Abstract
In humans, there is a strong beta (15-30 Hz) event-related desynchronization (ERD) that begins prior to movement, which has been tentatively linked to motor planning operations. The dynamics of this response are strongly modulated by whether a pending movement is cued, and the inherent parameters of the cue. However, previous studies have focused on the information content of cues, and not on parameters such as the timing of the cue relative to other events. Variations in such timing are critical, as they directly impact the amount of time that participants have to plan pending movements. In this study, participants performed finger-tapping sequences during magnetoencephalography (MEG), and we manipulated the amount of time (i.e., “long” versus “short”) between the presentation of the to-be-executed sequence, and the cue to initiate the sequence. We found that the beta ERD was stronger immediately after the cue to move in the contralateral postcentral gyrus and bilateral parietal cortices during the short compared to long planning time condition. During movement execution, the beta ERD was stronger in the premotor cortex and the supplementary motor area (SMA) in the short relative to long condition. Finally, peak-latency in the SMA significantly correlated with reaction time, such that the closer the peak beta ERD was to the cue to move, the quicker the participant responded. The results of this study establish that peri-movement beta ERD activity across the cortical motor circuit is highly sensitive to cue-related temporal factors, with a direct link to motor performance.
1. Introduction
Motor control is served by cortical oscillatory activity across a network of brain regions (Cheyne, Bakhtazad, & Gaetz, 2006; Cheyne, Bells, Ferrari, Gaetz, & Bostan, 2008; Gaetz, Edgar, Wang, & Roberts, 2011; Gaetz, Macdonald, Cheyne, & Snead, 2010; Heinrichs-Graham & Wilson, 2015; Heinrichs-Graham et al., 2014; Jurkiewicz, Gaetz, Bostan, & Cheyne, 2006; Muthukumaraswamy, 2010; Pfurtscheller & Lopes da Silva, 1999; Pfurtscheller, Neuper, Andrew, & Edlinger, 1997; Tzagarakis, Ince, Leuthold, & Pellizzer, 2010; Wilson, Heinrichs-Graham, & Becker, 2014; Wilson et al., 2010; Wilson, Slason, et al., 2011), especially in the alpha (8-14 Hz), beta (14-30 Hz), and gamma (60-90 Hz) frequencies. In particular, about 600 ms before movement onset, there is a strong beta event-related desynchronization (ERD) that is sustained throughout movement (Cheyne et al., 2006; Gaetz et al., 2010; Heinrichs-Graham et al., 2014; Jurkiewicz et al., 2006; Pfurtscheller & Lopes da Silva, 1999; Wilson, Heinrichs-Graham, et al., 2014; Wilson et al., 2010; Wilson, Slason, et al., 2011). This response is commonly referred to as the movement-related beta desynchronization or peri-movement beta ERD, and has been localized to the pre- and postcentral gyri, with weaker activity in parietal areas, cerebellum, supplementary motor area (SMA), and the premotor cortices (Cheyne et al., 2006; Gaetz et al., 2010; Heinrichs-Graham et al., 2014; Jurkiewicz et al., 2006; Pfurtscheller & Lopes da Silva, 1999; Wilson, Heinrichs-Graham, et al., 2014; Wilson et al., 2010; Wilson, Slason, et al., 2011).
Interestingly, the amplitude of the peri-movement beta ERD significantly differs depending on whether the pending movement is executed at the individual’s discretion or paced by an external cue (Kaiser, Lutzenberger, Preissl, Mosshammer, & Birbaumer, 2000; Rektor, Sochurkova, & Bockova, 2006). Consistent with these data, increased activation across the cortical motor network, but especially in the SMA, during self-paced compared to cued movement paradigms has been demonstrated using positron emission tomography (PET) (Jenkins, Jahanshahi, Jueptner, Passingham, & Brooks, 2000) and functional magnetic resonance imaging (fMRI) (Cunnington, Windischberger, Deecke, & Moser, 2002; Deiber, Honda, Ibanez, Sadato, & Hallett, 1999). Furthermore, if the movement is externally cued, the peri-movement beta ERD is sensitive to whether the cue is regularly paced or randomly paced (Alegre et al., 2003). For example, Alegre and colleagues (2003) recorded electroencephalography (EEG) while participants performed movements that were either externally paced from a cue presented at a fixed interval, or externally paced from a cue presented at a random interval. They found that beta ERD activity started prior to the cue in the fixed-interval paced condition, but began after the cue and was of smaller amplitude in the randomly-paced condition. Taken together, the premise that external cues modulate neuronal activity in motor-related areas is heavily supported.
Various EEG and MEG studies have attempted to establish the functional role(s) of the peri-movement beta ERD, and this oscillatory response has been tentatively linked with movement planning or selection (Doyle, Yarrow, & Brown, 2005; Grent-'t-Jong, Oostenveld, Jensen, Medendorp, & Praamstra, 2014; Heinrichs-Graham & Wilson, 2015; Kaiser, Birbaumer, & Lutzenberger, 2001; Kaiser, Ulrich, & Lutzenberger, 2003; Praamstra, Kourtis, & Nazarpour, 2009; Tzagarakis et al., 2010). In particular, there is increased peri-movement beta ERD when the direction of a pending movement is more certain (Tzagarakis et al., 2010), when lateralization of the movement cue is more direct (Doyle et al., 2005; Kaiser et al., 2003), and when the potential directions of a pending movement are more similar (Grent-'t-Jong et al., 2014; Praamstra et al., 2009). These and other studies draw a cumulative conclusion that the peri-movement beta ERD is directly affected by the parameter space of the pending movement.
While these studies are certainly valuable in their own right, these conclusions were drawn using paradigms with inherent differences in the pre-cue “instructional” stimulus that preceded the actual cue to initiate movement, and functioned to restrict the parameter space of the upcoming movement. For example, Doyle et al. (2005) used EEG and a pre-cued bilateral movement task. In their task, the pre-cue either fully predicted or gave no information about the nature of the pending movement. They found that when the pre-cue was fully predictive, the beta ERD was more lateralized to the contralateral motor cortex. In contrast, when the pre-cue gave no information, the beta ERD was of lower amplitude and bilateral (Doyle et al., 2005). In an MEG study by Tzagarakis and colleagues (2010), the movement cue was preceded by a pre-cue that depicted a variable number of potential movement directions. They found that, with a decreased number of potential movement directions (i.e., more certainty for the direction of the pending movement), there was an increase in beta ERD amplitude (Tzagarakis et al., 2010). Importantly, in these and other studies, the beta ERD response did not begin until after the pre-cue, and was of variable amplitude between the pre-cue and the cue to move, contingent on the degree of information that was embedded in the pre-cue. In other words, the amplitude of the beta ERD was directly affected by the reduction in the movement parameter space that was provided by the pre-cue, up until movement commenced.
These studies have provided critical insight on the role of the beta ERD in many aspects of movement planning, including directional certainty and movement selection. Furthermore, it is clear that the presence or absence of external cues is important in understanding beta ERD dynamics. However, whether features surrounding the cue independent of the parameter space of the pending movement modulate peri-movement beta oscillations remains incompletely understood. Such features include the amount of time between a pre-cue and cue to move, which for complex (and at least some simple) movements would serve as the “planning time” for the upcoming movement, given that complex movement selection is a time-intensive process. Thus, the amount and the predictability of time allocated to this movement selection process is likely to modulate a participant’s ability to perform a movement quickly and accurately (Haith, Huberdeau, & Krakauer, 2015; Lavergne, Vergilino-Perez, Collins, Orriols, & Dore-Mazars, 2008), and such modulation should be reflected in the neuronal processes that underlie motor planning. We posit that small differences in planning time (i.e., the time between when a motor sequence is presented and when it must be executed) will modulate beta ERD amplitude, and thus provide further evidence connecting this response to motor planning operations. To this end, we used a novel motor sequence paradigm and high-density MEG to study the effects of variable planning time on peri-movement beta oscillations. We manipulated the amount of time between the appearance of the to-be-executed movement sequence and the cue to initiate the movement, such that participants had either a short or a long time to plan the pending movement before execution. These conditions were pseudo-randomized, such that the amount of time between the sequence description and the cue to move was unpredictable. We hypothesized that cortical motor regions would exhibit a strong beta ERD throughout movement planning and execution, and that the temporal dynamics of this response would change as a function of cue timing, thereby connecting another crucial aspect of movement performance (i.e., the amount of time given to plan a movement and the predictability of such timing) with beta oscillatory dynamics.
2. Methods
2.1 Subject Selection
We studied 19 healthy, right-handed males (mean age: 26.00, range 19-30), all of whom were recruited from the local community. Exclusionary criteria included any medical illness affecting CNS function, neurological or psychiatric disorder, history of head trauma, current substance abuse, and the MEG Laboratory’s standard exclusion criteria (e.g., any type of ferromagnetic implanted material). After complete description of the study was given to participants, written informed consent was obtained following the guidelines of the University of Nebraska Medical Center’s Institutional Review Board, which approved the study protocol.
2.2 Experimental Paradigm and Stimuli
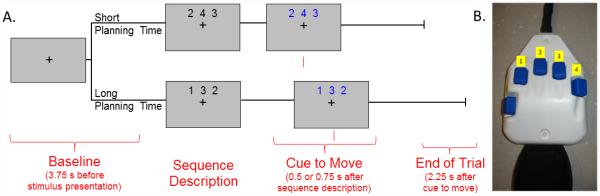
During MEG recording, participants were seated in a nonmagnetic chair within the magnetically-shielded room. Each participant rested their right hand on a custom-made five-finger button pad (see Figure 1b), while fixating on a crosshair presented centrally. This response pad was connected such that each button sent a unique signal (i.e., TTL pulse/trigger code) to the MEG system acquisition computer, and thus behavioral responses were temporally synced with the MEG data. This allowed accuracy, reaction times, and movement durations (in ms) to be computed offline. In order to create a sufficient baseline, participants initially fixated on a crosshair for 3.75 s before the beginning of each trial (Figure 1A). After this baseline period, a series of three numbers, each corresponding to a button on the response pad (Figure 1B), was presented simultaneously on the screen in black. These numbers remained on the screen for a variable period of time before the cue to move appeared. In the “short” planning time condition, these numbers changed color 0.5 s after appearing on the screen, which signaled the participant to initiate the tapping sequence. In the “long” planning time condition, these numbers changed color 0.75 s later, again signaling that the participant should initiate the tapping sequence. In other words, the participant was given either 0.5 s or 0.75 s to plan the movement before being cued to execute the movement, and thereafter the participant was given 2.25 s to complete the motor plan and return to rest (Figure 1A). At that time, the numbers disappeared and only the fixation crosshair remained. This series of events constituted one trial; Figure 1A depicts the total time course of a single trial. Participants performed a single pseudo-randomized block of trials, which ensured that the time between the sequence description and the cue to move was unpredictable to the participant. Further, the task contained the same set of sequences across conditions, and these sequences were controlled for several different variables. First, sequences were controlled for the first finger tapped, ensuring that any delays related to the ease at which a specific button could be pressed were equal, and thus could not skew reaction time data. Secondly, the sequences contained the same amount of total movement (i.e., three finger taps) and the same fingers tapped. A total of 80 trials per condition were completed (160 total trials), making overall MEG recording time about 16 minutes for the task.
Figure 1. Task paradigm.
a) Prior to the start of each trial, participants fixated on a crosshair for 3.75 s. Following this baseline period, a series of three numbers, each corresponding to a digit on the finger, appeared on the screen. These numbers changed color (black-to-blue) after a “long” or “short” period of time, which cued the participant to move. Participants were given either 0.5 s (“short”) or 0.75 s (“long”) to process the sequence description before the numbers changed color cueing the participant to move. Participants then had 2.25 s to complete the motor plan and return to rest. b) The button pad used during this task. Each button on the pad corresponded to a specific finger; the thumb was not used for task performance.
Besides this MEG task, participants completed an additional behavioral experiment to test for possible temporal predictability effects. Essentially, since there were two possible time points at which the participant was cued to move, our behavioral and neural metrics in the main experiment could be affected by inter-conditional differences in temporal predictability (i.e., if the cue to move did not occur at 0.5 s, then it was certain to occur at 0.75 s). Following this logic, participants may respond more quickly in the 0.75 s condition, irrespective of the extra time to plan, because they may be actively predicting the onset of the cue to move. Of course, such “stimulus predicting” would also affect our neural MEG data. To evaluate this possibility, participants performed a follow-up behavioral task that was identical to the MEG study described above, except that in this purely behavioral experiment participants were cued to move at either 0.75 s or 1.0 s. As such, the 0.75 s time period was less certain (i.e., 50% probability) and the 1.0 s cue was more certain (i.e., 100% probability), but in either case the movement sequence should have been fully planned, as 0.75 s is more than adequate time to plan a three-movement sequence and consequently the effect of extra planning time would be negligible. Thus, the pattern of behavioral results across these two experiments should help tease apart the effects of planning time and temporal predictability in the main MEG experiment.
2.3 MEG Data Acquisition & Coregistration with Structural MRI
All recordings were conducted in a one-layer magnetically-shielded room with active shielding engaged. Neuromagnetic responses were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1–330 Hz using a 306-sensor Elekta MEG system (Elekta, Helsinki, Finland). MEG data from each individual were corrected for head motion and subjected to noise reduction using the signal space separation method with a temporal extension (tSSS)(Taulu & Simola, 2006; Taulu, Simola, & Kajola, 2005). Each participant’s MEG data were then coregistered with high-resolution structural T1-weighted MRI data prior to the application of source space analyses (i.e., beamforming) using BESA MRI (Version 2.0). These neuroanatomic images were acquired with a Philips Achieva 3T X-series scanner using an eight-channel head coil and a 3D fast field echo sequence with the following parameters: TR: 8.09 ms; TE: 3.7 ms; field of view: 24 cm; slice thickness: 1 mm with no gap; in-plane resolution: 1.0 × 1.0 mm; sense factor: 1.5. The structural volumes were aligned parallel to the anterior and posterior commissures and transformed into standardized space. Following the beamformer analyses, each subject’s functional images were transformed into standardized space by using the transform that was previously applied to the structural MRI volume and spatially resampled.
2.4 MEG Time-Frequency Transformation and Statistics
Cardio-artifacts were removed from the data using signal-space projection (SSP), which was accounted for during source reconstruction (Uusitalo & Ilmoniemi, 1997). The continuous magnetic time series was divided into epochs of 6.5 s duration, with the baseline defined as −0.9 to −0.4 s before initial sequence description (Figure 1A). To determine which brain areas were modulated by cue timing and/or more directly linked to movement onset, epochs for each analysis were separately referenced to both the cue to move (movement cue = 0.0 s) and to movement onset (movement onset = 0.0 s). Importantly, baseline activity was referenced to the same time bin before sequence description (−0.9 s to −0.4 s) in both analyses. When movement onset was used as the reference, mean reaction times for each participant and condition were used to individually correct for differences in duration from the baseline period (i.e., before the sequence description), which is important because such differences could systematically bias the amplitude of the beta ERD. Epochs containing artifacts were rejected based on a fixed threshold method, supplemented with visual inspection.
Artifact-free epochs were transformed into the time-frequency domain using complex demodulation (resolution: 2.0 Hz, 25 ms from 4 to 50 Hz; (Papp & Ktonas, 1977)), and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized by dividing the power value of each time-frequency bin by the respective bin’s baseline power, which was calculated as the mean power during the −0.9 s to −0.4 s pre-sequence description time period.
The specific time-frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms across the entire array of gradiometers. Each data point in the spectrogram was initially evaluated using a mass univariate approach based on the general linear model. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two stage procedure was followed to control for Type 1 error. In the first stage, one-sample t-tests were conducted on each data point and the output spectrogram of t-values was thresholded at p < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations across all participants. In stage two, time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also above the (p < 0.05) threshold and within 2 cm of each other spatially, and then a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster values, and the significance level of the observed clusters (from stage one) were tested directly using this distribution (Ernst, 2004; Maris & Oostenveld, 2007). For each comparison, at least 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, time-frequency windows that contained a significant oscillatory event across all participants and conditions immediately following the cue to move, as well as immediately after movement onset, were subjected to the beamforming analysis.
2.5 MEG Imaging & Virtual Sensor Analysis
Cortical networks were imaged through an extension of the linearly constrained minimum variance vector beamformer (Van Veen, van Drongelen, Yuchtman, & Suzuki, 1997), which employs spatial filters in the frequency domain to calculate source power for the entire brain volume. The single images were derived from the cross spectral densities of all combinations of MEG gradiometers averaged over the time-frequency range of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. Following convention, the source power in these images was normalized per participant using a separately averaged noise period of equal duration and bandwidth from the baseline (i.e., before initial sequence description (Van Veen et al., 1997). MEG pre-processing and imaging used the Brain Electrical Source Analysis (BESA version 6.0) software.
Normalized source power was computed for the selected time-frequency bands over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. The effect of duration between the sequence description and the cue to move (“short” vs. “long”) was examined using a random effects analysis for the time-frequency bins of interest. Paired-sample t-tests were conducted to probe differences in peri-movement beta ERD as a function of planning time. As with the sensor-level analysis, a two-stage approach was used to control for Type 1 error. In the first stage, t-tests were conducted on each voxel and the output was thresholded at (p < 0.05) to create statistical parametric maps (SPMs) showing clusters of potentially significant activation. A cluster value was derived in stage two, for each cluster surviving stage one, by summing all of the t-values of all data points (voxels) within the cluster. Subsequently, permutation testing was used to derive a distribution of cluster-values, and the observed clusters were tested for significance using this distribution (Ernst, 2004; Maris & Oostenveld, 2007). For each comparison, at least 1,000 permutations were computed to build a distribution of cluster values. Following statistical analysis of the beamformer images, we extracted virtual sensors corresponding to the peak voxel for conditional effects (e.g., short > long planning time). Briefly, we identified the peak voxel of each task effect by conducting paired-sample t-tests between conditions (e.g., short vs. long). Each t-test yielded a SPM and we selected the voxel with the highest t-value per significant cluster for virtual sensor extraction. To create the virtual sensors, we applied the sensor weighting matrix derived through the forward computation to the preprocessed signal vector, which yielded a time series for the specific coordinate in source space. Note that this virtual sensor extraction was done per participant and condition individually, once the coordinates of interest (i.e., one per cluster) were known. These virtual sensors were used to evaluate the temporal dynamics of neuronal activity in brain regions where significant oscillatory modulations were detected. Finally, partial correlations (corrected for differences between conditions, when appropriate) between neurophysiological and behavioral results were computed to determine the relationship between brain activity and behavioral performance.
3. Results
3.1 Behavioral Results
All participants were able to successfully perform both tasks. Two participants were excluded from all statistical analyses due to excessive artifacts in their MEG data, leaving 17 total participants. We will start by describing results from the MEG task, followed by the control task. All participants performed generally well, with an overall accuracy of 96.80% (SD: 3.20%). There was no difference in accuracy between conditions, t(16) = 0.076, p = .94. However, participants had significantly slower reaction times (computed as the time between the cue to move and movement onset) in the short planning time condition compared to the long planning time condition, t(16) = 6.204, p < .0001. Conversely, participants had a shorter movement duration in the short condition than the long condition, t(16) = 4.83, p < .0001. In other words, participants were slower to initiate movement after being cued to move in the short compared to the long condition, but once they began the movement, they completed the sequence more quickly in the short relative to the long condition (Figure 2). In addition, there was a significant correlation between reaction time and movement duration (short condition: r(15) = .523, p = .031; long condition: r(15) = .539, p = .026), such that participants with shorter reaction times tended to complete the sequence faster, regardless of condition. In other words, some participants were generally quicker at completing sequences throughout the task (i.e., in both conditions; Figure 3).
Figure 2. Behavioral results.
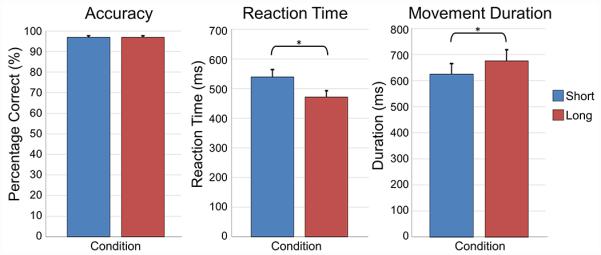
Participants performed generally well, and there was no significant difference in accuracy (percent correct) between the conditions (p = 0.94). In contrast, participants were significantly slower in the short compared to the long condition (p < .001), but interestingly had significantly shorter movement durations in the short condition relative to the long condition (p < .001). See legend for color descriptions. Error bars denote the standard error of the mean (SEM). * = p < 0.05
Figure 3. Correlation between movement duration and reaction time.
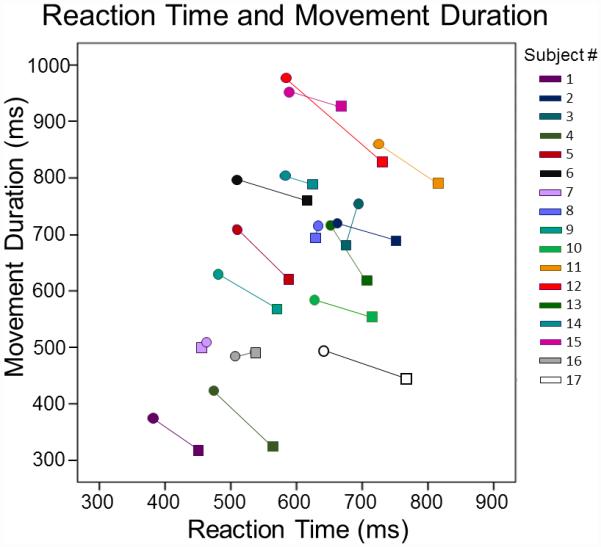
Movement duration (in ms) is shown on the y-axis, with reaction time (in ms) on the x-axis. Each participant’s data points are shown as a different color, with a line connecting their data for each condition (short condition: circle; long condition: square). There was a significant correlation between the movement duration and reaction time in each condition, such that participants who responded more quickly also completed each sequence more quickly across both conditions (p’s < .05).
As described in Section 2.2, we conducted a control behavioral experiment to evaluate whether temporal predictability differences between the 0.5 and 0.75 s conditions (i.e., if the cue to move did not occur at 0.5 s, then it was certain to occur at 0.75 s) were driving our key results. This experiment was identical to the main experiment except that the two conditions consisted of 0.75 and 1.0 s planning time windows. The results of this follow-up behavioral experiment were negative; there was no significant difference in reaction time between the 0.75 and 1.0 s planning time conditions, t(16) = 1.127, p = 0.241. In addition, we compared the reaction time data from the 0.75 s condition in each experiment, and found that there was no significant difference between reaction times in this condition between experiments, t(32) = 0.925, p = .362. As discussed below, these results provide evidence that the behavioral (and neural) differences found in our MEG study were driven, at least in part, by differences in planning time in the 0.75 s (versus 0.5 s) condition.
3.2 MEG Sensor-Level Results
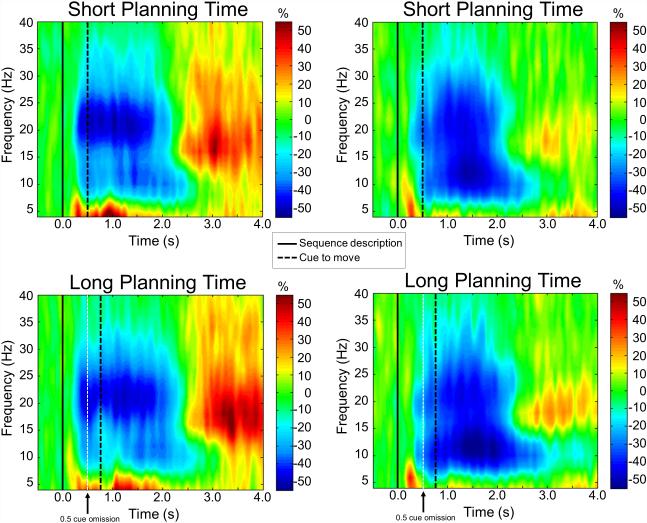
In order to ensure that peri-movement beta oscillatory activity was generated at the same time in both conditions and to determine the precise frequency band, sensor-level time-frequency spectrograms were first statistically examined using one-sample t-tests of the sensor-level plots of each condition separately using the sequence description onset as a reference (sequence description = 0.0 s). Results indicated significant peri-movement beta ERD in a large number of sensors around the sensorimotor cortices in the 16-26 Hz frequency range from about 0.2 s after initial sequence description (both p’s < .0001, corrected; see Figure 4). To determine when the beta ERD terminated, controlling for differences in trial length between conditions, statistical analyses of sensor-level spectrograms that used movement onset as reference (movement onset = 0.0 s) for each condition were performed separately. These analyses confirmed that, in both conditions, significant beta ERD dissipated approximately 1.0 s after movement termination (both p’s < .0001, corrected). In order to distinguish conditional differences in beta activity following the cue to move and during movement execution, the significant peri-movement beta ERD response period was divided into four temporally-distinct 16-26 Hz windows per condition. The first two bins corresponded to the time period just after the cue to move (movement cue 1: 0.1-0.3 s after the cue to move; movement cue 2: 0.3-0.5 s after the cue to move), while the third and fourth time bins corresponded to movement onset (movement onset 1: 0.0-0.2 s after movement onset; movement onset 2: 0.2-0.4 s after movement onset). These windows were independently imaged using beamforming to determine the precise brain regions generating significant oscillatory responses in each window per participant.
Figure 4. Group-averaged time-frequency spectra during the short and long planning time conditions.
Time (in s) is denoted on the x-axis, with the solid line indicating the sequence description onset and the dotted black line showing the cue to move onset (i.e., sequence description = 0.0 s). Frequency (in Hz) is shown on the y-axis. All signal power data is expressed as a percent difference from baseline, with the color legend shown to the right of each spectrogram. Data represent group-averaged gradiometer sensors near the left (left panel) and right (right panel) sensorimotor cortices, and were computed separately for the short (top panel) and long (bottom panel) planning time conditions. A white dotted line on the bottom panel at 0.5 s denotes where the short cue would have been.
3.3 Neuroanatomical Results
3.3.1 Beamforming analysis
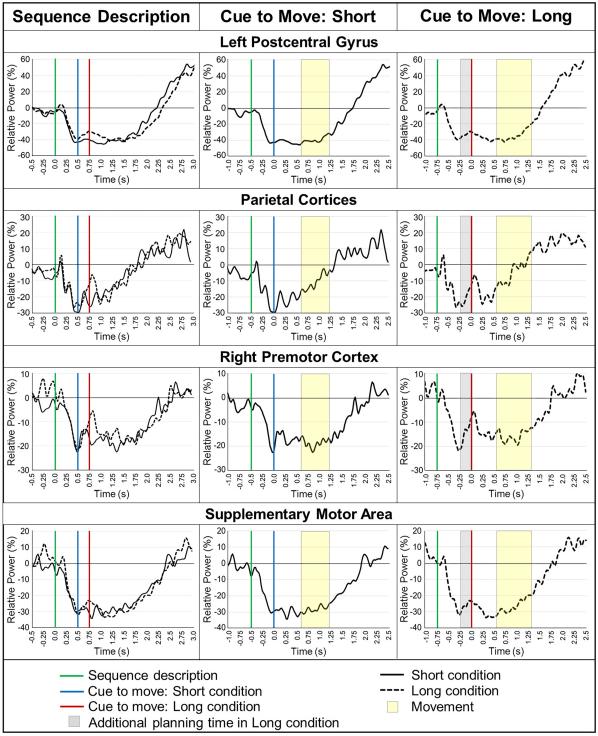
Analysis of task effects for both the short and long planning time conditions indicated significant beta (16-26 Hz) ERD in the bilateral primary sensorimotor cortices (stronger in the left), SMA, premotor cortices bilaterally, and bilateral superior parietal regions during all time bins (all p’s < .0001, corrected), consistent with previous literature. Statistical analysis of condition effects using paired-samples t-tests identified which regions of the motor network were differentially modulated by movement planning time (short vs. long), and/or during movement execution. During movement cue 1 (0.1-0.3 s after the cue to move), significantly stronger beta ERD responses were found in the left postcentral gyrus (p < .05, corrected) and bilateral parietal regions (p < .05, corrected) during the short condition relative to the long condition, and these differences in the parietal regions (but not the primary sensorimotor cortex) were sustained through the subsequent movement cue window (0.3-0.5 s; p < .05, corrected; Figure 5). In addition, during movement cue 2, there was significantly stronger beta ERD in the right premotor cortex in the short compared to the long condition (p < .05 corrected), and this increased beta ERD in the right ventral premotor cortex was also found in movement onset 1 (0.0-0.2 s following movement onset; p < .05, corrected; Figure 5). However, it should be noted that in the movement cue 2 time window, a large fraction of participants had already begun completing the sequence, and thus these time bins are not completely independent. Finally, marginally stronger beta ERD during the short relative to the long condition emerged in the SMA during this time window (0.0-0.2 s after, p = .059, corrected; p = .0006, uncorrected) and was sustained through the movement onset 2 period (0.2-0.4 s following movement onset, p = .070, corrected; p = .0008, uncorrected; Figure 5). No other regions were significantly different.
Figure 5. Brain regions with stronger beta ERD during the short planning time condition.
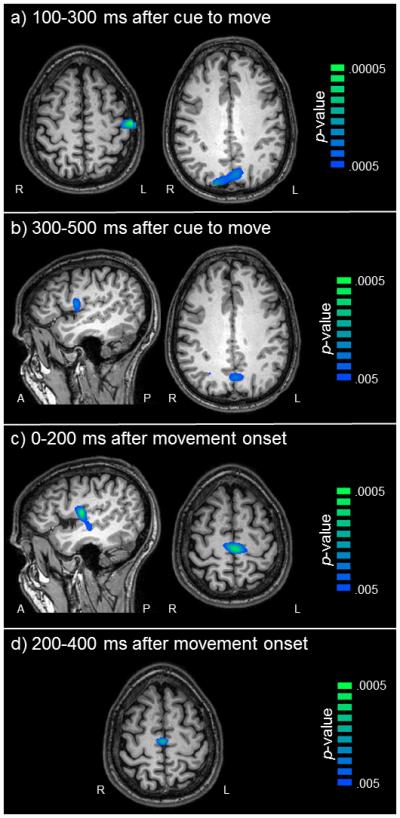
Color bars to the right denote image thresholds (in uncorrected p-values) and all images reflect areas of stronger beta ERD in the short compared to the long condition. Axial slices are shown in radiologic convention (right = left), and all sagittal slices are taken from the right hemisphere. a) Movement Cue 1. Participants exhibited stronger beta ERD in the left postcentral gyrus (left panel) and bilateral parietal regions (right panel) during the short condition from 0.1-0.3 s after the cue to move (p < 0.05, corrected). b) Movement Cue 2. Differences in the right premotor cortex emerged (left panel) and those in the parietal regions persisted from 0.3-0.5 s after the cue to move (right panel; p < 0.05, corrected). c) Movement Onset 1. Significant differences in the right premotor cortex (left panel) were also found during movement onset (p < 0.05, corrected) and marginal differences emerged in the supplementary motor area (SMA) from 0.0-0.2 s after movement onset (SMA; right panel; p = 0.059, corrected; p = .0006, uncorrected). d) Movement onset 2. Marginal differences in beta SMA activity continued 0.2-0.4 s after the onset of movement execution, (p = .07, corrected; p = .0008, uncorrected).
3.3.2 Time series analysis
We extracted the time series of the peak voxel corresponding to each conditional effect to more precisely examine the dynamics, and to identify the peak latency of the beta ERD response. The time series indicated that in both conditions, significant beta ERD began approximately 0.2 s after the initial sequence description. In the short condition, this beta ERD became progressively stronger throughout the motor planning period, sharply weakened after the onset of the cue to move, then re-desynchronized and was sustained throughout movement execution. Interestingly, in the long condition, the beta ERD also became weaker at around 0.5 s following the sequence description, despite no cue to move appearing (as in the short condition), and this resynchronization lasted about 0.1 s longer than was observed in the short condition. Subsequently, neural beta activity re-desynchronized after the long condition movement cue appeared (see Figure 6). Note that, unfortunately, this period of resynchronization in the long condition (0.5-0.75 s) cannot be directly compared between conditions, as this pre-cue time bin did not exist in the short planning time condition. However, it should be noted that in the long condition, the divergence in the last 0.1 s before the cue to move can be clearly identified by overlaying the peak voxel time courses yoked to the onset of the sequence description (i.e., in real time; see right panel in Figure 6).
Figure 6. Temporal evolution of the beta ERD response.
Virtual sensors were extracted from the peak voxel of each conditional effect to more precisely examine the dynamics of the beta ERD response in both the short (solid black line) and long (dotted black line) conditions. Time (in s) is denoted on the x-axis, while relative power (expressed as percentage from baseline) is shown on the y-axis. The left panel shows each response in real time (sequence description = 0.0 s), while the center and right panels show each response relative to their cue to move (cue to move = 0.0 s). The legend at the bottom defines the additional descriptors. Note that the time bin shown in grey cannot be directly compared between conditions because it did not exist in the short condition.
Finally, we correlated the peak latencies in each significant region with behavioral reaction time measures, while controlling for differences in behavioral measures across conditions. Briefly, we extracted the latency of the absolute peaks for our correlation; that is, if there were two peaks (as found in some participants), we used the larger of the two. Of note, not every participant had two distinct peaks in the long condition, and some (but not all) participants had two peaks for the short condition; thus, selecting the absolute peak seemed to be the best systematic approach. When there were two peaks, more often than not the second peak was of higher amplitude. There was a significant partial correlation between peak latency in the SMA and reaction time across both conditions (r(31) = −.339, p = .05), controlling for differences in reaction time between conditions. Basically, the later the peak beta ERD amplitude occurred relative to the actual cue to move, the faster the participants responded, regardless of condition.
4. Discussion
In this study, we utilized high-density MEG during a novel motor sequence task to study the effects of temporal factors on peri-movement beta oscillations. During the task, there was a variable amount of time between the appearance of the to-be-executed sequence description and the cue to initiate the movement, which corresponded to either a short time or a long time to plan the movement. Moreover, we utilized a complex movement sequence paradigm, as complex movements require much more planning and preparation than simple movements, and thus are more susceptible to changes in movement planning time and related parameters. Behaviorally, in the MEG experiment participants were slower to initiate movement in the short condition, but once movement was initiated, participants completed the movement faster in the short relative to the long condition. There were no behavioral differences in the follow-up control experiment. Our MEG results showed no conditional differences (i.e., long vs. short planning time) in the amplitude or latency of beta ERD in any brain region prior to the movement cue. However, shortly after the cue to move, there was increased beta suppression in the contralateral postcentral gyrus and parietal areas during the short condition compared to the long condition. The latter persisted until 0.5 s after the cue to move. Moreover, increased beta ERD in the short relative to the long condition emerged in the right ventral premotor cortex 0.3 s after the movement cue, and was also significant after movement onset. Finally, there was a marginal increase in beta ERD activity in the SMA throughout movement execution in the short compared to the long condition. Peak latency in the SMA was also inversely related to reaction time; that is, the later the maximum amplitude was relative to the movement cue, the shorter the reaction time, regardless of condition. This pattern of results lends itself to several interpretations. For example, the findings could reflect (a) insufficient time to plan the movement in the short condition, (b) motor inhibition in the long condition after the 0.5 s cue did not appear, and/or (c) temporal updating and/or reorienting of motor resources related to the omission of the 0.5 s cue. Below we discuss the significance of these data in understanding the effect of cue timing on the beta ERD, especially in regard to motor planning time and the predictability of movement cues.
As stated above, we found increased beta ERD in the postcentral gyrus and parietal areas in the short condition relative to the long condition shortly after the cue to move, with the differences in the parietal regions being sustained throughout movement preparation. Functionally, the postcentral gyrus serves as the primary somatosensory cortex, and it has been widely established that sensorimotor integration is pivotal to proper movements. Classic neurophysiology has also demonstrated that the posterior parietal cortex receives both sensory and motor information, and likely serves preparatory sensorimotor integration among other functions. Recent work using invasive recordings from the parietal cortices of behaving primates (Cui & Andersen, 2011), as well as fMRI (Thoenissen, Zilles, & Toni, 2002) and MEG (Park, Kim, & Chung, 2013) in humans, have shown increased activity in parietal areas during the motor planning and selection phases of motor sequencing paradigms. In our study, we posit that our experimental manipulation initially affected brain regions performing predictive sensorimotor integration, but that activity in these areas “normalized” (e.g., became similar between conditions) as this information was shared with secondary motor regions, which integrate this information into actual movement planning and execution.
Slightly before and during movement execution we found increased beta ERD in the right ventral premotor cortex, as well as a marginal increase in the SMA, in the short compared to the long condition. Many invasive studies in non-human primates have demonstrated that activity in the premotor cortex can be reliably linked to the sensory guidance of movement (Weinrich & Wise, 1982; Weinrich, Wise, & Mauritz, 1984). Thus, it is intuitive that there would be stronger decreases (i.e., ERD) in beta activity during conditions with shorter planning time and/or greater temporal uncertainty in the premotor cortex. Finally, the SMA has been reliably linked to the coordination and ordering of motor sequences (Ashe, Lungu, Basford, & Lu, 2006; Chan, Rao, Chen, Ye, & Zhang, 2006; Hoshi & Tanji, 2004; Nachev, Kennard, & Husain, 2008; Wilson, Kurz, & Arpin, 2014). Accordingly, it is again sensible that this area would exhibit significant differences as a function of planning time and/or the temporal predictability of a cued movement onset.
Taken together, these results suggest a broad modulation of the motor planning beta response throughout areas that serve motor control that is sensitive to the temporal factors surrounding the cue. One consequence of our experimental manipulation was that, in the short condition compared to the long condition, there was less time to plan the movement prior to execution. This decreased time to plan the movement may have resulted in a compensatory increase in beta ERD amplitude, indicative of more strenuous or extensive neuronal processing, in brain areas that serve the motor planning process. Basically, preparatory somatosensory and proprioceptive information had to be shared more rapidly with secondary motor planning regions during the short compared the long planning condition. This could explain the initial increase in beta ERD in the primary somatosensory regions and the posterior parietal regions that are hypothesized to function as sensory input and sensorimotor integration areas, respectively. Further, areas that specialize in motor planning and coordination such as the premotor cortex and the SMA were differentially active as a function of planning time just before and during movement.
We also found a significant correlation between the peak latency in the SMA and reaction time in both conditions. This may simply indicate that the later the SMA peak amplitude, the shorter the reaction time. However, the peak latency was calculated relative to the cue to move, and consequently was actually delayed by 250 ms in the long condition. Thus, an alternative view is that the closer the peak latency in the SMA was to the movement cue, regardless of whether the cue was 0.75 or 0.5 s after the sequence description, the quicker the participant moved after the onset of the cue. One possible interpretation of this SMA/reaction time link is that our dual-option motor cueing paradigm not only manipulated planning time, but may have also served as a temporal prediction evaluation. Basically, in the current study, participants were instructed to move as soon as the movement cue appeared, which occurred at one of two durations after presentation of the sequence description. Thus, if the movement cue did not appear by the estimated short planning time, it was guaranteed to occur at the long time. Many studies have linked parietal, premotor, frontal, and supplementary motor regions to predictive movement timing (Correa & Nobre, 2008; Coull, Frith, Buchel, & Nobre, 2000; Heinen & Liu, 1997; Janssen & Shadlen, 2005; Leon & Shadlen, 2003; Maimon & Assad, 2006), although these studies use single-neuron firing rates and/or event-related potentials, which are not directly comparable to the oscillatory responses analyzed in the current study. Nonetheless, the voxel time series extracted in the current study showed a slight decrease (resynchronization) in beta activity after the onset (and omission) of the short cue, which was of slightly larger amplitude in the long planning time condition (i.e., when the cue was absent) but clearly present in both. This response could be the oscillatory signature of a motor inhibition response, such that in the absence of the short delay cue, there was a slightly stronger resynchronization to inhibit movement until the later cue in the long condition.
Alternatively, studies have shown that regions of the motor network update temporal knowledge and reorient resources after the omission of an uncertain (short) cue, in preparation for a now-certain (long) cue (Coull et al., 2000). Such temporal updating and reorienting could also explain the slight resynchronization seen in the long condition. However, one problem with both the motor inhibition and reorienting interpretations is that participants in our study were completing complex sequences of movements that had to be deliberately planned. This is in contrast to the simple button presses or saccades that are commonly used in temporal prediction tasks. Performing these complex sequences required extensive motor planning and hence demanded significant attention, consuming cognitive resources that might otherwise be used to predict the onset of the cue, which would have been necessary for both the motor inhibition and the temporal updating/reorienting interpretations.
Unfortunately, our current results cannot distinguish between these possibilities (i.e., insufficient planning time, motor inhibition, updating/reorienting). Our behavioral data were more consistent with a planning time interpretation, whereas the neuronal time courses could be equally interpreted as a consequence of any of these three possibilities. Basically, there was a significant reaction time difference between conditions in our MEG experiment, whereby participants responded faster in the long relative to the short condition. This difference may indicate that motor planning was incomplete when the cue appeared in the short, but not the long condition. However, the alternative interpretation is that participants had completed their motor plan in both conditions, and that they responded faster in the long condition because the cue onset was perfectly certain, whereas it had 50/50 odds of appearing in the short condition (i.e., if the cue did not occur at 0.5 s, then it was certain to occur at 0.75 s). To sort out whether cue certainty was the critical difference, a follow-up behavioral study using longer delays to rule out insufficient planning time was conducted. The findings of this experiment indicated that there was no difference in reaction time between a 0.75 s delay condition (50% predictability) and 1.0 s delay condition (100% predictability). These data thereby suggest that the differences in temporal predictability alone were not enough to produce the reaction time differences that were observed in our MEG experiment. Nonetheless, this control experiment was only suggestive and the absence of a finding (i.e., faster reaction time to more certain cues) cannot serve to rule out a specific interpretation.
Regardless of the precise interpretation, the current data supported our main hypothesis that beta ERD dynamics are sensitive to the temporal characteristics of a pre-movement cue, independent of the parameters of the movement embedded in the cue itself. Future studies should clarify whether the beta ERD modulation and behavioral differences observed in the current study were primarily attributable to the temporal predictability of the cue or the absolute amount of motor planning time. Such studies could use a block design where the cue is delayed 0.5 and 0.75 s from the sequence description in separate blocks, which would produce 100% temporal certainty in each condition. An alternative would be to keep the pseudo-random presentation design, and indicate on the sequence description image which condition was upcoming (e.g., by changing the fixation color or font of the numbers) and thereby maintain 100% certainty in each condition. Such experiments will be essential to identifying the role of each parameter and thereby clarifying the functional role of the beta ERD in motor control. Lastly, and of special interest for follow-up, we observed a “bimodal” beta ERD in the time series of all significant brain regions, which was diminished in the short condition. In other words, there were two ERD peaks, one before and one after the movement cue in both conditions, albeit smaller in the short condition. Despite a slight resynchronization after the cue to move in the long condition, participants still had faster reaction times, suggesting that the initial increase in beta ERD was in preparation of a motor plan, not for movement execution. Future studies of the beta ERD should also consider this possible interpretation in their design.
This study was one of the first to investigate how cue-related temporal factors modulate cortical beta oscillations. We found significant increases in beta ERD amplitude in the short compared to the long condition shortly after the cue to move through movement execution in many primary and secondary motor regions, including the postcentral gyrus, parietal cortices, premotor cortex, and SMA. Taken together, these results provide crucial new information on the temporal dynamics within the cortical motor circuit during motor processing. A more precise understanding of the effects of environmental cues on motor network dynamics may offer critical insight into the mechanisms of therapeutic treatments for patients with physical injuries or movement disorders (Heinrichs-Graham et al., 2014; Kurz, Becker, Heinrichs-Graham, & Wilson, 2014; Wilson, Fleischer, Archer, Hayasaka, & Sawaki, 2011).
Acknowledgements
This work was supported by NIH grant R01 MH103220 (TWW), NSF grant #1539067 (TWW), the Shoemaker Prize from the University of Nebraska Foundation (TWW), a Kinman-Oldfield Award for Neurodegenerative Research from the University of Nebraska Foundation (TWW), and a grant from the Nebraska Banker’s Association. The Center for Magnetoencephalography at the University of Nebraska Medical Center was founded through an endowment from an anonymous donor. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Alegre M, Gurtubay IG, Labarga A, Iriarte J, Malanda A, Artieda J. Alpha and beta oscillatory changes during stimulus-induced movement paradigms: effect of stimulus predictability. Neuroreport. 2003;14(3):381–385. doi: 10.1097/00001756-200303030-00017. doi: 10.1097/01.wnr.0000059624.96928.c0. [DOI] [PubMed] [Google Scholar]
- Ashe J, Lungu OV, Basford AT, Lu X. Cortical control of motor sequences. Curr Opin Neurobiol. 2006;16(2):213–221. doi: 10.1016/j.conb.2006.03.008. doi: 10.1016/j.conb.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Chan RC, Rao H, Chen EE, Ye B, Zhang C. The neural basis of motor sequencing: an fMRI study of healthy subjects. Neurosci Lett. 2006;398(3):189–194. doi: 10.1016/j.neulet.2006.01.014. doi: 10.1016/j.neulet.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum Brain Mapp. 2006;27(3):213–229. doi: 10.1002/hbm.20178. doi: 10.1002/hbm.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage. 2008;42(1):332–342. doi: 10.1016/j.neuroimage.2008.04.178. doi: 10.1016/j.neuroimage.2008.04.178. [DOI] [PubMed] [Google Scholar]
- Correa A, Nobre AC. Neural modulation by regularity and passage of time. J Neurophysiol. 2008;100(3):1649–1655. doi: 10.1152/jn.90656.2008. doi: 10.1152/jn.90656.2008. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Buchel C, Nobre AC. Orienting attention in time: behavioural and neuroanatomical distinction between exogenous and endogenous shifts. Neuropsychologia. 2000;38(6):808–819. doi: 10.1016/s0028-3932(99)00132-3. [DOI] [PubMed] [Google Scholar]
- Cui H, Andersen RA. Different representations of potential and selected motor plans by distinct parietal areas. J Neurosci. 2011;31(49):18130–18136. doi: 10.1523/JNEUROSCI.6247-10.2011. doi: 10.1523/JNEUROSCI.6247-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage. 2002;15(2):373–385. doi: 10.1006/nimg.2001.0976. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M. Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol. 1999;81(6):3065–3077. doi: 10.1152/jn.1999.81.6.3065. [DOI] [PubMed] [Google Scholar]
- Doyle LM, Yarrow K, Brown P. Lateralization of event-related beta desynchronization in the EEG during pre-cued reaction time tasks. Clin Neurophysiol. 2005;116(8):1879–1888. doi: 10.1016/j.clinph.2005.03.017. doi: 10.1016/j.clinph.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Ernst MD. Permutation methods: A basis for exact inference. Stat Sci. 2004;19:676–685. [Google Scholar]
- Gaetz W, Edgar JC, Wang DJ, Roberts TP. Relating MEG measured motor cortical oscillations to resting gamma-aminobutyric acid (GABA) concentration. Neuroimage. 2011;55(2):616–621. doi: 10.1016/j.neuroimage.2010.12.077. doi: 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Macdonald M, Cheyne D, Snead OC. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. Neuroimage. 2010;51(2):792–807. doi: 10.1016/j.neuroimage.2010.01.077. doi: 10.1016/j.neuroimage.2010.01.077. [DOI] [PubMed] [Google Scholar]
- Grent-'t-Jong T, Oostenveld R, Jensen O, Medendorp WP, Praamstra P. Competitive interactions in sensorimotor cortex: oscillations express separation between alternative movement targets. J Neurophysiol. 2014;112(2):224–232. doi: 10.1152/jn.00127.2014. doi: 10.1152/jn.00127.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith AM, Huberdeau DM, Krakauer JW. The influence of movement preparation time on the expression of visuomotor learning and savings. J Neurosci. 2015;35(13):5109–5117. doi: 10.1523/JNEUROSCI.3869-14.2015. doi: 10.1523/JNEUROSCI.3869-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinen SJ, Liu M. Single-neuron activity in the dorsomedial frontal cortex during smooth-pursuit eye movements to predictable target motion. Vis Neurosci. 1997;14(5):853–865. doi: 10.1017/s0952523800011597. [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW. Coding complexity in the human motor circuit. Hum Brain Mapp. 2015 doi: 10.1002/hbm.23000. doi: 10.1002/hbm.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW, Santamaria PM, Heithoff SK, Torres-Russotto D, Hutter-Saunders JA, Gendelman HE. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson's disease. Cereb Cortex. 2014;24(10):2669–2678. doi: 10.1093/cercor/bht121. doi: 10.1093/cercor/bht121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi E, Tanji J. Differential roles of neuronal activity in the supplementary and presupplementary motor areas: from information retrieval to motor planning and execution. J Neurophysiol. 2004;92(6):3482–3499. doi: 10.1152/jn.00547.2004. doi: 10.1152/jn.00547.2004. [DOI] [PubMed] [Google Scholar]
- Janssen P, Shadlen MN. A representation of the hazard rate of elapsed time in macaque area LIP. Nat Neurosci. 2005;8(2):234–241. doi: 10.1038/nn1386. doi: 10.1038/nn1386. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. Pt 6. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage. 2006;32(3):1281–1289. doi: 10.1016/j.neuroimage.2006.06.005. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Birbaumer N, Lutzenberger W. Event-related beta desynchronization indicates timing of response selection in a delayed-response paradigm in humans. Neurosci Lett. 2001;312(3):149–152. doi: 10.1016/s0304-3940(01)02217-0. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W, Preissl H, Mosshammer D, Birbaumer N. Statistical probability mapping reveals high-frequency magnetoencephalographic activity in supplementary motor area during self-paced finger movements. Neurosci Lett. 2000;283(1):81–84. doi: 10.1016/s0304-3940(00)00921-6. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Ulrich R, Lutzenberger W. Dynamics of sensorimotor cortex activation to spatial sounds precueing ipsi- versus contralateral manual responses. Brain Res Cogn Brain Res. 2003;17(3):573–583. doi: 10.1016/s0926-6410(03)00171-x. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Becker KM, Heinrichs-Graham E, Wilson TW. Neurophysiological abnormalities in the sensorimotor cortices during the motor planning and movement execution stages of children with cerebral palsy. Dev Med Child Neurol. 2014;56(11):1072–1077. doi: 10.1111/dmcn.12513. doi: 10.1111/dmcn.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavergne L, Vergilino-Perez D, Collins T, Orriols E, Dore-Mazars K. The planning of a sequence of saccades in pro- and antisaccade tasks: influence of visual integration time and concurrent motor processing. Brain Res. 2008;1245:82–95. doi: 10.1016/j.brainres.2008.09.065. doi: 10.1016/j.brainres.2008.09.065. [DOI] [PubMed] [Google Scholar]
- Leon MI, Shadlen MN. Representation of time by neurons in the posterior parietal cortex of the macaque. Neuron. 2003;38(2):317–327. doi: 10.1016/s0896-6273(03)00185-5. [DOI] [PubMed] [Google Scholar]
- Maimon G, Assad JA. A cognitive signal for the proactive timing of action in macaque LIP. Nat Neurosci. 2006;9(7):948–955. doi: 10.1038/nn1716. doi: 10.1038/nn1716. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol. 2010;104(5):2873–2885. doi: 10.1152/jn.00607.2010. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9(11):856–869. doi: 10.1038/nrn2478. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–145. [PubMed] [Google Scholar]
- Park H, Kim JS, Chung CK. Differential beta-band event-related desynchronization during categorical action sequence planning. PLoS One. 2013;8(3):e59544. doi: 10.1371/journal.pone.0059544. doi: 10.1371/journal.pone.0059544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110(11):1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G. Foot and hand area mu rhythms. Int J Psychophysiol. 1997;26(1-3):121–135. doi: 10.1016/s0167-8760(97)00760-5. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Kourtis D, Nazarpour K. Simultaneous preparation of multiple potential movements: opposing effects of spatial proximity mediated by premotor and parietal cortex. J Neurophysiol. 2009;102(4):2084–2095. doi: 10.1152/jn.00413.2009. doi: 10.1152/jn.00413.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rektor I, Sochurkova D, Bockova M. Intracerebral ERD/ERS in voluntary movement and in cognitive visuomotor task. Prog Brain Res. 2006;159:311–330. doi: 10.1016/S0079-6123(06)59021-1. doi: 10.1016/S0079-6123(06)59021-1. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol. 2006;51(7):1759–1768. doi: 10.1088/0031-9155/51/7/008. doi: 10.1088/0031-9155/51/7/008. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M. Applications of the signal space separation method (SSS) IEEE Trans Signal Process. 2005;53(9):3359–3372. [Google Scholar]
- Thoenissen D, Zilles K, Toni I. Differential involvement of parietal and precentral regions in movement preparation and motor intention. J Neurosci. 2002;22(20):9024–9034. doi: 10.1523/JNEUROSCI.22-20-09024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G. Beta-band activity during motor planning reflects response uncertainty. J Neurosci. 2010;30(34):11270–11277. doi: 10.1523/JNEUROSCI.6026-09.2010. doi: 10.1523/JNEUROSCI.6026-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo MA, Ilmoniemi RJ. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput. 1997;35(2):135–140. doi: 10.1007/BF02534144. [DOI] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng. 1997;44(9):867–880. doi: 10.1109/10.623056. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Weinrich M, Wise SP. The premotor cortex of the monkey. J Neurosci. 1982;2(9):1329–1345. doi: 10.1523/JNEUROSCI.02-09-01329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinrich M, Wise SP, Mauritz KH. A neurophysiological study of the premotor cortex in the rhesus monkey. Brain. 1984;107:385–414. doi: 10.1093/brain/107.2.385. Pt 2. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Fleischer A, Archer D, Hayasaka S, Sawaki L. Oscillatory MEG motor activity reflects therapy-related plasticity in stroke patients. Neurorehabil Neural Repair. 2011;25(2):188–193. doi: 10.1177/1545968310378511. doi: 10.1177/1545968310378511. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Becker KM. Circadian modulation of motor-related beta oscillatory responses. Neuroimage. 2014;102P2:531–539. doi: 10.1016/j.neuroimage.2014.08.013. doi: 10.1016/j.neuroimage.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Kurz MJ, Arpin DJ. Functional specialization within the supplementary motor area: a fNIRS study of bimanual coordination. Neuroimage. 2014;85:445–450. doi: 10.1016/j.neuroimage.2013.04.112. Pt 1. doi: 10.1016/j.neuroimage.2013.04.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, Rojas DC. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn. 2010;73(2):75–84. doi: 10.1016/j.bandc.2010.03.001. doi: 10.1016/j.bandc.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev Neuropsychol. 2011;36(5):596–613. doi: 10.1080/87565641.2011.555573. doi: 10.1080/87565641.2011.555573. [DOI] [PMC free article] [PubMed] [Google Scholar]