Abstract
Introduction
Particulate matter (PM), specifically nickel (Ni) found on or in PM, has been associated with an increased risk of mortality in human population studies and significant increases in vascular inflammation, generation of reactive oxygen species, altered vasomotor tone, and potentiated atherosclerosis in murine exposures. Recently, murine inhalation of Ni nanoparticles have been shown to cause pulmonary inflammation which affects cardiovascular tissue and potentiates atherosclerosis. These adverse cardiovascular outcomes may be due to the effects of Ni on endothelial progenitor cells (EPCs), endogenous semi-pluripotent stem cells that aid in endothelial repair. Thus, we hypothesize that Ni nanoparticle exposures decrease cell count and cause impairments in function which may ultimately have significant effects on various cardiovascular diseases such as atherosclerosis.
Methods
Experiments involving inhaled Ni nanoparticle exposures(2 days/5 hrs/day at ~1000 μg/m3, 3 days/5 hrs/day at ~1000 μg/m3, and 5days/5 hrs/day at ~100 μg/m3), were performed in order to quantify bone marrow resident EPCs using flow cytometry in C57BL/6 mice. Plasma levels of SDF-1α and VEGF were assessed by ELISA and in vitro functional assessments of cultured EPCs were conducted.
Results and Conclusions
Significant EPC count differences between exposure and control groups for Ni nanoparticle exposures were observed. Differences in EPC tube formation and chemotaxis were also observed for the Ni nanoparticle exposed group. Plasma VEGF and SDF-1α differences were not statistically significant. In conclusion, this study shows that inhalation of Ni nanoparticles results in functionally impaired EPCs and reduced number in the bone marrow, which may lead to enhanced progression of atherosclerosis.
Introduction
Key studies involving the long term exposure of apolipoprotein-E deficient (ApoE −/−) mice to concentrated ambient air particles (CAPs) demonstrated significant increases in macrophage infiltration, vascular inflammation, generation of reactive oxygen species (ROS), altered vasomotor tone, and potentiated atherosclerosis (Sun et al. 2005, Chen, Nadziejko 2005). These findings drove additional studies evaluating the effects of ApoE −/− mice exposed for 5 months to CAPs as well as the examination of the National Mortality and Morbidity Air Pollution Study (NMMAPS) with associations to mortality (Lippmann et al. 2006). Back-trajectory analysis of the murine exposure study showed significant associations with nickel (Ni) and acute changes in heart rate and heart rate variability. Significant associations were also observed with daily mortality and Ni levels from the NMMAPS data. Taken together, these findings suggested that Ni, although a minor mass component, may be a major contributor responsible for the adverse effects resulting from exposure to ambient particulate matter.
In addition to the metals coated on or forming part of the core of particulate matter, engineered metal nanoparticles themselves are now being generated for use in a variety of consumer goods (Maynard et al. 2006). Emerging evidence has shown that inhalation of various nano-sized particles results in the generation of ROS that cause systemic inflammation and oxidative stress (Xia, Li & Nel 2009, Simeonova, Erdely 2009, Kennedy et al. 2009). Key findings in our laboratory have specifically shown that nickel (II) hydroxide nanoparticles can induce pulmonary inflammation through the activation of inflammatory mediators such as cytokines, chemokines, and metalloproteinases (Gillespie et al. 2010). Furthermore, the same chronic exposure also resulted in systemic effects such as increased gene expression of inflammatory markers in cardiovascular tissue (Kang GS, Gillespie P, Chen LC. 2010). In turn, it is possible that the ensuing systemic inflammation may adversely affect the number and function of endothelial progenitor cells (EPCs) resulting in a variety of cardiovascular disease states (Lamon, Hajjar 2008). The function and number of EPCs is relevant because it has been shown that EPCs are actively involved in endothelium repair of cardiovascular tissue and therefore play an important role in cardiovascular endothelium health and maintenance (Povsic, Goldschmidt-Clermont 2008, Zampetaki, Kirton & Xu 2008). Thus, we postulate that acute inhaled Ni nanoparticle exposures cause endothelial damage from which EPCs cannot adequately repair; likely by the release of functionally impaired EPCs from the bone marrow, by their reduction in numbers, or both.
Methods
Exposure
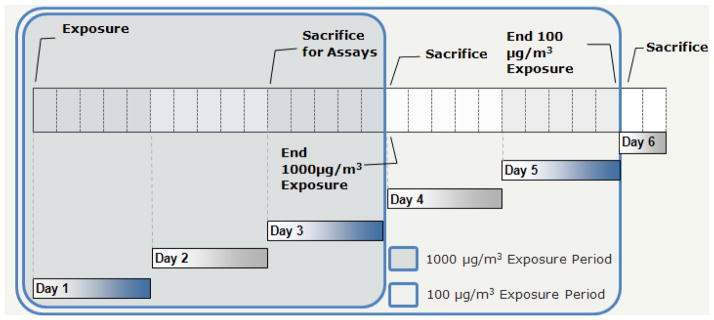
Three separate Ni nanoparticle exposures were performed for this preliminary study in order to determine the effects of the generated particles at several time points and concentrations (Figure 1). A 3 day, 5hr/day high Ni concentration exposure was performed in order to evaluate EPC numbers at a concentration of 725 μg/m3 while the Ni exposure to assess EPC function was performed at 1237 μg/m3 for 2 days/5 hrs/day (both nominally referred to as 1000 μg/m3 throughout text). In addition, a lower concentration, 86 μg/m3, Ni exposure was performed for 5 days/5hrs/day in order to assess EPC numbers exposed at a lower dose for a longer period of time (referred to as 100 μg/m3 throughout text). A full discussion of the particle generation and characterization for the Ni nanoparticles can be found in our previous study (Gillespie et al. 2010). Briefly, Ni nanoparticles were generated using a GFG-1000 Palas (Germany, Karlsruhe) spark generator and mice were exposed in a whole body chamber supplemented with 20% oxygen. Particle number concentration was determined with a TSI 3071 electrostatic classifier and size distribution was assessed with a 2010 condensation particle counter (St. Paul, MN) and via transmission electron microscopy (TEM) while particle mass was determined gravimetrically (Gillespie et al. 2010). All ethical guidelines, work, and procedures were approved and conducted under the NYU School of Medicine Institutional Animal Care and Use Committee. A total of 24 C57BL/6 mice (n=4/group/exposure) from Taconic Farms (Germantown, NY) were used and were 10–12 weeks old at time of first exposure. Animals were maintained on a 12 hr day and night cycle and provided food and water ad libitum except during exposure.
Figure 1.
Exposure time period diagram
Enumeration of EPCs
Flow Cytometry
One million bone marrow mononuclear cells were incubated with 0.25μg mouse Fc block (BD Pharmingen, California) for 5 minutes followed by incubation with FITC CD-34, PE VEGF-R2, and Alexa Fluour-647 CD-11b antibodies (BD Pharmingen, California) in the dark for 40 minutes on ice. Corresponding isotypes and single flurochorme antibodies were stained with 1M bone marrow cells as above. After staining, cells were washed with buffer and fixed with 0.25% paraformaldehyde before flurescence-activated cell sorting flow cytometry (FACS) analysis.
The EPCs, assessed as cells bearing CD34+/VEGF-R2+/CD11b− antigens were enumerated using a Becton Dickinson FACSCalibur system equipped with a 15 mW argon laser (488 nm) and a 17 mW HeNe laser (633 nm). The FACSCalibur was equipped with a forward and side scatter detector and 3 color channels excited by the 488 laser and one excited by the 633 laser. EPCs were quantified using FlowJo (v. 8, TreeStar, OR) by negatively gating on CD11b followed by positive gating for both CD34 and VEGF-R2.
Functional Assays
EPC Isolation
All mice were sacrificed the following morning after the last day of their exposure. The femora and tibiae were removed from the mice and the bone marrow was collected by repeated flushing with 1ml of PBS into each bone cavity. Marrow from four bones per mouse was collected into a single 15 mL polypropylene tube. The aspirate was placed on top of a ficoll layer (Histopaque 1083; Sigma Aldrich, MO) and spun at 300 g for 20 min at 4°C. Following density gradient separation, the bone marrow mononuclear cells fraction was removed and washed. The EPC rich mononuclear cell fraction was enumerated using flow cytometry or plated (1M cells/well) on human fibronectin (Sigma Aldrich, MO) coated 24-well plates in EGM-2MV media in a humidified 5% CO2 incubator at 37°C for 4 days to allow adherent cells to attach to the tissue culture surface. After the 4 day period, the culture plate was gently aspirated and the media was replaced every 3 days. An EPC phenotype was visually confirmed for the in vitro studies by use of fluorescence staining of the cells with DAPI, acLDL-DiI (Invitrogen, OR) and CD-31 (BD Pharmingen, California).
Chemotaxis
Cells were plated (2x104) in the upper of the two chambers separated by 8μm pores membrane (Corning Transwell, NY). VEGF (R&D Systems, MN) or media control was used in the lower chamber as a chemoattractant (chemotaxis) or to assess random cell movement (chemokenesis), respectively. After 24 hours, the top side of the chamber was wiped with a cotton swab to remove remaining cells and the number of migrated cells on the reverse side was counted in 6 random high powered fields (200x). Final results were expressed by subtracting the background media control values from the obtained values.
Tube Formation
The tubule assay was performed in phenol red free basement membrane matrix matrigel (BD Biosciences, California). EPCs were washed with PBS and then trypsinized following 10 days of incubation. Cells were seeded (2x104) per well in a 96 well plate and incubated for 24 hours. EPCs contributing to tubule formation were assessed by numeration of total tubule structures formed under 6 random high powered fields (200x). The numbers of EPCs contributing to the structure was not assessed due to the low number of tubes formed in the exposed group.
Plasma VEGFR-2 and SDF-1α
Approximately 1mL of whole blood was collected in a 2ml centrifuge tube containing EDTA by right ventricle puncture. Following centrifugation at 1200 g for 15 minutes, the plasma supernatant was drawn off and stored at −80°C until assayed. Levels of VEGFR-2 and SDF-1α, important proteins involved in releasing EPCs from the bone marrow, homing to sites of endothelial injury, and inducing differentiation, were assessed using ultra-sensitive ELISA kits in duplicate (R&D Systems, MN, and SABiosciences, MD, respectively).
Statistical Analysis
A two-tailed Student’s t-test was used to compare exposure groups and control groups using GraphPad Prism (v.4). Probability values (p) less than 0.05 were considered significant.
Results
Ni nanoparticles were previously characterized and reported by Gillespie et al. (2010). Briefly, TEM analysis and electrostatic classification revealed that the primary nanoparticles generated were approximately 5nm and that the count mean diameter (CMD) agglomerate which entered the chamber was 40nm. X-ray photoelectron spectroscopy (XPS) previously identified the spark generated Ni nanoparticles to be nickel (II) hydroxide (Ni(OH)2) in its core and on its surface.
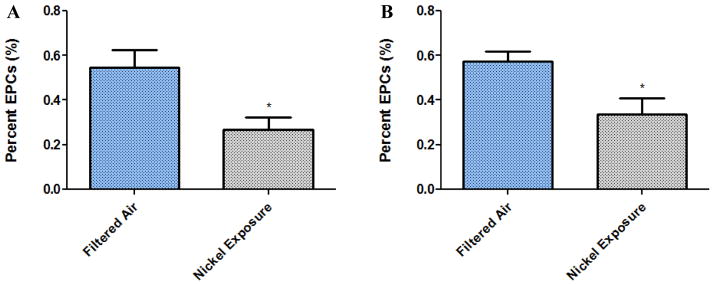
In this study, significant differences in bone marrow EPC counts (Figure 2), both moderate 3-day Ni exposure (p = 0.027) and low 5 day Ni concentration exposure (p = 0.031), were observed. Plasma levels of SDF-1α and VEGF (Table 1) from the Ni exposed mice were not significantly different compared to controls. In addition to the reduced bone marrow EPC counts, significantly functional differences in the cultured EPCs from the Ni nanoparticle exposed mice were also observed. Both tube formation and chemotaxis, important functions of EPCs with regards to endothelial cell repair, were impaired in the 2-day 1200 μg/m3 Ni nanoparticle exposure (Table 1). Figure 3 represents a typical tube formed.
Figure 2.
Percent EPCs (n=4). Quantified as CD-34+/VEGF-R2+/CD11b−. A) 3 Day High Nickel Exposure (1000μg/m3; p= 0.0273). B) 5 Day Low Nickel Exposure (100μg/m3; p= 0.0313).
Table 1.
Functional Assessment and Plasma Protein Results
| Experiment | Filtered Air (±SD) | Ni Exposed (±SD) | p Value |
|---|---|---|---|
| Plasma VEGF (pg/ml) | 26.1 ± 10.17 | 27.85 ± 5.5 | 0.886 |
| Plasma SDF-1α (pg/ml) | 918.1 ± 250.8 | 1158.0 ± 355.8 | 0.580 |
| Tube Formation (200X) | 1.89 ± 0.55 | 0.13 ± 0.08 | 0.016 |
| Chemotaxis (200X) | 4.78 ± 0.15 | 0.86 ± 0.24 | 0.0002 |
SDF, stromal-derived factor; VEGF, vascular endothelial growth factor.
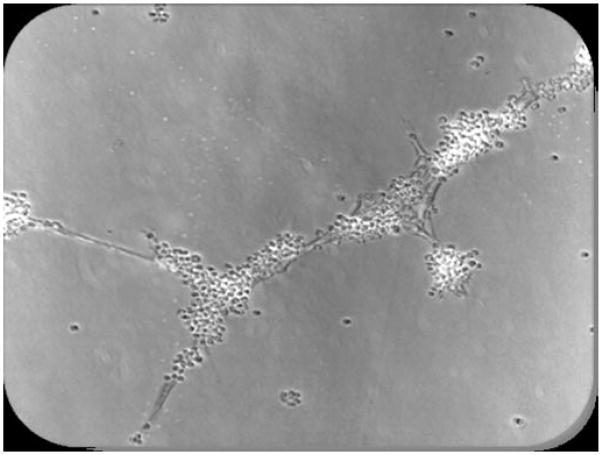
Figure 3.
Photographmicrograph of a typical tube formed in matrigel after 24 h.
Discussion
In human populations, decreased circulating EPC counts are associated with increased cardiovascular risk and a variety of cardiovascular disease states (Werner, Nickenig 2007, Werner et al. 2005, Hill et al. 2003, Chironi et al. 2007). Since EPCs are involved in the endothelium repair process they can be used as a marker of vascular reparative capacity (Povsic, Goldschmidt-Clermont 2008), and hence, if low EPCs are present, reparative capacity is diminished. The finding that both 5 day low Ni exposure and 3 day high Ni exposure caused a reduction in bone marrow EPCs and the 2 day high Ni exposure caused EPC functional impairments is particularly striking and suggestive of reduced reparative capacity after inhalation of the Ni nanoparticles. While the reversibility of these effects were not probed, one study found that some phenotypes of circulating EPCs slowly return to near-baseline levels 24h post-second hand smoking exposure (Heiss et al., 2008). Another study found that circulating EPCs from humans exposed to episodic increases in PM and mice exposed to CAPs both negatively correlated with PM exposure and that incurred vascular injury was reversible (O’Toole et al., 2010). However, to date, no study has assessed the effects of inhaled contaminants on bone marrow resident EPCs.
One hypothesis for this finding is that EPCs are being recruited out of the bone marrow to sites of injury. While this may be a normal response to injury, in a chronic exposure scenario, such as that which occurs in PM pollution, the resident EPCs may not be able to replenish themselves and therefore cannot adequately repair damaged endothelium. This may also explain the functional impairments observed in the cultured EPCs; it is possible that as EPCs are recruited from the bone marrow the remaining cells divide to attempt replenish their numbers. Hence more naïve cells, perhaps lacking mature and functionally superior phenotype, remain in the bone marrow; however, this would likely result in an increased or baseline EPC count, which was not observed. An alternative explanation is that the localized pulmonary inflammation or soluble Ni components adversely affect the EPCs causing cell death or apoptosis thus reducing both number and function of EPCs; however both possibilities are unconfirmed at this time.
Non-significant elevation in plasma VEGF and SDF-1α were observed and may be explained by the recovery period following exposure. As per our experimental protocol, mice were sacrificed the morning after the last day of exposure and thus plasma proteins involved in EPC mobilization, homing, and differentiation may have returned to baseline. Future studies should expand the exposure to longer period to simulate human exposure scenario, assess circulating EPCs in addition to bone marrow EPCs, attempt to quantify plasma proteins involved in EPC mobilization, homing, and differentiation, and lastly quantify atherosclerotic plaque sizes in mice.
Conclusions
Due to the growing use of nanoparticles in commercial goods and the lack of toxicological profiles for these compounds and to the ubiquity of human PM exposure, there is a pressing need to determine adverse health effect associated with their exposure. Research into understanding how and why Ni nanoparticles and metal containing PM cause adverse effects and its resultant cardiovascular outcomes is needed in order to protect the large number of people exposed to these compounds; especially the increasing worker population who manufacture nanoparticles. Since EPCs play a role in endothelial cell maintenance, their ability to recognize signals to home to sites of injury and form tubule networks is very important. Thus, both non-functioning EPCs and reduced count may be a contributing factor to the cardiovascular outcomes observed in both human populations and animals exposed to PM and nanoparticles (Sun et al. 2005, Lippmann et al. 2006, Gillespie et al. 2010, Kang GS, Gillespie P, Chen LC. 2010, Chen et al. 2010, Gunnison, Chen 2005, Lippmann et al. 2005). This study is the first of its kind to demonstrate an adverse effect to EPC number and function associated with nanoparticle exposure. Furthermore, the results of this study can help explain the observed long-term effects associated with metal containing PM exposure in both human and animal studies. In conclusion, we have shown that 3 days of high Ni exposure and 5 days of low Ni exposure results in a reduction of bone marrow resident EPCs while a 2-day high Ni exposure results in functional impairments in cultured EPCs.
Footnotes
Declaration of interest
Work supported by NIEHS R21ES016570, R01ES015495, and P30 ES00260. Personnel support by CIHR-DRA to EN Liberda.
References
- Chen LC, Hwang JS, Lall R, Thurston G, Lippmann M. Alteration of cardiac function in ApoE(−/−) mice by subchronic urban and regional inhalation exposure to concentrated ambient PM(2.5) Inhalation toxicology. 2010 doi: 10.3109/08958371003596579. [DOI] [PubMed] [Google Scholar]
- Chen LC, Nadziejko C. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. V. CAPs exacerbate aortic plaque development in hyperlipidemic mice. Inhalation toxicology. 2005;17(4–5):217–224. doi: 10.1080/08958370590912815. [DOI] [PubMed] [Google Scholar]
- Chironi G, Walch L, Pernollet MG, Gariepy J, Levenson J, Rendu F, Simon A. Decreased number of circulating CD34+KDR+ cells in asymptomatic subjects with preclinical atherosclerosis. Atherosclerosis. 2007;191(1):115–120. doi: 10.1016/j.atherosclerosis.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Gillespie PA, Kang GS, Elder A, Gelein R, Chen L, Moreira AL, Koberstein J, Tchou-Wong K, Gordon T, Chen LC. Pulmonary response after exposure to inhaled nickel hydroxide nanoparticles: Short and long-term studies in mice. Nanotoxicology. 2010;4(1):106–119. doi: 10.3109/17435390903470101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnison A, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. VI. Gene expression in heart and lung tissue. Inhalation toxicology. 2005;17(4–5):225–233. doi: 10.1080/08958370590912851. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. The New England journal of medicine. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Kang GS, Gillespie P, Chen LC. Long-term effects of inhaled nickel hydroxide nanoparticles on the progression of atherosclerosis. 2010 Submitted. [Google Scholar]
- Kennedy IM, Wilson D, Barakat AI HEI Health Review Committee. Uptake and inflammatory effects of nanoparticles in a human vascular endothelial cell line. Research report (Health Effects Institute) 2009;136:3–32. [PubMed] [Google Scholar]
- Lamon BD, Hajjar DP. Inflammation at the molecular interface of atherogenesis: an anthropological journey. The American journal of pathology. 2008;173(5):1253–1264. doi: 10.2353/ajpath.2008.080442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M, Hwang JS, Maciejczyk P, Chen LC. PM source apportionment for short-term cardiac function changes in ApoE−/− mice. Environmental health perspectives. 2005;113(11):1575–1579. doi: 10.1289/ehp.8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M, Ito K, Hwang JS, Maciejczyk P, Chen LC. Cardiovascular effects of nickel in ambient air. Environmental health perspectives. 2006;114(11):1662–1669. doi: 10.1289/ehp.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdorster G, Philbert MA, Ryan J, Seaton A, Stone V, Tinkle SS, Tran L, Walker NJ, Warheit DB. Safe handling of nanotechnology. Nature. 2006;444(7117):267–269. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- Povsic TJ, Goldschmidt-Clermont PJ. Endothelial progenitor cells: markers of vascular reparative capacity. Therapeutic advances in cardiovascular disease. 2008;2(3):199–213. doi: 10.1177/1753944708093412. [DOI] [PubMed] [Google Scholar]
- Simeonova PP, Erdely A. Engineered nanoparticle respiratory exposure and potential risks for cardiovascular toxicity: Predictive tests and biomarkers. Inhalation toxicology. 2009;21(S1):68–73. doi: 10.1080/08958370902942566. [DOI] [PubMed] [Google Scholar]
- Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA : the journal of the American Medical Association. 2005;294(23):3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. The New England journal of medicine. 2005;353(10):999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- Werner N, Nickenig G. Endothelial progenitor cells in health and atherosclerotic disease. Annals of Medicine. 2007;39(2):82–90. doi: 10.1080/07853890601073429. [DOI] [PubMed] [Google Scholar]
- Xia T, Li N, Nel AE. Potential health impact of nanoparticles. Annual Review of Public Health. 2009;30:137–150. doi: 10.1146/annurev.publhealth.031308.100155. [DOI] [PubMed] [Google Scholar]
- Zampetaki A, Kirton JP, Xu Q. Vascular repair by endothelial progenitor cells. Cardiovascular research. 2008;78(3):413–421. doi: 10.1093/cvr/cvn081. [DOI] [PubMed] [Google Scholar]