Abstract
Distracting stimuli in the environment can pull our attention away from our goal-directed tasks. fMRI studies have implicated regions in right frontal cortex as being particularly important for processing distractors (e.g., Demeter, Hernandez-Garcia, Sarter, & Lustig, 2011; de Fockert & Theeuwes, 2012). Less is known, however, about the timing and sequence of how right frontal or other brain regions respond selectively to distractors and how distractors impinge upon the cascade of processes related to detecting and processing behaviorally-relevant target stimuli. Here we used electroencephalography (EEG) and event-related potentials (ERPs) to investigate the neural consequences of a perceptually salient but task-irrelevant distractor on the detection of rare target stimuli embedded in a rapid, serial visual presentation (RSVP) stream. We found that distractors that occur during the presentation of a target interfere behaviorally with detection of those targets, reflected by reduced detection rates, and that these missed targets show a reduced amplitude of the long-latency, detection-related P3 component. We also found distractors elicited a right-lateralized frontal negativity beginning at 100 ms, whose amplitude negatively correlated across subjects with their distraction-related behavioral impairment. Finally, we also quantified the instantaneous amplitude of the steady-state visual evoked potentials (SSVEPs) elicited by the RSVP stream and found that the occurrence of a distractor resulted in a transient amplitude decrement of the SSVEP, presumably reflecting the pull of attention away from the RSVP stream when distracting stimuli occur in the environment.
Keywords: attention, distraction, EEG, ERP, SSVEP
Introduction
While focusing on a goal-directed task, such as writing, our attention may be pulled away from the task at hand by a salient, unexpected stimulus in the environment, such as a sound on your computer signifying the arrival of a new email message. While at times the ability of environmental stimuli to pull our attention away from other tasks can be behaviorally useful, like when a fire alarm signals that you should stop writing and evacuate the building, at other times these stimuli are simply behaviorally-irrelevant distractors impeding our ability to focus on a behaviorally-relevant task.
In the context of visual attention, the features or qualities that can make a stimulus distracting include being perceptually salient (e.g., Burnham, Neely, Naginsky, & Thomas, 2010; Theeuwes, 1991a, 1991b; Lamy, Tsal, & Egeth, 2003; Rauschenberger, 2003), having an abrupt onset or offset (e.g., Folk, Remington, & Wu, 2009; Grubb, White, Heeger, & Carrasco, 2015; Jonides & Irwin, 1981; Yantis & Jonides, 1984), having proximity to a target stimulus in time or space (e.g., Leonard, Balestreri, & Luck, 2015; Seibold & Rolke, 2014; Theeuwes, 1995), or sharing features with a target stimulus (e.g., Folk, Remington, & Johnston, 1992; Lamy, Leber, & Egeth, 2004). The boundary conditions of what properties make a stimulus behaviorally distracting and whether top-down, goal-oriented attention can override potential distractors have been intensely studied and debated, particularly in the additional-singleton paradigm literature (e.g., Theeuwes, 1992). For example, the presence of an irrelevant but perceptually salient distractor item has been shown to impair behavioral performance in identifying a relevant target stimulus. Similarly, in the contingent attentional capture literature (e.g. Folk, Leber, & Egeth, 2002; Folk et al., 1992) the capture of attention depends on whether the distractor stimulus shares a property that is part of participants’ task-relevant top-down “set”. The perceptual load of a task has also been shown to affect distractor processing (e.g., Elliott & Giesbrecht, 2010; Lavie, 2005).
In contrast to the relatively more extensive behavioral literature, the literature is much more sparse regarding the neural effects of distraction. In a recent event-related potential (ERP) study of visual search, Gaspar & McDonald (2014) found that successfully ignoring salient distractors was associated with the “PD” component, an ERP marker of attentional suppression. In the context of attentional blink paradigms, where processing of a first target in a rapid, serial visual presentation (RSVP) stream impairs detection of a second target depending on the timing between target stimuli, distractor stimuli in the stream that share features with the target have been shown to also reduce detection of the second target and to reduce the amplitude of the P3 ERP component associated with the processing of the second target (Pincham & Szucs, 2014). In the neuroimaging literature, fMRI studies have implicated regions in right frontal cortex as being especially important when distraction challenges attentional control, (e.g., de Fockert & Theeuwes, 2012; Demeter et al., 2011; Marini, Demeter, Roberts, Chelazzi, & Woldorff, 2016), but the precise role of this region and whether it activates directly in response to distractor stimuli themselves or more broadly to contexts where attentional control is needed is still unclear.
Here, we used electroencephalographic (EEG) recordings of brain activity to explore the neural effects of distraction on selective attention using a novel twist on a classic RSVP paradigm, which we have termed the distractor RSVP (dRSVP) task. In our task, participants centrally fixated while covertly attending to an RSVP stream just above fixation consisting of frequent nontarget letters and rare target numbers. Meanwhile, a task-irrelevant but perceptually salient checkerboard distractor stimulus was infrequently presented with unpredictable timing just below fixation. We were first interested in investigating how the timing of the distractor occurrence would affect the behavioral detection of target stimuli in the RSVP stream and what the neural consequences of distraction would be for the ERPs generated by target stimuli. Secondly, we were also interested in investigating the ERPs generated by the distractor stimuli themselves. On the basis of the neuroimaging literature (e.g., Demeter et al., 2011), we predicted distractors would evoke a right frontal neural response. Finally, we also sought to analyze the effect of distraction on the envelope of the instantaneous amplitude of the steady state visual evoked potential (SSVEP) generated by extrastriate visual cortical neurons in response to the flickering RSVP steam. An SSVEP is an oscillatory brain response at the same fundamental frequency as the driving stimulus, and previous work has demonstrated that SSVEP amplitudes increase when attention is directed towards the driving stimulus and decrease when attention is directed away from the driving stimulus (e.g., Andersen, Müller, & Martinovic, 2012; Müller et al., 2006; Müller et al., 1998). We predicted that distractors would temporarily pull participants’ attention away from the RSVP stream, which would be evidenced by a transient decrement in the SSVEP amplitude time-locked to the distractor occurrence.
Methods
Participants
Participants consisted of 20 young adults (8 female, age 21 to 36, mean 26.2 years). Participants had normal or corrected-to-normal vision and did not report any neurological disorders or conditions that could affect attention or memory. All participants underwent informed consent prior to beginning the study, and participants were financially compensated at a rate of $15/hour. Participant recruitment, consent, and experimental procedures were in accordance with protocols approved by Duke University’s Institutional Review Board.
dRSVP Task
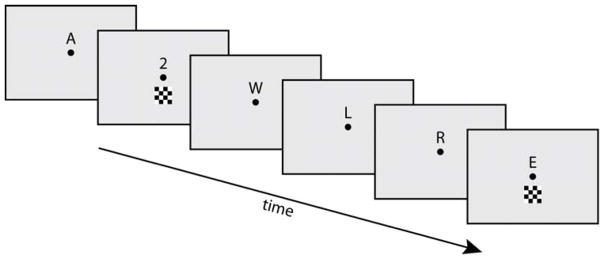
For the dRSVP task (Figure 1), participants maintained fixation on a central point while covertly attending to an RSVP stream presented just above fixation. The RSVP stream consisted of letters (Nontargets) and infrequent numbers (Targets; 8% of stimuli) presented at a rate of 116 ms duration per stimulus (8.6 Hz). All of the RSVP stimuli were black and the background screen was gray. Participants were instructed to buttonpress whenever they detected a number Target.. Meanwhile, a brief checkerboard Distractor stimulus occasionally and unpredictably flashed just below fixation. The black and white pattern of the checkerboard alternated with each presentation. The Distractor was presented with a random distribution (minimum time between distractors = 500 ms, maximum time = 1050 ms, flat distribution) for a duration of 33 ms. The Distractor stimulus onset could occur 0, 16, 33, 50, 66, 83, or 100 ms (i.e., at any screen refresh with a 60 Hz refresh rate) after the onset of a stimulus in the RSVP stream. This temporal jittering of the Distractor relative to the RSVP stimuli ensured that the SSVEP signal evoked by the RSVP task stream would be averaged out in the ERPs time-locked averaged to the Distractor onset (c.f., Crist, Wu, Karp, & Woldorff, 2008). Finally, all stimuli were kept along the vertical midline in order to facilitate extraction of predicted right-frontal components associated with distractor processing (see Introduction). Participants were familiarized with the task and all procedures, and then completed 15 three-min runs of the dRSVP task while EEG data were recorded. Three additional three-min runs of the RSVP task without any distractors were also collected.
Figure 1. dRSVP task.
Participants fixated on a central dot while covertly attending to an upper-field RSVP stream consisting of letters and infrequent numbers. Each stimulus in the RSVP stream was presented for 116 ms (8.6 Hz) with no gap between stimuli, and participants were instructed to buttonpress every time a number appeared. Meanwhile, below fixation, an irrelevant checkerboard distractor (33 ms duration) flashed every 500 – 1050 ms.
EEG Recording
EEG was recorded with a 63-channel active electrode system (Brain Vision actiCHamp, Brain Products, Gilching Süd, Germany) using a customized, extended coverage, elastic electrode cap (Woldorff, Liotti, Seabolt, Busse, Lancaster, & Fox, 2002; EASYCAP, Herrsching). These caps were designed to have an extended coverage of the head from just above the eyebrows to below the inion posteriorly and to have electrodes that are equally spaced across the cap. An electrode was placed below the left eye in order to monitor vertical eye movements, and two electrodes somewhat lateral to the left and right outer canthi were used for monitoring lateral eye movements. The scalp sites of our equidistant electrode custom cap are reported in terms of the closest location in the standard 10–10 system if within a couple of millimeters. For any electrodes further than a couple of millimeters from the related 10–10 electrode, the electrode is denoted with a subscript of “a,” “p,” “i,” or “s” for slightly “anterior,” “posterior,” “inferior,” or “superior,” respectively. Relevant electrodes are also specifically identified on schematic head figures in the Results section. The EEG (and EOG) data were recorded with a bandpass from DC to 138 Hz, sampled at 500 Hz per channel, and referenced to the right mastoid. Data were subsequently re-referenced offline to the algebraic average of the two mastoids.
Behavioral analyses
In order to examine the behavioral effects of distraction on target detection, Targets were categorized on the basis of when a Distractor onset occurred relative to the onset of the RSVP Target stimuli to create the following six Bins: Bin 1: −348 to −232 ms before the onset of a Target, Bin 2: −232 to −116 ms, Bin 3: −116 to 0 ms, Bin 4: 0 to 116 ms following the onset of a Target, Bin 5: 116 to 232 ms following, or Bin 6: 232 to 348 ms following. The width of each of these Bins (116 ms) corresponds to the duration of one stimulus in the RSVP stream. Accuracy and reaction times for detecting Targets were analyzed using repeated-measures ANOVAs with the factor of Bin (6 levels). The Greenhouse-Geisser sphericity correction was applied as needed. Corrected F and p values are reported, but degrees of freedom are rounded to integer values for easier reading. Significant ANOVA main effects were further queried using post-hoc t-tests where appropriate.
ERP analyses
ERP analyses were conducted using EEGLab and ERPLab software (Delorme & Makeig, 2004; Lopez-Calderon & Luck, 2014). Offline, data were bandpass filtered from 0.01 to 30 Hz using an infinite impulse response Butterworth noncausal filter with a 12 decibel per octave roll-off. Epochs with eye movements, blinks, or muscle movements were excluded from analyses using ERPLab’s automated artifact detection algorithms, with settings optimized for each participant. The number of epochs rejected did not systematically differ between conditions. For each subject, Detected Targets, Missed Targets, and Nontargets were categorized by when a Distractor stimulus occurred using the same timings as the six bins created for the behavioral analyses. ERPs were created by time-lock averaging the epoched EEG data to the onset of Detected Targets, Missed Targets, and Nontargets within each of the six bins, as well as to the onset of the Distractor stimuli. The selectively averaged ERPs were baseline corrected by subtracting the mean amplitude of the baseline period (−200 to 0 ms) from the ERP for each time-locked stimulus. Specific contrasts and subtractions to isolate functional activations are described in detail in the appropriate sections of the Results. ANOVAs on the mean amplitudes over specified time ranges were conducted in order to test statistical differences between responses to different stimulus conditions as described in further detail below. The Greenhouse-Geisser sphericity correction was applied as needed.
SSVEP Instantaneous Amplitude analyses
We hypothesized that distraction would temporarily pull participants’ attention away from the RSVP task stream, and that this temporary reduction in attention would be reflected by a decrease in the envelope of the instantaneous amplitude (IA) of the SSVEP generated by the steady-state RSVP stream. In order to test this hypothesis, we created epochs (−600 to 1000 ms) time-locked to Nontargets without a Distractor, to Nontargets with a Distractor 0 to 116 ms following the Nontarget onset, and to the Distractor stimuli. Epochs containing Target stimuli were excluded from this analysis. For the Nontarget-without-Distractor epochs, data from the three RSVP task runs without distraction were used. For the Nontarget-without-Distractor and Nontarget-with-Distractor conditions, the envelope of the IA of the SSVEP signal generated by the RSVP task was estimated by convolving the epoched EEG data with a complex Morlet wavelet (see also Gladwin, Lindsen, & de Jong, 2006). The frequency mean of the wavelet was set to 8.6 Hz, the driving frequency of the RSVP stream, and the standard deviation in the frequency domain was set to 2 Hz (equivalent to a standard deviation of 80 ms in the time domain). In order to control for the physical presence of the Distractor stimulus in the Nontarget-with-Distractor condition, we wanted to subtract out the Distractor’s contribution in the 8.6 Hz frequency band within the relevant time range from the Nontarget-with-Distractor IA values. To extract the content in the 8.6 Hz frequency band from the Distractor response, we convolved the complex Morlet wavelet (frequency mean = 8.6 Hz, standard deviation = 2 Hz) with the time-locked-averaged ERP response to the Distractor stimuli. As the Distractor could have occurred at seven possible onset times relative to the onset of a Nontarget stimulus (0, 16, 33, 50, 66, 83, or 100 ms later), we then convolved the resulting data with a seven-point delta function corresponding to these possible onset times. Finally, we subtracted these Distractor IA values from the IA values for the Nontarget-with-Distractor condition, giving us corrected IA values for this condition. We predicted the corrected Nontarget-with-Distractor condition would show a temporary reduction in the IA values, reflecting the brief pull of attention away from the task stream. In contrast, we predicted the Nontarget-without-Distractor condition would show a consistent IA over time.
Results
Behavior
Distractors that occur during the presentation of a target interfere with detection of those targets
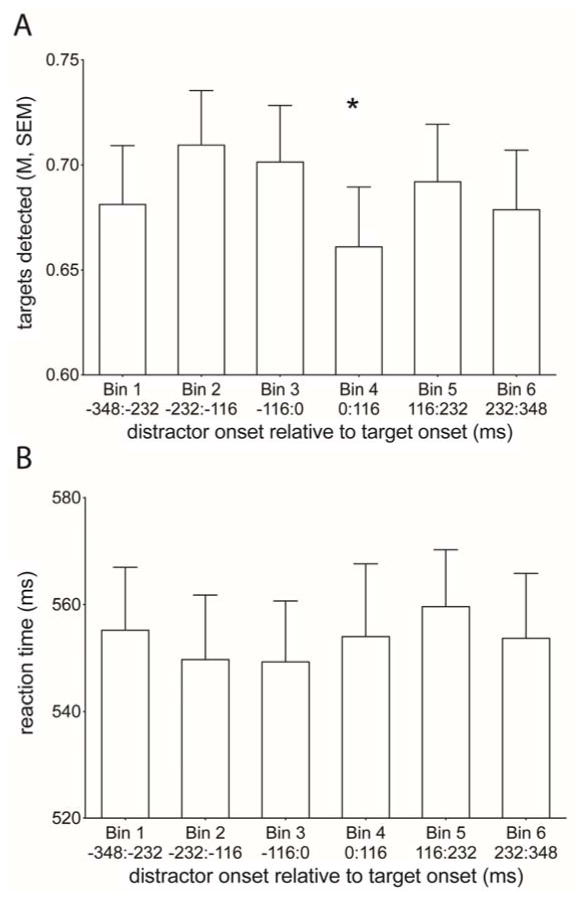
The behavioral results for the dRSVP task are shown in Figure 2. Figure 2A shows the proportion of Targets detected as a function of when a Distractor onset occurred relative to the onset of a Target stimulus. Overall, the proportion of Targets detected was 0.69 ± 0.03. There was a significant main effect of Bin on the proportion of Targets detected (F(5,95) = 5.73, p = 0.001). Pairwise comparisons of the Bins revealed that Targets were most likely to be missed when a Distractor onset occurred 0 to 116 ms following the Target onset (Bin 4; paired t-tests between Bin 4 and all other Bins, all p < 0.04). Accuracy was the highest for Bins 2 (Distractor −232 to −116 ms prior to Target) and 3 (Distractor −116 to 0 ms prior to Target; paired t-tests between Bins 2 and 3 and Bins 1, 4, and 6 all p < 0.04). There was no effect of Distractor onset time on the reaction times for successfully Detected Targets (Figure 2B; main effect of Bin, F(5,95) = 0.68, p = 0.64).
Figure 2. Target detection is impaired when a distractor is presented 0 to 116 ms following the target onset.
A) Bars represent the mean proportion of Targets detected as a function of when a Distractor onset occurred relative to the onset of a target stimulus. Error bars represent the between-subjects standard error around the mean. When a Distractor onset occurred 0 to 116 ms following the onset of a target stimulus, the detection of those Targets was significantly impaired compared to the proportion of Targets detected when Distractors were presented in other time windows (paired t-tests between Bin 4 and every other Bin all p < 0.04). B) Bars represent the mean reaction time for correctly detecting Targets as a function of when a Distractor onset occurred relative to the onset of a Target stimulus. Error bars represent the between-subjects standard error around the mean. There were no significant effects of the Distractor occurrence timing on the reaction times.
Electrophysiology
Distraction reduces P3 of Missed Targets
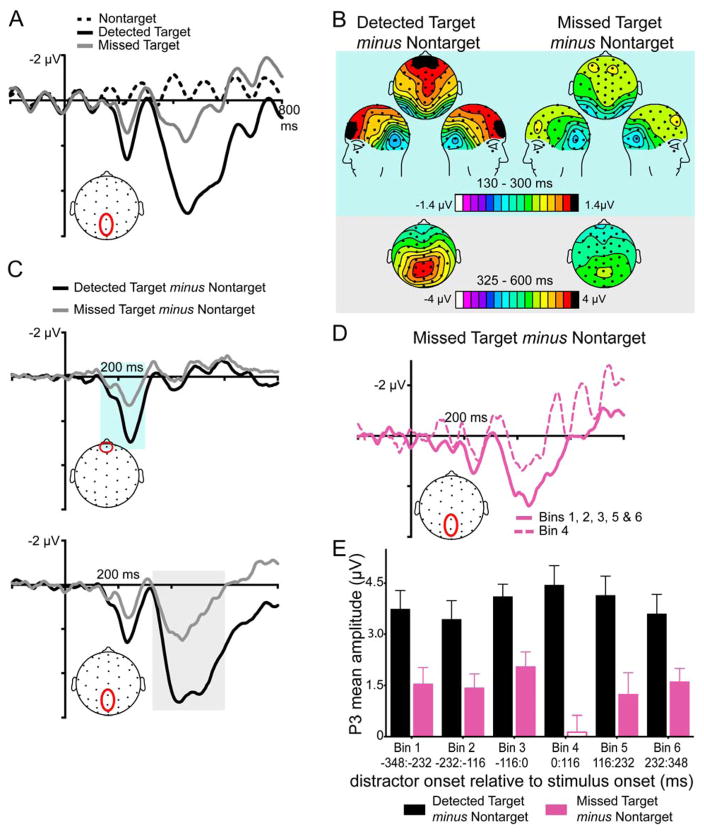
We next examined the effect of distraction on the neural processes related to target detection by analyzing the EEG data to create ERPs time-locked averaged to the onset of the Target and Nontarget stimuli. For these analyses, Targets were coded on the basis of whether they were successfully detected or missed behaviorally. Detected Target, Missed Target and Nontarget ERPs are shown in Figure 3 A. Detected Target, Missed Target, and Nontarget stimuli were categorized into the six time Bins outlined in the Methods section, based on when a Distractor onset occurred relative to the Target or Nontarget onset. In order to isolate the functional activity associated with target detection more specifically, we subtracted the Nontarget ERPs from the Detected and Missed Target ERPs, respectively, in order to create difference waves. These difference waves also controlled for the physical presence of the Distractor stimuli as both Targets and Nontargets were identically binned according to when Distractors occurred. We first examined these difference waves for Detected and Missed Targets, irrespective of when Distractors occurred, and then examined whether these ERPs varied as a function of when a Distractor stimulus onset occurred. As the targets were occurring relatively rarely (8% of total stimuli) and were task relevant, we predicted that they would elicit a P3 wave as is often seen in traditional oddball paradigms or in other RSVP tasks with rare targets (see review by Polich, 2007). We also hypothesized Missed Targets in our task would generate a smaller P3 than Detected Targets. Based on our behavioral data, we further hypothesized that Distractors that onset 0 to 116 ms following a Target would impair the neural processing of that Target, as reflected by a reduced amplitude P3, compared to when the Distractor onset occurred in the other relative time windows.
Figure 3. Effects of detection and distraction on target-related neural activity.
A) Detected Target, Missed Target and Nontarget ERP waves from a centroparietal ROI. To isolate target-related activity, as well as to subtract off the ongoing SSVEP activity overlapping on these event-related responses, we also calculated the difference waves between Detected Targets and Nontargets and Missed Targets and Nontargets. Targets that were behaviorally detected generated larger ERP responses than Targets that were ultimately missed. These effects were most evident in an early frontocentral positivity (130 to 300 ms, blue shading) and in a later large centroparietal positivity (P3 component, 325 to 600 ms, gray shading). Panel B) shows topographic scalp maps of the mean amplitudes extracted in these two latency windows for both Detected Targets minus Nontargets and Missed Targets minus Nontargets. Panel C) shows Detected Target minus Nontarget and Missed Target minus Nontarget difference waves from channel AFz and from a centroparietal ROI. For the P3 component, in addition to showing an effect of Detection, there was also an interaction between Detection and Bin, indicating the size of this component was also modulated by the timing of the distractor stimulus (see text for statistical details). Further analyses revealed that the P3 amplitude for Detected Targets minus Nontargets was unaffected by the timing of Distractors, but the P3 for Missed Targets minus Nontargets were smaller when Distractors onset 0 to 116 ms following Target onset (Bin 4). Panel D shows the ERP difference waves for Missed Targets minus Nontargets specifically for Bin 4 (dashed line) and for the average across the other five bins (solid line). Panel E) shows the mean amplitude from 325–600 ms for Detected Targets minus Nontargets (black bars) and Missed Targets minus Nontargets (pink bars). These data were categorized into the six Bins based on when a Distractor onset occurred relative to the onset of a Target. Error bars represent the between-subjects standard error around the mean.
Figure 3 illustrates the data from these analyses. Detected Targets minus Nontargets showed an early frontocentral positivity starting around 130 ms and peaking around 245 ms. This positivity was the largest over anterior channels. This effect was smaller and slightly less anterior in the Missed Targets minus Nontarget waves (Figure 3B and C). This early positivity may be related to the previously reported prefrontal positivity or “pP” component (Perri, Berchicci, Spinelli, & Di Russo, 2014; Perri, Berchicci, Lucci, Spinelli, & Di Russo, 2015a,b), also called the Go-P2 in the literature (Gajewski & Falkenstein, 2013). This component is thought to reflect stimulus-response mapping processes prior to response execution. As predicted, Detected Targets minus Nontargets also generated a large P3 component, as evidenced by a large positivity strongest over centroparietal channels and beginning around 325 ms. This positivity was strongly attenuated in the Missed Targets minus Nontarget difference waves (Figure 3B and C).
In order to more specifically investigate the effects of distraction on target-related activity, the Detected Target minus Nontarget difference waveforms and, separately, the Missed Target minus Nontarget difference waveforms were binned into the six time windows based on Distractor onset times. For the early frontocentral positivity, mean amplitudes from 130 to 300 ms were extracted from a region of interest (ROI) created by averaging the data from the frontocentral channels FCz, Cz, C1a, and C2a. These mean amplitudes were subjected to a repeated-measures omnibus ANOVA with the factors of Detection (Detected, Missed) and Bin (6 levels). For this frontocentral positivity, there was a main effect of Detection (F(1,95) = 29.75, p < 0.0001), but no effect of Bin and no interaction between Detection and Bin. Thus, while this component was larger when the Target was ultimately behaviorally detected than when it was not, the timing of the Distractor stimuli did not differentially affect the component.
Identical analyses were conducted on the P3 component by extracting the mean amplitudes from 325 ms to 600 ms from an ROI over centroparietal sites where this positivity was the strongest (channels Pz and CPz; see Figure 3A for a diagram). As before, these mean amplitudes were subjected to a repeated-measures omnibus ANOVA with the factors of Detection (Detected, Missed) and Bin (6 levels). Significant effects were further queried with repeated-measures ANOVAs and paired t-tests separately for the Detected Target minus Nontarget and Missed Target minus Nontarget data. The results of the omnibus ANOVA revealed a significant main effect of Detection, with the Detected Target minus Nontarget P3 mean amplitudes being larger than those observed for the Missed Target minus Nontarget condition (F(1,19) = 49.70, p < 0.01). There was no main effect of Bin (F(5,95) = 0.99, p = 0.43). The Detection by Bin interaction was significant (F(5,95) = 3.95, p < 0.01), indicating that the Distractors could have a differential effect on Detected and Missed Targets depending on when the Distractors occurred. In order to explore this interaction, we next conducted repeated-measures ANOVA with the factor of Bin (6 levels) separately for the Detected Target minus Nontarget data and the Missed Target minus Nontarget data. There was no main effect of Bin on the P3 amplitude for the Detected Target minus Nontarget condition (F(5,95) = 1.84, p = 0.11), suggesting that Targets that were successfully detected behaviorally were unaffected by the timing of the Distractor. However, for the Missed Target minus Nontarget condition, the effect of Bin was significant (F(5,95) = 2.41, p = 0.04; Figure 3D and E). For these data, the P3 amplitude for the Missed Target minus Nontarget condition was smallest for Bin 4, when the Distractor onset 0–116 ms following the onset of the Target and Nontarget stimuli (Figure 3D and E), paralleling the behavioral impairment results. Paired t-tests between Bin 4 and the other Bins revealed Bin 4 was significantly smaller than all other Bins (all p < 0.05), with the exception of Bin 5, the comparison to which did not reach significance (p = 0.09). There were no other significant differences on these components between bins.
Distraction induces a right-lateralized frontal negativity that correlates with reduced behavioral impairment
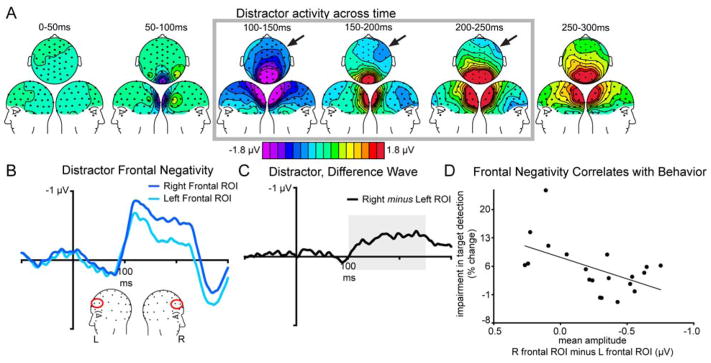
In order to investigate the neural activity associated with distractor processing, we created ERPs time-lock averaged to the onset of the Distractor stimuli (Figure 4). Figure 4A shows the topography of the mean amplitude of the ERP response to the Distractor over time. Notably, while our stimuli were all presented along the vertical midline, these data revealed a frontal negativity that appeared to be larger on the right side of the scalp than on the left (Figure 4A, outlined in gray and indicated by the black arrows). We explored this right-lateralized frontal negativity by creating ROIs over left and right frontal cortex (channels F3a, F3i and F4a, F4i, respectively) and then extracting from both ROIs the ERP response time-lock averaged to the Distractor onset (Figure 4B). Figure 4C illustrates the right ROI minus the left ROI ERP difference wave. The mean amplitude of this difference wave was calculated in 50 ms intervals between 0 and 1000 ms post-Distractor onset in order to determine where it was significantly greater than zero. This analysis revealed the difference wave was significantly greater than zero between 100 and 250 ms (all p < 0.04). It did not significantly differ from zero between 250 and 300 ms (t(19) = 2.05, p = 0.054). There were a few 50 ms intervals that again achieved significance after 300 ms, and then the difference wave was consistently no longer different from zero following 600 ms.
Figure 4. Distractors evoke a right-lateralized frontal negativity.
Panel A) shows topographic scalp maps of the mean amplitude over time of the ERP response time-lock averaged to the onset of the Distractor. A right-lateralized negativity between 100 and 250 ms was observed (gray outline box, activity indicated by black arrows). B) To explore this negativity, ERP responses time-lock averaged to the Distractor onset were extracted from ROIs created over left and right frontal channels. The right frontal ROI response (dark blue trace) was significantly more negative than the left frontal ROI response (light blue trace; paired t-test on the mean amplitudes of the ERP response between 100 and 250 ms, t(19) = 4.412, p < 0.01). Panel C) illustrates the right frontal ROI minus the left frontal ROI difference wave. The mean amplitude of this difference wave between 100 and 250 ms (shaded in gray) was extracted and used in a correlational analysis with behavior. D) The size of the mean amplitude difference between the right and left frontal ROIs negatively correlated with the amount of impairment individuals showed when the Distractor was presented 0–116 ms following the onset of a Target (see text for analysis details). That is, individuals who showed a bigger difference between the right and left frontal ROIs also showed less behavioral impairment in target detection when Distractors were presented.
As noted in the Introduction, previous neuroimaging work has indicated regions in right frontal cortex to be particularly important for distractor processing and for the implementation of top-down attentional mechanism in response to distractor challenges (e.g., Demeter et al., 2011). Given this, we next wanted to further explore the functional characteristics of this right-lateralized negativity induced by the distractor, in particular whether it correlated with participants’ behavioral performance on the RSVP task. As participants’ showed a selective impairment in the proportion of Targets detected when Distractors were presented 0–116 ms following Target onset (Bin 4), we calculated the percent change between participants’ Bin 4 accuracy and their average accuracy over the remaining five Bins in order to index the behavioral effect of distraction. We then correlated these behavioral data with the mean amplitude of the difference between the right and left frontal ROI ERP responses between 100 and 250 ms (Figure 4D). These data demonstrated that the right-lateralized negativity in the ERP response to Distractors was correlated with a reduction in behavioral impairment with distraction (r = −0.53, p = 0.02) – that is, the more right-lateralized the frontal response to the Distractor, the less the corresponding behavioral impairment.
Distraction pulls attention away from the RSVP stream, modulating the SSVEP instantaneous amplitude (IA)
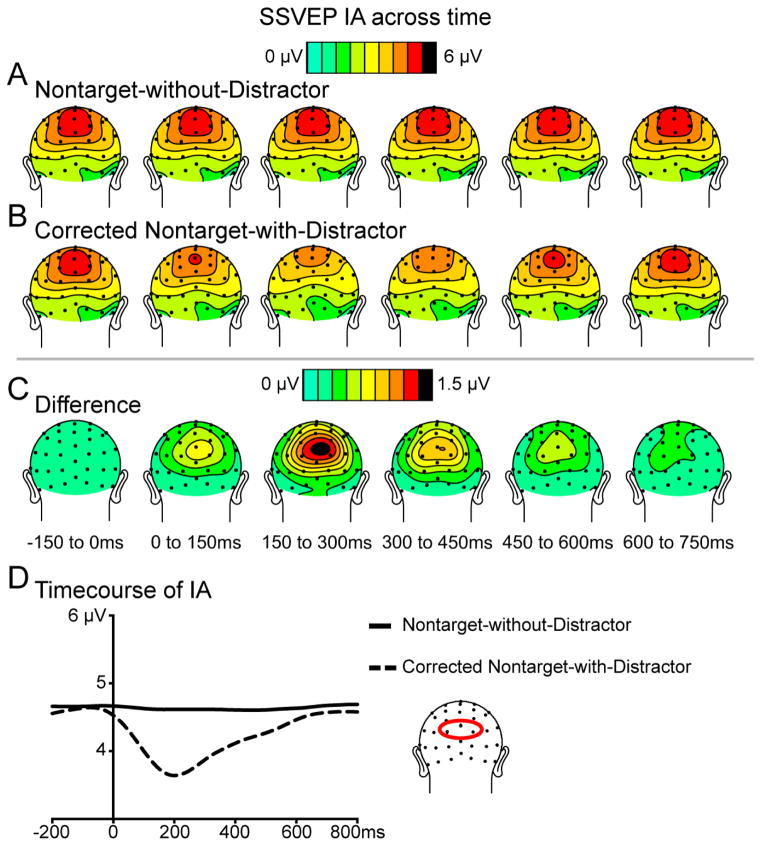
Figure 5 depicts topographic plots of the IA of the SSVEP over time (−150 to 750 ms relative to Nontarget onset) for the Nontarget-without-Distractor (Figure 5A) and the corrected Nontarget-with-Distractor conditions (Figure 5B). The largest IA values were observed over posterior channels, as would be expected for these visual SSVEPs. Averaging together the Nontarget-without-Distractor and corrected Nontarget-with-Distractor condition data revealed that the largest IA values of the averaged data occurred over channel Pz. To statistically analyze the effect of distraction on the IA values for both the Nontarget-without-Distractor and the corrected Nontarget-with-Distractor conditions, the IA from channel Pz was averaged within six 150 ms intervals (relative to the time-locked Nontarget onset: −150 to 0 ms, 0 to 150 ms, 150 to 300 ms, 300 to 450 ms, 450 to 600 ms, and 600 to 750 ms). These values were then subjected to a 2 × 6 repeated-measures ANOVA with the factors of Distraction and Time. This analysis revealed a significant Distraction by Time interaction (F(5,95) = 14.79, p < 0.01), as well as significant main effects of both Distraction (F(1,19) = 21.97, p < 0.01) and Time (F(5,95) = 24.84, p < 0.01). In order to further probe the Distraction by Time interaction, separate 1 × 6 ANOVAs were conducted within each of the two Nontarget conditions (see Table 1 A for means). In the absence of distraction, the IA of the envelope of the SSVEP evoked by the 8.6 Hz driving frequency of the RSVP task stream remained nearly constant over the course of the epoch (no main effect of Time, F(5,95) = 2.38, p = 0.11). In contrast, when a Distractor occurred 0–116 ms following the onset of a Nontarget, (with the sensory effect of the Distractor removed), there was a significant main effect of Time (F(5,95) = 21.97, p < 0.01). More specifically, the IA values decreased starting in the 0–150 ms interval, reaching their lowest point in the 150 to 300 ms interval, and then returned to their pre-distraction values by the final 600–750 ms interval. This reduction of the RSVP SSVEP following the Distractor occurrence suggests reduced attentional allocation to the RSVP stream.
Figure 5. The instantaneous amplitude (IA) of the SSVEP over time.
Topographic plots represent data from epochs time-locked (A) to the onset of Nontarget stimuli in the absence of Distractors, (B) to the onset of Nontargets when Distractors occurred (Distractor onsets 0–116 ms relative to Nontarget onset, corrected for sensory activity of the Distractor itself; see Methods for additional details), and (C) the difference between these two conditions. Note no pre-Distractor baseline correction was performed on these data. As is clear in the maps, the IAs were the largest over posterior channels. For the Nontarget-without-Distractor condition, data from 3 RSVP runs without distraction were used. D) The extracted timecourse of the IA. Data are the IA values over time averaged within a posterior ROI (illustrated). Without Distractors, the IA of the envelope of the SSVEP generated by the RSVP task stream remained nearly constant over time. In contrast, Distractor presentation resulted in a modulation of the SSVEP envelope (lower amplitude), implicating reduced attentional allocation to the RSVP stream.
Table 1. Instantaneous amplitudes (IA), averaged within 150 ms time intervals.
Data are means (standard error) of the IA in microvolts for the Nontarget-without-Distractor and corrected Nontarget-with-Distractor conditions. Significant differences between conditions are indicated by underlining (paired t-tests, all p < 0.05).
| A. Means for Channel Pz | ||||||
|---|---|---|---|---|---|---|
| Time Interval relative to Nontarget onset (ms) | ||||||
|
| ||||||
| Condition | −150 to 0 | 0 to 150 | 150 to 300 | 300 to 450 | 450 to 600 | 600 to 750 |
| Nontarget without Distractor | 5.73 (0.60) | 5.71 (0.60) | 5.75 (0.62) | 5.80 (0.63) | 5.81 (0.64) | 5.84 (0.64) |
| Corrected Nontarget with Distractor | 5.69 (0.61) | 5.26 (0.63) | 4.78 (0.59) | 5.13 (0.55) | 5.44 (0.58) | 5.67 (0.59) |
| B. Means for Channels POz, O1, and O2 | ||||||
|---|---|---|---|---|---|---|
| Time Interval relative to Nontarget onset (ms) | ||||||
|
| ||||||
| Condition | −150 to 0 | 0 to 150 | 150 to 300 | 300 to 450 | 450 to 600 | 600 to 750 |
| Nontarget without Distractor | 4.66 (0.55) | 4.64 (0.54) | 4.62 (0.54) | 4.61 (0.54) | 4.62 (0.55) | 4.66 (0.55) |
| Corrected Nontarget with Distractor | 4.62 (0.55) | 4.15 (0.56) | 3.71 (0.51) | 4.06 (0.46) | 4.32 (0.47) | 4.55 (0.50) |
As is evident from the topographic plots showing the difference between the Nontarget-without-Distractor and corrected Nontarget-with-Distractor conditions (Figure 5C), the greatest difference between these conditions was observed over channels POz, O1, and O2. For completeness, we created an ROI based on these channels as well. The timecourse of the IA from this ROI is plotted in Figure 5D. Subjecting this ROI to the same Distraction by Time repeated-measures ANOVA as run for channel Pz revealed a significant Distraction by Time interaction (F(5,95) = 21.64, p < 0.01; see Table 1B for means), as well as significant main effects of Distraction (F(1,19) = 22.61, p < 0.01) and Time (F(5,95) = 20.29, p < 0.01). Again, the Nontarget-without-Distraction condition showed a nearly constant SSVEP IA over time (no main effect of Time, F(5,95) = 1.02, p = 0.41), while the corrected Nontarget-with-Distractor condition showed a significant effect of Time (F(5,95) = 23.08, p < 0.01), with the IA values reaching their lowest point in the 150 to 300 ms interval.
Size of IA decrement correlates with behavioral impairment
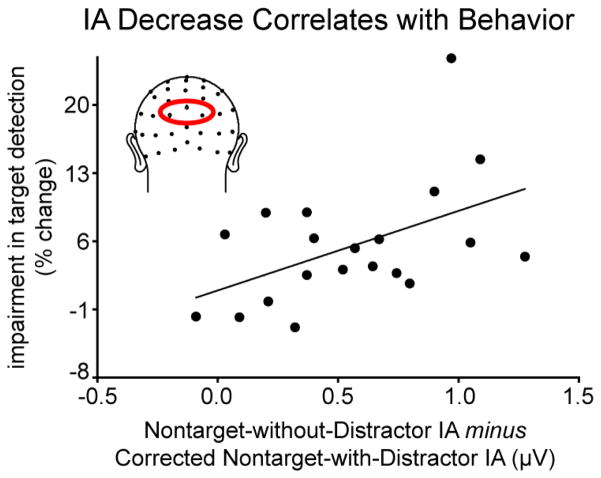
We next explored whether the distraction-related decrease in the SSVEP IA correlated with behavioral performance, using the same index of behavioral impairment with distraction as used in the previous correlational analysis. We hypothesized that more distracted individuals would have a larger decrease in IA with the occurrence of a Distractor and that this would correlate with a larger behavioral impairment. To test this hypothesis, we created a posterior ROI based on channels showing the greatest difference between the Nontarget-without-Distractor and corrected Nontarget-with-Distractor conditions (channels POz, O1, and O2; see Figure 6 for illustration). Within this ROI, the average IA from 0 to 600 ms was calculated for each condition. We found that the difference between the Nontarget-without-Distractor condition and the corrected Nontarget-with-Distractor condition correlated with participants’ behavioral impairment with distraction (r = 0.49, p = 0.03), such that the larger the decrease in the SSVEP IA observed with distraction, the greater the behavioral impairment in target detection.
Figure 6. Decrease in instantaneous amplitude (IA) correlates with behavioral impairment.
The decrease in IA of the RSVP SSVEP due to distraction correlated positively across subjects with participants’ impairment in target detection. For this analysis, the average IA was extracted from a posterior ROI (illustrated) from epochs time-locked to the onset of Nontargets with and without Distractors (latency used 0–600 ms). These values were then entered into a correlational analysis with the same accuracy measures used for Figure 4D (i.e. degree of impairment in the critical 0–116 ms bin window for Distractor occurrence). The results indicate that the larger the decrease seen in the IA with distraction, the greater the behavioral impairment also observed.
Discussion
In the present work, we presented a brief but salient, task-irrelevant distractor in a temporally unpredictable fashion while participants performed an RSVP task. We predicted that the distractor would temporarily pull participants’ attention away from the RSVP task, resulting in missed RSVP targets and a reduction in the IA of the SSVEP generated by the RSVP stream. We also predicted the distractor stimulus itself would elicit activity over right frontal cortex, in line with the existing attentional control neuroimaging literature. We found that distractors that occurred during the presentation of RSVP targets interfered with the detection of those targets, resulting in more missed targets when distractors onset 0–116 ms following the onset of a target than when distractors occurred either before or farther away in time from the presentation of a target. In line with these behavioral data, the P3 elicited by missed targets when distractors occurred in this time window were smaller in amplitude compared to missed targets with distractors that occurred in other time windows. Furthermore, time-lock averaging to the distractor stimulus itself revealed a frontal negativity that was larger over right frontal channels than over left frontal ones from 100–250 ms following the distractor. The difference in amplitude between the right and left frontal negativity (i.e., its right-sidedness) correlated with participants’ behavioral impairment such that the more right-lateralized this component was, the less behavioral impairment a participant showed for targets in the distraction-susceptible window of 0–116 ms following target onset. Finally, we also found that the SSVEP generated by the task stream decreased in amplitude when distractors were presented. We were able to perform this analysis and extract this result on epochs containing nontarget stimuli only, where there was no direct behavioral index of distraction. However, we found that the size of the IA decrease with distractors positively correlated across participants with their distraction-related behavioral impairment, suggesting that the more distracted individuals were by the irrelevant flashing checkerboard, the more their attention was pulled away from the RSVP stream and the more RSVP targets they missed.
Although the brief distractors employed here produced statistically significant impairments in target detection, the behavioral effects (change in targets detected) were nonetheless fairly small in magnitude. Notably, our distractor stimuli (checkerboards), which appeared in a different but nearby location relative to the task-relevant stimulus stream, did not share a target-defining feature with the targets in that stream. It has been theorized that distractors that share a target-defining feature capture attention because they correspond to the top-down “set” participants have established in order to facilitate target detection (contingent attentional capture; Folk et al., 1992; Folk et al., 2002). Accordingly, we might have been able to get larger behavioral effects of distraction if our distractors shared a distinct feature with our target stimuli (for example, if RSVP targets were red items among gray nontargets, with distractors also being red). However, as one of our aims was to look at the neural response to the distractor stimuli themselves, we elected to make the distractor featurally different than the targets. Related to this discussion, other ERP studies of behavioral distraction have also found novel, infrequent distractors can significantly impair attentional performance (e.g., Escera, Alho, Winkler & Näätänen, 1998; Berti & Schröger, 2001). In our study, it is possible that rarer distractors could have resulted in more behavioral distraction than our higher-frequency distractor stimuli. However, this would have also resulted in fewer trials per condition in our current design. Additionally, our RSVP stimuli were always presented above fixation and our distractors below fixation. Future work will be needed to explore whether and how changing the location of these stimuli affects behavior and task-related brain activity.
In our data, the distractor had the most influence on target detection when it occurred during the presentation of a target stimulus. Sato & Kawahara (2014) found similar behavioral effects using task-irrelevant face distractors, demonstrating that faces presented either simultaneously with targets or at a very short SOA relative to targets resulted in attentional capture, whereas the presentation of face distractors at longer SOAs relative to targets did not result in attentional capture. These authors argued that this pattern of results could be a consequence of people’s ability to quickly and efficiently process naturalistic stimuli, resulting in a rapid attentional engagement with and disengagement from the distractor stimuli. Our checkerboard distractor was not a naturalistic visual stimulus, but it was presented very briefly (33 ms duration), which could also trigger relatively rapid attentional shifting to the distractor and then quickly back to the relevant task stream. In contrast to the role of relative timing in the current data, the typical temporal pattern of capture effects by color singletons in the contingent attentional capture literature is quite different. For example, Folk et al. (2002) found peripheral color singletons presented approximately 200 ms before a uniquely colored central target in a stream of nontargets captured attention and resulted in impaired detection of those subsequent targets. Similarly, in the attentional blink literature (e.g., Raymond, Shapiro, & Arnell, 1992), the presentation of a target (T1) or a disruptor stimulus (typically also sharing some feature with targets) 150–500 ms before a second target (T2) disrupts detection of the T2. These different patterns in when distractor presentation have been observed to have the most behavioral impact suggests different mechanisms may underlie why or how these distractors operate to disrupt behavior. However, of note, Pincham & Szucs (2014) found task-set-colored distractors in an attentional blink paradigm reduced both accuracy for the T2 and the amplitude of the P3 component it elicited. As our data also found effects of distraction on target P3 amplitudes, this suggests that the downstream consequences of distraction on target processing may be similar across these different paradigms.
A particularly novel aspect of our findings is the right-lateralized frontal negativity from 100–250 ms that we observed in the ERPs time-locked to the distractors. Right frontal activity during attentional control tasks or in the presence of distraction has been demonstrated in fMRI studies (e.g., de Fockert & Theeuwes, 2012; Demeter et al., 2011; Leber, 2010; Marini et al., 2016; Serences et al., 2005), and the importance of this region in attentional control has been underscored in both human lesion (Japee, Holiday, Satyshur, Mukai, & Ungerleider, 2015) and animal lesion and neurochemical work (Passetti, Chudasama, & Robbins, 2002; St Peters, Demeter, Lustig, Bruno, & Sarter, 2011). While ERP studies have also contributed to the large body of evidence that frontal cortex in general is important for attentional control (e.g., Grent-’t-Jong & Woldorff, 2007; Shomstein, Kravitz, & Behrmann, 2012), many of these studies have made use of lateralized stimuli and/or experimental designs that would hinder the isolation of right-lateralized neural responses to distraction (for example, where distractors are temporally paired with targets, or where activity due to stimuli in the ipsilateral visual field is subtracted from activity due to stimuli in the contralateral visual field). In our experiment we presented our stimuli along the vertical midline and temporally-jittered the presentation of distractors relative to the onsets of the RSVP stimuli in order to facilitate extraction of hypothesized right-lateralized frontal components.
In Berti & Schröger (2001), infrequent distractor stimuli elicited ERP components related to early sensory processing, followed by P3a (~350 ms post-stimulus) and re-orienting negativity (RON; ~500 ms post-stimulus) components. This pattern was interpreted as distractor stimuli activating brain regions associated with detecting novel stimuli (as reflected by the P3a activity) and then triggering a re-orienting of attention away from the distractor (as reflected by the RON). In our experiment, distractor stimuli did not elicit a RON-like component, but rather showed a relatively sustained right-lateralized frontal negativity beginning around 100 ms following distractor onset. We also found that the amplitude of this component negatively correlated with distraction-related behavioral impairments across participants (larger for subjects with less distraction-related impairment), suggesting enhancement of this neural process is beneficial to attentional performance. It is possible that infrequent distractors may trigger reactive reorienting processes, while frequent distractors may trigger more proactive, sustained control mechanisms (see Marini et al., 2016, for a discussion on proactive vs reactive responses to distraction and Bledowski, Prvulovic, Hoechstetter, Scherg, Wibral, Goebel, & Linden, 2004 for a discussion of the brain regions associated with processing visual distractors and targets). The present work helps better connect the ERP attention literature to the findings observed with other techniques, particularly fMRI, while also marshaling the high temporal resolution of ERPs to reveal the timing of the right frontal activity for distraction resistance and its selective elicitation by the distractor itself.
In addition to our target- and distractor-related ERP findings, we also investigated how distraction affected the envelope of the SSVEP elicited by the constantly flickering RSVP stream. While the ERPs are transient brain responses to a particular event in time, the SSVEPs are a continuous brain response at the same frequency as the driving stimulus, in this case the 8.6 Hz RSVP stream. SSVEP amplitudes are known to be enhanced by attention and reduced when an input is suppressed (see review by Andersen, Müller, & Hillyard, 2011), and other studies have used the “frequency-tagging” of stimuli to study the effects of spatial and feature-based attention, including the time course of visual cortical facilitation following attentional cues (Müller et al., 1998), the ability to concurrently direct the attentional spotlight to more than one spatially distinct location (Müller, Malinowski, Gruber, & Hillyard, 2003), and the enhancement and suppression of inputs based on feature-selection (Andersen & Müller, 2010). Here, we found that distractor presentation resulted in a transient dip in the envelope of the SSVEP signal. This finding, combined with the positive correlation across participants between this amplitude decrease and the distractor-related behavioral impairments in target detection, provides neural evidence that the distractors temporarily pulled attention away from the RSVP task stream, resulting in reduced cortical facilitation of the task-relevant input stream. Future work could examine how manipulating other characteristics of the distractor, such as its spatial distance from the task stream or whether it had any task-related features, influences the distractor’s effect on the SSVEP signal, or how the presence of multiple distractors may influence the SSVEP signal.
In conclusion, we found that the presentation of a brief, task-irrelevant distractor pulled participants’ attention away from an on-going task stream. Distraction resulted in both a behavioral and neural impairment in target detection and processing, as well as reduced cortical responses of the ongoing task input stream. Distractors also elicited a temporally specific, right-lateralized frontal negativity that could be related to the right-lateralized attentional control and distractor-processing activations observed with fMRI. Future work will be necessary to determine what other types of distractors are capable of eliciting this right frontal ERP response and to confirm that this component is beneficial for attentional control. As many patient populations show reductions in their attentional control capabilities compared to healthy individuals, such as patients with schizophrenia (Demeter, Guthrie, Taylor, Sarter, & Lustig, 2013), Attention Deficit Hyperactivity Disorder (ADHD; Friedman-Hill et al., 2010) or Alzheimer’s (Perry & Hodges, 1999), it would also be of substantial interest to investigate how this right frontal component presents in these populations.
Acknowledgments
This work was supported by NIH grant NS051048 to M.G.W.
References
- Andersen SK, Müller MM. Behavioral performance follows the time course of neural facilitation and suppression during cued shifts of feature-selective attention. PNAS. 2010;107(31):13878–13882. doi: 10.1073/pnas.1002436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SK, Müller MM, Hillyard SA. Tracing the allocation of attention in visual scenes with steady-state evoked potentials. In: Posner MI, editor. Cognitive neuroscience of attention. 2. New York: Guilford; 2011. pp. 197–216. [Google Scholar]
- Andersen SK, Müller MM, Martinovic J. Bottom-up biases in feature-selective attention. J Neurosci. 2012;32(47):16953–16958. doi: 10.1523/JNEUROSCI.1767-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Hoechstetter K, Scherg M, Wibral M, Goebel R, Linden DEJ. Localizing P300 generators in visual target and distractor processing: a combined event-related potential and functional magnetic resonance imaging study. J Neurosci. 2004;24(42):9353–9360. doi: 10.1523/JNEUROSCI.1897-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham BR, Neely JH, Naginsky Y, Thomas M. Stimulus-driven attentional capture by a static discontinuity between perceptual groups. J Exp Psychol Hum Percept Perform. 2010;36(2):317–329. doi: 10.1037/a0015871. [DOI] [PubMed] [Google Scholar]
- Crist RE, Wu CT, Karp C, Woldorff MG. Face processing is gated by visual spatial attention. Frontiers in Human Neuroscience. 2008;1(10):1–6. doi: 10.3389/neuro.09/010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fockert JW, Theeuwes J. Role of frontal cortex in attentional capture by singleton distractors. Brain Cogn. 2012;80(3):367–373. doi: 10.1016/j.bandc.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Demeter E, Guthrie SK, Taylor SF, Sarter M, Lustig C. Increased distractor vulnerability but preserved vigilance in patients with schizophrenia: evidence from a translational Sustained Attention Task. Schizophr Res. 2013;144(1–3):136–141. doi: 10.1016/j.schres.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Demeter E, Hernandez-Garcia L, Sarter M, Lustig C. Challenges to attention: a continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. Neuroimage. 2011;54(2):1518–1529. doi: 10.1016/j.neuroimage.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JC, Giesbrecht B. Distractor suppression when attention fails: behavioral evidence for a flexible selective attention mechanism. PLoS One. 2015;10(4):e0126203. doi: 10.1371/journal.pone.0126203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escera C, Alho K, Winkler I, Näätänen R. Neural mechanisms of involuntary attention to acoustic novelty and change. J of Cog Neurosci. 1998;10(5):590–604. doi: 10.1162/089892998562997. [DOI] [PubMed] [Google Scholar]
- Folk CL, Leber AB, Egeth HE. Made you blink! Contingent attentional capture produces a spatial blink. Perception & Psychophysics. 2002;64(5):741–753. doi: 10.3758/bf03194741. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control setti. J Exp Psychol Hum Percept Perform. 1992;18(4):1030–1044. [PubMed] [Google Scholar]
- Folk CL, Remington RW, Wu SC. Additivity of abrupt onset effects supports nonspatial distraction, not the capture of spatial attention. Atten Percept Psychophys. 2009;71(2):308–313. doi: 10.3758/APP.71.2.308. [DOI] [PubMed] [Google Scholar]
- Friedman-Hill SR, Wagman MR, Gex SE, Pine DS, Leibenluft E, Ungerleider LG. What does distractibility in ADHD reveal about mechanisms for top-down attentional control? Cognition. 2010;15(1):93–103. doi: 10.1016/j.cognition.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski PD, Falkenstein M. Effects of task complexity on ERP components in Go/Nogo tasks. Int J Psychophysiol. 2013;87(3):273–8. doi: 10.1016/j.ijpsycho.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Gaspar JM, McDonald JJ. Suppression of salient objects prevents distraction in visual search. J Neurosci. 2014;34(16):5658–5666. doi: 10.1523/JNEUROSCI.4161-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladwin TE, Lindsen JP, de Jong R. Pre-stimulus EEG effects related to response speed, task switching and upcoming response hand. Biol Psychol. 2006;72(1):15–34. doi: 10.1016/j.biopsycho.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Grent-’t-Jong T, Woldorff MG. Timing and Sequence of Brain Activity in Top-Down Control of Visual-Spatial Attention. PLOS Biology. 2007;5:114–126. doi: 10.1371/journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MA, White AL, Heeger DJ, Carrasco M. Interactions between voluntary and involuntary attention modulate the quality and temporal dynamics of visual processing. Psychon Bull Rev. 2015;22(2):437–444. doi: 10.3758/s13423-014-0698-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japee S, Holiday K, Satyshur MD, Mukai I, Ungerleider LG. A role of right middle frontal gyrus in reorienting of attention: a case study. Front Syst Neurosci. 2015;9:23. doi: 10.3389/fnsys.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonides J, Irwin DE. Capturing attention. Cognition. 1981;10(1–3):145–150. doi: 10.1016/0010-0277(81)90038-x. [DOI] [PubMed] [Google Scholar]
- Lamy D, Leber A, Egeth HE. Effects of task relevance and stimulus-driven salience in feature-search mode. J Exp Psychol Hum Percept Perform. 2004;30(6):1019–1031. doi: 10.1037/0096-1523.30.6.1019. [DOI] [PubMed] [Google Scholar]
- Lamy D, Tsal Y, Egeth HE. Does a salient distractor capture attention early in processing? Psychon Bull Rev. 2003;10(3):621–629. doi: 10.3758/bf03196524. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused? Selective attention under load. Trends in Cog Sci. 2005;9(2):75–92. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Leber AB. Neural predictors of within-subject fluctuations in attentional control. J Neurosci. 2010;30(34):11458–11465. doi: 10.1523/JNEUROSCI.0809-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Balestreri A, Luck SJ. Interactions between space-based and feature-based attention. J Exp Psychol Hum Percept Perform. 2015;41(1):11–16. doi: 10.1037/xhp0000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci. 2014;8(213):1–14. doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini F, Demeter E, Roberts KC, Chelazzi L, Woldorff MG. Orchestrating proactive and reactive mechanisms for filtering distracting information: Brain-behavior relationships revealed by a mixed-design fMRI study. J Neurosci. doi: 10.1523/JNEUROSCI.2966-15.2016. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MM, Andersen S, Trujillo NJ, Valdes-Sosa P, Malinowski P, Hillyard SA. Feature-selective attention enhances color signals in early visual areas of the human brain. PNAS. 2006;103(38):14250–14254. doi: 10.1073/pnas.0606668103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MM, Malinowski P, Gruber T, Hillyard SA. Sustained division of hte attentional spotlight. Nature. 2003;424(6946):309–312. doi: 10.1038/nature01812. [DOI] [PubMed] [Google Scholar]
- Müller MM, Picton TW, Valdes-Sosa P, RIera J, Tedder-Sulejurvi WA, Hillyard SA. Effects of spatial selective attention on the steady-state visual evoked potential in the 20–28 Hz range. Cognitive Brain Research. 1998;6:249–261. doi: 10.1016/s0926-6410(97)00036-0. [DOI] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The Frontal Cortex of the Rat and Visual Attentional Performance: Dissociable Functions of Distinct Medial Prefrontal Subregions. Cerebral Cortex. 2002;12:1254–1268. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Perri RL, Berchicci M, Spinelli D, Di Russo F. Individual differences in response speed and accuracy are associated to specific brain activities of two interacting systems. Front Behav Neurosci. 2014;8:251. doi: 10.3389/fnbeh.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri RL, Berchicci M, Lucci G, Spinelli D, Di Russo F. Why do we make mistakes? Neurocognitive processes during the preparation-perception-action cycle and error-detection. Neuroimage. 2015;113:320–8. doi: 10.1016/j.neuroimage.2015.03.040. [DOI] [PubMed] [Google Scholar]
- Perri RL, Berchicci M, Lucci G, Spinelli D, Di Russo F. The premotor role of the prefrontal cortex in response consistency. Neuropsychology. 2015;29(5):767–75. doi: 10.1037/neu0000168. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease - A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- Pincham HL, Szucs D. Disruption reduces accuracy and P3b amplitudes in the attentional blink. Neurosci Lett. 2014;581:26–31. doi: 10.1016/j.neulet.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschenberger R. When something old becomes something new: spatiotemporal object continuity and attentional capture. J Exp Psychol Hum Percept Perform. 2003;29(3):600–615. doi: 10.1037/0096-1523.29.3.600. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink? J Exp Psychol Hum Percept Perform. 1992;18(3):849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Sato S, Kawahara JI. Attentional capture by completely task-irrelevant faces. Psychol Res. 2014 doi: 10.1007/s00426-014-0599-8. [DOI] [PubMed] [Google Scholar]
- Seibold VC, Rolke B. Does temporal preparation facilitate visual processing in a selective manner? Evidence from attentional capture. Acta Psychol (Amst) 2014;151:51–61. doi: 10.1016/j.actpsy.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of Voluntary and Stimulus-Driven Attentional Control in Human Cortex. Psychological Science. 2005;16(2):114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Shomstein S, Kravitz DJ, Behrmann M. Attentional control: temporal relationships within the fronto-parietal network. Neuropsychologia. 2012;50(6):1202–1210. doi: 10.1016/j.neuropsychologia.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Peters M, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J Neurosci. 2011;31(26):9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J. Cross-dimensional perceptual selectivity. Perception & Psychophysics. 1991a;50(2):184–193. doi: 10.3758/bf03212219. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Exogenous and endogenous control of attention: the effect of visual onsets and offsets. Perception & Psychophysics. 1991b;49(1):83–90. doi: 10.3758/bf03211619. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Perceptual selectivity for color and form. Perception & Psychophysics. 1992;51(6):599–606. doi: 10.3758/bf03211656. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Temporal and spatial characteristics of preattentive and attentive processing. Visual Cognition. 1995;2:221–233. [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: evidence from visual search. J Exp Psychol Hum Percept Perform. 1984;0(5):601–621. doi: 10.1037//0096-1523.10.5.601. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Liotti M, Seabolt M, Busse L, Lancaster JL, Fox PT. The temporal dynamics of the effects in occipital cortex of visual-spatial selective attention. Brain Res Cogn Brain Res. 2002;15(1):1–15. doi: 10.1016/s0926-6410(02)00212-4. [DOI] [PubMed] [Google Scholar]