Abstract
Alcoholic liver disease (ALD) develops in approximately 20% of alcoholics, with a higher prevalence in females. ALD progression is marked by fatty liver and hepatocyte necrosis, as well as apoptosis, inflammation, regenerating nodules, fibrosis, and cirrhosis 1. ALD develops via a complex process involving parenchymal and non-parenchymal cells, as well as recruitment of other cell types to the liver in response to damage and inflammation. Hepatocytes are damaged by ethanol, via generation of reactive oxygen species and induction of endoplasmic reticulum stress and mitochondrial dysfunction. Hepatocyte cell death via apoptosis and necrosis are markers of ethanol-induced liver injury. We review the mechanisms by which alcohol injures hepatocytes and the response of hepatic sinusoidal cells to alcohol-induced injury. We also discuss how recent insights into the pathogenesis of ALD will affect treatment and management of patients.
Keywords: alcoholic hepatitis, alcoholic liver disease, hepatic stellate cell
Ethanol leads to hepatocyte stress and injury through effects on hepatocytes and on intestinal epithelia. Toxicity is mediated through ethanol as well as ethanol metabolism and its metabolites. What are the molecular mechanisms of these processes, and how do they affect disease development and phenotype?
Oxidative Stress
Ethanol is metabolized through 2 major oxidative and 2 minor non-oxidative pathways. Ethanol is oxidized by alcohol dehydrogenase (ADH) and cytosolic aldehyde dehydrogenase 1 (ALDH1) and mitochondrial ALDH2 1, which converts NAD+ into NADH. Ethanol is also oxidized by cytochrome P450 family 2, subfamily E, polypeptide 1 (CYP2E1) and catalase, which generates reactive oxygen species (ROS) and leads to cellular damage 2. Although the liver metabolizes the most ethanol, other tissues can also metabolize alcohol 3. For example, chronic ethanol intake induces CYP2E1 expression in Kupffer cells 4, 5. In addition to oxidative metabolism, 2 non-oxidative pathways can metabolize small amounts of ethanol 3. However, the effects of these pathways on cell functions are not clear 6.
Ethanol induces oxidative stress via multiple pathways (reviewed in 7). In hepatocytes, increased oxidative stress directly damages mitochondria to induce cell death or sensitize hepatocytes to cell death in response to inflammatory cytokines. Increased oxidative stress in Kupffer cells also increases their sensitivity to lipopolysaccharide (LPS) 5. Livers from NADPH oxidase- and inducible nitric oxide synthase-knockout mice have decreased, whereas Cu,Zn-superoxide dismutase knockout-mice have increased, oxidative and nitrate-induced stress and injury after chronic ethanol feeding 8–10.
Strategies to downregulate oxidative stress pathways might therefore prevent or reduce development of ALD. Unfortunately, clinical trials in patients with ALD focusing on antioxidant therapies have generally not been successful. Rather than excluding a role for this approach in therapy, the results highlight the need to increase our understanding of oxidative stress and antioxidants, to guide appropriate selection of targets, as well as proper doses for humans. Other antioxidants that have received attention either in preclinical and small clinical trials and warrant further study include s-adenosyl methionine, betaine, folate, and methionine adenosyltransferase I, alpha (MAT1A).
Hypoxia
Alcohol consumption induces pericentral hypoxia due to increased oxygen consumption during ethanol metabolism 11, 12. Prolonged hypoxia promotes ROS production, impairs mitochondrial fatty acid oxidation, and stimulates expression of lipid synthesis genes, contributing to mitochondrial damage and cell death. Liver can adapt to hypoxia by activating transcription of genes that include hypoxia inducible factors (HIFs). Expression of HIF1A and HIF2A increases in liver with binge and chronic ethanol feeding. Unfortunately, although HIF1A protects the liver from hypoxia, a number of events also ensue that exacerbate alcohol-induced liver injury 13–15. Mice with hepatocyte-specific disruption of Hif1b (Arnt) are resistant to chronic plus binge alcohol-induced steatosis and liver injury 16. Activation of HIFs during alcohol-induced hypoxia therefore have complex effects on liver injury in mice. The roles of HIFs in human ALD have not been investigated.
Dysregulation of Lipid Synthesis
Alcohol consumption dysregulates lipid synthesis and metabolism, resulting in steatosis17. Alcohol exposure inhibits hepatic activity of sirtuin 1 (SIRT1), leading to increased acetylation and stability, as well as increased transcriptional activity of sterol regulatory element-binding protein-1 (SREBP1), which regulates lipogenesis 18, 19. SREBP2, lipin 1 20, 21, and ceramide also regulate lipogenesis. SIRT1 regulates fatty acid synthesis and oxidation; ethanol-induced decreases in SIRT1 affect multiple processes to lead to steatosis 22,23, 24. Because regulates lipid metabolism, it could be targeted to prevent and/or treat steatosis associated with ALD.
Mitochondria, Endoplasmic Reticulum (ER) stress, and Autophagy
Acute and chronic alcohol exposure alter liver mitochondria structure and function in animal models and humans 25. Alcohol exposure also damages mitochondrial DNA and ribosomes and decreases rates of mitochondrial respiration (state III) and oxygen consumption, leading to mitochondrial-mediated apoptosis 11, 26. However, liver can adapt to chronic alcohol-induced mitochondrial and metabolic stress by activating mitophagy 27, mitochondrial fusion, or mitochondrial respiration, as well as mitochondrial biogenesis (via peroxisome proliferator-activated receptor gamma, coactivator 1 alpha or PPARGC1A) in mice 28. So, mitochondrial plasticity allows for a balance between alcohol-induced mitochondrial damage and repair or biogenesis.
Alcohol metabolism can result in the formation of a variety of protein adducts, as well as impair proper protein folding in the ER, resulting in accumulation of misfolded protein and ER stress29. Cells can adapt to ER stress by activating the unfolded protein response (UPR). The UPR attenuates ER stress and restores ER homeostasis by decreasing general protein translation and increasing protein folding capacity (by promoting expression of chaperone proteins). The UPR also promotes degradation of misfolded proteins by ER-associated protein degradation (ERAD) via the proteasome or by ER stress-mediated compensatory autophagy 30, 31. However, chronic alcohol consumption inhibits hepatic proteasome activity 32. As a result, damaged or misfolded proteins accumulate in cells and form insoluble protein aggregates that are resistant to degradation by the proteasome and require removal by autophagy. In support of this model, autophagy was observed to be activated in mouse liver after acute or chronic alcohol exposure 33, 34. This process adds another layer of adaptive compensatory mechanisms for regulation of proteostasis in response to ER stress and impaired proteasome function after alcohol exposure.
Autophagy is a catabolic process involved in maintaining normal liver physiology and development of liver diseases 35. Autophagy involves the formation of double-membrane autophagosomes that traffic and fuse with lysosomes to form autolysosomes, where autophagic cargo are degraded. Chronic alcohol impairs vesicular function and trafficking 36–39. Cells can adapt to impaired vesicular function by promoting lysosome biogenesis and synthesis of early autophagosomes 40. Therefore, autophagy could either decrease or increase depending on the balance of impaired cellular trafficking and lysosomes, and the compensatory activation of de novo autophagosome synthesis and lysosome biogenesis after alcohol exposure. Although our knowledge in this area is far from complete, especially with regard to autophagy in human ALD, a number of newly developed and repurposed drugs have been shown to regulate the autophagy process and may eventually warrant evaluation for human ALD.
Interactions of Apoptosis, Necrosis, and Autophagy
Alcohol consumption leads to hepatocyte death via apoptosis, necrosis, or necroptosis (programmed necrosis). Ethanol induces apoptosis via the extrinsic (death-receptor regulated) or intrinsic (mitochondrial) pathways 41–43. Necroptosis is similar in nature to necrosis but is initiated by death receptor activation and mediated by receptor-interacting protein kinase 1 (RIP1) and 3 (RIP3), and the downstream mixed lineage kinase domain-like protein (MLKL) 44–46.
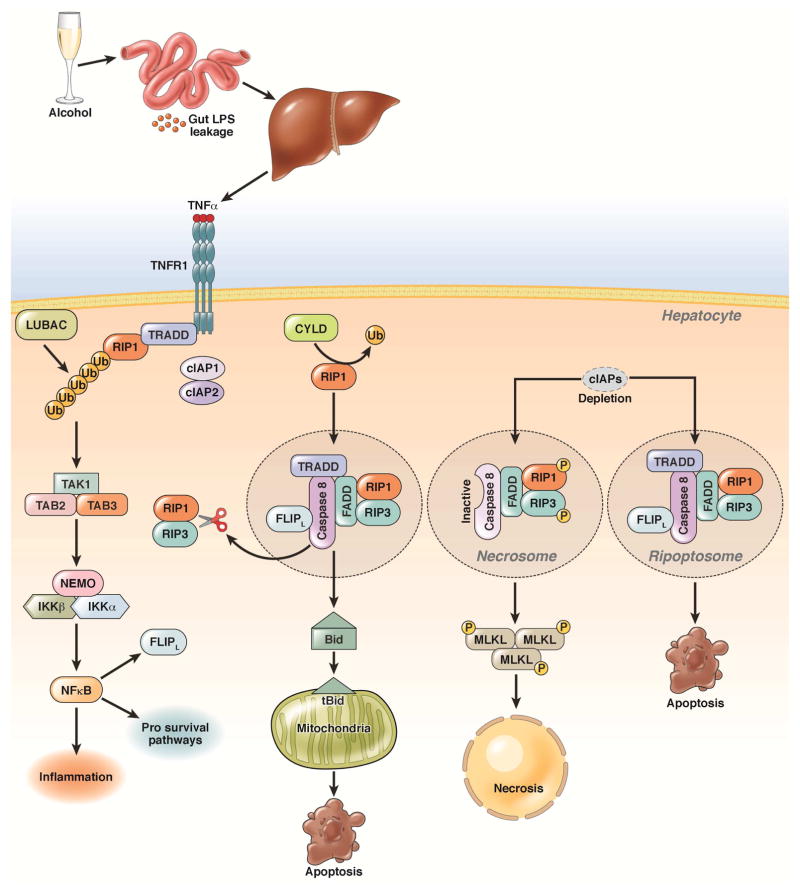
Although multiple pathways can contribute to hepatocyte cell death in the context of ethanol exposure, tumor necrosis factor-α (TNF)-induced cell death has been the most well studied. After TNF binds to its receptor (TNFR1), it recruits downstream factors (see Figure 1)47. Formation of specific down-stream effector complexes determines whether TNF induces hepatocyte survival, apoptosis or necroptosis47. Recent reviews have summarized mechanisms that regulate formation of these effector complexes 48, 49. RIP1 and RIP3 could have multiple roles in regulating apoptosis, necroptosis, cell survival, and inflammation (Figure 1) 50–52.
Figure 1. Pathways of alcohol-induced apoptosis and necrosis.
Alcohol consumption increases intestinal permeability resulting in increased influx of LPS into the liver. LPS activates Kupffer cells to produce TNF. TNF binds to its receptor (TNFR1), which recruits TNFR-associated death domain (TRADD), RIP1, cellular inhibitor of apoptosis proteins 1 (cIAP1/2), and linear ubiquitin chain assembly complex (LUBAC) and promotes the linear ubiquitination of RIP1. This leads to activation of NF-κB by the ubiquitin chain-dependent recruitment of transforming growth factor beta-activated kinase 1/MAP3K7 binding protein 2 (TAB2) and 3 (TAB3), transforming growth factor beta-activated kinase 1 (TAK1), and the I-κB kinase (IKK) complex. CYLD removes the ubiquitin chain from RIP1, and de-ubiquitinated RIP1 interacts with TRADD, fas-associated protein with a death domain (FADD), caspase-8, and the CASP8 and FADD-like apoptosis regulator (CFLAR), resulting in caspase-8 activation. Activated caspase-8 cleaves Bid to activate the mitochondrial apoptotic pathway and induce apoptosis; it also cleaves RIP1 and RIP3 to inactivate RIP1–RIP3-mediated necroptosis. When cIAPs are depleted and caspase-8 is inhibited, RIP1 and RIP3 interact via RHIM domains to from the amyloid-like necrosome. Auto- and trans-phosphorylated RIP1 and RIP3 then recruit downstream MLKL to initiate necroptosis. In the absence of cIAPs, RIP1, RIP3, TRADD, caspase-8, and CFLAR form a complex called the ripoptosome, which activates caspase-8 and apoptosis and requires RIP1 kinase activity.
Because TNF mediates ethanol-induced hepatocyte death, as well as inflammation, its pathway has been a longstanding therapeutic target for human ALD. Unfortunately, results from studies of mice were not translated into success in clinical trials. In fact, inhibition of TNF in patients with ALD had detrimental effects. Findings from mouse studies are difficult to translate to humans. We require a more detailed understanding of TNF signaling pathways—particularly in the context of alcohol-induced steatosis and inflammation.
Strategies to disrupt apoptosis have also been tested in clinical trials—trials with caspase inhibitors are in planning stages. However, in contrast to TNF, results of studies of early stages of steatosis or inflammation do not uniformly indicate that apoptosis contributes to liver injury. Although hepatocyte apoptosis has been observed in mice fed alcohol and in humans with ALD, inhibition of apoptosis either by disruption of the Bid gene or a pharmacologic pan-caspase inhibitor (VX166) did not attenuate alcohol-induced liver injury and steatosis in mice 53. Therefore, other forms of cell death are also involved in the pathogenesis of ALD. Increased levels of RIP3 protein were found in mouse livers after chronic ethanol feeding, and in livers of alcoholic patients 54. Moreover, RIP3-knockout mice have less liver injury, steatosis, and inflammation than control mice after chronic ethanol feeding 54. Studies of mice with liver-specific disruption of Rip1 or Rip3 mice, fed with alcohol, could help clarify the role of RIP1 and RIP3 in alcohol-induced necrosis and liver pathogenesis.
How would apoptosis and necrosis occur during the pathogenesis of ALD if apoptosis normally suppresses necrosis, via caspase-mediated cleavage of RIP3? Liver has unique zones with different levels of oxygen, nutrients, and metabolic enzymes. Induction of autophagy varies among the different zones of mouse liver after acetaminophen administration 55. It is therefore possible that apoptosis and necrosis also occur in different zones of the liver. Future studies are needed to elucidate these processes.
In addition to the mutual regulation of apoptosis and necrosis, autophagy and cell death can regulate each other. Many cell death stimuli induce cell death (apoptosis and necrosis) and autophagy at the same time. Autophagy might protect against ALD by selectively removing damaged organelles (such as mitochondria) and lipid droplets, as well as by releasing ER stress 27, 34. Although autophagy can protect against cell death, apoptosis can suppress autophagy, inducing caspase-mediated cleavage of essential autophagy proteins such as Beclin 1 56. Moreover, RIP1 also represses basal autophagy by activating ERK and subsequently inhibits TFEB-mediated expression of autophagy-related and lysosome genes 57.
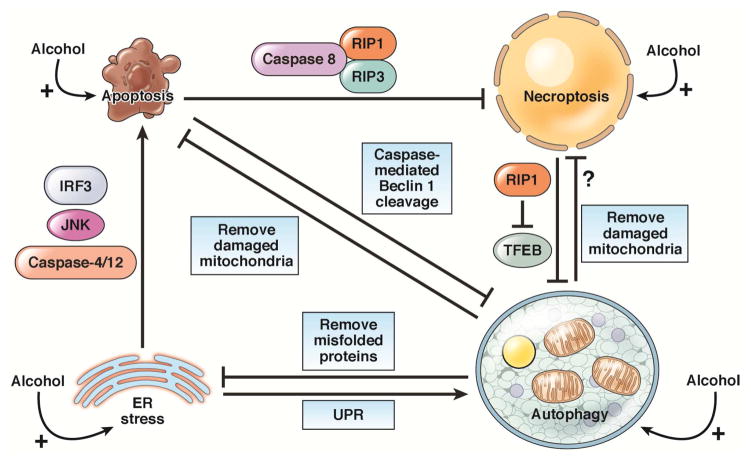
So, there is a complicated regulatory network among autophagy, apoptosis, and necroptosis. After alcohol exposure, a cell’s fate is decided by the balance of autophagy vs apoptosis, necroptosis, or mitochondrial stress, as well as UPR vs ER stress. Disruption of the balance from cell survival (UPR and autophagy) towards cell injury (apoptosis or necroptosis) eventually leads to liver injury after alcohol exposure (Figure 2). Therefore, despite the negative results observed with TNF inhibitors in clinical trials of patients with ALD, studies of the pathways of hepatocyte apoptosis and necrosis will continue to provide new therapeutic targets. These might include strategies to inhibit caspases or factors in the TNF pathway that mediate liver injury.
Figure 2. Interactions among apoptosis, necrosis, ER stress, and autophagy.
Apoptosis suppresses necroptosis via caspase-8 cleavage and inactivation of RIP1 and RIP3. Apoptosis also inhibits autophagy by caspase-mediated cleavage of Beclin 1. The necroptosis protein RIP1 negatively regulates TFEB to repress autophagy. ER stress induces apoptosis by activating caspase-12 and -4, JNK, and the interferon regulator factor 3 (IRF3) signaling pathway. ER stress can also induce autophagy via UPR signaling. Autophagy inhibits apoptosis and necrosis by removing damaged mitochondria. Autophagy also relieves ER stress by degrading misfolded proteins and protein aggregates.
Interactions Between Liver and Intestine
High-dose acute and chronic consumption of ethanol can damage the intestinal mucosa, causing loss of epithelial cells from villi tips and hemorrhagic erosions in the lamina propria 58. Ethanol also affects the diversity of the intestinal microbiome (see Bajaj and Gillevet, ref XX). Junction proteins, including those in tight and adherens junctions, are disassembled by alcohol and acetaldehyde 59,59. Alterations include redistribution of occludin and zonula occludens from intercellular junctions and dissociation from the actin cytoskeleton. Other alterations include disruption of the adherens junctions via redistribution of E-cadherin and β-catenin from intercellular junctions 59. Ethanol can also modulate the peri-junctional actin and myosin filaments through activation of myosin light-chain kinase 58. Ethanol activates nuclear factor (NF)-kB, leading to instability of the F-actin cytoskeleton and disruption of the intestinal barrier 58. Additionally, ethanol initiates barrier dysfunction by affecting the transcription of intestinal circadian clock genes and activity of microRNAs 58. Ethanol metabolism also induces oxidative stress, partly by increasing expression of CYP2E1, which increases gut permeability by damaging cells and modifying proteins 58, 60.
Disruption of the tight junction protein complex has pathologic potential, because it allows exogenous and endogenous agents to cross the epithelial barrier and initiate or perpetuate systemic inflammation. Experimental animal models of acute and chronic ethanol exposure have associated increased permeability to gastrointestinal macromolecules and pathogen-associated molecular patterns with inflammatory conditions and organ injury 60–62. Alcoholic patients without advanced-stage liver disease have increased intestinal permeability to macromolecules, so increased gut permeability appears to be caused by ethanol, rather than as a consequence of advanced alcoholic liver disease 63. Drugs are being developed to reduce gut permeability for treatment of a number of luminal diseases, such as celiac disease and inflammatory bowel disease. These might also be used in patients with early-stage ALD, and reduce or reverse disease progression before severe liver injury and inflammation.
Hepatocyte Signaling in Response to Injury
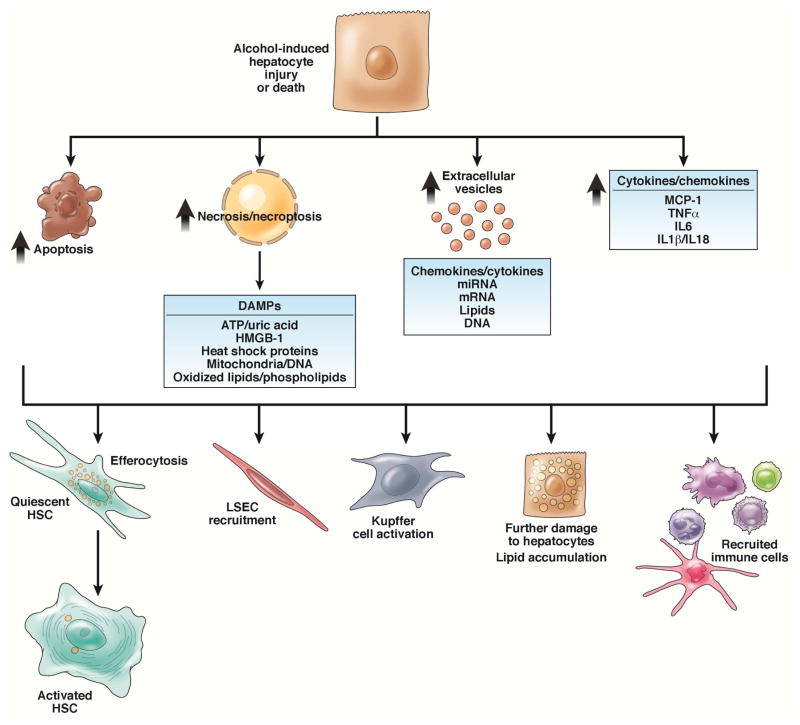
When hepatocytes are injured or stressed, they communicate with other cells in their local environment, as well as to more distant organs, such as the bone marrow. These signals facilitate a protective or wound-healing response. We review some of the mechanisms by which hepatocytes communicate injury and coordinate either maintenance of a healthy liver or progression of chronic disease (see Figure 3).
Figure 3. Injured hepatocytes signal to cells within the hepatic sinusoid.
Cells within the hepatic sinusoid respond to hepatocytes dying via apoptosis and necrosis/necroptosis, either via efferocytosis or phagocytosis of apoptotic bodies or DAMP-mediated signaling. Injured hepatocytes can also signal to their surrounding environment via the release of extracellular vesicles, containing a number of regulatory factors, or the direct expression of cytokines and chemokines.
Release of danger-associated molecular patterns (DAMPs)
Injured and dying hepatocytes release DAMPs (metabolites, proteins, carbohydrates and organelles), which signal to surrounding cells (Figure 3). DAMPs are primarily released from necrotic, necroptotic, or pyroptotic cells, but also undergo regulated release 64. DAMPs can have paracrine or endocrine effects within the hepatic sinusoid, where they interact with immune cells, liver endothelial cells, and hepatic stellate cells (HSCs). For example, ethanol exposure results in the release of high mobility group box 1 (HMGB1) from hepatocytes; 65 disruption of Hmgb1 in hepatocytes of mice prevents ethanol-induced hepatic steatosis 66. HMGB1 also recruits HSCs and liver endothelial cells to sites of ethanol-induced injury 65.
Although apoptotic hepatocytes release fewer DAMPs than necrotic cells, they can activate quiescent HSCs 67. ATP and uric acid, released from hepatocytes in response to ethanol, activated inflammasomes in immune cells in the hepatic sinusoid 68. In humans, a single dose of alcohol increased serum levels of ATP and uric acid, indicating that DAMPs also signal to distant organs 68. Agents that target the DAMP-mediated signaling may be effective at limiting the transition from hepatocyte injury to exacerbated inflammation and fibrosis in patients with ALD. For example, antagonists of the interleukin 1 beta (IL1B) receptor, such as anakinra and ramonaband, which broadly target inflammasome activity, are under evaluation in patients with alcohol-associated hepatitis.
Cytokine and chemokine signaling
Hepatocytes also produce cytokines and chemokines. Although immune cells produce the greatest levels of cytokines and chemokines, under some conditions, hepatocytes release IL1B and IL18, by upregulating components of the inflammasome 69, 70. Hepatocytes also produce other inflammatory cytokines, including TNF and IL6. Chemokine (C-C motif) ligand 2 (CCL2 or MCP1) is a hypoxia-sensitive chemokine produced by immune cells and hepatocytes in response to chronic ethanol exposure 71,14. MCP1 also acts as a steatokine, increasing the accumulation of triglycerides in hepatocytes via the liver X receptor (nuclear receptor subfamily 1, group H, member 3) α 72. Many compounds have been identified that target specific chemokines, but these have not been tested in trials of patients with ALD.
Extracellular vesicles
Recent studies have focused on extracellular vesicles as a mechanism to mediate alcohol-related liver injury 73, 74. Extracellular vesicles include exosomes, microparticles, and apoptotic bodies; these are distinguished based on size and marker proteins 75. Ethanol stimulates release of extracellular vesicles from hepatocytes in vivo and in vitro, 73 and extracellular vesicles are increased in blood samples of patients with alcoholic hepatitis 73, 74. Putative extracellular vesicle-associated molecules include microRNAs and proteins, such as CD40 ligand; these might contribute to inflammatory responses that develop after alcohol-induced hepatocyte injury. Strategies to prevent release of extracellular vesicles, such as those that target exocytic actin, the molecular motor machinery, or specific molecules within extracellular vesicles, might be developed as therapeutics.
Innate Immune Responses to Alcohol and Injured Hepatocytes
The innate immune response is activated during acute and chronic exposure to ethanol and contributes to alcoholic liver disease. It is a relatively low-grade form of inflammation that does not resolve quickly. Ethanol intake affects many cellular and soluble components of the innate immune system.
Kupffer cells and macrophage
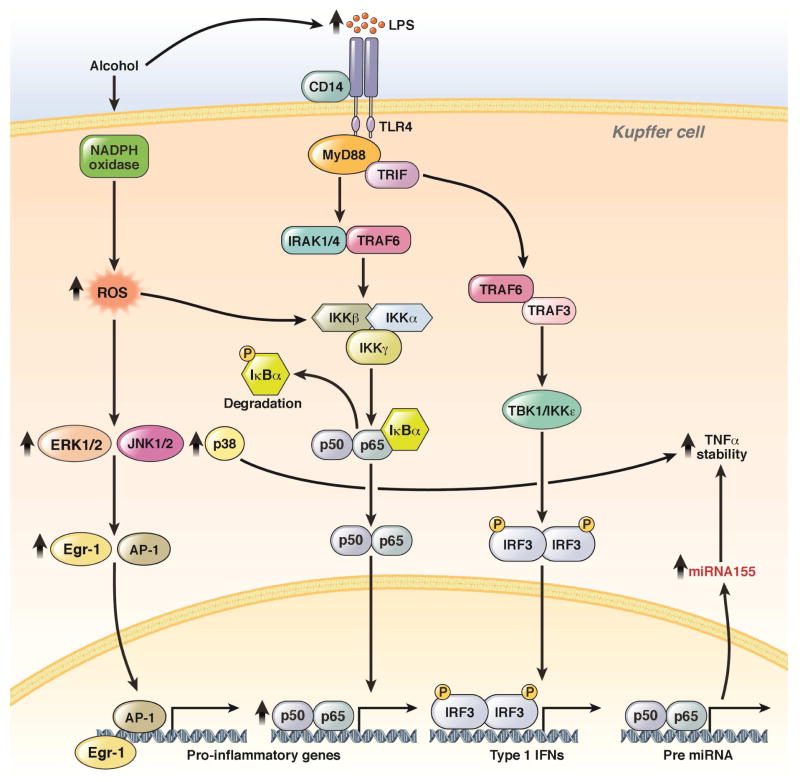
Kupffer cells, which are macrophages of the liver, are important mediators of ALD. Following short-term and chronic exposure to alcohol, receptor-dependent signal transduction is dysregulated in these cells. Kupffer cells become sensitized to LPS and signal via TLR4, increasing production of inflammatory cytokines, chemokines, and other mediators of inflammation (reviewed in 76, see Figure 4). Production of these inflammatory mediators is regulated at transcriptional and post-transcriptional levels, including mRNA stability and cytokine secretion.
Figure 4. Sensitization of TLR4-mediated signal transduction in hepatic macrophages.
Chronic ethanol feeding not only increases the amount of circulating gut-derived endotoxin (LPS), but also sensitizes Kupffer cells to TLR4-mediated signaling via MyD88-dependent and –independent pathways. These processes increase expression of TLR4 and increase free radical formation by NADPH oxidase and CYP2E1. Increased ROS then increases TLR4-mediated signaling via MyD88-dependent activation of MAPKs and NF-κB. These signaling pathways integrate to increase transcription of cytokines, as well as the mRNA stability of TNF. This sensitization depends on ethanol-induced changes expression of microRNAs—particularly related to the stabilization of TNF mRNA. MyD8-independent signaling pathways activate STAT transcription factors and promote interferon production to regulate other immune cells in the liver.
MicroRNAs determine the sensitivity of Kupffer cells to LPS 77. For example, expression of microRNA155 is up-regulated in Kupffer cells and contributes to ethanol-induced stabilization of TNF mRNA 78. Multiple microRNAs are affected by ethanol; 17, 79 studies should address the role of additional microRNAs in regulation of inflammatory responses to ethanol.
Although responses of Kupffer cells to pattern-associated recognition receptors have been well studied, less is known about the sensitivity of Kupffer cells to DAMPs. Given our growing knowledge of the types of DAMPs released by injured hepatocytes during ethanol exposure, studies of DAMP-mediated responses in Kupffer cells will be important.
The relative expression of inflammatory vs anti-inflammatory mediators depends, at least in part, on the state of Kupffer cells and polarization of macrophages in the liver. Alcohol shifts the balance of the M1 vs the M2 phenotype towards M1, characterized by increased production of inflammatory cytokines and ROS 80, 81. The mechanisms of this shift are not well understood, but metabolic reprogramming via NOTCH1 signaling has been implicated in ethanol-induced on M1 polarization 82. Although gamma-secretase inhibitors inhibit the NOTCH pathway, researchers are developing more-selective agents that target NOTCH. Agents such as antibody-tagged corticosteroids, which selectively target macrophages, are also under evaluation and might reduce hepatic inflammation in patients with ALD.
Anti-inflammatory pathways
Multiple anti-inflammatory mediators control the resolution of inflammation 83, 84. Chronic ethanol exposure may impair the ability of Kupffer cells to activate and/or respond to anti-inflammatory mediators. For example, the sensitivity of Kupffer cells to anti-inflammatory mediators that act via cAMP, such as adenosine and PGE2, is decreased in chronic ethanol-fed rats 85 and associated with an increase in PDE4. Adiponectin is another anti-inflammatory molecule that may be decreased after chronic ethanol feeding 86. Incubation of Kupffer cells from ethanol-fed rodents with adiponectin normalizes TLR4-medated signaling via MYD88-dependent and -independent pathways, via induction of heme-oxygenase 1 87, 88. Compounds such as PDE4 inhibitors, which promote resolution of inflammation, are being tested in trials of ALD.
Recruitment of immune cells to the liver
Although resident macrophages are important regulators of innate immune responses during the progression of ALD, peripheral monocytes and neutrophils are also recruited to the liver during disease progression. They are recruited by a variety of chemokines, which have salutary as well as detrimental effects. For example, macrophage inhibitor factor (MIF), a pluripotent cytokine and chemokine, recruits peripheral Ly6C+ monocytes to livers of mice during development of chronic ethanol-induced injury and carbon tetrachloride-induced fibrosis 89, 90. MIF-mediated recruitment of monocytes contributes to inflammation and damage, but protects against fibrosis. This is likely because these cells activate scar-associated macrophages, which promote resolution of fibrosis 89, 91. During alcohol-induced liver injury, chemokines of CXC and CC families recruit neutrophils, monocytes, and T and B cells 71, 92. Adhesion molecules, such as E-selectin, also mediate infiltration of the liver by neutrophils during ethanol exposure 93.
Adaptive Immune Responses
Alcohol can impair the adaptive immune system, depending on the severity of ALD 94. Patients with advanced ALD have circulating T cells and antibodies against epitopes generated by hydroxyethyl free radicals, produced during ethanol oxidation, as well as end products of lipid peroxidation 95. IL17-producing T helper cells contribute to development of ALD by promoting infiltration of liver by neutrophils 96. Regulatory T cells might also mediate progression of ALD—patients with alcohol-associated hepatitis have reduced numbers of circulating regulatory T cells97.
Dendritic cells and natural killer (NK) cells
Hepatic dendritic cells are important antigen presenting cells in liver. Chronic ethanol consumption disrupts dendritic cell function, likely contributing to impaired cellular immune responses. For example, myeloid dendritic cells isolated from ethanol-fed mice have enhanced interleukin (IL) 1β and IL-10 production, but decreased secretion of TNF, IL12, interferon gamma, and IL6 98, 99. Similarly, myeloid dendritic cells generated in presence of ethanol produce increased amounts of IL10 production and express lower levels of the co-stimulatory molecules CD80 and CD86, compared to myeloid dendritic cells not exposed to ethanol100.
NK regulate development liver fibrosis as well as hepatic immune tolerance. NK cells prevent fibrosis by killing activated stellate cells. Depletion of NK cells increases experimental liver fibrosis in mice 101. NK cells indirectly maintain hepatic tolerance through interactions with dendritic cells 102. However, NK cells also contribute to neutrophil infiltration of liver in mouse models of ALD. 103 Early-phase clinical trials are being designed to alter activities of NK cells in patients with ALD.
Fibrogenesis
Fibrogenesis culminates from alcohol-induced hepatocyte injury and its associated immune response. Mechanisms of fibrogenesis in ALD are probably similar to those of other fibrogenic liver diseases in that they are likely to be mediated by activated HSCs. During fibrogenesis, HSCs migrate, proliferate, and increase matrix deposition. HSC activation occurs via multiple pathways (see 104), although some of these might be more specifically associated with development of ALD compared to in other diseases. For example, alcohol-induced dysbiosis of the intestinal microbiota and increased gut permeability appear to selectively activate the TLR4 signaling pathway, which leads to HSC activation 105. Acetaldehyde has also been implicated in HSC activation and collagen expression.
Our mechanistic understanding of fibrogenesis in response to alcohol-related liver injury has been hindered by the lack of animal models that recapitulate human disease. Nonetheless, studies have identified several potential pathways for therapeutic intervention. For example, IL22, a hepato-protective cytokine produced by inflammatory cells that acts on hepatocytes and HSC 106, attenuates HSC activation and matrix production. Macrophages regulate fibrosis and may be therapeutic targets. Macrophages acquire a phenotype that promotes inflammation (polarization) in response to alcohol 107. However, alternative phenotypes of macrophages, such as scar-associated macrophages, are important for fibrosis resolution 108. The precise roles of macrophages in fibrogenesis and fibrosis resolution in the context of ALD require further study. Studies of natural killer cells indicated these cells may be an interesting target for therapy of alcohol-related fibrogenesis 109. Finally, cell signaling within the hepatic sinusoid is implicated in fibrogenesis, including communication via molecules contained within extracellular vesicles . Interestingly, some contents of extracellular vesicles can activate HSC, whereas other cargo promote their quiescence 110.
Clinical Manifestations and Pathogenesis
Clinical trials.gov lists over 100 clinical trials related to ALD. However, we know little about the genetic and epigenetic factors that determine why some individuals who drink in excess do not develop histologic lesions beyond steatosis. Nonetheless, the increase in clinical trial activity is encouraging, given the dearth of trials for this disease in prior years.
From our understanding of the molecular and cellular pathogenesis of the disease, it is clear that the progression of ALD can be distinguished from other forms of chronic liver injury, except for non-alcoholic steatohepatitis (NASH), which produces liver lesions similar to those found in patients with ALD, based on histopathologic analysis. ALD is believed to progress from steatosis to inflammation and eventually to fibrosis. Histologic features of steatosis can be detected even after short alcohol binges by healthy individuals. Histologic markers of inflammation, which indicate alcoholic hepatitis, include Mallory hyaline, mega-mitochondria, balloon degeneration of hepatocytes, and infiltration by polymorphonuclear leukocytes. Advanced-stage disease is characterized by fibrosis. In reality, many of these stages of ALD occur concurrently. However, in many patients with advanced fibrosis, early-stage lesions can no longer be detected by histologic analysis, so the alcohol-associated etiology might not be obvious. What strategies are being developed to target these specific lesions? For more comprehensive reviews focused see 111, 112.
Steatosis is the earliest histopathologic marker of ALD. It cannot distinguish patients from ALD with those from non-alcoholic liver disease, so excess alcohol consumption must be confirmed. For patients with this lesion, interventions aimed at decreasing harmful drinking behaviors are an important first step. Ethanol metabolism mediates steatosis and subsequent injury. Increased oxidative stress, due, at least in part, to ethanol metabolism, as well as alcohol-induced changes in gut integrity and microbiome might also be targeted at this early stage. In fact, the link between gut permeability and pathogenesis of liver disease is more established for ALD than for other liver diseases. Therapeutic strategies to target these processes include neutralization of gut bacterial products (endotoxin), restitution of intestinal permeability (with zinc), and realignment of changes in microbiome (with probiotics or antibiotics) 111. Increasing knowledge of how lipid droplet biology contributes to steatosis is likely to lead to therapies that act on steatosis itself, although steatosis itself might not have any detrimental effects on liver function.
As disease progresses, the situation becomes more complex. Inflammation spirals out of control, and hepatocyte injury and death continue. Agents that block a single inflammatory cytokines, such as TNF, have not been shown to be effective in patients with ALD. This is probably because most cytokines have inflammatory and hepato-protective activities. TNF is required for liver regeneration and protection from infection, which are each required for recovery from any severe liver insult. In fact, stimulating regeneration could be an important and understudied approach for treatment of ALD. Similarly, steroids, which reduce inflammation systemically, increase the risk of infection and sepsis.
Strategies aimed at decreasing hepatocyte cell death, by either apoptosis or necroptosis, are also of interest, but will need to be developed for specific stages and cell types. For example, agents that prevent programmed cell death of hepatocytes might also prevent apoptosis of immune cells, which would exacerbate inflammation, or prevent death of activated HSCs, which would promote fibrosis.
Recent preclinical studies have focused on the cytokine IL22 113, 114. IL22 is produced by inflammatory cells and induces regeneration of hepatocytes via STAT proteins. IL22 might therefore be developed to promote hepatocyte regeneration in patients with ALD. IL22 has also been proposed to prevent HSC activation. Its lack of inhibitory effect on inflammatory cells indicates that it may not induce immunosuppression, although there have no clinical studies of IL22’s effects.
Another compound of interest is anakinra, which is already approved for treatment of rheumatologic disorders. Anakinra disrupts IL1B signaling, which is required for induction of sterile necrosis, in which hepatocyte injury leads to recruitment of inflammatory cells. Studies of this compound are underway in patients with alcoholic hepatitis.
Future Directions
Unregulated inflammation stimulates a dysregulated wound healing response that leads to the development of fibrosis and eventual cirrhosis in response to chronic heavy ethanol consumption. In humans, long-term abstinence can attenuate this process. Potential therapies for early-stage ALD could overlap with those for liver cirrhosis of other etiologies. With the increasing prevalence of NASH, a number of trials are underway to target NASH-induced fibrosis. These provide an opportunity to evaluate compounds that might have effects in patients with ALD as well, given their similarities in histopathology. One example is the FXR agonist obeticholic acid 115. This agent has been shown to have effect in patients with NASH. In mouse models, it prevents and even induces regression of fibrosis and reduces portal hypertension.
Our understanding of the mechanisms by which alcohol induced steatosis, inflammation, and fibrosis continues to grow at a rapid rate. New advances in animal models of ALD could more effectively guide identification of targets and development of new therapeutic agents for ALD. Combination therapies against multiple aspects of this complex disease might also be effective. These could include drugs that improve gut and kidney function, normalize (rather than eliminate) the immune response, and promote hepatocyte survival or regeneration. The National Institute on Alcohol Abuse and Alcoholism has increased support for clinical trials of ALD, which could lead to new treatments for ALD.
Acknowledgments
GRANT SUPPORT: Supported by National Institutes of Health (NIH) U01 AA02189 and R01 AA011975 – LEN; (NIH) R01 AA020518; DK1021242; COBRE P20 RR021940 – WXD; (NIH) K99 AA023266 – GC; and (NIH) U01 AA 21788 - VHS.
ABBREVIATIONS
- ADH
alcohol dehydrogenase
- ALDH1
aldehyde dehydrogenase 1
- NAD
nicotinamide adenine dinucleotide
- CYP2E1
cytochrome P450 family 2, subfamily E, polypeptide 1
- ROS
reactive oxygen species
- LPS
lipopolysaccharide
- TNF
tumor necrosis factor-α
- ALD
alcoholic liver disease
- HIFs
hypoxia inducible factors
- SREBP1
sterol regulatory element-binding protein-1
- PPARGC1A
proliferator-activated receptor gamma, coactivator 1 alpha
- ER
endoplasmic reticulum
- UPR
unfolded protein response
- ERAD
ER-associated protein degradation
- IKK
I-κB kinase
- IRF3
interferon regulator factor 3
- RIP
receptor-interacting protein kinase
- MIF
macrophage inhibitor factor
- MLKL
mixed-lineage kinase domain-like protein
- SIRT1
sirtuin 1
- TNFR1
TNF receptor 1
- TRADD
TNFR-associated death domain
- TRAF2
TNFR-associated factor 2
- cIAP1
cellular inhibitor of apoptosis proteins 1
- TAK1
transforming growth factor β-activated kinase 1
- FADD
fas-associated protein with a death domain
- CFLAR
CASP8 and FADD-like apoptosis regulator
- TAB2
TGF-beta activated kinase 1/MAP3K7 binding protein 2
Footnotes
DISCLOSURES: The authors disclose no conflicts.
AUTHOR CONTRIBUTIONS: All authors contributed to writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crabb DW, Matsumoto M, Chang D, et al. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc Nutr Soc. 2004;63:49–63. doi: 10.1079/pns2003327. [DOI] [PubMed] [Google Scholar]
- 2.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–38. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Wang S, Ni HM, et al. Autophagy in alcohol-induced multiorgan injury: mechanisms and potential therapeutic targets. Biomed Res Int. 2014;2014:498491. doi: 10.1155/2014/498491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Q, Mak KM, Lieber CS. Cytochrome P4502E1 primes macrophages to increase TNF-alpha production in response to lipopolysaccharide. Am J Physiol Gastrointest Liver Physiol. 2005;289:G95–107. doi: 10.1152/ajpgi.00383.2004. [DOI] [PubMed] [Google Scholar]
- 5.Thakur V, Pritchard MT, McMullen MR, et al. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348–56. doi: 10.1189/jlb.1005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lange LG, Sobel BE. Mitochondrial dysfunction induced by fatty acid ethyl esters, myocardial metabolites of ethanol. J Clin Invest. 1983;72:724–31. doi: 10.1172/JCI111022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 8.Kono H, Rusyn I, Yin M, et al. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867–72. doi: 10.1172/JCI9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kessova IG, Ho YS, Thung S, et al. Alcohol-induced liver injury in mice lacking Cu, Zn-superoxide dismutase. Hepatology. 2003;38:1136–45. doi: 10.1053/jhep.2003.50450. [DOI] [PubMed] [Google Scholar]
- 10.McKim SE, Gabele E, Isayama F, et al. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology. 2003;125:1834–44. doi: 10.1053/j.gastro.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Zelickson BR, Benavides GA, Johnson MS, et al. Nitric oxide and hypoxia exacerbate alcohol-induced mitochondrial dysfunction in hepatocytes. Biochim Biophys Acta. 2011;1807:1573–82. doi: 10.1016/j.bbabio.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arteel GE, Iimuro Y, Yin M, et al. Chronic enteral ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology. 1997;25:920–6. doi: 10.1002/hep.510250422. [DOI] [PubMed] [Google Scholar]
- 13.Nishiyama Y, Goda N, Kanai M, et al. HIF-1alpha induction suppresses excessive lipid accumulation in alcoholic fatty liver in mice. J Hepatol. 2012;56:441–7. doi: 10.1016/j.jhep.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Nath B, Levin I, Csak T, et al. Hepatocyte-specific hypoxia-inducible factor-1alpha is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53:1526–37. doi: 10.1002/hep.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehal WZ. HIF-1alpha is a major and complex player in alcohol induced liver diseases. J Hepatol. 2012;56:311–2. doi: 10.1016/j.jhep.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Ni HM, Bhakta A, Wang S, et al. Role of hypoxia inducing factor-1beta in alcohol-induced autophagy, steatosis and liver injury in mice. PLoS One. 2014;9:e115849. doi: 10.1371/journal.pone.0115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You M, Liang X, Ajmo JM, et al. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008;294:G892–8. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- 19.Ponugoti B, Kim DH, Xiao Z, et al. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285:33959–70. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin H, Hu M, Liang X, et al. Deletion of SIRT1 from hepatocytes in mice disrupts lipin-1 signaling and aggravates alcoholic fatty liver. Gastroenterology. 2014;146:801–11. doi: 10.1053/j.gastro.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu M, Yin H, Mitra MS, et al. Hepatic-specific lipin-1 deficiency exacerbates experimental alcohol-induced steatohepatitis in mice. Hepatology. 2013;58:1953–63. doi: 10.1002/hep.26589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purushotham A, Schug TT, Xu Q, et al. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009;9:327–38. doi: 10.1016/j.cmet.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You M, Matsumoto M, Pacold CM, et al. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. 2004;127:1798–808. doi: 10.1053/j.gastro.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 24.Ajmo JM, Liang X, Rogers CQ, et al. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–42. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Ruiz C, Kaplowitz N, Fernandez-Checa JC. Role of Mitochondria in Alcoholic Liver Disease. Curr Pathobiol Rep. 2013;1:159–168. doi: 10.1007/s40139-013-0021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King AL, Swain TM, Mao Z, et al. Involvement of the mitochondrial permeability transition pore in chronic ethanol-mediated liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2014;306:G265–77. doi: 10.1152/ajpgi.00278.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams JA, Ni HM, Ding Y, et al. Parkin regulates mitophagy and mitochondrial function to protect against alcohol-induced liver injury and steatosis in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G324–40. doi: 10.1152/ajpgi.00108.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han D, Ybanez MD, Johnson HS, et al. Dynamic adaptation of liver mitochondria to chronic alcohol feeding in mice: biogenesis, remodeling, and functional alterations. J Biol Chem. 2012;287:42165–79. doi: 10.1074/jbc.M112.377374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–99. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 30.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 31.Ding WX, Yin XM. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy. 2008;4:141–50. doi: 10.4161/auto.5190. [DOI] [PubMed] [Google Scholar]
- 32.Donohue TM, Jr, Zetterman RK, Zhang-Gouillon ZQ, et al. Peptidase activities of the multicatalytic protease in rat liver after voluntary and intragastric ethanol administration. Hepatology. 1998;28:486–91. doi: 10.1002/hep.510280228. [DOI] [PubMed] [Google Scholar]
- 33.Lin CW, Zhang H, Li M, et al. Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice. J Hepatol. 2013;58:993–9. doi: 10.1016/j.jhep.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding WX, Li M, Chen X, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:1740–52. doi: 10.1053/j.gastro.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Czaja MJ, Ding WX, Donohue TM, Jr, et al. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9:1131–58. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joseph RA, Shepard BD, Kannarkat GT, et al. Microtubule acetylation and stability may explain alcohol-induced alterations in hepatic protein trafficking. Hepatology. 2008;47:1745–53. doi: 10.1002/hep.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clemens DL, Casey CA, Sorrell MF, et al. Ethanol oxidation mediates impaired hepatic receptor-mediated enocytosis. Alcohol Clin Exp Res. 1998;22:778–9. doi: 10.1111/j.1530-0277.1998.tb03866.x. [DOI] [PubMed] [Google Scholar]
- 38.Casey CA, Wiegert RL, Tuma DJ. Chronic ethanol administration impairs ATP-dependent acidification of endosomes in rat liver. Biochem Biophys Res Commun. 1993;195:1127–33. doi: 10.1006/bbrc.1993.2161. [DOI] [PubMed] [Google Scholar]
- 39.Kharbanda KK, McVicker DL, Zetterman RK, et al. Ethanol consumption reduces the proteolytic capacity and protease activities of hepatic lysosomes. Biochim Biophys Acta. 1995;1245:421–9. doi: 10.1016/0304-4165(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 40.Settembre C, De Cegli R, Mansueto G, et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat Cell Biol. 2013;15:647–58. doi: 10.1038/ncb2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165–94. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casey CA, Nanji A, Cederbaum AI, et al. Alcoholic liver disease and apoptosis. Alcohol Clin Exp Res. 2001;25:49S–53S. doi: 10.1097/00000374-200105051-00009. [DOI] [PubMed] [Google Scholar]
- 43.Feldstein AE, Gores GJ. Apoptosis in alcoholic and nonalcoholic steatohepatitis. Front Biosci. 2005;10:3093–9. doi: 10.2741/1765. [DOI] [PubMed] [Google Scholar]
- 44.He S, Wang L, Miao L, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–11. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 46.Cho YS, Challa S, Moquin D, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–23. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol. 2011;12:439–52. doi: 10.1038/nrm3143. [DOI] [PubMed] [Google Scholar]
- 48.Weinlich R, Green DR. The two faces of receptor interacting protein kinase-1. Mol Cell. 2014;56:469–80. doi: 10.1016/j.molcel.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, et al. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–47. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 50.Mandal P, Berger SB, Pillay S, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell. 2014;56:481–95. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tenev T, Bianchi K, Darding M, et al. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–48. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Dillon CP, Weinlich R, Rodriguez DA, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roychowdhury S, Chiang DJ, Mandal P, et al. Inhibition of apoptosis protects mice from ethanol-mediated acceleration of early markers of CCl4-induced fibrosis but not steatosis or inflammation. Alcohol Clin Exp Res. 2012;36:1139–47. doi: 10.1111/j.1530-0277.2011.01720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roychowdhury S, McMullen MR, Pisano SG, et al. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57:1773–83. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ni HM, Williams JA, Jaeschke H, et al. Zonated induction of autophagy and mitochondrial spheroids limits acetaminophen-induced necrosis in the liver. Redox Biol. 2013;1:427–32. doi: 10.1016/j.redox.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, Wang P, Sun Q, et al. Following cytochrome c release, autophagy is inhibited during chemotherapy-induced apoptosis by caspase 8-mediated cleavage of Beclin 1. Cancer Res. 2011;71:3625–34. doi: 10.1158/0008-5472.CAN-10-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yonekawa T, Gamez G, Kim J, et al. RIP1 negatively regulates basal autophagic flux through TFEB to control sensitivity to apoptosis. EMBO Rep. 2015;16:700–8. doi: 10.15252/embr.201439496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elamin EE, Masclee AA, Dekker J, et al. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr Rev. 2013;71:483–99. doi: 10.1111/nure.12027. [DOI] [PubMed] [Google Scholar]
- 59.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–44. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forsyth CB, Farhadi A, Jakate SM, et al. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163–72. doi: 10.1016/j.alcohol.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez-Reimers E, Santolaria-Fernandez F, Martin-Gonzalez MC, et al. Alcoholism: a systemic proinflammatory condition. World J Gastroenterol. 2014;20:14660–71. doi: 10.3748/wjg.v20.i40.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang XP, Lei F, Du F, et al. Protection of Gastrointestinal Mucosa from Acute Heavy Alcohol Consumption: The Effect of Berberine and Its Correlation with TLR2, 4/IL1beta-TNFalpha Signaling. PLoS One. 2015;10:e0134044. doi: 10.1371/journal.pone.0134044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bode C, Bode JC. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17:575–92. doi: 10.1016/s1521-6918(03)00034-9. [DOI] [PubMed] [Google Scholar]
- 64.Pittman K, Kubes P. Damage-associated molecular patterns control neutrophil recruitment. J Innate Immun. 2013;5:315–23. doi: 10.1159/000347132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seo YS, Kwon JH, Yaqoob U, et al. HMGB1 recruits hepatic stellate cells and liver endothelial cells to sites of ethanol-induced parenchymal cell injury. Am J Physiol Gastrointest Liver Physiol. 2013;305:G838–48. doi: 10.1152/ajpgi.00151.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ge X, Antoine DJ, Lu Y, et al. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD) J Biol Chem. 2014;289:22672–91. doi: 10.1074/jbc.M114.552141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang JX, Venugopal S, Serizawa N, et al. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology. 2010;139:1375–84. doi: 10.1053/j.gastro.2010.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petrasek J, Iracheta-Vellve A, Saha B, et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J Leukoc Biol. 2015;98:249–56. doi: 10.1189/jlb.3AB1214-590R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szabo G, Petrasek J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol. 2015;12:387–400. doi: 10.1038/nrgastro.2015.94. [DOI] [PubMed] [Google Scholar]
- 70.DeSantis DA, Lee P, Doerner SK, et al. Genetic resistance to liver fibrosis on A/J mouse chromosome 17. Alcohol Clin Exp Res. 2013;37:1668–79. doi: 10.1111/acer.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mandrekar P, Ambade A, Lim A, et al. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185–97. doi: 10.1002/hep.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Na TY, Han YH, Ka NL, et al. 22-S-Hydroxycholesterol protects against ethanol-induced liver injury by blocking the auto/paracrine activation of MCP-1 mediated by LXRalpha. J Pathol. 2015;235:710–20. doi: 10.1002/path.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Momen-Heravi F, Bala S, Kodys K, et al. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci Rep. 2015;5:9991. doi: 10.1038/srep09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Verma VK, Li H, Wang R, et al. Alcohol stimulates macrophage activation through caspase dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2015 Nov 26; doi: 10.1016/j.jhep.2015.11.020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.SELA, Mager I, Breakefield XO, et al. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–57. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 76.Wang HJ, Gao B, Zakhari S, et al. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343–68. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Connell RM, Taganov KD, Boldin MP, et al. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A. 2007;104:1604–9. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bala S, Marcos M, Kodys K, et al. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286:1436–44. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szabo G, Petrasek J, Bala S. Innate immunity and alcoholic liver disease. Dig Dis. 2012;30(Suppl 1):55–60. doi: 10.1159/000341126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wan J, Benkdane M, Teixeira-Clerc F, et al. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130–42. doi: 10.1002/hep.26607. [DOI] [PubMed] [Google Scholar]
- 81.Mandal P, Pratt BT, Barnes M, et al. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem. 2011;286:13460–9. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu J, Chi F, Guo T, et al. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J Clin Invest. 2015;125:1579–90. doi: 10.1172/JCI76468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schmidt-Weber CB, Blaser K. Regulation and role of transforming growth factor-beta in immune tolerance induction and inflammation. Curr Opin Immunol. 2004;16:709–16. doi: 10.1016/j.coi.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 84.Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat Immunol. 2005;6:1198–205. doi: 10.1038/ni1274. [DOI] [PubMed] [Google Scholar]
- 85.Aldred A, Nagy LE. Ethanol dissociates hormone-stimulated cAMP production from inhibition of TNF-alpha production in rat Kupffer cells. Am J Physiol. 1999;276:G98–G106. doi: 10.1152/ajpgi.1999.276.1.G98. [DOI] [PubMed] [Google Scholar]
- 86.Huang H, Park PH, McMullen MR, et al. Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J Gastroenterol Hepatol. 2008;23(Suppl 1):S50–3. doi: 10.1111/j.1440-1746.2007.05284.x. [DOI] [PubMed] [Google Scholar]
- 87.Mandal P, Roychowdhury S, Park PH, et al. Adiponectin and heme oxygenase-1 suppress TLR4/MyD88-independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. J Immunol. 2010;185:4928–37. doi: 10.4049/jimmunol.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bakhautdin B, Das D, Mandal P, et al. Protective role of HO-1 and carbon monoxide in ethanol-induced hepatocyte cell death and liver injury in mice. J Hepatol. 2014;61:1029–37. doi: 10.1016/j.jhep.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barnes MA, McMullen MR, Roychowdhury S, et al. Macrophage migration inhibitory factor contributes to ethanol-induced liver injury by mediating cell injury, steatohepatitis, and steatosis. Hepatology. 2013;57:1980–91. doi: 10.1002/hep.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barnes MA, McMullen MR, Roychowdhury S, et al. Macrophage migration inhibitory factor is required for recruitment of scar-associated macrophages during liver fibrosis. J Leukoc Biol. 2015;97:161–9. doi: 10.1189/jlb.3A0614-280R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramachandran P, Pellicoro A, Vernon MA, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–95. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chang B, Xu MJ, Zhou Z, et al. Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: An important role for CXCL1. Hepatology. 2015;62:1070–85. doi: 10.1002/hep.27921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology. 2013;58:1814–23. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220–32. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Albano E, Vidali M. Immune mechanisms in alcoholic liver disease. Genes Nutr. 2010;5:141–7. doi: 10.1007/s12263-009-0151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Laso JF, Madruga IJ, Orfao A. Cytokines and alcohol liver disease. In: Sherman DIN, Preedy VR, Watson RR, editors. Ethanol and the Liver: Mechanisms and Management. London: Taylor and Francis; 2002. pp. 206–219. [Google Scholar]
- 97.Almeida J, Polvorosa MA, Gonzalez-Quintela A, et al. Decreased peripheral blood CD4+/CD25+ regulatory T cells in patients with alcoholic hepatitis. Alcohol Clin Exp Res. 2013;37:1361–9. doi: 10.1111/acer.12095. [DOI] [PubMed] [Google Scholar]
- 98.Aloman C, Gehring S, Wintermeyer P, et al. Chronic ethanol consumption impairs cellular immune responses against HCV NS5 protein due to dendritic cell dysfunction. Gastroenterology. 2007;132:698–708. doi: 10.1053/j.gastro.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 99.Eken A, Ortiz V, Wands JR. Ethanol inhibits antigen presentation by dendritic cells. Clin Vaccine Immunol. 2011;18:1157–66. doi: 10.1128/CVI.05029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mandrekar P, Catalano D, Dolganiuc A, et al. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004;173:3398–407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- 101.Gao B, Radaeva S. Natural killer and natural killer T cells in liver fibrosis. Biochim Biophys Acta. 2013;1832:1061–9. doi: 10.1016/j.bbadis.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang W, Sun R, Zhou R, et al. TLR-9 activation aggravates concanavalin A-induced hepatitis via promoting accumulation and activation of liver CD4+ NKT cells. J Immunol. 2009;182:3768–74. doi: 10.4049/jimmunol.0800973. [DOI] [PubMed] [Google Scholar]
- 103.Mathews S, Feng D, Maricic I, et al. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration. Cell Mol Immunol. 2015 doi: 10.1038/cmi.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pellicoro A, Ramachandran P, Iredale JP, et al. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–94. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 105.Inokuchi S, Tsukamoto H, Park E, et al. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res. 2011;35:1509–18. doi: 10.1111/j.1530-0277.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kong X, Feng D, Wang H, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150–9. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao B, Seki E, Brenner DA, et al. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516–25. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang L, Kwon J, Popov Y, et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146:1339–50. e1. doi: 10.1053/j.gastro.2014.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maricic I, Sheng H, Marrero I, et al. Inhibition of type I natural killer T cells by retinoids or following sulfatide-mediated activation of type II natural killer T cells attenuates alcoholic liver disease in mice. Hepatology. 2015;61:1357–69. doi: 10.1002/hep.27632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen L, Chen R, Kemper S, et al. Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression: Role of exosomes in horizontal transfer of Twist1. Am J Physiol Gastrointest Liver Physiol. 2015;309:G491–9. doi: 10.1152/ajpgi.00140.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Orman ES, Odena G, Bataller R. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol. 2013;28(Suppl 1):77–84. doi: 10.1111/jgh.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol. 2015;62:S38–46. doi: 10.1016/j.jhep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kong X, Feng D, Mathews S, et al. Hepatoprotective and anti-fibrotic functions of interleukin-22: therapeutic potential for the treatment of alcoholic liver disease. J Gastroenterol Hepatol. 2013;28(Suppl 1):56–60. doi: 10.1111/jgh.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao B, Shah VH. Combination therapy: New hope for alcoholic hepatitis? Clin Res Hepatol Gastroenterol. 2015;39(Suppl 1):S7–S11. doi: 10.1016/j.clinre.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gawrieh S, Chalasani N. Pharmacotherapy for Nonalcoholic Fatty Liver Disease. Semin Liver Dis. 2015;35:338–48. doi: 10.1055/s-0035-1562951. [DOI] [PMC free article] [PubMed] [Google Scholar]