Abstract
Background/Objectives
Information about the safety of physical activity (PA) programs in older adults with mobility limitations is critically important to inform clinicians and others responsible for their clinical management. We conducted a safety analysis of study hospitalization data and determined whether the intervention was differentially associated with categories of hospitalizations or subgroups of participants.
Design
Multicenter RCT. Participants randomized to a PA or health education (HE) program for an average of 2.6 years.
Setting
8 field centers.
Participants
1,635 sedentary men and women aged 70-89 years with lower extremity physical limitations, but able to walk 400-m in ≤15 min.
Interventions
Structured, moderate-intensity PA (n = 818) at a center (2x/wk) and at home (3-4x/wk) that included aerobic, strength, balance, and flexibility training or HE (n = 817) of educational workshops and upper extremity stretching exercises.
Main Outcomes and Measures
All cause in-patient hospitalizations ascertained at six-month intervals.
Results
49.1% of PA and 44.4% of HE (risk difference=4.68%, 95%CI −0.18 to 9.54; HR=1.16, 95%CI 1.00 to 1.34) reported a total of 1458 hospitalizations. The intervention effect on incident hospitalization did not differ by race, sex, SPPB, age, and history of CVD/diabetes. PA was associated with an increase in the rates of hospitalization within the middle baseline gait speed category, compared to HE: <0.8m/s, HR[95%CI] 0.93[0.76-1.14]; 0.8–1.0m/s, 1.54[1.23-1.94]; >1.0m/s, 1.05[0.67-1.65]; interaction p=0.005).
Conclusion/Relevance
A PA program in older adults at risk for mobility disability did not lead to a differential risk of specific types of hospitalizations compared to a HE group, overall. Baseline gait speed may be a marker for risk of hospitalization during a PA intervention, as individuals with moderate baseline gait speed in the PA group had slightly higher rates of hospitalizations, compared to HE.
Keywords: physical activity, older adults, mobility disability, safety, hospitalizations
INTRODUCTION
Previously, we reported that a structured moderate intensity physical activity (PA) program compared with a health education (HE) program, reduced major mobility disability over an average follow-up of 2.6 years among older adults at risk of disability1. Another potential benefit of a PA intervention suggested by observational studies of older persons is reduced risk for hospitalization, and fewer admissions and bed stays2-6. However, in the LIFE study, a randomized controlled trial, we found a marginal non-significant difference in the overall proportion of randomized participants reporting hospitalizations at assessment visits in the PA group compared to the HE group (48.4% in PA vs. 44.1% in HE, RR=1.10; 95% CI 0.99-1.22); a comparison that was closely scrutinized during follow-up as a safety outcome by the LIFE Data Safety and Monitoring Board1. Given the importance of this finding related to participant safety and the prescription of PA in older adults with mobility limitations and chronic disease burden, it is important to explore the issue in greater detail to inform clinicians responsible for the clinical management of this rapidly growing segment of the population.
Therefore we conducted a detailed post-hoc analysis of the LIFE Study hospitalization data and examined whether subgroups of the LIFE participants might be at higher risk for hospitalizations. We used the clinically relevant baseline sub-groups previously identified in the main outcomes paper. We were also interested in whether the effect of the intervention on hospitalizations differed based on baseline physical function. For example, poorer lower extremity function has been associated with increased hospitalizations7-9 and in the LIFE Study, individuals with poorer baseline lower extremity physical function appeared to benefit more from the PA intervention1 compared to those with higher levels of function.
The specific aims of this study were: 1) to examine whether a PA intervention compared with a HE intervention was associated with a higher risk of hospitalization-related outcomes: length of stay (LOS), LOS >1 day, and specific types of hospitalizations using a standardized classification scheme; 2) to examine whether the effect of the intervention on initial hospitalization differed by baseline subgroups. We also provide an exploration of an intervention by baseline predictor interaction that was found in the course of our analyses.
METHODS
Trial design and participants
The LIFE study was a multicenter, single-blinded, parallel randomized trial conducted at 8 field centers across the U.S (see on-line Appendix) between February 2010 and December 2013. The study protocol was approved by the institutional review board at each field center and written informed consent was provided by all participants. Details of the LIFE Study design and inclusion/exclusion criteria10, recruitment and baseline characteristics of the sample11, and main outcomes manuscript1 have been published. Eligibility criteria included: 1) age 70-89 years; 2) sedentary status; 3) mobility limitations but able to walk 400 meters in <15 minutes; and 4) no major cognitive impairment.
Interventions
The PA intervention focused on walking, with a goal of 150 min/week, and also incorporated strength, flexibility, and balance training10. The intervention included attendance at two center-based visits per week and home-based activity 3-4 times per week for the duration of the study. The PA sessions were individualized and progressed towards a goal of 30-40 min of walking daily at moderate intensity, 10 min of lower extremity strength training, 10 min of balance training, and 5-10 min of flexibility exercises. The Borg Rating of Perceived Exertion scale12 that ranges from 6 to 20, was used to measure intensity of activity. The participants began with lighter intensity and gradually increased intensity over the first 2-3 weeks of the intervention to 13 (activity perception “somewhat hard”) for walking and 15-16 for strength exercises.
The HE intervention involved weekly workshops during the first 26 weeks, and then monthly sessions thereafter (bi-monthly attendance was optional). Workshops included topics relevant to older adults, such as how to negotiate the health care system, how to travel safely, preventive services and screenings recommended at different ages, where to go for reliable health information, and nutrition, but there was no discussion of PA. Each workshop also included a 5-10 minute instructor-led program of gentle upper extremity flexibility exercises.
Baseline Demographics, Clinical Characteristics
The baseline characteristics of the LIFE participants have been reported in detail and are presented in the on-line appendix (eTable S1)11. Physical function was assessed with the Short Physical Performance Battery13 and the 400-m walk test14. We converted the time to walk 400-m to gait speed (m/s).
Outcome Assessment
The primary outcome for the study was all cause in-patient hospitalizations ascertained at six month intervals. Outcome assessors were blinded to the intervention assignment and all hospitalizations were considered serious adverse events (SAEs). Also, participants were asked to notify study personnel about any hospitalization in between follow-up interviews. Medical record technicians and adjudicators could also identify additional hospitalizations upon review of medical records. For each hospitalization, medical records were obtained and abstracted for codes, diagnoses, procedures, and length of stay. Medical Officers at each site assigned presence or absence of each diagnosis using standardized criteria. We used the Medical Dictionary for Regulatory Activities (MedDRA®) classification scheme, to examine categories of related medical conditions at the System Organ Class level (SOC, 26 categories). In all analyses, hospitalizations were considered an event when they were reported to masked staff (staff who were blinded to intervention group assignment) at a scheduled assessment visit or found through medical record technicians and adjudicators, regardless of whether the event was also reported at any point during follow-up to an unmasked staff member. Because PA participants had more contact with unmasked interventionists who could report SAEs, we focus here on hospitalizations reported to masked staff at assessment visits scheduled at equal follow-up intervals in both groups, providing a comparison between randomized groups that is less affected by ascertainment and reporting biases.
Statistical Considerations
All analyses were performed in SAS 9.4. Baseline characteristics were summarized by intervention group using mean (SD) and proportions.
To address aim 1, the number and percent of participants reporting hospitalization at assessment visits was summarized as a composite of hospitalizations for all causes, and by the components of the composite with causes defined by MedDRA® SOC. Seventeen randomized participants [11 (1.3%) of 818 from PA, 6 (0.7%) of 817 from HE] that did not have any follow-up assessment visits where hospitalizations could be reported to masked staff are excluded from these analyses. For this reason, the overall percentages of hospitalized participants reported in each group will be slightly larger than that previously reported1. We calculated 95% confidence intervals on the differences between intervention groups in the proportion of participants hospitalized using exact confidence intervals when the number of hospitalizations within a MedDRA® SOC category was small. The number of events per person year of follow-up was calculated within intervention group overall, and by MedDRA® SOC category, as the total number of events divided by the total person years of follow-up. Because single participants could contribute multiple events to the numerator, when appropriate, confidence intervals were calculated using a negative binomial model and accounted for the non-independence of events within participants. Rate ratios, comparing event rates between intervention groups, were obtained from these models.
Length of stay was summarized with medians (IQR) and number of hospitalizations with length of stay >1 day are described by intervention group in terms of the proportion of all hospitalizations. We used zero-inflated negative binomial models adjusted for sex and clinic (both used to stratify randomization) to examine the effect of intervention arm on length of stay for the initial hospitalization.
Time until the initial hospitalization was defined as time from randomization until the date of the first hospital admission. Censoring dates were defined as the last assessment visit date with an outcome assessment. Cumulative hazard curves were used to express the cumulative risk of any hospitalization versus follow-up time.
Analyses addressing aim 2 used Cox regression models, stratified by clinical site and gender, to estimate the intervention hazard ratio for initial hospitalizations and to explore whether the intervention effect was homogeneous across levels of baseline subgroups. Subgroup effects were evaluated by entering the interaction term between the baseline factor and the intervention effect into the model. When evaluating for subgroup effects of gender, we no longer stratified the baseline hazard on this factor. Subgroup levels were classified as follows: age (<80, ≥80 years), race (Non-Hispanic White, Other), gender, baseline SPPB (<8, ≥8), baseline 400-m walk gait speed (<0.8 m/s, ≥0.8 m/s), history of CVD, and history of diabetes. We found a significant interaction between baseline gait speed and the intervention and explored this relationship over the continuous range of gait speed by fitting a B-spline curve, which allows us to fit a smoothed non-linear relationship, to gait speed within the Cox regression model.
RESULTS
As previously reported11 and shown in eTable 1, the PA and HE groups were well balanced on the baseline variables. The table also shows that the number of participants in the PA and HE groups was balanced across the three gait speed categories.
Hospitalization Events and Reasons for Hospitalizations
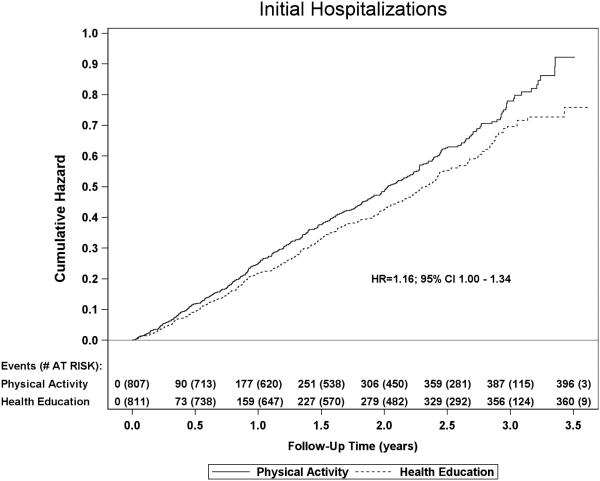
There were 1458 hospitalizations reported during the trial (Table 1). Overall, 49.1% of PA participants and 44.4% of HE participants reported a hospitalization during an average of 2.6 years of follow-up, corresponding to an absolute difference of −4.68% (95% CI −9.54 to 0.18) or 36 total participants between the two groups. This difference equates to a relative risk of 1.106 (95% CI 0.996 to 1.227). The number of total hospitalizations per year of follow-up was 0.378 for the PA group and 0.324 for the HE group, with a rate ratio of 1.16 (95% CI 1.002 to 1.353; p=0.047) and a difference of 96 hospitalizations between the two groups. The cumulative hazard curves for the first reported incidence of in-patient hospitalization are shown in Figure 1.
Table 1.
Total hospitalizations by treatment group and by MedDRA System Organ Class category
| Physical Activity (N=807) | Health Education (N=811) | |||||||
|---|---|---|---|---|---|---|---|---|
| MedDRA System Organ Class | Participants (% of participants) |
Total Events |
Events per participant year (95% CI) |
Particip ants (% of particip ants) |
Tot al Eve nts |
Events per participant year (95% CI) |
Difference In % of Participan ts (95% CL) |
Rate Ratio (95% CI) |
| ALL Inpatient Hospitalizations | 396 (49.1%) | 777 |
0.378 (0.340,
0.419) |
360
(44.4%) |
681 |
0.324 (0.291,
0.361) |
−4.68 (−
9.54, 0.18) |
1.16 (1.00,
1.35) |
| Blood And Lymphatic System Disorders | 11 (1.4%) | 16 |
0.009 (0.004,
0.022) |
9 (1.1%) | 12 |
0.006 (0.003,
0.014) |
−0.25 (−
1.33, 0.82) |
1.45 (0.44,
4.78) |
| Cardiac Disorders | 77 (9.5%) | 116 |
0.054 (0.042,
0.070) |
70
(8.6%) |
97 |
0.045 (0.034,
0.060) |
−0.91 (−
3.71, 1.89) |
1.21 (0.83,
1.76) |
| Ear And Labyrinth Disorders | 5 (0.6%) | 5 |
0.002 (0.001,
0.006) |
6 (0.7%) | 6 |
0.003 (0.001,
0.006) |
0.12 (−4.72,
4.97) |
0.84 (0.26,
2.76) |
| Endocrine Disorders | 4 (0.5%) | 5 |
0.002 (0.001,
0.006) |
1 (0.1%) | 1 |
0.000 (0.000,
0.003) |
−0.37 (−
5.21, 4.48) |
5.05 (0.59,
43.26) |
| Gastrointestinal Disorders | 59 (7.3%) | 81 |
0.038 (0.029,
0.050) |
47
(5.8%) |
53 |
0.025 (0.018,
0.034) |
−1.52 (−
3.93, 0.90) |
1.50 (0.99,
2.28) |
| General Disorders And Administration Site Conditions |
19 (2.4%) | 24 |
0.011 (0.006,
0.017) |
19 (2.3%) | 21 |
0.009 (0.005,
0.015) |
−0.01 (−
1.49, 1.46) |
1.16 (0.58,
2.32) |
| Hepatobiliary Disorders | 8 (1.0%) | 9 |
0.004 (0.002,
0.010) |
6 (0.7%) | 7 |
0.003 (0.001,
0.008) |
−0.25 (−
1.15, 0.65) |
1.29 (0.36,
4.70) |
| Immune System Disorders | 5 (0.6%) | 7 |
0.003 (0.001,
0.008) |
3 (0.4%) | 3 |
0.001 (0.000,
0.005) |
−0.24 (−
5.09, 4.60) |
2.44 (0.48,
12.40) |
| Infections And Infestations | 61 (7.6%) | 73 |
0.035 (0.027,
0.045) |
49
(6.0%) |
57 |
0.027 (0.020,
0.035) |
−1.52 (−
3.97, 0.94) |
1.30 (0.88,
1.93) |
| Injury, Poisoning And Procedural Complications |
54 (6.7%) | 58 |
0.027 (0.021,
0.036) |
56
(6.9%) |
63 |
0.029 (0.023,
0.038) |
0.21 (−2.24,
2.67) |
0.93 (0.64,
1.36) |
| Investigations | 3 (0.4%) | 4 |
0.002 (0.001,
0.005) |
2 (0.2%) | 2 |
0.001 (0.000,
0.004) |
−0.12 (−
4.97, 4.72) |
2.02 (0.37,
11.03) |
| Metabolism And Nutrition Disorders | 22 (2.7%) | 25 |
0.012 (0.008,
0.017) |
21
(2.6%) |
21 |
0.010 (0.006,
0.015) |
−0.14 (−
1.70, 1.43) |
1.20 (0.67,
2.15) |
| Musculoskeletal And Connective Tissue Disorders |
50 (6.2%) | 54 |
0.025 (0.019,
0.034) |
55
(6.8%) |
64 |
0.029 (0.022,
0.038) |
0.59 (−1.81,
2.99) |
0.87 (0.59,
1.29) |
| Neoplasms Benign, Malignant And Unspecified (Incl Cysts And Polyps) |
17 (2.1%) | 17 |
0.008 (0.005,
0.014) |
17
(2.1%) |
20 |
0.009 (0.006,
0.015) |
−0.01 (−
1.41, 1.39) |
0.87 (0.43,
1.76) |
| Nervous System Disorders | 67 (8.3%) | 83 |
0.039 (0.030,
0.051) |
55
(6.8%) |
66 |
0.031 (0.024,
0.041) |
−1.52 (−
4.09, 1.05) |
1.26 (0.86,
1.85) |
| Psychiatric Disorders | 7 (0.9%) | 7 |
0.003 (0.001,
0.007) |
3 (0.4%) | 3 |
0.001 (0.000,
0.004) |
−0.49 (−
5.33, 4.36) |
2.34 (0.61,
9.07) |
| Renal And Urinary Disorders | 14 (1.7%) | 15 |
0.006 (0.004,
0.011) |
16
(2.0%) |
16 |
0.007 (0.004,
0.011) |
0.24 (−1.08,
1.55) |
0.94 (0.46,
1.90) |
| Reproductive System And Breast Disorders | 4 (0.5%) | 4 |
0.002 (0.001,
0.005) |
3 (0.4%) | 3 |
0.001 (0.000,
0.004) |
−0.12 (−
4.97, 4.72) |
1.35 (0.30,
6.03) |
| Respiratory, Thoracic And Mediastinal Disorders |
48 (5.9%) | 60 |
0.030 (0.022,
0.042) |
36
(4.4%) |
44 |
0.021 (0.015,
0.031) |
−1.51 (−
3.67, 0.65) |
1.43 (0.87,
2.33) |
| Skin And Subcutaneous Tissue Disorders | 2 (0.2%) | 2 |
0.001 (0.000,
0.004) |
7
(0.9%) |
9 |
0.004 (0.002,
0.010) |
0.61 (−4.23,
5.45) |
0.22 (0.04,
1.19) |
| Surgical And Medical Procedures | 68 (8.4%) | 76 |
0.036 (0.028,
0.046) |
73
(9.0%) |
84 |
0.039 (0.031,
0.049) |
0.57 (−2.17,
3.32) |
0.91 (0.65,
1.28) |
| Vascular Disorders | 33 (4.1%) | 36 |
0.017 (0.011,
0.025) |
26
(3.2%) |
29 |
0.014 (0.009,
0.021) |
−0.88 (−
2.71, 0.94) |
1.25 (0.69,
2.29) |
Figure 1.
Cumulative hazards for initial in-patient hospitalization by treatment group.
We also examined the components of the all-cause hospitalization outcome using MedDRA® SOC category to define the types of hospitalizations (Table 1). There were 22 MedDRA® SOC categories with 4 or more total hospitalizations in either the PA or HE group. Hospitalizations for cardiac disorders accounted for the greatest number of total events (N=213, 116 in PA, 97 in HE). MedDRA® SOC categories with rate ratios >2.0 had a very small number of events. Note that all the MedDRA® SOC category confidence intervals included 1.00; only gastrointestinal disorders came close to having a lower bound of >1.0.
Length of Stay
The median and inter-quartile range for initial hospitalization length of stay was 5 days (IQR = 2 to 10.5) in the PA group and 5 days (IQR=3 to 10.5) in the HE group. We found no significant difference between groups in the length of initial hospitalization (p=0.29). Of the 777 hospitalizations in the PA group, 624 (79.0%) were >1 day and of the 681 hospitalizations in the HE group, 545 (80.0%) were >1 day.
Evaluation of the Overall Hazard Ratio for Hospitalization
Using Cox regression, the PA intervention was estimated to have increased the hazard rate for initial hospitalization by 16% compared to the HE intervention (HR=1.16; 95% CI 1.005 to 1.338; p=0.043).
Evaluation of the Intervention Effect among Baseline Subgroups
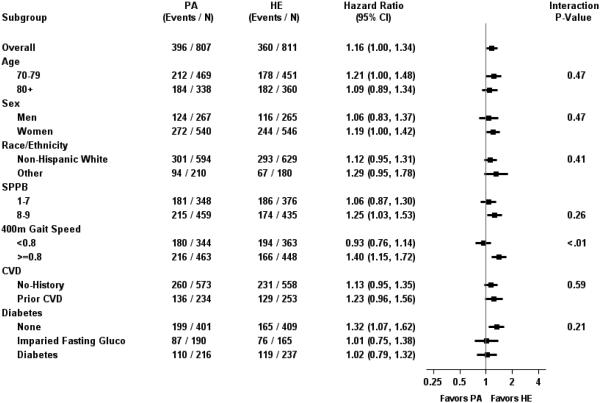
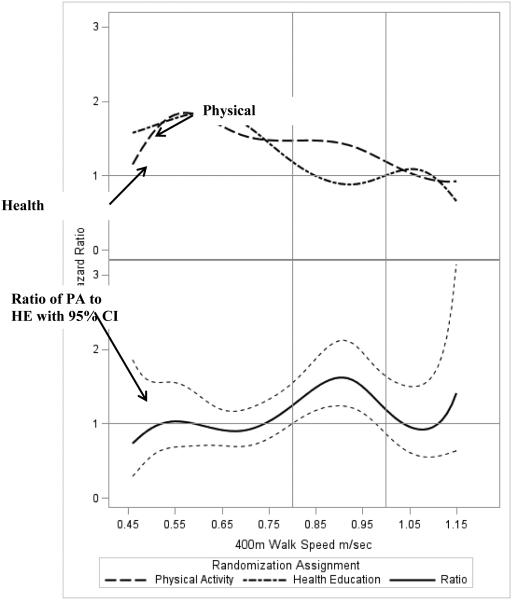
With the exception of 400-m gait speed (p<0.01), there were no differences in the effect of the intervention on hospitalizations among levels of baseline subgroups (Figure 2). For baseline gait speed, the risk of hospitalization was elevated by 40% (HR=1.40; 95% CI 1.15 to 1.72) among those with baseline gait speed ≥0.8 m/s. We further evaluated this relationship by fitting a B-spline to continuous baseline gait speed (Figure 3). The top panel of Figure 3 illustrates hazard ratios in the PA and HE groups relative to a person in the HE group with a baseline gait speed of 1.0 m/s (i.e. the reference group). Generally, risk of hospitalization decreased with increasing gait speed in both groups, with risk leveling off for PA participants with gait speed between 0.75 m/s and 0.95 m/s. The bottom panel provides hazard ratios (95% CI) for the PA group relative to the HE group for baseline walking speed and represents the ratio of the corresponding points on the lines in the top panel. For example, a participant in the PA group with a walking speed of 0.80 m/s has a 47% (i.e. HR=1.47) increase in risk of hospitalization compared to a participant in the HE group with a walking speed of 1.0 m/s. In contrast, a participant in the HE group with a walking speed of 0.80 m/sec has a 19% (i.e. HR=1.19) increase relative to the participant walking at 1.0 m/sec in the HE group (top panel). Using this example, it follows, that at 0.80 m/s, the PA participant has a 24% (=1.47/1.19) increased risk compared to an HE participant also walking at that speed (bottom panel). There was elevated risk in PA compared to HE among those with baseline walking speed between 0.8 and 1.0 m/s, with the lower bound of the 95% confidence interval generally being >1 in this interval.
Figure 2.
Hazard Ratios (95% CI) for Hospitalizations among Baseline Subgroups
Figure 3.
B-Spline Curve Illustrating the Relationship Between Baseline Gait Speed and Hospitalizations
Exploration of Baseline Gait Speed and Intervention Interaction Effect
In these exploratory analyses (Available On-line), we asked if the observed interaction between baseline gait speed (more hospitalizations for PA in the 0.8-1.0 m/s category) was consistent across diagnostic categories and field centers as well as over time throughout the study. We also examined whether the differences were attributable to greater PA in the 0.8-1.0 m/s gait speed category.
As reported above, there was an absolute difference of 36 total participants between the two groups. As shown in eTable S2, in the lowest gait speed category, the difference was 14 participants in the opposite direction to the overall result, (180 in PA, 194 in HE). In the highest speed category, the difference was 8 participants in the same direction as the overall result (43 in PA, 35 in HE). Therefore, in the middle category the difference was 42 participants (an absolute difference of 12.8%) with 173 in PA and 131 in HE.
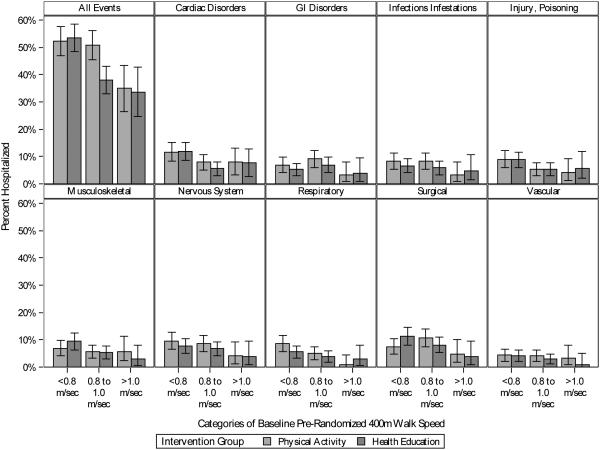
Within the 0.8–1.0 m/s group, the PA group reported more hospitalizations than the HE group in 8 of 9 MedDRA® SOC categories (Figure 4 and eTable 2). Therefore, the result is not being driven by one particular component of the composite, all-cause hospitalization outcome. Also, as shown in eTable S3 and eFigure S1, the nominally, larger percentage of hospitalizations in the PA versus HE group was observed in the middle gait speed category in 7 of 8 field centers, a result that indicates consistency of these differences. We found no indication that participants in the 0.8–1.0 m/s category were doing any more or less activity than expected, based on their functional capacity, compared to the other two categories (eFigure S2). We observed a stepwise and consistent pattern of PA behavior across all three baseline gait speed categories. Finally, across the duration of the study, hazard rates were similar for the PA and HE groups in the <0.8 m/s and >1.0 m/s gait speed categories (eFigure S3). For those with a gait speed of 0.8–1.0 m/s, the PA group had an initial increase in hazard which then stabilized and was consistently higher than the HE group, resulting in accumulation of a larger number of events. We examined MedDRA® SOC categories for hospitalizations that occurred within the first three months following randomization for the PA group (18 events) and HE group (10 events). The hospitalizations were distributed across 15 categories, with no clustering of events in any one category.
Figure 4.
Hospitalization rates by categories of 400-m walk gait speed at baseline, overall and by MedDRA SOC categories.
DISCUSSION
We had previously reported a marginal difference in the risk of hospitalizations in the PA group compared to the HE group, a result that was close to reaching conventional levels of statistical significance1. The independent Data Safety and Monitoring Board convened for the LIFE Study scrutinized the adverse event data closely but did not see evidence of harm sufficient to discontinue the LIFE Study. We previously concluded that “Further studies are needed to assess the effects of physical activity on mortality and hospitalizations in vulnerable older adults”1. The goal of this study was to conduct a detailed post-hoc analysis of the LIFE Study hospitalization data and determine whether subgroups of the LIFE participants might be at higher risk for hospitalizations.
In summary, the absolute difference between the PA and HE groups both in the number of participants reporting a hospitalization (36 participants) and the absolute number of hospitalizations (96 hospitalizations) was small, when considering the 1458 in-patient hospitalizations reported during the trial. The hazard ratio reported herein provided similar conclusions to the analysis of risk presented in the main outcomes paper1 even after the more complete adjustment for factors used to stratify randomization (site, gender), with the lower bound on the hazard ratio equal to 1.005 (p=0.043). There was considerable heterogeneity in the reasons for the hospitalizations as evidenced by the MedDRA® SOC categories. When considering MedDRA® SOC categories with a sufficient number of events to make an informed judgment, we found scant evidence that the PA intervention led to greater rates of hospitalizations for a particular SOC category. The LIFE Study was an 8 site trial and the overall results on hospitalizations were generally consistent across the field centers. Finally, our post-hoc analysis identified only gait speed during the self-paced 400-m walk test at baseline as modifying the intervention effect on hospitalizations.
The largest number of hospitalizations were related to cardiac disorders with 19 more events in the PA compared to the HE group, not surprising given the participants’ age and the fact that, at baseline, the participants were sedentary. One might hypothesize that the higher, but non-significant, number of hospitalizations for cardiac disorders in the PA group was related to the PA intervention producing symptoms that revealed clinical conditions requiring a hospital admission for a precautionary evaluation or in-patient procedure. Interestingly, we did not see evidence for an increase in the rate ratio for musculoskeletal and connective tissue disorders (0.87) as a result of the PA intervention; there were 10 fewer events in the PA compared to the HE group in a total of 118 hospitalizations. With the exception of gastrointestinal disorders (1.50), the difference in the number of hospitalizations between the two groups for all other MedDRA® SOC categories was less than 20 events. We find no compelling evidence that the PA intervention led to a higher incidence of hospitalization within a specific MedDRA® SOC category when the data were examined in aggregate.
We found no evidence that a PA intervention reduced length of stay. In cross-sectional and observational studies of older adults, regular PA has been shown to significantly reduce healthcare utilization and costs, including length of stay2-5, 15-17. However, Davies and colleagues18 reported in a recent Cochrane review, that there was only a non-significant trend towards a reduction in the number of patients experiencing hospital admissions with exercise in randomized controlled trials of exercise-based interventions with six months follow-up or longer compared to usual medical care.
To provide some context to the gait speed categories that were used in our analyses, we note that working groups have proposed using cut point values of 0.8 m/s and 1.0m/s to define slow gait speed19, 20. A recent review focused on geriatric clinical settings, reported that over short distances (2-15 m) a gait speed estimate for usual pace of 0.58 m/s (95% confidence interval [CI]: 0.49–0.67) and 0.89 m/s (95% CI: 0.75–1.02) for maximal pace, while gait speed at usual pace in acute care settings was 0.46 m/s (95% CI: 0.34–0.57), which was significantly slower than the gait speed of 0.74 m/s (95% CI: 0.65–0.83) recorded in outpatient settings.21. Cesari et al.22 reported that a 6-m usual gait speed of less than 1 m/s identifies persons at high risk of health-related outcomes within a cohort of well-functioning older people. Finally, Studenski23 synthesized a wide literature base and a task force report and suggested the following gait speed categories that might be used in diagnostic testing: < 0.6 m/s - seriously abnormal; 0.6 to 1.0 - mildly abnormal; 1.0 to 1.4 – normal; 1.4 or higher - superior.
The strong interaction between baseline 400-m walk gait speed and treatment showed that individuals in the middle gait speed category accounted for the bulk of the imbalance in hospitalizations between the PA and HE groups. Overall, this remained true when we examined hospitalizations in the three gait speed categories at each site and for each MedDRA® SOC category. Potentially, individuals in the <0.8 m/s category may have exercised at a lower intensity which did not unmask physical symptoms, whereas individuals in the >1.0 m/s category, who were likely healthier and may have walked at a higher intensity, found the additional stress or disruption of homeostasis manageable. The 0.8-1.0 m/s category is a potentially more unstable category who have the cardiovascular and neuromuscular capacity to walk at a relatively high intensity but have poorer overall health so that the stress of moderate intensity PA may unmask latent physical symptoms leading to the small between treatment imbalance in hospitalizations.
However, when we examined the data for differences between the three gait speed categories for MedDRA® SOC category, field center, PA dose, and hazard rates, the overwhelming conclusion was that none of these factors furthered our understanding of the imbalance in hospitalizations within the middle gait speed category. Rather we observed remarkable consistency in the pattern of hospitalizations and the hazard rates, while the PA dose revealed nothing unusual across the three gait speed categories.
The results should be considered in the context of the study’s strengths and limitations. First, the LIFE Study was a randomized controlled trial which allowed a comparison of hospitalization data for the PA and HE groups, thereby strengthening causal inferences. However, our findings may not be applicable to other groups of older individuals, for example, older adults with no mobility limitations. Our analyses were prompted by repeated monitoring of the LIFE study for the purposes of safety, and as such, there was no control for Type I error, i.e., a false positive finding, across this repeated monitoring. In addition, we acknowledge that subgroup analyses of clinical trials have high risks of false findings. Therefore the association between the middle gait speed with increased hospitalization in the PA group relative to the HE group could be a spurious finding and not be present were the subgroups larger. However, as a safety endpoint, these results may have identified a group that needs more attention during implementation of a PA intervention. We only examined events reported to masked staff which we believe reduced the potential detection bias related to reporting of hospitalizations by unmasked interventionists. However, the more frequent contacts between PA group intervention staff and participants compared to the HE group may have led to the recognition of additional health conditions leading to referrals and slightly more in-patient procedures, a possible referral bias. However, this would not explain the interaction between 400-m walk gait speed and the intervention effect. Finally, a limitation of the 400-m walk test is that it may not be feasible to conduct in all clinical environments or patient populations. Simonsick and colleagues24 reported that walking speed during a 2 minute walk test, used as a warm-up prior to the 400-m walk test, was similar (p =0.78) to the speed during the 400-m walk test completed “as quickly as possible, at a pace you can maintain” suggesting that shorter distance/duration tests might be used to determine walking speed. Walking speeds measured during a 4 m walk and a 400-m walk at usual pace have been shown to be correlated,14 but we remain cautious about substituting short walk tests for the 400-m walk test.
In summary, we did not find compelling evidence that a PA program in older adults at high risk for mobility disability placed participants at risk for hospitalizations that involved specific types of conditions; yet, at the same time, we found no support for the hypothesis that a PA intervention reduces the rate of hospitalizations in our sample. We found no evidence of higher risk for hospitalizations between PA and HE for subgroups based on age, sex, race, or CVD and diabetes. Interestingly, we did observe that individuals with gait speeds of 0.8-1.0 m/s accounted for the majority of the imbalance in hospitalizations between the two intervention groups, albeit the difference was small (a difference of 42 participants in PA compared to HE, 12.8% absolute difference). The 400-m gait speed did appear to identify a different subgroup of participants compared to the SPPB since we did not observe a significant difference in hospitalizations in participants with an SPPB score of <8 compared to 8-9 between the PA and HE group in our subgroup analyses (Figure 2). Gait speed might be a marker that clinical intervention staff can use to identify individuals who have the physical capacity to ramp up intensity and duration of exercise but may be vulnerable with respect to health and chronic conditions.
Supplementary Material
ACKNOWLEDGMENTS
MedDRA® trademark is owned by International Federation of Pharmaceutical Manufacturers & Associations on behalf of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use.
RESEARCH INVESTIGATORS FOR THE LIFE STUDY
The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH.
The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1 P30 AG028740), Wake Forest University (1 P30 AG21332),Tufts University (1P30AG031679), University of Pittsburgh (P30 AG024827), and Yale University (P30AG021342) and the NIH/NCRR CTSA at Stanford University (UL1 RR025744).
Tufts University is also supported by the Boston Rehabilitation Outcomes Center (1R24HD065688-01A1).
Footnotes
The LIFE investigators are listed in the On-line Appendix.
Trial Registration: ClinicalTrials.gov identifier: NCT01072500
Conflict of Interest: All authors were supported by an NIH grant to work on this study.
Roger A. Fielding: received grants/funds-NIH, USDA, Nestle, Regeneron; Honoraria-Nestle, Regeneron; Consultant-Eli Lilly, Regereron, Nestle; Stocks-Pronutria, Myosyntax, Inside Trader; Board Member-Cytokinetics, Pronutria, Myosyntax, Inside Trader, Ammonett
Authors Contributions: All authors were involved in the study concept and design, acquisition of data, analysis and interpretation and preparation/review of the manuscript.
Sponsor’s Role: The NIH sponsor was a voting member (1 vote out of 12 votes) of the LIFE Steering Committee, which approved the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
LIFE investigators are also partially supported by the following:
Dr. Thomas Gill (Yale University) is the recipient of an Academic Leadership Award (K07AG3587) from the National Institute on Aging.
Dr. Carlos Fragoso (Spirometry Reading Center, Yale University) is the recipient of a Career Development Award from the Department of Veterans Affairs.
Dr. Roger Fielding (Tufts University) is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-0-014. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
Administrative Coordinating Center, University of Florida, Gainesville, FL
Marco Pahor, MD – Principal Investigator of the LIFE Study
Jack M. Guralnik, MD, PhD – Co-Investigator of the LIFE Study (University of Maryland School of Medicine, Baltimore, MD)
Christiaan Leeuwenburgh, PhD
Connie Caudle
Lauren Crump, MPH
Latonia Holmes
Jocelyn Lee, PhD
Ching-ju Lu, MPH
Data Management, Analysis and Quality Control Center, Wake Forest University, Winston Salem, NC
Michael E. Miller, PhD – DMAQC Principal Investigator
Mark A. Espeland, PhD – DMAQC Co-Investigator
Walter T. Ambrosius, PhD
William Applegate, MD
Daniel P. Beavers, PhD, MS
Robert P. Byington, PhD, MPH, FAHA
Delilah Cook, CCRP
Curt D. Furberg, MD, PhD
Lea N. Harvin, BS
Leora Henkin, MPH, Med
John Hepler, MA
Fang-Chi Hsu, PhD
Laura Lovato, MS
Wesley Roberson, BSBA
Julia Rushing, BSPH, MStat
Scott Rushing, BS
Cynthia L. Stowe, MPM
Michael P. Walkup, MS
Don Hire, BS
W. Jack Rejeski, PhD
Jeffrey A. Katula, PhD, MA
Peter H. Brubaker, PhD
Shannon L. Mihalko, PhD
Janine M. Jennings, PhD
National Institutes of Health, Bethesda, MD
Evan C. Hadley, MD (National Institute on Aging)
Sergei Romashkan, MD, PhD (National Institute on Aging)
Kushang V. Patel, PhD (National Institute on Aging)
National Heart, Lung and Blood Institute, Bethesda, MD
Denise Bonds, MD, MPH
Field Centers
Northwestern University, Chicago, IL
Mary M. McDermott, MD – Field Center Principal Investigator
Bonnie Spring, PhD – Field Center Co-Investigator
Joshua Hauser, MD – Field Center Co-Investigator
Diana Kerwin, MD – Field Center Co-Investigator
Kathryn Domanchuk, BS
Rex Graff, MS
Alvito Rego, MA
Pennington Biomedical Research Center, Baton Rouge, LA
Timothy S. Church, MD, PhD, MPH – Field Center Principal Investigator
Steven N. Blair, PED (University of South Carolina)
Valerie H. Myers, PhD
Ron Monce, PA-C
Nathan E. Britt, NP
Melissa Nauta Harris, BS
Ami Parks McGucken, MPA, BS
Ruben Rodarte, MBA, MS, BS
Heidi K. Millet, MPA, BS
Catrine Tudor-Locke, PhD, FACSM
Ben P. Butitta, BS
Sheletta G. Donatto, MS, RD, LDN, CDE
Shannon H. Cocreham, BS
Stanford University, Palo Alto, CA
Abby C. King, PhD – Field Center Principal Investigator
Cynthia M. Castro, PhD
William L. Haskell, PhD
Randall S. Stafford, MD, PhD
Leslie A. Pruitt, PhD
Kathy Berra, MSN, NP-C, FAAN
Veronica Yank, MD
Tufts University, Boston, MA
Roger A. Fielding, PhD – Field Center Principal Investigator
Miriam E. Nelson, PhD – Field Center Co-Investigator
Sara C. Folta, PhD – Field Center Co-Investigator
Edward M. Phillips, MD
Christine K. Liu, MD
Erica C. McDavitt, MS
Kieran F. Reid, PhD, MPH
Won S. Kim, BS
Vince E. Beard, BS
University of Florida, Gainesville, FL
Todd M. Manini, PhD – Field Center Principal Investigator
Marco Pahor, MD – Field Center Co-Investigator
Stephen D. Anton, PhD
Susan Nayfield, MD
Thomas W. Buford, PhD
Michael Marsiske, PhD
Bhanuprasad D. Sandesara, MD
Jeffrey D. Knaggs, BS
Megan S. Lorow, BS
William C. Marena, MT, CCRC
Irina Korytov, MD
Holly L. Morris, MSN, RN, CCRC (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Margo Fitch, PT (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Floris F. Singletary, MS, CCC-SLP (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Jackie Causer, BSH, RN (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Katie A. Radcliff, MA (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
University of Pittsburgh, Pittsburgh, PA
Anne B. Newman, MD, MPH – Field Center Principal Investigator
Stephanie A. Studenski, MD, MPH – Field Center Co-Investigator
Bret H. Goodpaster, PhD
Nancy W. Glynn, PhD
Oscar Lopez, MD
Neelesh K. Nadkarni, MD, PhD
Kathy Williams, RN, BSEd, MHSA
Mark A. Newman, PhD
George Grove, MS
Janet T. Bonk, MPH, RN
Jennifer Rush, MPH
Piera Kost, BA (deceased)
Diane G. Ives, MPH
Wake Forest University, Winston Salem, NC
Stephen B. Kritchevsky, Ph.D. – Field Center Principal Investigator
Anthony P. Marsh, PhD – Field Center Co-Investigator
Tina E. Brinkley, PhD
Jamehl S. Demons, MD
Kaycee M. Sink, MD, MAS
Kimberly Kennedy, BA, CCRC
Rachel Shertzer-Skinner, MA, CCRC
Abbie Wrights, MS
Rose Fries, RN, CCRC
Deborah Barr, MA, RHEd, CHES
Yale University, New Haven, CT
Thomas M. Gill, MD – Field Center Principal Investigator
Robert S. Axtell, PhD, FACSM – Field Center Co-Investigator (Southern Connecticut State University, Exercise Science Department)
Susan S. Kashaf, MD, MPH (VA Connecticut Healthcare System)
Nathalie de Rekeneire, MD, MS
Joanne M. McGloin, MDiv, MS, MBA
Karen C. Wu, RN
Denise M. Shepard, RN, MBA
Barbara Fennelly, MA, RN
Lynne P. Iannone, MS, CCRP
Raeleen Mautner, PhD
Theresa Sweeney Barnett, MS, APRN
Sean N. Halpin, MA
Matthew J. Brennan, MA
Julie A. Bugaj, MS
Maria A. Zenoni, MS
Bridget M. Mignosa, AS
Cognition Coordinating Center, Wake Forest University, Winston Salem, NC
Jeff Williamson, MD, MHS – Center Principal Investigator
Kaycee M Sink, MD, MAS – Center Co-Investigator
Hugh C. Hendrie, MB, ChB, DSc (Indiana University)
Stephen R. Rapp, PhD
Joe Verghese, MB, BS (Albert Einstein College of Medicine of Yeshiva University)
Nancy Woolard
Mark Espeland, PhD
Janine Jennings, PhD
Electrocardiogram Reading Center, University of Florida, Gainesville, FL
Carl J. Pepine MD, MACC
Mario Ariet, PhD
Eileen Handberg, PhD, ARNP
Daniel Deluca, BS
James Hill, MD, MS, FACC
Anita Szady, MD
Spirometry Reading Center, Yale University, New Haven, CT
Geoffrey L. Chupp, MD
Gail M. Flynn, RCP, CRFT
Thomas M. Gill, MD
John L. Hankinson, PhD (Hankinson Consulting, Inc.)
Carlos A. Vaz Fragoso, MD
Cost Effectiveness Analysis Center
Erik J. Groessl, PhD (University of California, San Diego and VA San Diego Healthcare System)
Robert M. Kaplan, PhD (Office of Behavioral and Social Sciences Research, National Institutes of Health)
REFERENCES
- 1.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li CL, Sheu JT, Wang TA, et al. The relationship between healthy lifestyle and hospital utilization among adults with diabetes: Results from a national cohort in Taiwan. Asia Pac J Public Health. 2015;27:303–313. doi: 10.1177/1010539514524817. [DOI] [PubMed] [Google Scholar]
- 3.Perkins AJ, Clark DO. Assessing the association of walking with health services use and costs among socioeconomically disadvantaged older adults. Prev Med. 2001;32:492–501. doi: 10.1006/pmed.2001.0832. [DOI] [PubMed] [Google Scholar]
- 4.Martin MY, Powell MP, Peel C, et al. Leisure-time physical activity and health-care utilization in older adults. J Aging Phys Act. 2006;14:392–410. doi: 10.1123/japa.14.4.392. [DOI] [PubMed] [Google Scholar]
- 5.LaCroix AZ, Leveille SG, Hecht JA, et al. Does walking decrease the risk of cardiovascular disease hospitalizations and death in older adults? J Am Geriatr Soc. 1996;44:113–120. doi: 10.1111/j.1532-5415.1996.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 6.Woolcott JC, Ashe MC, Miller WC, et al. Does physical activity reduce seniors' need for healthcare?: A study of 24 281 Canadians. Br J Sports Med. 2010;44:902–904. doi: 10.1136/bjsm.2008.057216. [DOI] [PubMed] [Google Scholar]
- 7.Penninx BW, Ferrucci L, Leveille SG, et al. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 8.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 9.Hardy SE, Kang Y, Studenski SA, et al. Ability to walk 1/4 mile predicts subsequent disability, mortality, and health care costs. J GenInternMed. 2011;26:130–135. doi: 10.1007/s11606-010-1543-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: Design and methods. J Gerontol A Biol Sci Med Sci. 2011;66A:1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68:1549–1558. doi: 10.1093/gerona/glt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 13.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 14.Rolland YM, Cesari M, Miller ME, et al. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52:972–976. doi: 10.1111/j.1532-5415.2004.52267.x. [DOI] [PubMed] [Google Scholar]
- 15.Li CL, Chu SJ, Sheu JT, et al. Impact of physical activity on hospitalization in older adults: A nationwide cohort from Taiwan. Arch Gerontol Geriatr. 2011;53:141–145. doi: 10.1016/j.archger.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Liu-Ambrose TY, Ashe MC, Marra C, et al. Chronic Conditions Research T. Independent and inverse association of healthcare utilisation with physical activity in older adults with multiple chronic conditions. Br J Sports Med. 2010;44:1024–1028. doi: 10.1136/bjsm.2008.046458. [DOI] [PubMed] [Google Scholar]
- 17.Metsios GS, Stavropoulos-Kalinoglou A, Treharne GJ, et al. Disease activity and low physical activity associate with number of hospital admissions and length of hospitalisation in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13:R108. doi: 10.1186/ar3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies EJ, Moxham T, Rees K, et al. Exercise based rehabilitation for heart failure. Cochrane Database System Rev. 2010:CD003331. doi: 10.1002/14651858.CD003331.pub3. [DOI] [PubMed] [Google Scholar]
- 19.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peel NM, Kuys SS, Klein K. Gait speed as a measure in geriatric assessment in clinical settings: A systematic review. J Gerontol A Biol Sci Med Sci. 2013;68:39–46. doi: 10.1093/gerona/gls174. [DOI] [PubMed] [Google Scholar]
- 22.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 23.Studenski S. Bradypedia: Is gait speed ready for cinical use? J Nutr Health Aging. 2009;13:878–880. doi: 10.1007/s12603-009-0245-0. [DOI] [PubMed] [Google Scholar]
- 24.Simonsick EM, Montgomery PS, Newman AB, et al. Measuring fitness in healthy older adults: The Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49:1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.