Abstract
Objective
Children born preterm are at elevated risk for several developmental and health concerns. Early sleep patterns may be associated with these concerns. The current study assesses the associations between toddler circadian sleep/activity patterns and later developmental, behavioral, attentional, and health concerns in this at-risk population.
Methods
We examined circadian sleep/activity patterns at 2 years of age in 99 children born preterm. Child cognitive skills were tested at 3 years of age, and behavior, attention, and health concerns were reported at 3 and 6 years of age. First, sleep/activity data collected via actigraphy were assessed using time series analyses (TSA). For this, we assessed how each child’s sleep/activity pattern compared to a specified 24-hour circadian cycle (SCC) with an adjustment for daytime napping. Second, in a series of regression models child sleep/activity parameters from the TSA were assessed with child gender, prematurity, and family sociodemographic assets as covariates.
Results
Toddlers with patterns that closely aligned with the SCC had higher abbreviated IQ scores at 3 years of age. Additionally, at 6 years these children had a lower risk for illness-related medical visits. Higher toddler average activity level was associated with fewer teacher-reported ADHD symptoms and a lower risk for illness-related medical visits.
Conclusion
The novel approach used in this study to index child circadian patterns provides a pattern-based analysis of sleep/activity, which may prove to be developmentally consequential. With replication, these findings may help practitioners promote optimal cognitive and health development via circadian sleep supports in infants born preterm.
Keywords: preterm, sleep, cognitive skills, attention, behavior problems, health concerns, circadian
Circadian Sleep Patterns in Toddlers Born Preterm: Longitudinal Associations with Developmental and Health Concerns
Individual differences in circadian sleep patterns are well documented; however, the developmental sequela associated with these differences is not well understood in young children. In infants born preterm, distinct circadian patterns have been reported (when compared to those born at term), and preterm infants are at elevated risk for developmental and health difficulties later in life. Building on the preterm infant sleep literature and the growing research base on the developmental correlates of circadian sleep cycles, the present study assesses if circadian sleep patterns are associated with later developmental, behavioral, and/or health concerns in children born preterm.
Premature Infant Sleep
In infancy, premature infants engage in longer, lighter, and more active sleep than infants born at term,1–4 and they develop a 24-hour circadian cycle earlier.1 As preterm infants develop, their sleep patterns gradually begin to resemble the patterns of infants born at term, although their sleep tends to be more variable and less consistent across the first year of life when compared to infants born at term.5 During their neonatal intensive care unit (NICU) stay, sleep patterns of preterm infants appear to follow a distinctly different pattern than sleep at other stages of development,6,1 and these differences may predict later developmental outcomes.7
Several studies have assessed circadian sleep patterns in preterm infants to inform NICU lighting practices relative to gestational age.8 In the NICU, preterm infants develop a 24-hour activity/rest cycle at an earlier gestational age than infants born at term.1 In adolescents born preterm, studies of circadian patterns report a bias toward an early chronotype or advanced sleep phase when compared to those born at term.9,10 Similarly, the Helsinki Study reported higher rates of an early chronotype in adults who were born preterm.11 In sum, these studies document circadian differences in individuals born preterm, but little is known about any affiliated developmental or health difficulties. Additionally, there is a marked shortage of research in early childhood. The present study fills both of these gaps with a sleep assessment at two years of age (adjusted for gestational age) and indices of developmental functioning and health later in life.
Developmental Correlates of Circadian Patterns
The most common correlates of circadian patterns in healthy children/adolescents (born at term) are attentional difficulties and behavioral problems. Several studies have documented that children with later bedtimes, later chronotypes, or later family lifestyle patterns have more attentional and behavioral problems.12–16 Additionally, studies in adolescence document an increased risk for health-related issues, anti-social behaviors, and a decline in school performance with later bedtimes.17,18 For example, Merikanto and colleagues17 documented more self-reported health concerns (i.e., headaches, bodily pains, abdominal irritation) and more missed school with later bedtimes. In young children there are far fewer studies; but a similar pattern emerges with associations between irregular or late bedtimes and elevated behavioral problems.19 The present study builds on this literature but assesses circadian sleep patterns in a distinctly different manner.
Assessing Circadian Patterns
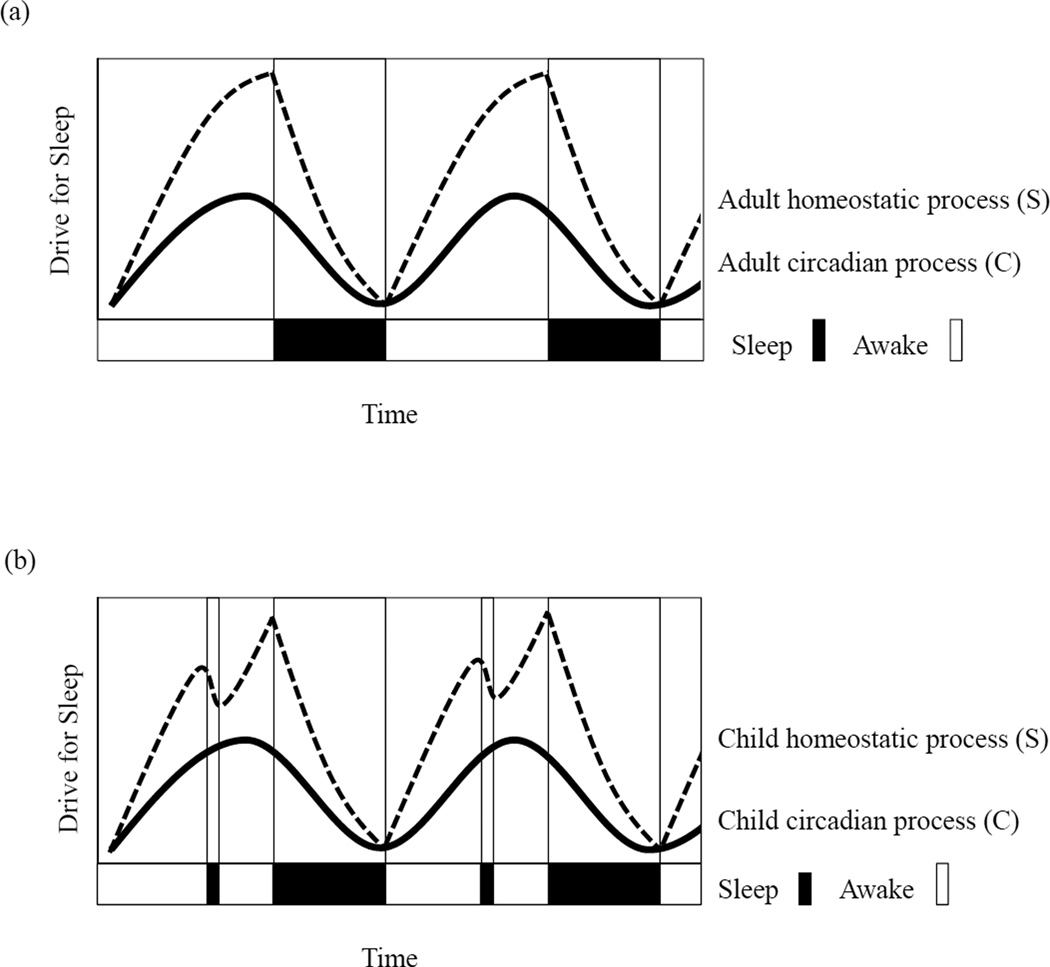
Theoretical models of sleep-wake regulation highlight the robust influence of homeostatic and circadian sleep processes; however, researchers and clinicians readily recognize challenges in assessing these patterns in children.20 The most commonly used model in sleep research is the two-process model of sleep regulation. In this model, sleep/wake regulation is a function of two biological processes – homeostatic (process S) and circadian (process C). The homeostatic drive (or need) for sleep builds the longer a person is awake, and the circadian drive for sleep is aligned roughly with 24-hour day/night cycles. For adults and children, circadian patterns (process C) follow a relatively consistent sinusoidal pattern (Figure 1a). For homeostatic patterns (process S), children follow a distinctly different pattern because sleep pressures rise more quickly in children (Figure 1b). This increased daytime sleep drive often culminates in a midday nap. Researchers often grapple with how to model or capture meaningful elements of child sleep patterns that reflect both of these processes. Some researchers assess amount or variability in sleep, but this method does not adequately capture patterns over time. To more readily index sleep patterns, other studies have assessed bedtime or morning rise time19 or have categorized children based on family lifestyle/sleep timing elements,16 all of which may capture circadian but not homostatic processes. Recently, researchers have started applying times series analysis (TSA) to actigraphically collected data which allows for sinusoidial modeling of activity/rest patterns using specified oscillations.21 This approach captures circadian cycling well, but like other methods, it does not adequately account for the homeostatic pressures present in young children. The current study builds on this approach by using TSA with two sinusoidal waves. We modeled circadian processes using a sinusoidal oscillation with a 24-hour period and homeostatic processes using an attenuated oscillation with a 12-hour period. Figure 2 provides a visual depiction of the employed TSA with the two specified oscillations (Figure 2c), and a conceptual depiction of the combined wave form (Figure 2d). This approach will allow us to assess the regularity of a child’s 24-hour circadian cycle (process C) with an adjustment for daytime napping (process S).
Figure 1.
The two-process model of sleep illustrated for adults (a) and young children (b).
Figure 2.
A visual depiction of the employed data processing approach: (a) raw actigraphic data for five 24-hour periods, (b) 60-minute smoothing of the same data, (c) overlay of the two specified oscillations; one with a 24-hour period (single solid line) and an attenuated 12-hour period (double solid line), and (d) a conceptual depiction of the combined wave form estimated within the cosinor models (dotted line).
Hypotheses
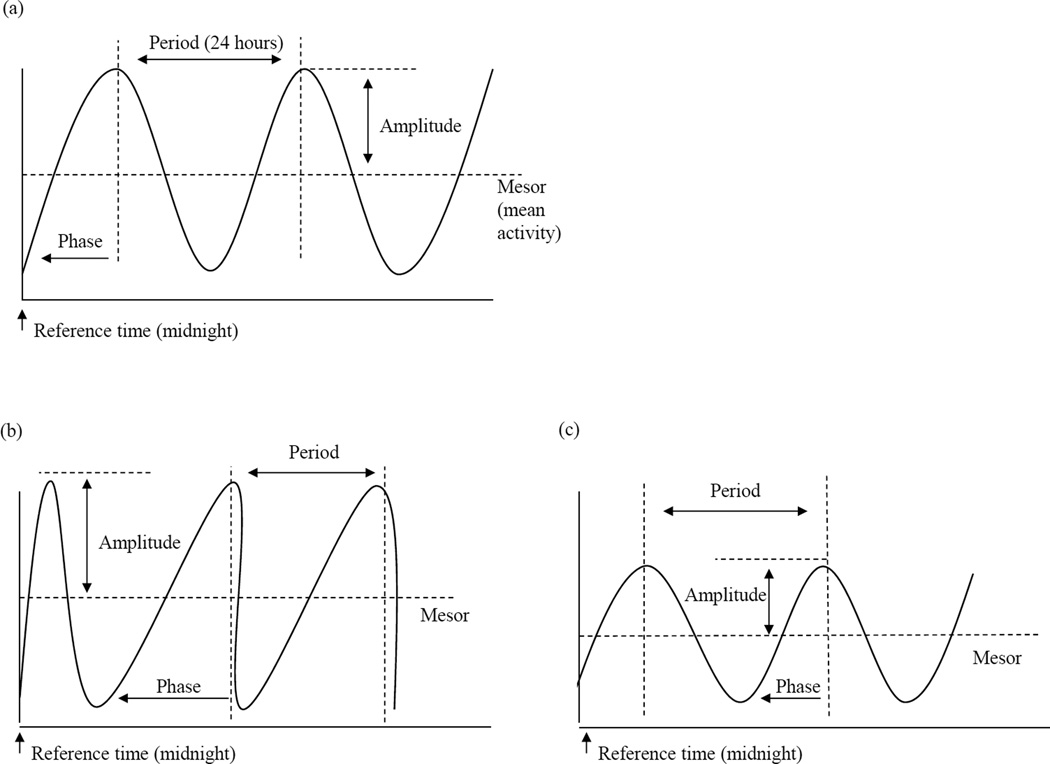
Building on previous sleep research, we anticipated that toddler circadian patterns would be associated with later cognitive, behavioral, attention, and health concerns. Toddler circadian patterns will be evaluated using three wave form or pattern parameters (mesor, amplitude, phase; see Figure 3a) and overall goodness-of-fit to a specified circadian sleep pattern. This specified pattern follows established norms for circadian patterns (roughly 24 hours per cycle) and a developmentally appropriate dip in activity (or sleep) approximately 6 hours after waking (roughly12 hours per cycle). We hypothesize toddlers with sleep patterns that do not consistently align with this pattern (low overall goodness-of-fit) will have less optimal outcomes (i.e., lower cognitive skills, more behavioral and attentional problems, and more health concerns). With respect to wave form parameters (Figure 3a), we hypothesized that higher average activity (mesor) and variability (amplitude) will be associated with less optimal outcomes. For phase, we hypothesized that shorter phase or more abrupt transitions from sleep to highest activity would be associated with less optimal outcomes.
Figure 3.
Diagram of a single wave model of circadian rhythm (24-hour) with estimated parameters (a), an example wave form with relatively higher mesor and amplitude and longer phase (b), and an example wave form with relatively lower mesor and amplitude and a shorter phase (c).
METHODS
Participants
Participants included 99 infants born preterm but without significant neurological complications. A total of 181 mothers and their infants were initially recruited from three neonatal intensive care units (NICUs) in the central region of the United States between 2005 and 2008 as part of a larger longitudinal study focusing on infants born preterm or low birthweight. Data from four of the initially enrolled 181 families were removed because we later discovered that a grade IV IVH had occurred and/or the child was diagnosed with cerebral palsy. Six additional cases were removed because the infants were born ≥ 36 weeks, despite having low birthweights. The present study includes a subset of these infants who participated in the sleep portion of the study. Enrollment into the sleep study was based on time of entry into the study. Participant family characteristics paralleled the population of the region in education and poverty, although participant families were more racially diverse (for additional details see Poehlmann-Tynan et al, 2015).22 Additional descriptive statistics are provided in Table 1.
Table 1.
Demographic Information for Particpant Subgroups at 3 and 6 Years
| 3 Years | |||
|---|---|---|---|
| n = 99 | Range or Frequency | M or % | SD |
| Child | |||
| Gestational age (weeks) | 25.00 – 35.86 | 31.45 | 3.05 |
| Birthweight (grams) | 680 – 3328 | 1735.17 | 572.22 |
| Neonatal Medical Risks | 0 – 9 | 3.05 | 2.28 |
| Gender (% male) | 53% | .50 | |
| Caucasian | 73 | 74% | |
| African American | 7 | 7% | |
| Latino | 2 | 2% | |
| Asian | 1 | 1% | |
| More than one Racial Heritage | 16 | 16% | |
| Family | |||
| Mother Age (years) | 17 – 42 | 30.20 | 6.21 |
| Mother Education (years) | 8 – 21 | 14.34 | 2.62 |
| Household Annual Income | 6,000 – 200,000 | 58,412.57 | 36,452.87 |
| Children in Home | 1–11 | 2.00 | 1.36 |
| Sociodemographic Assets | −5.01 – 5.36 | 0.19 | 2.82 |
| n = 68 | 6 Years | ||
| Child | |||
| Gestational age (weeks) | 25.00 – 35.86 | 31.62 | 2.89 |
| Birthweight (grams) | 710 – 3328 | 1763.49 | 572.22 |
| Neonatal Medical Risks | 0 – 9 | 2.97 | 2.17 |
| Gender (% male) | 53% | .50 | |
| Caucasian | 55 | 81% | |
| African American | 2 | 3% | |
| Latino | 1 | 2% | |
| Asian | 1 | 1% | |
| More than one Racial Heritage | 9 | 13% | |
| Family | |||
| Mother Age (years) | 18 – 42 | 31.19 | 6.10 |
| Mother Education (years) | 10 – 21 | 15.06 | 2.40 |
| Household Annual Income | 6,000 – 200,000 | 66,495.24 | 38,002.14 |
| Children in Home | 1–5 | 1.9 | 1.06 |
| Sociodemographic Assets | −4.63 – 5.36 | 0.81 | 2.16 |
Measures
Infant Prematurity and Medical Risks
Infant medical records were reviewed to collect infant prematurity and medical intervention/complications data. Because infant birthweight and gestational age were highly correlated (r = .88, p < .001), we standardized and summed them to generate an index of infant prematurity, with lower scores representing more prematurity. We also created a 10 item neonatal medical risk variable. For this index we dichotomized each of the following into 1 if present and 0 if absent (% indicates the percentage of the sample with this medical risk): diagnosis of apnea (68%), respiratory distress (52%), chronic lung disease (11%), gastroesophageal reflux (9%), multiple birth (19%), supplementary oxygen at NICU discharge (10%), apnea monitor at NICU discharge (44%), 5 minute Apgar score less than 6 (3%), ventilation during NICU stay (52%), and NICU stay of more than 30 days (37%). Data screening revealed that the infant prematurity and neonatal medical risk indices were highly correlated, (r = .70, p < .001). It is probable that this strong association would create analytic issues; therefore, we retained only infant prematurity in the final models, but information on infant medical complications/risk are provided in Table 1.
Behavior Problems
The preschool form of the Child Behavior Checklist (CBCL)23 was used to assess children’s behavior and sleep problems at 3 years of age and the school-age CBCL form was used at 6 years of age. The CBCL is a commonly used standardized behavior rating scale that is completed by an adult with whom the child lives.24–27 The CBCL lists 99 problem behaviors. Parents rated each problem behavior on a three point scale, not true (0), somewhat or sometimes true (1), or very true or often true (2), in reference to the child’s behaviors that occurred during the past two months. Raw scores were summed and converted into standardized T scores which ranged from 50 to 94. The present study utilized the Internalizing, Externalizing, and Total Problems scales. The CBCL has high internal consistency, with Cronbach’s alphas ranging from .78 to .97, and it has been used with children at risk for developmental concerns.28
Attention Problems
Child attention problems were indexed with three measures. First, the Conners 3-P and the Connors 3-T were used to assess parent and teacher ratings of child functioning including symptoms of inattention, hyperactivity/impulsivity, learning problems/executive functioning, depression, anxiety, aggression, peer relationships, and family relationships at 6 years of age. Specifically, the hyperactivity/impulsivity scale includes items corresponding to the core symptoms of ADHD.29 The Connors 3 was normed on a sample including 1,200 parents and 1,200 teachers from the 2000 U.S. Census. It has strong internal consistency (Cronbach’s alpha ranged from .71 to .98) and construct validity.29 Additionally, the attention problems and the ADHD subscales of the CBCL were used to index parent-reports of attention problems at 3 and 6 years of age.
Circadian Patterns
Child movements were recorded in one-minute epochs using a micromini-motionlogger® (Ambulatory Monitoring, Incorporated) and interpreted as sleep or awake using the Sadeh algorithm provided in Action-W version 2.7.3. Following actigraphy best practice guidelines, actigraphs were worn for 7 consecutive 24-hour periods, and a parent-report sleep log was used to aid in interpreting the actigraphy data.30 Actigraphy is commonly used to index child activity/sleep patterns in healthy children, children with developmental concerns, and children born preterm.31,32 A minimum of five nights were required for analyses. The work of Thomas and Burr33 has demonstrated that only 48 hours of data are required to assess circadian patterns via a cosinor model; therefore, we feel five nights are adequate. Each child’s raw minute-by-minute activity scores were smoothed using a moving average with a 60-minute window and then subjected to the cosinor model.
Cognitive Skills
Child cognitive skills at 3 years of age were estimated using the Abbreviated Battery IQ scale (ABIQ) from the Stanford-Binet Intelligence Scales, 5th edition.34 The ABIQ comprises the sum of the Nonverbal Fluid Reasoning and Verbal Knowledge scaled scores. We assessed each child’s ABIQ age equivalent and their ABIQ based on his/her chronological age and corrected age (adjusted for gestational age).
Illness-related Health Care Visits
A general health questionnaire was completed by the child’s primary caregiver at 3 and 6 years of age. The questionnaire assessed number of: (1) emergency room (ER) visits, (2) hospital in-patient stays, (3) acute care visits, and (4) all illness-related medical visits for the year. The first three categories are distinct, but the fourth category (total illness-related medical visits) includes all of the other three and all other illness-related medical care visits (i.e., general practitioner visits). Child illness-related medical visits ranged from 0 to 31 at 3 years of age and 0 to 20 visits at 6 years. The data for illness-related health care visits had a positive skew and followed a Poisson distribution with most children having only a few medical visits. These data were assessed using a Poisson regression with incidence rate ratios (IRR) to account for this distribution. Additional medical care visit information is provided in Table 5.
Table 5.
Descriptive Statistics and Poisson Regression Incidence Rate Ratios (IRR) for Child Illness-related Health Care Visits
| 3 Years | |||||
|---|---|---|---|---|---|
| n = 92 | Total Medical | Acute Care | Hospital | ER | |
| Range, M (SD) | 0 – 31, 3.32 (4.74) | 0 – 6, 0.73 (1.27) | 0 – 3, 0.14 (0.43) | 0 – 3, 0.17 (0.52) | |
| R2 | fit to PCP | 0.940 | 1.041 | 1.921* | 1.061 |
| Mesor | average activity | 0.853* | 0.699** | 0.454 | 0.929 |
| amp1 | amplitude for 24-hour cycle | 0.935 | 0.919 | 2.423* | 0.888 |
| amp2 | amplitude for 12-hour cycle | 0.970 | 1.299* | 0.451 | 0.592+ |
| phs1 | phase for 24-hour cycle | 0.954 | 0.965 | 1.020 | 1.019 |
| phs2 | phase for 12-hour cycle | 1.158* | 1.304 | 1.298 | 1.548 |
| Sociodemographic assets | 1.096 | 1.108 | 1.053 | 1.219 | |
| Infant prematurity index | 0.999 | 0.889+ | 0.535** | 0.919 | |
| Infant gender | 1.134 | 1.184 | 7.397* | 1.631 | |
| Number of children in home | 1.157* | 1.270+ | 2.054+ | 1.685* | |
| pseudo r2 | 0.05 | 0.08 | 0.15 | 0.09 | |
| n = 54 | 6 Years | ||||
| Range, M (SD) | 0 – 20, 5.04 (7.43) | 0 – 20, 3.83 (4.55) | 0 –16, 0.37 (2.18) | 0 – 20, 0.76 (2.78) | |
| R2 | fit to PCP | 0.749*** | 0.877+ | 0.613 | 0.430** |
| Mesor | average activity | 0.768*** | 1.008 | 0.179** | 0.573*** |
| amp1 | amplitude for 24-hour cycle | 0.865* | 0.697*** | 1.490 | 1.405 |
| amp2 | amplitude for 12-hour cycle | 1.032 | 0.994 | 0.431 | 1.077 |
| phs1 | phase for 24-hour cycle | 0.967 | 1.028 | 0.327+ | 1.043 |
| phs2 | phase for 12-hour cycle | 0.926 | 0.975 | 1.490 | 0.855 |
| sociodemographic assets | 0.902* | 0.973 | 0.261* | 0.824 | |
| Infant prematurity index | 0.873*** | 0.943 | 0.458* | 0.794* | |
| Infant gender | 1.181 | 1.045 | 2.098 | 1.590 | |
| Number of children in home | 1.272*** | 1.219** | 1.718 | 1.274 | |
| psuedor2 | 0.15 | 0.10 | 0.60 | 0.41 | |
p< .10,
p< 0.05,
p< 0.01,
p< 0.001,
PCP = prototypical circadian pattern,
all models control for sociodemographic assets, prematurity, child gender, and number of children in the home,
r2 modelis for the sleep variables only
Sociodemographic Assets
Mothers completed a demographic questionnaire at hospital discharge, which included information about maternal age, maternal education, family income, and household composition. Because maternal age, education, and income were highly correlated, these variables were standardized and summed to generate a sociodemographic asset index (descriptive statistics provided in Table 1).
Procedure
Multiple methods were used to collect data prior to hospital discharge, and 2, 3, and 6 years of age. At hospital discharge, infant birthweight and gestational age were collected from hospital records, and mothers completed a demographic questionnaire. At 2 years of age, toddler sleep was assessed via actigraphy and a parent-report diary. Child concerns were assessed at 3 years of age using parent-reports of health (illness-related medical visits), attention, and behavior problems. Additionally, at 3 years of age each child completed a laboratory visit which included an assessment of cognitive skills. At 6 years of age, parents once again reported on child illness-related medical visits, attention, and behavior problems and teachers were asked to report on any attentional problems.
Statistical Analysis
The employed statistical approach included two steps. First, a two-period cosinor model35 was estimated for each child using his/her actigraph data. The raw actigraph data (Figure 2a) was first smoothed using a moving average with a 60-minute window (Figure 2b). Cosinor models were used to extract parameters on each child. These parameters included mesor, amplitutes for each period, phases for each period, and the R2 (overall fit to the specified model). Cosinor parameters were orthoganalized to avoid multi-collinearity. Second, estimated parameters from each child’s cosinor model were used to predict cognitive, behavioral, attention, and health concerns at 3 and 6 years of age with covariates that included household sociodemographic assets, prematurity, infant gender, and number of children in the home. Linear regression was used for continuous outcomes, and Poisson regression was used for count data (i.e., illness-related health care visits).
Interpretation of Cosinor Parameters
The employed TSA (cosinor models) included estimates for several elements of a wave form. In Figure 3a, a sample 24-hour cosinor model is provided with each of the estimated parameters: mesor, amplitude, and phase. To align with typical human circadian cycles, the period was set at 24 hours, but the other wave form elements (mesor, amplitude, and phase) where allowed to vary from child to child. Period refers to the distance between peak activity or the time elapsed for one complete cycle. The mesor measures the rhythm-adjusted mean in activity levels. Mesor therefore may be interpreted as the average activity level over time. The amplitude measures half the distance between the minimum activity (sleep) and peak activity fitted by the function. Amplitude can be interpreted as the intra-individual heterogeneity in activity (i.e., the half-range between sleep and peak activity). Phase measures the time to peak activity (or wake time or sleep onset) from a reference time point. Phase can shift forward or back when wake or sleep onset moves in relation to the reference time (y axis). While a change in mesor would shift the curve vertically, a change in phase would shift the curve horizontally such that a different part of the cycle crosses the y-axis. Figure 3b depicts a sample wave form with a relatively longer phase, higher mesor, and higher amplitude. Figure 3c is a sample wave form with a relatively short phase, lower mesor, and lower amplitude.
We use the same reference time for all children, midnight, so that the estimated phase is measured using the same reference point. We created an average or age-appropriate pattern with a 24-hour circadian ‘wave’ and a second attenuated oscillation with a 12-hour period to represent the homeostatic ‘wave’ (Figure 2c). This pattern was built based on existent research that demonstrates that most humans follow a roughly 24-hour circadian cycle and young children (between 2 and 3 years of age) typically nap roughly 6 hours after waking (in the afternoon). With the addition of the second wave we aimed to account for the expected midday nap or drop in activity. We labeled this the specified 24-hour circadian pattern (SCC) with an adjustment for daytime napping. We interpreted this as circadian because the circadian oscillation was the predominate wave. We used R2 as a measure of how well each child’s smoothed data fit the SCC.
RESULTS
Behavior Problems
Behavior problem scores at ages 3 and 6 (Table 2) were not associated with most of the circadian rhythm parameters with the exception of the amplitude on the 12-hour cycle. This implies that children with more night activity at age 2, including possible awakenings, were more likely to have higher externalizing and total problem behaviors at age 3 as indexed by the CBCL. For example, higher amplitude (by one standard deviation) for the 12-hour period was associated with a 0.23 standard deviation increase in total problem behavior at age 3. At age 6, a similar effect emerged for internalizing behavior problems. None of the sleep parameters were associated with internalizing problems at 3 years of age or externalizing problems and total problems at 6 years of age. Additionally, more family sociodemographic assets were associated with fewer parent-reported externalizing and total problems at 3 years of age and fewer externalizing problems at 6 years of age.
Table 2.
Regression Models for Behavior Problems at 3 and 6 Years
| 3Years | ||||
|---|---|---|---|---|
| Internalizing | Externalizing | Total | ||
| n = 91 | βstd | βstd | βstd | |
| R2 | fit to PCP | −0.011 | −0.054 | −0.013 |
| Mesor | average activity | −0.101 | 0.015 | −0.031 |
| amp1 | amplitude for 24-hour cycle | 0.038 | −0.053 | −0.017 |
| amp2 | amplitude for 12-hour cycle | 0.204* | 0.230* | 0.26* |
| phs1 | phase for 24-hour cycle | 0.102 | 0.021 | 0.086 |
| phs2 | phase for 12hour cycle | 0.100 | 0.031 | 0.037 |
| SES | sociodemographic assets | −0.165 | −0.274* | −0.249* |
| r2b | 0.10 | 0.10 | 0.12 | |
| n = 66 | 6 Years | |||
| R2 | fit to PCP | −0.156 | −0.017 | −0.134 |
| Mesor | average activity | 0.058 | −0.051 | −0.036 |
| amp1 | amplitude for 24-hour cycle | 0.080 | 0.109 | 0.109 |
| amp2 | amplitude for 12-hour cycle | 0.253* | 0.107 | 0.168 |
| phs1 | phase for 24-hour cycle | −0.015 | −0.077 | −0.066 |
| phs2 | phase for 12hour cycle | 0.001 | 0.025 | 0.053 |
| SES | Sociodemographic assets | −0.284+ | −0.311* | −0.303+ |
| r2b | 0.10 | 0.02 | 0.06 | |
p< 0.05,
p< .10,
PCP = prototypical circadian pattern,
all models control for sociodemographic assets, prematurity, child gender, and number of children in the home,
r2 modelis for the sleep variables only.
Attentional Problems
Unexpectedly, child fit to the SCC was not associated with child attentional difficulties or ADHD symptoms at 3 or 6 years of age (Table 3). Family sociodemographic assets were the most robust predictor at 3 years of age with more assets preceding fewer parent-reported attentional problems. Elevated teacher-reports of child ADHD symptoms at 6 years of age were associated with lower MESOR or lower average activity at two years of age.
Table 3.
Regression Modelsa for Attention Problems and ADHD Symptoms at 3 and 6 Years
| 3 Years | |||||
|---|---|---|---|---|---|
| ADHD (CBCL) |
Attention (CBCL) |
ADHD (Connors 3-T) |
ADHD (Connors 3-P) |
||
| n = 90 | βstd | βstd | βstd | βstd | |
| R2 | fit to PCP | −0.190 | −0.116+ | ||
| Mesor | average activity | −0.072 | −0.064 | ||
| amp1 | amplitude for 24-hour cycle | −0.062 | −0.058 | ||
| amp2 | amplitude for 12-hour cycle | 0.096 | 0.084 | ||
| phs1 | phase for 24-hour cycle | 0.038 | 0.066 | ||
| phs2 | phase for 12-hour cycle | −0.005 | −0.071 | ||
| SES | sociodemographic assets | −0.259* | −0.280* | ||
| r2b | 0.05 | 0.05 | |||
| 6 Years | |||||
| n | 66 | 66 | 43 | 62 | |
| R2 | fit to PCP | −0.175 | −0.190 | 0.005 | −0.210 |
| Mesor | average activity | 0.034 | −0.037 | −0.400* | 0.062 |
| amp1 | amplitude for 24-hour cycle | −0.026 | 0.134 | 0.243 | 0.133 |
| amp2 | amplitude for 12-hour cycle | −0.081 | −0.030 | −0.164 | 0.067 |
| phs1 | phase for 24-hour cycle | 0.114 | 0.120 | −0.273 | 0.018 |
| phs2 | phase for 12-hour cycle | 0.134 | 0.003 | 0.192 | 0.051 |
| SES | sociodemographic assets | −0.037 | 0.002 | −0.118 | −0.030 |
| r2b | 0.08 | 0.08 | 0.22 | 0.08 | |
p< .10,
p< 0.05,
PCP = prototypical circadian pattern, CBCL = Child Behavior Checklist, PCP = prototypical circadian pattern,
all models control for sociodemographic assets, prematurity, child gender, and number of children in the home,
r2 modelis for the sleep variables only.
Cognitive Skills
At 3 years of age, higher child ABIQ scores were associated with better fit to the SCC (Table 4). An R2 value that is one standard deviation higher (about 15% more variance explained) is associated with approximately one quarter standard deviation increase in ABIQ scores. Children from families with more sociodemographic assets also had higher ABIQ scores.
Table 4.
Regression Modelsa for Cognitive Skills at 3 Years
| 3 Years | ||||
|---|---|---|---|---|
| ABIQ chron |
ABIQ corrected |
age equivalent |
||
| n = 90 | βstd | βstd | βstd | |
| R2 | fit to PCP | 0.244* | 0.235* | 0.226* |
| Mesor | average activity | −0.054 | −0.078 | −0.021 |
| amp1 | amplitude for 24-hour cycle | −0.090 | −0.081 | −0.112 |
| amp2 | amplitude for 12-hour cycle | 0.016 | 0.006 | 0.002 |
| phs1 | phase for 24-hour cycle | −0.155 | −0.136 | −0.131 |
| phs2 | phase for 12-hour cycle | 0.037 | 0.020 | 0.010 |
| SES | sociodemographic assets | 0.355* | 0.329** | 0.384* |
| r2b | 0.16 | 0.15 | 0.11 | |
p< 0.05,
p< 0.01,
ABIQ = abbreviated intelligence quotient, PCP = prototypical circadian pattern,
all models control for sociodemographic assets, prematurity, child gender, and number of children in the home,
r2 modelis for the sleep variables only
Illness-related Health Care Visits
Several illness-related health care visits at ages 3 and 6 years were associated with circadian parameters (Table 5). Unexpectedly, good fit to the SCC was associated with more hospital visits at 3 years of age. Additionally, at age 3, higher average activity (mesor) was associated with lower risk for total medical visits, acute care visits, and hospital visits. For example, a one unit higher average activity level was associated with a risk rate less than half (Hospital IRR = 0.45) that of someone with a unit lower activity level. For amplitude (variability), distinctly different patterns emerged at 3 and 6 years of age. At 3 years of age, higher amplitudes were associated with increased risk for acute care and hospital visits. Specifically, higher 24-hour amplitude was associated with a higher risk for hospital visits (IRR = 2.42), and a higher 12-hour amplitude was associated with a higher risk for acute care visits (IRR = 1.29). These findings are consistent with our hypotheses; however, contrary to expectations, at 6 years of age higher amplitude was associated with fewer acute care and total medical visits. Additionally, boys and children who were born more premature were at elevated risk for hospital visits.
At 6 years of age, better fit to the SCC at 2 years predicted a lower risk for total medical visits and ER visits. Likewise, higher average activity predicted a lower risk for total medical visits, hospital visits, and ER visits. Children who were born later and weighted more at birth had a lower risk for total medical care visits, hospital visits, and ER visits. Similarly, children from families with more sociodemographic assets had a lower risk for total medical visits and hospital visits.
DISCUSSION
For young children born preterm, toddler circadian sleep patterns were associated with their later cognitive testing and illness-related health care visits. At two years of age, children with sleep/activity cycles that consistently maintained a roughly 24-hour cycle had higher abbreviated IQ scores at 3 years and a reduced risk for total medical and ER visits at 6 years of age. In this at-risk sample, a higher average level of activity at 2 years of age appeared to be protective and was associated with few teacher-reported ADHD symptoms and a lower risk for illness-related health care visits. However, not all of our findings followed the expected pattern. Contrary to expectations, at 3 years of age higher goodness-of-fit to our specified circadian cycle was associated with a greater risk for hospital visits, and at 6 years of age higher amplitude (variability) was associated with a lower risk for acute care and total medical visits. Overall, the findings from this study are not entirely consistent and indicate that replication is warranted before generating clinical recommendations. The following sections will consider our study findings within the larger body of literature in this area.
Child behavior problems are often associated with child sleep problems and later child/family choronotypes.12–16 In the present study, we did not find this; however, we did not directly assess sleep problems or child chronotype. Our modeling technique provided a strong test of circadian cycle regularity. However, because our models all started at midnight, this provided an indirect assessment of circadian cycle timing or chronotype. Children who did not have low-levels of activity at midnight (i.e., those who were awake later) did not have good fit to the SCC. Another reason our results may not have reflected the expected association between behavior problems and sleep pertains to this modeling strategy. Children could have a poor fit to the SCC for multiple reasons (e.g., cycle length irregularity, high activity levels at night, long daytime naps) and not all of these may be associated with behavior problems. For example, children and adults born preterm tend to have an early chronotype,9–11 and this early chronotype is often associated with positive or more adaptive behavioral features. In the present study, poor fit to the SCC may reflect preterm toddlers with an early chronotype or those who have cycle sleep irregularity. This difference in the underlying mechanism for poor fit may be masking our expected effect. Alternatively, our findings could reflect that objectively measured sleep patterns are not robustly associated with perceived behavior problems. This is consistent with previous literature as many studies that report associations between sleep and behavior problems are comparing perceived problems in both areas.36,37
Our findings regarding average activity level are intriguing. At first glance it seems counter intuitive that higher average activity levels would be associated with positive developmental and health outcomes. However, this likely reflects the high-risk nature of this sample. A portion of infants born preterm remain medically fragile for much of their early development, and children with low levels of activity may reflect these less robust infants/toddlers. The mesor or activity index in the present study reflects both activity at night and during the day. Although low levels of activity at night are generally acknowledged as adaptive, low levels of activity during the day could reflect medical fragility, lethargy, or other developmental processes. With replication, this information may inform future screening efforts to identify infants who are at elevated risk for less than optimal long-term outcomes.
For children born preterm, attention problems and ADHD symptoms are among the most common concerns raised by parents and teachers (and documented by research).37 Given the strong literature base linking sleep and daytime attention,39 we anticipated toddler sleep behaviors would be associated with later attentional problems and ADHD symptoms. We did not find support for this. Only one association emerged between teacher ADHD symptom reports and activity. As noted earlier, this was not in the expected direction with a significant association between lower average activity and higher ADHD symptom reports. It is possible the attention problems faced by children born preterm reflect elements of neurological maturity at birth and are less influenced by sleep. Gössel-Symank and colleagues40 recommend following rest-activity patterns to identify children at increased risk for attentional problems, but our study did not provide support for this recommendation.
In the present study, family sociodemographic assets were one of the most consistent predictors of later cognitive, behavior, attention, and health concerns. At three years of age, children from families with more sociodemographic assets had higher ABIQ scores and fewer parent-reported attention, ADHD, externalizing, and total problems on the CBCL. At six years of age, these children continued to have fewer parent-reported externalizing problems. Additionally, they had fewer total medical visits and hospitalizations at 6 years of age. A long line of research highlights the strong developmental impact of poverty in early childhood and this impact appears to be even stronger for infants born preterm.41–43 The current study is consistent with this line of literature and demonstrates family sociodemographic assets (at time of birth) may be used to identify children at the highest risk for later developmental and health concerns.
Limitations
In the present study we aimed to model both circadian and homeostatic processes in sleep using two sinusoidal waves within a TSA framework. In theory this was sound, but in practice the influence of the second (smaller) oscillation was minor, and it did not provide a model of daytime napping or a midday drop in activity. Our interpretation of the models reflects this (we focus on the larger 24-hour circadian wave), but this is a clear limitation of the present study. Future studies that employ a TSA approach can build on our model, but additional parameters may be needed to more accurately reflect the dynamic nature of the circadian and homeostatic processes in young children (i.e., napping). A second limitation is our rather large and non-random attrition from 3 to 6 years. Families who did not participate in the study at 6 years of age had fewer sociodemographic assets and were less likely to be Caucasian (Table 1; see Poehlmann-Tynan et al., 2015 for more details).22 This attrition pattern may limit the study generalizability and the presented findings may not reflect non-Caucasian families or those with fewer sociodemographic assets. Finally, the bidirectional nature of sleep and developmental/health concerns should be acknowledged. Although sleep and developmental/health concerns were temporally ordered in the present study, this is not sufficient to imply causation. Developmental and health concerns are often linked with concurrent sleep problems/patterns, and the presented associations may not be predictive.
CONCLUSION
The novel approach used in the present study to assess circadian sleep patterns provides clinicians and researchers a pattern-based method to assess sleep patterns over time. In children born preterm, toddler circadian sleep patterns may inform later cognitive skills and health risks. With replication, the present study could provide a mechanism for physicians and early childhood interventionists to promote development and health in this at-risk population. By educating parents on the importance of regular sleep habits, physicians and interventionists may ameliorate the modifiable risk factor of sleep dysregulation. When supporting healthy sleep habits, interventionists should consider the larger literature base (not just this study) and recognize that each child’s optimal sleep pattern will reflect several factors (e.g., genetics, family context) and may not follow the specified 24-hour circadian cycle used in this study.
Supplementary Material
Acknowledgments
Funding support provided by National Institutes of Health (R01 HD44163; F31 HD051035, PI: Poehlmann).
Footnotes
All authors have no conflicts of interest to report.
Contributor Information
A. J. Schwichtenberg, College of Health and Human Sciences, Department of Human Development and Family Studies, Department of Psychological Sciences, Purdue University, West Lafayette, IN
Sharon Christ, College of Health and Human Sciences, Department of Human Development and Family Studies, Department of Statistics, Purdue University, West Lafayette, IN
Emily Abel, College of Health and Human Sciences, Department of Human Development and Family Studies, Purdue University, West Lafayette, IN
Julie A. Poehlmann-Tynan, School of Human Ecology, Department of Human Development and Family Studies, University of Wisconsin, Madison, WI
References
- 1.Guyer C, Huber R, Fontijn J, et al. Very preterm infants show earlier emergence of 24-hour sleep-wake rhythms compared to term infants. Early Hum Dev. 2015;91:37–42. doi: 10.1016/j.earlhumdev.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Huang J, Zhong Z, Wang M, et al. Circadian modulation of dopamine levels and dopaminergic neuron development contributes to attention deficiency and hyperactive behavior. J. Neurosci. 2015;35:2572–2587. doi: 10.1523/JNEUROSCI.2551-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scher MS, Johnson MW, Ludington SM, et al. Physiologic brain dysmaturity in late preterm infants. Pediatr Res. 2011;70:524–528. doi: 10.1203/PDR.0b013e31822f24af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergara ER, Bigsby R. Developmental and therapeutic interventions in the NICU. Baltimore, MD: Paul H Brookes Publishing Company; 2004. [Google Scholar]
- 5.Anders TF, Keener M. Developmental course of nighttime sleep-wake patterns in full-term and premature infants during the first year of life. Sleep. 1985;8:173–192. doi: 10.1093/sleep/8.3.173. [DOI] [PubMed] [Google Scholar]
- 6.Arnardottir H, Thornorsteinsson H, Karlsson KG. Dynamics of sleep-wake cyclicity at night across the human lifespan. Front Neurol. 2010;1:156–166. doi: 10.3389/fneur.2010.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisman O, Magori-Cohen R, Louzoun Y, et al. Sleep-wake transitions in premature neonates predict early development. Pediatr. 2011;128:706–714. doi: 10.1542/peds.2011-0047. [DOI] [PubMed] [Google Scholar]
- 8.Dorn F, Wirth L, Gorbey S, et al. Influence of acoustic stimulation on the circadian and ultradian rhythm of premature infants. Chronobiol Int. 2014;31:1062–1074. doi: 10.3109/07420528.2014.948183. [DOI] [PubMed] [Google Scholar]
- 9.Hibbs AM, Storfer-Isser A, Rosen C, et al. Advanced sleep phase in adolescents born preterm. Behav Sleep Med. 2014;12:412–424. doi: 10.1080/15402002.2013.825838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natale V, Sansavini A, Trombini E, et al. Relationship between preterm birth and circadian typology in adolescence. Neurosci Lett. 2005;382:139–142. doi: 10.1016/j.neulet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Björkqvist J, Paavonen J, Andersson S, et al. Advanced sleep-wake rhythm in adults born prematurely: confirmation by actigraphy-based assessment in the helsinki study of very low birth weight adults. Sleep Med. 2014;15:1101–1106. doi: 10.1016/j.sleep.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 12.Gelbmann G, Kuhn-Natriashvili S, Pazhedath TJ, et al. Morningness: protective factor for sleep-related and emotional problems in childhood and adolescence? Chronobiol Int. 2012;29:898–910. doi: 10.3109/07420528.2012.686946. [DOI] [PubMed] [Google Scholar]
- 13.Giannotti F, Cortesi F, Sebastiani T, et al. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11:191–199. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein D, Hahn CS, Hasher L, et al. Time of day, intellectual performance, and behavioral problems in morning versus evening type adolescents: is there a synchrony effect? Pers indiv Differ. 2007;42:431–440. doi: 10.1016/j.paid.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pieters S, Van Der Vorst H, Burk WJ, Wiers RW, et al. Puberty-dependent sleep regulation and alchohol use in early adolescents. Alcohol Clin Exp Res. 2010;34:1512–1518. doi: 10.1111/j.1530-0277.2010.01235.x. [DOI] [PubMed] [Google Scholar]
- 16.Yokomaku A, Misao K, Omoto F, et al. A study of the association between sleep habits and problematic behaviors in preschool children. Chronobiol Int. 2008;25:549–564. doi: 10.1080/07420520802261705. [DOI] [PubMed] [Google Scholar]
- 17.Merikanto I, Lahti T, Puusniekka R, et al. Late bedtimes weaken school performance and predispose adolescents to health hazards. Sleep Med. 2013;14:1105–1111. doi: 10.1016/j.sleep.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Schlarb AA, Sopp R, Ambiel D, et al. Chronotype-related differences in childhood and adolescent aggression and antisocial behavior: a review of the literature. Chronobiol Int. 2014;31:1–16. doi: 10.3109/07420528.2013.829846. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi K, Yorifuji T, Yamakawa M, et al. Poor toddler-age sleep schedules predict school-age behavioral disorders in a longitudinal survey. Brain Dev. 2015;37:572–578. doi: 10.1016/j.braindev.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Jenni OG, LeBourgeois MK. Understanding sleep-wake behavior and sleep disorders in children: the value of a model. Curr Opin Psychiatry. 2006;19:282–287. doi: 10.1097/01.yco.0000218599.32969.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas KA, Burr RL, Spieker S, et al. Mother-infant circadian rhythm: development of individual patterns and dyadic synchrony. Early Hum Dev. 2014;90:885–890. doi: 10.1016/j.earlhumdev.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poehlmann-Tynan J, Gerstein ED, Burnson C, et al. Risk and resilience in preterm children at age 6. Dev & Psychopath. 2015:843–858. doi: 10.1017/S095457941400087X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Achenbach TM, Rescorla LA. Manual for the ASEBA preschool forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families; 2000. [Google Scholar]
- 24.Aronen ET, Paavonen EJ, Fjällberg M, et al. Sleep and psychiatric symptoms in school-age children. J Am Acad Child Adolesc Psychiatry. 2000;39:502–508. doi: 10.1097/00004583-200004000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Goodlin-Jones B, Tang K, Liu J, et al. Sleep problems, sleepiness and daytime behavior in preschool-age children. J Child Psychol Psychiatry. 2009;50:1532–1540. doi: 10.1111/j.1469-7610.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 26.Lavigne JV, Arend R, Rosenbaum D, et al. Sleep and behavior problems among preschoolers. J Dev Behav Pediatr. 1999;20:164–169. doi: 10.1097/00004703-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Paavonen EJ, Porkka-Heiskanen T, Lahikainen AR. Sleep quality, duration and behavioral symptoms among 5–6 year-old children. Eur Child Adolesc Psychiatry. 2009;18:747–754. doi: 10.1007/s00787-009-0033-8. [DOI] [PubMed] [Google Scholar]
- 28.Yu JW, Buka SL, McCormick MC, et al. Behavioral problems and the effects of early intervention on eight-year-old children with learning disabilities. Matern Child Health J. 2006;10:329–338. doi: 10.1007/s10995-005-0066-7. [DOI] [PubMed] [Google Scholar]
- 29.Conners KC. Conners 3rd edition. Toronto, Canada: Multi-Health Systems; 2008. [Google Scholar]
- 30.Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin N. 2006;12:23–30. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Anders T, Iosif A, Schwichtenberg AJ, et al. Sleep and daytime functioning: a short-term longitudinal study of three preschool-age comparison groups. Am J Intellect Dev Disabil. 2012;117:275–290. doi: 10.1352/1944-7558-117.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asaka Y, Takada S. Activity-based assessment of the sleep behaviors of VLBW preterm infants and full-term infants at around 12 months of age. Brain Dev. 2010;32:150–155. doi: 10.1016/j.braindev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Thomas KA, Burr RL. Circadian research in mothers and infants: how many days of actigraphy data are needed to fit cosinor parameters? J Nurs Meas. 2008;16:201–206. doi: 10.1891/1061-3749.16.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roid GH. Stanford binet intelligence scales. 5th. Itasca, IL: Riverside Publishing; 2003. [Google Scholar]
- 35.Refinetti R, Lissen GC, Halburg F. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 2007;38:275–325. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reid GJ, Hong RY, Wade TJ. The relation between common sleep problems and emotional and behavioral problems among 2 and 3-year-olds in the context of known risk factors for psychopathology. J Sleep Res. 2009;18:49–59. doi: 10.1111/j.1365-2869.2008.00692.x. [DOI] [PubMed] [Google Scholar]
- 37.Sivertsen B, Harvey AG, Reichborn-Kjennerud T, et al. Later emotional and behavioral problems associated with sleep problems in toddlers: a longitudinal study. JAMA Pediatr. 2015;169:575–582. doi: 10.1001/jamapediatrics.2015.0187. [DOI] [PubMed] [Google Scholar]
- 38.Bhutta AT, Cleves MA, Casey PH, et al. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA Pediatr. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 39.Sadeh A, De Marcas G, Guri Y, et al. Infant sleep predicts attention regulation and behavior problems at 3–4 years of age. Dev Neuropsychol. 2015;40:122–137. doi: 10.1080/87565641.2014.973498. [DOI] [PubMed] [Google Scholar]
- 40.Gössel-Symank R, Grimmer I, Korte J, et al. Actigraphic monitoring of the activity-rest behavior of preterm and full-term infants at 20 months of age. Chronobiol Int. 2004;21:661–671. doi: 10.1081/cbi-120039208. [DOI] [PubMed] [Google Scholar]
- 41.Bradley R, Whiteside L, Mundfrom D, et al. Early indications of resilience and their relation to experiences in the home environments of low birthweight, premature children living in poverty. Child Dev. 1994;65:346–360. [PubMed] [Google Scholar]
- 42.Brumberg H, Shah S. Born early and born poor: An eco-bio-developmental model for poverty and preterm birth. J Neonatal Perinatal Med. 2015;8:179–187. doi: 10.3233/NPM-15814098. [DOI] [PubMed] [Google Scholar]
- 43.Potijk M, Kerstjens J, Bos A, et al. Developmental delay in moderately preterm-born children with low socioeconomic status: risks multiply. J Pediatr. 2013;163:1289–1295. doi: 10.1016/j.jpeds.2013.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.