Abstract
The TLC-based Generally Useful Estimate of Solvent Systems (GUESS) method was employed for countercurrent chromatography solvent system selection, in order to separate the three synthetic isomers: 3-O-methylpyridoxine, 4′-O-methylpyridoxine (ginkgotoxin) and 5′-O-methylpyridoxine. The Rf values of the three isomers indicated that ChMWat +2 (chloroformmethanol-water 10:5:5, v/v/v) was appropriate for the countercurrent chromatography. The isomer separation was highly selective and demonstrated that the TLC-based GUESS method can accelerate solvent system selection for countercurrent chromatography. Accordingly, the study re-emphasizes the practicality of TLC as a tool to facilitate the rapid development of new countercurrent and centrifugal partition chromatography methods for this solvent system. Purity and structure characterization of all samples was performed by quantitative 1H NMR.
Keywords: Thin layer chromatography, Countercurrent separation, Generally useful estimate of solvent systems, Quantitative 1H NMR, Ginkgotoxin isomers, Ginkgo biloba
1. Introduction
Countercurrent and centrifugal partition chromatography (CCC and CPC respectively) and related technologies are liquid only countercurrent separation methods, which minimize compound losses created by adsorption or degradation common in standard column chromatography and, therefore, sample recovery approaches 100% [1]. CCC, CPC and related technologies have been used for the isolation of natural products [2], separation of isomers [3], and purification of organic synthetic products [4]. An appropriate solvent system is the key for high resolution in CCC and CPC separations [5]. The solvent system selection procedure is, however, multistep and time-consuming [6]. For simplification of the solvent system selection, a TLC-based Generally Useful Estimate of Solvent Systems (GUESS) method was proposed for a chloroform-methanol-water solvent system [7].
TLC is typically performed on the collected fractions in order to confirm their chemical composition. TLC analysis of countercurrent separation fractions shows that the distribution of analytes is strongly polarity-related [8]. This inspired the development of the TLC-based GUESS method [7], which established the correspondence between the partition coefficient (K value) of a compound from a partitioning experiment and its TLC retention factor (Rf value). Interestingly, even though the original GUESS publication from 2005 has been cited over 100 times, one of its key aspects, i.e., the simplification of solvent system selection by rapid and easily performed TLC, has rarely been reported. Thus, the present study was performed as an extension of the original GUESS method, and demonstrates that TLC-based solvent system selection may be applied in a single step to different set of analytes than what was proposed in the original article. The present study was also motivated by a recent study on 4′-O-methylpyridoxine or ginkgotoxin (2), which established a CCC- and qHNMR-based threshold assay for this botanical negative marker [9]. The synthesis and purification of the ginkgotoxin regioisomers, 3-O-methylpyridoxine (1) and 5-O-methylpyridoxine (3), enables their targeted CCC-based detection in complex (natural) mixtures with the aim of providing a better understanding of these alleged anti-vitamins [10].
In order to confirm the suitability and advantages of the TLC-based GUESS method for a new and challenging separation problem, the three regioisomers in Fig. 1 were chosen as target analytes. With closely related polarity characteristics as probes, the outcome demonstrates how GUESS guidance accelerates the optimization of CCC conditions and simplifies the workflow to accommodate diverse structural classes.
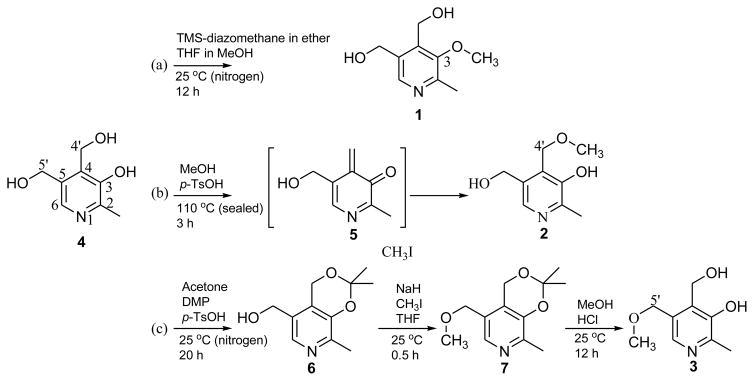
Fig. 1.
Synthesis scheme of three isomers; 4 is pyridoxine (hydrochloride). The final products of the reactions are 1(a), 2(b), and 3(c).
2. Experimental
2.1. Materials
All reagents and CDCl3 (99.8 atom % D) and DMSO-d6 (99.9 atom % D) were obtained from Sigma-Aldrich Inc. (St. Louis, MO, USA). All analytical grade solvents were purchased from Pharmco-AAPER (Crookfield, CT, USA) and redistilled before use.
2.2. Synthesis of three isomers
3-O-methylpyridoxine (1) [11], 4′-O-methylpyridoxine (2) [9], and 5′-O-methylpyridoxine (3) [12,13] were synthesized as shown in Fig. 1. The products from the synthetic preparations were purified separately by silica gel column chromatography or by precipitation as indicated by the literature methods. Characterization of the three isomers was performed by High Resolution-Electro Spray Ionization Mass Spectrometry (HR-ESI-MS; calibrant: reserpine) on a Waters Synapt Mass Spectrometer (Waters, Milford, MA, USA) and NMR on a DPX-400 spectrometer (Bruker, Billerica, MA, USA). HR-ESI-MS data and NMR spectra and may be found in the Supplementary Information S1 – S15.
2.3. Countercurrent chromatography (CCC) procedure
The TLC-based GUESS method was performed on silica gel TLC plates (Macherey-Nagel, Bethlehem, PA, USA) with three solvent systems based on chloroform-methanol-water [7] (Table 1). The CCC separation was performed as described in the Supplementary Information S16. Throughout the separation, the stationary phase volume retention ratio (Sf value), the partition coefficient (K value) and the eluting phase (phase metering apparatus, PMA value) were monitored by the CherryOne operating system [14]. Each synthetic product was purified by CCC (ChMWat +2, 10:5:5, v/v/v). The resulting fractions were analyzed by both TLC and NMR. The purity of each synthetic product was determined by quantitative 1H NMR (qHNMR) as described in the Supplementary Information S2–S14. For the CCC resolution study, a sample was prepared by combining 1 (0.8 mg), 2 (2 mg), and 3 (0.5 mg).
Table 1.
Corresponding pairs of selected chloroform-methanol-water (ChMWat; with polarity indices) countercurrent chromatography (CCC) solvent systems and chloroform-methanol-water TLC solvent systems (SSCs) [21,33], along with TLC Rf values.
| CCC conditions | TLC conditions | TLC Rf values | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| ChMWat | CHCl3:CH3OH:H2O | SSC | CHCl3:CH3OH:H2O | 1 | 2 | 3 |
| +1 | 100:40:60 | 5 | 90:10:0.5 | 0.16 | 0.16 | 0.20 |
| +2 | 100:50:50 | 6 | 85:15:0.5 | 0.48 | 0.48 | 0.54 |
| +3 | 100:60:40 | 7 | 80:19:1.0 | 0.63 | 0.65 | 0.69 |
2.4. Quantitative 1H NMR analysis (qHNMR)
The sample preparation, qHNMR acquisition and information processing were performed as described in the Supplementary Information S1. All data were obtained based on NMR peak area (integration) and purity was calculated based on the 100% method [15,16]
3. Result and Discussion
3.1. Synthesis and characterization of three ginkgotoxin positional isomers
The synthesis of 3-O-methylpyridoxine (1), 4′-O-methylpyridoxine or ginkgotoxin (2), and 5′-O-methylpyridoxine (3) was undertaken as the compounds were not commercially available. The starting material was pyridoxine (4), which was methylated to 1(a), 2(b), and 3(c) (Fig. 1). Characterization and quantification of 1, 2, and 3 was achieved via qHNMR throughout this study, as UV absorption and MS based spectrometry have intrinsic limitations for isomer identification [17]. Representative 1H NMR spectra of the three synthetic products are shown in Fig. 2. The structural elucidation, which included obtaining the 1D 1H NMR spectra in both CDCl3-d and DMSO-d6 as well as 2D NMR methods is described in the Supplementary Information S1. The qHNMR analysis revealed that CCC achieved superior purification compared to silica gel column chromatography. In particular, CCC achieved the purification of 1, 2, and 3 at 92%, 98%, and 97% purity, respectively. The complete qHNMR purity assignment data sets are found in the Supplementary Information S2–S14. Altogether, these results demonstrate that CCC compares very favorably to classical purification methods.
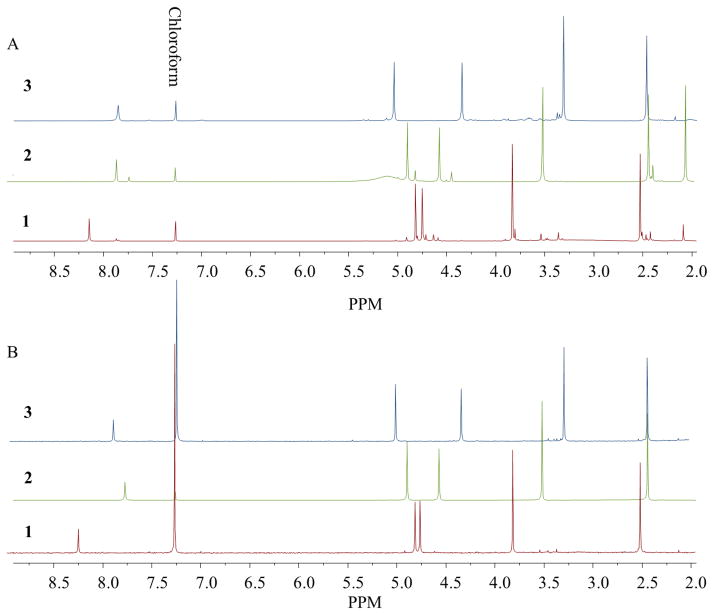
Fig. 2.
Comparative qHNMR purity analysis of materials of the three isomers obtained by different purification methods. A. Silica gel column chromatography (1 and 2) and no further treatment beyond precipitation (3) led to purity values of 84%, 81%, and 96% for 1, 2, and 3 respectively. B. After countercurrent chromatography, the purities increased to 92%, 98%, and 97% for 1, 2, and 3 respectively.
3.2. Solvent system selection procedure
The first priority of any CCC and CPC procedure is proper solvent system selection. Based on initial experiments with standard CCS solvent system families (HEMWat, HterAcWat, EBuWat, ChMWat), solvent systems based on chloroform-methanol-water (ChMWat) were explored further as they matched best the polarities of the three isomers. The Rf values obtained from the chloroform-methanol-water based TLC solvent systems 5, 6, and 7 from the GUESS method [7] are listed in Table 1. An Rf value of 0.5 is considered optimal and presumptively corresponds to a K value of unity [7] The TLC system CHCl3:CH3OH:H2O 6 (85:15:0.5, v/v/v) yielded Rf values in the vicinity of 0.5 for all three isomers. According to the GUESS method, this TLC system corresponds to ChMWat +2 (10:5:5, v/v/v, Table 1) as its matching CCC system, whose organic phase has a similar composition to that of the TLC solvent system. Therefore, this solvent system was prioritized for the CCC experiment.
Table 1 shows that the solvent systems adjacent to ChMWat +2 were predicted to move the analytes outside of the interval of optimal separation and/or result in insufficient analyte resolution. In ChMWat +1, the Rf values showed a significant attraction to the stationary phase over the mobile phase and were too close (0.16 to 0.20) to predict sufficient analyte resolution. The Rf values of the three analytes in ChMWat +3 all different, which indicated that the selectivity of the corresponding ChMWat solvent system could potentially be suitable to resolve these analytes. However, the Rf values indicated that the analytes show a preference for the mobile phase rather than the stationary phase. Therefore, the decision to test ChMWat +2 followed established GUESS rationales, according to which priority should be given to a solvent system that offers the best polarity match and may be associated with optimum resolution under CCC conditions.
3.3. Countercurrent chromatography separation
Once the solvent system was selected, the three isomers were successfully separated by CCC and their relative abundances determined by qHNMR (Fig. 3). None of the fractions contained more than a single isomer according to the limits of NMR detection. Even though 1 and 2 had very close Rf values, ChMWat +2 (10:5:5, v/v/v) achieved the desired resolution without any extra adjustment. The elution profile showed that the K values are 2.1, 1.5, and 1.0 for 1, 2, and 3, respectively. Because it is the normal phase mode, the elution order is the same as their Rf distribution on silica gel TLC. In this case, determination of K values by partitioning experiments was avoided and the GUESS method showed powerful potential for the TLC prediction of solvent system selection.
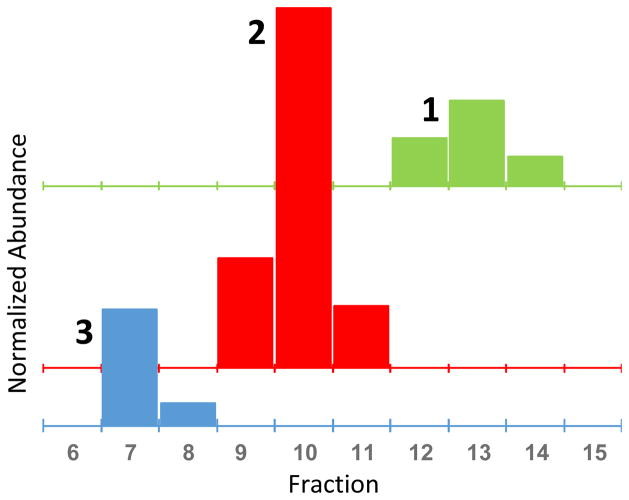
Fig. 3.
Off-line qHNMR analysis of the discrete countercurrent chromatography fractions (x-axis) for the isolation of the three isomers, shown as separate bar graphs for each isolate 1–3. The scale of the Y-axis is arbitrary and normalized to the residual solvent signal of CHCl3. The K values of the analytes 1, 2, and 3 were 2.1, 1.5, and 1.0 respectively, eluted under normal phase conditions. While the discrete sample rate of the qHNMR data points does not allow reconstruction of the Gaussian peak shapes, the lack of detectable amounts of 1 in fractions of 2, of 2 in fractions of 3, and vice versa, demonstrates the baseline separation of all analytes.
Real time parameters (Sf, K, and phase metering apparatus values), determined by the CherryOne operating system, indicated that the selected solvent system was appropriate [14]. The phase metering apparatus (PMA) employed electrical permittivity to monitor, in real-time, which of the two phases eluted from the CCC column during the entire run. The dielectric property of a material to transmit an applied electric field is expressed as electrical permittivity. Real time Sf (Fig. 4) showed that elution from the pre-equilibrated column occurred without stationary phase loss until 96 min, when the extrusion stage began. The dip occurring in the real time PMA curve (Fig. 4) from 115 min until the end of the run indicated the elution of ionic constituents at the very end of the extrusion stage. The real-time K values (Fig. 4) were also monitored by the CherryOne system.
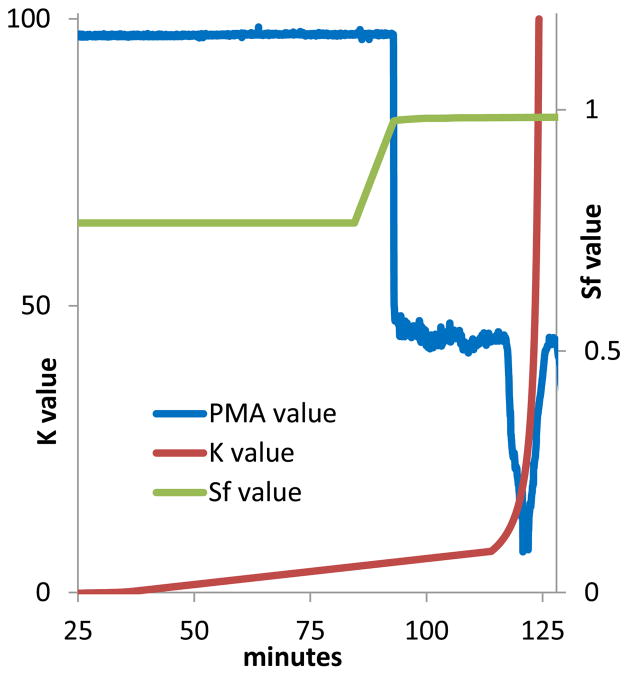
Fig. 4.
An example of CherryOne system operating output. Real time phase monitoring of Sf shows that 0 to 85 min is an equilibrium state with an Sf value of 0.77. At 84 min the extrusion process was initiated by pumping the upper stationary phase that displaced the lower mobile phase in the column without stopping the rotation. Real time measurement of K monitors the gradual increase in partition coefficient values as elution proceeds as well as the precipitous rise in K values during extrusion. Phase metering apparatus (PMA) values show that the original stationary phase is extruded beginning at 93 min. The signal reduction occurring in the phase metering apparatus curve from 115 until 127 min is caused by ionic constituents being extruded in the aqueous phase.
4. Conclusion
This study demonstrates that the TLC-based GUESS method is practical and has potential to reduce the solvent system selection time required to purify target analytes by CCC. One advantage of the GUESS approach is the simplification of the method development for determining target compound K values which would require a large number of partitioning experiments and the follow-up detections. The purity advantage of CCC was demonstrated by use of qHNMR. The use of off-line qHNMR analysis in the present study also demonstrated that qHNMR is well-suited for a more routine implementation as purity assay for a variety of analytes [18].
While the use of chloroform as a co-solvent in CCC separations is discouraged due to its cost and toxicity, the current study with a chloroform-containing solvent illustrates the concept of the TLC-based GUESS method for reducing solvent selection time. Potentially this concept can be extended to other solvent systems in the future such as those in the popular category of ethyl acetate-n-butanol-water. Therefore, the organic phase of the equilibrated solvent system (or a close approximation of the organic phase composition) of any biphasic solvent system may be used for TLC-based solvent system selection [19]. In summary, the GUESS method provides a practical and resource efficient means of rapidly establishing optimal conditions for target analytes. Taking into account the practical experience of the authors from the last decade, the present results imply that the approach of “GUESSing” CCC and CPC conditions by use of easy-to-perform TLC analysis has significant merit. The outcome also emphasizes that the GUESS scheme is not only useful for the classification of solvent systems, but provides a direct and very practical means for CCC and CPC practitioners to achieve desired separations.
Supplementary Material
The synthetic scheme for ginkgotoxin and two positional isomers is described.
TLC-based Generally Useful Estimate of Solvent Systems method is reintroduced.
Countercurrent chromatography is used to purify three ginkgotoxin positional isomers.
Purity and structure characterization of all samples was done by quantitative 1H NMR.
Acknowledgments
Support from the team at Cherry Instruments - Wrightwood Technology (Chicago, IL) in particular by Samuel Pro and Warren Friedel, is gratefully acknowledged. The authors are also grateful to Dr. Clemens Erdelmeier of Dr. Wilmar Schwabe Pharmaceuticals, (Karlsruhe, Germany) for his generous contribution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
J. Brent Friesen, Email: jbfriesen@dom.edu.
Shao-Nong Chen, Email: sc4sa@uic.edu.
References
- 1.Ito Y. High-speed countercurrent chromatography. Nature. 1987;326:419–420. doi: 10.1038/326419a0. [DOI] [PubMed] [Google Scholar]
- 2.Friesen JB, McAlpine JB, Chen SN, Pauli GF. Countercurrent separation of natural products: an update. J Nat Prod. 2015;78:1765–1796. doi: 10.1021/np501065h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroder M, Vetter W. Investigation of unsaponifiable matter of plant oils and isolation of eight phytosterols by means of high-speed counter-current chromatography. J Chromatogr A. 2012;1237:96–105. doi: 10.1016/j.chroma.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 4.Ito Y. pH-zone-refining counter-current chromatography: origin, mechanism, procedure and applications. J Chromatogr A. 2013;1271:71–85. doi: 10.1016/j.chroma.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Friesen JB, McAlpine JB, Pauli GF. Solvent system selection strategies in countercurrent separation. Planta Med. 2015 doi: 10.1055/s-0035-1546246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumner N. Developing counter current chromatography to meet the needs of pharmaceutical discovery. J Chromatogr A. 2011;1218:6107–6113. doi: 10.1016/j.chroma.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Friesen JB, Pauli GF. G.U.E.S.S.—A generally useful estimate of solvent systems for CCC. J Liq Chromatogr Relat Technol. 2005;28:2777–2806. [Google Scholar]
- 8.Hostettmann KHKM, Sticher O. Application of droplet counter current chromatography to the isolation of natural product. J Chromatogr A. 1979;186:529–534. [Google Scholar]
- 9.Liu Y, Chen SN, McAlpine JB, Klein LL, Friesen JB, Lankin DC, Pauli GF. Quantification of a botanical negative marker without an identical standard: ginkgotoxin in Ginkgo biloba. J Nat Prod. 2014;77:611–617. doi: 10.1021/np400874z. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi D, Yoshimura T, Johno A, Ishikawa M, Sasaki K, Wada K. Decrease in pyridoxal-5′-phosphate concentration and increase in pyridoxal concentration in rat plasma by 4′-O-methylpyridoxine administration. Nutr Res. 2015;35:637–642. doi: 10.1016/j.nutres.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Hill RE, Rowell FJ, Gupra RN, Spenser ID. Biosynthesis of vitamin B6. J Biol Chem. 1972;247:1869–1883. [PubMed] [Google Scholar]
- 12.Morozowich NL, Weikel AL, Nichol JL, Chen C, Nair LS, Laurencin CT, Allcock HR. Polyphosphazenes containing vitamin substituents: synthesis, characterization, and hydrolytic sensitivity. Macromolecules. 2011;44:1355–1365. [Google Scholar]
- 13.Zhang M, Zhang X, Li J, Guo Q, Xiao Q. A new pyridoxal derivative for transamination of N-terminus of proteins. Chinese J Chem. 2011;29:1715–1721. [Google Scholar]
- 14.Pauli GF, Pro SM, Chadwick LR, Burdick T, Pro L, Friedl W, Novak N, Maltby J, Qiu F, Friesen JB. Real-time volumetric phase monitoring: advancing chemical analysis by countercurrent separation. Anal Chem. 2015;87:7418–7425. doi: 10.1021/acs.analchem.5b01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pauli GF, Jaki BU, Lankin DC. A routine experimental protocol for qHNMR illustrated with Taxol. J Nat Prod. 2007;70:589–595. doi: 10.1021/np060535r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pauli GF, Godecke T, Jaki BU, Lankin DC. Quantitative H-1 NMR. Development and Potential of an Analytical Method: An Update. J Nat Prod. 2012;75:834–851. doi: 10.1021/np200993k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pauli GF. qNMR--a versatile concept for the validation of natural product reference compounds. Phytochem Anal. 2001;12:28–42. doi: 10.1002/1099-1565(200101/02)12:1<28::AID-PCA549>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 18.Pauli GF, Chen SN, Simmler C, Lankin DC, Gödecke T, Jaki BU, Friesen JB, McAlpine JB, Napolitano JG. Importance of purity evaluation and the potential of quantitative 1H NMR as a purity assay. J Med Chem. 2014;57:9220–9231. doi: 10.1021/jm500734a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang CY, Yang Y, Aisa HA, Xin XL, Ma HR, Yili A, Zhao YX. Bioassay-guided isolation of antioxidants from Astragalus altaicus by combination of chromatographic techniques. J Sep Sci. 2012;35:977–983. doi: 10.1002/jssc.201101104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.