Abstract
Obesity and diabetes have reached epidemic proportions in the past few years. In 2011–2012, more than a third of the US population was obese. Although recent trend data indicate that the epidemic has “leveled off”, prevalence of abdominal obesity continues to rise, especially among adults. As seen for obesity, the past few decades have seen a doubling of the diabetes incidence with an increasing number of type 2 diabetes cases being diagnosed in children. Significant racial and ethnic disparities exist in the prevalence and trends of obesity and diabetes. In general, in both adults and children, non-Hispanic blacks and Mexican Americans appear to be at a high risk than their non-Hispanic white counterparts. Secular changes in agricultural policies, diet, food environment, physical activity, and sleep have all contributed to the upward trends in the “diabesity” epidemic. Despite marginal improvements in physical activity and the US diet, the food environment has changed drastically to an “obesogenic” one with increased portion sizes and limited access to healthy food choices especially for disadvantaged populations. Interventions that improve the food environment are critical as both obesity and diabetes raise the risk of cardiovascular disease by nearly two-fold. Among those with type 2 diabetes, significant sex differences occur in the risk of cardiovascular disease such that diabetes completely eliminates or attenuates the advantages of being female. Given the substantial burden of obesity and diabetes, future research efforts should adopt a translational approach to find sustainable and holistic solutions in preventing these costly diseases.
Keywords: diabetes, cardiovascular disease, epidemiology, obesity, diet and lifestyle
1. INTRODUCTION
Obesity and diabetes have emerged as enormous public health problems not only in the United States (US) but also globally. In fact, globally, obesity is a bigger health crisis than hunger, and is the leading cause of death and disabilities around the world with burdens expected to increase in coming years. In the US, diabetes affects nearly 1 in 10 adults, with a majority (90%–95%) of the cases being type 2 diabetes (T2D). Although historically thought to be an adult disease, increases in body weight among children and adolescents have resulted in an increasing number of T2D cases being diagnosed, especially among Black (57.8%) and Hispanic youths (46.1%)1. The associated costs of obesity and diabetes are steep. Obese individuals pay on average 42% more for healthcare costs than normal-weight individuals while diabetics pay more than twice (2.3) as much as those without diabetes. In 2008, the annual medical cost of obesity was estimated to be at $147 billion while the total excess costs related to the current prevalence of adolescent overweight and obesity is estimated to be greater at $254 billion2. In 2012, diabetes cost the US taxpayers $245 billion representing a 41% increase from costs in 20073. The economic burden associated with diagnosed diabetes, undiagnosed diabetes, gestational diabetes, and prediabetes is much higher and exceeded $322 billion in 2012 representing a 48% increase from 20074.
Both obesity and diabetes are related multifactorial, complex diseases and a large proportion of the cases are preventable. Both conditions significantly raise the risk for cardiovascular disease (CVD) and stroke. In fact, the American Heart Association has identified body mass index of <25 kg/m2 and a fasting plasma glucose concentration of <100 mg/dL as part of a construct of ideal cardiovascular health5. A first step in preventing these costly diseases is an understanding of the reasons behind their unprecedented growth in the past few decades. In this paper, we present trends in obesity and diabetes, explore secular changes in the drivers of this epidemic, and finally evaluate the increased risk of CVD associated with these conditions.
2. PREVALENCE AND TRENDS IN OBESITY
2.1. Adult obesity
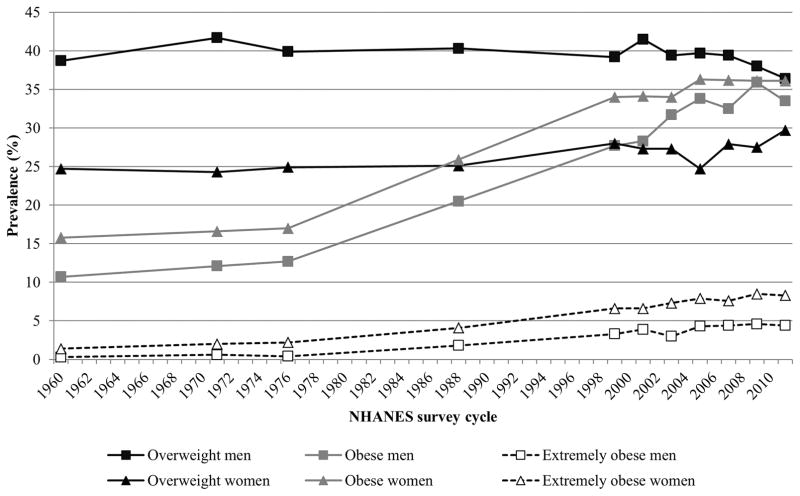
Obesity has reached epidemic proportions in the US. Long standing and well established surveys such as the Behavioral Risk Factor Surveillance System (BRFSS), the National Health Interview Survey (NHIS), and the National Health and Nutrition Examination Survey (NHANES) have documented the dramatic increases in the prevalence of both overweight and obesity from as early as the 1960s. Data from successive cross-sectional and nationally representative NHANES surveys showed a dramatic increase in the prevalence of obesity from 12.8% to 22.5% between 1960–1994 (Figure 1). These upward trends continued between the years 1990–2000 and 2009–2010 when the prevalence of obesity continued to increase by 17.8%6. However, since 2003–2004, there have been no significant changes in obesity prevalence among US adults suggesting a “leveling off” of the obesity epidemic6–8. In examining trend data collected between 1970s and 2004, notable differences were observed in annual increase rates. Overweight and obesity increased much faster in adults than in children and in women than in men. When considering obesity alone, African-American women had the highest rate of increase (annual average increase of 0.88%) compared to other ethnic groups. If these secular trends continue, it is estimated that, by 2030, 86.3% of adults will be overweight or obese and 51.1% obese and by the year 2048, all American adults would be either overweight or obese9. Similar forecasts were predicted using the BRFSS 1990–2008 data where a 33% increase in the prevalence of obesity is forecasted over the next 2 decades10. The most recent NHANES data indicate that we are well on our way in reaching these predictions. In 2011–2012, the age-adjusted combined prevalence of overweight and obesity among adults, aged ≥20 years, was nearly 70% in women while the prevalence of obesity alone was 35%. Like before, the highest prevalence rates for obesity were seen in non-Hispanic black (56.6%) and Hispanic women (44.4%)8. Similar disparities were seen for other body composition measures from NHANES. Among men, non-Hispanic blacks had a lower percent of body fat (25.8%) than non-Hispanic whites (28.3%) and Mexican Americans (28.9%). Among women, Mexican Americans had higher mean percent body fat (41.6%) than non-Hispanic whites (39.7%) or Blacks (40.9%)11.
Figure 1.
Trends in age-adjusted prevalence of overweight and obesity categories in US adults aged 20–74 years, 1960–2012. Overweight is defined as a BMI≥25.0 kg/m2 and <30.0 kg/m2. Obese is defined as BMI ≥30.0 kg/m2. Extremely obese is defined as BMI ≥40.0 kg/m2. Data are derived from (1960–1962), NHANES I (1971–1974), NHANES II (1976–1980), NHANES III (1988–1994), and NHANES (1999–2012). Data sources: Ogden et al8 and Fryar et al123.
2.2. Childhood and Adolescent Obesity
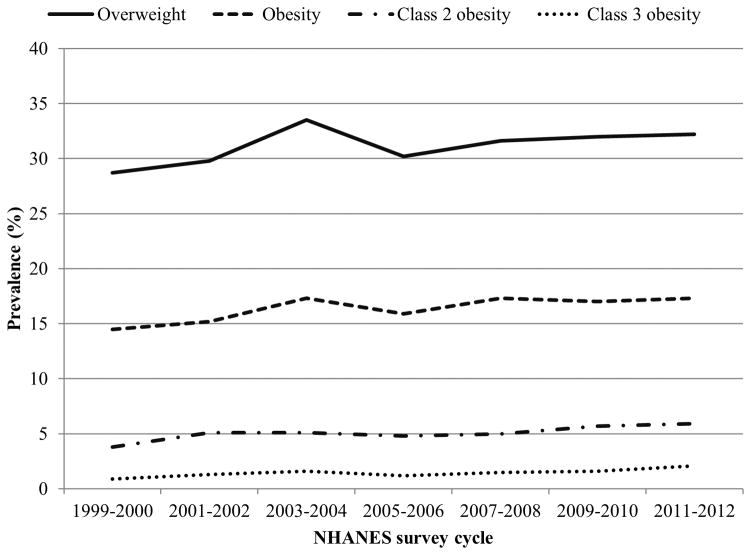
In children and adolescents, aged 2–19 years, overweight is defined as a body mass index (BMI) between the 85th and 95th percentile of the Centers for Disease Control and Prevention (CDC) sex-specific BMI for age growth charts released in the year 2000. Obesity is defined as a BMI greater than the 95th percentile12. Based on the CDC growth charts, in 2012, nearly 17% of children and adolescents were obese and about 32% were overweight or obese. Although these percentages were not significantly different from 2009–2010, trend analyses over a 14-year period showed a significant increase in obesity prevalence from 14.5% in 1999–2000 to 17.3% in 2009–2010. The greatest increases were seen for class 2 (defined as BMI ≥ 120% of the 95th percentile or BMI ≥ 35; 3.8%–5.9%) and class 3 obesity (defined as BMI ≥ 140% of the 95th percentile or BMI ≥ 40; 0.9%–2.1%) (Figure 2). Prevalence rates of overweight, obesity, and class 2 obesity increased significantly among Hispanic females and black males. Although the overall proportion of children with obesity appears to be somewhat leveling off, the rates of severe obesity in children continue to rise, particularly in select ethnic groups13. Given the high prevalence rates of childhood obesity and that a significant proportion of obese adolescents grow up to be obese adults 14, it is imperative to address the obesity epidemic. In fact, in 2010, the White House has rightfully established the first ever Task Force on Childhood Obesity to end the problem of childhood obesity within a generation15 and the one year report indicates significant progress in communities across the nation16.
Figure 2.
Trends in overweight and obesity prevalence among children and adolescents, aged 2–19 years, by NHANES cycle. Overweight is defined as BMI ≥85th percentile for age and sex and obesity defined as BMI ≥95th percentile for age and sex. Class 2 obesity is defined as a BMI >120% of the 95th percentile for age and sex or a BMI ≥35, whichever is lower. Class 3 obesity is defined as a BMI >140% of the 95th percentile for age and sex or a BMI ≥40, whichever is lower. Data source: Skinner et al13
2.3. Abdominal obesity
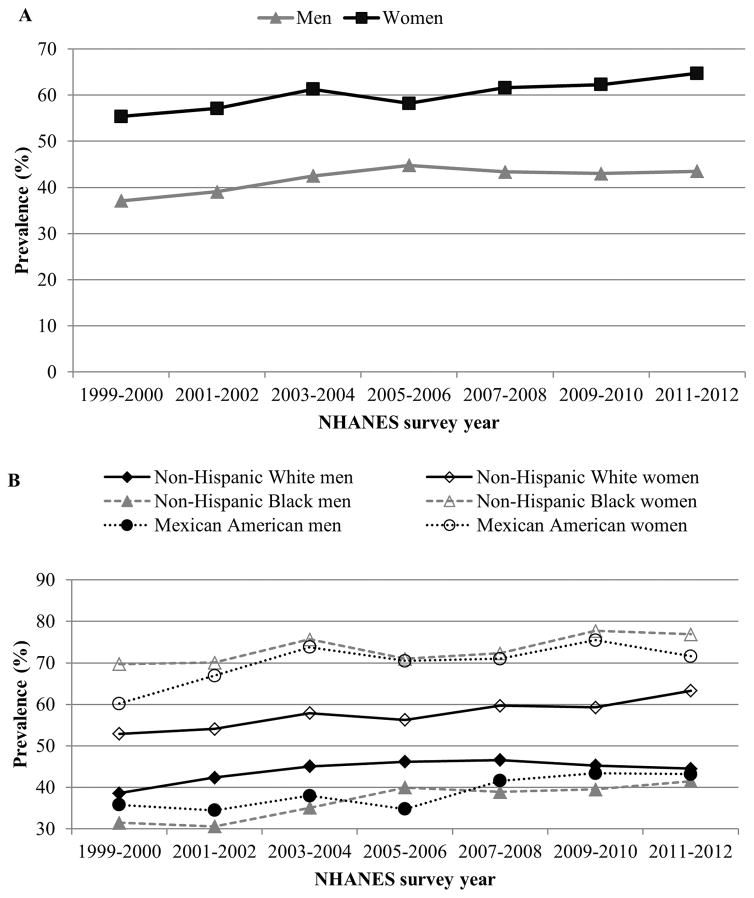
Abdominal obesity is a known risk factor for CVD independent of BMI17, 18 and is thought to affect disease risk through increased insulin resistance19. Waist circumference is a simple and reliable measure of abdominal obesity and is particularly useful as a marker of disease among patients who are categorized as normal or overweight20. Utilizing nationally representative data over a 40 year period from NHANES, Okosun et al21 found that mean waist circumference increased progressively and significantly in the US population from 1960–1962 to 1999–2000 (9.9 cm in men and by 23.2 cm in women). Most importantly, these upward trends were observed in normal weight, underweight, and obese categories. More recent trend data from 1999–2012 continued to show concerning upward trends in waist circumference22. The age-adjusted mean waist circumference increased from 95.5 cm in 1999–2000 to 98.5 cm in 2011–2012. Likewise, the overall age-adjusted prevalence of abdominal obesity increased significantly from 46.4% in 1999–2000 to 54.2% in 2011–2012. Significant increases were found in both genders, non-Hispanic whites, non-Hispanic blacks, and Mexican Americans. Across all years, prevalence was higher in men compared to women (Figure 3A). Non-Hispanic black women (76.9%) and Mexican American women (71.6%) had the highest prevalence rates compared to other ethnic groups (Figure 3B)22. Of note, increases in waist circumference have occurred independent of changes in BMI suggesting that abdominal obesity continues its upward trend despite a plateauing in general obesity. Between 1999–2000 and 2011–2012, independent of BMI, mean waist circumference increased by 0.2 cm in men and 2.4 cm in women23. These concerning trend data indicate the need for continuous measurement of waist circumference in clinical care and for policies aimed at curbing abdominal obesity.
Figure 3.
Trends in age-adjusted prevalence of abdominal obesity (defined as waist circumference >88 cm in women and >102 cm in men) among adults by sex (panel A) and race (panel B), NHANES 1999–2012. Data source: Ford ES et al 201422
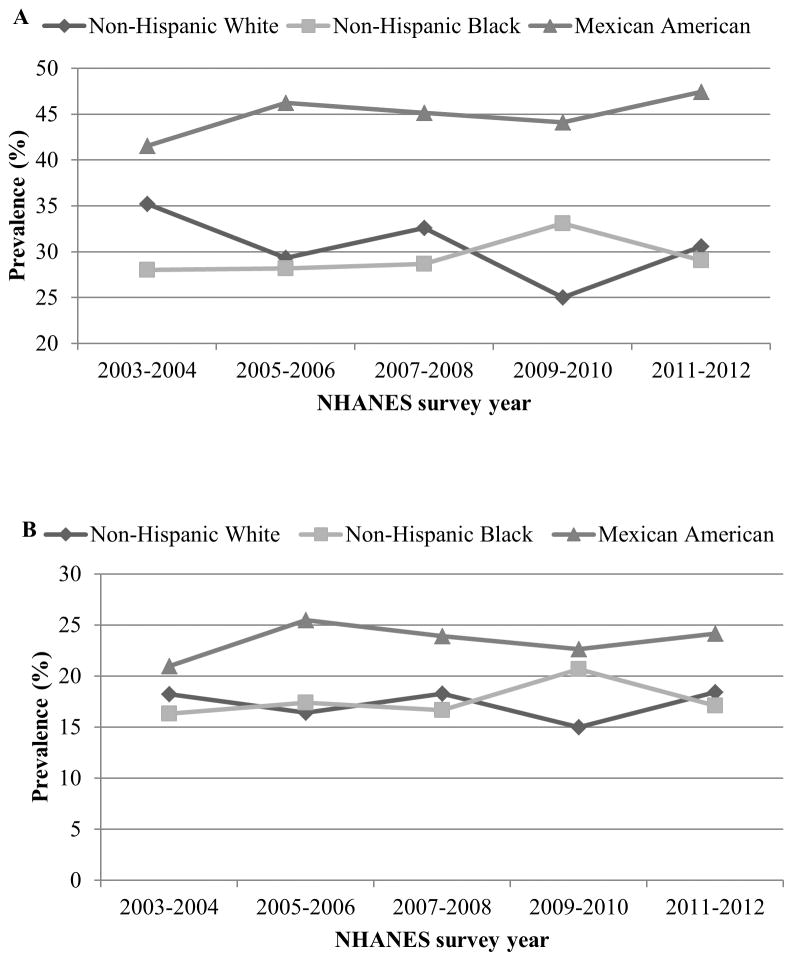
In children and adolescents, aged 2–18 years, the age and sex-specific 90th percentile values of waist circumference in NHANES III were used as cutoff values to identify those with abdominal obesity24. Waist/height ratio of ≥0.5 is another metric for defining abdominal obesity in youth, aged 6–18 years as this cut-off may overestimate prevalence of abdominal obesity in children younger than 6 years13. Upward trends in abdominal obesity among children and adolescents were much steeper than those in adults. Between 1988–1994 and 1999–2004, prevalence of abdominal obesity in children and adolescents increased by 65.4% (from 10.5% to 17.4%) for boys and by 69.4% (from 10.5% to 17.8%) for girls25. However, unlike their adult counterparts, abdominal obesity (using waist/height ratio as a metric) remained stable between 2003–2004 and 2011–2012 in all age, sex, and ethnic groups except for non-Hispanic whites whose prevalence in 2011–2012 (35.2%) was significantly lower than in the years 2003–2004 (30.6%) (Figures 4A and 4B). Still, prevalence rates in 2011–2012 continued to remain high with nearly 20% of children and adolescents being abdominally obese using waist circumference as the defining criteria26. Mexican American children had consistently higher prevalence rates compared to other ethnic groups (Figures 4A and 4B).
Figure 4.
Trends in the prevalence of abdominal obesity among US children and adolescents aged 2–18 y by race, NHANES 2003–2004 to 2011–2012. Abdominal obesity is defined as waist circumference ≥ gender- and age-specific 90th percentile based on NHANES III (1988–1994) data (Panel A) in all 2–18 year olds or a waist to hip ratio ≥0.5 among youth aged 6 to 18 years (Panel B). Data Source: Xi B et al 201426
3. PREVALENCE AND TRENDS IN DIABETES
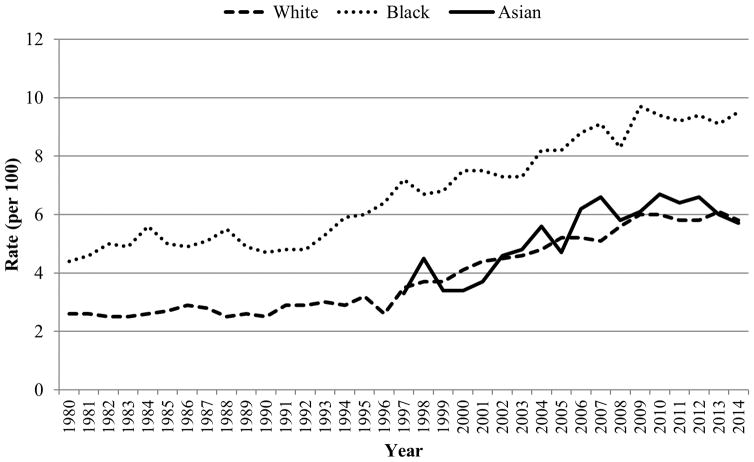
Obesity is a major risk factor for T2D27–30 and trends in prevalence and incidence of T2D have closely mirrored those of obesity. The most recent CDC report shows that from 1980–2014, the age-adjusted incidence of diagnosed diabetes nearly doubled from 3.5 to 6.6 per 1000 population. Between 1990 and 2008, rates more than doubled from 3.8 to 8.5 per 1000 and increases were seen in both sexes. However, from 2008–2014, age-adjusted incidence significantly declined from 8.5 to 6.6 per 1000. Yet, racial disparities continued to persist such that incidence rates were lower among whites than among blacks or Hispanics. However, from 1997 to 2014, age-adjusted incidence did not significantly change among blacks. Among whites, the numbers significantly increased from 1997 to 2008 and significantly decreased from 2008 to 2014 (Figure 5)31. In examining trends in prevalence data from 1988–1994 and 2011–2012, diabetes increased significantly over time in all age groups, in both sexes, in every racial/ethnic group, and in all education categories (P<0.05). Increases were particularly steep among non-Hispanic blacks (16.3% to 20.6%) and Mexican Americans (17.5% to 20.5%). On the other hand, the proportion of people with undiagnosed diabetes significantly decreased. However, when trends were examined by BMI categories, diabetes only increased among those who were obese (18.0% to 20.1%) suggesting that much of the increase in the prevalence of diabetes is due to the increasing prevalence of obesity32. In fact, 85.2% of people with T2D are overweight or obese33. If present trends continue, it is estimated that 1 in 3 Americans will have diabetes by 205034. Of particular concern is the high prevalence of diabetes among Asians who despite having a lower BMI are 30%–50% more likely to develop diabetes than their white counterparts35. In fact, prospective data from Kaiser Permanente confirm that Pacific Islanders, South Asians, and Filipinos have the highest prevalence (18.3%, 15.9%, and 16.1%, respectively) of diabetes among all racial/ethnic groups, including minorities traditionally considered high risk such as African Americans, Latinos, and Native Americans36.
Figure 5.
Trends in age-adjusted rates of diagnosed diabetes per 100 civilian, non-institutionalized population in the US, by Race, 1980–201431. Data source: Centers for Disease Control and Prevention, National Center for Health Statistics, Division of Health Interview Statistics, data from the National Health Interview Survey
Prediabetes (defined as hemoglobin A1c of 5.7 to <6.5% or fasting glucose 100 to <126 mg/dL) is associated with an increased risk of developing T2D. The most recent NHANES 2011–2012 survey data indicate that the prevalence of prediabetes was 36.5% among adults, aged ≥20 years. Highest prevalence rates were seen among those aged ≥65 years (54.6%) and among men (39.1%) compared to women (33.8%). Although no significant differences were seen across race/ethnic groups, non-Hispanic blacks had the highest prevalence numbers (38.8%)32. Between 1999–2002 and 2007–2010, concomitant with secular changes in diabetes, prevalence of prediabetes increased from 29.2% to 36.2%37 highlighting the need for initiating prevention efforts much before the start of the disease process.
In recent years, T2D has emerged as a pediatric problem and accounts for up to 45% of new cases in children and adolescents38. Among youth aged 10–19 years, the overall prevalence of T2D increased by 35% from 0.34 per 1000 in 2001 to 0.46 per 1000 in 2009. As with adults, significant increases were seen in both sexes, and in whites, blacks, and Hispanics. While no increases were observed among American Indians, prevalence rates in this group were very high (1.20 per 1000 in 2009)39. Despite reports of a significant decline in incidence, prevalence rates continue to remain high and policy efforts to curb the diabetes epidemic should focus on high risk minority groups and in children and adolescents.
4. MAJOR DRIVERS OF THE OBESITY AND DIABETES EPIDEMICS
Although obesity has traditionally been considered to be a disease of energy imbalance, its etiology is highly complex and involves interplay between genetic, environmental, physiological, behavioral, social, and economic factors. As obesity is a primary risk factor for T2D and because both diseases share common etiologies, we discuss below how secular changes in the major risk factors contributed to the “diabesity” epidemic.
4.1. Changes in agricultural policies
In recent years, changes in US agricultural policies may have played a role in the obesity epidemic. In particular, the US farm bill which was created to subsidize farmers’ income and provide a secure food supply for Americans has morphed into a system that may have directly contributed to the obesity epidemic. For example, 70%–80% of all farm subsidies are directed toward 8 “commodity” crops (corn, wheat, cotton, soybeans, rice, barley, oats, and sorghum) and farmers growing “specialty crops” like fruits and vegetables are penalized if they receive federal farm payments for these crops. As a result, “commodity” crops cover nearly 70% of US cropland and their abundance has undoubtedly contributed to their low cost. This in turn has changed corporate behavior and consumer consumption patterns towards those that favor the obesity and diabetes epidemic40, 41. For example, the overabundance of corn has led to the emergence of inexpensive corn-based sweeteners such as high fructose corn syrup (HFCS) which comprises of ~55% fructose and ~45% sucrose (as opposed to table sugar which is 50% sucrose and 50% fructose). In the past few decades, HFCS has slowly replaced sucrose and it now represents >40% of caloric sweeteners added to foods and is the sole caloric sweetener in carbonated beverages in the US. Indeed, food consumption data from the USDA indicate that consumption of HFCS increased >1000% between 1970 and 1990. The average daily consumption of HFCS exceeds 130 kcal for all Americans aged ≥2 y, and the top 20% of consumers of caloric sweeteners ingest 316 kcal from HFCS/d. This increased consumption has mirrored the rapid increase in obesity in the US42. Moreover, countries with larger availability of HFCS have a higher prevalence of diabetes that is independent of obesity43.
4.2. Changes in physical activity and sleep
Regular physical activity has been shown to be associated with decreased risk for obesity and other chronic diseases such as CVD and T2D among adults, irrespective of their gender or ethnicity44. Physical activity trend data have shown marginal improvements over the years. National estimates of leisure-time physical activity during 1990–1998 using data obtained from BRFSS show that the proportion of adults reporting no physical activity decreased from 30.7% in 1990 to 28.7% in 199845. While nearly 40% of adults engaged in no leisure time physical activity in 1997, only one in 3 adults reported doing so in 2013 meeting the 2020 target of 32.6%46. However, the picture remains unpromising for youth. Recent data from national surveys and longitudinal studies indicate that active commuting, high school physical education, and outdoor play (in 3–12 year olds) has declined over time. At the same time, in 2013, more than a third (41.3%) of children reported spending 3 or more hours per day on an average school day playing video or computer games or used a computer for something that was not school work47. Increases in time spent watching television (per hour per day) are independently associated with weight gain (0.31 lb)48. Further, use of an electronic device has been known to be disruptive to sleep49. It is noteworthy that among racial/ethnic minority children, the presence of a bedroom TV alone, irrespective of screen time, was associated with shorter sleep from infancy to mid childhood50. Sleep, in turn, is a recognized risk factor for T2D. In a meta-analysis of prospective studies representing a total of 18,443 incident T2D cases and 482,502 participants, compared with 7-h sleep duration per day, each 1-h shorter sleep duration was associated with a 9% higher risk (95% confidence interval (CI): 4%–15%) for T2D among individuals who slept <7 h per day51. Most recently, in the Nurses’ Health Study, we observed that compared with women who had no change, decreases in sleep duration were adversely associated with changes in diet quality and physical activity, while increases were associated with greater weight gain52. The low levels of physical activity combined with increases in screen time and the accompanying sleep “debt” contribute to a higher risk of T2D both directly and indirectly through their effects on body weight.
4.3. Changes in diet and the food environment
Diet is an important modifiable risk factor for the prevention of non-communicable diseases including obesity, CVD, T2D, and certain cancers. In fact, in the US, dietary risks account for 26% of deaths and 14% of disability adjusted life years53. Therefore, improvement in diet represents a huge potential for disease reduction either directly or indirectly through improvements in intermediate risk factors such as blood pressure, fasting glucose, and weight gain. The top dietary risk factors in the US are diets low in fruits, low in nuts and seeds, high in sodium, high in processed meats, low in vegetables, and high in trans fats53. Like physical activity, an examination of dietary trends in the past century has shown that the US diet has been slowly improving. Using the Diet Quality Index as an overall metric of diet quality in a nationally representative sample of US adults, Popkin et al54 found that overall dietary quality improved over a quarter century between 1965 to 1989–1991 in all socioeconomic and racial/ethnic groups. More recently, to capture the effect of changes in the food supply, national economy, and food policy on diet quality, Wang et al55 applied the Alternate Healthy Eating Index (AHEI) 2010, a 11-dimension dietary quality index, to investigate recent trends in dietary quality in the US adult population. The authors found that from 1999 to 2010, the quality of the US diet improved modestly overall. Yet, the greatest improvements were seen among those with a higher socioeconomic status and a healthier BMI suggesting that disparities in diet quality worsened over the decade. However, diet quality of the US population remains far from optimal. For example, despite a trend toward an overall important in AHEI-2010 scores for fruit, vegetables, whole grains, nuts and legumes, and long-chain omega-3 fatty acids, the top dietary risk factors in the US, consumption remained low. In fact, more than half of the gain in diet quality was due to a large reduction in consumption of trans fat which was the result of several policy changes during that period. The authors estimated that improvements in dietary quality between 1999 and 2012 prevented 1.1 million premature deaths and resulted in 12.6% fewer T2D cases and 8.6% fewer CVD cases56.
Increasing consumption of fruits and vegetables among the US population is especially important given strong evidence that healthy dietary patterns such as those rich in fruits and vegetables are associated with less long term weight gain57 while those characterized by meat and fried foods are associated with greater weight gain57, 58. A more recent analysis from US health professionals pointed that 4-year weight change was positively and strongly associated with increased daily servings of potato chips, potatoes, sugar sweetened beverages (SSBs), unprocessed red meats, and processed meats and inversely associated with intake of vegetables, whole grains, fruits, nuts, and yogurt48, 59. In addition, to specific foods, poor carbohydrate quality also appears to influence subsequent weight gain59 and T2D risk60. A considerable amount of attention has focused on added sugar intake primarily because of their caloric contributions to the diet. Data from the Minnesota Heart Survey indicate that added sugar increased by 54% in women between 1980 to 1982 and 2000 to 2002, but declined somewhat in 2007 to 2009 with men following a similar pattern. Concurrent with these increases, BMI increased in both genders and all age and weight groups61. Sugar sweetened beverages are the single largest source of added sugar intake and the top source of energy intake in the US diet62. A quantitative synthesis of the literature has shown that they are implicated in weight gain in both adults and children63. Most recently, we showed that SSB consumption was associated with a higher risk of T2D, independent of its effect on weight gain64. Although national trends indicate that consumption of SSBs has reduced in the past decade, youth and adults continue to consume ~150 kcal/d on average from these beverages. At the same time, consumption of non-traditional SSBs such as sports/energy drinks has increased65, 66. These trends can largely be attributed to changes in the food environment. Although diet and physical activity are the primary determinants of obesity, the built environment can determine the level of exposure to these risk factors. Over the past few decades, the food landscape has changed to an “obesogenic” one with increased availability of energy-dense foods and larger portion sizes. In the last 30 years (between 1977 and 2003–2006), total energy intake increased by 570 kcal/d among US adults, aged 19 years and older. Although the number of daily eating occasions did increase over this period (+1.1), increases in total energy intake were primarily due to increases in portion sizes (15 kcal/d/y)67. Similar increases in total energy intake (108 kcal/d) and eating out occasions (+1.2) were noted among children between 1977 and 2005–201068. When examining trends by age and race/ethnicity, Piernas and Popkin69 found that adolescents were more susceptible to increased portion sizes than younger children. For example, among children aged, 7–18-years, higher energy intakes at meals coincided with larger portion sizes of SSBs, French fries, or salty snacks. Adults living in neighborhoods with a high density of fast-food restaurants or “food deserts” were nearly twice (OR=1.89, 95% CI, 1.01–3.50) as likely to be obese compared to those in areas with a low density of fast-food restaurants70. As a result, the White House Task Force on Childhood Obesity Report to the President identified access to healthy, affordable food and reducing food “deserts” as key to solving the childhood obesity epidemic. To achieve this, the Healthy Food Financing Initiative was launched in 2010 as a key part of the “Let’s Move!” initiative. With a goal of eliminating food deserts across the country over the next seven years, this multi-million dollar initiative aims to expand access to nutritious food in food deserts through the establishment of healthy food retail outlets, including developing and equipping grocery stores, small retailers, corner stores and farmers markets selling healthy food.
Taken together, these data underscore the urgent need for not only improving dietary quality but changing the food landscape such that “making the healthy choice the easy choice”. To achieve this, it is crucial for legislators and policy makers to adopt a holistic approach in curbing the existing “diabesity” epidemic.
4.4. Genetics and gene-environment interactions
It is no surprise that obesity has an underlying genetic component. Recent genome wide association studies have identified 97 BMI associated loci which together account for ~2.7% of BMI variation. When the cumulative effect of the 97 loci were constructed into a genetic-susceptibility score, there was an average modest increase of 0.1 kg/m2 per BMI-increasing allele or 260–320 g higher body weight in adults of 160–180 cm in height71. Given these modest increases, variations in these genes alone cannot explain the dramatic increases in obesity prevalence. However, changes in the environment have dictated how disease risk is modified in individuals with different genetic makeup. Accordingly, a bulk of the literature has rightfully focused on gene-environment interactions. For example, in children and adolescents, lower dietary protein intake was found to attenuate the association between FTO genotype and adiposity72. In another recent study, Qi et al73 documented an interaction between fried food consumption and a genetic risk score based on 32 BMI-associated variants on BMI. Compared to consumption of fried foods less than once per week, the likelihood of obesity per 10 risk alleles was nearly 3-fold (odds ratio (OR)=2.72, 95% CI: 2.12–3.48) for fried food consumption four or more times a week. Using the same genetic risk score, for every increment of 10 risk alleles, the authors noted a nearly 5-fold higher risk of obesity among those consuming one or more servings of SSBs per day74. These results indicate that unhealthy dietary habits may amplify genetic effects of obesity.
In addition to gene-environment interactions, a substantial body of evidence supports the role of early life exposures to famine75, maternal malnutrition (including both undernutrition and overnutrition)76, 77, maternal smoking status78, maternal diabetes prior to pregnancy79, gestational diabetes80, and maternal obesity81 in influencing offspring’s subsequent risk of obesity and T2D, through mechanisms often referred to as “developmental programming”. These “programs” can result in permanent structural changes of various organs and tissues, alter responses to environmental stimuli, and induce epigenetic changes in gene expression82. The importance of the early life environment in determining obesity and diabetes risk later in life presents a unique opportunity for intervention and prevention of these costly diseases83.
5. OBESITY AND CARDIOVASCULAR DISEASE
Obesity is a chronic metabolic disease that has a substantial influence on the cardiovascular system84. Obesity produces a variety of structural and functional adaptations to the cardiovascular system, including lower cardiac output, increased peripheral resistance, increased left ventricular mass, left ventricular wall thickness, internal dimension, and poorer left ventricular systolic function85. Obesity is also known to influence coronary risk indirectly through its effect on related comorbidities such as dyslipidemia, hypertension, glucose intolerance, endothelial dysfunction, and inflammation86, 87. National secular trends in CVD risk factors show that although marginal improvements have occurred in all weight groups, risk factors continue to be higher in obese and overweight individuals88, 89. In addition to its indirect effects on CVD risk, early epidemiologic evidence from the Framingham Heart Study90, the Nurses’ Health Study91, and the Manitoba study92 established obesity as an independent risk factor for CVD. In the Nurses’ Health Study, obesity was associated with a nearly 2-fold higher risk (RR=1.9, 95% CI: 1.3–2.6) of coronary heart disease (CHD) even after adjustment for hypertension, diabetes, high serum cholesterol, and parental history of myocardial infarction. Such independent associations were observed in both men and women and even with small increases in BMI86. In a recent systematic review and meta-analysis of 1.2 million participants and 37,488 incident CHD cases, each unit increment in BMI (kg/m2) was associated with a higher risk of CHD in women (hazard ratio (HR)=1.04, 95% CI: 1.03–1.05) and men (HR=1.05, 95% CI: 1.04–1.07). Compared with people of a normal weight, obesity was associated with a nearly 60% higher CHD (HR=1.61, 95% CI: 1.42–1.82 in women; HR=1.60, 95% CI: 1.43–1.79 in men)93. From a pathophysiological standpoint, several mechanisms underlie obesity-induced atherosclerosis94. As reviewed by Lovren et al94, these include an adipokine imbalance due to release of pro-inflammatory adipokines by the visceral adipose tissue, increased oxidative stress due to elevation in free radicals production by the adipose tissue, impaired autophagy, endothelial dysfunction, and activation of adipose tissue macrophages, T cells, and B cells within fat deposits all of which lead to fibrous plaques and lesions. Most recently, gut microbiota found in obese individuals were found to induce low-grade chronic inflammation in the host, thereby suggesting a novel link between the microbiome and atherosclerotic risk.
5.1. Metabolically healthy obesity: The fat but fit hypothesis
Despite the widely established metabolic effects of obesity at the population level, substantial heterogeneity exists in individual responses to obesity. Findings from epidemiological studies indicate that a subgroup of obese individuals present with a normal metabolic profile, have a substantially lower risk of metabolic complications than other obese individuals, or are not a higher risk of CVD than their non-obese counterparts. This group has been described as having “metabolically healthy obesity” (MHO). Although the definition of MHO varies considerably in the literature, accepted criteria include, absence of abdominal obesity, absence of metabolic syndrome components, insulin sensitivity, and a high level of cardiorespiratory fitness95. The proportion of population considered to be MHO varies based on the criteria used to define it. National estimates from NHANES indicate that the proportion of US adults who are obese yet have a high cardiovascular fitness level (fat but fit) was 8.9% while another 17.4% were overweight and high fit (defined as age and sex-specific cut-offs of VO2 max ≥60th percentile of the Aerobics Center Longitudinal Study)96. Data from the1999–2004 NHANES survey indicate that among US adults, aged ≥20 years, nearly 16 million adults (or 23.5%) of normal-weight were metabolically abnormal (defined as ≥2 cardiometabolic abnormalities), whereas nearly 36 million (51.3%) of overweight adults and about 20 million (31.7%) obese adults were metabolically healthy97. Significant ethnic disparities exist in the prevalence of the MHO phenotype such that non-Hispanic blacks (38.9%) have a higher prevalence compared to non-Hispanic whites (30.8%)97. Nearly a third of obese children and adolescents (BMI >85th percentile), aged 8–17 years, can be classified as MHO98. Accumulating evidence from prospective cohort studies show that although individuals with the MHO phenotype have a higher risk of incident CVD and T2D compared to those who are normal weight, they are at a lower risk than their metabolically unhealthy counterparts99–101. Still, those with MHO have a greater prevalence of subclinical markers of disease progression, including greater intima media thickness102, 103 and coronary artery calcification103, 104 than normal weight metabolic healthy individuals. Data from animal and human studies point to several potential mechanisms that underlie the development of the MHO phenotype95. These include preserved insulin sensitivity, increased adipogenesis in subcutaneous adipose tissue, lower amount of lipid deposition in the liver, a metabolically beneficial adipokine pattern, and decreased mitochondrial iron transport into the mitochondrial matrix95, 105, 106. From a clinical and public health standpoint, it is critical to understand if the MHO phenotype is fixed or if it represents a transient state where the metabolic function of MHO individuals may change into that of a metabolically high-risk individual over time. Prospective data indicate that the MHO is, in fact, a dynamic concept and that over time, a significant proportion progress into a metabolically at risk profile and are more likely to develop T2D 99, CVD100, and chronic kidney disease107. Due to the transient nature of this sub-phenotype, prevention strategies that result in moderate weight loss may reverse disease progression from MHO to healthy normal weight105. Finally, the existence of MHO phenotype underscores the need for clinicians to consider other metabolic markers in addition to BMI alone. Genetic association studies of large cohorts of individuals with the MHO phenotype and those who are metabolically at risk obese could provide insights regarding protective genes.
6. DIABETES AND CARDIOVASCULAR DISEASE
6.1. Epidemiological evidence
Cardiovascular disease is the leading cause of morbidity and mortality among individuals with T2D accounting for 68% of all diabetic deaths2. Between 1997–2005, NHIS data indicate that the number of persons aged ≥35 years with diagnosed diabetes who reported having CVD increased 36%. However, the age-adjusted prevalence decreased 11% indicating an increase in the number of patients diagnosed with diabetes that exceeded the increase in CVD prevalence108. This excess risk disproportionately affects women109 such that diabetes completely eliminates or attenuates the advantages of being female. In two quantitative summaries of data from 64 cohort studies, women with diabetes were found to have a 44% greater risk of incident CHD and 27% greater risk of stroke compared with men with diabetes. Among women, the risk of incident CHD events associated with diabetes was 2.82 (95% CI: 2.35–3.38) while the corresponding risk estimates were 2.16 (95% CI: 1.82–2.56) in men110. For stroke associated with diabetes, the pooled maximum-adjusted relative risk (RR) was 2·28 (95% CI 1·93–2·69) in women and 1·83 (1·60–2·08) in men111. Diabetes also raises the risk of other vascular endpoints. Evidence from case-control studies and prospective data indicate that diabetes is associated with a higher risk of venous thromboembolism (pooled HR=1.35, 95% CI: 1.17–1.55)112 and sudden cardiac death (HR=2.18, 95% CI: 1.89–2.52)113 with risk estimates being similar in men and women. In a meta-analysis of 7 prospective studies comprising of over 1.6 million participants, diabetes was associated with a 24% higher risk of atrial fibrillation (95% CI: 6%–44%) and the population attributable fraction of atrial fibrillation owing to diabetes was 2.5%114. In the Emerging Risk Factors Collaboration, diabetes was associated with greater than two-fold risk (HR=2.32; 95% CI, 2.11–2.56) of death from vascular causes115. However, rates of diabetes related complications have substantially declined in the past two decades (between 1990 and 2010) with acute myocardial infarctions and stroke declining by more than half. Still, the burden of diabetes continues to be enormous due to its high prevalence116.
6.2. Lifestyle and dietary determinants of cardiovascular disease among those with type 2 diabetes
Despite advances in pharmacotherapy, diet and lifestyle remain the cornerstone in preventing cardiovascular complications among those with T2D. A recent meta-analysis of 16 RCT’s found that lifestyle interventions (such as increased physical activity, reduced caloric intake, dietary education, and counseling and education regarding treatment adherence or disease monitoring) had favorable effects on various CVD risk factors. The standardized difference in means of change from baseline significantly favored the intervention for BMI (−0.29; 95% CI: −0.52 to −0.06), HbA1c (−0.37; 95% CI: −0.59 to −0.14), systolic blood pressure (−0.16: 95% CI: −0.29 to −0.03), and diastolic blood pressure (−0.27, 95% CI: −0.41 to −0.12). However, no differences between the intervention and control groups in high density lipoprotein-cholesterol (HDL-C) and low density lipoprotein-cholesterol (LDL-C) concentrations were documented117. In another meta-analysis of 20 RCTs, walking as a form of physical activity was found to significantly decrease HbA1c by 0.50% (95% CI: −0.78% to −0.21%), BMI by 0.91 kg/m2 (95% CI: −1.22 to −0.59), and diastolic blood pressure by 1.97 mm Hg (−3.94 to −0.0). However, like before, no effects were noted for systolic blood pressure, HDL-C, or LDL-C118. Taken together, these quantitative summaries suggest that interventions that focus on weight reduction and improvements in physical activity have beneficial effects on glycemic control and improve the cardiometabolic profile. In fact, in the landmark Look AHEAD trial, an intensive lifestyle intervention that promoted weight loss through decreased caloric intake and increased physical activity produced greater reductions in HbA1c and improvements in fitness and all CVD risk factors, except for LDL-C among overweight or obese adults with T2D. Despite these improvements, after a median follow-up of 9.6 years, the intervention did not significantly reduce the rate of CVD events, the primary outcome of the trial119. On the other hand, in the PREDIMED trial, a Mediterranean diet supplemented with extra-virgin olive oil reduced the incidence of major CVD events by 31% (95% CI: 3%–50%) among those with diabetes120. Similarly, in the Da Qing Diabetes Prevention Study, a 6-year lifestyle intervention (of diet and/or exercise) lowered CVD mortality by 41% (HR=0.59, 95% CI 0.36–0.96) after 23 years of follow-up among Chinese adults with impaired glucose tolerance121. Altogether, these results suggest that even short-term lifestyle changes have long-term consequences in the prevention of chronic disease and provide justification for implementing public health policies that focus on risk factor control early on.
7. CONCLUSIONS
While the most recent national data for obesity and diabetes are somewhat encouraging, their high prevalence combined with their gigantic costs highlight the urgent need for comprehensive measures in preventing these diseases. Obesity and T2D are complex diseases. Such complexity calls for a multi-pronged solution that ranges from cellular and metabolic investigations to community-level interventions to curb their increases. At a cellular level, recent advances in systems levels tools and “omics” technologies have enabled researchers to understand the underlying biological pathways in disease progression. In particular, the field of metabolomics offers a unique opportunity to not only explore how complex interactions between modifiable risk factors and the human organism affect future disease risk but to also identify new targets for disease prevention. At the community level, successful studies like the Shape Up Somerville have demonstrated that a comprehensive approach that engages all sectors and levels of the community, focuses on engaging at risk populations, increases access to healthy food and physical activity opportunities has lasting effects in curbing the obesity epidemic122. Finally, policy measures such as a tax on SSBs could prevent thousands of new cases of obesity and diabetes and save considerable medical costs. Clearly, no single approach can effectively tackle these epidemics. The need of the hour is a translational approach that spans from the basic sciences to community-based participatory research and involves all the relevant stakeholders – the research community, clinicians, policy makers, and the general public.
Supplementary Material
Acknowledgments
Source of funding: Drs. Bhupathiraju and Hu are supported by grant P30 DK46200 from the National Institutes of Health
Non-standard Abbreviations and Acronyms
- AHEI
Alternate Healthy Eating Index
- BMI
body mass index
- BRFSS
Behavioral Risk Factor Surveillance System
- CDC
Centers for Disease Control and Prevention
- CHD
coronary heart disease
- CI
confidence interval
- CVD
cardiovascular disease
- HDL-C
high density lipoprotein-cholesterol
- HFCS
high fructose corn syrup
- HR
hazard ratio
- LDL-C
low density lipoprotein-cholesterol
- MHO
metabolically healthy obesity
- NHANES
National Health and Nutrition Examination Survey
- NHIS
National Health Interview Survey
- OR
odds ratio
- RCTs
randomized controlled trials
- RR
relative risk
- SSBs
Sugar sweetened beverages
- T2D
type 2 diabetes
- US
United States
Footnotes
Disclosures to report: None
References
- 1.Dabelea D, Bell RA, D’Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the united states. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.Economic costs of diabetes in the U.S. In 2012. Diabetes care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dall TM, Yang W, Halder P, Pang B, Massoudi M, Wintfeld N, Semilla AP, Franz J, Hogan PF. The economic burden of elevated blood glucose levels in 2012: Diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes care. 2014;37:3172–3179. doi: 10.2337/dc14-1036. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The american heart association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 6.An R. Prevalence and trends of adult obesity in the us, 1999–2012. ISRN obesity. 2014;2014:185132. doi: 10.1155/2014/185132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among us children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all americans become overweight or obese? Estimating the progression and cost of the us obesity epidemic. Obesity (Silver Spring) 2008;16:2323–2330. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 10.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. American journal of preventive medicine. 2012;42:563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Li C, Ford ES, Zhao G, Balluz LS, Giles WH. Estimates of body composition with dual-energy x-ray absorptiometry in adults. The American journal of clinical nutrition. 2009;90:1457–1465. doi: 10.3945/ajcn.2009.28141. [DOI] [PubMed] [Google Scholar]
- 12.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. National health statistics reports. 2010:1–5. [PubMed] [Google Scholar]
- 13.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the united states, 1999–2012. JAMA pediatrics. 2014;168:561–566. doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 14.Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: A systematic review of the literature. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2008;9:474–488. doi: 10.1111/j.1467-789X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 15.White House Task Force on Childhood Obesity Report to the President. [Accessed on November 15, 2015];Solving the problem of childhood obesity within a generation. 2010 doi: 10.1089/bfm.2010.9980. http://www.letsmove.gov/sites/letsmove.gov/files/TaskForce_on_Childhood_Obesity_May2010_FullReport.pdf. [DOI] [PubMed]
- 16.White House Task Force on Childhood Obesity. [Accessed on November 15, 2015];One year progress report. 2011 http://www.letsmove.gov/sites/letsmove.gov/files/Obesity_update_report.pdf.
- 17.Casanueva FF, Moreno B, Rodriguez-Azeredo R, Massien C, Conthe P, Formiguera X, Barrios V, Balkau B. Relationship of abdominal obesity with cardiovascular disease, diabetes and hyperlipidaemia in spain. Clinical endocrinology. 2010;73:35–40. doi: 10.1111/j.1365-2265.2009.03727.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee SY, Chang HJ, Sung J, Kim KJ, Shin S, Cho IJ, Shim CY, Hong GR, Chung N. The impact of obesity on subclinical coronary atherosclerosis according to the risk of cardiovascular disease. Obesity (Silver Spring) 2014;22:1762–1768. doi: 10.1002/oby.20760. [DOI] [PubMed] [Google Scholar]
- 19.Muller MJ, Lagerpusch M, Enderle J, Schautz B, Heller M, Bosy-Westphal A. Beyond the body mass index: Tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012;13(Suppl 2):6–13. doi: 10.1111/j.1467-789X.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 20.NHLBI Obesity Education Initiative Expert Panel on the Indentification E, and Treatment of Obesity in Adults. The practical guide to the identification, evaluation, and treatment of overweight and obesity in adults. 2000 [Google Scholar]
- 21.Okosun IS, Chandra KM, Boev A, Boltri JM, Choi ST, Parish DC, Dever GE. Abdominal adiposity in u.S. Adults: Prevalence and trends, 1960–2000. Preventive medicine. 2004;39:197–206. doi: 10.1016/j.ypmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Ford ES, Maynard LM, Li C. Trends in mean waist circumference and abdominal obesity among us adults, 1999–2012. JAMA. 2014;312:1151–1153. doi: 10.1001/jama.2014.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman DS, Ford ES. Are the recent secular increases in the waist circumference of adults independent of changes in bmi? The American journal of clinical nutrition. 2015;101:425–431. doi: 10.3945/ajcn.114.094672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third national health and nutrition examination survey, 1988–1994. Archives of pediatrics & adolescent medicine. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Ford ES, Mokdad AH, Cook S. Recent trends in waist circumference and waist-height ratio among us children and adolescents. Pediatrics. 2006;118:e1390–1398. doi: 10.1542/peds.2006-1062. [DOI] [PubMed] [Google Scholar]
- 26.Xi B, Mi J, Zhao M, Zhang T, Jia C, Li J, Zeng T, Steffen LM. Trends in abdominal obesity among u.S. Children and adolescents. Pediatrics. 2014;134:e334–339. doi: 10.1542/peds.2014-0970. [DOI] [PubMed] [Google Scholar]
- 27.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. The New England journal of medicine. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 28.Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett-Connor EL, Dowse GK, Haffner SM, Pettitt DJ, Sorkin JD, Muller DC, Collins VR, Hamman RF. Predictors of progression from impaired glucose tolerance to niddm: An analysis of six prospective studies. Diabetes. 1997;46:701–710. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, Sellers TA, Lazovich D, Prineas RJ. Associations of general and abdominal obesity with multiple health outcomes in older women: The iowa women’s health study. Archives of internal medicine. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 30.Dagenais GR, Auger P, Bogaty P, Gerstein H, Lonn E, Yi Q, Yusuf S. Increased occurrence of diabetes in people with ischemic cardiovascular disease and general and abdominal obesity. The Canadian journal of cardiology. 2003;19:1387–1391. [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. [Accessed on December 4, 2015];Diabetes public health resource. 2015 http://www.cdc.gov/diabetes/statistics/prevalence_national.htm.
- 32.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the united states, 1988–2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 33.Report CMaMW. Prevalence of overweight and obesity among adults with diagnosed diabetes --- united states, 1988–1994 and 1999–2002. 2004;53(45):1066–1068. [PubMed] [Google Scholar]
- 34.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the us adult population: Dynamic modeling of incidence, mortality, and prediabetes prevalence. Population health metrics. 2010;8:29. doi: 10.1186/1478-7954-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JW, Brancati FL, Yeh HC. Trends in the prevalence of type 2 diabetes in asians versus whites: Results from the united states national health interview survey, 1997–2008. Diabetes care. 2011;34:353–357. doi: 10.2337/dc10-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karter AJ, Schillinger D, Adams AS, Moffet HH, Liu J, Adler NE, Kanaya AM. Elevated rates of diabetes in pacific islanders and asian subgroups: The diabetes study of northern california (distance) Diabetes care. 2013;36:574–579. doi: 10.2337/dc12-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bullard KM, Saydah SH, Imperatore G, Cowie CC, Gregg EW, Geiss LS, Cheng YJ, Rolka DB, Williams DE, Caspersen CJ. Secular changes in u.S. Prediabetes prevalence defined by hemoglobin a1c and fasting plasma glucose: National health and nutrition examination surveys, 1999–2010. Diabetes care. 2013;36:2286–2293. doi: 10.2337/dc12-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamadi A, Cooke DW. Type 2 diabetes mellitus in children and adolescents. Adolescent medicine: state of the art reviews. 2010;21:103–119. x. [PubMed] [Google Scholar]
- 39.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller M, Schoonover H, Wallinga D. Considering the contribution of u.S. Food and agricultural policy to the obesity epidemic: Overview and opportunities. 2007. [Google Scholar]
- 41.Jackson RJ, Minjares R, Naumoff KS, Shrimali BP, Martin LK. Agriculture policy is health policy. Journal of hunger & environmental nutrition. 2009;4:393–408. doi: 10.1080/19320240903321367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. The American journal of clinical nutrition. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 43.Goran MI, Ulijaszek SJ, Ventura EE. High fructose corn syrup and diabetes prevalence: A global perspective. Global public health. 2013;8:55–64. doi: 10.1080/17441692.2012.736257. [DOI] [PubMed] [Google Scholar]
- 44.US Department of Health and Human Services. Physical activity guidelines for americans. 2008. [Google Scholar]
- 45.From the centers for disease control and prevention. Physical activity trends--united states, 1990–1998. JAMA. 2001;285:1835. [PubMed] [Google Scholar]
- 46.Office of Disease Prevention and Health Promotion. [Accessed on December 4, 2015];HealthyPeople.gov. 2015 http://www.healthypeople.gov/2020/data-search/Search-the-Data?f%5B%5D=field_topic_area%3A3504&pop=&ci=&se=
- 47.Kann L, Kinchen S, Shanklin SL, et al. Youth risk behavior surveillance--united states, 2013. MMWR Surveill Summ. 2014;63(Suppl 4):1–168. [PubMed] [Google Scholar]
- 48.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. The New England journal of medicine. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hysing M, Pallesen S, Stormark KM, Jakobsen R, Lundervold AJ, Sivertsen B. Sleep and use of electronic devices in adolescence: Results from a large population-based study. BMJ open. 2015;5:e006748. doi: 10.1136/bmjopen-2014-006748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cespedes EM, Gillman MW, Kleinman K, Rifas-Shiman SL, Redline S, Taveras EM. Television viewing, bedroom television, and sleep duration from infancy to mid-childhood. Pediatrics. 2014;133:e1163–1171. doi: 10.1542/peds.2013-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, Rong Y, Jackson CL, Hu FB, Liu L. Sleep duration and risk of type 2 diabetes: A meta-analysis of prospective studies. Diabetes care. 2015;38:529–537. doi: 10.2337/dc14-2073. [DOI] [PubMed] [Google Scholar]
- 52.Cespedes EM, Bhupathiraju SN, Li Y, Rosner B, Redline S, Hu FB. Long-term changes in sleep duration, energy balance and risk of type 2 diabetes. Diabetologia. 2015 doi: 10.1007/s00125-015-3775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray CJ, Atkinson C, Bhalla K, et al. The state of us health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Popkin BM, Siega-Riz AM, Haines PS. A comparison of dietary trends among racial and socioeconomic groups in the united states. The New England journal of medicine. 1996;335:716–720. doi: 10.1056/NEJM199609053351006. [DOI] [PubMed] [Google Scholar]
- 55.Wang DD, Leung CW, Li Y, Ding EL, Chiuve SE, Hu FB, Willett WC. Trends in dietary quality among adults in the united states, 1999 through 2010. JAMA internal medicine. 2014;174:1587–1595. doi: 10.1001/jamainternmed.2014.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang DD, Li Y, Chiuve SE, Hu FB, Willett WC. Improvements in us diet helped reduce disease burden and lower premature deaths, 1999–2012; overall diet remains poor. Health Aff (Millwood) 2015;34:1916–1922. doi: 10.1377/hlthaff.2015.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwingshackl L, Hoffmann G, Kalle-Uhlmann T, Arregui M, Buijsse B, Boeing H. Fruit and vegetable consumption and changes in anthropometric variables in adult populations: A systematic review and meta-analysis of prospective cohort studies. PloS one. 2015;10:e0140846. doi: 10.1371/journal.pone.0140846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boggs DA, Palmer JR, Spiegelman D, Stampfer MJ, Adams-Campbell LL, Rosenberg L. Dietary patterns and 14-y weight gain in african american women. The American journal of clinical nutrition. 2011;94:86–94. doi: 10.3945/ajcn.111.013482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith JD, Hou T, Ludwig DS, Rimm EB, Willett W, Hu FB, Mozaffarian D. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: Results from 3 prospective cohorts. The American journal of clinical nutrition. 2015;101:1216–1224. doi: 10.3945/ajcn.114.100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.AlEssa HB, Bhupathiraju SN, Malik VS, Wedick NM, Campos H, Rosner B, Willett WC, Hu FB. Carbohydrate quality and quantity and risk of type 2 diabetes in us women. The American journal of clinical nutrition. 2015 doi: 10.3945/ajcn.115.116558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Steffen LM, Zhou X, Harnack L, Luepker RV. Consistency between increasing trends in added-sugar intake and body mass index among adults: The minnesota heart survey, 1980–1982 to 2007–2009. American journal of public health. 2013;103:501–507. doi: 10.2105/AJPH.2011.300562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu FB. Resolved: There is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14:606–619. doi: 10.1111/obr.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. The American journal of clinical nutrition. 2013;98:1084–1102. doi: 10.3945/ajcn.113.058362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imamura F, O’Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. doi: 10.1136/bmj.h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han E, Powell LM. Consumption patterns of sugar-sweetened beverages in the united states. Journal of the Academy of Nutrition and Dietetics. 2013;113:43–53. doi: 10.1016/j.jand.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mesirow MS, Welsh JA. Changing beverage consumption patterns have resulted in fewer liquid calories in the diets of us children: National health and nutrition examination survey 2001–2010. Journal of the Academy of Nutrition and Dietetics. 2015;115:559–566. e554. doi: 10.1016/j.jand.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 67.Duffey KJ, Popkin BM. Energy density, portion size, and eating occasions: Contributions to increased energy intake in the united states, 1977–2006. PLoS medicine. 2011;8:e1001050. doi: 10.1371/journal.pmed.1001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duffey KJ, Popkin BM. Causes of increased energy intake among children in the u.S., 1977–2010. American journal of preventive medicine. 2013;44:e1–8. doi: 10.1016/j.amepre.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piernas C, Popkin BM. Increased portion sizes from energy-dense foods affect total energy intake at eating occasions in us children and adolescents: Patterns and trends by age group and sociodemographic characteristics, 1977–2006. The American journal of clinical nutrition. 2011;94:1324–1332. doi: 10.3945/ajcn.110.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li F, Harmer P, Cardinal BJ, Bosworth M, Johnson-Shelton D. Obesity and the built environment: Does the density of neighborhood fast-food outlets matter? American journal of health promotion : AJHP. 2009;23:203–209. doi: 10.4278/ajhp.071214133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qi Q, Downer MK, Kilpelainen TO, et al. Dietary intake, fto genetic variants, and adiposity: A combined analysis of over 16,000 children and adolescents. Diabetes. 2015;64:2467–2476. doi: 10.2337/db14-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qi Q, Chu AY, Kang JH, et al. Fried food consumption, genetic risk, and body mass index: Gene-diet interaction analysis in three us cohort studies. BMJ. 2014;348:g1610. doi: 10.1136/bmj.g1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, Ridker PM, Hunter DJ, Willett WC, Rimm EB, Chasman DI, Hu FB, Qi L. Sugar-sweetened beverages and genetic risk of obesity. The New England journal of medicine. 2012;367:1387–1396. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roseboom TJ, Painter RC, van Abeelen AF, Veenendaal MV, de Rooij SR. Hungry in the womb: What are the consequences? Lessons from the dutch famine. Maturitas. 2011;70:141–145. doi: 10.1016/j.maturitas.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez-Twinn DS, Ozanne SE. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiology & behavior. 2006;88:234–243. doi: 10.1016/j.physbeh.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 77.Renault KM, Carlsen EM, Norgaard K, Nilas L, Pryds O, Secher NJ, Cortes D, Beck Jensen JE, Olsen SF, Halldorsson TI. Intake of carbohydrates during pregnancy in obese women is associated with fat mass in the newborn offspring. The American journal of clinical nutrition. 2015 doi: 10.3945/ajcn.115.110551. [DOI] [PubMed] [Google Scholar]
- 78.Riedel C, Schonberger K, Yang S, Koshy G, Chen YC, Gopinath B, Ziebarth S, von Kries R. Parental smoking and childhood obesity: Higher effect estimates for maternal smoking in pregnancy compared with paternal smoking--a meta-analysis. International journal of epidemiology. 2014;43:1593–1606. doi: 10.1093/ije/dyu150. [DOI] [PubMed] [Google Scholar]
- 79.Chernausek SD, Arslanian S, Caprio S, Copeland KC, El Ghormli L, Kelsey MM, Koontz MB, Orsi CM, Wilfley D. Relationship between parental diabetes and presentation of metabolic and glycemic function in youth with type 2 diabetes: Baseline findings from the today trial. Diabetes care. 2015 doi: 10.2337/dc15-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim SY, Sharma AJ, Callaghan WM. Gestational diabetes and childhood obesity: What is the link? Current opinion in obstetrics & gynecology. 2012;24:376–381. doi: 10.1097/GCO.0b013e328359f0f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaar JL, Crume T, Brinton JT, Bischoff KJ, McDuffie R, Dabelea D. Maternal obesity, gestational weight gain, and offspring adiposity: The exploring perinatal outcomes among children study. The Journal of pediatrics. 2014;165:509–515. doi: 10.1016/j.jpeds.2014.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith CJ, Ryckman KK. Epigenetic and developmental influences on the risk of obesity, diabetes, and metabolic syndrome. Diabetes, metabolic syndrome and obesity : targets and therapy. 2015;8:295–302. doi: 10.2147/DMSO.S61296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Examining a developmental approach to childhood obesity: The fetal and early childhood years: Workshop summary. Washington (DC): 2015. [PubMed] [Google Scholar]
- 84.Poirier P, Eckel RH. Obesity and cardiovascular disease. Current atherosclerosis reports. 2002;4:448–453. doi: 10.1007/s11883-002-0049-8. [DOI] [PubMed] [Google Scholar]
- 85.Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Progress in cardiovascular diseases. 2014;56:369–381. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 86.Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: The framingham experience. Archives of internal medicine. 2002;162:1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 87.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. European cytokine network. 2006;17:4–12. [PubMed] [Google Scholar]
- 88.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, Narayan KM, Williamson DF. Secular trends in cardiovascular disease risk factors according to body mass index in us adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 89.Saydah S, Bullard KM, Cheng Y, Ali MK, Gregg EW, Geiss L, Imperatore G. Trends in cardiovascular disease risk factors by obesity level in adults in the united states, nhanes 1999–2010. Obesity (Silver Spring) 2014;22:1888–1895. doi: 10.1002/oby.20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the framingham heart study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 91.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, Speizer FE, Hennekens CH. A prospective study of obesity and risk of coronary heart disease in women. The New England journal of medicine. 1990;322:882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 92.Rabkin SW, Mathewson FA, Hsu PH. Relation of body weight to development of ischemic heart disease in a cohort of young north american men after a 26 year observation period: The manitoba study. The American journal of cardiology. 1977;39:452–458. doi: 10.1016/s0002-9149(77)80104-5. [DOI] [PubMed] [Google Scholar]
- 93.Mongraw-Chaffin ML, Peters SA, Huxley RR, Woodward M. The sex-specific association between bmi and coronary heart disease: A systematic review and meta-analysis of 95 cohorts with 1.2 million participants. The lancet. Diabetes & endocrinology. 2015;3:437–449. doi: 10.1016/S2213-8587(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lovren F, Teoh H, Verma S. Obesity and atherosclerosis: Mechanistic insights. The Canadian journal of cardiology. 2015;31:177–183. doi: 10.1016/j.cjca.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 95.Stefan N, Haring HU, Hu FB, Schulze MB. Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. The lancet. Diabetes & endocrinology. 2013;1:152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 96.Duncan GE. The “fit but fat” concept revisited: Population-based estimates using nhanes. The international journal of behavioral nutrition and physical activity. 2010;7:47. doi: 10.1186/1479-5868-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, Sowers MR. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: Prevalence and correlates of 2 phenotypes among the us population (nhanes 1999–2004) Archives of internal medicine. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 98.Prince RL, Kuk JL, Ambler KA, Dhaliwal J, Ball GD. Predictors of metabolically healthy obesity in children. Diabetes care. 2014;37:1462–1468. doi: 10.2337/dc13-1697. [DOI] [PubMed] [Google Scholar]
- 99.Appleton SL, Seaborn CJ, Visvanathan R, Hill CL, Gill TK, Taylor AW, Adams RJ. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: A cohort study. Diabetes care. 2013;36:2388–2394. doi: 10.2337/dc12-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hinnouho GM, Czernichow S, Dugravot A, Nabi H, Brunner EJ, Kivimaki M, Singh-Manoux A. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: The whitehall ii cohort study. European heart journal. 2015;36:551–559. doi: 10.1093/eurheartj/ehu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bell JA, Kivimaki M, Hamer M. Metabolically healthy obesity and risk of incident type 2 diabetes: A meta-analysis of prospective cohort studies. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2014;15:504–515. doi: 10.1111/obr.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marini MA, Succurro E, Frontoni S, Hribal ML, Andreozzi F, Lauro R, Perticone F, Sesti G. Metabolically healthy but obese women have an intermediate cardiovascular risk profile between healthy nonobese women and obese insulin-resistant women. Diabetes care. 2007;30:2145–2147. doi: 10.2337/dc07-0419. [DOI] [PubMed] [Google Scholar]
- 103.Khan UI, Wang D, Thurston RC, Sowers M, Sutton-Tyrrell K, Matthews KA, Barinas-Mitchell E, Wildman RP. Burden of subclinical cardiovascular disease in “metabolically benign” and “at-risk” overweight and obese women: The study of women’s health across the nation (swan) Atherosclerosis. 2011;217:179–186. doi: 10.1016/j.atherosclerosis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kwon BJ, Kim DW, Her SH, Kim DB, Jang SW, Cho EJ, Ihm SH, Kim HY, Youn HJ, Seung KB, Kim JH, Rho TH. Metabolically obese status with normal weight is associated with both the prevalence and severity of angiographic coronary artery disease. Metabolism: clinical and experimental. 2013;62:952–960. doi: 10.1016/j.metabol.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 105.Bluher M. Are metabolically healthy obese individuals really healthy? European journal of endocrinology/European Federation of Endocrine Societies. 2014;171:R209–219. doi: 10.1530/EJE-14-0540. [DOI] [PubMed] [Google Scholar]
- 106.Vukovic R, Milenkovic T, Mitrovic K, Todorovic S, Plavsic L, Vukovic A, Zdravkovic D. Preserved insulin sensitivity predicts metabolically healthy obese phenotype in children and adolescents. European journal of pediatrics. 2015;174:1649–1655. doi: 10.1007/s00431-015-2587-4. [DOI] [PubMed] [Google Scholar]
- 107.Chang Y, Ryu S, Choi Y, et al. Metabolically healthy obesity and development of chronic kidney disease: A cohort study. Annals of internal medicine. 2016 doi: 10.7326/M15-1323. [DOI] [PubMed] [Google Scholar]
- 108.Prevalence of self-reported cardiovascular disease among persons aged > or =35 years with diabetes--united states, 1997–2005. MMWR. Morbidity and mortality weekly report. 2007;56:1129–1132. [PubMed] [Google Scholar]
- 109.Regensteiner JG, Golden S, Huebschmann AG, et al. Sex differences in the cardiovascular consequences of diabetes mellitus: A scientific statement from the american heart association. Circulation. 2015 doi: 10.1161/CIR.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 110.Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: A systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57:1542–1551. doi: 10.1007/s00125-014-3260-6. [DOI] [PubMed] [Google Scholar]
- 111.Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973–1980. doi: 10.1016/S0140-6736(14)60040-4. [DOI] [PubMed] [Google Scholar]
- 112.Bai J, Ding X, Du X, Zhao X, Wang Z, Ma Z. Diabetes is associated with increased risk of venous thromboembolism: A systematic review and meta-analysis. Thrombosis research. 2015;135:90–95. doi: 10.1016/j.thromres.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 113.Zaccardi F, Khan H, Laukkanen JA. Diabetes mellitus and risk of sudden cardiac death: A systematic review and meta-analysis. International journal of cardiology. 2014;177:535–537. doi: 10.1016/j.ijcard.2014.08.105. [DOI] [PubMed] [Google Scholar]
- 114.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. The American journal of cardiology. 2011;108:56–62. doi: 10.1016/j.amjcard.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Seshasai SR, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. The New England journal of medicine. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the united states, 1990–2010. The New England journal of medicine. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 117.Chen L, Pei JH, Kuang J, Chen HM, Chen Z, Li ZW, Yang HZ. Effect of lifestyle intervention in patients with type 2 diabetes: A meta-analysis. Metabolism: clinical and experimental. 2015;64:338–347. doi: 10.1016/j.metabol.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 118.Qiu S, Cai X, Schumann U, Velders M, Sun Z, Steinacker JM. Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: A meta-analysis. PloS one. 2014;9:e109767. doi: 10.1371/journal.pone.0109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with a mediterranean diet. The New England journal of medicine. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 121.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the da qing diabetes prevention study: A 23-year follow-up study. The lancet. Diabetes & endocrinology. 2014;2:474–480. doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 122.Economos CD, Hyatt RR, Must A, Goldberg JP, Kuder J, Naumova EN, Collins JJ, Nelson ME. Shape up somerville two-year results: A community-based environmental change intervention sustains weight reduction in children. Preventive medicine. 2013;57:322–327. doi: 10.1016/j.ypmed.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 123.Fryar CD, Carroll MD, Ogden CL. [Accessed on November 24, 2015]; http://www.cdc.gov/nchs/data/hestat/obesity_adult_09_10/obesity_adult_09_10.htm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.