Abstract
Background
Ventilator-associated pneumonia (VAP) is among the most common health care associated infections in the intensive care unit, and is associated with significant morbidity and mortality. Existing VAP prevention intervention bundles vary widely on the interventions included, as well as in the approaches used to develop these bundles.
Objective
To develop a new VAP prevention bundle using a systematic approach that elicits clinician perceptions on which interventions are most important and feasible to implement.
Methods
We identified potential interventions to include through a review of current guidelines and literature. We implemented a two-step modified Delphi method to gain consensus on the final list of interventions. An interdisciplinary group of clinical experts participated in the Delphi process, which was guided by a technical expert panel.
Results
We identified 65 possible interventions. Through the Delphi method, we narrowed that list to 19 interventions that included 5 process and 14 structural measures.
Conclusion
We described a structured approach for developing a new VAP prevention bundle. Obtaining clinician input on what interventions to include increases the likelihood that providers will adhere to the bundle.
Keywords: ventilator-associated pneumonia, quality improvement, prevention, bundle
Background
Ventilator-associated pneumonia (VAP) is among the most common health care associated infections in the intensive care unit (ICU), and is associated with significant morbidity and mortality.1-3 Between 10-20% of patients ventilated for a duration of over 48 hours develop VAP.1 In addition, VAP is associated with a longer duration of mechanical ventilation, hospital length of stay (LOS), ICU LOS, and higher hospital charges.1,4,5
Several published guidelines summarize effective interventions and infection control practices and provide recommendations to prevent VAP.6-9 Some of these guidelines are now close to 10 years old and fail to include more recent evidence. Some recommendations are inconsistent across these guidelines. Furthermore, despite these guidelines, many patients do not receive the recommended interventions as translation of evidence into practice remains challenging.10 Effective strategies to increase adherence to the guidelines and reduce related public health consequences associated with VAP are paramount.
One commonly used approach to increase adherence to VAP guidelines is to aggregate care processes into a “bundle” of care. The use of bundles improves process reliability and clinical outcomes.11 Implementation of bundles designed to improve care for mechanically ventilated patients has been associated with significant reductions in VAP rates.11-15 Nevertheless, the specific care processes included in these bundles vary,16,17 and the approach to select specific bundle components in the US has not been well articulated. Furthermore, the most widely use ventilator bundle in the US was originally developed to reduce various complications associated with mechanical ventilation, not just VAP.11,18 As a result, concerns exist regarding the internal validity of this bundle and its use as a potential quality indicator for reducing VAP rates.16,18
In this manuscript, we describe a systematic approach for developing a VAP prevention bundle. Specifically, we focus on the process we used to summarize guideline recommendations, and systematically seek clinician perspectives in identifying interventions for inclusion in a new VAP prevention bundle. The Institutional Review Board at The Johns Hopkins University School of Medicine approved this study.
Methods
We reviewed current VAP prevention guidelines and recently published literature to identify candidate interventions, convened an interdisciplinary group of experts, and implemented a two-step modified Delphi method to gain consensus on a final set of interventions to include in our VAP prevention bundle. To provide guidance throughout the bundle development process, we convened a fifteen-member technical expert panel (TEP) in August 2011. The TEP was made up of experts from the disciplines of Critical Care, Pulmonary, and Infectious Disease, as well as researchers with an expertise in basic measurement and implementation science. The TEP met via a conference call on a quarterly basis and as needed.
Review of VAP Prevention Guidelines and Literature
We examined and summarized all interventions listed in VAP prevention guidelines published during the last 15 years by the American Thoracic Society (ATS),6 Society for Healthcare Epidemiology of America/Infectious Diseases Society of America (SHEA/IDSA),8 Canadian Critical Trials Group (CCTG),7 and Centers for Disease Control and Prevention (CDC).9 We tracked articles cited in each of these sources back through three to four generations to identify additional original research, scientific reviews, and meta-analyses.
Using relevant keywords (Appendix A – all appendices are available upon request), we searched the literature to identify relevant articles published following the release of each guideline referenced above, as well as articles not cited in the guidelines. Additionally, we researched articles referencing particular interventions to identify competing findings or opinions in the field. To ensure that we received information published by all health care provider types, we searched PubMed, CINAHL and Google Scholar.
We sorted the VAP prevention interventions into five topic groups, based on the framework used in the SHEA/IDSA guideline.8 These groups were: 1) prevention of transmission of bacteria; 2) aspiration prevention; 3) reduce colonization of the aerodigestive tract; 4) prophylactic procedures for prevention of pneumonia; and, 5) minimize contamination of equipment. We chose to limit categorization of each intervention to the most appropriate group, although some could have been included in more than one group.
Modified Delphi Technique
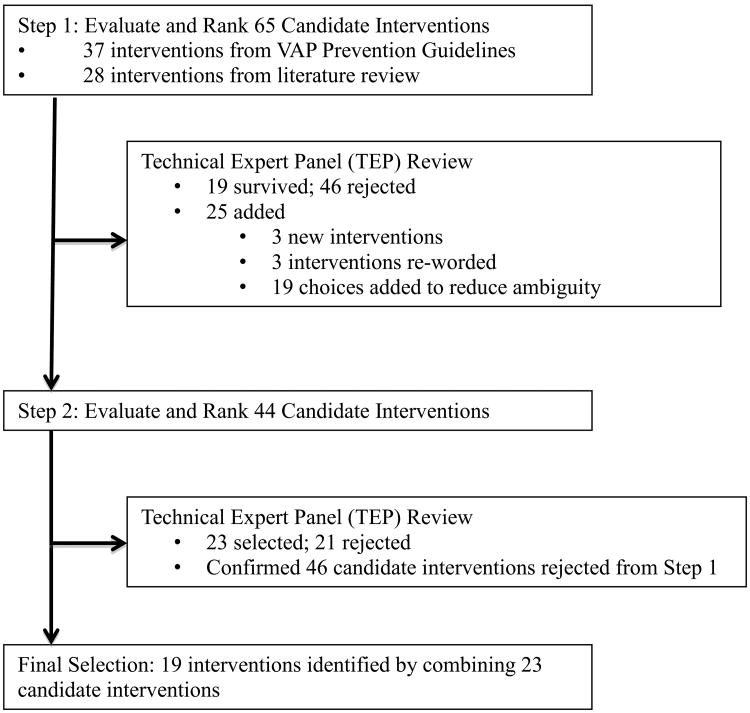
We used a two-step modified Delphi method developed by the RAND Corporation to determine which interventions to include in our proposed VAP prevention bundle (Figure 1). The modified Delphi method obtains a reliable consensus among a group of experts by eliciting individual opinions on the subject of interest, providing feedback about these initial opinions to the participants, allowing the opportunity for individual reassessment, and assuring anonymity of individual responses. The Delphi method allows participants to express their opinions independently and avoid confrontation that can hinder arriving at an accurate consensus. Each participant's opinion has an equal weight in the consensus reached by the group.19,20
Figure 1. Schematic of Two-Step Modified Delphi Method Used to Develop Proposed VAP Prevention Bundle.
An interdisciplinary group of clinical experts completed the two-step modified Delphi method. We first recruited known experts in the field of VAP prevention, and then used a “snowball” invitation process. Clinicians who had agreed to participate were asked to forward information regarding the project to other clinicians they felt might be interested in participating in the project. We also emailed potential participants via listservs of national professional societies, including the Society of Critical Care Medicine, Society of Healthcare Epidemiology of America, and American Association for Respiratory Care. Participants were self-selected, based on their own interest and expertise. We restricted participation predominantly to US providers to capture American perceptions of VAP prevention interventions. We collected from each Delphi participant demographic information including age, gender, health care role, primary department, size of hospital, hospital location (urban, suburban, and rural), experience treating VAP, perceived knowledge of both original and current literature on VAP prevention, and potential conflicts of interest.
Delphi participants completed two rounds of rating VAP prevention interventions. Prior to each round, we provided participants with the list of VAP prevention interventions, organized by the 5-group framework described earlier, a summary of recommendations in the guideline for each specific intervention, and pertinent references. In the list of interventions, we intentionally presented all identified interventions, including those that were mutually exclusive interventions, such as “Use the supine position” and “Avoid the supine position.” This thorough presentation of interventions allowed participants an opportunity to express their preferences during the rating process. Participants rated the interventions using a web-based instrument. For each of the five groups of candidate interventions, we provided text boxes in which participants could write explanations, suggestions, or other comments about the interventions being considered.
In the first round of rating, we instructed participants to rate each intervention based on their perceived importance of the intervention in preventing VAP. Participants used a Likert scale of 1 to 9, where 1 was least important and 9 was the most important. We also offered “A Can't Rate (C/R)” option to participants to use if they perceived that they did not have a clear opinion or had insufficient knowledge of the intervention, or if the intervention as presented in the web-based instrument was ambiguous.
Using Stata software (version 9.1), we calculated mean values (with standard deviations) to summarize the overall rating given to each candidate intervention. The TEP reviewed these results and identified the mean cutoff point to determine the short list of candidate interventions for Delphi participants to rate in the second round. After the first round of rating, and based on feedback from the Delphi participants and the TEP, we modified several existing interventions and added new interventions. The TEP made all decisions based on a consensus.
In preparation for the second round of rating, the Delphi participants received a packet with the results of the first round of rating; a summary of comments from participants and responses from the TEP; and lists of the surviving, revised, and dropped interventions from the first round of rating. We instructed participants to rate the surviving and revised list of interventions based on their perceived importance of each intervention in preventing VAP prevention, and on the feasibility of implementing each intervention. The participants rated each intervention using a Likert scale of 1 to 9, where 1 meant that they would definitely exclude the intervention from the VAP prevention bundle, and 9 meant that they would definitely include the intervention in the VAP prevention bundle. They were also asked to indicate whether we should reinstate the interventions dropped after the first round of rating. We wanted to ensure that the Delphi participants had the opportunity to re-evaluate results from the first round of ratings.
Using Stata software (version 9.1), we calculated mean values (with standard deviations) to summarize the rating results. The TEP reviewed the results of the second round of ratings and again chose the cutoff point to determine which interventions to include in our proposed VAP prevention bundle. We sent these rating results and the proposed VAP prevention bundle of interventions to the Delphi participants. We invited all TEP and Delphi participants to participate in two telephone conference calls to review the results of the ratings and discuss findings.
Categorization of Process and Structural Interventions
The TEP further categorized the proposed interventions in the VAP prevention bundle as process or structural measures. We achieved consensus through an iterative process in which the TEP members, on the basis of their knowledge and clinical experience, considered the likely focus of improvement efforts in preventing VAP. For example, variation in adherence to process interventions would likely reflect individual provider performance, and adherence would likely need to be frequently evaluated. Variation in adherence to structural interventions would likely reflect hospital, local ICU, nursing, or respiratory therapy policies and procedures, and adherence could likely be evaluated less frequently.
Results
Guideline and Primary Literature Review
We identified 65 candidate interventions (Appendix B). Of these, 37 came from existing guidelines. We identified the remaining 28 through the literature review.
Interdisciplinary VAP Prevention Committee (VPC) and Delphi Method
Overall, 171 health care providers agreed to participate in the Delphi method. Nine of these participants reported a possible conflict of interest.
Delphi Results
In the first round of ratings, 155 (90.6%) participants responded. The mean score (standard deviation) ranged (on 1-9 point Likert scale) from 8.80 (0.57) for the “Make a daily assessment of readiness to wean” intervention to 1.48 (1.10) for the “Routinely administer IV immunoglobulins or white-cell stimulating factors” intervention. All interventions received at least one rating of 1 and one rating of 9 on the Likert scale (Appendix C).
The TEP established a cutoff point at the mean score of ≥ 6.00. The TEP's goal was to achieve a significant reduction in the number of surviving interventions, but not exclude potentially important interventions. As a result, the TEP selected 19 interventions that were part of the first round of rating for inclusion in the second round of rating, and added 25 interventions. The addition of the 25 new interventions were due to comments from the Delphi participants and the TEP. Of these 25, 3 were new interventions and 3 were interventions reworded to eliminate ambiguity. The remaining 19 focused on when the intervention should be used (for example, “Use silver-coated endotracheal tubes” was changed to 3 separate candidate interventions: 1) “Routinely use silver-coated endotracheal tubes;” 2) “Use silver-coated endotracheal tubes for patients who are at a high risk for VAP;” and, 3) “Use silver-coated endotracheal tubes in units where VAP rates are high”). The second round of rating included a total of 44 candidate interventions.
In the second round of ratings, we only asked the 155 individuals who responded in the first round to participate. In this second round, 143 (92.3%) participants responded. The mean (standard deviation) scores ranged from 8.83 (0.43) for the “Make a daily assessment of readiness to wean” intervention to 3.38 (2.17) for the “Routinely use silver-coated endotracheal tubes” intervention (Appendix D). All interventions received at least one rating of 9 on the Likert scale. All dropped interventions received at least one vote for reinstatement; however, none of the interventions met the a priori threshold of 25% (maximum 17%) to be reinstated for further consideration.
After the first round of rating, the TEP established a mean score of ≥ 6.8 as the cutoff point for selecting the final set of interventions to include in the VAP prevention bundle. Some interventions were in competition with similar interventions which had received higher ratings. For example, “Use chlorhexidine gluconate when performing oral care” (mean of 7.8) was rated higher than “Use antiseptics when performing oral care” (mean of 6.7).
The second round of rating yielded 23 candidate interventions. The TEP further modified selected interventions based on clinical and implementation experience, with the intent of reducing ambiguity of each intervention during improvement efforts. For example, “Use a sedation protocol with sedation vacation” was combined with “Use a validated sedation scale at least daily (i.e. RASS)” to create the final intervention: “Use a sedation protocol with a sedation vacation and validated sedation scale at least daily.” After these modifications, 19 interventions remained: 5 were process measures and 14 were structural measures (Table 1).
Table 1. Process and Structural Measures in Proposed VAP Prevention Bundle.
| Process Measures | Structural Measures |
|---|---|
|
| |
|
|
We sent the results of the second round of rating to all TEP members and Delphi participants, and discussed the final bundle of interventions during debriefing calls. The main topics discussed during these calls were challenges associated with diagnosing VAP, proposed changes to the CDC's VAP surveillance definition, and the next steps in implementing our VAP prevention bundle and evaluating its impact on VAP rates. During these telephone calls, the Delphi participants did not express concerns about the results of the modified Delphi method, and many thought the new VAP prevention bundle was important and advanced the field.
Discussion
We have described a systematic approach to elicit clinician perceptions regarding which interventions are most important to include in a VAP prevention bundle. The Delphi participants, including 171 interdisciplinary members, reviewed 65 recommendations from published VAP prevention guidelines and primary literature, and identified 23 interventions as the most important and feasible to implement. Following additional modifications, 19 interventions reflecting bedside care and structure related processes were included in the proposed VAP prevention bundle. The bundle's focus includes five bedside care processes, which are evidence-based and manageable interventions, and which were selected by a large and diverse group of clinical experts that kept the bundle's implementation in mind.
The implementation of bundles of care processes has been associated with significant and sustained reductions in health care associated conditions, including central-line associated bloodstream infections21 and VAP.11-14,22 While a causal inference remains elusive, these bundles are likely effective, because they provide a clear and manageable set of expectations in a complex health care environment. Bundles help providers translate evidence into practice by summarizing and simplifying the strongest evidence, and providing reminders to adhere to evidence-based practices. The goal-oriented and multifaceted nature of bundles also provoke providers to adapt the care delivery system, specifically implement structural changes, and improve teamwork, resulting in improved patient outcomes.11
The process of developing a VAP prevention bundle is important given the increasing focus on VAP prevention as a national priority and because existing clinical guidelines make different recommendations, some of which may be contradictory. These differences likely reflect that guidelines are published at different times and based on the literature available at the time. In addition, the primary literature may be interpreted differently by expert panels. Furthermore, many guidelines lack practical advice to assist readers with implementation of the recommendations.23
While implementation of care bundles has been associated with improved outcomes, there are currently no published standards for bundle development. Concerns exist regarding existing VAP prevention bundles. Specifically, current VAP prevention bundles vary widely with respect to care practices and approach to development. The widely used Institute for Healthcare Improvement's (IHI) ventilator bundle, for example, was developed by a national “collaborative faculty's review of interventions a patient on mechanical ventilation should receive.”11 Though not specifically termed bundles, 14 evidence-based recommendations for VAP prevention, diagnosis, and treatment implemented in 11 Canadian ICUs, and an 8-item multi-faceted intervention implemented at an academic French ICU were developed by “multidisciplinary” panels.12,22 No further details were published regarding the development of the Canadian or French bundles, aside from reviewing available literature. In addition, a 7-item VAP prevention protocol decreased VAP in trauma patients, but the origins of this protocol are not described.14 In contrast, the development of a European VAP prevention bundle that uses multi-criteria decision analysis (MCDA) is described in detail;4 however, this bundle may not reflect the perceptions of US providers.
We described a structured approach for developing a proposed VAP prevention bundle. This approach has many advantages compared with other methodologies. First, the modified Delphi method minimized the potential impact of variability in guideline recommendations by galvanizing expert opinion from a very large and diverse group of experts and building a consensus regarding the most effective VAP prevention interventions. Variation in local practice was mitigated by involving experts and providers from multiple institutions, including those in community, rural, and academic settings around the US. By presenting the Delphi participants with a comprehensive list of candidate interventions, we anchored the process in the established body of VAP prevention knowledge. Participants responded anonymously, allowing open expression and critique of opinions, while eliminating the prospect of control by a few participants and biases from group interaction, which often occur during in-person panel discussions. The structured format to elicit and distill expert opinion permitted more formal control of information content and flow. The Delphi participants received full feedback from each round of the ratings, which gave them the opportunity to further comment on or modify their own selections. Consensus was achieved harmoniously, and the trail to consensus regarding bundle items is well-defined and retraceable.
In addition to content experts, our Delphi participants included front-line providers, specifically, physicians, nurses, respiratory therapists, and infection preventionists. Clinician perceptions regarding interventions provide important insights into the likelihood of provider compliance and engagement in efforts to improve intervention adherence. The number and breadth of health care providers who participated, both by type and experience, enriches the content, scope, and credibility of the final bundle.
Our approach has several limitations. First, our Delphi method included a relatively small number of all practicing clinicians. Nevertheless, 171 interdisciplinary members represent a large number of participants representing diverse specialties and settings, which provides confidence that the results reflect clinical priorities in the field. In addition, a 90% participation rate in each round of rating is exceptional,24 suggesting that the participants were committed to the process and thoughtful in performing their ratings.25 Nevertheless, it is possible the selected interventions may have slightly varied if a different panel of experts and providers participated.
Second, because we did not conduct a formal systematic review of the literature, it is possible that we missed an important VAP prevention intervention. To minimize this risk, we summarized all recommendations from published VAP prevention guidelines and recent primary literature. In addition, we provided Delphi participants an opportunity to nominate interventions we may have missed for inclusion in the first round of rating.
Third, we may have introduced misclassification (i.e., incorrectly including or dropping an intervention). To minimize the risk for misclassification, we provided an opportunity for Delphi participants to re-evaluate interventions dropped following the first round of rating, and to evaluate additional alternatives that emerged from the first round. The conference calls we held after completing the Delphi method also allowed the participants and our TEP members to discuss concerns about the interventions included in the VAP prevention bundle.
Finally, the validity of the proposed VAP prevention bundle will ultimately be determined by the providers' use of this bundle to evaluate current performance and successfully implement interventions to reduce the preventable morbidity, mortality, and costs of care associated with VAP. Further work is needed to develop explicit definitions and data collection tools for each bundle element, and to pilot test the proposed VAP prevention bundle to evaluate the feasibility of implementation and impact on outcomes.
Conclusion
We have presented a structured approach to elicit and distill clinical expert opinion in developing a new VAP Prevention Bundle. The number and breadth of health care providers who participated in the study, both by type and experience, also enriched the content, scope and credibility of the final VAP prevention bundle. Obtaining clinician input on what interventions to include increases the likelihood that providers will adhere to the bundle.
Highlights.
A new VAP prevention bundle using a systematic approach to elicit clinician perceptions on potential interventions for ventilator-associated pneumonia was developed.
65 potential interventions were identified through an extensive literature review.
A two-step Delphi method was implemented to gain consensus on the final list of interventions.
155 clinicians with expertise and interest in ventilator-associated pneumonia treatment and prevention participated in the process of consensus development.
5 process and 14 structural, or policy-driven, interventions were identified.
Acknowledgments
The authors thank Vipra Ghimire, MPH, for reviewing and editing the manuscript.
Footnotes
Competing Interests: Dr. Sean Berenholtz receives support from the National Institutes of Health (NIH) and Agency for Healthcare Research and Quality for grants and contracts focused on improving patient safety and quality, including VAP prevention; and receives honoraria and travel expenses from various hospitals and hospital associations for consulting and speaking on topics related to improving patient safety and quality. The other authors of this manuscript do not have any competing interest to disclose. This work has been funded by the NIH, grant R01HL105903.
Authors' Contributions: KS carried out the literature and guideline review, coordinated the modified Delphi method, and helped draft the manuscript. NR interpreted the results of the modified Delphi method and helped draft the manuscript. NW and HT carried out the literature and guideline review, implemented the modified Delphi method and carried out data collection. DF participated in the design of the study, and analysis and interpretation of its results. SB conceived of the study, participated in its design, interpretation of results, and helped draft the manuscript. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nishi Rawat, Email: nrawat1@jhmi.edu.
Noah C. Weiner, Email: weiner.noah@gmail.com.
Haddis G. Tujuba, Email: haddistujuba@gmail.com.
Donna Farley, Email: Donna.farley43@gmail.com.
Sean Berenholtz, Email: sberenho@jhmi.edu.
References
- 1.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: A systematic review. Crit Care Med. 2005;33(10):2184–2193. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 2.Bassetti M, Taramasso L, Giacobbe DR, Pelosi P. Management of ventilator-associated pneumonia: Epidemiology, diagnosis and antimicrobial therapy. Expert Rev Anti Infect Ther. 2012;10(5):585–596. doi: 10.1586/eri.12.36. [DOI] [PubMed] [Google Scholar]
- 3.Agrafiotis M, Siempos II, Ntaidou TK, Falagas ME. Attributable mortality of ventilator-associated pneumonia: A meta-analysis. Int J Tuberc Lung Dis. 2011;15(9):1154–63, i-v. doi: 10.5588/ijtld.10.0498. [DOI] [PubMed] [Google Scholar]
- 4.Rello J, Lode H, Cornaglia G, Masterton R VAP Care Bundle Contributors. A European care bundle for prevention of ventilator-associated pneumonia. Intensive Care Med. 2010;36(5):773–780. doi: 10.1007/s00134-010-1841-5. [DOI] [PubMed] [Google Scholar]
- 5.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 7.Muscedere J, Dodek P, Keenan S, et al. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia: Prevention. J Crit Care. 2008;23(1):126–137. doi: 10.1016/j.jcrc.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Coffin S, MD, Klompas M, MD, Classen D, MD, et al. Strategies to prevent Ventilator-Associated pneumonia in acute care hospitals. Infection Control and Hospital Epidemiology. 2008;29:S31–S40. doi: 10.1086/591062. S1, A Compendium of Strategies to Prevent Healthcare-Associated Infections in Acute Care Hospitals. [DOI] [PubMed] [Google Scholar]
- 9.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing healthcare-associated pneumonia, 2003: Recommendations of CDC and the healthcare infection control practices advisory committee. MMWR Recomm Rep. 2004;53:1–36. [PubMed] [Google Scholar]
- 10.Scales DC. Pneumonia in the ICU: A lethal or VAPid complication? Am J Respir Crit Care Med. 2011;184(10):1097–1098. doi: 10.1164/rccm.201109-1589ED. [DOI] [PubMed] [Google Scholar]
- 11.Resar R, Pronovost P, Haraden C, Simmonds T, Rainey T, Nolan T. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. Jt Comm J Qual Patient Saf. 2005;31(5):243–248. doi: 10.1016/s1553-7250(05)31031-2. [DOI] [PubMed] [Google Scholar]
- 12.Bouadma L, Deslandes E, Lolom I, et al. Long-term impact of a multifaceted prevention program on ventilator-associated pneumonia in a medical intensive care unit. Clin Infect Dis. 2010;51(10):1115–1122. doi: 10.1086/656737. [DOI] [PubMed] [Google Scholar]
- 13.Berenholtz SM, Pham JC, Thompson DA, et al. Collaborative cohort study of an intervention to reduce ventilator-associated pneumonia in the intensive care unit. Infect Control Hosp Epidemiol. 2011;32(4):305–314. doi: 10.1086/658938. [DOI] [PubMed] [Google Scholar]
- 14.Heimes J, Braxton C, Nazir N, et al. Implementation and enforcement of ventilator-associated pneumonia prevention strategies in trauma patients. Surg Infect (Larchmt) 2011;12(2) doi: 10.1089/sur.2009.028. [DOI] [PubMed] [Google Scholar]
- 15.Sinuff T, Muscedere J, Cook DJ, et al. Implementation of clinical practice guidelines for ventilator-associated pneumonia: A multicenter prospective study. Crit Care Med. 2013;41(1):15–23. doi: 10.1097/CCM.0b013e318265e874. [DOI] [PubMed] [Google Scholar]
- 16.Kollef MH. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med. 2004;32(6):1396–1405. doi: 10.1097/01.ccm.0000128569.09113.fb. [DOI] [PubMed] [Google Scholar]
- 17.Klompas M. Ventilator-associated pneumonia: Is zero possible? Clin Infect Dis. 2010;51(10):1123–1126. doi: 10.1086/656738. [DOI] [PubMed] [Google Scholar]
- 18.Zilberberg MD, Shorr AF, Kollef MH. Implementing quality improvements in the intensive care unit: Ventilator bundle as an example. Crit Care Med. 2009;37(1):305–309. doi: 10.1097/CCM.0b013e3181926623. [DOI] [PubMed] [Google Scholar]
- 19.Linstone HA, Turoff M. The Delphi method: Techniques and applications. London: Addison-Wesley; 1975. [Google Scholar]
- 20.Brown BB. Delphi process: A methodology used for the elicitation of opinions of experts. 1968 [Google Scholar]
- 21.Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: A model for large scale knowledge translation. BMJ. 2008;337:a1714. doi: 10.1136/bmj.a1714. [DOI] [PubMed] [Google Scholar]
- 22.Sinuff T, Muscedere J, Cook D, Dodek P, Heyland D Canadian Critical Care Trials Group. Ventilator-associated pneumonia: Improving outcomes through guideline implementation. J Crit Care. 2008;23(1):118–125. doi: 10.1016/j.jcrc.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Goutier J, Holzmueller C, Edwards K, Klompas M, Speck K, Berenholtz S. Strategies to enhance adoption of ventilator-associated pneumonia interventions: A systematic literature review. Infect Control Hosp Epidemiol. 2014 doi: 10.1086/677152. In press. [DOI] [PubMed] [Google Scholar]
- 24.Witkin BR, Altschuld JW. Planning and conducting needs assessment: A practical guide. United States of America: Sage Publications, Inc; 1984. [Google Scholar]
- 25.Ludwig B. Predicting the future: Have you considered using the Delphi methodology? Journal of Extension. 1997;35(5) May, 17, 2013. [Google Scholar]