Abstract
Pulmonary and cardiovascular dysfunctions are leading causes of morbidity and mortality in patients with chronic Spinal Cord Injury (SCI). Impaired respiratory motor function and decreased Baroreflex Sensitivity (BS) are predictors for the development of cardiopulmonary disease. This observational case-controlled clinical study was undertaken to investigate if respiratory motor control deficits in individuals with SCI affect their ability to perform the Valsalva maneuver, and to determine if a sustained Maximum Expiratory Pressure (MEP) effort can serve as an acceptable maneuver for determination of the BS in the event that the Valsalva maneuver cannot be performed. The BS outcomes (ms/mmHg) were obtained using continuous beat-to-beat arterial blood pressure (BP) and heart rate (HR) recordings during Valsalva or MEP maneuvers in thirty nine individuals with chronic C3-T12 SCI. Twenty one participants (54%) reported signs of intolerance during the Valsalva maneuver and only 15 individuals (39%) were able to complete this task. Cervical level of injury was a significant risk factor (p = .001) for failing to complete the Valsalva maneuver, and motor-complete injury was a significant risk factor for symptoms of intolerance (p = .04). Twenty eight participants (72%) were able to perform the MEP maneuver; the other 11 participants failed to exceed the standard airway pressure threshold of 27 cmH2O. Neither level nor completeness of injury were significant risk factors for failure of MEP maneuver. When the required airway pressure was sustained, there were no significant differences between BS outcomes obtained during Valsalva and MEP maneuvers. The results of this study indicate that individuals with high-level and motor-complete SCI are at increased risk of not completing the Valsalva maneuver and that baroreflex-mediated responses can be evaluated by using sustained MEP maneuver when the Valsalva maneuver cannot be performed.
Keywords: Spinal cord injury, Respiratory function, Valsalva maneuver, Autonomic regulation, Baroreflex
Introduction
In the United States, among the 275,000 people who are living with chronic SCI (Devivo, 2012; NSCISC, 2014), cardiovascular and pulmonary dysfunctions are leading causes of morbidity and mortality (Eigenbrodt et al., 2000; Frankel et al., 1998; Garshick et al., 2005; Walter et al., 2002). After SCI, impairment to descending sympathetic pathways causes decreased baroreflex sensitivity (BS) associated with poor baroreflex-mediated cardiovascular responses and deterioration in the inability to control heart rate (HR) in response to blood pressure (BP) changes (Grimm et al., 1998; Phillips et al., 2012; Wecht et al., 2003). This is pertinent in a population predisposed to cardiovascular disease, as analysis of BS is predictive of future cardiovascular events (Koutelou et al., 2009; La Rovere et al., 1998): a blunted baroreceptor reflex can increase the risk of myocardial infarction and stroke due to poorly regulated BP (La Rovere et al., 2008). Accurate assessment of cardiovascular function via baroreceptor control is therefore an important tool in the management of cardiovascular dysfunction secondary to SCI.
Several non-invasive methods have been developed for evaluating BS, including the analysis of reflex responses to pharmacological or mechanical manipulations of baroreceptors, or analysis of spontaneously occurring changes in BP and HR (Osterhues et al., 2000). A standard approach that can be used to measure spontaneous BS without major redistribution of blood volumes is the Valsalva maneuver (Gorlin et al., 1957; Grimm et al., 1998; Novak, 2011; Porth et al., 1984), a forced expiration with a closed mouth and nose, during which an airway pressure of at least 27 cmH2O (20 mmHg) is sustained for 15 – 20s (Benarroch et al., 1991; Porth et al., 1984; Walker and Cutting, 2010). The increase in intrathoracic pressure decreases venous return and cardiac output which results in cardiovagal tone withdrawal during initial 4 – 7s (early phase II), followed by an increased sympathetic vasomotor activity during consecutive 13 – 16s (late phase II), and increased parasympathetic cardiac activity upon airway pressure release (phase IV) (Daroff and Aminoff, 2014; Kihara et al., 1998; Novak, 2011; Persson and Kirchheim, 1991; Sandroni et al., 1991). This maneuver is repeatable, non-invasive, and easily administered bedside and therefore is a common clinical test used in both the non-injured and SCI populations (Airaksinen et al., 1993; Grimm et al., 1998; Phillips et al., 2012; Previnaire et al., 2012; Rostagno et al., 1999; Vogel et al., 2005). Adequate performance of the Valsalva maneuver requires considerable expiratory effort associated with recruitment of expiratory muscles. In SCI, however, the ability to recruit these muscles for forced expiration is impaired and depends on the level of injury, with cervical and upper thoracic levels experiencing significantly diminished airway pressure generation as a result (Ovechkin et al., 2010; Schilero et al., 2009). It would logically follow that patients with cervical and high-thoracic injuries would have trouble performing the Valsalva maneuver. However, there are no reports in the literature to indicate whether SCI would render the Valsalva maneuver difficult (Grimm et al., 1998; Johnson et al., 1969; Previnaire et al., 2012). Our previous work has shown that the majority of individuals with chronic SCI are able to generate substantial and sustained airway pressure during the MEP maneuver (Aslan et al., 2013; Ovechkin et al., 2010).
The purpose of this study was thus to investigate if individuals with SCI are able to perform the Valsalva maneuver, and to determine if the MEP maneuver can serve as an acceptable replacement for determination of BS in the event that the Valsalva maneuver could not be performed. To be consistent with the clinical standards for the Valsalva maneuver, the MEP effort required participants to sustain an expiratory airway pressure above the standard threshold level of 27 cmH2O, equal in duration to early phase II of the Valsalva maneuver (Kihara et al., 1998; Novak, 2011; Persson and Kirchheim, 1991; Sandroni et al., 1991). We hypothesized that the BS responses to the MEP maneuver during phases II and IV are representative of those observed during early phase II and phase IV of Valsalva maneuver.
2. Methods
2.1 Demographic and clinical characteristics
Research participants were recruited from the outpatient pool at Frazier Rehabilitation and Neuroscience Institute in Louisville, KY, or were referred to the study by a clinician. After approval by the University of Louisville Institutional Review Board, participants were deemed eligible to participate after a clinical evaluation found them to be ventilator-independent and free of cardiovascular or pulmonary diseases unrelated to SCI. Eleven subjects were female and twenty eight were male, 67 ± 88 (mean ± SD) months post injury, 38 ± 13 years of age. The International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI) (Kirshblum et al., 2011) was used to determine the neurological level and clinical severity of the spinal cord lesion according to the American Spinal Cord Injury Association Impairment Scale (AIS) (Kirshblum et al., 2011). For this study, 23 SCI participants were classified as clinically motor-complete (AIS-A or AIS-B) and 16 were motor-incomplete (AIS-C or AIS-D), with neurological levels of SCI ranging from C3 to T12. (Table 1).
Table 1.
Participant Demographics and Performance of Valsalva and MEP maneuvers
| Subjects (n=39) | Age (years) | Sex | Height (in) | Weight (Ibs) | Level of SCI | AIS category | Time after SCI (mo) | Completion | Signs of intolerance of Valsalva | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Valsalva | MEP | |||||||||||
| Cervical (n=27) | Complete (n=16) | A58 | 40 | M | 70 | 230 | C3 | A | 21 | N | N | P |
| B18 | 56 | M | 72 | 155 | C3 | B | 29 | N | Y | AD | ||
| A54 | 32 | F | 68 | 125 | C4 | A | 114 | N | Y | |||
| A64 | 49 | M | 75 | 155 | C4 | A | 405 | N | N | AD | ||
| A65 | 29 | M | 68 | 180 | C4 | A | 10 | N | Y | |||
| B06 | 42 | F | 67 | 123 | C4 | B | 70 | N | N | |||
| B16 | 60 | M | 71 | 220 | C4 | B | 31 | N | Y | |||
| B21 | 30 | M | 73 | 165 | C4 | B | 64 | N | Y | |||
| B22 | 51 | M | 70 | 180 | C4 | B | 37 | N | Y | P | ||
| A67 | 30 | F | 65 | 128 | C5 | A | 48 | N | N | P | ||
| A71 | 25 | F | 68 | 195 | C5 | A | 85 | Y | Y | AD | ||
| B11 | 25 | M | 70 | 185 | C5 | B | 119 | N | N | P | ||
| B19 | 40 | M | 74 | 177 | C6 | B | 6 | N | Y | S | ||
| A68 | 34 | M | 70 | 125 | C7 | A | 33 | Y | Y | P | ||
| B24 | 21 | M | 67 | 125 | C7 | B | 25 | N | N | P | ||
| A69 | 28 | M | 72 | 165 | C8 | A | 44 | N | Y | P | ||
| Incomplete (n=11) | C33 | 59 | M | 69 | 145 | C2 | C | 6 | N | Y | ||
| D35 | 60 | M | 71 | 220 | C2 | D | 35 | Y | Y | |||
| D38 | 42 | M | 70 | 245 | C2 | D | 155 | Y | Y | |||
| C27 | 58 | M | 70 | 190 | C4 | C | 47 | N | N | |||
| C30 | 19 | F | 67 | 94 | C4 | C | 12 | N | N | P | ||
| C31 | 24 | F | 66 | 108 | C4 | C | 27 | N | N | AR | ||
| C34 | 20 | M | 16 | 140 | C4 | C | 53 | N | N | P | ||
| C38 | 18 | M | 72 | 130 | C5 | D | 420 | N | Y | P | ||
| D42 | 72 | M | 71 | 230 | C5 | D | 33 | Y | Y | P, AR | ||
| C26 | 33 | M | 72 | 165 | C6 | C | 4 | N | Y | S | ||
| D40 | 33 | M | 31 | 135 | C7 | D | 39 | Y | Y | |||
| Thoracic (n=12) | Complete (n=7) | B20 | 28 | M | 65 | 128 | T2 | B | 53 | N | Y | S |
| A57 | 27 | F | 73 | 165 | T4 | A | 45 | N | N | p | ||
| A59 | 26 | M | 73 | 140 | T4 | A | 27 | Y | Y | AD | ||
| A70 | 32 | M | 74 | 210 | T4 | A | 50 | Y | Y | |||
| B25 | 43 | F | 61 | 125 | T4 | B | 52 | N | Y | P, AR | ||
| A66 | 48 | M | 72 | 170 | T6 | A | 82 | Y | Y | |||
| A55 | 35 | M | 68 | 165 | T11 | A | 50 | Y | Y | |||
| Incomplete (n=5) | C32 | 35 | M | 76 | 201 | T2 | C | 67 | Y | Y | ||
| C28 | 29 | M | 70 | 160 | T5 | C | 56 | Y | Y | |||
| D41 | 44 | F | 65 | 180 | T8 | D | 40 | Y | Y | |||
| C24 | 40 | F | 68 | 125 | T11 | C | 110 | Y | Y | |||
| C39 | 45 | F | 68 | 135 | T11 | C | 26 | Y | Y | |||
| Mean ± SD | 38 ± 13 | N/A | 69 ± 7 | 163 ± 37 | N/A | N/A | 67 ± 88 | N/A | N/A | N/A | ||
Signs of intolerance of Valsalva maneuver: presyncope (P), syncope (S), arrhythmias (AR), and/or bouts of autonomic dysreflexia (AD)
2.2 Protocol
Participants were assessed in a seated position with no compression garments or abdominal binders. To perform the Valsalva and the MEP maneuvers, the participants were instructed to inspire to total lung capacity and blew through a three-way valve system (Airlife 001504) that incorporates a 1.5 mm diameter leak to prevent glottic closure and to reduce buccal muscle contraction (Griffiths and McConnell, 2007; Smyth et al., 1984). Cheeks were not held during the maneuvers. The thresholds for successful completion of Valsalva maneuver included generation of 27cmH2O, a required minimum to create enough intrathoracic pressure to substantially reduce venous return to the heart, sustained for 15s (Benarroch et al., 1991). To ensure that these requirements were met, the research subjects were asked to sustain 30cmH2O for a period of 20s. To be consistent with standard requirements for airway pressure generation and length of early phase II of Valsalva maneuver, successful completion of the MEP maneuver required generation of 27cmH2O or more to be sustained for 5s. Each Valsalva and MEP maneuver was repeated three times, with 1-min relaxation period in between maneuvers to allow BP to return to baseline levels. Research subjects were instructed to breathe normally and refrain from nonessential movement or talking between attempts. The attempt was discontinued if symptoms of arterial hypotension, presyncope, syncope, arrhythmia, and/or bouts of autonomic dysreflexia (“signs of intolerance”) were self-reported and/or examiner-identified. Participants were asked during the rest between maneuvers if they experienced any nausea, blurred or spotty vision, dizziness, or tingling in the limbs (categorized as “presyncope,”) or a loss of consciousness (categorized as “syncope”).
2.3 Data acquisition
Data were converted from analog to digital signals using a Powerlab 16/35 system, recorded by LabChart 7 (AD Instruments, Denver, CO). To record airway pressure an MP45 low-pressure transducer system (Validyne Engineering, Northridge, CA) connected to the three-way valve system (Airlife 001504) was used. During the Valsalva maneuver, in order to sustain the required airway pressure, an additional tube was connected to a M4102 pressure gauge (Boehringer Laboratories, Phoenixville, PA) which allowed the participant to monitor his or her airway pressure.
Systolic BP (SBP), diastolic BP (DBP), and HR were acquired continuously from a finger cuff using Portapres-2 (Finapres Medical System B.A., Netherlands) and ML880 PowerLab 16/30 (ADInstruments, Colorado Springs, CO) systems. The ML132 three-lead II electrodes (ADInstruments) were used to record the electrocardiogram (ECG). The finger BP waveforms were calibrated using brachial BP values that were assessed simultaneously by V100 cuff system (GE’s Dinamap Patient Monitor, Boston, MA) (Currie et al., 2015). Hemodynamic variables were acquired at 1000 Hz using LabChart 7 (AD Instruments, Denver, CO). Unidentified data sets were evaluated for consistence with airway pressure/duration threshold requirements, arrhythmias, and symptoms of intolerance.
2.4 Baroreflex assessment
The SBP values were plotted against the following R-R interval (RRI) using a linear regression analysis and a Pearson correlation. The resulting slope (ms/mmHg) and Pearson correlation coefficient (r) quantify BS by describing how rapidly (slope) and consistently (r) the receptors change HR in response to a change in SBP (Goldstein et al., 1982; Porth et al., 1984; Smith et al., 1987a; Smith et al., 1987b). SBP and RRI values were calculated by Labchart from the BP waveform and ECG, respectively. The phases were excluded from analysis if they triggered spasms, autonomic dysreflexia, or coughs. The mean BS of all acceptable Valsalva and MEP attempts for each subject was included in the analysis. We evaluated the period beginning with the first three consecutively increasing RRIs and ending with the peak SBP. All periods with fewer than 5 heart beats were excluded from the analysis (Palmero et al., 1981; Smith et al., 1987a).
2.5 Statistical analysis
Logistic regression models were used to determine factors that influenced the odds of failure and odds of a sign or signs of intolerance. A Valsalva maneuver was deemed a failure in the model if an airway pressure of 27cmH2O was not sustained for at least 15s; a MEP maneuver was deemed a failure in the model if an airway pressure of 27cmH2O was not sustained for at least 5s. Signs of intolerance were grouped into one dichotomous variable and were considered present if the participant experienced symptoms of syncope, presyncope, autonomic dysreflexia, or ECG arrhythmias during or within 30s following the maneuvers. Factors of interest were neurological level of injury (cervical or thoracic), and AIS impairment category (motor-complete or motor-incomplete). Covariates for all logistic regression models included sex, height, weight, age, and time after injury (months).
Linear regressions of SBP and RRI during phases II and IV were used to determine the BS (ms/mmHg); slopes with an r > .80 were included for analysis. BS outcomes from phases II and IV of the Valsalva maneuver were compared to phases II and IV of the MEP maneuver with a two-way ANOVA with a within-subjects correction (n=15). To be included for comparison, the Valsalva maneuvers must have been sustained without episodes of autonomic dysreflexia or arrhythmias (Benarroch et al., 1991).
To compare BS outcomes of the MEP to further determine accuracy and eliminate a sampling bias, participants were sorted into groups based on performance of the Valsalva maneuver (yes or no) and standard airway pressure generation (yes ≥ 27 cmH2O ≥ no). This allowed us to compare BS responses of those that could perform the Valsalva maneuver (group 1, n=15): i.e., those participants most likely to have identical BS outcomes between the Valsalva and MEP maneuvers) to participants that could not perform a Valsalva maneuver but could sustain the minimum required pressure (group 2, n=13). An ANOVA was used to test for significance; and data are presented as mean ± SD. Significance was set to α < .05. All analyses were performed using the open-source R 3.0.2 statistical computing software (R Development Core Team, 2013).
Results
3.1 Valsalva and MEP maneuvers
Of the 39 participants that attempted a Valsalva maneuver, 15 subjects (38%; predominantly with low-level motor-incomplete SCI) were able to perform it for the required duration (19 ± 2s) and pressure generation (36 ± 6cmH2O): 11 without signs of intolerance and 4 with symptoms of presyncope or autonomic dysreflexia during the maneuver. 24 participants (62%) were not able to complete the Valsalva maneuver: 15 experienced either syncope, presyncope, and/or ECG arrhythmias; in 2 participants the Valsalva maneuver triggered bouts of autonomic dysreflexia. The remaining 7 participants experienced no signs of intolerance, but were unable to sustain the maneuver for the minimum requirement of 15 sec (Table 1). The logistic regression analysis determined: 1) a significant relationship between cervical injuries and increased risk of failure of the Valsalva maneuver when controlling for AIS category; 2) irrespective of injury level, significant risk of signs of intolerance during this maneuver is associated with motor-completeness of SCI; and finally 3) individuals with cervical motor-complete injuries were at significant risk of failure of the Valsalva maneuver when compared to those with cervical, motor-incomplete injuries (Table 2). The remaining covariates were found to have neither a significant relationship with odds of failure nor sign of intolerance. The MEP maneuver was performed by all participants with no signs of intolerance; airway pressure above threshold (59 ± 24cmH2O) was sustained for 6 ± 0.7s in 17 of 27 participants with cervical SCI and 11 of 12 subjects with thoracic SCI (Table 1). Finally, the logistic regression analysis determined no significant risk of insufficient airway pressure generation during the MEP maneuver (Table 2).
Table 2.
Logistic Regression Models and Risk of Event Occurrence
| Risk Factor | Failure of Valsalva Maneuver | Intolerance of Valsalva Maneuver | Failure of MEP Maneuver | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | p-value | Odds Ratio | 95% Confidence Interval | p-value | Odds Ratio | 95% Confidence Interval | p-value | |
| Cervical vs. Thoracic* SCI |
23 | 3 – 155 | 0.002 | 3 | 1 – 17 | 0.13 | 8 | 1 – 151 | 0.1 |
| Motor-complete vs. Motor-Incomplete* SCI |
5 | 1 – 36 | 0.09 | 5 | 1 – 20 | 0.04 | 2 | 0.3 – 8 | 0.5 |
| Cervical motor-complete vs. Cervical motor-incomplete*† SCI |
8 | 2 – 56 | 0.02 | 3 | 1 – 14 | 0.07 | 3 | 1 – 12 | 0.15 |
| Thoracic motor-complete vs. Thoracic motor-Incomplete* SCI |
< 1 | 0.1 – 2 | 0.21 | 1 | 0.3 – 8 | 0.73 | < 1 | 0.1 – 2 | 0.32 |
Represents reference factor
3.2 Baroreflex sensitivity (BS)
There were no significant differences found between the BS obtained from phase II or phase IV when comparing between maneuvers (Fig. 2). An ANOVA of the data that included one outlier above three SDs from the mean (Maronna et al., 2006) determined that there were no significant differences between BS outcomes of the MEP and participants grouped by Valsalva ability and airway pressure generation, thus participants that could not sustain a Valsalva maneuver still generated a BS during early phase II that was not significantly different from the responses of those that successfully completed the Valsalva maneuver (Table 3).
Fig. 2.
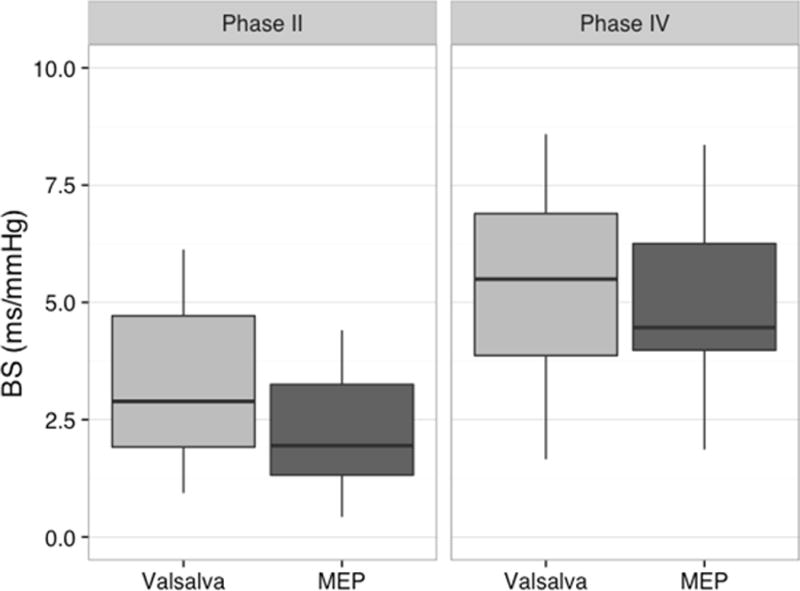
Box and whisker plots of baroreflex sensitivity (BS) during phases II and IV of the Valsalva and maximum expiratory pressure (MEP) maneuvers (n=15). Note that there were no significant differences between values obtained during related phases of the Valsalva and MEP maneuvers.
Table 3.
Baroreflex Sensitivity (BS) During Phase II and Phase IV of the MEP maneuver
| Groups | MEP Phase | |
|---|---|---|
| II | IV | |
| 1: BS of participants that could sustain a Valsalva maneuver, and generate an ≥ 27cmH2O | 2.7 ± 1.3 | 4.7 ± 1.9 |
| 2: BS of participants that could not sustain a Valsalva maneuver, but could generate ≥ 27cmH2O | 3.0 ± 3.0 | 5.8 ± 2.3 |
Discussion
The results of this study indicate that the MEP maneuver engages the cardiac loop of the baroreceptor reflex in the same manner as the Valsalva maneuver. We have demonstrated for the first time that assessment of baroreflex responses during the MEP maneuver can be a valuable tool to evaluate autonomic regulation in persons with respiratory motor control deficits secondary to SCI.
Our review of the literature found others who used the Valsalva maneuver to test BS in the SCI population, but there was no mention of the difficulty, if any, that their patients experienced while performing the maneuver. A study of AIS-motor complete tetraplegics and paraplegics reported successful performance of the Valsalva maneuver, even in participants that experienced bouts of orthostatic hypotension during head-up tilt tests (Johnson et al., 1969); while others report use of the Valsalva maneuver in tetraplegic (Previnaire et al., 2012) and paraplegic (Grimm et al., 1998) participants to assess cardiovascular reflex integrity. However, our results indicate that the Valsalva maneuver is difficult for injured persons to perform: less than a third of our participants were able to perform it, and half the participants experienced signs of intolerance during or following the maneuver. The logistic regression found that independent of AIS score cervical participants were 40 times more likely to fail at the Valsalva maneuver than participants with thoracic injuries (p = .002). Within the cervical group, individuals with motor-complete SCI were eight times more likely to fail this maneuver than individuals with motor-incomplete injury (p = .02). This is most likely due to the decreased respiratory motor control seen in cervical participants, particularly those with cervical motor-complete injuries, who have few to no unimpaired forced expiratory muscles available to assist with a sustained airway pressure generation. Upper thoracic participants also had trouble performing the Valsalva maneuver, though the differences vs. those with cervical lesions were not significant: 4 of 8 did not succeed and experienced syncope (1), presyncope (1), or autonomic dysreflexia (2). We suspect that a relationship probably actually exists between upper thoracic lesions and inability to perform the Valsalva maneuver that could be elucidated with more participants. The respiratory motor control deficits in SCI therefore make the Valsalva maneuver difficult to sustain, which would cloud interpretation of the results, as BS outcomes are dependent on a steady airway pressure (Benarroch et al., 1991).
There was a significant relationship between motor-complete SCI and signs of intolerance (p < .05), indicating that all motor-complete participants, irrespective of level of injury, were equally likely to experience a sign of intolerance following the Valsalva maneuver. This is likely due to the increased vasomotor tone required during late phase II to maintain BP: thoracic motor-complete participants would still have impaired sympathetic output to the peripheral vasculature, increasing the risk of presyncope, syncope, or autonomic dysreflexia following the maneuver. The Valsalva maneuver is therefore not as easily performed in the SCI population as the literature suggested: 2 out of 3 participants were unable to perform it, and half of those that attempted it experienced an adverse event.
Performance of the MEP maneuver, however, elicited no signs of intolerance, and our analysis demonstrated that it generated BS outcomes similar to the Valsalva maneuver provided the maximum airway pressure was above 27cmH2O: no significant differences were detected between BS slopes when comparing similar phases between maneuvers, and even those that could not sustain a Valsalva maneuver generated similar BS outcomes during the MEP maneuver. The MEP maneuver is most likely eliciting the same reflex responses despite the shorter duration because of the increased airway pressure generation and its subsequent decrease in venous return, which could more efficiently engage the reflexes. Our results further confirm that the magnitude of BS slope depends not upon the duration of the maneuvers but the changes in intrathoracic pressure that decrease cardiac output (Benarroch et al., 1991). The airway pressure during phase II of MEP maneuver was significantly higher compared to the pressure during Valsalva maneuver, however, BS outcomes during these maneuvers were not significantly different. Although there is no upper limit for the airway pressure generation for Valsalva maneuver, it is necessary to validate normative BS responses during MEP maneuver at specific air pressure levels before it can be used as a standard tool.
It is difficult to pinpoint the exact reason for the autonomic dysfunction in our group of participants: phases II and IV of Valsalva maneuver have been attributed to the sympathetic and parasympathetic activation, respectively, but it is not so easy to isolate sympathetic from parasympathetic activity, nor cardiac response from vascular response in persons with SCI. There is a delay in sympathetic engagement following onset of the maneuver (as many as 3 seconds) (Benarroch et al., 1991) and varying degrees of sympathetic impairment and vagal withdrawal common in SCI (Previnaire et al., 2009; Wecht et al., 2006). In addition, recruitment of the vasculature depends upon on airway pressure generation and available sympathetic vasomotor efferents (Benarroch et al., 1991), both of which can be impaired in SCI; in total, this would subsequently affect the BS obtained during phases II and IV. It is therefore safest to assume that the BS outcomes obtained from our participants, in both the Valsalva and MEP maneuvers, result from available sympathetic engagement combined with vagal withdrawal of HR, with minimal recruitment of the vasculature.
While the BS outcomes from the MEP can be used to quantify HR response and demonstrate BS impairment in SCI, the validation of this maneuver has not been achieved, particularly because there is no upper limit to airway pressure generated during the maneuver. Because of that, it is not known if increases in airway pressure, particularly those above what our participants were able to generate, would impede venous return more and subsequently increase sympathetic engagement, altering BS outcomes. We would hope to compare the BS outcomes generated in non-injured, healthy persons, to see if and how BS changes with increases in airway pressure. The creation of a standard MEP response would not only better elucidate the balance of sympathetic and parasympathetic activity, but it would allow the MEP maneuver to be utilized in a variety of cardiopulmonary diseases that would make performance of the Valsalva maneuver difficult.
Assessment of baroreceptor activity is an important clinical tool as it not only describes autonomic and cardiovascular function, but also is predictive of future cardiovascular events (Koutelou et al., 2009; La Rovere et al., 1998). The MEP maneuver is a viable and novel addition to the battery of autonomic tests currently used to assess BS in SCI, especially in case of severe high-level injuries where patients would be incapable of performing the Valsalva maneuver, and for whom accurate assessment of BS is crucial.
Study limitations
Unequal distribution of neurological injury levels limited this study: when we attempted to compare outcomes between cervical, upper-, and lower-thoracic injuries, there were no significant differences found, despite the assumption that the amount of spared autonomic pathways between groups is different. This could most likely be remedied with a greater sample size, particularly in the thoracic SCI group. Finally, we did not measure the amount of damage to sympathetic networks, which decreased the precision of analysis.
Conclusion
The results of this study indicate that individuals with high-level and motor-complete SCI are at increased risk of not completing the Valsalva maneuver. Baroreflex-mediated responses can be evaluated by using sustained MEP maneuver when the Valsalva maneuver cannot be performed.
Fig. 1.
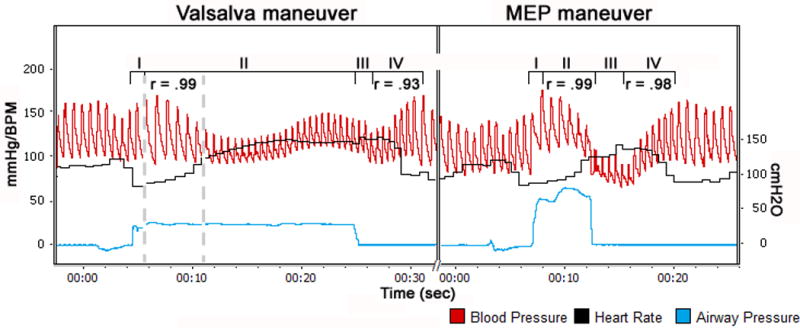
Changes in arterial blood pressure (red); airway pressure (blue) and heart rate (black) during Valsalva maneuver (left) and Maximum Expiratory Pressure (MEP) maneuver (right) in individual with T11 AIS-A SCI (A55). Phases are labeled above the BP traces. Dashed vertical lines represent early phase II of the Valsalva maneuver. Correlation coefficients (r) between beat-to-beat systolic blood pressure and R-R interval are given for each phase.
Acknowledgments
We express our deep appreciation to Steven R. Williams, MD; Carie Z. Tolfo, PT, DPT and Jamie Ochsner, PT for the clinical support of this study; to Eddie Brown, Manpreet Chopra, and Goutam Singh for data collection and processing; and to Paul Ditterline for statistical assistance. This work was funded by Kentucky Spinal Cord and Head Injury Research Trust 9-10A; Christopher and Dana Reeve Foundation OA2-0802; Craig H. Neilsen Foundation 1000056824; U.S. Department of Education and Department of Health and Human Services H133N110007; and National Institutes of Health 1R01HL103750 and P30GM103507 grants.
References
- Airaksinen KE, Hartikainen JE, Niemela MJ, Huikuri HV, Mussalo HM, Tahvanainen KU. Valsalva manoeuvre in the assessment of baroreflex sensitivity in patients with coronary artery disease. Eur Heart J. 1993;14:1519–1523. doi: 10.1093/eurheartj/14.11.1519. [DOI] [PubMed] [Google Scholar]
- Aslan SC, Chopra MK, McKay WB, Folz RJ, Ovechkin AV. Evaluation of Respiratory Muscle Activation Using Respiratory Motor Control Assessment (RMCA) in Individuals with Chronic Spinal Cord Injury. J Vis Exp. 2013;77:1–10. doi: 10.3791/50178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE, Opfer-Gehrking TL, Low PA. Use of the photoplethysmographic technique to analyze the valsalva maneuver in normal man. Muscle & Nerve. 1991;14:1165–1172. doi: 10.1002/mus.880141204. [DOI] [PubMed] [Google Scholar]
- Currie KD, Wong SC, Warburton DE, Krassioukov AV. Reliability of the sit-up test in individuals with spinal cord injury. J Spinal Cord Med. 2015 doi: 10.1179/2045772315Y.0000000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daroff RB, Aminoff MJ. Encyclopedia of the Neurological Sciences. 2nd. Academic Press; 2014. 1 online resource (4740 pages) [Google Scholar]
- Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D. Orthostatic hypotension as a risk factor for stroke: the atherosclerosis risk in communities (ARIC) study, 1987–1996. Stroke. 2000;31:2307–2313. doi: 10.1161/01.str.31.10.2307. [DOI] [PubMed] [Google Scholar]
- Frankel HL, Coll JR, Charlifue SW, Whiteneck GG, Gardner BP, Jamous MA, Krishnan KR, Nuseibeh I, Savic G, Sett P. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord. 1998;36:266–274. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- Garshick E, Kelley A, Cohen SA, Garrison A, Tun CG, Gagnon D, Brown R. A prospective assessment of mortality in chronic spinal cord injury. Spinal Cord. 2005;43:408–416. doi: 10.1038/sj.sc.3101729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Horwitz D, Keiser HR. Comparison of techniques for measuring baroreflex sensitivity in man. Circulation. 1982;66:432–439. doi: 10.1161/01.cir.66.2.432. [DOI] [PubMed] [Google Scholar]
- Gorlin R, Knowles JH, Storey CF. The Valsalva maneuver as a test of cardiac function; pathologic physiology and clinical significance. Am J Med. 1957;22:197–212. doi: 10.1016/0002-9343(57)90004-9. [DOI] [PubMed] [Google Scholar]
- Griffiths LA, McConnell AK. The influence of inspiratory and expiratory muscle training upon rowing performance. Eur J Appl Physiol. 2007;99:457–466. doi: 10.1007/s00421-006-0367-6. [DOI] [PubMed] [Google Scholar]
- Grimm DR, Almenoff PL, Bauman WA, De Meersman RE. Baroreceptor sensitivity response to phase IV of the Valsalva maneuver in spinal cord injury. Clin Auton Res. 1998;8:111–118. doi: 10.1007/BF02267821. [DOI] [PubMed] [Google Scholar]
- Johnson RH, Smith AC, Spalding JM. Blood pressure response to standing and to Valsalva’s manoeuvre: independence of the two mechanisms in neurological diseases including cervical cord lesions. Clinical science. 1969;36:77–86. [PubMed] [Google Scholar]
- Kihara M, Takahashi M, Nishimoto K, Okuda K, Matsui T, Yamakawai T, Okumura A. Autonomic dysfunction in elderly bedfast patients. Age Ageing. 1998;27:551–555. doi: 10.1093/ageing/27.5.551. [DOI] [PubMed] [Google Scholar]
- Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Mulcahey MJ, Schmidt-Read M, Waring W. International standards for neurological classification of spinal cord injury (revised 2011) J Spinal Cord Med. 2011;34:535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutelou M, Katsikis A, Flevari P, Theodorakis G, Livanis E, Georgiadis M, Voudris V, Kremastinos D. Predictive value of cardiac autonomic indexes and MIBG washout in ICD recipients with mild to moderate heart failure. Annals of Nuclear Medicine. 2009;23:677–684. doi: 10.1007/s12149-009-0289-6. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Raczak G. Baroreflex sensitivity: measurement and clinical implications. Ann Noninvasive Electrocardiol. 2008;13:191–207. doi: 10.1111/j.1542-474X.2008.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maronna RA, Yohai VJ, Martin RD. Robust statistics: theory and methods. John Wiley & Sons; Chichester: 2006. [Google Scholar]
- Novak P. Assessment of sympathetic index from the Valsalva maneuver. Neurology. 2011;76:2010–2016. doi: 10.1212/WNL.0b013e31821e5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSCISC. The 2014 Annual Statistical Report for the Spinal Cord Injury Model Systems. University of Alabama; Birmingham: 2014. [Google Scholar]
- Osterhues HH, Hombach V, Moss AJ, SpringerLink (Online service) Developments in Cardiovascular Medicine. Springer; Netherlands, Dordrecht: 2000. Advances in Noninvasive Electrocardiographic Monitoring Techniques. 1 online resource. [Google Scholar]
- Ovechkin A, Vitaz T, Terson de Paleville D, Aslan S, McKay W. Evaluation of respiratory muscle activation in individuals with chronic spinal cord injury. Respir Physiol Neurobiol. 2010;173:171–178. doi: 10.1016/j.resp.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Palmero HA, Caeiro TF, Iosa DJ, Bas J. Baroreceptor reflex sensitivity index derived from Phase 4 of te Valsalva maneuver. Hypertension. 1981;3:II-134–137. doi: 10.1161/01.hyp.3.6_pt_2.ii-134. [DOI] [PubMed] [Google Scholar]
- Persson PB, Kirchheim HR. Baroreceptor reflexes: integrative functions and clinical aspects. Springer-Verlag, Berlin; London: 1991. [Google Scholar]
- Phillips AA, Krassioukov AV, Ainslie PN, Warburton DE. Baroreflex function after spinal cord injury. J Neurotrauma. 2012;29:2431–2445. doi: 10.1089/neu.2012.2507. [DOI] [PubMed] [Google Scholar]
- Porth CJ, Bamrah VS, Tristani FE, Smith JJ. The Valsalva maneuver: mechanisms and clinical implications. Heart Lung. 1984;13:507–518. [PubMed] [Google Scholar]
- Previnaire JG, Soler JM, El Masri W, Denys P. Assessment of the sympathetic level of lesion in patients with spinal cord injury. Spinal Cord. 2009;47:122–127. doi: 10.1038/sc.2008.87. [DOI] [PubMed] [Google Scholar]
- Previnaire JG, Soler JM, Leclercq V, Denys P. Severity of autonomic dysfunction in patients with complete spinal cord injury. Clin Auton Res. 2012;22:9–15. doi: 10.1007/s10286-011-0132-8. [DOI] [PubMed] [Google Scholar]
- Rostagno C, Felici M, Caciolli S, Olivo G, Comeglio M, Galanti G, Serneri GG. Decreased baroreflex sensitivity assessed from phase IV of Valsalva maneuver in mild congestive heart failure. Angiology. 1999;50:655–664. doi: 10.1177/000331979905000806. [DOI] [PubMed] [Google Scholar]
- Sandroni P, Benarroch EE, Low PA. Pharmacological dissection of components of the Valsalva maneuver in adrenergic failure. J Appl Physiol. 1991;71:1563–1567. doi: 10.1152/jappl.1991.71.4.1563. [DOI] [PubMed] [Google Scholar]
- Schilero GJ, Spungen AM, Bauman WA, Radulovic M, Lesser M. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol. 2009;166:129–141. doi: 10.1016/j.resp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Smith SA, Salih MM, Littler WA. Assessment of beat to beat changes in cardiac output during the Valsalva manoeuvre using electrical bioimpedance cardiography. Clin Sci (Lond) 1987a;72:423–428. doi: 10.1042/cs0720423. [DOI] [PubMed] [Google Scholar]
- Smith SA, Stallard TJ, Salih MM, Littler WA. Can sinoaortic baroreceptor heart rate reflex sensitivity be determined from phase IV of the Valsalva manoeuvre? Cardiovasc Res. 1987b;21:422–427. doi: 10.1093/cvr/21.6.422. [DOI] [PubMed] [Google Scholar]
- Smyth RJ, Chapman KR, Rebuck AS. Maximal inspiratory and expiratory pressures in adolescents. Normal values. Chest. 1984;86:568–572. doi: 10.1378/chest.86.4.568. [DOI] [PubMed] [Google Scholar]
- Vogel ER, Sandroni P, Low PA. Blood pressure recovery from Valsalva maneuver in patients with autonomic failure. Neurology. 2005;65:1533–1537. doi: 10.1212/01.wnl.0000184504.13173.ef. [DOI] [PubMed] [Google Scholar]
- Walker S, Cutting P. Impact of a modified Valsalva manoeuvre in the termination of paroxysmal supraventricular tachycardia. Emerg Med J. 2010;27:287–291. doi: 10.1136/emj.2009.073866. [DOI] [PubMed] [Google Scholar]
- Walter JS, Sacks J, Othman R, Rankin AZ, Nemchausky B, Chintam R, Wheeler JS. A database of self-reported secondary medical problems among VA spinal cord injury patients: its role in clinical care and management. J Rehabil Res Dev. 2002;39:53–61. [PubMed] [Google Scholar]
- Wecht JM, de Meersman RE, Weir JP, Spungen AM, Bauman WA. Cardiac autonomic responses to progressive head-up tilt in individuals with paraplegia. Clin Auton Res. 2003;13:433–438. doi: 10.1007/s10286-003-0115-5. [DOI] [PubMed] [Google Scholar]
- Wecht JM, Weir JP, Bauman WA. Blunted heart rate response to vagal withdrawal in persons with tetraplegia. Clin Auton Res. 2006;16:378–383. doi: 10.1007/s10286-006-0367-y. [DOI] [PubMed] [Google Scholar]


