Abstract
Background
The International Serial Transverse Enteroplasty (STEP) Data Registry is a voluntary online database created in 2004 to collect information on patients undergoing the STEP procedure. The aim of this study was to identify preoperative factors significantly associated with 1) transplantation or death, or 2) attainment of enteral autonomy following STEP.
Study Design
Data were collected from September 2004 to January 2010. Univariate and multivariate logistic regression analyses were applied to determine predictors of transplantation/death or enteral autonomy post-STEP. Time to reach full enteral nutrition was estimated using a Kaplan-Meier curve.
Results
Fourteen of the 111 patients in the Registry were excluded due to inadequate follow-up. Of the remaining 97 patients, 11 patients died, and 5 progressed to intestinal transplantation. On multivariate analysis, higher direct bilirubin and shorter pre-STEP bowel length were independently predictive of progression to transplantation or death (p = .05 and p < .001, respectively). Of the 78 patients who were ≥7 days of age and required parenteral nutrition (PN) at the time of STEP, 37 (47%) achieved enteral autonomy after the first STEP. Longer pre-STEP bowel length was also independently associated with enteral autonomy (p = .002). The median time to reach enteral autonomy based on Kaplan-Meier analysis was 21 months (95% CI: 12-30).
Conclusions
Overall mortality post-STEP was 11%. Pre-STEP risk factors for progressing to transplantation or death were higher direct bilirubin and shorter bowel length. Among patients who underwent STEP for short bowel syndrome, 47% attained full enteral nutrition post-STEP. Patients with longer pre-STEP bowel length were significantly more likely to achieve enteral autonomy.
INTRODUCTION
The Serial Transverse Enteroplasty (STEP) procedure was first reported in 20031,2. Clinically it has been used to increase intestinal length in children with short bowel syndrome (SBS)2, ameliorate bacterial overgrowth3 and primarily lengthen and taper dilated proximal bowel in neonates with intestinal atresia and marginal bowel length4, 5. The International STEP Data Registry was established in 2004 to serve as a secure, online database to collect information on patients undergoing this novel procedure. The first report of the International STEP Data Registry contained 38 patients from 10 different centers, and focused on immediate postoperative outcomes and complications, with a median follow-up time of just over one year6.
This report aims to provide data on the long-term follow-up of patients who have undergone the STEP procedure. Specifically, we sought to determine: 1) pre-operative factors predictive of death or small bowel transplantation after STEP, and 2) pre-operative factors that would predict the ability to achieve full enteral autonomy after the STEP procedure in the subgroup of patients with SBS.
METHODS
The International STEP Data Registry is maintained as a secure, password protected online database (http://www.stepoperation.org) by the surgical research staff at Children's Hospital Boston. Patient information is obtained through direct data entry by the participating surgeon or with the assistance of registry personnel. The registry has maintained the approval of the Investigational Review Board (IRB) at Children's Hospital Boston; participants from other institutions are encouraged to obtain approval from their institutional IRB as necessary.
For this study, registry data collected between September 2004 and January 2010 were used for analysis. Attempts were made to contact all participating surgeons in the two months prior to data analysis to ensure each patient's information was current and accurate. Data collected in the registry includes pre-, intra-, and post-operative information on patients undergoing the STEP procedure and has been detailed previously6. The pre-operative data includes date of birth, gestational age, gender, race, primary diagnosis, co-existing medical conditions, weight prior to surgery and presence of the ileocecal valve. Baseline preoperative serum levels of bilirubin and INR, and the percentage of calories taken via the enteral route were also recorded. Intra-operative data includes date of STEP, pre- and post-operative small intestinal length and width, and the number of staple firings during the procedure. Post-operative data includes complications and percent enteral nutrition. Multiple follow-up points can be created for a single patient.
Statistical analyses were conducted using the Statistical Package for Social Sciences for Windows (SPSS). The one-sample Kolmogorov-Smirnoff test was used to determine if sample data were normally distributed. Normally distributed data were reported as means (+/− standard deviation (SD)). Tests for skewed data were performed using a one-sample Kolmogorov-Smirnoff test. The Mann-Whitney Test was used to compare non-parametric data using means and inter-quartile ranges (IQR). Univariate and multivariate logistic regression analyses were conducted to determine predictors of death/transplantation and enteral autonomy. Kaplan-Meier analysis was used to estimate the time needed to wean from parenteral nutrition after the STEP procedure. Significance was set at p < .05.
RESULTS
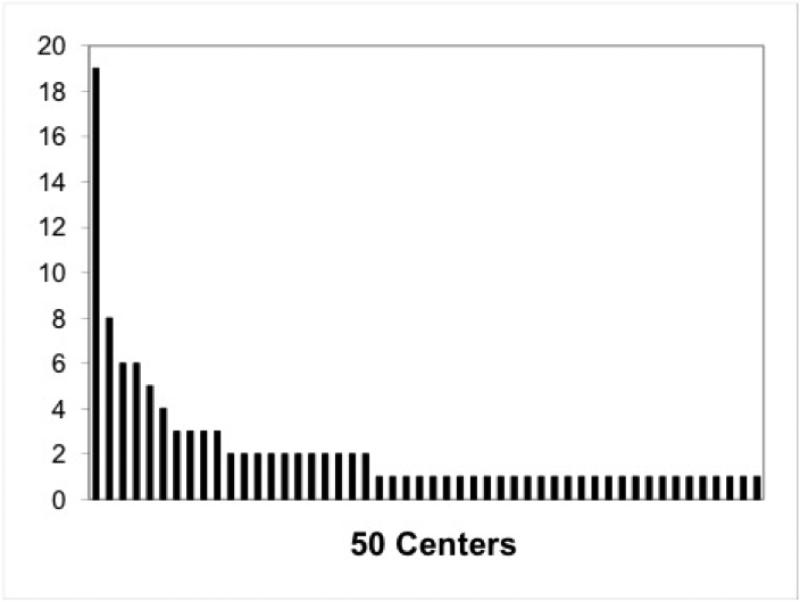
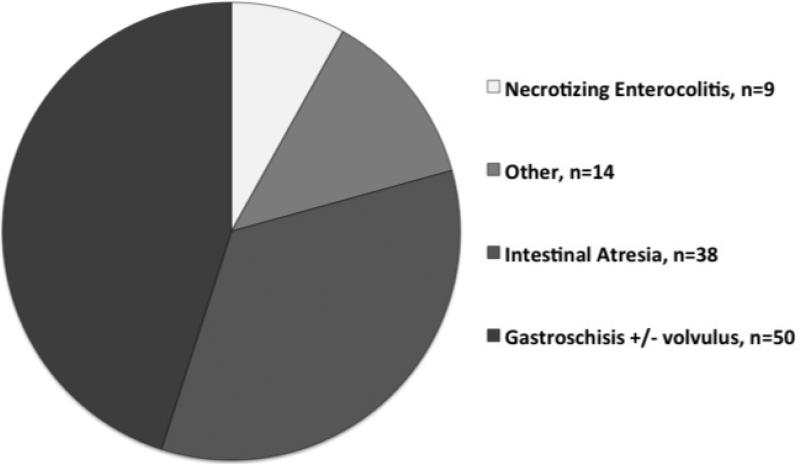
Fifty centers, representing 13 countries and 25 U.S. states (including Washington DC and Puerto Rico) submitted a total of 111 patients to the STEP Registry between September 2004 and January 2010; including the 38 patients previously reported in the first Registry update6. Figure 1 demonstrates the number of STEP procedures completed at each center. There was a significant concentration of experience, with only 20% of centers reporting 3 or more STEP procedures but 54% of the procedures being performed in those centers. The baseline primary diagnosis for all patients is shown in Figure 2. Actual indications for STEP were poorly reported. Fourteen patients had inadequate operative and postoperative data and were therefore excluded from subsequent analysis. Demographic and clinical data for the remaining 97 patients are shown in Table 1.
Figure 1.
Volume of serial transverse enteroplasty procedures at each of the 50 participating centers.
Figure 2.
Primary diagnosis in 111 patients enrolled in the International Serial Transverse Enteroplasty Data Registry. White slice, necrotizing enterocolitis, n=9; light gray slice, other, n=14; dark gray slice, intestinal atresia, n=38; black slice, gastroschisis +/− volvulus, n=50.
Table 1.
Demographic and clinical characteristics of the 97 patients with follow up after first STEP in the International STEP Data Registry
| Sex, male, n (%) | 55 (57) |
| Ethnicity, n (%) | |
| Black | 11 (11) |
| White | 57 (59) |
| Asian | 6 (6) |
| Hispanic | 16 (17) |
| Unknown | 7 (7) |
| Gestational age, wk, mean +/− SD, n = 82 | 35 +/− 3 |
| Age at STEP, mo, median (IQR), n = 97 | 6.6 (2.4 - 37.8) |
| Weight at STEP, kg, median (IQR), n = 81 | 5.7 (3.7 - 14.8) |
| Preoperative enteral nutrition, %, median (IQR), n = 87 | 10 (0 - 55) |
| Laboratory values at STEP | |
| Total bilirubin, mg/dL, median (IQR), n = 73 | 1.9 (0.5 - 8.0) |
| Direct bilirubin, mg/dL, median (IQR), n = 59 | 1.2 (0.1 - 5.9) |
| INR, mean +/− SD, n = 50 | 1.17 +/− 0.21 |
| Operative data, cm, median (IQR) [range] | |
| Pre-STEP bowel length, n = 92 | 49 (28 - 85) [7 – 200] |
| Pre-STEP bowel width, n = 80 | 5.3 (4.0 - 7.0) [2 – 24] |
| Post-STEP bowel length, n = 81 | 75 (49 - 117) [17 −325] |
| Post-STEP bowel width, n = 84 | 2.0 (1.5 - 2.5) [0.8 – 5] |
| Staple firings, n = 94 | 10 (7 - 17) [2 – 35] |
| Presence of ileocecal valve, n (%), n = 95 | 30 (31) |
IQR, Interquartile Range (25-75%); STEP, Serial Transverse Enteroplasty.
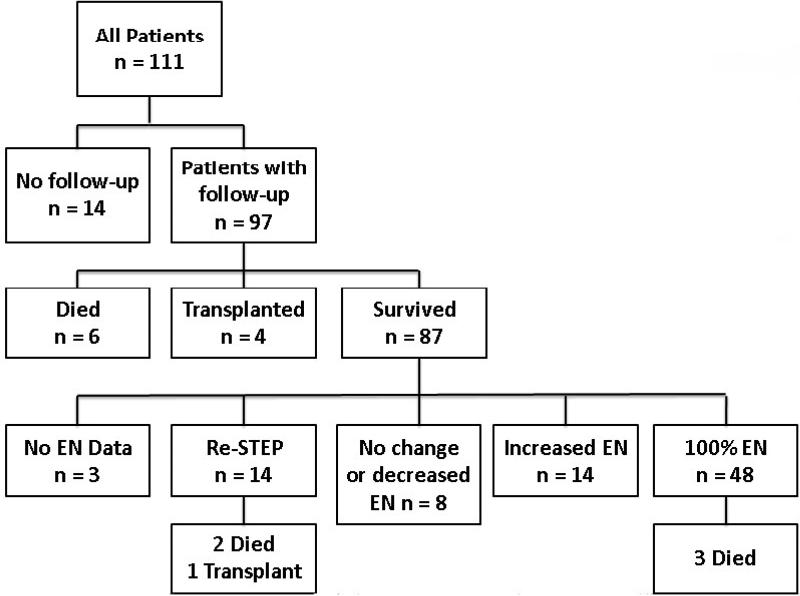
Figure 3 is a schematic representation of outcomes following STEP. 97 patients had adequate follow-up for analysis following initial STEP; of these, 6 patients died and 4 patients progressed to small intestinal transplantation. Of the 87 transplant-free survivors of the first STEP, 48 (55%) progressed to enteral autonomy, 14 (16%) had increased enteral tolerance without full autonomy, 8 (9%) had no change or decreased enteral tolerance, and 14 (16%) underwent a second STEP. Three patients with incomplete enteral data were excluded from further analysis. Of the 14 patients who underwent a second STEP procedure, two patients died and one progressed to intestinal transplantation. A total of 11 patients died, however three of these reached full enteral nutrition prior to death.
Figure 3.
Outcomes of the 111 patients undergoing a first STEP procedure. EN, enteral nutrition, STEP, serial transverse enteroplasty procedure.
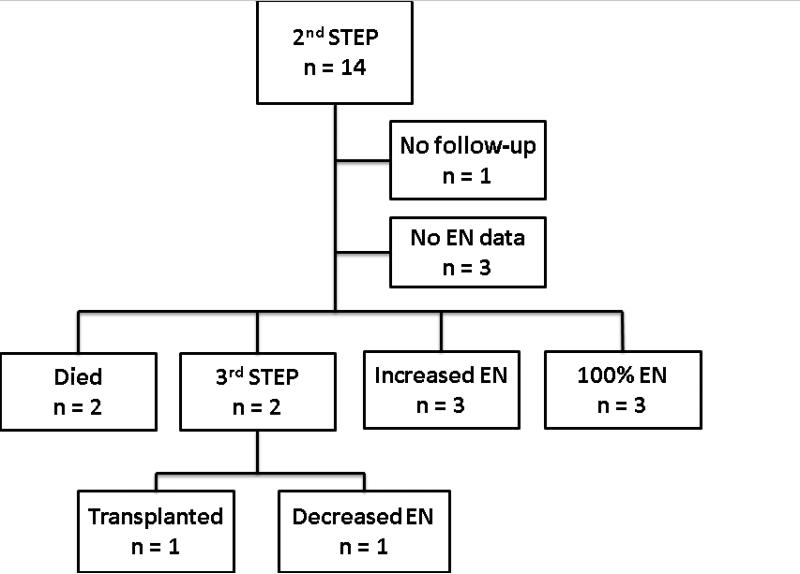
Figure 4 shows the outcomes of the 14 patients who underwent a second STEP. One patient had no follow-up after the second STEP, and 3 patients had no enteral data available after the second STEP. Of the remaining 10 patients, 2 died and 2 went on to a third STEP. As of last follow up, the remaining 6 patients had either reached enteral autonomy (50%) or increased their enteral intake (50%). Of the two patients who underwent a third STEP, one eventually underwent small bowel transplantation, and the other had a decrease in enteral tolerance at last follow-up.
Figure 4.
Outcomes of the 14 patients undergoing a second STEP procedure. EN, enteral nutrition, STEP, serial transverse enteroplasty procedure.
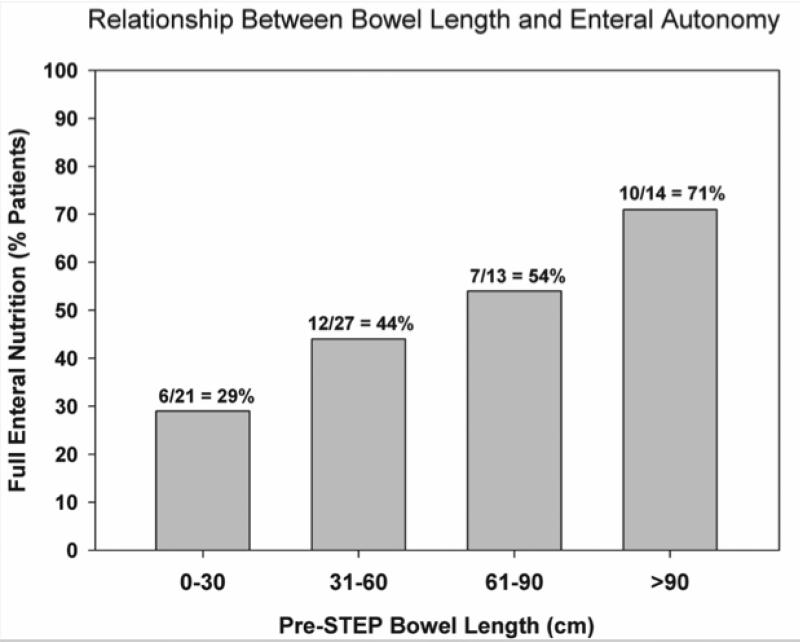
To determine pre-operative factors associated with progression to death or small intestinal transplant after STEP, patients were subdivided into transplant-free survivors (n=81) and those who died or were transplanted (n=16). Baseline comparisons of these two groups are shown in Table 2. Univariate analysis demonstrated no statistical difference between the two groups with regard to gender, ethnicity, gestational age, diagnosis, weight at STEP, serum INR, or presence of the ileocecal valve. Statistically significant preoperative variables identified by univariate analysis included serum total and direct bilirubin concentrations, bowel length and bowel width. Patients who progressed to death or transplantation had higher serum total and direct bilirubin concentrations, shorter pre-STEP bowel length and smaller pre-STEP bowel width when compared with transplant-free survivors. All preoperative variables with a p-value < .15 (age at STEP, pre-operative percent enteral nutrition, serum total and direct bilirubin, pre-STEP bowel length and width) were included in a multivariate analysis. On logistic regression analysis only higher serum direct bilirubin (p = .05) and shorter pre-STEP bowel length (p < .001) were independently associated with death or small bowel transplantation after first STEP. A subgroup analysis was performed to identify factors associated with the ability to achieve full enteral autonomy following the STEP procedure. We excluded 14 patients without follow-up, 12 patients who were on full enteral nutrition prior to the first STEP, and 7 patients who underwent STEP at <7 days of age. We assumed that patients who were on full enteral nutrition underwent STEP for bacterial overgrowth and those who had the STEP at < 7 days of age were neonates with intestinal atresia, dilated proximal bowel, and marginal bowel length. The outcomes for the remaining 78 patients who underwent STEP for short bowel syndrome with inability to wean from PN are detailed in Table 3. Thirty-seven patients (47%) reached enteral autonomy, while 41 patients remained on PN. On univariate analysis, pre-STEP bowel length, post-STEP bowel length, and number of staple firings were significantly associated with the ability to wean from PN. Only longer pre-STEP bowel length was independently associated with attainment of enteral autonomy after STEP on multivariate analysis (p= 0.002). Figure 5 demonstrates this relationship between preoperative bowel length and the ability to achieve enteral autonomy. Kaplan-Meier modeling was used to estimate the time to full enteral nutrition after STEP for the 37 patients who eventually reached full enteral autonomy (Figure 6).
Table 2.
Comparison of Demographic and Clinical Characteristics of 97 Patients in the International STEP Data Registry with Respect to Poor Outcomes (Death or Transplantation)
| Death/transplant (n = 16) | Survival (n = 81) | p Value | |
|---|---|---|---|
| Sex, male, n (%) | 7 (44) | 48 (59) | 0.28 |
| Ethnicity, n (%) | 0.25 | ||
| Black | 3 (20) | 8 (11) | |
| White | 7 (47) | 50 (67) | |
| Asian | 0 (0) | 6 (8) | |
| Hispanic | 5 (33) | 11 (15) | |
| Unknown | 1 (6) | 6 (7) | |
| Gestational age, wk, mean +/− SD, n = 82 | 34 +/− 3 | 35 +/− 3 | 0.55 |
| Age at STEP, mo, median (IQR), n = 97 | 3.9 (1.4 - 8.2) | 6.6 (2.4 - 37.8) | 0.11 |
| Weight at STEP, kg, median (IQR), n = 81 | 5.0 (3.3 - 7.0) | 5.7 (3.7 - 14.8) | 0.27 |
| Preoperative enteral nutrition, %, median (IQR), n = 87 | 0 (0 - 16) | 10 (0 - 55) | 0.09 |
| Laboratory values at STEP | |||
| Total bilirubin, mg/dL, median (IQR), n = 73 | 9.0 (2.0 - 15.3) | 1.9 (0.5 - 8.0) | 0.00 |
| Direct bilirubin, mg/dL, median (IQR), n=59 | 7.3 (1.6 - 9.5) | 1.2 (0.1 - 5.9) | 0.01 |
| INR mean +/− SD, n = 50 | 1.15 +/− 0.19 | 1.17 +/− 0.21 | 0.81 |
| Operative data, cm, median (IQR) | |||
| Pre-STEP bowel length, n = 92 | 30 (18 - 40) | 49 (28 - 85) | 0.01 |
| Pre-STEP bowel width, n = 80 | 4.0 (3.8 - 6.0) | 5.3 (4.0 - 7.0) | 0.04 |
| Post-STEP bowel length, n = 81 | 52 (34 - 77) | 75 (49 - 117) | 0.02 |
| Post-STEP bowel width, n = 84 | 1.8 (1.0 - 2.0) | 2.0 (1.5 - 2.5) | 0.06 |
| Staple firings, n = 94 | 9 (5 - 13) | 10 (7 - 17) | 0.10 |
| Presence of ileocecal valve, n (%), n = 95 | 5 (31) | 25 (32) | 0.99 |
IQR, Interquartile Range (25-75%); STEP, Serial Transverse Enteroplasty.
Table 3.
Demographics of Patients in the International STEP Data Registry who Did and Did Not Reach 100% Enteral Nutrition
| EN100 (n = 37) | Still on PN (n = 41) | p Value | |
|---|---|---|---|
| Sex, male, n (%) | 22 (60) | 22 (54) | 0.65 |
| Ethnicity, n (%) | 0.19 | ||
| Black | 4 (11) | 6 (15) | |
| White | 25 (68) | 22 (54) | |
| Asian | 2 (5) | 0 (0) | |
| Hispanic | 3 (8) | 10 (24) | |
| Unknown | 3 (8) | 3 (7) | |
| Gestational age, wk, mean +/− SD, n = 65 | 35 +/− 4 | 35 +/− 3 | 0.80 |
| Age at STEP, mo, median (IQR), n = 76 | 9 (3 -53 ) | 6.0 (3 - 21) | 0.16 |
| Weight at STEP, kg, median (IQR), n = 66 | 8.0 (5.0 - 18.0) | 5.4 (3.9 - 8.2) | 0.07 |
| Preoperative enteral nutrition (%), median (IQR), n = 67 | 12 (0 - 45) | 5 (0 - 30) | 0.32 |
| Laboratory values at STEP | |||
| Total bilirubin (mg/dL), median (IQR), n = 62 | 1.1 (0.4 - 9.1) | 2.1 (0.6 - 8.3) | 0.36 |
| Direct bilirubin (mg/dL), median (IQR), n = 51 | 1.2 (0.1 - 6.1) | 1.6 (0.3 - 6.3) | 0.63 |
| INR, mean +/− SD, n = 43 | 1.18 +/− 0.14 | 1.15 +/− 0.26 | 0.73 |
| Operative Data (cm), median (IQR) | |||
| Pre-STEP bowel length, n = 75 | 60 (45 - 106) | 36 (23 - 64) | <0.01 |
| Pre-STEP bowel width, n = 62 | 5 (4 - 7) | 6 (4.0 - 7.0) | 0.54 |
| Post-STEP bowel length, n = 65 | 97 (68 - 183) | 60 (39 - 102) | <0.01 |
| Post-STEP bowel width, n = 66 | 2.0 (1.5 - 2.3) | 2.0 (1.5 - 2.5) | 0.76 |
| Staple firings, n = 75 | 14 (8 - 20) | 10 (7 - 14) | 0.03 |
| Presence of ileocecal valve, n (%), n = 74 | 12 (34) | 10 (24) | 0.45 |
Patients without follow-up, patients with STEP at <7 d of age, and those on EN100 preoperatively were excluded.
EN100, 100% enteral nutrition; IQR, Interquartile Range (25-75%); PN, parenteral nutrition. STEP, Serial Transverse Enteroplasty.
Figure 5.
Relationship between preoperative bowel length and the ability to achieve enteral autonomy after the STEP procedure among the 75 patients in whom preoperative bowel length data were available. STEP, serial transverse enteroplasty procedure.
Figure 6.
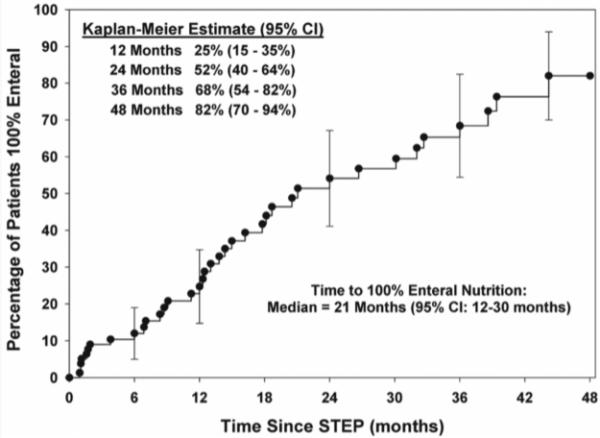
Time to achievement of 100% enteral nutrition after STEP in patients with short bowel syndrome who achieved full enteral nutrition (n=37). STEP, serial transverse enteroplasty procedure.
DISCUSSION
The STEP procedure was developed in 2003 as a novel bowel lengthening procedure, and has since been shown to be safe and effective in the short and intermediate term, and as a repeat procedure 1,7-10. In contrast to longitudinal intestinal lengthening (LILT), which decreases the bowel circumference by half regardless of the degree of dilation, STEP allows for the creation of uniform intestinal caliber in patients with variable degrees of bowel dilation. Further potential advantages of STEP include the absence of intestinal anastomoses, decreased risk of vascular compromise, and reduced technical complexity when compared with other bowel lengthening procedures.
The largest experience with LILT was reported as a review of 8 published studies in 2006, and showed survival ranging from 30-100% with PN weaning rates of 33-100% among survivors11. The largest single center experience with STEP was published by Sudan et al in 2007. They reported 21 patients who underwent 34 STEP procedures, with a 95% overall survival and PN weaning rate of 60%12. Additionally, Oliveira et al recently reported five year outcomes in 12 patients undergoing primary STEP; transplant-free survival was 66% (8/12) and 7 of those 8 patients achieved enteral autonomy by four years following STEP13. When compared with these findings, the cohort of patients with SBS from the International STEP Data Registry demonstrated overall survival of 89% after first STEP, with approximately half of surviving patients progressing to enteral autonomy. An additional 17% of patients with SBS had improved enteral tolerance after first STEP, but had not reached autonomy at the time of data collection for this report. Follow up of registry patients undergoing a repeat STEP yielded an 80% survival rate, with 37% of patients achieving full enteral autonomy and an additional 37% with improved enteral tolerance. The mechanism for this salutary effect in patients undergoing STEP may include improved absorption of enteral nutrients, decreased bacterial overgrowth, improved motility, and enhanced intestinal adaptation6,14,15. Similar to that seen in LILT, preoperative bilirubin and baseline intestinal length were found to be indicators for progression to intestinal transplantation or death16.
Limitations of the registry include its retrospective nature and potential for selection and reporting bias. The voluntary nature of the database precludes guaranteed follow-up for all patients, as evidenced by the 14 patients for which no follow-up data was available. Additionally, the registry does not provide a co-temporal control group. Nonetheless, this remains the largest review of patients undergoing autologous intestinal reconstruction surgery (AIRS).
In the cohort of patients who reached full enteral tolerance after STEP, the median time to full EN was 21 months. This suggests that small bowel continues to adapt post-STEP, a concept that is supported by animal data14. Decreasing caloric requirements may also play a role in weaning from PN over time. During infancy, the total caloric requirements can be 120-140 Kcal/kg/day whereas a healthy teenager or young adult may only require 35 Kcal/kg/day or less. This four-fold decrease in total caloric needs with increasing age may allow a child with a relatively fixed absorptive capacity to eventually achieve full enteral autonomy, even after many years.
The data in this largest series of STEP patients to date supports several clinical observations and allows us to hypothesize about the mechanism of action of the STEP procedure. First, it seems intuitive that patients with more bowel will be more likely to benefit from the STEP procedure, but why might this be true? The current analysis of the STEP Registry data demonstrates that both bowel length and width are important determinants of transplant-free survival and bowel length is an important predictor of the ability to wean from PN following the STEP procedure. Using basic geometric approximations, these bowel measurements can simply be thought of as a surrogate for the actual Mucosal Surface Area (aMSA) of intestine available to a particular patient. A sudden and massive loss of aMSA may result in a decreased absorptive capacity below a threshold level required to maintain full enteral nutrition, thus resulting in SBS. As intestinal adaptation progresses, some patients will increase aMSA through both microscopic villous hypertrophy as well as macroscopic intestinal growth (primarily bowel dilatation) such that functional Mucosal Surface Area (fMSA) exceeds the threshold level required to attain full enteral autonomy (Figure 7A).
Figure 7.
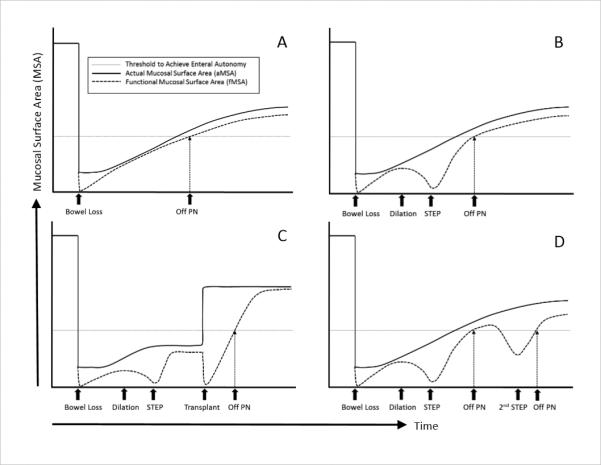
Hypothetical model describing interaction between actual mucosal surface area (aMSA) and functional mucosal surface area (fMSA). (A) Patient who is able to adapt and wean from PN. (B) Patient who has enough aMSA but is unable to wean due to poor fMSA secondary to bowel dilation; good candidate for STEP. (C) Patient who will never be able to wean due to insufficient aMSA; intestinal transplant candidate. (D) Patient who initially weans from PN but who then redilates; good candidate for second STEP. PN, parenteral nutrition; STEP, serial transverse enteroplasty procedure. Actual mucosal surface area, solid line; functional mucosal surface area, dashed line; threshold to achieve enteral autonomy, horizontal dotted line.
However, despite the increased aMSA that can be seen as a result of bowel dilatation in some cases, the bowel may become dysfunctional as a result of poor motility and/or bacterial overgrowth. In these cases, aMSA may significantly exceed fMSA due to the detrimental effects of having dilated poorly functional bowel. In this select group of patients with dysfunctional, dilated bowel, the STEP procedure may increase the fMSA to a level above the threshold required to allow for full enteral autonomy. The goal of the STEP is therefore to allow for the optimization of fMSA such that fMSA approaches the maximal limit as determined by the aMSA (Figure 7B). Unfortunately, a subset of patients with massive intestinal loss may never adapt adequately to even have enough aMSA to wean from PN, and therefore even optimizing intestinal function with a STEP procedure will not be enough to allow weaning from PN. In these cases, the only currently available method to increase the aMSA and fMSA to above the threshold level to achieve full enteral autonomy is intestinal transplantation (Figure 7C). Finally, a subset of these patients who initially do well after a STEP procedure will redilate as adaptation progresses. This may lead to a fall in fMSA requiring reinstitution of PN. In these cases, a second STEP may again increase the fMSA to a level that allows for full enteral autonomy (Figure 7D). This model emphasizes the concept that the primary mechanism of action for the STEP is really optimization of the bowel width to allow for the maximal functioning of existing aMSA.
Conclusions: The International STEP Data Registry has accrued data from 111 patients from 50 centers representing 13 countries and 25 U.S. states since its inception in 2004. The overall survival of patients following the STEP procedure was 89%. Five patients eventually required small bowel transplantation after STEP. Shorter pre-operative small bowel length and higher serum direct bilirubin levels were found to be significantly associated with death or progression to small bowel transplantation after STEP. Of the 78 patients who underwent STEP for PN dependence, longer pre-operative small bowel length was the only factor that predicted the attainment of enteral autonomy. Because the median time to enteral autonomy for this cohort was 21 months, any patient who is considered for STEP should have adequate pre-operative bowel length, reversible liver disease, and the ability to survive the time necessary to reach enteral autonomy.
Acknowledgment
We would like to thank the many surgeons and medical centers who participated in this project. Participating hospitals are: Hospital Italiano de Buenos Aires, Buenos Aires, Argentina; Hospital de Niños Sor Marı̒a Ludovica, La Plata, Buenos Aires, Argentina; Children's Hospital at Westmead, Sydney, NSW, Australia; BC Children's Hospital, Vancouver, BC, Canada; McMaster Children's Hospital, Hamilton, ON, Canada; Charles University and Motol University Hospital, Prague, Czech Republic; Hôpital Jeanne de Flandre, Universite Lille, Lille, France; National Center for Child Health and Development, Tokyo, Japan; Medical University of Gdan̒sk, Gdan̒sk, Poland; Hospital de São João, Porto, Portugal; University Pediatric Hospital, San Juan, Puerto Rico; Hamad Medical Corporation, Doha, Qatar; Pusan National University Children's Hospital, Seoul, South Korea; Hospital Universitari Vall d'Hebron, Barcelona, Spain; Sheffield Children's Hospital, Sheffield, UK; Leeds General Infirmary, Leeds, West Yorkshire, UK; University of Southampton, Southampton, Hampshire, UK; The Newcastle upon Tyne Hospitals, Newcastle, UK; University of South Alabama, Mobile, AL, USA; Saguaro Children's Surgery, Mesa, AZ, USA; Phoenix Children's Hospital, Phoenix, AZ, USA; Children's Hospital of Oakland, Oakland, CA, USA; Kaiser Permanente Los Angeles, Los Angeles, CA, USA; University of California, San Francisco, San Francisco, CA, USA; St. Mary's Hospital, Grand Junction, CO, USA; Georgetown University Hospital, Washington, D.C., USA; University of Miami, Miami, FL, USA; Emory University, Atlanta, GA, USA; Kapi'olani Pediatric Surgery, Honolulu, HI, USA; Pediatric Surgery of Idaho, Boise, ID, USA; Boston Children's Hospital, Boston, MA, USA; Children's Hospital of Michigan, Detroit, MI, USA; C.S. Mott Children's Hospital at the University of Michigan, Ann Arbor, MI, USA; Minneapolis Pediatric Surgery Associates, Minneapolis, MN, USA; Duke University, Durham, NC, USA; Children's Hospital of New Jersey, Newark, NJ, USA; Morgan Stanley Children's Hospital of New York Presbyterian, New York, NY, USA; Schneider Children's Hospital, New Hyde Park, NY, USA; Nationwide Children's Hospital, Columbus, OH, USA; Northwest Kaiser Permanente, Portland, OR, USA; Penn State University, Hershey, PA, USA; Monroe Carell Jr. Children's Hospital at Vanderbilt, Nashville, TN, USA; Baylor College of Medicine, Houston, TX, USA; Austin Pediatric Surgical Association, Austin, TX, USA; University of Utah, Salt Lake City, UT, USA; Intermountain Primary Children's Medical Center, Salt Lake City, UT, USA; Pediatric Surgical Group, Annandale, VA, USA; Seattle Children's Hospital, Seattle, WA, USA; University of Wisconsin, Madison, WI, USA; Gunderson Lutheran Hospital, La Crosse, WI, USA.
Footnotes
Disclosure Information: Authors have nothing to disclose. Timothy J Eberlein, Editor-in-Chief, has nothing to disclose.
Preliminary data from this study presented at the annual meeting of the American Academy of Pediatrics, San Francisco, CA, October, 2010.
REFERENCES
- 1.Kim HB, Fauza D, Garza J, et al. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg. 2003;38:425–429. doi: 10.1053/jpsu.2003.50073. [DOI] [PubMed] [Google Scholar]
- 2.Kim HB, Lee PW, Garza J, et al. Serial transverse enteroplasty for short bowel syndrome: a case report. J Pediatr Surg. 2003;38:881–885. doi: 10.1016/s0022-3468(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 3.Modi BP, Langer M, Duggan C, et al. Serial transverse enteroplasty for management of refractory D-lactic acidosis in short bowel syndrome. J Pediatr Gastroenterol Nutr. 2006;43:395–397. doi: 10.1097/01.mpg.0000228116.52229.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ismail A, Alkadhi A, Alnagaar O, et al. Serial transverse enteroplasty in intestinal atresia management. J Pediatr Surg. 2005;40:E5–6. doi: 10.1016/j.jpedsurg.2004.10.059. [DOI] [PubMed] [Google Scholar]
- 5.Wales PW, Dutta S. Serial transverse enteroplasty as primary therapy for neonates with proximal jejunal atresia. J Pediatr Surg. 2005;40:E31–34. doi: 10.1016/j.jpedsurg.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Modi BP, Javid PJ, Jaksic T, et al. First report of the international serial transverse enteroplasty data registry: indications, efficacy, and complications. J Am Coll Surg. 2007;204:365–371. doi: 10.1016/j.jamcollsurg.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Javid PJ, Kim HB, Duggan CP, et al. Serial transverse enteroplasty is associated with successful short-term outcomes in infants with short bowel syndrome. J Pediatr Surg. 2005;40:1019–1023. doi: 10.1016/j.jpedsurg.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 8.Wales PW, de Silva N, Langer JC, et al. Intermediate outcomes after serial transverse enteroplasty in children with short bowel syndrome. J Pediatr Surg. 2007;42:1804–1810. doi: 10.1016/j.jpedsurg.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Andres AM, Thompson J, Grant W, et al. Repeat surgical bowel lengthening with the STEP procedure. Transplantation. 2008;85:1294–1299. doi: 10.1097/TP.0b013e31817268ca. [DOI] [PubMed] [Google Scholar]
- 10.Piper H, Modi BP, Kim HB, et al. The second STEP: the feasibility of repeat serial transverse enteroplasty. J Pediatr Surg. 2006;41:1951–1956. doi: 10.1016/j.jpedsurg.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Bianchi A. From the cradle to enteral autonomy: the role of autologous gastrointestinal reconstruction. Gastroenterology. 2006;130:S138–146. doi: 10.1053/j.gastro.2005.09.070. [DOI] [PubMed] [Google Scholar]
- 12.Sudan D, Thompson J, Botha J, et al. Comparison of intestinal lengthening procedures for patients with short bowel syndrome. Ann Surg. 2007;246:593–601. doi: 10.1097/SLA.0b013e318155aa0c. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira C, de Silva N, Wales PW. Five-year outcomes after serial transverse enteroplasty in children with short bowel syndrome. J Pediatr Surg. 2012;47:931–937. doi: 10.1016/j.jpedsurg.2012.01.049. [DOI] [PubMed] [Google Scholar]
- 14.Chang RW, Javid PJ, Oh JT, et al. Serial transverse enteroplasty increases intestinal function in a model of short bowel syndrome. Ann Surg. 2006;243:223–228. doi: 10.1097/01.sla.0000197704.76166.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modi BP, Ching YA, Langer M, et al. Preservation of intestinal motility after the serial transverse enteroplasty procedure in a large animal model of short bowel syndrome. J Pediatr Surg. 2009;44:229–235. doi: 10.1016/j.jpedsurg.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi A. Longitudinal intestinal lengthening and tailoring: results in 20 children. J R Soc Med. 1997;90:429–432. doi: 10.1177/014107689709000804. [DOI] [PMC free article] [PubMed] [Google Scholar]