Fibrillation Decision Support Tool (AFDST). Among patients between the ages of 31 and 50, the proportion with discordant care ranges between 50% and 70.
Table 2a describes in what manner treatment was discordant among women and men compared with therapy recommended by the AFDST. Among 326 women with discordant treatment 99% (322/326) was due to under-treatment and 1% (4/326) was due to over-treatment (patients receiving either aspirin or OAT when no antithrombotic therapy was recommended, or OAT when only aspirin was recommended). Among 338 men with discordant treatment 81% (274/338) was due to under-treatment, while 19% (64/338) was due to over-treatment.
Table 3 describes in what manner treatment was discordant among patients <85 and ≥85 years of age. Among 89 elderly patients with discordant treatment, 98% (87/89) of discordance was due to under-treatment and 2% (2/89) was due to over-treatment; whereas in those < 85 years of age, 88% (509/575) was due to under-treatment and 12% (66/575) was due to over-treatment.
We developed a multivariable logistic regression model to explore the impact of gender and age in order to investigate the hypothesis that female gender and advanced age were associated with an increased risk of discordant therapy. While the impact of female gender supported our initial hypothesis (Beta coefficient 0.36, p = 0.0007), we were surprised to find the opposite effect of increasing age (Beta coefficient −0.024, p < 0.0001).
DISCUSSION
In our study of 1,585 adults, similar proportions of women and men were receiving OAT. However, when looking at discordance between actual treatment and recommendations of either an Atrial Fibrillation Decision Support Tool or the most recent AHA/ACC/HRS guideline, women with AF continue to receive less aggressive treatment to prevent stroke.
The sad irony is that female gender is associated with an increased risk of AF-related stroke. Thus, for any given set of risk factors, a woman has a higher risk of AF-related stroke than a man. While previously published studies have come to varying conclusions with regards to the presence or absence of gender differences in antithrombotic therapy for patients with AF4, 5, 11-13, 31, 32,33, our study adds to the more contemporary evidence that gender differences in treatment are still occurring in some academic medical center settings, and we must remain vigilant in addressing them.
Numerous past studies have found advanced age to be a predictor of decreased use of oral anticoagulants.14, 31-36 This is an issue since a growing proportion of patients with AF are elderly. It is estimated that by the year 2050, 50% of the roughly 5.6 million people with AF will be over the age of 80 years.1 Clearly, physicians have concerns about frailty and fall risk, cognitive deficits that might interfere with medication adherence, polypharmacy, and an increased risk of intracranial hemorrhage among others in the elderly. However, given the generally increased risk of AF-related stroke in older patients, the absolute gain in decreased stroke risk afforded by OAT may be greater in the elderly. In our cohort, we found no significant difference in the use of OAT in patients older or younger than 85 years of age. However, this does address whether such treatment is appropriate or not. When we looked at treatment compared with the recommendations of the AFDST, which takes both stroke and bleeding risk into account, we found similar proportions of patients older or younger than 85 years of age were under-treated. However, of some concern, a significant proportion of undertreated elderly patients were receiving aspirin as the sole antithrombotic agent. In interviews with physicians trying to understand why AFDST or guideline-appropriate therapy was not being given to these under-treated elderly patients, physicians cited concerns about bleeding risk. They felt that they “needed to do something” and that while aspirin is less effective than OAT, it has a lower associated bleeding risk. Recent data evaluating the efficacy of aspirin has shown a declining efficacy with increasing age, such that over the age of 77 years, aspirin has minimal if any impact on reducing AF-related stroke risk.37 Furthermore, bleeding risk associated with aspirin is often underappreciated. The net result of treating elderly patients with aspirin is that we expose them to a significant risk of major hemorrhage while providing virtual no benefit in terms of stroke prevention. In 2007, the Birmingham Atrial Fibrillation Treatment of the Aged Study (BAFTA) of 973 patients aged 75 years or older, randomized to treatment with either warfarin or aspirin (75 mg), found the relative risk of stroke among patients receiving warfarin was 0.46 (95% CI, 0.26-0.79) compared with aspirin.38 Furthermore, rates of major hemorrhage, including intracranial and extracranial bleeds, were not different between the warfarin- and aspirin-treated arms of the study. It has been proposed that the lack of difference in rates of major bleeding may have been due to selection bias in that over four-fifths of recruited patients were already taking either aspirin or warfarin. Thus, having already “survived” exposure to antithrombotic therapy, they may have been a population pre-selected to be at lower risk of major bleeding.39 Nonetheless, anticoagulation of elderly patients with AF is beneficial unless there are compelling contraindications or the patient decides that the benefits are not worth the risk and hassle.
Four NOACs have been recently approved for use in the United States for AF.40-43 While they all possess somewhat different profiles, in general they are equally if not more effective than warfarin, and generally have a lower risk of major hemorrhage, in particular, intracerebral hemorrhage, and do not require routine monitoring.44 Clinicians have questioned the generalizability of these results to a more elderly population. A recent meta-analysis evaluated evidence from 10 randomized controlled trials focusing on an elderly population of patients ≥ 75 years of age.24 In the 4 trials of patients with AF, the NOACs were more effective than warfarin in preventing stroke in the elderly. In the full meta-analysis of all 10 studies, NOACs did not cause excess bleeding in the elderly. Similar results were noted in a summary of subgroup analyses of elderly patients in the phase 3 RCTs comparing warfarin with NOAC therapy.45 Perhaps the NOACs can fill the niche now occupied inappropriately by aspirin therapy for elderly patients with AF.
Finally, contrary to our initial hypothesis, a larger proportion of patients under the age of 85 were receiving treatment that was discordant from the recommendation of the decision support tool. This was due largely to over-treatment of younger patients between the ages of 31 and 50.
CONCLUSIONS
We found that women in our cohort of AF patients receiving primary care in an integrated healthcare system of an academic medical center are regularly under-treated with antithrombotic therapy. Somewhat surprisingly, compared with older patients, a larger proportion of patients < 85 years of age are receiving treatment that is discordant from recommended therapy. Furthermore, in women and the elderly the major reason for discordant therapy is under-treatment; whereas in men and younger patients, a larger proportion of discordance is due to over-treatment.
What actions should these findings prompt? Physicians should understand that female gender is a significant and independent risk factor for AF-related stroke and factor this into their decision-making about thromboprophylaxis. While there are many reasons to be circumspect about treating older patients with OAT, we are under-treating these patients. Furthermore, in our efforts to avoid harm by treating older patients with aspirin instead of OAT we are actually exposing them to a significant risk of bleeding with little to no benefit relative to reduction of AF-related stroke risk. Finally, although they comprise a small proportion of patients with AF, we are over-treating young patients who are at minimal risk of AF-related stroke.
ACKNOWLEDGMENTS
Funding Source: Support for this study came from the Pfizer Educational Group, Bristol-Myers Squibb/Pfizer Education Consortium, and NIH/NCATS grant 8 UL1 TR000077-05.
Sponsor’s Role: The funding sources had no role in study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Biography
Gregory Y.H. Lip has served as a consultant for Bayer, Astellas, Merck, Sanofi, BMS/Pfizer, Daiichi-Sankyo, Biotronik, Medtronic, Portola and Boehringer Ingelheim and has been on the speakers bureau for Bayer, BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic and Sanofi Aventis.
Matthew L. Flaherty has served as a consultant to Boehringer Ingelheim, and has served on an advisory board for, as a consultant to, and on a speaker’s program for CSL Behring.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Mark H. Eckman, Gregory Y.H. Lip, Ruth E. Wise, Matthew L. Flaherty, Dawn Kleindorfer, Carlos Aguilar, Anthony Leonard, Alexandru Costea, John Kues participated in the conception and design of the project; Megan Sullivan, Lora Arduser, Nita Walker, Barbara Speer, John Kues, Peter Baker, Robert Ireton, Dave Hoskins, Brett M. Harnett were responsible for acquisition of data; Mark H. Eckman, Anthony Leonard, Lora Arduser were responsible for data analysis and interpretation; Mark H. Eckman drafted the original article; Mark H. Eckman, Gregory Y.H. Lip, Ruth E. Wise, Barbara Speer, Megan Sullivan, Nita Walker, Brett Kissela, Matthew L. Flaherty, Dawn Kleindorfer, Peter Baker, Robert Ireton, Dave Hoskins, Brett M. Harnett, Carlos Aguilar, Anthony Leonard, Lora Arduser, Dylan Steen, Alexandru Costea, John Kues contributed to critical revisions, intellectual content, and approved the final draft.
Antithrombotic therapy – includes oral anticoagulants and antiplatelet drugs (aspirin, clopidogrel, prasugrel).
Although the AHA/ACC/HRS guideline makes no special mention of women with CHA2DS2VASc scores of 1, whose only risk factor is female gender, we have used the European Guideline recommendation suggesting no antithrombotic therapy for these patients due to very low stroke risk.
Contributor Information
Mark H. Eckman, Division of General Internal Medicine and Center for Clinical Effectiveness, University of Cincinnati, Cincinnati, OH; Center for Health Informatics, University of Cincinnati, Cincinnati, OH.
Gregory Y.H. Lip, University of Birmingham Centre for Cardiovascular Sciences, Birmingham, United Kingdom of Great Britain and Northern Ireland.
Ruth E. Wise, Division of General Internal Medicine and Center for Clinical Effectiveness, University of Cincinnati, Cincinnati, OH.
Barbara Speer, Department of Family and Community Medicine, University of Cincinnati, Cincinnati, OH.
Nita Walker, Division of General Internal Medicine and Center for Clinical Effectiveness, University of Cincinnati, Cincinnati, OH.
Brett Kissela, Department of Neurology, University of Cincinnati, Cincinnati, OH.
Matthew L. Flaherty, Department of Neurology, University of Cincinnati, Cincinnati, OH.
Dawn Kleindorfer, Department of Neurology, University of Cincinnati, Cincinnati, OH.
Peter Baker, Center for Health Informatics, University of Cincinnati, Cincinnati, OH.
Robert Ireton, Center for Health Informatics, University of Cincinnati, Cincinnati, OH.
Dave Hoskins, Center for Health Informatics, University of Cincinnati, Cincinnati, OH.
Brett M. Harnett, Center for Health Informatics, University of Cincinnati, Cincinnati, OH.
Carlos Aguilar, Center for Health Informatics, University of Cincinnati, Cincinnati, OH.
Anthony Leonard, Department of Family and Community Medicine, University of Cincinnati, Cincinnati, OH.
Lora Arduser, Department of English, University of Cincinnati, Cincinnati, OH.
Dylan Steen, Division of Cardiology, University of Cincinnati, Cincinnati, OH.
Alexandru Costea, Division of Cardiology, University of Cincinnati, Cincinnati, OH.
John Kues, Department of Family and Community Medicine, University of Cincinnati, Cincinnati, OH.
REFERENCES
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, D'Agostino RB, Belanger AJ, et al. Probability of stroke: A risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 3.Beyth RJ, Antani MR, Covinsky KE, et al. Why isn't warfarin prescribed to patients with nonrheumatic atrial fibrillation? J Gen Intern Med. 1996;11:721–728. doi: 10.1007/BF02598985. [DOI] [PubMed] [Google Scholar]
- 4.Gage BF, Boechler M, Doggette AL, et al. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–827. doi: 10.1161/01.str.31.4.822. [DOI] [PubMed] [Google Scholar]
- 5.Humphries KH, Kerr CR, Connolly SJ, et al. New-onset atrial fibrillation: Sex differences in presentation, treatment, and outcome. Circulation. 2001;103:2365–2370. doi: 10.1161/01.cir.103.19.2365. [DOI] [PubMed] [Google Scholar]
- 6.Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: A systematic review. Am J Med. 2010;123:638–645. e634. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Ewen E, Zhang Z, Simon TA, et al. Patterns of warfarin use and subsequent outcomes in atrial fibrillation in primary care practices. Vasc Health Risk Manag. 2012;8:587–598. doi: 10.2147/VHRM.S34280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305:2080–2087. doi: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates SM, Greer IA, Hirsh J, et al. Use of antithrombotic agents during pregnancy: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:627S–644S. doi: 10.1378/chest.126.3_suppl.627S. [DOI] [PubMed] [Google Scholar]
- 10.Frost L, Johnsen SP, Pedersen L, et al. Atrial fibrillation or flutter and stroke: A Danish population-based study of the effectiveness of oral anticoagulation in clinical practice. J Intern Med. 2002;252:64–69. doi: 10.1046/j.1365-2796.2002.01009.x. [DOI] [PubMed] [Google Scholar]
- 11.Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: The anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–934. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 12.Sudlow M, Thomson R, Thwaites B, et al. Prevalence of atrial fibrillation and eligibility for anticoagulants in the community. Lancet. 1998;352:1167–1171. doi: 10.1016/S0140-6736(98)01401-9. [DOI] [PubMed] [Google Scholar]
- 13.Fang MC, Singer DE, Chang Y, et al. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: The AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) study. Circulation. 2005;112:1687–1691. doi: 10.1161/CIRCULATIONAHA.105.553438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCrory DC, Matchar DB, Samsa G, et al. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med. 1995;155:277–281. [PubMed] [Google Scholar]
- 15.Fang MC, Stafford RS, Ruskin JN, et al. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med. 2004;164:55–60. doi: 10.1001/archinte.164.1.55. [DOI] [PubMed] [Google Scholar]
- 16.McCormick D, Gurwitz JH, Goldberg RJ, et al. Prevalence and quality of warfarin use for patients with atrial fibrillation in the long-term care setting. Arch Intern Med. 2001;161:2458–2463. doi: 10.1001/archinte.161.20.2458. [DOI] [PubMed] [Google Scholar]
- 17.Bungard TJ, Ghali WA, McAlister FA, et al. Physicians' perceptions of the benefits and risks of warfarin for patients with nonvalvular atrial fibrillation. CMAJ. 2001;165:301–302. [PMC free article] [PubMed] [Google Scholar]
- 18.Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–46. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 19.Monette J, Gurwitz JH, Rochon PA, et al. Physician attitudes concerning warfarin for stroke prevention in atrial fibrillation: Results of a survey of long-term care practitioners. J Am Geriatr Soc. 1997;45:1060–1065. doi: 10.1111/j.1532-5415.1997.tb05967.x. [DOI] [PubMed] [Google Scholar]
- 20.Deedwania PC. New oral anticoagulants in elderly patients with atrial fibrillation. Am J Med. 2013;126:289–296. doi: 10.1016/j.amjmed.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Harper P, Young L, Merriman E. Bleeding risk with dabigatran in the frail elderly. N Engl J Med. 2012;366:864–866. doi: 10.1056/NEJMc1112874. [DOI] [PubMed] [Google Scholar]
- 22.Legrand M, Mateo J, Aribaud A, et al. The use of dabigatran in elderly patients. Arch Intern Med. 2011;171:1285–1286. doi: 10.1001/archinternmed.2011.314. [DOI] [PubMed] [Google Scholar]
- 23.Wychowski MK, Kouides PA. Dabigatran-induced gastrointestinal bleeding in an elderly patient with moderate renal impairment. Ann Pharmacother. 2012;46:e10. doi: 10.1345/aph.1Q747. [DOI] [PubMed] [Google Scholar]
- 24.Sardar P, Chatterjee S, Chaudhari S, et al. New oral anticoagulants in elderly adults: Evidence from a meta-analysis of randomized trials. J Am Geriatr Soc. 2014;62:857–864. doi: 10.1111/jgs.12799. [DOI] [PubMed] [Google Scholar]
- 25.January CT, Wann LS, Alpert JS, et al. AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014 doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: Nationwide cohort study. BMJ. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: The Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 28.Eckman MH, Wise RE, Speer B, et al. Integrating real-time clinical information to provide estimates of net clinical benefit of antithrombotic therapy for patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2014;7:680–686. doi: 10.1161/CIRCOUTCOMES.114.001163. [DOI] [PubMed] [Google Scholar]
- 29.Eckman MH, Singer DE, Rosand J, et al. Moving the tipping point: The decision to anticoagulate patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2011;4:14–21. doi: 10.1161/CIRCOUTCOMES.110.958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 31.Fang MC, Go AS, Hylek EM, et al. Age and the risk of warfarin-associated hemorrhage: The anticoagulation and risk factors in atrial fibrillation study. J Am Geriatr Soc. 2006;54:1231–1236. doi: 10.1111/j.1532-5415.2006.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quilliam BJ, Lapane KL. Clinical correlates and drug treatment of residents with stroke in long-term care. Stroke. 2001;32:1385–1393. doi: 10.1161/01.str.32.6.1385. [DOI] [PubMed] [Google Scholar]
- 33.Friberg L, Hammar N, Ringh M, et al. Stroke prophylaxis in atrial fibrillation: Who gets it and who does not? Report from the Stockholm Cohort-study on Atrial Fibrillation (SCAF-study) Eur Heart J. 2006;27:1954–1964. doi: 10.1093/eurheartj/ehl146. [DOI] [PubMed] [Google Scholar]
- 34.Hylek EM, D'Antonio J, Evans-Molina C, et al. Translating the results of randomized trials into clinical practice: The challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37:1075–1080. doi: 10.1161/01.STR.0000209239.71702.ce. [DOI] [PubMed] [Google Scholar]
- 35.Gallagher AM, Rietbrock S, Plumb J, et al. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: Do the appropriate patients receive stroke prophylaxis? J Thromb Haemost. 2008;6:1500–1506. doi: 10.1111/j.1538-7836.2008.03059.x. [DOI] [PubMed] [Google Scholar]
- 36.Perez I, Melbourn A, Kalra L. Use of antithrombotic measures for stroke prevention in atrial fibrillation. Heart. 1999;82:570–574. doi: 10.1136/hrt.82.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Walraven C, Hart RG, Connolly S, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation: The atrial fibrillation investigators. Stroke. 2009;40:1410–1416. doi: 10.1161/STROKEAHA.108.526988. [DOI] [PubMed] [Google Scholar]
- 38.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): A randomised controlled trial. Lancet. 2007;370:493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 39.Garcia D, Hylek E. Stroke prevention in elderly patients with atrial fibrillation. Lancet. 2007;370:460–461. doi: 10.1016/S0140-6736(07)61208-2. [DOI] [PubMed] [Google Scholar]
- 40.Flaker G, Ezekowitz M, Yusuf S, et al. Efficacy and safety of dabigatran compared to warfarin in patients with paroxysmal, persistent, and permanent atrial fibrillation: Results from the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) study. J Am Coll Cardiol. 2012;59:854–855. doi: 10.1016/j.jacc.2011.10.896. [DOI] [PubMed] [Google Scholar]
- 41.Halperin JL, Hankey GJ, Wojdyla DM, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) Circulation. 2014;130:138–146. doi: 10.1161/CIRCULATIONAHA.113.005008. [DOI] [PubMed] [Google Scholar]
- 42.Avezum A, Lopes RD, Schulte PJ, et al. Apixaban compared with warfarin in patients with atrial fibrillation and valvular heart disease: Findings from the ARISTOTLE Trial. Circulation. 2015;132:624–632. doi: 10.1161/CIRCULATIONAHA.114.014807. [DOI] [PubMed] [Google Scholar]
- 43.Johnson SA, Rondina MT. Edoxaban was noninferior to warfarin for preventing stroke or systemic embolism in atrial fibrillation. Ann Intern Med. 2014;160:JC7. doi: 10.7326/0003-4819-160-6-201403180-02007. [DOI] [PubMed] [Google Scholar]
- 44.Miller CS, Grandi SM, Shimony A, et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol. 2012;110:453–460. doi: 10.1016/j.amjcard.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 45.Barco S, Cheung YW, Eikelboom JW, et al. New oral anticoagulants in elderly patients. Best Pract Res Clin Haematol. 2013;26:215–224. doi: 10.1016/j.beha.2013.07.011. [DOI] [PubMed] [Google Scholar]