Abstract
Objective
Metrics of diffusion tensor imaging (DTI) and magnetization transfer imaging (MTI) can detect diffuse axonal injury in traumatic brain injury (TBI). The relationship between the changes of these imaging measures and the underlying pathologies is still relatively unknown. This study investigated the radiological-pathological correlation between these imaging techniques and immunohistochemistry using a closed head rat model of TBI.
Methods
TBI was performed on female rats followed longitudinally by MRI out to 30 days post-injury, with a subset of animals selected for histopathological analyses. A MRI-based finite element analysis was generated to characterize the pattern of the mechanical insult and estimate the extent of brain injury to direct the pathological correlation with imaging findings.
Results
DTI axial diffusivity and fractional anisotropy (FA) were sensitive to axonal integrity, while radial diffusivity showed significant correlation to the myelin compactness. FA was correlated to astrogliosis in the gray matter while mean diffusivity was correlated to increased cellularity. Secondary inflammatory responses also partly affected the changes of these DTI metrics. The magnetization transfer ratio (MTR) at 3.5 ppm demonstrated a strong correlation with both axon and myelin integrity. Decrease in MTR at 20 ppm correlated with the extent of astrogliosis in both gray and white matter.
Interpretation
While conventional T2-weighted MRI did not detect abnormalities following TBI, DTI and MTI afforded complementary insight into the underlying pathologies reflecting varying injury states over time, thus may substitute for histology to reveal DAI pathologies in vivo. This correlation of MRI and histology furthers understanding of the microscopic pathology underlying DTI and MTI changes in TBI.
Keywords: Diffusion Tensor Imaging, Traumatic Brain Injury, Finite Element Analysis
Introduction
In the United States, approximately 5.3 million people live with disability following traumatic brain injury (TBI).1 Three of four patients experience mild TBI, termed a “silent epidemic” in part because many cases are not recognized with current imaging technologies lacking sensitivity in detecting mild neuronal damage.2 The clinical diagnosis of TBI primarily relies on patients’ self-reporting than neurological or imaging examination.3 Frequently in TBI, the inertia shearing force between the gray-white matter junctions stretches axons resulting in diffusive axonal injury (DAI) in brain and this diffuse injury pattern is usually irregular, without hemorrhage, and difficult to detect.4 Evidence suggests that although the acute care needs of mild TBI patients are fewer than those with moderate and severe TBI, these patients are still at risk for sequelae if exposed to repeated head injury that could result in permanent disability or a higher risk of neurodegeneration.5
Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) contrast mechanism sensitive to the coherence of fibrous structures that can detect DAI in TBI patients6, 7 and experimental studies.8–10 Interpretation of DTI metrics in TBI patients are controversial with some studies indicating “reduced” fractional anisotropy (FA) and “elevated” mean diffusivity (MD) in white matter on DTI,6, 11 while others report “increased” FA and “decreased” MD.7, 12, 13 Reasons for these confounding results partly originate from using cross-sectional studies at single time points to draw conclusions about DTI metrics at potentially disparate times during the injury/repair time course. The heterogeneity of diffuse injury patterns in the mild TBI patient further complicates the correlation of the DTI data with pathological findings.2, 14 Magnetization transfer imaging (MTI) also has been applied to mild TBI, and the magnetization transfer ratio (MTR) offers increased sensitivity over conventional MR imaging to detect DAI in patients at risk for cognitive deficits consequent to mild TBI.15, 16 The specificity of MTR measures to the DAI injury patterns and the underlying morphological correlation in mild TBI are also unknown.15, 17 Radiological-pathological correlations of DTI and MTI measures are needed to characterize the DAI natural history and substantiate the sensitivity and specificity of these MRI metrics in relationship to subtle abnormalities in TBI.
Most studies of the relationship between radiological metrics and pathology have been characterized in moderate-to-severe focal TBI animal models, such as controlled cortical impact (CCI),18, 19 or lateral fluid percussion (LFP).20 In milder forms of experimental brain trauma (i.e. mild TBI or concussion), the radiological-pathological correlation has been difficult because of subtle morphological changes and limited imaging resolution.8, 10 The purpose of this study was to investigate the relation between the observed changes of radiological metrics and the underlying DAI pathophysiology using a modification of the Marmarou weight drop model of TBI in female rats.21 This impact-acceleration injury model commonly results in DAI, inflammation, astrogliosis, cortical neuronal swelling and loss in the brain parenchyma,22 thus providing a useful platform for pathological correlation with imaging results facilitating the interpretation of the changes of DTI and MTI data for mild TBI. To improve the radiological-pathological correlation in DAI associated with the impact-acceleration model, a MRI-based finite element analysis (FEA) model was built to evaluate the injury-associated biomechanics and predict the primary injury pattern in the brain.23 The FEA results were then used to guide subsequent radiological and pathological analysis to examine DTI and MTI data in association with significant microscopic abnormalities in mild TBI.
Materials and Methods
Animal Studies
All studies were approved by the animal care and use committee at our institution, and experiments were performed according to the National Research Council’s Guide for the Care and Use of Laboratory Animals. Female 8-week-old Wistar rats purchased from Charles River Laboratory (Wilmington, MA) and Harlan Laboratory (Indianapolis, IN) were used in this study to address the injury response in an understudied group (females) in preclinical TBI studies. Rats underwent baseline MRI screening on a 7T scanner (Bruker, Billerica, MA) using a radiofrequency quadrature coil (Doty Scientific, Columbia, SC) as previously described.24 Forty-five rats (n=29 from Charles River, n=16 from Harlan) of a total 75 of animals screened were identified within normal limits based on our previous guidelines24 and were subsequently used in the TBI study.
Weight Drop Closed Head Injury Model and Finite Element Analysis
Forty rats underwent a modified Marmarou weight drop closed head injury model for TBI.21 Another five animals without injury served as the controls for this study (Fig. 1A). Rats were first anesthetized in a sealed acrylic chamber with an isoflurane/oxygen mixture (4.5–5%) and administered buprenorphine (0.3 mg/kg) subcutaneously 20 minutes prior to impact. For the injured group, the anesthetized animals’ scalps were shaved and then placed on a polyurethane foam block (density: 13.8 kg/m3; Foam to Size, Ashland, VA) with the dimension of 15cm height × 20cm width × 45cm length. The isoflurane/oxygen mixture (1.5–2.0%) was delivered through a custom-made nose cone. To prevent skull fractures, a round stainless steel disk (helmet) of 10mm in diameter and 3mm in thickness was strapped on top of the shaved head by an elastic band and positioned midline between bregma and lambda (see Fig 1B). Immediately before dropping the weight, the nose cone for delivering anesthesia was removed for clearance. TBI was induced by freely dropping a custom-made 450g impactor guided by a brass tube from a distance of 2m from the disk. The foam bed together with the rat was rapidly moved away from underneath the tube to ensure a single hit. The injured rats were allowed to breathe spontaneously after impact and monitored postinjury until they awakened and could move normally.
Figure 1.
(A) Radiological and pathological experiment timeline of mild traumatic brain injury. (B–F) The experimental setup (B) and the corresponding finite element analysis (FEA) model geometry and mesh (C to F) of the impact-acceleration model of TBI. The model was created based on the anatomical MRI consisting of the scalp, muscle, skull, dura, gray and white matter, ventricle and spinal cord along with the rest of body. A metal helmet was attached to an elastic band strapped on top of the skull. (G) The experimental real-time images (upper row in G) and the FEA simulation results (lower row in G) showed that the mechanical insult generates peak maximum principal strain rate (MPSR) right after the impact to the corpus callosum (left arrow). At 40 milliseconds, a second peak of MPSR appeared also at the corpus callosum region (right arrow) when the head reached nadir. (H) The total impact duration completed within 200 milliseconds, where the FEA simulation showed comparable displacement trajectories to the in-situ measured head displacement. IHC = immunohistochemistry.
For a subset of animals, the impact was digitally filmed by an EX-ZR1100 high-speed camera (Casio Computer Co., Tokyo, Japan) at 1,000 frames per second. The tissue-level injury response of the weight drop injury was assessed by FEA simulation using Abaqus v6.10 (SIMULIA, Providence, RI). The material properties and boundary conditions for the FEA model were adapted from previous literature (Supplemental Table 1).25–30 In brief, the Ogden hyperelastic model was used for brain and spinal cord to simulate the biomechanical behavior during the impact,25, 27, 28 while the Mooney-Rivlin model was applied to the cerebral spinal fluid compartment with low shear-to-bulk modulus to mimic its fluid-like behavior.30 A 2nd-order polynomial hyperelastic strain energy function was utilized to model scalp, skin and muscle. The rest of components were simulated by linear elastic model. The final FEA model of the impact-acceleration TBI consisted of 89,739 nodes and 360,691 elements (see Fig 1). The distribution of von Mises stress, maximum principal strain and strain rate (MPSR) were calculated for the duration of impact. A model convergence test was performed and validated by the in-situ impact trajectory recorded from the high-speed camera. The MPSR were averaged for volumetric mapping as the injury predictor to evaluate the DAI locations in the brain (Fig 2A).31, 32
Figure 2.
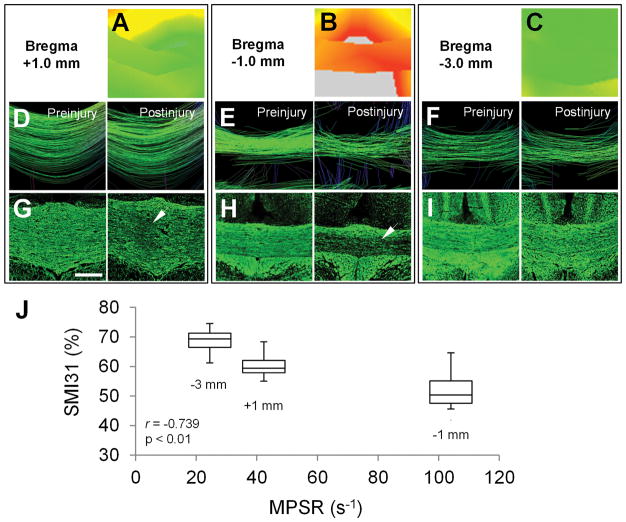
(A) The volumetric mapping of the averaged maximum principal strain rate estimated the possible injury location over the entire brain and guided the radiological-pathological correlation analysis. (B–G) The magnetic resonance imaging and immunohistochemistry data were acquired from multiple regions of interest at bregma +1.0mm (B, E), −1.0mm (C, F) and −3.0mm (D, G). The corpus callosum (CC) and cortex (CT) data were used for assessing the pathological correlation. The dashed squares in A indicate the regions evaluated in Figure 3. AC = anterior commissure; CP = cerebral peduncle; EC = external capsule; OT = optic tract; ST = striatum. Scale bar = 2mm.
In vivo MRI and Data Analysis
In vivo MRI was conducted on animals at five injury time-points: prior to TBI (Baseline, n = 45), 1 day postinjury (DPI) (n = 40); 10 DPI (n = 35); 20 DPI (n = 30), and 30 DPI (n = 25). Five rats per imaging time point were randomly picked for the cross-sectional immunohistochemistry (IHC) examination (see Fig 1A). Animals were anesthetized using an isoflurane/oxygen mixture (4.5–5% isoflurane for induction and 1.5–2.0% for maintenance) through a custom-made nose cone and placed in the scanner. Throughout MRI scans, warm water was circulated under the animals to keep them warm at 37°C; a steady respiratory rate was monitored using a pressure sensor (SA Instruments, Stony Brook, NY) and maintained at 40 to 50 breaths per minute by controlling the level of isoflurane/oxygen mixture. At each time point, T2-weighted images were first acquired by rapid acquisition with refocused echoes (RARE) sequence: repetition time (TR) = 3.8s, echo time (TE) = 15ms, RARE factor = 8, in-plane resolution = 100μm × 100μm, with 0.5mm thickness. Three-dimensional (3D) T2*-weighted images were also acquired to evaluate the presence of hemorrhage or subdural hematoma using multiple gradient echo (MGE): TR = 60ms, TE = 3.18ms, echo spacing = 3.25ms, voxel size 200μm3 (isotropic). T2* maps were created by fitting the magnitude images of 14 echo MGE data to an exponential function on a pixel-by-pixel basis. 3D DTI data was acquired using spin echo echo-planar imaging (EPI) sequence with TR = 700ms, TE = 37ms; segment = 6, Δ = 15ms; δ = 5ms; b-value = 0 and 800s/mm2, with 15 diffusion encoding directions. The voxel size of DTI was identical with MGE. Diffusion-weighted images were corrected for B0 susceptibility induced EPI distortion, eddy current distortions, and motion distortion with b-matrix reorientation using Tortoise.33 After correction, the diffusion tensor was used to calculate DTI parameters, FA, MD, axial diffusivity (AD), and radial diffusivity (RD). MTI was acquired by 2D RARE sequence with (MS) and without (M0) magnetization transfer (MT) preparation pulses added before excitation: TR = 5000ms, TE = 11.56ms, RARE factor = 8, pixel size = 200μm2, slice thickness = 0.5mm. Two commonly used MT saturation frequency offsets were chosen to compare the specificity of detecting DAI: 6000Hz (MTR-20)34–36 and 1,050Hz (MTR-3.5),17, 37, 38 saturation pulse amplitude = 4μT, duration = 1ms, and pulse number = 20. MTR maps were calculated by (M0-MS)/M0.
Based on the FEA results, a comprehensive region of interest (ROI) analysis was performed on 3 locations at bregma +1.0mm, −1.0mm, and −3mm to include various predicted DAI severities. ROIs were carefully delineated for corpus callosum (CC), cortex (CT), anterior commissure (AC), cerebral peduncle (CP), external capsule (EC), optic tract (OT) and striatum (ST; see Fig 2B–D). Values of DTI metrics acquired from three consecutive imaging slices were averaged for each anatomical region. A voxel-by-voxel temporal-spatial statistical analysis was performed for the FA and MTR-3.5 maps to test the sensitivity and specificity of examining the microstructural abnormalities in the entire brain following the injury over time. Maps of the injured brains acquired longitudinally were segmented and registered to a common anatomical image space using Oxford Centre for Functional MRI of the Brain’s Linear Image Registration Tool (FLIRT)39 and tract-based spatial statistics (TBSS).40 Each of the rats’ aligned maps was projected onto the mean skeleton, and the resulting data were fed into voxel-wise cross-subject statistics by repeated measures analysis of variance (ANOVA).40 The null distribution for the data in the TBSS statistics was built over 10,000 permutations and the results are shown as voxel-wise significance level (p value) < 0.05. For the multiple comparison correction within the data, cluster-level inference at t > 2.7, p < 0.05, and false discovery rate theory41 at q < 0.05 were used. Except for those processed by the aforementioned software, all other imaging data were processed via in house Matlab (Mathwork, Natick, MA) developed programs.
Immunohistochemistry Analysis
After imaging, twenty-five rat brains in the cross-sectional group were extracted and cryosectioned at 10μm for IHC analysis per published protocol.42 The following primary antibodies were used: ionized calcium-binding adaptor molecule 1 (Iba1; Cat. 01919741, Wako, Richmond, VA) at 1/200; glial fibrillary acidic protein (GFAP; Cat. ab7260, Millipore, Billerica, MA) at 1/1500; phosphorylated neurofilament H (SMI31; Cat. SMI31-R, Covance, Princeton, NJ) at 1/1500; hexaribonucleotide binding protein-3 (NeuN; Cat. mab377, Millipore) at 1/1000; myelin basic protein (MBP) at 1/1000 (Cat. ab24567, Abcam, Cambridge, MA). Secondary antibodies from Abcam were used at 1/200 as follows: for MBP, SMI31 and NeuN: goat anti-mouseF(ab′) IgG- H&L Dylight 594 (Cat. ab96881); for Iba1, and GFAP: goat anti-rabbit F(ab′) IgG- H&L Dylight 594 (Cat. ab102293). Apoptotic cells were stained by in situ direct DNA fragmentation (TUNEL) detection kit (Cat. 11684809910, Sigma Aldrich, St. Louis, MO). The IHC data were quantified independently by three investigators (R. A.W., J.D.L., T.-W.T.) from the corresponding locations matching to the MRI data (see Fig 2E–G) to cover various DAI extents for correlation analysis from all five TBI time points (n = 75) via threshold of the normal staining intensity determined by the control staining.24 The intraclass correlation coefficient for the IHC quantifications among investigators was >0.9 fulfilling the required minimum for good reliability. The IHC quantification from each investigator was then averaged to represent the percentage of positive fluorescent staining in the 20X images.
Statistical Analysis
Data were analyzed using one-way ANOVA with repeated measures by Prism v6.0c (GraphPad Software, Inc., La Jolla, CA). Bonferroni’s correction for multiple comparison was used to examine the difference between groups with the significant level predetermined at 0.05. Pearson correlation analysis was performed to delineate the possible pathological correlation between the MRI and IHC data. All data are reported as mean ± standard deviation.
Results
After TBI, the majority of rats regained consciousness and returned to normal behavior within five minutes of injury, with no other observable symptoms. A subset of animals experienced brief seizures (<15 seconds of either focal tail twitching [8 rats] or generalized seizures [5 rats]) immediately after impact followed by normal recovery. The rats with immediate post-traumatic seizure activity did not manifest prolonged recovery times. In the study cohort, neurological impairments (lethargy, weakness, slowed righting response) were rarely present after 5 minutes and did not persist beyond 10 minutes postinjury. All rats survived after TBI experiments. There was no evidence of skull fracture, subdural hematoma or hemorrhage on the acute T2*-weighted images and at time of euthanasia. The FEA simulation demonstrated the propagation of MPSR through the brain in this impact-acceleration model over time (see Fig 1G) and predicted peak MPSR for guiding the radiological-pathological correlation analysis (see Fig 2). Various levels of MPSR were shown in CC at bregma +1.0mm, −1.0mm, and −3.0mm, where the maximal MPSR appeared at bregma −1.0mm (Fig 3). The DTI tractography and SMI31 staining showed comparable results at the corresponding locations demonstrating different levels of axonal injury associated with the MPSR. The SMI31 quantification exhibited maximal loss of axonal integrity in CC at bregma −1.0mm to minimum axonal damage further from the site of highest MPSR.
Figure 3.
(A–C) Magnified from the locations denoted in Figure 2A, the maximum principal strain rate (MPSR) maps show that the corpus callosum (CC) endured the highest MPSR at bregma −1.0mm, where the diffuse axonal injury most likely occurred. (D–I) Correspondingly, the DTI tractography (D–F) and the phosphorylated neurofilament H (SMI31) neurofilament staining (G to I) also show that the most loss of white matter tracts and neurofilament staining were at bregma −1.0mm (arrow in H). Moderate injury signs are shown in the CC at bregma +1.0mm (arrow in G) and only few injuries were seen in the CC at bregma −3.0mm (I). (J) A strong negative correlation (r = −0.739, p = 0.002) is seen between the positive SMI31 staining and averaged MPSR. Scale bar = 400 μm.
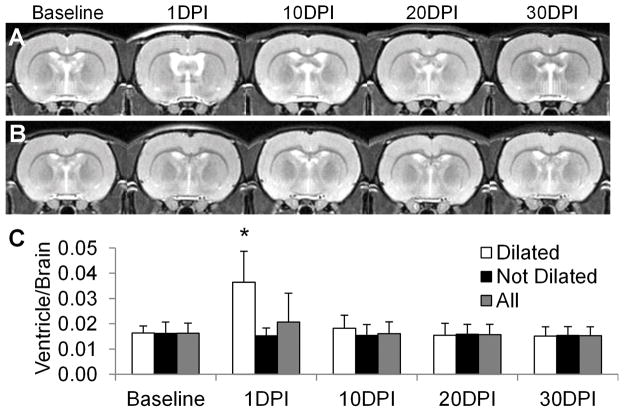
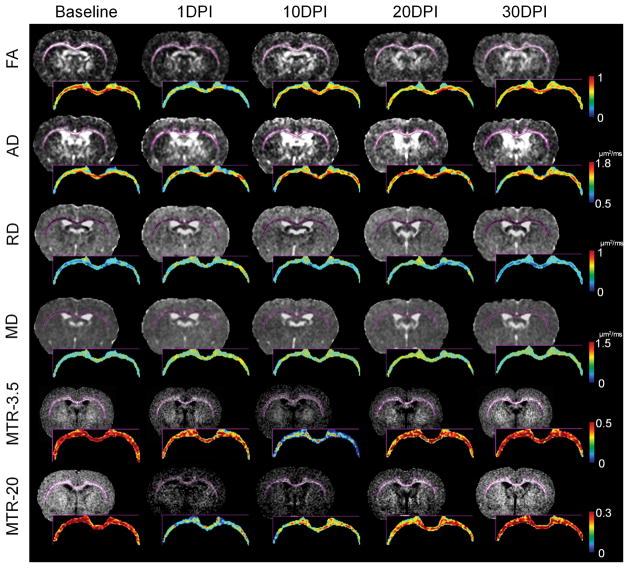
Following injury, ventricle dilation developed on 9 of the 40 injured brains at 1 DPI but rapidly resolved, with no significant changes in overall ventricular size over time after TBI (Fig 4). Longitudinal DTI and MTI maps demonstrate significant decreases of FA, AD and MTR-20 at 1DPI in CC (p<0.01), whereas RD and MD show increases in several discrete regions over time (Fig 5). MTR-3.5 decreased after injury and reached the nadir at 10 DPI (p < 0.01) for all structures. The group average data from the comprehensive ROI analysis are listed in Supplemental Table 2. In the major white matter tracts, AC, CC, and EC exhibited significantly decreased FA and AD at 1 DPI (p < 0.05), while OT and CP showed delayed decreases of FA and AD at 30 DPI (p < 0.05). In contrast, CT showed significantly increased FA at 30 DPI (p < 0.05), and increased MD at 20 DPI (p < 0.05). ST only showed significant changes in MTR-20 at 1 DPI (p < 0.01) and MTR-3.5 at 10 DPI (p < 0.05).
Figure 4.
(A) Longitudinal anatomical T2-weighted images of the brain following traumatic brain injury. Ventricle dilation was apparent at 1 day postinjury (DPI) in 9 of the 40 injured rats. (B) In the majority of injured brains (31 of 40), there was no evidence of ventricular dilation on T2-weighted images. (C) The volume quantification of the ventricle/brain was not significant when averaging all injured animals. *p < 0.05
Figure 5.
Longitudinal diffusion tensor imaging and magnetization transfer ratio (MTR) maps of the traumatic brain injury brain at bregma −1.0mm. Corpus callosum and external capsule were segmented for color mapping to highlight the pattern of diffusive axonal injury over time. Decreases of fractional anisotropy (FA), axial diffusivity (AD), and MTR at 20ppm are clearly seen immediately after injury at 1 day postinjury (DPI). Radial diffusivity (RD) and mean diffusivity (MD) only show changes in multiple scattering locations. MTR at 3.5ppm had a delayed decrease at 10 DPI.
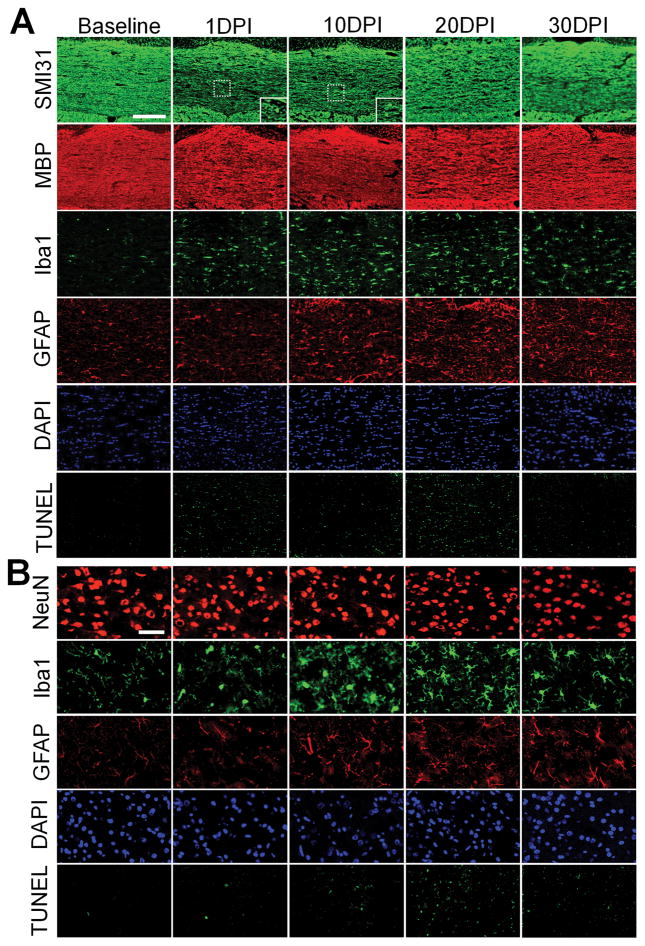
Immunohistochemical staining of the CC after TBI over time demonstrates changes consistent with white matter damage (Fig 6A). Significant loss (p < 0.01) of neurofilament SMI31 staining occurred at 1 DPI and 10 DPI in association with axonal beading, followed by a few scattered cavities with localized fiber degeneration at 20 DPI, and then recovered close to baseline levels by 30 DPI. MBP staining demonstrates insignificant loss of signal at 1 DPI, 10 DPI and 30 DPI over the CC. MBP staining demonstrates patchy loss of signal over the CC from 1 DPI to 20 DPI, becoming more condensed by 30 DPI. Iba1 staining both significantly increased (p < 0.01) at 1 DPI then reached maximum at 10 DPI to 20 DPI. GFAP increased over the 30-day time course (p < 0.01). DAPI increased significantly after 10 DPI (p < 0.01). The TUNEL staining significantly increased (p < 0.01) following injury at all time points and reached maximum at 20 DPI. Apoptotic cell death declined at 30 DPI yet was significantly higher (p < 0.01) than observations at baseline. IHC staining indicative of microgliosis and astrogliosis in the CT increased significantly (p<0.05) along a similar time course to the CC, while changes in NeuN and DAPI staining were not statistically significant (see Fig 6B). A similar trend of apoptotic cell death was seen in the CT when compared to the TUNEL staining in the CC over time. The group averaged values of the IHC quantification are presented in Supplemental Table 3. The heatmap summarizes the fold changes in MRI and IHC measures over time (Fig 7). Pearson correlation analysis shows relationships between MRI metrics and IHC quantification in both CC and CT (Table 1). SM131 staining significantly correlates with AD, FA, RD, and MTR-3.5, while MBP staining correlates with RD and MTR-3.5. MTR-20 shows statistically significant correlation with GFAP staining in both CC and CT, as dose FA in CT but not CC.
Figure 6.
Representative images of the immunofluorescent staining of (A) corpus callosum and (B) cortex show diffusive axonal injury pathologies in traumatic brain injury model. Phosphorylated neurofilament H (SMI31) staining clearly shows axonal injury at 1 day postinjury (DPI) and gradually recovers afterward, whereas myelin basic protein (MBP) staining shows low degree of perturbation during the injury time course (A). Neurite beadings are clear seen in the magnified images at 1 DPI and 10 DPI. Ionized calcium-binding adaptor molecule 1 (Iba1) staining displays microgliosis peaks at 10 DPI, and decreases after 30 days. Glial fibrillary acidic protein (GFAP) progressively increases up to day 30. 4,6-Diamidino-2-phenylindole (DAPI) shows a trend of increased cellularity over time. Hexaribonucleotide binding protein 3 (NeuN) staining shows a trend of decreased staining intensity over time although not significantly (B). Iba1 staining displays the maximum microgliosis appearing at 10 DPI and 20 DPI, and then normalizes after 30 DPI. GFAP progressively increases to day 30. DAPI shows a consistent trend but with a slight decrease at 10 DPI. An increased pattern of mild-to-moderate apoptotic cell death is seen in the TUNEL staining from 1 DPI to 20 DPI. The apoptotic cell death is largely declined at 30 DPI. The TUNEL positive staining shows more in the corpus callosum demonstrating the pattern of diffuse axonal injury. The images were captured from the corpus callosum and cortex at the bregma −1.0 mm denoted in Figure 2F. Scale bar = 200 μm (A), 50 μm (B).
Figure 7.
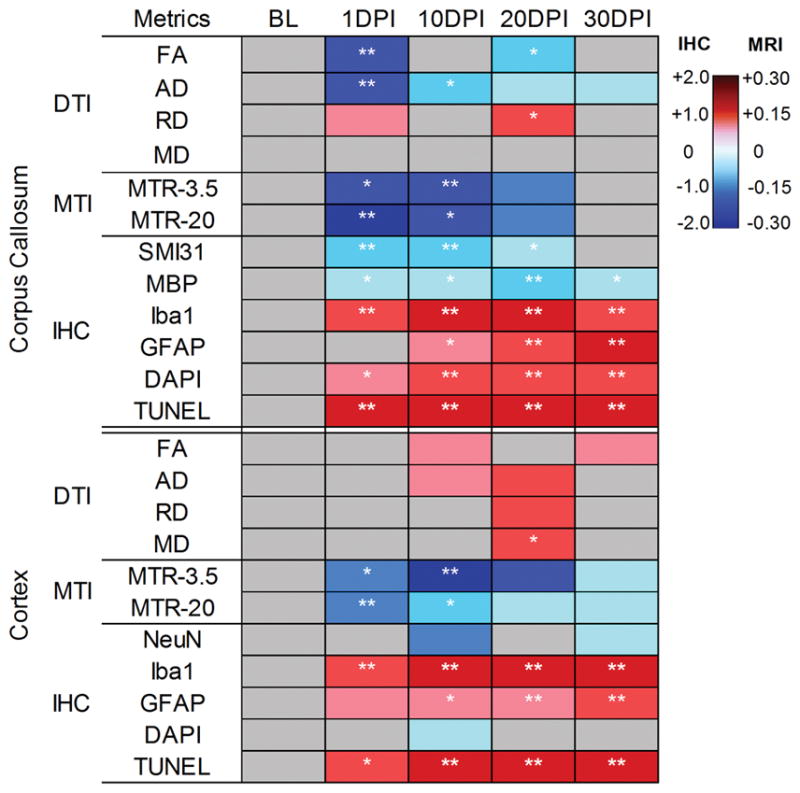
Heat map depicting the fold changes to the baseline (BL) values on thediffusion tensor imaging (DTI), magnetization transfer imaging (MTI), and immunohistochemistry (IHC) metrics of the corpus callosum and cortex following traumatic brain injury over time. Blue indicates decreased and red highlights increased values. *p < 0.05, **p < 0.01. AD 5 axial diffusivity; DAPI 5 4,6-diamidino- 2-phenylindole; DPI 5 days postinjury; FA 5 fractional anisotropy; GFAP 5 glial fibrillary acidic protein; Iba1 5 ionized calcium-binding adaptor molecule 1; MBP 5 myelin basic protein; MD 5 mean diffusivity; MRI 5 magnetic resonance imaging; MTR 5 magnetization transfer ratio; RD 5 radial diffusivity; SMI31 5 phosphorylated neurofilament H.
Table 1.
Pearson correlation analysis of the MRI metrics versus IHC quantifications (n=75).
| r | Corpus Callosum
|
Cortex
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SMI31 | MBP | GFAP | Iba1 | DAPI | NeuN | GFAP | Iba1 | DAPI | |
| FA | 0.66** | 0.13 | −0.18 | −0.36* | −0.25 | −0.04 | 0.63** | −0.06 | −0.13 |
| AD | 0.65** | −0.01 | −0.26 | −0.23 | −0.66** | 0.03 | 0.20 | 0.26 | 0.51** |
| RD | −0.39** | −0.46** | 0.28 | 0.50** | 0.31* | 0.05 | 0.31* | 0.24 | 0.49** |
| MD | 0.24 | −0.17 | 0.02 | −0.30* | −0.25 | 0.34* | 0.29* | 0.24 | 0.59** |
| MTR-3.5 | 0.51** | 0.38** | −0.07 | −0.25 | 0.07 | 0.221 | −0.16 | 0.02 | −0.06 |
| MTR-20 | 0.23 | 0.28 | 0.64** | 0.05 | −0.16 | −0.09 | 0.42** | −0.12 | −0.19 |
p < 0.01,
p < 0.05.
AD = axial diffusivity; DAPI = 4,6-diamidino-2-phenylindole; FA = fractional anisotropy; GFAP = glial fibrillary acidic protein; Iba1 = ionized calcium-binding adaptor molecule 1; MBP = myelin basic protein; MD = mean diffusivity; MTR = magnetization transfer ratio; NeuN = hexaribonucleotide binding protein 3; RD = radial diffusivity; SMI31 = phosphorylated neurofilament H.
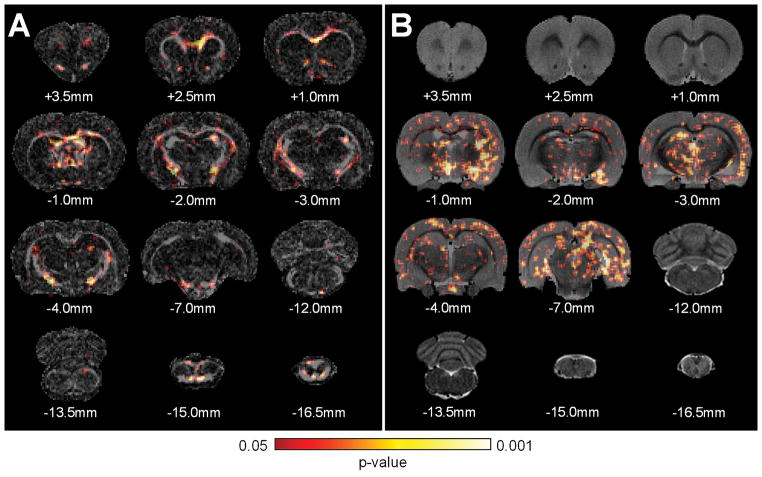
Creation of a significance map based upon the voxelwise statistical tests allows visualization of the spatial patterns of FA and MTR-3.5 changes across all of the injured groups compared to the baseline images. A distinct pattern of abnormalities related to TBI over time emerges from the voxelwise temporal-spatial statistical maps between FA and MTR-3.5 (Fig 8). The decreases in FA were more evident in white matter tracts, suggesting specificity to white matter injury in DAI. The extent of MTR-3.5 detected abnormalities is found in both white and gray matter regions.
Figure 8.
The significance maps of the (A) diffusion tensor imaging (DTI) fractional anisotropy (FA) and (B) magnetization transfer ratio (MTR) at 3.5ppm over the 30-day period after traumatic brain injury. The decreased DTI FA was most prominent in the major white matter tracts, suggesting superior specificity for detecting diffusive axonal injury. The changes of MTR at 3.5ppm not only showed in the white matter tracts, but also in the gray matter regions, including cortex, striatum, thalamus, and hippocampus. Subscripted indices are relative to the bregma.
Discussion
The current study demonstrates the radiological-pathological correlation between changes in DTI and MTI metrics in a modified Marmarou weight drop TBI model in female rats. Key findings of this study are as follows: (1) FEA modeling can be used to predict the location and extent of injury after TBI and help guide subsequent ROI analysis to uncover the subtle DAI in mild TBI; (2) DTI metrics colocalize and correlate with IHC staining indicative of axonal injury; secondary inflammatory responses, that is, microgliosis, astrogliosis, and increased cellularity, also contribute to the changes of DTI metrics; (3) MTI is sensitive in reflecting the consequences of TBI in brain, but is less specific than DTI for showing DAI in white matter.
Demonstration of the radiological-pathological correlations after TBI is complicated by the heterogeneity of injury and insufficient imaging resolution for comparison.14, 43 Several studies have reported few radiological-pathological correlations with DTI measures of axonal injury in moderate to severe CCI models. Beta-amyloid precursor protein correlated with decreases in AD and FA when compared to contralateral uninjured brain.18 In the LFP model, a decrease in FA and increase in RD was consistent with axonal degeneration and demyelination in the CC.44 Astrogliosis in the cortex has also been associated with increased FA in CCI19 and blast injury models.45 In the mild TBI model, DTI tractography showed fiber discontinuity within 6 hours of weight drop injury in the CC whereas MBP and Bielschowsky staining showing fiber disorganization.9 FA and AD were significantly reduced in CC corresponding to pathological axonal injury and neurofilament triplet-l protein staining in a rotating model of mild TBI.8 Using a similar impact-acceleration model of mild TBI, FA and AD were negatively correlated to the increase of beta-amyloid precursor protein stained axon in CC and pyramidal tract.46 These reports were limited because they were cross-sectional and short duration (ie, <7 days) investigations following injury and the correlations were made to axonal injury without considering the influence of secondary pathologies.
In the current study, the radiological-pathological correlation was investigated more thoroughly to include the influence of primary and secondary pathologies on the DTI and MTI metrics in mild TBI over time. Histological examination revealed that axonal injury without substantial demyelination was present during the early acute and subacute phases following TBI, and there was recovery by day 30. Following the initial injury, inflammation and reactive astrogliosis occurred in postinjury white and gray matter areas without significant neuronal loss. The correlation indicated that significant decreases in FA and AD, with minimal changes in RD, were mainly secondary to DAI during the acute injury phase. After the subacute phase, except for a strong correlation with axonal injury, FA and AD were also altered by the microgliosis and increased cellularity in CC. RD not only showed a strong correlation with myelin content, but also displayed a strong reaction to axonal integrity and microgliosis. These results suggest that DTI metrics were very sensitive and specific to DAI in the acute phase. Nevertheless, the changes of DTI metrics were also affected by the inflammatory TBI pathologies in the secondary phase.47, 48
In support of our observations, previous studies immediately following white matter injury observed decreases in AD and FA paralleled with initial shearing and disintegrated white matter structures (i.e. axonal skeleton, microtubules, and neurofilaments) along with associated neurite beading, fragmentation, transection, end-bulb formation, disrupted glia cells, and debris.8, 14, 18, 49, 50 At 10 DPI, AD in CC remains decreased in conjunction with decreased staining for SMI31 and MBP. These results may reflect the disappearance of the axonal skeletons and membranes, degradation of the myelin sheath, and/or the influx of inflammatory cells.48 By 30 DPI, the SMI31 and MBP staining documented recovery of white matter tracts in the injured CC, that was reflected in the normalization of AD and FA towards baseline in comparison to 1DPI changes.51
DTI has been reported to be sensitive to white matter injury, as visualized in the temporal-spatial statistics maps (see Fig 8A). However, DTI can also detect gray matter injury after TBI, with increased FA in gray matter correlating with astrogliosis in CCI injury19 and blast TBI.45 In the current model, we also observed increased astrogliosis correlating with FA in the CT. We postulate that the increase in FA was due to the astrocytic processes forming a more coherent pathway for diffusion between extracellular spaces.19, 45 MD increased by 20DPI and primarily correlated in cortex with increased cellularity and astrocytosis, and with microgliosis in CC.
MTR correlates with changes in myelin integrity in diseases including multiple sclerosis34, 35 and Alzheimer’s disease.52 In the MTI literature, the most common offset frequencies used for MT saturation pulse to detect white matter injury ranged from 1kHz to 2kHz on a 1.5T scanner, corresponding to 15ppm to 35ppm from the water peak.16, 34, 35, 53 Comparing mild TBI patients to healthy controls, MTR was decreased in the splenium of the CC using 18.8ppm offset frequency.16 In mild-moderate TBI patients, MTR was also lower in white matter compared to healthy controls using a 31.3ppm offset frequency and was correlated to persistent neurologic deficits.53 In contrast, MTI using near-resonance saturation offset frequency (3–10 ppm) demonstrated increased contrast of diseased tissue, due to the relative lack of suppression of the diseased white matter compared with normal brain tissue.37 In a pig model of rotational brain injury, changes in MTR at 7ppm correlated with axonal pathology.17 MTR at 10ppm correlated with the number of Luxol fast blue (LFB) stained myelinated axons in the CC of a rat model of therapeutically induced remyelination.38 In a reversible demyelination model with no axonal loss, MTR at 12.5ppm showed a strong correlation to MBP immunoreactivity, as well as a negative correlation to the percentage of GFAP immunoreactivity.54
The current study compared MTRs derived from the commonly used saturation frequency offset at 20 ppm and the near-resonance saturation offset frequency at 3.5 ppm. MTRs obtained at different saturation frequencies were useful in detecting neuropathologies. The MTR at 20ppm showed a strong correlation to increased GFAP staining over time and a weak correlation to SMI31 and MBP staining. The MTR at 3.5 ppm demonstrated enhanced correlation to the decrease in percentage area of fluorescence with both SMI31 and MBP, which was comparable to previous studies on postmortem MS brain.34, 35 The significantly decreased MTR within the subacute phase and its subsequent normalization to baseline at later time points in the present study may reflect resolution of edema.55 The temporal-spatial statistical maps demonstrated that MTR at 3.5ppm displayed a wider extent of abnormalities in both grey and white matter along the TBI injury progress, suggesting MTI was sensitive in reflecting the consequences of TBI in brain, but is less specific than DTI for the detection of DAI in white matter.
The difficulties of studying mild DAI patterns in preclinical studies underscores the obstacles that have been encountered in translating advanced imaging techniques to the clinic.56 The FEA simulation allows for the prediction of the possible locations of DAI by examining the biomechanical predictors of injury based on strain rate in TBI (Fig 3). The current FEA results agree with previous IHC study demonstrating that DAI in the impact-acceleration rat model was maximal in CC at bregma −1.0mm.23 Examination of MPSR distribution and other mechanical factors (eg von Mises stress and strain) in this TBI model helped ascertain probable injury patterns prior to the injury and improved the accuracy of the radiological-pathological correlation.32, 57
The current study focused on the radiological-pathological correlation based on DTI and MTI. Performing extensive behavioral assessments was beyond the scope of the present study and therefore no conclusions can be drawn about associations among radiological-pathological correlations and neurobehavioral outcomes. The original Maramrou weight drop model used 450g weight and 2.0m drop height to generate severe injury with 58.6% mortality rate on adult male Sprague-Dawley rats. In comparison, the current model caused mild injury using the same weight and height on the young adult female Wistar rats and 0% mortality. The milder form of TBI in the current study was identified by the SMI31 staining showing recoverable axonal injury and the TUNEL staining demonstrating limited mild-to-moderate apoptotic cell death (see Fig 6). The axonal damage and apoptotic cell death were clearly restored in the chronic phase at 30 DPI suggesting that the rats recovered in the current TBI model. Several factors in the experiment setup could possibly contribute to generate the milder form of TBI with this modified model. First, because most TBI rodent studies have historically been performed on male animals,58 introducing a potential sex bias in the reporting of results, we used female animals as part of the NIH initiative to balance the sex of animals in preclinical studies.59 Second, we modified the helmet installation by using a strap to fasten the stainless disk on the rat’s head, instead of firmly affixing it by adhesive to the skull vault for the ease of performing longitudinal MRI scans. Third, a brass tube was used to guide the weight drop, which may contribute different friction coefficient from the Plexiglas tube used in the original Marmarou model. Lastly, baseline MRI screening was performed on every rat prior to the TBI experiment to exclude the effect from abnormal baseline condition (e.g. ventriculomegaly)24, 60 that could introduce inconsistencies in the experiment results. A similar DTI study9 using male Wistar rats after weight drop injury found decreases in FA (14%), MD (39%), AD (42%), and RD (20%) at 6 hrs after impact using a 500g weight and a 1.5m drop height. In comparison, our results detected decreases in FA (18%), MD (6%), and AD (16%), but increased RD (10%) at 24 hrs after injury using a 450g weight and 2.0m drop height in female Wistar rats. Whether the variations in DTI metrics between these two studies are secondary to the timing after injury, the difference in drop height, and/or differences in the response to injury between male and female Wistar rats is currently unknown and require further investigation.
Although we have demonstrated modest correlation between imaging results and pathological findings, cautions are needed in extrapolating the current results to a clinical population. The clinical diagnosis of TBI encompasses diverse forms of injury, degree of trauma, injury locations and severity,61 and humans are more variable in genetic and environmental backgrounds than laboratory animals. The increased variation in clinical populations will make detection of subtle MRI imaging findings more difficult, and is further complicated by a lack of available human tissue for direct radiological-pathological correlation.
Conclusion
The current study demonstrates that after TBI, DTI and MTI can reveal changes that correlate with histopathology consistent with DAI. Our data support the hypotheses that AD and RD primarily reflect the extent of axon and myelin integrity, whereas FA and MD are more sensitive to increases in cellularity, water content and overall microstructural architecture. The secondary inflammatory response also introduces moderate effects to the changes of these parameters. Furthermore, MTR at 3.5ppm saturation offset shows correlation to both axon and myelin integrity, and MTR at 20ppm was related to astrogliosis. In conclusion, this study indicates that DTI and MTR are sensitive and may be useful in detecting injuries associated with mild TBI.
Supplementary Material
Supplemental Table 1. The material properties adapted in the finite element analysis of the impact-acceleration model of TBI.25–30 G: Shear Modulus, G0: instantaneous shear modulus, G∞: long-term shear modulus, Gi: relative relaxations, ti: relaxation time constants, ν: Poisson’s ratio, α and D: temperature-dependent material parameters.
Supplemental Table 2. Group averaged values of the DTI and MTI parameters quantified from the ROIs at anterior commissure, corpus callosum, cortex, cerebral peduncle, external capsule, striatum and optic tract (n=45). * p<0.05, ** p<0.01
Supplemental Table 3. Group averaged values of the IHC quantification (n=5). The data were acquired from the staining slices at bregma −1.0mm. * p<0.05, ** p<0.01
Acknowledgments
This work was supported by funding from the Department of Defense through the Center for Neuroscience and Regenerative Medicine (Henry M. Jackson Foundation Award #300604-8.01-60855 (JAF) and #305500-8.01-60855 (LCT)) and from the Intramural Research Programs of the Clinical Center and of the National Institute of Biomedical Imaging and Bioengineering at the National Institutes of Health.
Footnotes
Author Contributions
Guarantor of integrity of entire study: J.A. Frank; Study concepts/design: T-W Tu, L.C. Turtzo, J.A. Frank; data acquisition: T-W Tu, R.A. Williams, J.D. Lescher, N Jikaria; data analysis/interpretation: all authors; statistical analysis: T-W Tu, R.A. Williams, J.D. Lescher; manuscript drafting: T-W Tu; manuscript editing: T-W Tu, L.C. Turtzo, J. Frank; manuscript final approval: all authors.
References
- 1.Roozenbeek B, Maas AI, Menon DK. Changing patterns in the epidemiology of traumatic brain injury. Nature reviews Neurology. 2013 Apr;9(4):231–6. doi: 10.1038/nrneurol.2013.22. [DOI] [PubMed] [Google Scholar]
- 2.Belanger HG, Vanderploeg RD, Curtiss G, Warden DL. Recent neuroimaging techniques in mild traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2007 Winter;19(1):5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Borg J, Holm L, Cassidy JD, et al. Diagnostic procedures in mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of rehabilitation medicine. 2004 Feb;(43 Suppl):61–75. doi: 10.1080/16501960410023822. [DOI] [PubMed] [Google Scholar]
- 4.Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. The Journal of head trauma rehabilitation. 2003 Jul-Aug;18(4):307–16. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Sundman MH, Hall EE, Chen NK. Examining the relationship between head trauma and neurodegenerative disease: A review of epidemiology, pathology and neuroimaging techniques. Journal of Alzheimer’s disease & Parkinsonism. 2014 Jan 31;4 doi: 10.4172/2161-0460.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 2005 Aug;103(2):298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 7.Mayer AR, Ling J, Mannell MV, et al. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010 Feb 23;74(8):643–50. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Li XY, Feng DF, Gu L. Quantitative evaluation of microscopic injury with diffusion tensor imaging in a rat model of diffuse axonal injury. Eur J Neurosci. 2011 Mar;33(5):933–45. doi: 10.1111/j.1460-9568.2010.07573.x. [DOI] [PubMed] [Google Scholar]
- 9.van de Looij Y, Mauconduit F, Beaumont M, et al. Diffusion tensor imaging of diffuse axonal injury in a rat brain trauma model. NMR in biomedicine. 2010 Jan;25(1):93–103. doi: 10.1002/nbm.1721. [DOI] [PubMed] [Google Scholar]
- 10.Bennett RE, Mac Donald CL, Brody DL. Diffusion tensor imaging detects axonal injury in a mouse model of repetitive closed-skull traumatic brain injury. Neuroscience letters. 2012 Apr 4;513(2):160–5. doi: 10.1016/j.neulet.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutgers DR, Toulgoat F, Cazejust J, Fillard P, Lasjaunias P, Ducreux D. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. AJNR Am J Neuroradiol. 2008 Mar;29(3):514–9. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma. 2007 Sep;24(9):1447–59. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- 13.Ling JM, Pena A, Yeo RA, et al. Biomarkers of increased diffusion anisotropy in semi-acute mild traumatic brain injury: a longitudinal perspective. Brain. 2012 Apr;135(Pt 4):1281–92. doi: 10.1093/brain/aws073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain imaging and behavior. 2012 Jun;6(2):108–36. doi: 10.1007/s11682-011-9145-0. [DOI] [PubMed] [Google Scholar]
- 15.Sinson G, Bagley LJ, Cecil KM, et al. Magnetization transfer imaging and proton MR spectroscopy in the evaluation of axonal injury: correlation with clinical outcome after traumatic brain injury. AJNR Am J Neuroradiol. 2001 Jan;22(1):143–51. [PMC free article] [PubMed] [Google Scholar]
- 16.McGowan JC, Yang JH, Plotkin RC, et al. Magnetization transfer imaging in the detection of injury associated with mild head trauma. AJNR Am J Neuroradiol. 2000 May;21(5):875–80. [PMC free article] [PubMed] [Google Scholar]
- 17.McGowan JC, McCormack TM, Grossman RI, et al. Diffuse axonal pathology detected with magnetization transfer imaging following brain injury in the pig. Magn Reson Med. 1999 Apr;41(4):727–33. doi: 10.1002/(sici)1522-2594(199904)41:4<727::aid-mrm11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007 May;205(1):116–31. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain : a journal of neurology. 2011 Aug;134(Pt 8):2248–60. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albensi BC, Knoblach SM, Chew BG, O’Reilly MP, Faden AI, Pekar JJ. Diffusion and high resolution MRI of traumatic brain injury in rats: time course and correlation with histology. Exp Neurol. 2000 Mar;162(1):61–72. doi: 10.1006/exnr.2000.7256. [DOI] [PubMed] [Google Scholar]
- 21.Foda MA, Marmarou A. A new model of diffuse brain injury in rats. Part II: Morphological characterization. Journal of neurosurgery. 1994 Feb;80(2):301–13. doi: 10.3171/jns.1994.80.2.0301. [DOI] [PubMed] [Google Scholar]
- 22.Povlishock JT, Marmarou A, McIntosh T, Trojanowski JQ, Moroi J. Impact acceleration injury in the rat: evidence for focal axolemmal change and related neurofilament sidearm alteration. J Neuropathol Exp Neurol. 1997 Apr;56(4):347–59. [PubMed] [Google Scholar]
- 23.Li Y, Zhang L, Kallakuri S, Zhou R, Cavanaugh JM. Quantitative relationship between axonal injury and mechanical response in a rodent head impact acceleration model. J Neurotrauma. 2011 Sep;28(9):1767–82. doi: 10.1089/neu.2010.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu TW, Turtzo LC, Williams RA, Lescher JD, Dean DD, Frank JA. Imaging of spontaneous ventriculomegaly and vascular malformations in Wistar rats: implications for preclinical research. Journal of neuropathology and experimental neurology. 2014 Dec;73(12):1152–65. doi: 10.1097/NEN.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gefen A, Gefen N, Zhu Q, Raghupathi R, Margulies SS. Age-dependent changes in material properties of the brain and braincase of the rat. J Neurotrauma. 2003 Nov;20(11):1163–77. doi: 10.1089/089771503770802853. [DOI] [PubMed] [Google Scholar]
- 26.Goldsmith W, Plunkett J. A biomechanical analysis of the causes of traumatic brain injury in infants and children. Am J Forensic Med Pathol. 2004 Jun;25(2):89–100. doi: 10.1097/01.paf.0000127407.28071.63. [DOI] [PubMed] [Google Scholar]
- 27.Mao H, Jin X, Zhang L, et al. Finite element analysis of controlled cortical impact-induced cell loss. J Neurotrauma. 2010 May;27(5):877–88. doi: 10.1089/neu.2008.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maikos JT, Elias RA, Shreiber DI. Mechanical properties of dura mater from the rat brain and spinal cord. J Neurotrauma. 2008 Jan;25(1):38–51. doi: 10.1089/neu.2007.0348. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Gurao M, Yang KH, King AI. Material characterization and computer model simulation of low density polyurethane foam used in a rodent traumatic brain injury model. J Neurosci Methods. 2011 May 15;198(1):93–8. doi: 10.1016/j.jneumeth.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maikos JT, Qian Z, Metaxas D, Shreiber DI. Finite element analysis of spinal cord injury in the rat. Journal of neurotrauma. 2008 Jul;25(7):795–816. doi: 10.1089/neu.2007.0423. [DOI] [PubMed] [Google Scholar]
- 31.McAllister TW, Ford JC, Ji S, et al. Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann Biomed Eng. 2011 Jan;40(1):127–40. doi: 10.1007/s10439-011-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Mao H, Yang KH, Abel T, Meaney DF. A modified controlled cortical impact technique to model mild traumatic brain injury mechanics in mice. Frontiers in neurology. 2014;5:100. doi: 10.3389/fneur.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irfanoglu MO, Machiraju R, Sammet S, Pierpaoli C, Knopp MV. Automatic deformable diffusion tensor registration for fiber population analysis. Med Image Comput Comput Assist Interv. 2008;11(Pt 2):1014–22. doi: 10.1007/978-3-540-85990-1_122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmierer K, Scaravilli F, Altmann DR, Barker GJ, Miller DH. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann Neurol. 2004 Sep;56(3):407–15. doi: 10.1002/ana.20202. [DOI] [PubMed] [Google Scholar]
- 35.van Waesberghe JH, Kamphorst W, De Groot CJ, et al. Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Annals of neurology. 1999 Nov;46(5):747–54. doi: 10.1002/1531-8249(199911)46:5<747::aid-ana10>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 36.Horsfield MA, Barker GJ, Barkhof F, Miller DH, Thompson AJ, Filippi M. Guidelines for using quantitative magnetization transfer magnetic resonance imaging for monitoring treatment of multiple sclerosis. Journal of magnetic resonance imaging : JMRI. 2003 Apr;17(4):389–97. doi: 10.1002/jmri.10266. [DOI] [PubMed] [Google Scholar]
- 37.Ulmer JL, Mathews VP, Hamilton CA, Elster AD, Moran PR. Magnetization transfer or spin-lock? An investigation of off-resonance saturation pulse imaging with varying frequency offsets. AJNR American journal of neuroradiology. 1996 May;17(5):805–19. [PMC free article] [PubMed] [Google Scholar]
- 38.Deloire-Grassin MS, Brochet B, Quesson B, et al. In vivo evaluation of remyelination in rat brain by magnetization transfer imaging. J Neurol Sci. 2000 Sep 1;178(1):10–6. doi: 10.1016/s0022-510x(00)00331-2. [DOI] [PubMed] [Google Scholar]
- 39.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001 Jun;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 40.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006 Jul 15;31(4):1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002 Apr;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 42.Turtzo LC, Lescher J, Janes L, Dean DD, Budde MD, Frank JA. Macrophagic and microglial responses after focal traumatic brain injury in the female rat. J Neuroinflammation. 2014;11:82. doi: 10.1186/1742-2094-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long JA, Watts LT, Chemello J, Huang S, Shen Q, Duong TQ. Multiparametric and longitudinal MRI characterization of mild traumatic brain injury in rats. Journal of neurotrauma. 2015 Apr 15;32(8):598–607. doi: 10.1089/neu.2014.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laitinen T, Sierra A, Bolkvadze T, Pitkanen A, Grohn O. Diffusion tensor imaging detects chronic microstructural changes in white and gray matter after traumatic brain injury in rat. Front Neurosci. 2015;9:128. doi: 10.3389/fnins.2015.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Budde MD, Shah A, McCrea M, Cullinan WE, Pintar FA, Stemper BD. Primary blast traumatic brain injury in the rat: relating diffusion tensor imaging and behavior. Front Neurol. 2013;4:154. doi: 10.3389/fneur.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li S, Sun Y, Shan D, et al. Temporal profiles of axonal injury following impact acceleration traumatic brain injury in rats--a comparative study with diffusion tensor imaging and morphological analysis. International journal of legal medicine. 2013 Jan;127(1):159–67. doi: 10.1007/s00414-012-0712-8. [DOI] [PubMed] [Google Scholar]
- 47.Lin M, He H, Schifitto G, Zhong J. Simulation of changes in diffusion related to different pathologies at cellular level after traumatic brain injury. Magnetic resonance in medicine. 2015 Aug 10; doi: 10.1002/mrm.25816. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain : a journal of neurology. 2011 Dec;134(Pt 12):3590–601. doi: 10.1093/brain/awr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Budde MD, Frank JA. Neurite beading is sufficient to decrease the apparent diffusion coefficient after ischemic stroke. Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14472–7. doi: 10.1073/pnas.1004841107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007 Oct 31;27(44):11869–76. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie M, Tobin JE, Budde MD, et al. Rostrocaudal analysis of corpus callosum demyelination and axon damage across disease stages refines diffusion tensor imaging correlations with pathological features. J Neuropathol Exp Neurol. 2010 Jul;69(7):704–16. doi: 10.1097/NEN.0b013e3181e3de90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Flier WM, van den Heuvel DM, Weverling-Rijnsburger AW, et al. Magnetization transfer imaging in normal aging, mild cognitive impairment, and Alzheimer’s disease. Annals of neurology. 2002 Jul;52(1):62–7. doi: 10.1002/ana.10244. [DOI] [PubMed] [Google Scholar]
- 53.Bagley LJ, McGowan JC, Grossman RI, et al. Magnetization transfer imaging of traumatic brain injury. J Magn Reson Imaging. 2000 Jan;11(1):1–8. doi: 10.1002/(sici)1522-2586(200001)11:1<1::aid-jmri1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 54.Zaaraoui W, Deloire M, Merle M, et al. Monitoring demyelination and remyelination by magnetization transfer imaging in the mouse brain at 9.4 T. Magma. 2008 Sep;21(5):357–62. doi: 10.1007/s10334-008-0141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barzo P, Marmarou A, Fatouros P, Hayasaki K, Corwin F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg. 1997 Dec;87(6):900–7. doi: 10.3171/jns.1997.87.6.0900. [DOI] [PubMed] [Google Scholar]
- 56.Wintermark M, Sanelli PC, Anzai Y, Tsiouris AJ, Whitlow CT. Imaging evidence and recommendations for traumatic brain injury: advanced neuro- and neurovascular imaging techniques. AJNR Am J Neuroradiol. 2014 Feb;36(2):E1–E11. doi: 10.3174/ajnr.A4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ueno K, Melvin JW, Li L, Lighthall JW. Development of tissue level brain injury criteria by finite element analysis. J Neurotrauma. 1995 Aug;12(4):695–706. doi: 10.1089/neu.1995.12.695. [DOI] [PubMed] [Google Scholar]
- 58.Turtzo LC, Budde MD, Dean DD, et al. Failure of intravenous or intracardiac delivery of mesenchymal stromal cells to improve outcomes after focal traumatic brain injury in the female rat. PLoS One. 2015;10(5):e0126551. doi: 10.1371/journal.pone.0126551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCullough LD, de Vries GJ, Miller VM, Becker JB, Sandberg K, McCarthy MM. NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biology of sex differences. 2014;5:15. doi: 10.1186/s13293-014-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tu TW, Lescher JD, Williams RA, Jikaria N, Turtzo LC, Frank JA. Abnormal injury response in spontaneous mild ventriculomegaly Wistar rat brains: a pathological correlation study of diffusion tensor and magnetization transfer imaging. Journal of neurotrauma. 2016 Feb 23; doi: 10.1089/neu.2015.4355. ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singleton RH, Povlishock JT. Identification and characterization of heterogeneous neuronal injury and death in regions of diffuse brain injury: evidence for multiple independent injury phenotypes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004 Apr 7;24(14):3543–53. doi: 10.1523/JNEUROSCI.5048-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. The material properties adapted in the finite element analysis of the impact-acceleration model of TBI.25–30 G: Shear Modulus, G0: instantaneous shear modulus, G∞: long-term shear modulus, Gi: relative relaxations, ti: relaxation time constants, ν: Poisson’s ratio, α and D: temperature-dependent material parameters.
Supplemental Table 2. Group averaged values of the DTI and MTI parameters quantified from the ROIs at anterior commissure, corpus callosum, cortex, cerebral peduncle, external capsule, striatum and optic tract (n=45). * p<0.05, ** p<0.01
Supplemental Table 3. Group averaged values of the IHC quantification (n=5). The data were acquired from the staining slices at bregma −1.0mm. * p<0.05, ** p<0.01