Abstract
In diving birds and mammals, bradycardia and peripheral vasoconstriction potentially isolate muscle from the circulation. During complete ischemia, ATP production is dependent on the size of the myoglobin oxygen (O2) store and the concentrations of phosphocreatine (PCr) and glycogen (Gly). Therefore, we measured PCr and Gly concentrations in the primary underwater locomotory muscle of emperor penguin and modeled the depletion of muscle O2 and those energy stores under conditions of complete ischemia and a previously determined muscle metabolic rate. We also analyzed stroke rate to assess muscle workload variation during dives and evaluate potential limitations on the model. Measured PCr and Gly concentrations, 20.8 and 54.6 mmol kg−1, respectively, were similar to published values for non-diving animals. The model demonstrated that PCr and Gly provide a large anaerobic energy store, even for dives longer than 20 min. Stroke rate varied throughout the dive profile indicating muscle workload was not constant during dives as was assumed in the model. The stroke rate during the first 30 seconds of dives increased with increased dive depth. In extremely long dives, lower overall stroke rates were observed. Although O2 consumption and energy store depletion may vary during dives, the model demonstrated that PCr and Gly, even at concentrations typical of terrestrial birds and mammals, are a significant anaerobic energy store and can play an important role in the emperor penguin’s ability to perform long dives.
INTRODUCTION
In diving birds and mammals, bradycardia and peripheral vasoconstriction may reduce perfusion-dependent metabolic rates of organs and potentially isolate muscle from the circulation during forced submersion (Blix et al. 1983; Scholander 1940; Scholander et al. 1942; Zapol et al. 1979). If locomotory muscle is isolated from the circulation during a dive due to extreme vasoconstriction, a continuous supply of ATP must be generated locally as muscles continue to work propelling the animal through the water. Under these conditions, the oxygen (O2) necessary to generate ATP during aerobic metabolism is provided by the myoglobin (Mb)-bound O2 store. It has been suggested that the depletion of the Mb-O2 store is the basis for the aerobic dive limit (ADL, the dive duration beyond which post-dive blood lactate begins to accumulate) (Kooyman et al. 1983; Williams et al. 2011). In dives beyond the ADL, ATP levels can be maintained anaerobically through phosphocreatine (PCr) hydrolysis and glycolysis. If muscle is completely ischemic for an entire dive, making ATP production for muscular work dependent on the Mb-O2, PCr and glycogen (Gly) stores, then dive performance may be limited by the magnitude of these stores and muscle workload during the dive. Despite the potentially critical role of these anaerobic energy stores in diving birds and mammals, there is a dearth of information on the magnitude of these stores and their potential contribution to ATP production under anaerobic conditions.
It is well-established that diving birds and mammals have increased concentrations of myoglobin (Mb) in locomotory muscles, with Mb concentrations of some diving animals tenfold higher than terrestrial species (Burns et al. 2010; Noren et al. 2001). Gly is important not only for glycolysis, but can also provide a substrate during aerobic metabolism. The importance of the Gly store in the muscle metabolism of diving birds and mammals is further suggested by the accumulation of lactate, likely from glycolysis, after dives or forced submersions (Kooyman et al. 1983; Kooyman et al. 1980; Ponganis et al. 1997b; Scholander et al. 1942; Shaffer et al. 1997; Williams et al. 1999). Accordingly, increased concentrations of Gly in locomotory muscles of diving birds and mammals might also be expected. Yet, Gly concentrations in diving birds, including fasting emperor penguins, and mammals examined to date are not elevated above terrestrial animals (Groscolas and Rodriguez 1982; Kerem and Elsner 1973; Scholander et al. 1942). Thus, while Gly concentrations may not be elevated in diving animals, Gly clearly plays a role in the muscle metabolism of diving animals. However, few studies have examined the Gly store’s potential contribution to anaerobic ATP production during dives.
Less is known about the magnitude and role of PCr in diving birds and mammals. In humans, PCr hydrolysis plays a critical role in ATP production during the transition from rest to exercise (Barstow et al. 1994; Haseler et al. 2004; Marsh et al. 1993; McCann et al. 1995). However, the potential role of PCr in anaerobic metabolism in diving birds and mammals was initially thought to be insignificant compared to that of anaerobic glycolysis (Scholander et al. 1942). Studies examining total creatine (Cr) or indirectly measuring PCr with calibrated nuclear magnetic resonance (NMR) suggest that PCr concentrations in diving birds and mammals are not elevated above concentrations in terrestrial species (Blix 1971; Stephenson and Jones 1992). Although if PCr concentrations in Weddell seals are assumed to be similar to values in terrestrial animals, the PCr store could still provide up to one-third more ATP than the Mb-O2 store (Butler and Jones 1997). However, PCr concentrations have not yet been directly measured in any diving animal. Thus, the potential role and even magnitude of the PCr store in anaerobic metabolism during dives remains unclear.
Dive performance will be affected not only by the magnitude of the Gly and PCr stores and their contribution to ATP production, but also by the depletion rate of these stores. Previous studies examining depletion of the Mb-O2, PCr and Gly stores on forcibly submerged animals necessarily excluded the effect of locomotory muscle workload on muscle metabolic rate (Scholander et al. 1942; Stephenson and Jones 1992). Muscle metabolic rate of freely diving birds and mammals has rarely been measured and, even if measured, variations in locomotory muscle workload may change muscle metabolic rate, affecting depletion patterns of the anaerobic energy stores during dives (Williams et al. 2000; Williams et al. 2004). For example, gliding periods or stroke-and-glide patterns reduce locomotory costs during dives (Williams et al. 2000). Thus, an examination of stroke rate during diving is essential for a more complete understanding of muscle metabolism and depletion of the Mb-O2 and anaerobic energy stores.
To address these deficiencies in current knowledge, a comprehensive analysis of the potential contributions from aerobic and anaerobic metabolism during dives of a freely diving bird or mammal is needed. The emperor penguin (Aptenodytes forsteri Gray 1844) is a paragon of avian divers with dives ranging between 2 and 12 min. Most dives at sea are below the measured ADL of 5.6 min (Kooyman and Kooyman 1995; Ponganis et al. 1997b), however, dives longer than 20 min have been observed on rare occasions (Ponganis et al. 2007; Wienecke et al. 2007). Recent studies suggest locomotory muscle is isolated from the circulation in at least some dives of emperor penguins (Ponganis et al. 2007; Meir et al. 2008; Williams et al. 2011). In addition, the diving physiology of emperor penguins is well-studied, including the recent measure of locomotory muscle O2 depletion rate (12.4 ml O2 kg−1 muscle) in emperor penguins freely diving at an isolated dive hole in McMurdo Sound, Antarctica (Williams et al. 2011). For these reasons, the emperor penguin makes an ideal model to study magnitude and depletion of anaerobic energy stores and stroke rate.
The goals of this research were to 1) quantify the magnitude of the Mb-O2 and anaerobic energy stores in the emperor penguin primary locomotory muscle, the pectoralis-supracoracoideus complex, 2) model the intramuscular depletion of Mb-O2, PCr and Gly and the accumulation of lactate during dives of different durations based on the anaerobic energy stores determined in the present study and the previously determined mean diving locomotory muscle metabolic rate (Williams et al. 2011), and 3) analyze the rate of stroke rate (wing beats) during dives at sea in order to assess the limitations of the model due to potential variation of muscle workload and metabolic rate throughout a dive. We hypothesized that 1) PCr and Gly concentrations in the emperor penguin would not be elevated above values in terrestrial species, 2) the estimated intracellular Gly depletion and lactate accumulation during routine dives beyond the ADL would be minimal, consistent with the short surface intervals observed after such dives (Kooyman and Kooyman 1995; Wienecke et al. 2007) and 3) stroke rate would vary throughout the dive (Sato et al. 2002; van Dam et al. 2002).
MATERIALS AND METHODS
Mb-O2 and Metabolite Concentrations
Experimental approach
Non-breeding emperor penguins (8 to 15 per season, N = 10 for this experiment) were captured on the sea ice of McMurdo Sound, Antarctica in the austral springs of 2007 and 2008. Penguins were transported to a sea ice camp (Penguin Ranch) with a corral and two isolated dive holes on McMurdo Sound (77° 41’, 165° 59’). Penguins dived freely through the isolated dive holes as previously described (Kooyman et al. 1992). They foraged on sub-ice fish, squid and other prey as verified by underwater visual observations and guano deposits. Once experiments were completed, and within 6 weeks of capture, all penguins were released at the sea ice edge. All procedures were approved under a UCSD Animal Subjects Committee protocol and a US Antarctic Treaty permit.
Muscle biopsy sampling
Muscle biopsies were obtained from ten penguins (mass: 23.6 ± 1.9 kg; range: 21–26 kg) under isoflurane anesthesia (Kooyman et al. 1992) using a Bergstrom muscle biopsy needle (Bergstrom 1962). All muscle biopsies were taken from the pectoralis-supracoracoideus complex, 3–4 cm lateral to midline at the level of the axilla. Biopsy samples for Mb, PCr, creatine (Cr), Gly, lactate and water content analysis were obtained from a muscle depth of 6 – 8 cm and frozen immediately in liquid nitrogen (N2). Samples for water content analysis were weighed prior to being frozen. Total mass of all muscle biopsy samples taken from any one penguin ranged from approximately 300 mg to 600 mg. Samples were stored at (−)80° C until analysis, which was within 30 days.
To verify the biopsy method did not alter resting muscle condition, lactate values were determined in separate control samples in 4 penguins (Lowry and Passoneau 1972). Biopsy samples used for Gly analysis were taken approximately 48 hours after penguins were captured (while wandering the sea ice) and before diving began at Penguin Ranch. For other samples, biopsies were taken either 12.7 ± 5.3 hours after diving or at the same time Gly samples were taken.
Myoglobin assay
Frozen muscle samples were thawed, cleaned of fat or connective tissue and weighed. Muscle Mb concentration was determined spectrophotometrically using a modified Reynafarje method (Reynafarje 1963). After preliminary results showed a high Mb concentration for emperor penguin samples, the dilution factor was changed from 19.25 ml gram−1 tissue to 60 ml gram−1 tissue. The homogenate was centrifuged at 13,000 g at 0° C for 90 min (Eppendorf Centrifuge 5402 w/ rotor F-45-18-11, Hamburg, Germany). Spectrophotometric measurements were taken at 538 nm and 568 nm on a Shimadzu UV 2501PC spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Equine Mb standard (Sigma-Aldrich, St. Louis, MO) and blanks were run on all assays. All Mb assays (n = 6) were run in duplicate.
Sample preparation for metabolite assays and water content analysis
Muscle tissue samples were lyophilized for 24 hours (bench-top vacuum freeze-dryer, Virtis, Gardiner, NY, USA) and then reweighed. Water content was determined by the difference between the original biopsy wet weight (ww) and the freeze-dried weight (dry weight, dw). For PCr, Cr and Gly assays, non-muscle material (e.g., blood, connective tissue, feather particles) was removed from samples under a dissecting microscope. Samples were minced and then divided for analysis (2–4 mg dw for Gly, 4–10 mg dw for PCr and Cr).
Extraction and neutralization for metabolite assays
For PCr and Cr assays, extraction followed procedures outlined in Lowry and Passoneau (1972). Briefly, 0.05 ml of 2M perchloric acid (PCA) (pre-cooled in ice) per 1 mg tissue sample was added and then the sample mixed at 0° C for 10 min using a vortex mixer. Samples were spun at 15,000 g at 5° C for 10 min and supernatant was removed. The supernatant was cooled in dry ice until a slurry-type consistency was obtained. Then, 0.05 ml of 2M KHCO3 per 1 mg tissue was added to the tube. The sample was spun again at 15,000 g at 5° C for 10 min (Lowry and Passoneau 1972).
For Gly assays, 0.1 ml of 2N HCl was added per 1 mg tissue sample. The sample was weighed and then incubated at 100° C for 2 hours. Samples were reweighed and deionized H2O added to obtain the original weight. An equal volume of 2N NaOH was added and the samples spun at 15,000 g for 10 min.
Metabolite assays
PCr, Cr and Gly concentrations were determined using the Lowry and Passoneau (1972) method, with the following modifications. The PCr assay was particularly sensitive to pH. Each individual sample pH was checked after extraction and adjusted to a pH of 8.1 with additional KHCO3 or PCA as necessary. Any resulting changes in volume were accounted for in calculations. To stabilize the creatine kinase enzyme, the enzyme was mixed and stored in a 0.25M glycyl-glycine solution at 5 mg/ml. In addition, 0.1% albumin was added to the reaction buffer to prevent inactivation of creatine kinase due to dilution. Standards and blanks were run on all assays. All assays were run in duplicate.
PCr and Cr were assayed in 7 penguins, and Gly in 5 penguins. Sample sizes for PCr and Cr (n=7), Gly (n=5) and Mb (n=6) differed due to size of muscle samples and available extraction volumes for metabolite assays. Results for lactate, PCr, Cr and Gly assays are reported in mmol kg−1 muscle ww, using the conversion factor obtained from the water content analysis. Mb concentration is reported as g 100 g−1.
Metabolite assay validation
In order to validate sample preparation, extraction and assay methods, all metabolite assays were run on muscle samples from Holtzman rats (Rattus norvegicus) supplied by another lab. Muscle biopsies were obtained from the biceps femoris or gluteus maximus muscle after injection of a lethal dose of pentobarbital or, for PCr samples, while under isoflurane anesthesia.
Mb-O2 Store and Anaerobic Energy Store Depletion during Dives
Mb-O2 and metabolites were converted to ATP equivalents in order to (1) compare potential contributions from aerobic metabolism and anaerobic stores and (2) calculate depletion of those stores under the model. The ATP equivalents, in mmol kg−1 muscle, were calculated to be: (1) 0.268 ATP per ml O2; (2) 37 ATP per mmol Gly (glucosyl unit) used as a substrate during aerobic respiration; (3) 1 ATP per mmol PCr; and (4) 3 ATP per mmol Gly (glucosyl unit) used during anaerobic respiration (Hochachka and Somero 1984). The ATP equivalent for the muscle metabolic rate, 12.4 ml O2 min−1, was 3.32 ATP min−1. The ATP equivalent for O2 (0.268 ATP per ml O2) was calculated from ATP produced during aerobic metabolism using glycogen and intracellular fat as metabolic substrates. The exact percentage of fat and glycogen used in aerobic metabolism during diving is unknown. The assumption that glycogen provided 67% of the substrate and the remainder provided by intracellular fat was based on moderate exercise and the efficiency of glycolysis during hypoxia (Hochachka and Somero, 1984). However the difference between P:O ratios of fat and glycogen is minor: 6.2 for Gly vs 5.6 for fat (palmitate).
Using the PCr and Gly concentrations determined in this study, the modeling of depletion of the Mb-O2, PCr and Gly stores during dives was based on the following assumptions: (1) no muscle blood flow occurred during the dives (Meir et al. 2008; Ponganis et al. 2009; Ponganis et al. 2007; Williams et al. 2011); (2) locomotory muscle metabolic rate was constant after the first minute at a rate of 12.4 ml O2 kg−1 muscle min−1 (Williams et al. 2011); (3) in addition to a muscle metabolic rate of 12.4 ml O2 kg−1 muscle min−1 during the first min, PCr declined by 30% during the first minute based on the work-rest transition phase requirements demonstrated in exercise physiology studies (Barstow et al. 1994; Haseler et al. 2004; Marsh et al. 1993; McCann et al. 1995); (4) Mb-O2 depletion continued to provide the required ATP until 6 min into a dive; during that time, aerobic metabolism of glucose from the Gly store provided 67% of the metabolic substrate; it is assumed that intracellular fat provided the remainder (Hochachka and Somero 1984); (5) once Mb-O2 declined to 15–20% saturation, PCr and Gly depletion, at a 1:2 ratio (Kemp et al. 2001), provided 80% of ATP requirements. This assumption was based on a previously shown rise in lactate prior to complete Mb-O2 depletion (Scholander et al. 1942); (6) after Mb-O2 was fully desaturated, PCr hydrolysis and glycolysis provided 100% of ATP requirements, with Gly accounting for twice the metabolic fuel compared to PCr (Kemp et al. 2001); (7) it was assumed that PCr would not decline below 15% of resting value (Harris et al. 1986) and, thereafter, Gly contributed 100% of ATP requirements; and, (8) lactate levels in the locomotory muscle were assumed to be 2.3 mmol kg−1 muscle prior to dives, based on results from control values obtained in this study; any accumulation of lactate was assumed to be directly related to anaerobic glycolysis.
The ATP equivalents available from depletion of Mb-O2, breakdown of PCr, and anaerobic conversion of Gly to lactate were calculated on the basis of the concentrations measured in the biopsy samples. In addition, using the above assumptions, depletion of the Mb-O2, PCr and Gly stores were calculated for dives up to 23.1 min. Dives up to 12 min represent regular dive durations of emperor penguins and the 23.1 min dive represents the longest dive observed at the isolated dive hole (Ponganis et al. 2007).
Stroke Rate during Dives at Sea
Penguin capture and instrument attachment
Emperor penguins were captured as they departed on foraging trips from the Cape Washington colony in Antarctica (74°39′ S, 165°24′ E), in the austral spring of 2005. Multi-sensor data loggers (W1000-PD2GT, Little Leonardo Ltd, Tokyo) were attached to seven penguins (mass: 24.6 ± 2.6 kg; range: 22 – 29 kg) with waterproof tape (Tesa tape 4651, Tesa SE, Hamburg, Germany) and cable ties as previously described (Sato et al 2002; Sato et al. 2011; Wilson et al. 1997). The W1000-PD2GT loggers were 22 mm in diameter, 122 mm in length, with a mass of 73 g in air. They recorded depth and temperature at a sampling rate of 1 Hz, and two-dimensional acceleration at a sampling rate of 16 Hz. VHF transmitters (Model MM130, ATS, Isanti, MN, USA) were also attached using cable ties in order to relocate penguins after returning from foraging trips. Returning instrumented penguins were recaptured and instruments removed.
Data processing
Dive data were processed, graphed, and statistically analyzed using IGOR Pro (WaveMetrics, Inc., Lake Oswego, OR, USA), Origin (version 7.5, OriginLab Corp., Northampton, MA, USA), SPSS (version 11.5, SPSS, Inc. Chicago, IL, USA) and Microsoft Excel (Redmond, WA, USA). Several custom-developed macros were used in IGOR Pro to analyze dive and stroke rate data. Dives were defined as submergences two m or deeper and two min or longer in duration. Dive durations of two min or longer were necessary in order to examine stroke rate profiles throughout dives and model usage of Mb-O2 and anaerobic energy stores during dives. Dives were divided into 7 categories of dive depth (0–25, 25–50, 50–100, 100–200, 200–300, 300–400 and >400m). Post-dive surface intervals were calculated from the end of the previous dive to the beginning of the next dive and, as a result, may include short (<2 min) and shallow submergences (<2 m). Post-dive surface intervals greater than 60 min (n = 42) were excluded from the analysis as it appeared the penguin may have emerged from the water during these long surface intervals (range: 67 – 653 min).
Peaks occur in the longitudinal acceleration data as a result of the thrust generated from wing strokes (van Dam et al. 2002; Sato et al. 2002; Sato et al. 2005). Each peak was marked and counted from a custom-made peak detection script and then visually confirmed. Dive stroke rate for each dive was calculated from the total number of peaks for each dive divided by dive duration. Instantaneous stroke rate for 30-sec periods was determined using a custom-made macro and reported as a mean of all beat-to-beat intervals within a 30-sec period. The depth and time of the final glide during ascent to the surface were identified by marking the last stroke during each dive. Start-of-glide depth was expressed as a percentage calculated by dividing the depth of the last stroke by the maximum dive depth. The percentage of time gliding was determined by dividing the time between the last stroke and end of dive by the dive duration.
Statistics
Values are expressed as mean ± s.d., unless otherwise noted. Grand means were calculated using the means of data from each penguin. Linear regression was used to describe the relationship for each penguin between (1) stroke rate and maximum dive depth; (2) stroke rate and dive duration; (3) stroke rate during the first 30-sec period and maximum dive depth; and, (4) ascent gliding time and maximum dive depth. Residuals from the four regression analyses for each individual penguin were analyzed to assess normality and homogeneity. Data were log-transformed to correct instances of non-normal distribution or heterogeneity of residuals. To examine stroke rate within dives of different depths, the mean instantaneous stroke frequency for all dives in each dive depth category at 30-sec intervals was calculated. Statistical significance was assumed at P < 0.05.
RESULTS
Mb-O2 and Energy Store Concentrations
Water content
Water content of emperor penguin muscle tissue was 68.9 ± 1.5%, n=9, which agrees with previously published data on emperor penguin pectoralis muscle (68.7%) (Groscolas and Rodriguez, 1982). Therefore, in converting dw results of metabolite assays to ww, a conversion factor of 3.2 was used.
Lactate and myoglobin
Emperor penguin muscle lactate values from control samples were 2.3 ± 0.6 mmol kg−1 muscle (n=4), which are in the same range (2 – 4 mmol kg−1 muscle) as resting muscle lactate values in humans, seals and dogs (Eklof et al. 1981; Harris et al. 1986; Scholander et al. 1942; Tesch 1980). A resting muscle lactate concentration of 2.3 mmol kg−1 muscle was used in the model. Mb concentration was 6.5 ± 0.2 g 100g−1 (n=6). With an Mb concentration of 6.5 g 100g−1 and an O2-binding capacity of 1.34 ml O2 g−1 Mb, the Mb-O2 store for the model was calculated to be 86 ml O2 kg−1 muscle.
Metabolites
Mean PCr and Cr concentrations in emperor penguins were 20.8 ± 1.3 mmol kg−1 muscle (ww) and 12.0 ± 2.6 mmol kg−1 muscle (ww), respectively (Table 1). Mean Gly concentration was 54.6 ± 8.1 mmol kg−1 muscle (Table 1). Variance between duplicates was typically less than 10% and no samples were excluded due to high variance.
Table 1.
Phosphocreatine (PCr), creatine (Cr), and total Cr concentrations in muscles of mammals and birds
| Species | PCr | Cr | Total Cr | Glycogen | Reference |
|---|---|---|---|---|---|
| Emperor penguin | 21 | 12 | 33 | 55 | This study |
| Pekin duck | 25–32 |
Stephenson and Jones 1992; Stephenson et al. 1997 |
|||
| Starling | 16 | 13 | 29 | Beis and Newholme 1975 | |
| Pigeon | 14 | 18 | 32 | Beis and Newholme 1975 | |
| Eider | 35 | Blix 1971 | |||
| Rat | 14–23 | 9 | 23–31 | 34 | This study; Beis and Newholme 1975; Brault et al. 2003 |
| Cheetah | 26 | 40 | Williams et al. 1997 | ||
| Dogs | 18–24 | 33–105 | Harris et al. 1986 | ||
| Hooded seal | 31 | Blix 1971 | |||
| Weddell seal | 33–61 | Kerem et al. 1973 | |||
| Fasting emperor penguin | 30–60 | Groscolas and Rodriguez 1982 | |||
| Chicken | 62 | Edwards et al. 1999 | |||
| Bottlenose dolphin | 59–67 | Goforth 1986 |
Note. Concentrations shown in millimoles per kilogram of muscle.
PCr and Gly control values in rats (13.7 ± 1.8 mmol kg−1 muscle ww and 33.7 ± 0.8 mmol kg−1 muscle ww, respectively, n=3) were comparable to published values in rat muscle (Brault et al. 2003; Saltin and Gollnick 1983).
Aerobic and Anaerobic Contributions to Locomotory Muscle Metabolism during Dives
Aerobic metabolism, based on depletion of the Mb-O2 store, provides only 11% of the total ATP potentially available in isolated locomotory muscle, whereas anaerobic metabolism provides 89%, over eight times more total ATP than the Mb-O2 store. Within the anaerobic store, and Gly provides the majority of total ATP potentially available at 76%, while PCr provides 13%.
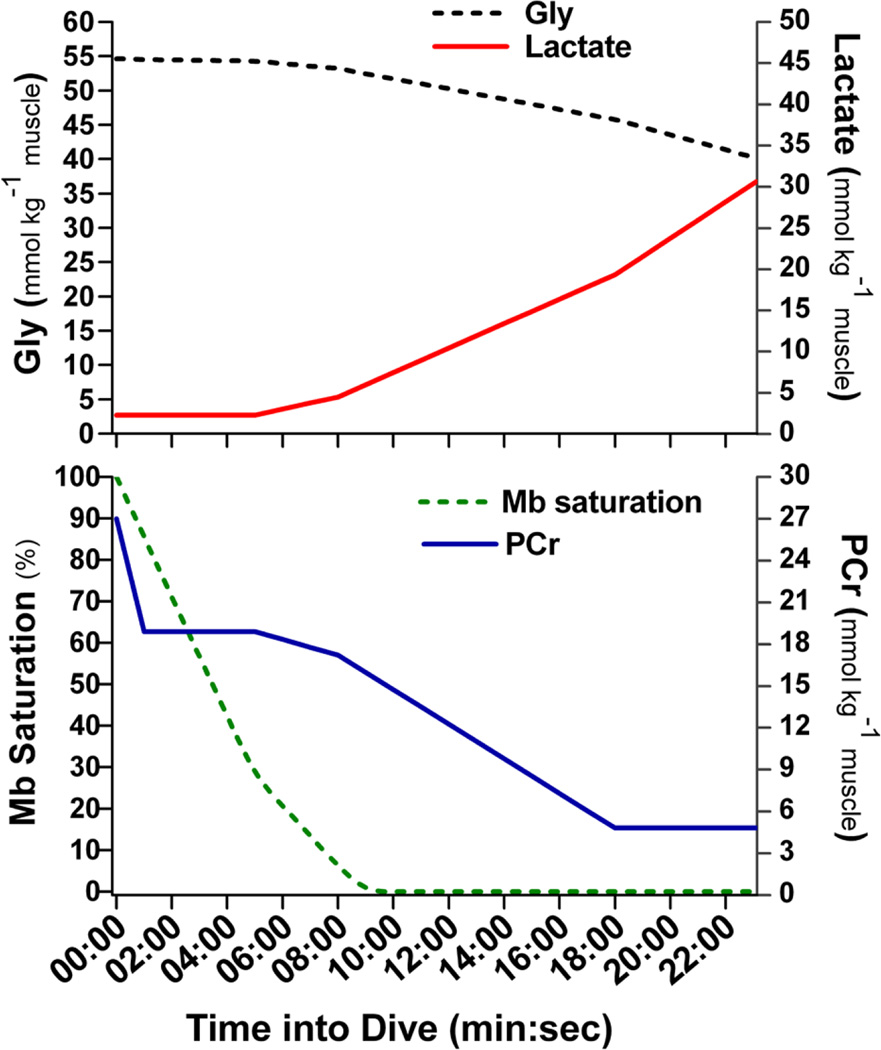
A theoretical time-course of depletion of the Mb-O2, PCr and Gly stores and accumulation of lactate during a 23.1 min dive is shown in Fig. 1. Gly store is less than 27% depleted after a theoretical 23.1 min dive.
Figure 1.
Model results showing changes in myoglobin (Mb) saturation, phosphocreatine (PCr) store, glycogen (Gly) store and muscle lactate in a hypothetical 23.1 min dive. Myoglobin is nearly completely desaturated within the first six min. Under the model, the PCr and Gly stores produce almost all of the required ATP after the first 6 min of long dives. (red line Mb saturation, blue line PCr, black line glycogen, green line lactate).
Stroke Rate during Dives at Sea
General dive data
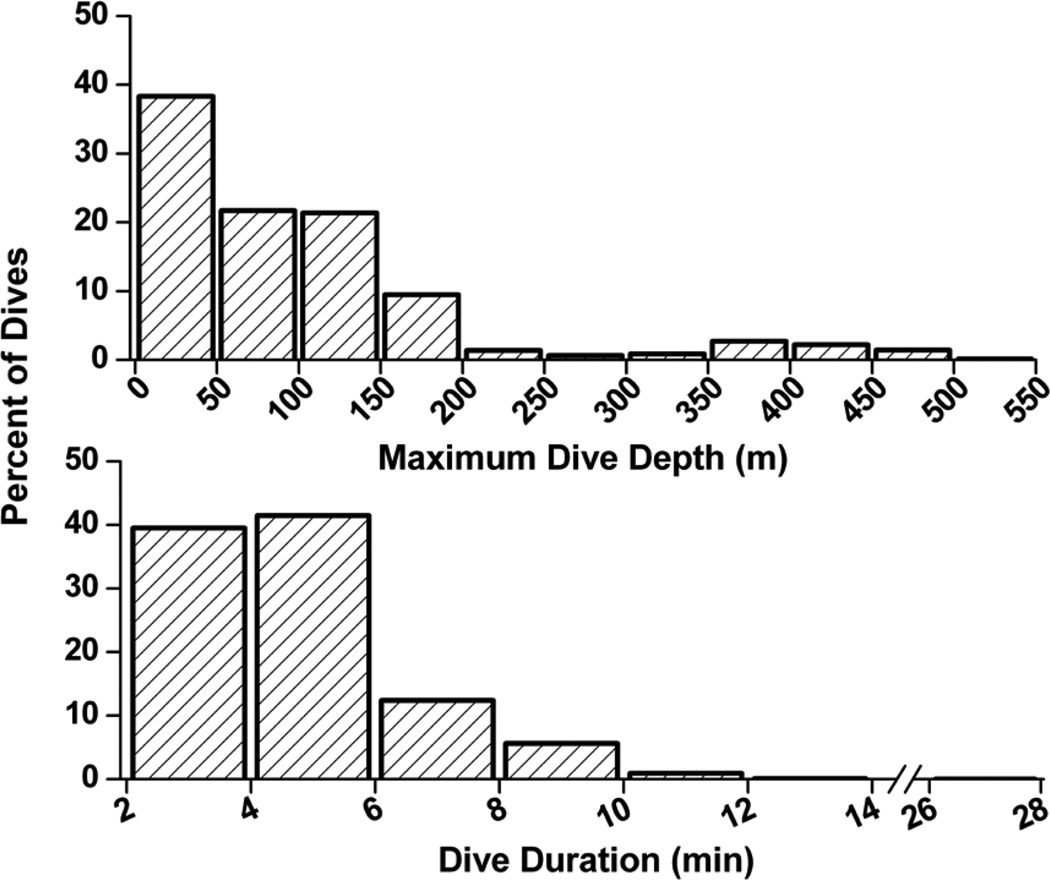
Stroke rate and dive data were obtained from seven birds for a total of 3250 dives > 2 min duration (Table 2). Dive durations from all dives ranged from 2.0 to 27.6 min (Fig. 2). Twenty-seven percent of dives were longer than the 5.6 min ADL, and 1% of dives were greater than 10 min. The grand mean dive duration was 4.7 ± 0.5 min. Maximum dive depth for all dives ranged from 7.8 to 513.5 m (Fig. 2), with a grand mean dive depth of 102.9 ± 28.6 m. Forty percent of dives had a maximum depth of 100 m or deeper, and 9% of dives were deeper than 200 m (Fig. 2). Grand mean stroke rate was 0.67 ± 0.05 Hz, with a range of 0.32 to 1.04 Hz for all dives. Mean post-dive surface intervals ranged from 1.7 to 3.3 min, with a grand mean of 2.6 ± 0.6 min (Table 2).
Table 2.
Body mass, number of dives, dive depth, dive duration, stroke rate, and postdive surface interval of emperor penguins diving at Cape Washington
| Penguin | Body mass (kg) |
No. dives |
Mean maximum depth (m) |
Mean dive duration (min) |
Mean stroke rate (strokes s−1) |
Mean postdive surface interval (min) |
|---|---|---|---|---|---|---|
| 1 | 29.0 | 424 | 54.5 ± 61.2 (9–357.5) | 3.7 ± 1.4 (2.0–8.4) | .59 ± .10 (.35–1.0) | 2.0 ± 3.8 (.18–46.13) |
| 2 | 23.0 | 148 | 132.2 ± 94.5 (15.3–418.0) | 5.1 ± 1.8 (2.0–12.2) | .67 ± .10 (.42–1.01) | 2.9 ± 3.7 (.45–29.70) |
| 3 | 21.5 | 452 | 91.8 ± 56.9 (10.0–423) | 4.6 ± 1.8 (2.0–12.7) | .73 ± .08 (.44–.96) | 1.7 ± 1.8 (.37–28.85) |
| 4 | 23.5 | 502 | 91.2 ± 97.0 (11.5–513.5) | 4.7 ± 1.9 (2.0–11.5) | .66 ± .06 (.4–.87) | 2.8 ± 3.9 (.30–29.93) |
| 7 | 24.0 | 498 | 127.1 ± 124.4 (11.3–475.8) | 4.9 ± 2.1 (2.0–10.2) | .72 ± .08 (.41–1.04) | 3.3 ± 5.0 (.08–48.92) |
| 8 | 27.5 | 447 | 92.7 ± 105.9 (9.5–501.8) | 4.6 ± 2.2 (2.0–27.7) | .65 ± .09 (.32–.92) | 2.5 ± 4.4 (.02–34.38) |
| 9 | 24.0 | 474 | 130.6 ± 112.9 (7.8–499.5) | 5.1 ± 1.9 (2.0–11.0) | .66 ± .10 (.36–1.02) | 3.2 ± 4.3 (.22–34.38) |
| Grand mean | 24.6 ± 2.6 | 464 ± 29 | 102.9 ± 28.6 | 4.7 ± .5 | .67 ± .05 | 2.6 ± .6 |
Note. Surface interval after the 27.6-min dive in penguin 8 was 8.4 h. All surface intervals greater than 60 min were excluded from calculation of mean postdive surface interval. Data shown as mean ± SD (range).
Figure 2.
Distribution of dive duration (top) and maximum dive depth (bottom) of dives from emperor penguins at sea (N=3250 dives).
Stroke rate, dive depth and dive duration
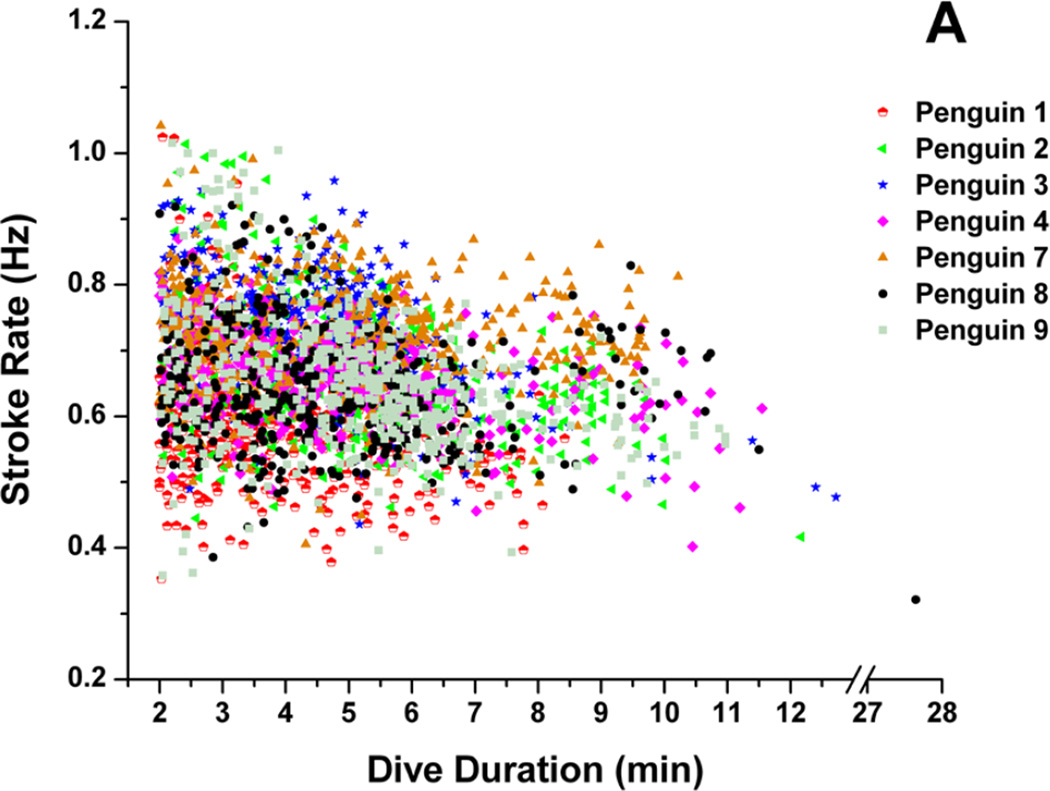
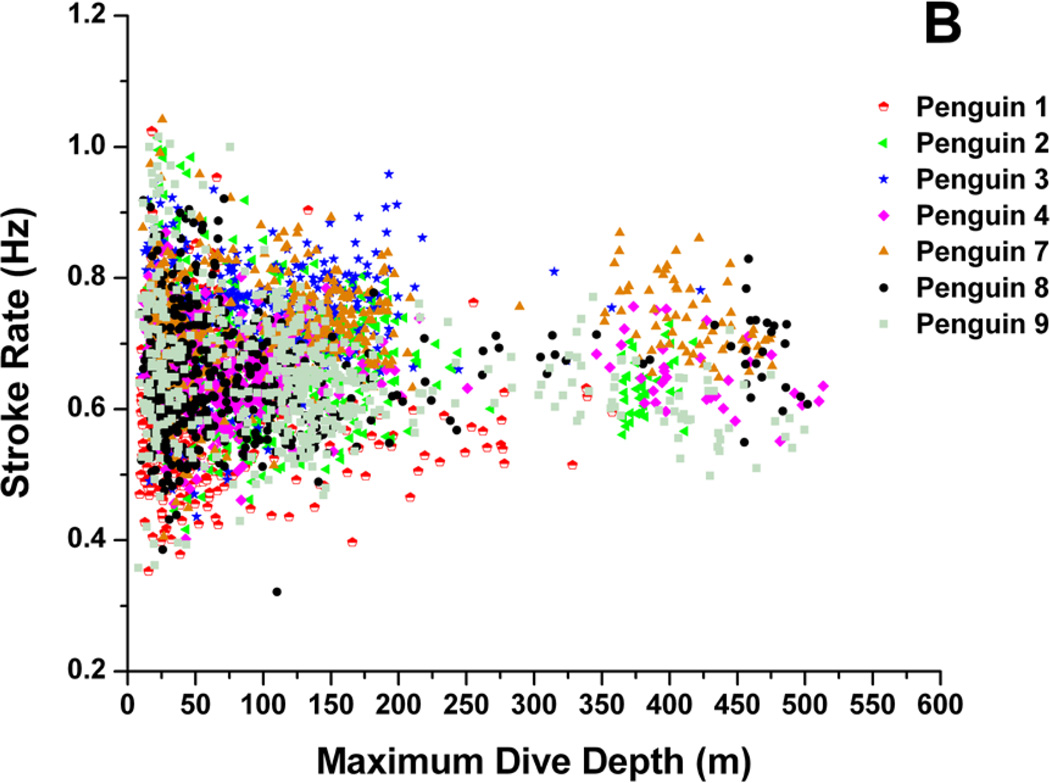
In six out of seven penguins, there was a weak, but significant inverse relationship between dive stroke rate and dive duration, (Fig. 3a) (r2 = 0.03 to 0.21, p<0.01). Dive stroke rate was very weakly related to dive depth in four of seven penguins (Fig. 3b) (r2 = 0.01 to 0.05, p <0.001 to p=0.01).
Figure 3.
Relationship between (A) dive stroke rate and dive duration and (B) dive stroke rate and maximum dive depth in seven penguins.
Stroke rate within dives
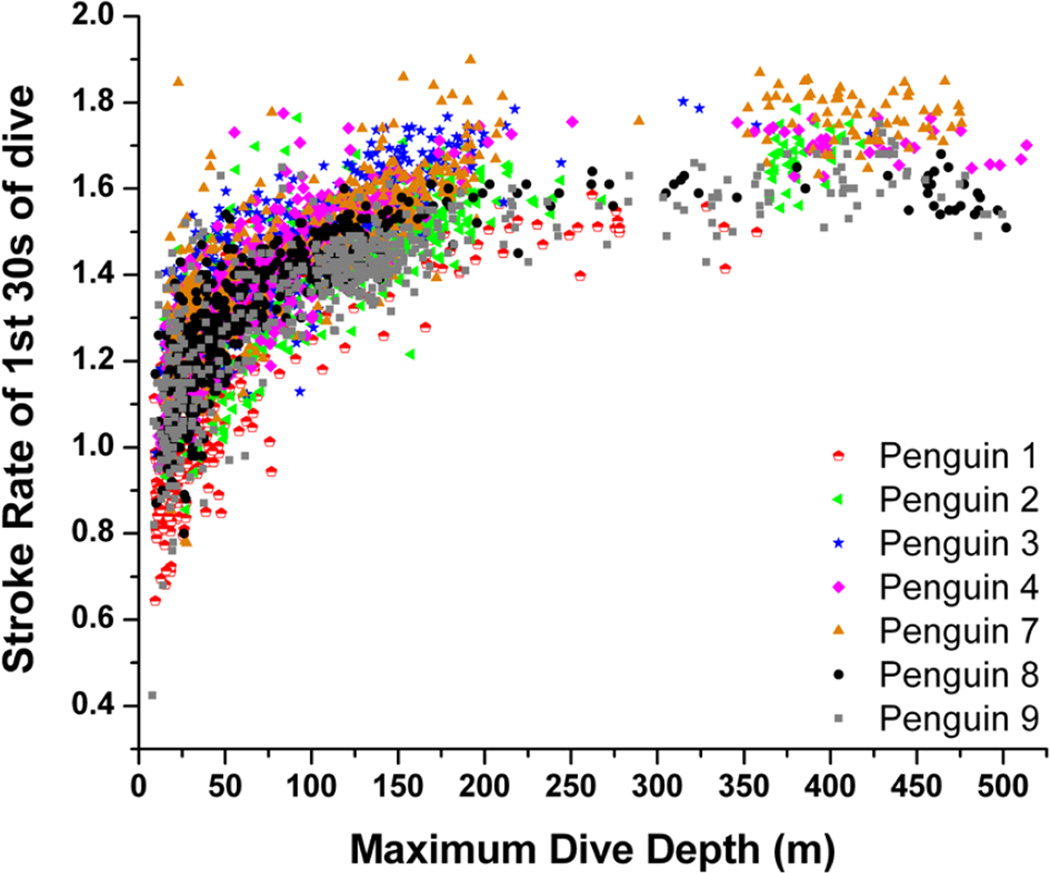
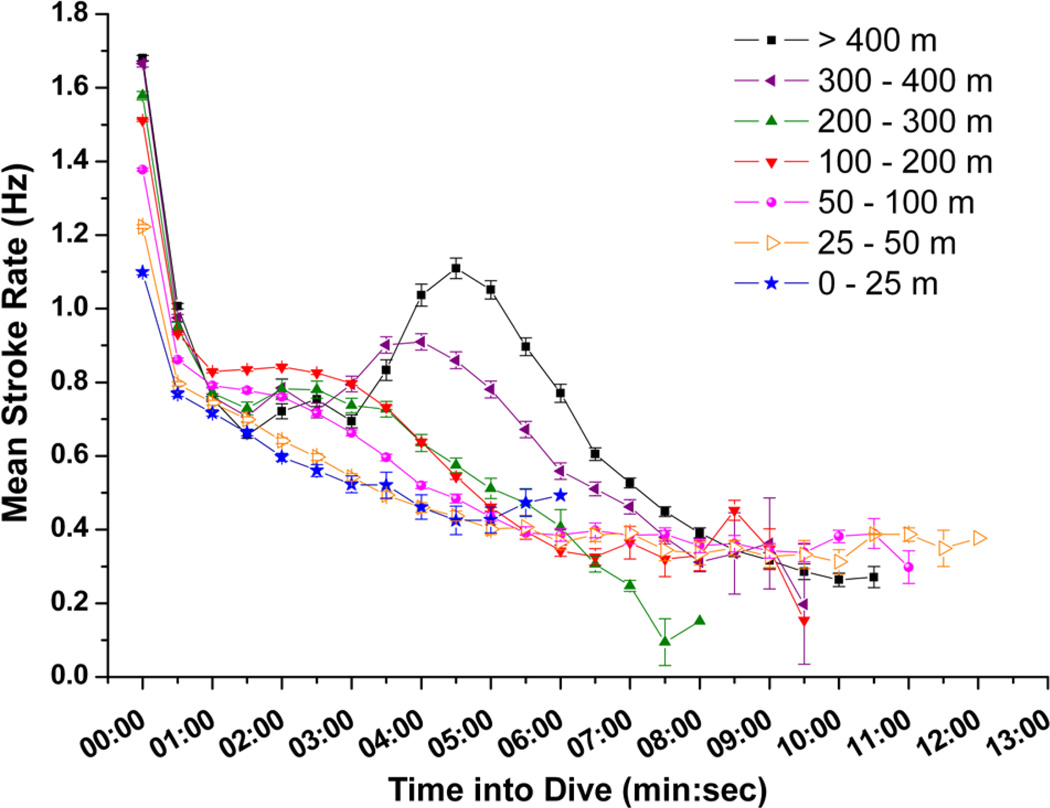
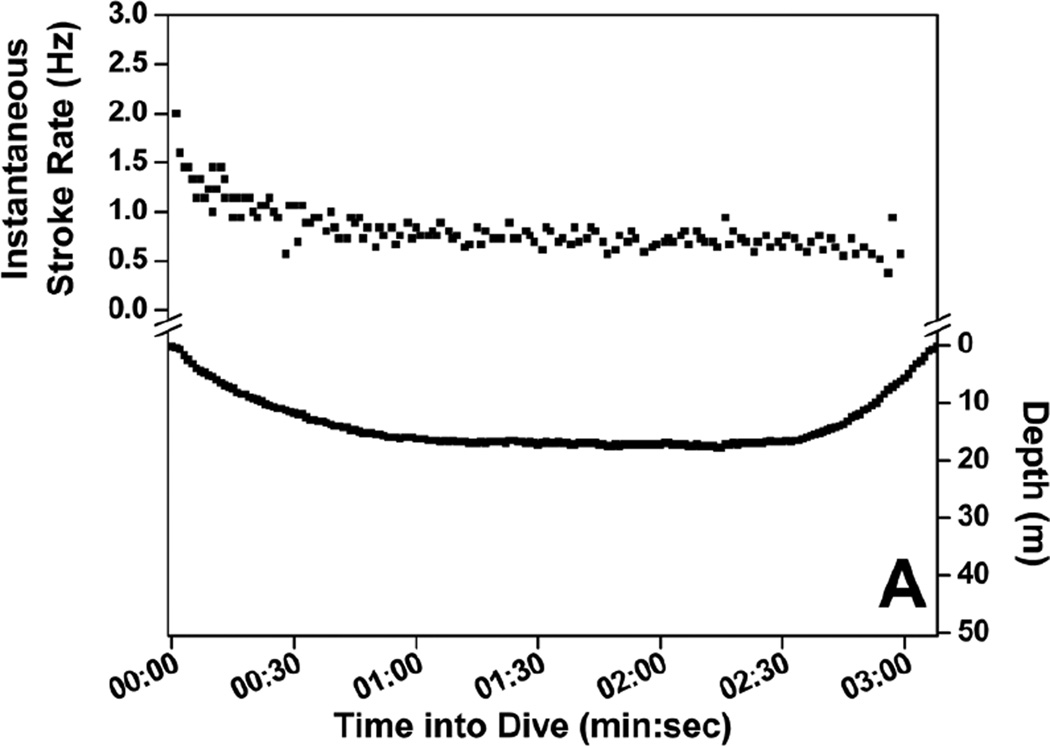
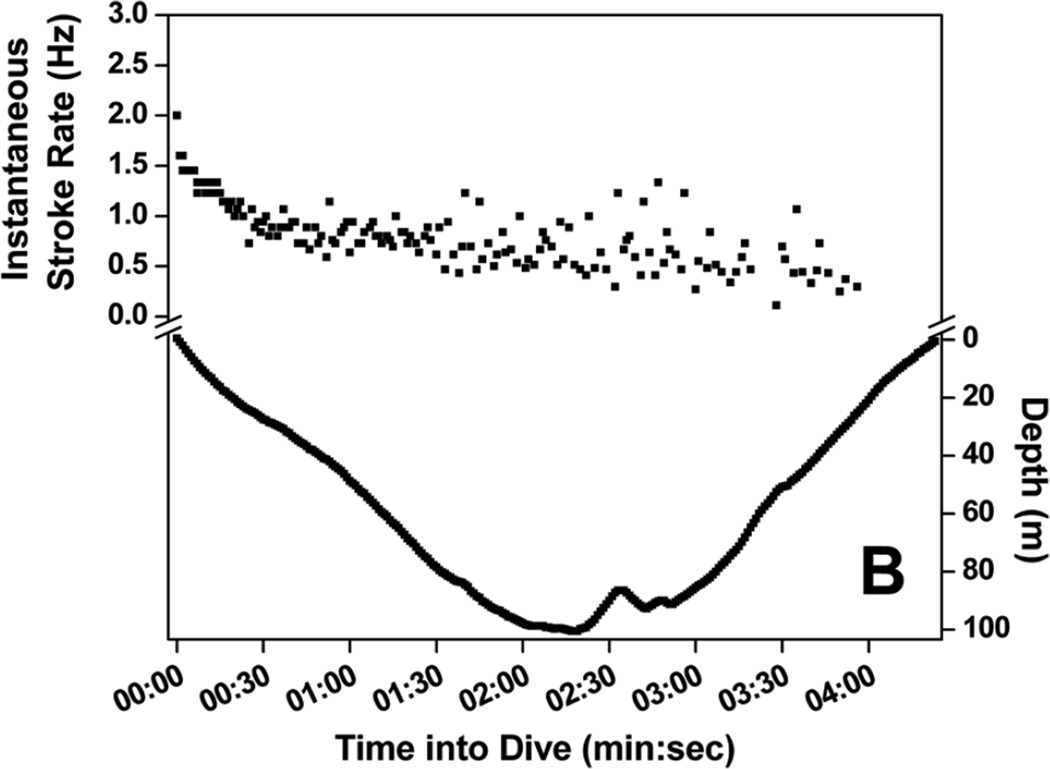
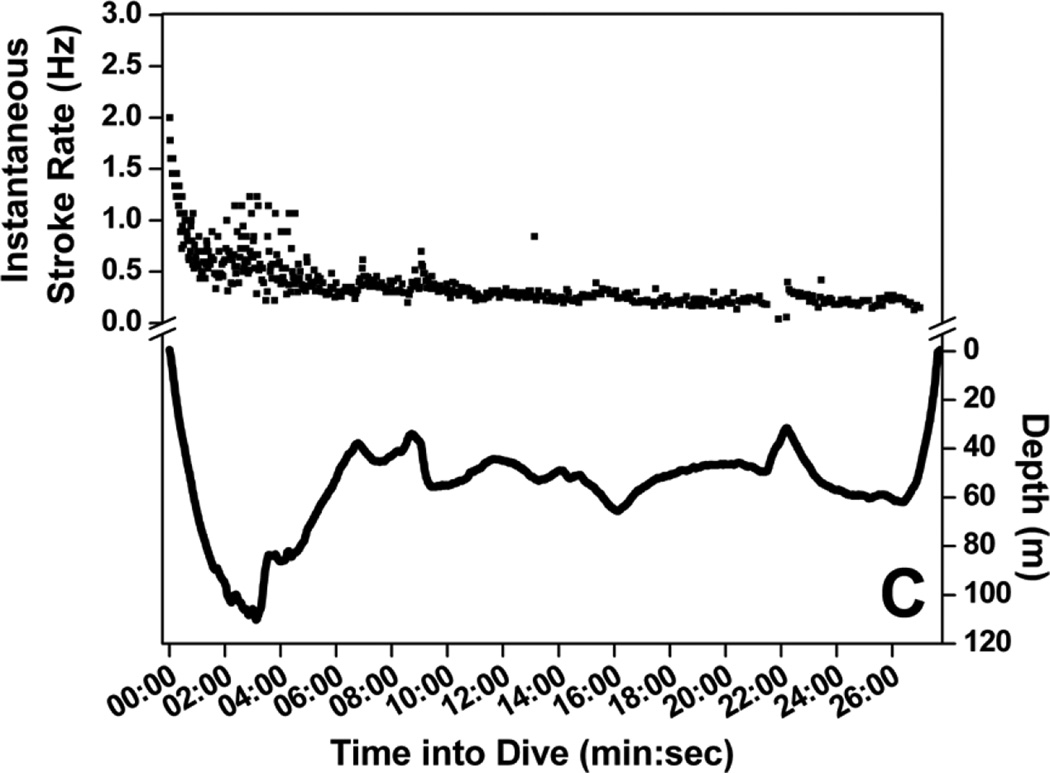
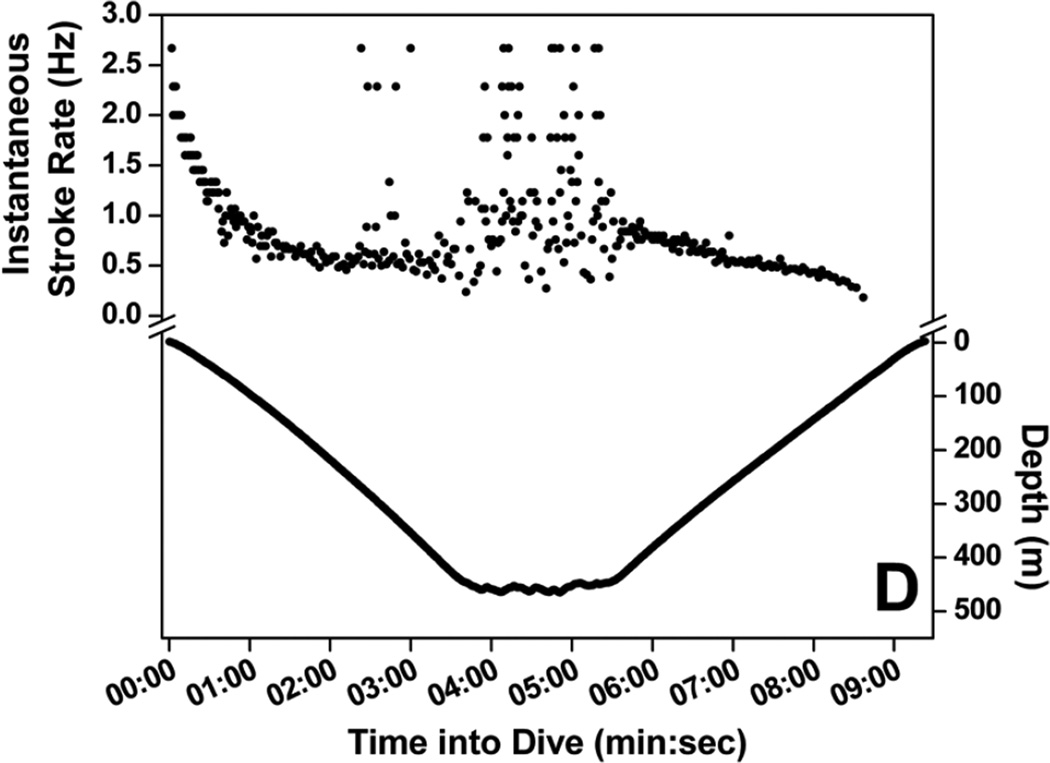
Stroke rate for the first 30-sec of dives increased with increasing maximum dive depth (Fig. 4) up to 200 m, where initial stroke rate leveled off and remained high, above 1.4 Hz (Fig. 4). A regression relationship between initial stroke rate and dive depth was significant in all seven penguins (r2 = 0.62 to 0.76, P <0.001). Mean stroke rate at 30-sec intervals of dives representing seven depth categories are shown in Fig. 5. Instantaneous stroke rate profiles in three dives of varying depth and in the 27.6 min dive, the longest reported dive for an emperor penguin (Sato et al. 2011) are shown in Fig. 6.
Figure 4.
Relationship between stroke rate for the first 30-sec of dives and maximum dive depth. Note first 30-sec stroke rate increases with increasing dive depth up to 200 m where stroke rate remains high, near 1.7 Hz
Figure 5.
Profiles of mean stroke rate at 30-sec intervals of dives for seven depth categories (0 – 25 m, 25 – 50 m, 50 – 100 m, 100 – 200 m, 200 – 300 m, 300 – 400 m and > 400 m). Standard error bars shown. The 27.6 min dive is excluded from this analysis because it was the only dive beyond 13 min. Instantaneous stroke rate for the 27.6 min dive is shown separately in Fig. 6c.
Figure 6.
Instantaneous stroke rate profiles of four dives: (A) 18 m, 3.1 min dive; (B) 101 m, 4.6 min dive; (C) 110 m, 27.6 min dive; and (D) 465 m, 9.3 min dive.
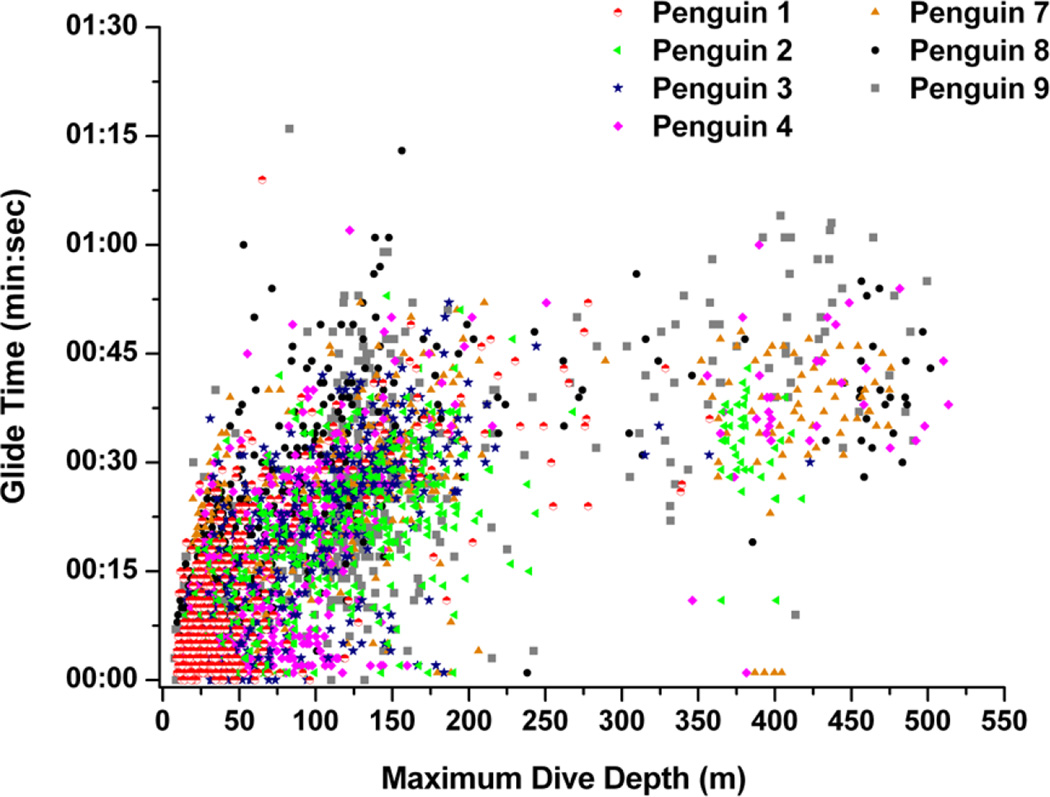
Mean depth of the last stroke before surfacing occurred between 12 and 28 m, or at 21 to 30% of maximum dive depth for all penguins. Grand mean last-stroke depth occurred at 22 ± 6 m and at 24.5 ± 2.9 % of maximum depth. Mean gliding time during the ascent ranged from 12 to 23 seconds, with a mean of 19 ± 4 seconds for all penguins. This accounted for an average of 5.0 to 8.2% of dive duration, with a grand mean of 6.7 ± 1.0 % of dive duration. Gliding time was significantly and positively related to maximum dive depth for each penguin (r2 = 0.34 to 0.55, P < 0.001) (Fig. 7).
Figure 7.
Relationship between ascent glide time and maximum dive depth.
DISCUSSION
Mb-O2 and Energy Store Concentrations
Myoglobin
Elevated Mb concentrations in locomotory muscle is one of the most striking adaptations of diving birds and mammals, with Mb concentrations often tenfold higher than their terrestrial counterparts (Burns et al. 2010; Noren et al. 2001). High Mb concentrations provide a significant O2 store for diving. Previous studies demonstrated an extremely high Mb concentration in emperor penguin locomotory muscle, which was confirmed in the present study (Ponganis et al. 1997a).
Phosphocreatine and creatine
Although muscle O2 stores have been measured in many diving species, few studies have examined concentrations of the anaerobic muscle energy sources, particularly PCr concentrations. We report the first PCr concentration directly measured in a diving animal; it is comparable to most terrestrial animals and in Pekin ducks, where PCr concentration was measured indirectly with calibrated NMR measurements (Stephenson and Jones 1992) (Table 1). In examining total Cr in seals and terrestrial animals, it was initially proposed that an elevated PCr concentration in seals might be beneficial for long dives because it would provide not only additional fuel for extending dives, but would also reduce acidification of muscle resulting from anaerobic metabolism (Blix 1971). However, as Blix found for total Cr in seals and eiders, emperor penguins do not have elevated PCr or Cr concentrations in their primary locomotory muscle (Table 1). Furthermore, elevated PCr concentrations may not be advantageous. In a transgenic mouse model, elevated PCr and Cr concentrations in cardiac muscle were associated with decreased glycolytic capacity, cardiac hypertrophy, and heart failure (Phillips et al. 2010; Wallis et al. 2005).
It has been reported that PCr concentrations determined from muscle biopsy samples may underestimate the true PCr concentration. When determined in vivo by 31P mass spectrometry, PCr concentrations are greater than those determined from muscle biopsies (Brault et al. 2003). This is likely due to breakdown of PCr to Cr during the biopsy procedure despite rapid freezing of the sample (Brault et al. 2003). Analysis of biopsy samples taken after inhibition of creatine kinase with iodoacetamide resulted in a 6 mmol kg−1 (20 – 30 %) increase in measured PCr concentrations in resting muscle (Brault et al. 2003). Correcting the assayed PCr concentration in emperor penguin locomotory muscle by this amount would result in a concentration of 27 mmol kg−1 muscle We believe this value, which is less than total Cr concentration (33 mmol kg−1 muscle), is an appropriate value since not all Cr is available to form PCr (Brault et al. 2003). Accordingly, a PCr concentration of 27 mmol kg−1 muscle was used in the modeling experiment.
Glycogen
Locomotory muscle Gly concentrations vary significantly within and between species. In general, skeletal muscle Gly concentrations in diving birds and mammals (31 to 80 mmol kg−1 muscle) are highly varied and similar to concentrations in most terrestrial animals (26 to 71 mmol kg−1 muscle) (Edwards et al. 1999; Goforth 1986; Groscolas and Rodriguez 1982; Kerem et al. 1973; Saltin and Gollnick 1983). Gly concentrations determined in this study are not notably different from fasting emperor penguins, other birds or mammals (Table 1). This Gly concentration (54.6 mmol kg−1 muscle) was used as the initial value for the Gly store in the model.
Mb-O2, PCr and Gly Depletion during Dives
Large anaerobic energy stores
The conversion of the Mb-O2 and anaerobic energy stores into ATP equivalents demonstrates the extremely large anaerobic store available during dives if all stores were depleted. ATP produced by aerobic metabolism in locomotory muscle isolated from the circulation is limited by O2 availability (86 ml O2 kg−1 muscle) to 23 mmol ATP kg−1 muscle. The PCr store, if completely depleted, would provide 27 mmol ATP kg−1 muscle, slightly more than from aerobic metabolism. The Gly store could potentially provide more than seven times the ATP produced from aerobic metabolism, with a maximum capacity of 163 mmol ATP kg−1 muscle. However, it is unlikely the entire Gly store would be consumed, due to the extremely high accumulated lactate (110 mmol lactate kg−1 muscle) that would result. Although rarely examined, this analysis demonstrates that anaerobic stores constitute surprisingly large energy reserves for diving penguins.
Potential contribution of anaerobic stores
The model results illustrate a potential depletion profile of anaerobic energy stores and highlight the role these stores play in emperor penguin diving (Fig. 1). PCr and Gly concentrations are not elevated above values in terrestrial species (Table 1), yet these stores provide a considerable anaerobic energy reservoir in the locomotory muscle of emperor penguins during dives. As illustrated in Fig. 1, under the model, the PCr and Gly stores produce almost all of the required ATP after the first 6 min of long dives. At the end of 8 min dives, when the Mb-O2 store is substantially depleted, PCr concentration is still above 50% of its pre-dive level and the Gly store is less than 3% depleted (Fig. 1). Even after dives twice as long as the 5.6 min ADL, 44% of the PCr store remains and the Gly store is over 90% of its pre-dive level (Fig. 1). Despite the availability of PCr and Gly for longer dives, most emperor penguin dives at sea are less than the ADL (Table 2) (Kooyman and Kooyman 1995; Wienecke et al. 2007).
Extreme dives of emperor penguins
Penguins do, on occasion, dive for extraordinarily long durations and, during these dives, the large anaerobic stores become essential (Fig. 1). In more extreme dives, such as the 23.1 min dive recorded at the isolated dive hole (Ponganis et al. 2007), both the Mb-O2 and PCr stores would be nearly depleted under the model, but the Gly store would remain more than 70% of pre-dive level (Fig. 1). The rate and magnitude of the depletion of muscle Mb-O2 and anaerobic energy stores, as well as lactate accumulation, modeled for a hypothetical 23.1 min dive is illustrated in Fig. 1. At the end of this dive, muscle lactate concentration would be 30.6 mmol kg−1 muscle. Although quite high, this value is much less than the muscle lactate concentration observed in a seal forcibly submerged for 15 min, 42 mmol kg−1 muscle (Scholander et al. 1942) and in a thoroughbred horse at a full gallop, 50 mmol kg−1 muscle (Snow et al. 1985).
A dive this long is clearly a rare event. In over 150,000 dives at sea, including those discussed below, less than 0.1% of dives were longer than 12 min in duration and only three were longer than 20 min (Kooyman and Kooyman 1995; Wienecke et al. 2007). Some of these extreme dives may be a consequence of diving in heavy pack ice, where openings in the ice may close at any time, requiring a submerged bird to extend its dive to find a new breathing hole (Kooyman and Kooyman 1995). Thus, the ability to endure long duration dives may be critical for the habitat in which emperor penguins dive; and, from the model, it is apparent that these extraordinarily long dives are well-within the anaerobic capacities of emperor penguin locomotory muscle.
Recovery rates and surface intervals
After the 23.1 min dive, as well as after the now longest dive recorded for an emperor penguin (27.6 min), no diving occurred for at least several hours (Ponganis et al. 2009; Sato et al. 2011). However, in other extreme dives, surface intervals were less than an hour (Wienecke et al. 2007). Most post-dive surface intervals only last a few minutes (Table 2) (Kooyman and Kooyman 1995). In emperor penguins, the Mb-O2 store resaturates within several minutes after dives (Williams et al. 2011). PCr recovery is similarly rapid in exercise studies, where PCr concentrations recovered from 25 to 70% depletion to near resting levels within one to two min (Blei et al. 1993; Forbes et al. 2009; McCully et al. 1994). Thus, Mb-O2 and PCr recovery should occur within most post-dive surface intervals. Although it is not known how washout of muscle lactate translates to blood lactate concentrations, the decline in blood lactate concentration is also fairly rapid in emperor penguins, near 0.6 mM min−1 (Ponganis et al. 1997b).
However, Gly resynthesis can be much slower and resynthesis times vary widely between and within species. While recovery from Gly depletion in penguins has not been studied, Gly recovery in humans is biphasic and dependent several factors, including post-exercise food consumption and Gly depletion level (Jentjens and Jeukendrup 2003). After strenuous exercise, Gly resynthesis in humans varied between 2 and 8 mmol kg−1 muscle hr−1 depending on the type and timing of food consumption (Blom et al. 1987; Ivy et al. 1988). Gly recovery in horses can be two to three times longer than in humans (Waller and Lindinger 2010). In contrast, full recovery from 50% Gly depletion in rats after moderate exercise occurred within 30 min (Garetto et al. 1984). Since 99% of emperor penguin dives at sea are less than 12 min, Gly depletion is minor after most dives (Fig. 1). However, repeated Gly-depleting dives may be significant and, depending on recovery rates in penguins, extended surface intervals may be required to replenish the Gly store.
Model provides a reasonable framework for depletion of the Mb-O2 and anaerobic energy stores
The assumptions in the model are based on diving or exercise physiology studies and the model results are in accord with findings from past diving physiology studies of emperor penguins. For example, the rate of Mb-O2 depletion, based on a recent study (Williams et al. 2011), and rates of anaerobic energy stores depletion used in the model (Fig. 1) are consistent with onset of post-dive blood lactate accumulation only in dives beyond the ADL (Ponganis et al. 1997b). The concurrence of PCr and Gly depletion, after the Mb-O2 store is substantially depleted, has been demonstrated in exercise studies (Kemp et al. 2001), and, in forcibly submerged ducks, where both glycolysis and PCr hydrolysis began within the first two min of the submergence (Stephenson and Jones 1992). If the entire PCr store was consumed before glycolysis started, lactate accumulation would not begin until dives 11 min or longer, which is inconsistent with the ADL concept, or the rise in blood lactate concentration observed in dives longer than 5–6 min (Ponganis et al. 1997b). Further, although depletion of PCr before glycolysis began would slow the rate of pH decline since PCr hydrolysis consumes H+ as part of its reaction, this is likely not necessary due to the high buffering capacity of the locomotory muscle in diving birds and mammals (Castellini and Somero 1981; Mill and Baldwin 1983).
The model provides a general framework for analyzing the depletion of Mb-O2, PCr and Gly stores, but necessarily does not account for all variation within dives. For example, the model assumes no locomotory muscle blood flow during dives. However, muscle blood flow does appear to occur in the middle of some dives (Williams et al. 2011). If locomotory muscle is perfused during a dive, depletion of the three stores (Mb-O2, PCr and Gly) and lactate accumulation would be reduced depending on the amount of muscle blood flow. Similarly, the model uses a muscle metabolic rate based on a constant, relatively high stroke rate (Williams et al. 2011). As discussed below, stroke rate may vary between and within dives.
In conclusion, from this model, most dives at sea are aerobic in nature and do not significantly deplete anaerobic energy stores. When penguins do dive longer they have substantial anaerobic energy stores for extremely long dives.
Dive and Stroke Rate Patterns at Sea
Dive behavior
Dives in this study were typical of dives in earlier studies of penguins at sea (Kooyman and Kooyman 1995; Wienecke et al. 2007). Despite the exclusion of dives less than 2 min in duration, grand mean dive duration was 4.7 min (Table 2), well below the ADL of 5.6 min. These results reinforce the conclusion that while at sea, most dives are less than the ADL. However, included in this analysis is a 27.6 min dive, the longest reported dive for an emperor penguin (Sato et al. 2011).
Stroke rate and locomotory muscle workload
Only a weak relationship was found between dive stroke rate and dive duration in six penguins (Fig. 3a). However, in examining dives greater than 12 min, stroke rates were less than 0.5 Hz (Fig. 3a), well below the grand mean of 0.67 Hz. The relationship between dive depth and dive stroke rate, although statistically significant in three penguins, was not biologically significant (Fig. 3b). These results suggest that, rather than examining stroke rate of individual dives, stroke rate changes within dives may be more indicative of variations in locomotory muscle workload during diving. In addition to stroke rate, stroke amplitude and stroke thrust are variables that also probably contribute to muscle workload. However, these have not been measured in any freely diving penguins.
Within dives, stroke rate was highest at the beginning of dives (Figs. 4, 5), as penguins worked to overcome their positive buoyancy (van Dam et al. 2002). This high initial stroke rate increased with deeper maximum dive depths (Fig. 4). For example, in dives less than 25 m, nearly all dives had an initial stroke rate less than or equal to 1.4 Hz. In contrast, in dives > 200 m, there was only one dive with an initial stroke rate of less than or equal to 1.4 Hz (Fig. 4). These results indicate that, not only is locomotory muscle workload highest at the beginning of dives, but also that it increases as maximum dive depth increases. In addition, it suggests penguins anticipate the depth of dives at the very beginning of the dive, stroking significantly faster for dives that will go to deeper depths. These increased stroke rates may be secondary to large diving air volumes and greater buoyancy at the start of deeper dives (Sato et al. 2002; Sato et al. 2011). From these data, it is clear that stroke rate, and thus locomotory muscle workload, varies significantly at the beginning of dives (Figs. 4, 5) and this variation changes with maximum dive depth.
Stroke rate also increased in the middle of deep dives. In dives deeper than 400 m, stroke rate was above 1.0 Hz at 4 to 5 min into dives (Fig. 5). Figure 6d shows a characteristic example of these deep dives with increased instantaneous stroke rate when the bird reached near maximum depth. Depth profiles revealed small variations at the bottom of these dives coincident with higher instantaneous stroke rate, suggesting foraging on a mid-water fish, the Antarctic silverfish Pleuragramma antarcticum (Kooyman and Kooyman 1995). Similar increases in stroke rate also occurred in the middle of 300 to 400 m dives, although the increases were not as great (Fig. 5). These high stroke rates did not usually occur in the middle of shallower dives (Figs 5, 6).
Finally, stroke rate, and thus locomotory muscle workload, declined to low levels at the end of most dives (Fig. 5). In all depth categories, stroke rate at the end of dives was near or less than 0.4 Hz (Fig. 5). Unlike penguins diving at an isolated dive hole (van Dam et al. 2002), penguins at sea glide during final ascents (Fig. 7) (Sato et al. 2002). In emperor penguins diving at sea, gliding time increased with maximum depth (Fig. 7). Gliding during the ascent began at about 25% of maximum dive depth and accounted for 5 to 8 % of the dive duration, a cost-saving strategy during ascents (Williams et al. 2000).
The longest recorded dive of an emperor penguin, 27.6 min, provides an informative example of the variation in stroke rate throughout a dive (Fig. 6c). Instantaneous stroke rate started out near 2 Hz, decreased rapidly until the penguin approached 100 m, when a secondary increase in stroke rate to above 1 Hz occurred but then quickly declined to less than 0.5 Hz. After the penguin ascended to about 60 m, instantaneous stroke rate remained near or less than 0.3 Hz throughout the remainder of the dive. The overall stroke rate for this dive was 0.32 Hz, less than half the mean stroke rate of dives from which the muscle metabolic rate was measured (0.74 Hz) (Williams et al. 2011).
Stroke rate variation: implications for the model
The modeling of depletion of Mb-O2 and anaerobic energy stores provides only a basic framework to examine what occurs in locomotory muscle during dives. After the first minute of diving, the model assumed a constant muscle metabolic rate. However, stroke rate profiles demonstrated that for all dive depths, stroke rate, and thus locomotory muscle workload, is not constant throughout a dive. Initial stroke rate increased with increased maximum dive depth, and stroke rate often changed in the middle of dives (Figs 4, 5). In addition, stroke rate decreased at the end of almost all dives (Fig. 5). During these changes in stroke rate, locomotory muscle workload, as well as depletion rates of Mb-O2, PCr and Gly, would also change. In extremely long dives, which have much lower stroke rates, the model may overestimate PCr and Gly depletion. Thus, while the model provides a general framework of muscle metabolism during dives, locomotory muscle metabolic rate is likely not constant during diving and, as a result, consumption of the Mb-O2 store and depletion of the PCr and Gly stores will vary from the general model depending on, among other factors, stroke rate within and among dives.
Conclusion
The most compelling finding of this study is that anaerobic energy stores have eight times the capacity of the Mb-O2 store to produce ATP during dives of emperor penguins. Although the measured PCr and Gly concentrations are typical of non-diving animals, they provide significant anaerobic energy reserves. Our simple model of muscle metabolism during dives demonstrates that most dives at sea are aerobic in nature and do not significantly deplete anaerobic energy stores. Even after 12-min dives, the PCr and Gly stores are only 66% and less than 10% depleted, respectively. These anaerobic stores are sufficient to supply energy for dives beyond 20 min. However, extremely long dives and serial long dives will likely require extended surface intervals for complete Gly resynthesis. Our analysis of stroke rate demonstrates the limitations of this model in that locomotory muscle metabolic rate is likely not constant during dives at sea. Stroke rate varies both within dives and among dive types.
LITERATURE CITED
- Barstow TJ, Buchthal S, Zanconato S, Cooper DM. Muscle energetics and pulmonary oxygen-uptake kinetics during moderate exercise. J. Appl. Physiol. 1994;77:1742–1749. doi: 10.1152/jappl.1994.77.4.1742. [DOI] [PubMed] [Google Scholar]
- Beis I, Newsholme EA. Contents of adenine-nucleotides, phosphagens and some glycolytic intermediates in resting muscles from vertebrates and invertebrates. Biochem. J. 1975;152:23–32. doi: 10.1042/bj1520023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J. Muscle electrolytes in man. Scand. J. Clin. Lab. Invest. 1962;14:1–110. [Google Scholar]
- Blei ML, Conley KE, Kushmerick MJ. Separate measures of ATP utilization and recovery in human skeletal muscle. J. Physiol. (Cambridge) 1993;465:203–222. doi: 10.1113/jphysiol.1993.sp019673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blix AS Creatine in diving animals - A comparative study. Comp. Biochem. Physiol. A. 1971;40:805–807. doi: 10.1016/0300-9629(71)90266-0. [DOI] [PubMed] [Google Scholar]
- Blix AS, Elsner R, Kjekshus JK. Cardiac output and its distribution through capillaries and A-V shunts in diving seals. Acta Physiol. Scand. 1983;118:109–116. doi: 10.1111/j.1748-1716.1983.tb07250.x. [DOI] [PubMed] [Google Scholar]
- Blom PCS, Hostmark AT, Vaage O, Kardel KR, Maehlum S. Effect of different post-exercise sugar diets on the rate of muscle glycogen synthesis. Med. Sci. Sports Exerc. 1987;19:491–496. [PubMed] [Google Scholar]
- Brault JJ, Abraham KA, Terjung RL. Phosphocreatine content of freeze-clamped muscle: influence of creatine kinase inhibition. J. Appl. Physiol. 2003;94:1751–1756. doi: 10.1152/japplphysiol.01070.2002. [DOI] [PubMed] [Google Scholar]
- Burns JM, Skomp N, Bishop N, Lestyk K, Hammill M. Development of aerobic and anaerobic metabolism in cardiac and skeletal muscles from harp and hooded seals. J. Exp. Biol. 2010;213:740–748. doi: 10.1242/jeb.037929. [DOI] [PubMed] [Google Scholar]
- Butler PJ, Jones DR. The physiology of diving of birds and mammals. Physiol. Rev. 1997;77:837–899. doi: 10.1152/physrev.1997.77.3.837. [DOI] [PubMed] [Google Scholar]
- Castellini MA, Somero GN. Buffering capacity of vertebrate muscle: correlations with potentials for anaerobic function. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1981;143:191–198. [Google Scholar]
- Edwards MR, McMurty JP, Vasilatos-Youken R. Relative insensitivity of avian skeletal muscle glycogen to nutritive status. Domest. Anim. Endocrinol. 1999;16:239–247. doi: 10.1016/s0739-7240(99)00013-2. [DOI] [PubMed] [Google Scholar]
- Eklof B, Neglen P, Thomson D. Temporary incomplete ischemia of the legs induced by aortic clamping in man effects on central hemodynamics and skeletal muscle metabolism by adrenergic block. Ann. Surg. 1981;193:89–98. doi: 10.1097/00000658-198101000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes SC, Paganini AT, Slade JM, Towse TF, Meyer RA. Phosphocreatine recovery kinetics following low- and high-intensity exercise in human triceps surae and rat posterior hindlimb muscles. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009;296:R161–R170. doi: 10.1152/ajpregu.90704.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garetto LP, Richter EA, Goodman MN, Ruderman NB. Enhanced muscle glucose-metabolism after exercise in the rat - the 2 phases. Am. J. Physiol. 1984;246:E471–E475. doi: 10.1152/ajpendo.1984.246.6.E471. [DOI] [PubMed] [Google Scholar]
- Goforth HW. Ph.D Dissertation. Los Angeles: University of California Los Angeles; 1986. Glycogenolytic responses and force production characteristics of a bottlenose dolphin (Tursiops truncatus) while exercising against a force transducer. [Google Scholar]
- Groscolas R, Rodriguez A. Glucose and lactate kinetics and interrelations in an Antarctic bird (emperor penguin) Am. J. Physiol. 1982;242:R458–R464. doi: 10.1152/ajpregu.1982.242.5.R458. [DOI] [PubMed] [Google Scholar]
- Harris K, Walker PM, Mickle DAG, Harding R, Guytley R, Wilson GJ, Kuzon B, McKee N, Romaschin AD. Metabolic response of skeletal muscle to ischemia. Am. J. Physiol. 1986;250:H213–H220. doi: 10.1152/ajpheart.1986.250.2.H213. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Kindig CA, Richardson RS, Hogan MC. The role of oxygen in determining phosphocreatine onset kinetics in exercising humans. J. Physiol. (Oxford) 2004;558:985–992. doi: 10.1113/jphysiol.2004.062042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Somero GN. Biochemical Adaptation. Princeton, N. J.: Princeton University Press; 1984. [Google Scholar]
- Ivy JL, Katz AL, Cutler CL, Sherman WM, Coyle EF. Muscle glycogen synthesis after exercise effect of time of carbohydrate ingestion. J. Appl. Physiol. 1988;64:1480–1485. doi: 10.1152/jappl.1988.64.4.1480. [DOI] [PubMed] [Google Scholar]
- Jentjens R, Jeukendrup AE. Determinants of post-exercise glycogen synthesis during short-term recovery. Sports Med. 2003;33:117–144. doi: 10.2165/00007256-200333020-00004. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Roussel M, Bendahan D, Le Fur Y, Cozzone PJ. Interrelations of ATP synthesis and proton handling in ischaemically exercising human forearm muscle studied by 31P magnetic resonance spectroscopy. J. Physiol. (Cambridge) 2001;535:901–928. doi: 10.1111/j.1469-7793.2001.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem D, Elsner R. Cerebral tolerance to asphyxial hypoxia in harbor seal. Respir. Physiol. 1973;19:188–200. doi: 10.1016/0034-5687(73)90077-7. [DOI] [PubMed] [Google Scholar]
- Kerem D, Hammond DD, Elsner R. Tissue glycogen levels in Weddell seal, Leptonychotes-weddelli - Possible adaptation to asphyxial hypoxia. Comp. Biochem. Physiol. 1973;45:731–736. doi: 10.1016/0300-9629(73)90076-5. [DOI] [PubMed] [Google Scholar]
- Kooyman GL, Castellini MA, Davis RW, Maue RA. Aerobic diving limits of immature Weddell seals Leptonychotes-weddelli. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1983;151:171–174. [Google Scholar]
- Kooyman GL, Kooyman TG. Diving behavior of emperor penguins nurturing chicks at Coulman Island, Antarctica. The Condor. 1995;97:536–549. [Google Scholar]
- Kooyman GL, Ponganis PJ, Castellini MA, Ponganis EP, Ponganis KV, Thorson PH, Eckert SA, Le Maho Y. Heart rates and swim speeds of emperor penguins diving under sea ice. J. Exp. Biol. 1992;165:161–180. doi: 10.1242/jeb.165.1.161. [DOI] [PubMed] [Google Scholar]
- Kooyman GL, Wahrenbrock EA, Castellini MA, Davis RW, Sinnett EE. Aerobic and anaerobic metabolism during voluntary diving in Weddell seals Leptonychotes-weddelli evidence of preferred pathways from blood chemistry and behavior. J. Comp. Physiol. (B) 1980;138:335–346. [Google Scholar]
- Lowry OH, Passoneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. [Google Scholar]
- Marsh GD, Paterson DH, Potwarka JJ, Thompson RT. Transient changes in muscle high-energy phosphates during moderate exercise. J. Appl. Physiol. 1993;75:648–656. doi: 10.1152/jappl.1993.75.2.648. [DOI] [PubMed] [Google Scholar]
- McCann DJ, Mole PA, Caton JR. Phosphocreatine kinetics in humans during exercise and recovery. Med. Sci. Sports Exerc. 1995;27:378–389. [PubMed] [Google Scholar]
- McCully KK, Iotti S, Kendrick K, Wang Z, Posner JD, Leigh J, Chance B. Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J. Appl. Physiol. 1994;77:5–10. doi: 10.1152/jappl.1994.77.1.5. [DOI] [PubMed] [Google Scholar]
- Meir JU, Stockard TK, Williams CL, Ponganis KV, Ponganis PJ. Heart rate regulation and extreme bradycardia in diving emperor penguins . Exp. Biol. 2008;211:1169–1179. doi: 10.1242/jeb.013235. [DOI] [PubMed] [Google Scholar]
- Mill GK, Baldwin J. Biochemical correlates of swimming and diving behavior in the little blue penguin Eudyptula minor. Physiol. Zool. 1983;56:242–254. [Google Scholar]
- Noren SR, Williams TM, Pabst DA, McLellan WA, Dearolf JL. Development of diving in marine endotherms: preparing the skeletal muscles of dolphins, penguins, and seals for activity during submergence. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2001;171:127–134. doi: 10.1007/s003600000161. [DOI] [PubMed] [Google Scholar]
- Phillips D, ten Hove M, Schneider JE, Wu CO, Sebag-Montefiore L, Aponte AM, Lygate CA, Wallis J, Clarke K, Watkins H, Balaban RS, Neubauer S. Mice over-expressing the myocardial creatine transporter develop progressive heart failure and show decreased glycolytic capacity. J. Mol. Cell. Cardiol. 2010;48:582–590. doi: 10.1016/j.yjmcc.2009.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponganis PJ, Costello ML, Starke LN, Mathieu-Costello O, Kooyman GL. Structural and biochemical characteristics of locomotory muscles of emperor penguins, Aptenodytes forsteri. Respir. Physiol. 1997a;109:73–80. doi: 10.1016/s0034-5687(97)84031-5. [DOI] [PubMed] [Google Scholar]
- Ponganis PJ, Kooyman GL, Starke LN, Kooyman CA, Kooyman TG. Post-dive blood lactate concentrations in emperor penguins, Aptenodytes forsteri. J. Exp. Biol. 1997b;200:1623–1626. doi: 10.1242/jeb.200.11.1623. [DOI] [PubMed] [Google Scholar]
- Ponganis PJ, Stockard TK, Meir JU, Williams CL, Ponganis KV, Howard R. O2 store management in diving emperor penguins. J. Exp. Biol. 2009;212:217–224. doi: 10.1242/jeb.026096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponganis PJ, Stockard TK, Meir JU, Williams CL, Ponganis KV, van Dam RP, Howard R. Returning on empty: extreme blood O2 depletion underlies dive capacity of emperor penguins. J. Exp. Biol. 2007;210:4279–4285. doi: 10.1242/jeb.011221. [DOI] [PubMed] [Google Scholar]
- Reynafarje B. Simplified method for the determination of myoglobin. J. Lab. Clin. Med. 1963;61:139–145. [PubMed] [Google Scholar]
- Saltin B, Gollnick PD. Skeletal muscle adaptability: significance for metabolism and performance. In: Peachy LD, Adrian RH, Geiger SR, editors. Handbook of Physiology. Baltimore: Williams and Wilkinson; 1983. pp. 555–631. [Google Scholar]
- Sato K, Naito Y, Kato A, Niizuma Y, Watanuki Y, Charrassin JB, Bost CA, Handrich Y, Le Maho Y. Buoyancy and maximal diving depth in penguins: do they control inhaling air volume? J. Exp. Biol. 2002;205:1189–1197. doi: 10.1242/jeb.205.9.1189. [DOI] [PubMed] [Google Scholar]
- Sato K, Ponganis PJ, Habara Y, Naito Y. Emperor penguins adjust swim speed according to the above-water height of ice holes through which they exit. J. Exp. Biol. 2005;208:2549–2554. doi: 10.1242/jeb.01665. [DOI] [PubMed] [Google Scholar]
- Sato K, Shiomi K, Marshall G, Kooyman GL, Ponganis PJ. Stroke rates and diving air volumes of emperor penguins: Implications for dive performance. J. Exp. Biol. 2011;214:2854–2863. doi: 10.1242/jeb.055723. [DOI] [PubMed] [Google Scholar]
- Scholander PF. Experimental investigations on the respiratory function in diving mammals and birds. Hvalrådets Skr. 1940;22:1–131. [Google Scholar]
- Scholander PF, Irving L, Grinnell SW. Aerobic and anaerobic changes in seal muscle during diving. J. Biol. Chem. 1942;142:431–440. [Google Scholar]
- Shaffer SA, Costa DP, Williams TM, Ridgway SH. Diving and swimming performance of white whales, Delphinapterus leucas: an assessment of plasma lactate and blood gas levels and respiratory rates. J. Exp. Biol. 1997;200:3091–3099. doi: 10.1242/jeb.200.24.3091. [DOI] [PubMed] [Google Scholar]
- Snow DH, Harris RC, Gash SP. Metabolic responses of equine muscle to intermittent maximal exercise. J. Appl. Physiol. 1985;58:1689–1697. doi: 10.1152/jappl.1985.58.5.1689. [DOI] [PubMed] [Google Scholar]
- Stephenson R, Jones DR. Metabolic responses to forced dives in Pekin duck measured by indirect calorimetry and 31P-MRS. Am. J. Physiol. 1992;262:R1309–R1317. doi: 10.1152/ajpregu.1992.263.6.R1309. [DOI] [PubMed] [Google Scholar]
- Stephenson R, Jones DR, Kasserra CE, Lemaire C. The effects of skeletal muscle contractions and paralysis on physiological responses to head immersion in Pekin duck. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 1997;118:765–770. [Google Scholar]
- Tesch P. Muscle fatigue in man with special reference to lactate accumulation during short-term intense exercise. Acta Physiol. Scand. Supp. 1980;480:1–40. [PubMed] [Google Scholar]
- van Dam RP, Ponganis PJ, Ponganis KV, Levenson DH, Marshall G. Stroke frequencies of emperor penguins diving under sea ice. J. Exp. Biol. 2002;205:3769–3774. doi: 10.1242/jeb.205.24.3769. [DOI] [PubMed] [Google Scholar]
- Waller AP, Lindinger MI. Nutritional aspects of post exercise skeletal muscle glycogen synthesis in horses: A comparative review. Equine Vet. J. 2010;42:274–281. doi: 10.2746/042516409X479603. [DOI] [PubMed] [Google Scholar]
- Wallis J, Lygate CA, Fischer A, ten Hove M, Schneider JE, Sebag-Montefiore L, Dawson D, Hulbert K, Zhang W, Zhang MH, Watkins H, Clarke K, Neubauer S. Supranormal myocardial creatine and phosphocreatine concentrations lead to cardiac hypertrophy and heart failure - Insights from creatine transporter-overexpressing transgenic mice. Circ. 2005;112:3131–3139. doi: 10.1161/CIRCULATIONAHA.105.572990. [DOI] [PubMed] [Google Scholar]
- Wienecke B, Robertson G, Kirkwood R, Lawton K. Extreme dives by free-ranging emperor penguins. Polar Biology. 2007;30:133–142. [Google Scholar]
- Williams CL, Meir JU, Ponganis PJ. What triggers the aerobic dive limit? Patterns of muscle oxygen depletion during dives of emperor penguins. J. Exp. Biol. 2011;214:1802–1812. doi: 10.1242/jeb.052233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TM, Davis RW, Fuiman LA, Francis J, Le Boeuf BJ, Horning M, Calambokidis J, Croll DA. Sink or swim: Strategies for cost-efficient diving by marine mammals. Science. 2000;288:133–136. doi: 10.1126/science.288.5463.133. [DOI] [PubMed] [Google Scholar]
- Williams TM, Dobson GP, Matheiu-Costello O, Morsbach D, Worley MB, Phillips JA. Skeletal muscle histology and biochemistry of an elite sprinter, the African cheetah. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 1997;167:527–535. doi: 10.1007/s003600050105. [DOI] [PubMed] [Google Scholar]
- Williams TM, Fuiman LA, Horning M, Davis RW. The cost of foraging by a marine predator, the Weddell seal Leptonychotes weddellii: pricing by the stroke. J. Exp. Biol. 2004;207:973–982. doi: 10.1242/jeb.00822. [DOI] [PubMed] [Google Scholar]
- Williams TM, Haun JE, Friedl WA. The diving physiology of bottlenose dolphins (Tursiops truncatus). I. Balancing the demands of exercise for energy conservation at depth. J. Exp. Biol. 1999;202:2739–2748. doi: 10.1242/jeb.202.20.2739. [DOI] [PubMed] [Google Scholar]
- Wilson RP, Putz K, Peters G, Culik B, Scolaro JA, Charrassin JB, Ropert-Coudert Y. Long-term attachment of transmitting and recording devices to penguins and other seabirds. Wildl. Soc. Bull. 1997;25:101–106. [Google Scholar]
- Zapol WM, Liggins GC, Schneider RC, Qvist J, Snider MT, Creasy RK, Hochachka PW. Regional blood flow during simulated diving in the conscious Weddell seal. J. Appl. Physiol. 1979;47:968–973. doi: 10.1152/jappl.1979.47.5.968. [DOI] [PubMed] [Google Scholar]