Dear editor
We read with interest the letter by Yang et al. reporting a human case of influenza A(H7N9) infection during summer in the first epidemic wave in China, 2013.1 Since then, human H7N9 infections were sporadically identified during summer.2 Winter 2014/15 saw a third epidemic wave of human infections with A(H7N9) avian influenza viruses (AIVs) in China, following Spring 2013 and Winter-spring 2013/2014. As visiting LPMs which were contaminated by A(H7N9) was identified as a major risk factor for human infections,3 understanding AIV activity in poultry will further describe human infection risk at different times. While AIV subtype such as H5, exhibited clear seasonality in China,4 the seasonality of A(H7N9) virus activity in poultry has not been previously characterized.
Retail and wholesale markets are two major types of LPMs in China. The retail LPMs typically process significantly less numbers of poultry when compared to the wholesale markets, favor mixing of different poultry species and may support extended holding time for unsold poultry. It is possible that the different settings at retail or wholesale LPMs may lead to different AIV dynamics and risks for human A(H7N9) infections. To monitor AIV activity, environmental surveillance at the LPMs has been implemented by the Guangdong Provincial Center for Disease Control and Prevention since 2005. In response to the A(H7N9) epidemic in humans, enhanced environmental surveillance at multiple human-poultry interfaces including retail and wholesale LPMs and poultry farms has been progressively implemented since April 2013. We characterized the seasonality of circulating AIV subtypes H7 and H9 and investigated the link between AIV activity at the human-poultry interface with the incidence of human A(H7N9) infections in Guangdong province from 2013–2015.
From January 2011 to April 2015, 26,935 environmental swab samples were collected monthly from 345 retail LPMs, 24 wholesale LPMs, and 15 poultry farms distributed in 21 cities in Guangdong province as described.5 The median monthly number of samples collected were 99 (range: 25–2,932), 40 (range: 9–403) and 42 (range: 8–141) for retail, wholesale LPMs and farms respectively. Samples collected for the purpose of investigation of human H7N9 cases were excluded in this study. During 2011–2014 with complete annual data, the highest influenza A positive rates were detected at the retail LPMs, ranging from 29.4 – 41.9%, followed by 0.0 – 24.9% at the wholesale LPMs, and 0.3 – 4.4% at the poultry farms (Table 1). All specimens positive for influenza A virus M gene were further subtyped (H9 or H7) using gene specific primers and probes designed by China CDC.6 Overall, we observed that the detection rate for influenza A virus of H9 subtype at the retail LPMs were comparable from 2011 to 2014, ranging from 13.3–20.1% (Table 1). Consistent with the surveillance data reported previously, no influenza A virus of H7 subtype was detected in the LPMs in Guangdong prior to April 2013,7 with increased detection rates noted in winter 2013–2014 and 2014–2015 (Figure 1A).
Table 1.
Detection of influenza A viruses at the poultry-human interface, January 2011 – April 2015
| Retail LPMsa | Wholesale LPMsb | Poultry farmsc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Flu A | H9 | H7 | No. swab |
Flu A | H9 | H7 | No. swab |
Flu A | H9 | H7 | No. swab |
|
| Jan–Dec, 2011 | 41.9% | 13.3% | ND | 444 | 0.0% | 0.0% | ND | 80 | 4.4% | 4.4% | ND | 138 |
| Jan–Dec, 2012 | 29.4% | 16.0% | ND | 930 | 2.0% | 0.0% | ND | 148 | 0.9% | 0.5% | ND | 217 |
| Jan–Dec, 2013 | 38.3% | 20.1% | 3.4% | 2,008 | 5.7% | 2.1% | 0.0% | 385 | 0.3% | 0.3% | 0.0% | 396 |
| Jan–Dec, 2014 | 32.7% | 16.2% | 7.8% | 8,631 | 24.9% | 15.4% | 3.2% | 1,661 | 0.7% | 0.5% | 0.0% | 598 |
| Jan–Apr, 2015d | 22.0% | 11.9% | 5.4% | 9,880 | 31.5% | 20.7% | 6.2% | 1,050 | 0.3% | 0.3% | 0.0% | 369 |
LPM: live poultry market; ND, not determined.
Swabs were collected monthly at the retail LPMs from January 2011 – April 2015
Swabs were collected once every three months in 2011, once every other month in 2012, and monthly from January 2013 – April 2015 at the wholesale LPMs
Swabs were collected once every three months or once every other month from January 2011 to April 2013, and monthly from April 2013 – April 2015
Detection rates of influenza A and H7 in retail and wholesale LPMs are expected to be higher in this period, comparing to other time of the year
Figure 1.
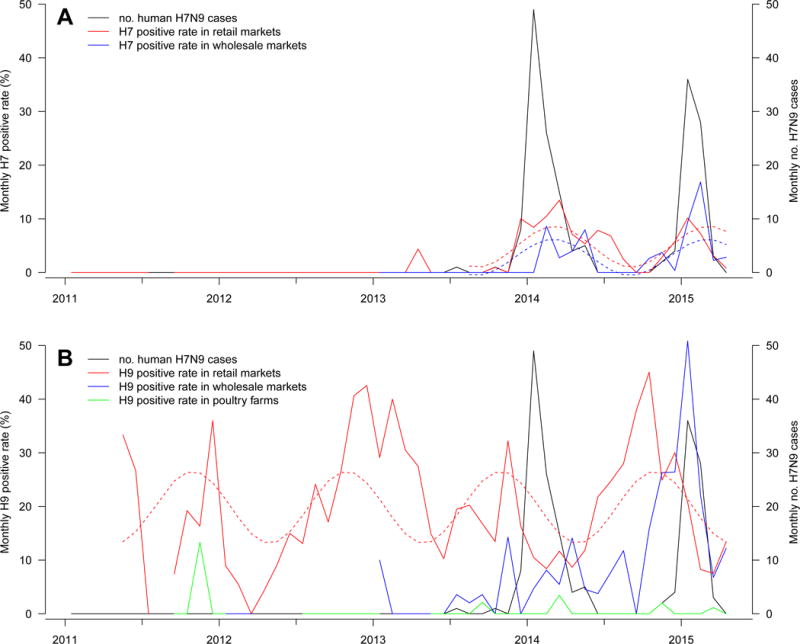
Monthly positive rates at the retail, wholesale LPMs and poultry farms for (a) subtype H7 and (b) subtype H9, and monthly number of human H7N9 infections in Guangdong, January 2011 – April 2015.
Dotted lines show the fitted trends using sinusoidal function. Influenza A H7 subtype was not detected at poultry farms.
We explored the seasonality of influenza A virus subtypes H7 and H9 using the positive rates detected at the retail and wholesale LPMs from August 2013 – April 2015, by fitting linear regression models with trigonometric terms. Data from April-July in 2013 were excluded as the presence of H7 during this period most likely reflected the introduction of H7 into the poultry in Guangdong, rather than seasonality. Based on two years of data, we estimated that H7 positive rates peaked in March, with an indication that H7 activities are in phase in retail LPMs (estimated peak at 3.1 month) and wholesale LPMs (estimated peak at 3.0 month). There was no obvious seasonality for H9 virus at the wholesale LPMs, but at retail LPMs there was peak activities around November (estimated peak at 10.9 month). Overall, the results support seasonality for the H7N9 virus in poultry markets, which peaked around March in Guangdong since the virus has been established locally in 2013.
To assess the impact of the presence of H7N9 at the poultry-human interface on human infection, we fitted negative binomial regression models to link the monthly number of H7N9 human cases to H7 positive rates at the retail and wholesale LPMs. We restricted the analysis to April 2013 to April 2015 when H7 was detected. H7 was not detected at the poultry farms in the whole study period. Human H7N9 infections were significantly associated with H7 detection rates at the retail LPMs (relative risk (RR)=1.60, 95% CI, 1.38–1.86) but not associated with that at the wholesale LPMs (RR= 1.16, 95% CI, 0.96–1.41) (Figure 1A). A sensitivity analysis excluding 6 H7N9 cases with occupational exposure obtained similar results. Similar analysis on monthly H9 positive rates found negative association in retail LPMs (RR=0.88, 95% CI, 0.80–0.96) and no significant association (RR=1.04, 95% CI, 0.97–1.12) with wholesale LPMs (Figure 1B). Of note, there was a moderate negative association (spearman correlation ρ = −0.50) between H7 and H9 detection rates in retail LPMs, but a moderate positive association (ρ = 0.64) in wholesale LPMs. Overall, the data suggests that the retail but not the wholesale LPMs are strongly associated with human H7N9 infection risk.
We found that A(H7N9) virus activity peaked around March in both retail and wholesale LPMs, which is considerably later than the usual peak of A(H5N1) virus in December – January in China.4 While poultry exposure confers significant risk for human infections of avian influenza, contact with own backyard poultry is associated with increased risk for infection of A(H5N1) but not for A(H7N9).3 Our results highlight the A(H7N9) activity over winter extending to spring, and the noticeable differential human infection risk to the H7N9 detection rate in retail and wholesale LPMs. The peak timing of A(H7N9) activity was different from other AIVs such as A(H5N1)4, 8 and human seasonal influenza.9 Hence, vigilance in control measures and surveillance in LPMs should be maintained over spring. Given the synchrony of H7 detection in retail and wholesale LPMs but low detection in farms, concerted control measures should be implemented in both LPM settings. Long-term control strategy should also identify the key sites for viral amplification.
Acknowledgments
We thank Qiqi Zhang for technical assistance. This study was supported by grants from the Science and Technology Planning Project of Guangzhou City, China (2014J4100091 and 2013J4200020), Science and Technology Planning Project of Guangdong Province (2013B020307006), JW was supported by Medical Scientific Research Foundation of Guangdong Province, China (A2012078). CWK was supported by 12th five-year-major-projects of China’s Ministry of Public Health (2012zx10004-213). BJC and EHYL were supported by the Harvard Center for Communicable Disease Dynamics from the National Institute of General Medical Sciences (U54 GM088558). EHYL, BJC, and HLY were supported by commissioned grants from the Health and Medical Research Fund of the Health, Welfare and Food Bureau of the Hong Kong SAR Government, and the Area of Excellence Scheme of the Hong Kong University Grants Committee (AoE/M-12/06).
Footnotes
Conflict of interest
BJC has received research funding from MedImmune Inc and Sanofi Pasteur and consults for Crucell NV.
Authors’ contribution
Jie Wu, Jinyan Lin and Changwen Ke designed the study. Lirong Zou, Hongbin Zhang, Yinchao Song and Guofeng Huang collected and collated the data. Eric HY Lau and Hongbin Zhang analyzed the data. Jie Wu, Eric HY Lau, Hui-Ling Yen, Qinbin Xing and Haojie Zhong interpreted the data. Jie Wu, Eric HY Lau, Min Kang and Hui-Ling Yen drafted the manuscript. Benjamin J Cowling and Qinbin Xing edited the manuscript. All authors reviewed and revised the manuscript.
References
- 1.Yang P, Wang Q, Pang X, Chen L, Tian L, Deng Y. A case of avian influenza A (H7N9) virus occurring in the summer season, China. The Journal of infection. 2013;67:624–5. doi: 10.1016/j.jinf.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO Risk Assessment of Human infections with avian influenza A(H7N9) virus. Available at: http://www.who.int/influenza/human_animal_interface/influenza_h7n9/RiskAssessment_H7N9_23Feb20115.pdf?ua=1. Last accessed Aug 20, 2015.
- 3.Liu B, Havers F, Chen E, et al. Risk factors for influenza A(H7N9) disease–China, 2013. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;59:787–94. doi: 10.1093/cid/ciu423. [DOI] [PubMed] [Google Scholar]
- 4.Park AW, Glass K. Dynamic patterns of avian and human influenza in east and southeast Asia. The Lancet Infectious diseases. 2007;7:543–8. doi: 10.1016/S1473-3099(07)70186-X. [DOI] [PubMed] [Google Scholar]
- 5.Kang M, He JF, Song T et al. Environmental Sampling for Avian Influenza A(H7N9) in Live-Poultry Markets in Guangdong, China. PloS one. 2015;10 doi: 10.1371/journal.pone.0126335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Real-time RT-PCR Protocol for the Detection of Avian Influenza A(H7N9) Virus. Available at: http://www.who.int/influenza/gisrs_laboratory/cnic_realtime_rt_pcr_protocol_a_h7n9.pdf. Last accessed Jyly 9, 2015.
- 7.Ke C, Lu J, Wu J, et al. Circulation of Reassortant Influenza A(H7N9) Viruses in Poultry and Humans, Guangdong Province, China, 2013. Emerging infectious diseases. 2014;20:2034–40. doi: 10.3201/eid2012.140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepin KM, Wang J, Webb CT, et al. Multiannual patterns of influenza A transmission in Chinese live bird market systems. Influenza Other Resp. 2013;7:97–107. doi: 10.1111/j.1750-2659.2012.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu HJ, Alonso WJ, Feng LZ, et al. Characterization of Regional Influenza Seasonality Patterns in China and Implications for Vaccination Strategies: Spatio-Temporal Modeling of Surveillance Data. Plos Med. 2013;10 doi: 10.1371/journal.pmed.1001552. [DOI] [PMC free article] [PubMed] [Google Scholar]


