Abstract
Anxiety symptoms are common in later life and are associated with diverse adverse health outcomes. Little is known about how genetic and environmental influences on anxiety symptoms might vary across older adulthood. The purpose of this study was to explore change and stability of contributions to anxiety symptoms across older adulthood. We examined data from the Swedish Adoption/Twin Study of Aging (SATSA). Between the years 1984 and 2010, 2,021 participants (including 753 complete twin pairs) completed up to seven assessments containing two measures of anxiety symptoms. Longitudinal genetic simplex models were fit to examine the stability and change in genetic and environmental influences. Amplification of genetic factors at ages 75–80 suggests tentative new genetic contributions to anxiety symptoms. These findings suggest that the heritability of anxiety symptoms may increase later in life. Physiological factors associated with aging are discussed as potential factors explaining this increase.
Keywords: Anxiety, older adults, longitudinal, genetics
Significant anxiety symptoms are common in later life affecting as many as 32 percent of non-depressed community dwelling older adults (Braam et al. 2014). Late-life anxiety symptoms have been linked with many adverse outcomes such as death ideation (Van Orden et al. 2013), nursing home placement (Gibbons et al. 2002), poor cognitive performance (Beaudreau et al. 2013), functional impairment (Brenes et al. 2008; Porensky et al. 2009), and poor general health (Losada et al. 2014).
Anxiety disorders tend to be chronic across the lifespan (Goncalves and Byrne 2012; Kessler et al. 2005). Onset of anxiety disorders in later life, however, does exist and is not uncommon. Chou (2009) found, in a large epidemiological study, that almost half of the older adults with generalized anxiety disorder (GAD) had onset after the age of 55. In more recent research, Zhang et al. (2015), examining data from a large prospective study of community-dwelling older adults, found that 8.4% of older adults developed GAD over a span of 12 years. Interestingly, 80% of those cases were first episode cases suggesting that later life onset of GAD is more common than previously assumed. Overall GAD may have a bimodal onset with a peak of onset earlier in life as well as increased rate of incidence after the age of 50 (Le Roux et al. 2005). Symptoms of anxiety also tend to be relatively stable; earlier analyses of the same sample as the present study found minimal fluctuation in symptoms over a span of six years in older adulthood (Wetherell et al. 2001). Thus, anxiety symptoms are stable longitudinally with some evidence of later life onset for some adults.
Behavioral genetic twin studies afford the means of distinguishing between the effects of genetics and those that are attributable to environmental influences. Twin studies examining the contribution of genes to the etiology of anxiety suggest that genetic factors explain approximately 34–46% of the variance in anxiety symptoms in younger adults (Kendler et al. 1986) and 35% of the variance in health anxiety in middle aged adults (Taylor, Thordarson, Jang, & Asmundson, 2006). Likewise, the various anxiety disorders have similar heritability (Hettema et al. 2005).
Stability and change in anxiety symptoms across older adulthood may be due to either genetic or environmental contributions, or both. Compared to younger adults, a number of potential new sources of genetic or environmental variance in anxiety symptoms arise later in life. These include physiological factors (e.g. biological aspects of normal aging, chronic illnesses, and cognitive impairment) and social factors (e.g. role transitions associated with aging, loss of independence, caregiving for significant other, and bereavement) that are more unique to the presentation of anxiety later in life (Wolitzky-Taylor et al. 2010). These new influences could also lead to onset of anxiety in older adults who have no prior history of anxiety symptoms.
The extent to which age moderates the heritability of anxiety symptoms in older adulthood remains relatively understudied. This is an important question as elucidating the etiology of anxiety in later life may aid in identifying targets for future intervention and prevention efforts. A longitudinal examination of individuals across the ages of 20 to over 70 found the new genetic influences on anxiety symptoms at age 30 in women (Gillespie et al. 2004). Due to sample considerations, this study had to group women aged 70 and older together, and men aged 60 and older together, resulting in limited sensitivity in examining the potential for new genetic and environmental influences within middle and late older adulthood.
As anxiety and depression are often co-morbid and share similar genetic influences (Kendler et al. 1987), the literature on depression may suggest how the heritability of anxiety symptoms change across older adulthood. Some research examining the variability of depressive disorders and depressive symptoms across the lifespan suggest that new genetic influences may emerge later in life. The Gillespie et al. (2004) study found new genetic influences on depressive symptoms at age 40 for women. Gatz et al. (1992) found the heritability of depressive symptoms was greater in adults older than 60 than in adults younger than 60. Comparing across two time points, Carmelli et al. (2000) found genetic influences on depression in men increased in later life by approximately 25%. The depression literature is mixed, however, as some studies have reported no moderating effect of age on heritability of depressive symptoms (Johnson et al. 2002; McGue and Christensen 2003; McGue and Christensen 2013).
The purpose of this study was to examine the change and stability of genetic and environmental contributions to anxiety symptoms across 26 years in older adults from ages 50 to 85. We sought to identify if new genetic or environmental influences were contributing to the etiology of anxiety symptoms throughout older adulthood, and hypothesized that new genetic factors would contribute to the etiology of anxiety in later life.
Materials and methods
Participants
Data were drawn from the Swedish Adoption Twin Study of Aging (SATSA). The SATSA study is a subset of the Swedish Twin Registry. The registry includes data from all same-sex twin pairs born in Sweden between 1886 and 1958 and is representative of the Swedish population (Cederlof and Lorich 1978). SATSA contains all twins from the Swedish registry who were reared apart, as well as twins reared together who were matched with twins reared apart on gender, county of birth, and age. A total of 2,136 participants completed at least one measure of anxiety. Because this study was interested in examining genetic and environmental influences across older adulthood we excluded participants who never reached age 50 during the study period (N = 75). Due to the small number of participants aged 85 or older at the initial anxiety assessment these participants were excluded from our analyses (N = 15). Participants were assessed at Q1 (1984) and again in 1987 (Q2), 1990 (Q3), 1993 (Q4), 2004 (Q5), 2007 (Q6), and 2010 (Q7). Therefore, over the 26-year follow-up period, participants were assessed as many as seven times. In total, 2,021 participants (753 complete twin pairs) completed at least one measurement of anxiety symptoms and were between the ages of 50 and 85 years old. Of these 2,021 participants: 89 (4.40%) completed all seven anxiety assessments, 133 (6.58%) completed six, 183 (9.05%) completed five, 504 (24.94%) completed four, 355 (17.57%) completed three, 331 (16.38%) completed two, and 426 (21.08%) completed only one assessment. This dataset is particularly advantageous as a means for investigating our research question due to the fact that half of the twin pairs were reared apart, increasing the power to examine the contribution of shared rearing environment influences. For greater detail on the SATSA methods and sample, please see Finkel & Pedersen (2004) or Pedersen et al. (1991).
Measures
Anxiety was measured using two scales. The primary phenotype for this study was state anxiety as measured using the 10-item state anxiety subscale of the State-Trait Personality Inventory (STPI; Spielberger 1979). The STPI administered in SATSA used a 5-point response scale (“Fits me exactly” to “Does not fit me at all”). The STPI has been shown to be reliable and valid with community dwelling older adults (Potvin et al. 2011). The STPI was administered at Q1, Q2, Q3, Q4, and Q7.
Anxiety was also measured at all Qs using a secondary anxiety scale, that we call the Anxiety Personality Questionnaire (APQ), derived via data harmonization from anxiety items of the neuroticism scale of the Eysenck Personality Inventory (EPI; Eysenck and Eysenck 1968). The EPI neuroticism scale contains nine items which participants rate dichotomously (No = 0, Yes = 1) to indicate if they have been experiencing particular thoughts or feelings. Data from the Q1 assessment was utilized to find the best set of EPI neuroticism items that correspond to the STPI items and then transform the score of these neuroticism items into STPI score units. Harmonization used the random equivalence-equating method (Bond and Fox 2007). Rasch analyses demonstrated that three neuroticism items (sensitive, happy or sad without reason, and worry after embarrassing oneself in a social situation) exhibited poor fit with the STPI and were removed from the scale. Subsequent Rasch analyses with the six remaining neuroticism items (“anxious”, “make decisions late”, “tired”, “deep in thought”, “restless”, “nervous”) exhibited acceptable face validity, person and item level psychometric properties, internal reliability (Cronbach’s alpha = 0.66), and infit/outfit mean squares. A crosswalk conversion table based on common person ability measures for each set of items permitted computing an APQ score that matched the raw score of the six EPI items with the latent trait score values from the STPI. Creating a second harmonized measure of anxiety permitted us more times of measurement because APQ scores were available at all assessment points.
The STPI is a measure of state anxiety. Participants are asked to rate how they have been feeling over the past 2 weeks. The APQ is a measure of trait anxiety in that participants were asked to answer how they generally feel. It is likely that the APQ was capturing mean anxiety over a long period of time while the STPI was capturing more current levels of anxiety. Prior work in SATSA (Wetherell et al., 2001) has found that although the STPI is assessing state anxiety symptoms are relatively stable (autoregressive factor loading = 0.67) over time.
Zygosity and rearing status
Zygosity was determined using responses to standard questionnaire items as to physical similarity, and confirmed with standard serological or genetic marker assays for a subset of twins. Twins were classified as being reared apart if they were separated before the age of 11 years old. Over 80% of twins reared apart were separated before the age of five (Pedersen et al., 1991).
Statistical Analyses
Preliminary analyses were run prior to genetic model fitting. Due to sample size considerations we were unable to fit the simplex models separately for males and females. Because prior research suggests that the genetic and environmental determinants of anxiety may vary by gender (Gillespie et al. 2004), we controlled for sex effects by residualizing sex effects using regression (McGue and Bouchard 1984). For descriptive purposes we examined the differences between men and women on mean anxiety as well as the cross sectional heritability of anxiety symptoms at the Q1 assessment. Additionally, the STPI (skew= 1.15, kurtosis=1.09) and APQ (skew =1.64, kurtosis=2.02) were significantly skewed so a square root transformation was used to decrease the skew of the data (skew after transformation = 0.73 and 1.29; kurtosis = −0.02 and 0.80). Lastly, to examine the possible effect of differential attrition and missing data over the study period we compared participants who completed only 1–2 assessments and participants who had completed at least 3 assessments.
Standard biometrical genetic model-fitting methods were implemented. Twin studies allow for the decomposition of the variance of a phenotype into the following components: the additive genetic variance (A), shared rearing variance (S), correlated environmental variance (C), and non-shared environmental variance including error (E). In the ACSE models four sets of equations were used. Equations are based on whether the twin pair is monozygotic reared together (covMZT = Va + Vc + Vs), monozygotic reared apart (covMZA = Va + Vc), dizygotic reared together (covDZT = 0.5*Va + Vc + Vs), or dizygotic reared apart (covDZA = 0.5*Va + Vc). The additive genetic coefficient for monozygotic (MZ) twins was set to 1.0 while for dizygotic (DZ) twins it was set to 0.50, as MZ pairs share 100% of the additive effects of their segregating genes while DZ pairs share 50% on average. The intrapair correlations were suggestive of possible dominant genetic effects. Therefore, we also examined an ADSE model. It is mathematically impossible to estimate all ACDSE components in the same model, therefore, separate ACSE and ADSE models were fit.
Participant age at each measurement was calculated and classified to be in one of seven age buckets: 50–54.99, 55–59.9, 60–64.9, 65–69.9, 70–74.9, 75–79.9, and 80–84.9. If participants were assessed twice during one of these periods only the first observation was used. The total number of twin pairs aged 85 and older was small (N = only 12 complete twin pairs with STPI data). Due to the limited number of participants in this age group simplex models did not include twins 85 and older. See Table I for the total number of complete and partial twin pairs by zygosity, rearing status, and age bucket. Participants may potentially be in multiple age buckets, therefore numbers do not add up to 2,021. Additionally, because the APQ was administered at more time points there is a discrepancy between number of assessment points for the STPI and APQ.
Table I.
Number of complete and incomplete twin pairs who completed a STPI assessment by age interval, zygosity, and rearing status.
| 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | 75–79 | 80–84 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ●● | ●○ | ●● | ●○ | ●● | ●○ | ●● | ●○ | ●● | ●○ | ●● | ●○ | ●● | ●○ | |
| Variable: STPI | ||||||||||||||
| 1.MZ | 53 | 24 | 68 | 26 | 76 | 42 | 82 | 70 | 73 | 76 | 41 | 74 | 17 | 56 |
| 2.DZ | 93 | 71 | 106 | 82 | 128 | 112 | 160 | 137 | 128 | 183 | 86 | 142 | 28 | 123 |
| 1.MZT | 18 | 12 | 24 | 15 | 24 | 23 | 29 | 37 | 34 | 33 | 20 | 32 | 8 | 25 |
| 2.MZA | 35 | 12 | 44 | 11 | 52 | 19 | 53 | 33 | 39 | 43 | 21 | 42 | 9 | 31 |
| 3.DZT | 57 | 42 | 66 | 49 | 71 | 68 | 77 | 81 | 56 | 100 | 38 | 77 | 11 | 70 |
| 4.DZA | 36 | 29 | 40 | 33 | 57 | 44 | 83 | 56 | 72 | 83 | 48 | 65 | 17 | 53 |
| Total | 146 | 95 | 174 | 108 | 204 | 154 | 242 | 207 | 201 | 259 | 127 | 216 | 45 | 179 |
| Variable: APQ | ||||||||||||||
| 1.MZ | 60 | 33 | 92 | 27 | 102 | 49 | 109 | 75 | 101 | 93 | 67 | 98 | 39 | 71 |
| 2.DZ | 125 | 75 | 144 | 96 | 179 | 138 | 223 | 175 | 191 | 223 | 125 | 196 | 57 | 182 |
| 1.MZT | 20 | 17 | 32 | 14 | 34 | 26 | 43 | 39 | 42 | 41 | 31 | 38 | 19 | 27 |
| 2.MZA | 40 | 16 | 60 | 13 | 68 | 23 | 66 | 36 | 59 | 52 | 36 | 60 | 20 | 44 |
| 3.DZT | 69 | 45 | 87 | 57 | 95 | 87 | 113 | 104 | 90 | 130 | 64 | 109 | 30 | 98 |
| 4.DZA | 56 | 30 | 57 | 39 | 84 | 51 | 110 | 71 | 101 | 93 | 61 | 87 | 27 | 84 |
| Total | 185 | 108 | 236 | 123 | 281 | 187 | 332 | 250 | 292 | 316 | 192 | 294 | 96 | 253 |
Note: Single participants may be in multiple intervals; discrepancy between STPI and APQ is due extra APQ timepoints ●● = complete twin pair; ●○ = incomplete twin pair; STPI=State Trait subscale from the State Trait Personality Inventory; APQ= Anxiety Personality Questionnaire; MZ = Monozygotic twin pair; DZ = Dizygotic twin pair; MZT = Monozygotic pair raised together; MZA = Monozygotic pair raised apart; DZT = Dizygotic pair raised together; DZA = Dizygotic pair raised apart
Longitudinal simplex models
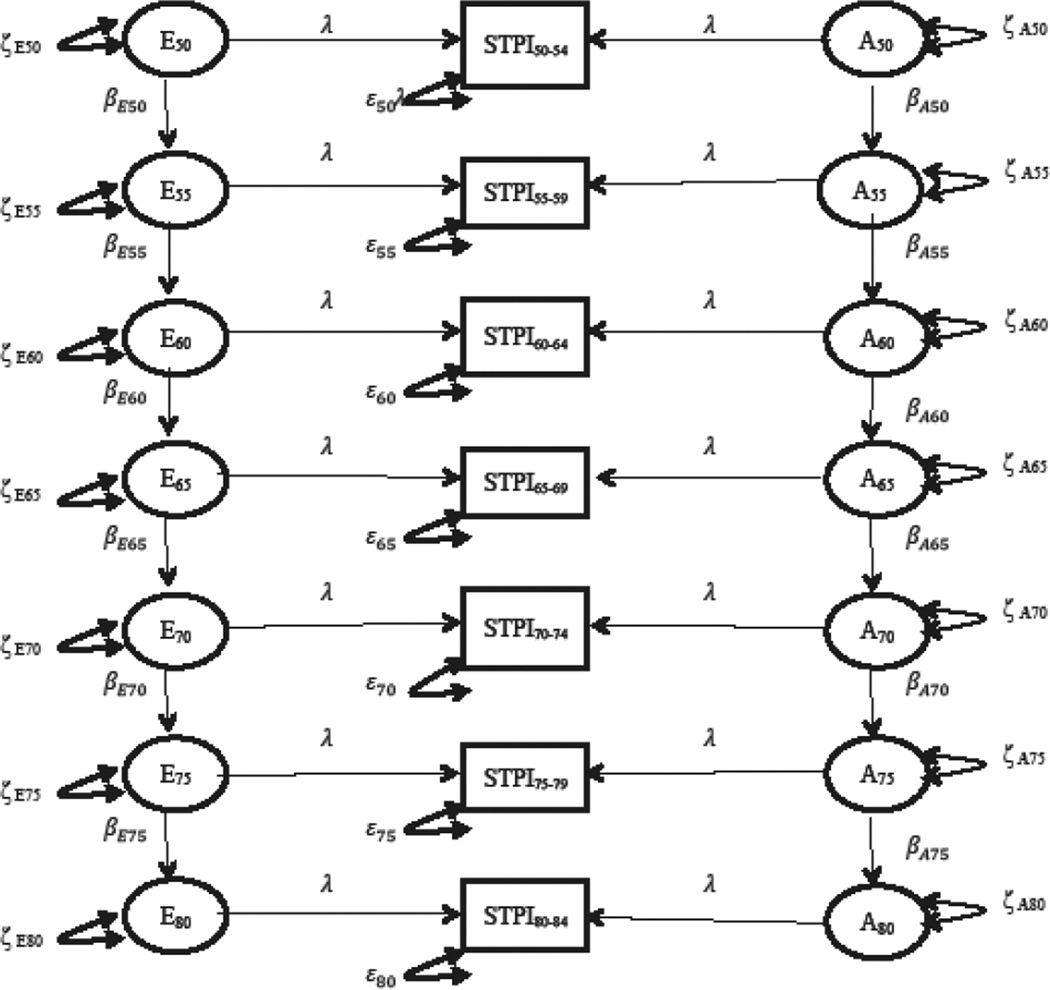
Simplex models were fit to examine the stability of genetic and environmental influences on anxiety symptoms over time. Simplex models allow for the analysis of the longitudinal nature of the data and enables inferences of temporal causation (Boomsma et al. 1989). Simplex models also discriminate between genetic/environmental factors that are persistent across time and factors that are unique to a certain age. Genetic simplex models are first-order autoregressive models in that scores are predicted on the basis of the previous time point. Figure 1 displays the full simplex model with parameter labels. The anxiety score at age 55–59.99 can be expressed by the following equation: anx55–59 = β50 * anx54 + ζ55–59. In this equation the anx55–59 term represents the latent variable of anxiety for those 55–59 years old. The β50 is the regression of the latent factor on the preceding latent factor of anxiety for those 50–54 years old (anx50). A beta that is significantly greater than 1.0 represents amplifications of previous influences to the next age. Similarly, a beta significantly less than 1.0 represents a degradation of prior influences. The parameter ζ55–59 is the new genetic innovation for those between the ages of 55–59. The equation for the measurement model for those ages 50–54 was written as ANX50 = λ50*anx50 + ε50. In this equation ANX50 represents the raw anxiety score for someone in this age group, λ50 represents the factor loading of this raw score (λ was always constrained to 1.00) on the latent variable anx50, and ε50 represents the measurement error of the observed variables. The innovation term (ζ) in the structural equation represents genetic or environmental factors that significantly explain variance at that age group as well as every subsequent age. For example, if genetic innovations at the ages of 50–54 are observed, assuming betas are non-zero, those genetic innovations would continuously explain variance at all subsequent time points. The genetic innovation at the 50–54 ages would also theoretically represent continuation of genetic effects from even younger ages. Likewise, if significant genetic innovations were observed at the ages of 70–74, this would indicate that new genetic influences explain additional variance in anxiety at this age as well as all subsequent ages. These genetic factors in this age 70–74 innovation, however, would not explain variance in participants younger than 70. The error terms for the last two time points were constrained to be equal. This constraint is needed in order for the model to be identified and converge.
Figure 1.
Depiction of the parameter estimated in the full AE STPI simplex model. A = additive genetic effects; E = unique environmental effects; STPI = STPI for each age bucket; single headed arrows=path coefficients; double headed arrow = variance components. λ is constrained to 1.0.
The full model consisted of estimating all ACSE components. In total, the full ACSE model estimated 65 parameters consisting of: six error terms (ε54 – ε75), seven means (µ54 – µ80), seven innovations (ζ) and six regression coefficients (β) for each source of variation (A, C, S, or E). First, we examined the effect of shared rearing (S) by dropping this term from the model and estimating an ACE model. Second, the effect of correlated (C) environmental parameters was examined by dropping these terms from the model and estimating an AE model. Next, we examined the effect of dropping the additive genetic parameter (A) on model fit by dropping this term and estimating an E model. Model comparisons were done by the log-likelihood ratio difference test. Full information maximum likelihood was used as FIML estimation provides optimal error rates compared with alternative estimators when missing data is present (Enders and Bandalos 2001). Parameters were kept in the model if dropping the parameter from the model resulted in significantly worse fit (p <0.05) when compared to the full model.
After determining if an ACSE, ACE, AE, or E model best explained the data, we examined the effect of genetic and environmental innovations. First, an omnibus test of the genetic and environmental innovations was conducted in which all genetic and environmental innovation terms except for age 50–54 were dropped from the model. Next, we followed the model comparison steps implemented in past research utilizing simplex modeling to examine anxiety symptoms across the lifespan (Gillespie et al. 2004). Replicating some steps from Gillespie (2004), genetic and environmental innovations specific to each age group were examined by systematically dropping each genetic and environmental innovation term one by one for each time point starting at the age 55–59 grouping. Again, the log-likelihood ratio test was used to compare the omnibus and pairwise sub-models to the best fitting model from the prior step. Innovations were kept in the model if dropping the parameter resulted in significantly worse model fit. Last, to test for amplification or degradation of contributions, we examined if transmission factors were significantly different than 1.0. We did this by first conducting an omnibus test by constraining all transmission betas to equal 1.0. The omnibus test was followed by separate comparisons constraining each transmission beta to equal 1.0 in a stepwise fashion starting with the β50 transmission term. The log-likelihood ratio test was used to compare the constrained model to the best fitting model from the prior step. The goal was to develop the most parsimonious model that best explains the data. Models were fit for each anxiety measure separately using the program OpenMx version 2.2 (Boker et al. 2011). Sensitivity analyses were also conducted to examine the extent differential dropout and missing data impacted our results. We re-ran the STPI and APQ simplex models with the subsample of participants completing three or more assessments. These sensitivity analyses will have even less power, therefore we examined both pattern of estimates and statistical tests.
Results
Sample characteristics
The average age of participants at the Q1 assessment was 60.1 (SD = 14.0) years old. The majority of the sample was female (57.9%, N = 1111) and the average STPI score at Q1 was 19.0 (SD = 7.9) while the average APQ score was 19.4 (SD = 9.9). The STPI and APQ means were not significantly different for MZ and DZ twins. Table II presents the means and twin correlations for the STPI and APQ by age group and zygosity. In Table II participants may be present in multiple age groups. On average women had higher STPI scores (Women M = 18.8 vs. Men M = 17.6; p <0.01) greater overall raw variance on the STPI (Variance women = 0.72 vs. Variance men = 0.55) with STPI being more heritable in women (31.2%) compared to men (22.7%). When comparing participants who completed more than two assessments to participants with one or two assessments, those participants completing more assessments were significantly younger (60.8 years old; SD = 7.8 vs. 67.4 years old; SD = 10.1) and had lower scores on the STPI (M = 18.5; SD = 7.5, vs. M = 19.6; SD = 8.7).
Table II.
Twin correlations and means for STPI and APQ by age group and zygosity.
| Age | MZ r |
DZ r |
MZ Mean (SD) |
DZ Mean (SD) |
t | p |
|---|---|---|---|---|---|---|
| Variable: STPI | ||||||
| 50 | 0.39 0.13–0.60 |
−0.05 −0.25–0.15 |
19.31 (8.22) |
17.96 (7.81) |
1.58 | 0.12 |
| 55 | 0.43 0.21–0.60 |
0.22 0.03–0.39 |
17.66 (7.08) |
17.82 (7.68) |
−0.22 | 0.83 |
| 60 | 0.33 0.11–0.51 |
0.22 0.05–0.38 |
18.73 (7.58) |
17.78 (6.92) |
1.50 | 0.14 |
| 65 | 0.32 0.11–0.50 |
0.23 0.08–0.37 |
18.68 (8.23) |
18.35 (7.43) |
0.53 | 0.60 |
| 70 | 0.31 0.07–0.50 |
0.22 0.05–0.38 |
19.08 (7.82) |
18.89 (7.74) |
−0.29 | 0.77 |
| 75 | 0.58 0.32–0.75 |
0.41 0.21–0.57 |
19.55 (7.68) |
19.43 (8.37) |
0.15 | 0.88 |
| 80 | 0.77 0.44–0.91 |
0.50 0.15–0.73 |
20.11 (7.37) |
18.89 (7.17) |
1.36 | 0.17 |
| Variable: APQ | ||||||
| 50 | 0.40 0.16–0.59 |
0.26 0.08–0.41 |
19.05 (9.69) |
17.52 (8.22) |
1.79 | 0.08 |
| 55 | 0.30 0.10–0.47 |
0.34 0.19–0.48 |
19.56 (9.35) |
18.14 (9.30) |
1.78 | 0.08 |
| 60 | 0.43 0.26–0.58 |
0.29 0.15–0.42 |
18.10 (9.69) |
17.89 (8.79) |
0.30 | 0.77 |
| 65 | 0.22 0.03–0.39 |
0.28 0.15–0.39 |
18.36 (10.31) |
17.62 (8.58) |
1.14 | 0.26 |
| 70 | 0.34 0.16–0.50 |
0.34 0.20–0.46 |
18.53 (9.74) |
18.02 (9.06) |
0.77 | 0.44 |
| 75 | 0.61 0.43–0.74 |
0.45 0.29–0.58 |
18.44 (8.92) |
18.56 (9.37) |
−0.16 | 0.87 |
| 80 | 0.68 0.46–0.82 |
0.39 0.16–0.59 |
18.85 (9.00) |
19.20 (9.43) |
−0.37 | 0.72 |
Note: Single participants may be in multiple age groups
STPI simplex models
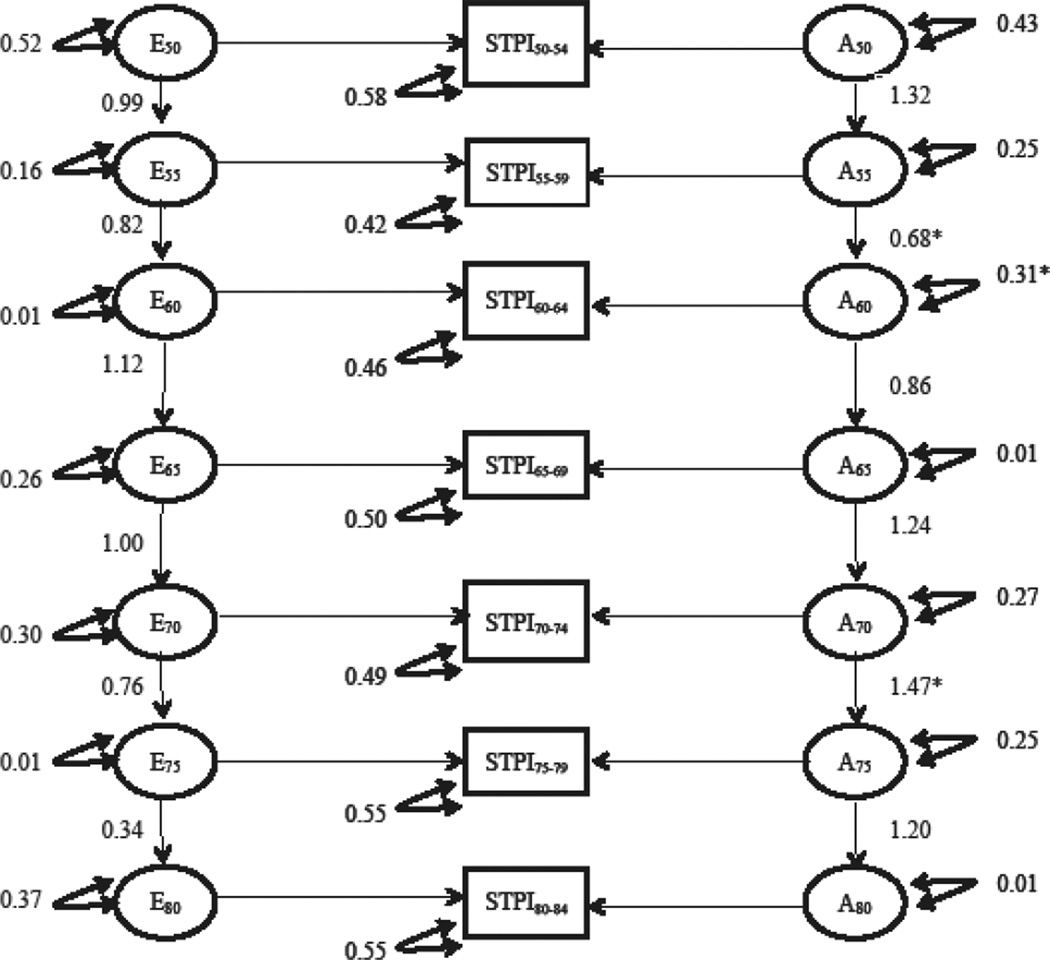
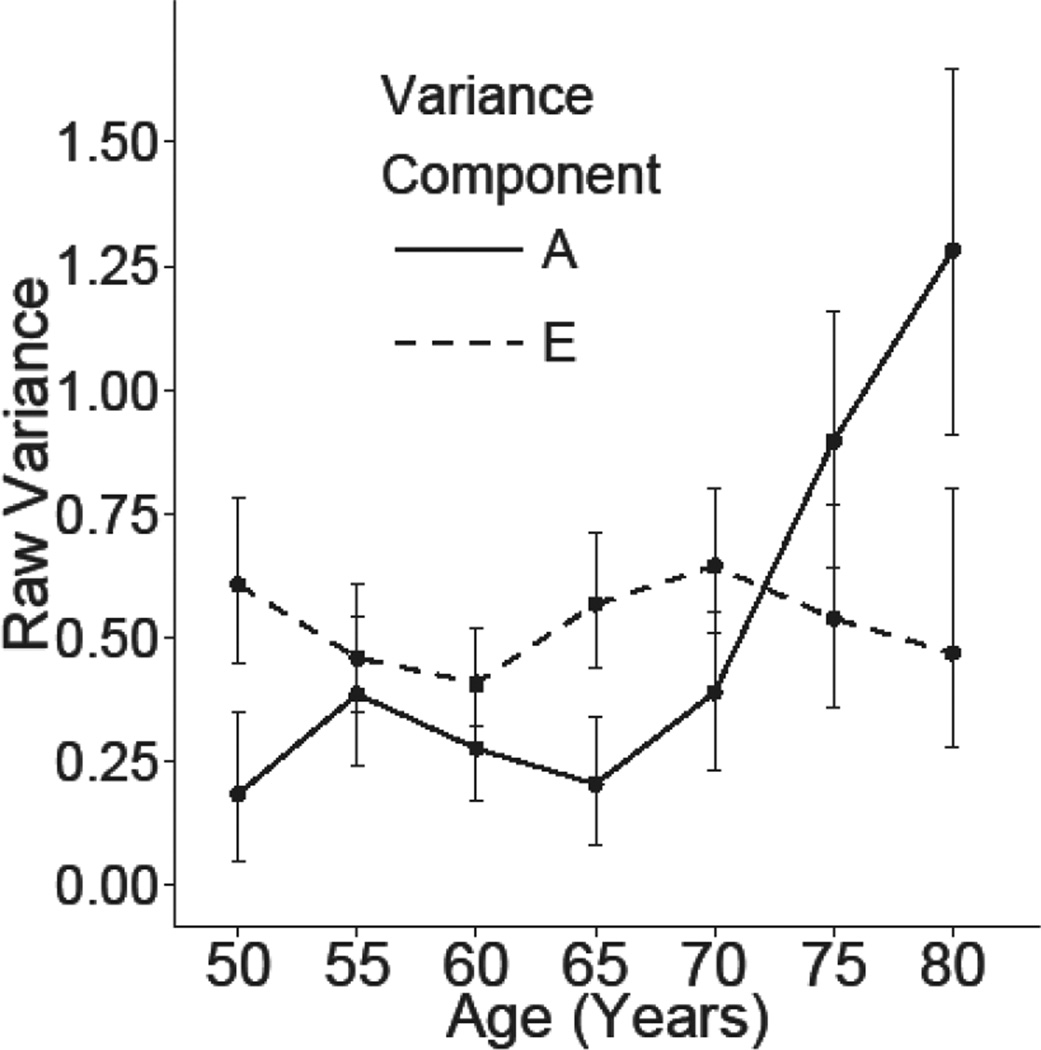
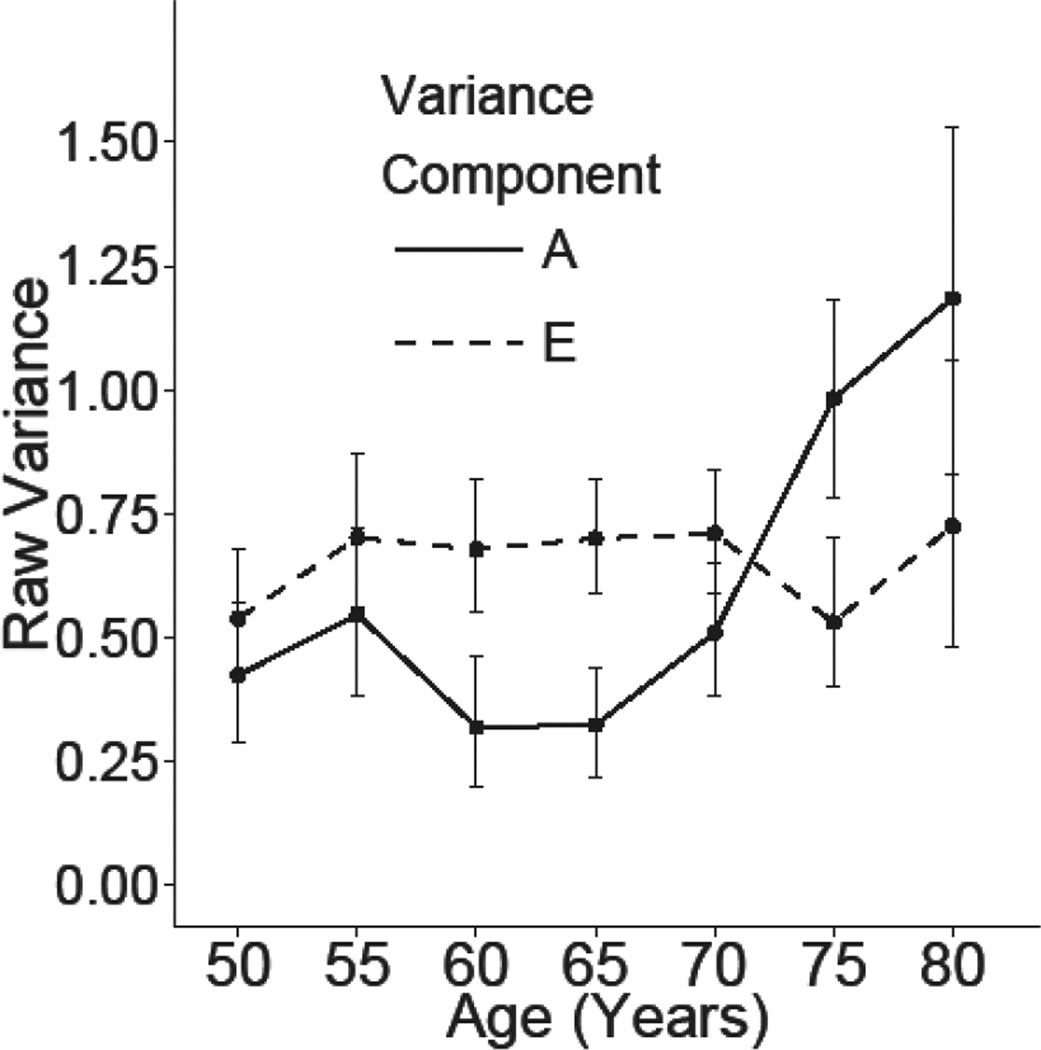
Table III presents a summary of the model fitting results for the longitudinal simplex model of the STPI. Six separate full models were fitted: an ACSE model, an ADSE, an ACE, an ADE, an ASE model, an AE model, and an E model. Model comparisons suggested that we were able to drop the both rearing and shared environment variance components (Δχ2 (26) = 31.13, p = 0.22), as well as the dominant genetic effects Δχ2 (13) = 9.48, p =0.74. Dropping the additive genetic component resulted in significantly worse model fit (Δχ2 (13) = 104.38, p = <0.01). Figure 2 presents the parameter estimates from the full AE model for the STPI and figure 3 presents a graph of the raw variance components accounted for by genetic and environmental factors estimated from the full AE model at each age group. As described previously, the estimates of raw variance are a function of the innovations at each age bucket and the autoregressive effect from the prior age bucket. The estimated additive genetic variance is relatively stable from ages 50–54 until ages 70–74 at which the estimates increase over the following 10 years. The estimated heritability of the STPI at each age bucket were as follows: 23% ages 50–54, 46% ages 55–59, 40% ages 60–64, 26% ages 65–69, 38% ages 70–74, 62% ages 75–79, and 73% age 80–84.
Table III.
Model fit statistics of STPI simplex models.
| Model | −2LL | df | AIC | Compare | Δ −2LL |
Δ df | p |
|---|---|---|---|---|---|---|---|
| 1.ACSE | 8546.57 | 3431 | 1685 | - | - | - | - |
| 2.ADSE | 8545.13 | 3431 | 1683 | - | - | - | - |
| 3.ACE | 8568.50 | 3444 | 1681 | 1 | 21.93 | 13 | 0.06 |
| 4.ASE | 8554.61 | 3444 | 1667 | 1 | 8.04 | 13 | 0.84 |
| 5.AE | 8577.70 | 3457 | 1664 | 1 | 31.13 | 26 | 0.22 |
| 6.E | 8682.08 | 3470 | 1742 | 4 | 104.38 | 13 | <0.01 |
| AE simplex sub-models | |||||||
| Tests of innovation parameters | |||||||
| Drop Ai55-Ai80 | 8585.63 | 3463 | 1660 | 5 | 7.94 | 6 | 0.24 |
| Drop Ai55 | 8577.95 | 3458 | 1662 | 5 | 0.26 | 1 | 0.61 |
| Drop Ai60 | 8583.60 | 3458 | 1668 | 5 | 5.90 | 1 | 0.02 |
| Drop Ai65 | 8577.89 | 3458 | 1662 | 5 | 0.20 | 1 | 0.66 |
| Drop Ai70 | 8578.69 | 3458 | 1663 | 5 | 0.99 | 1 | 0.32 |
| Drop Ai75 | 8577.94 | 3458 | 1662 | 5 | 0.25 | 1 | 0.62 |
| Drop Ai80 | 8577.70 | 3458 | 1662 | 5 | <0.01 | 1 | 0.99 |
| Drop Ei55-Ei80 | 8580.24 | 3463 | 1654 | 5 | 2.54 | 6 | 0.86 |
| Drop Ei55 | 8577.70 | 3458 | 1662 | 5 | <0.01 | 1 | 0.99 |
| Drop Ei60 | 8578.03 | 3458 | 1662 | 5 | 0.34 | 1 | 0.56 |
| Drop Ei65 | 8578.71 | 3458 | 1663 | 5 | 1.01 | 1 | 0.32 |
| Drop Ei70 | 8578.17 | 3458 | 1662 | 5 | 0.47 | 1 | 0.49 |
| Drop Ei75 | 8577.70 | 3458 | 1662 | 5 | <0.01 | 1 | 0.99 |
| Drop Ei80 | 8578.56 | 3458 | 1663 | 5 | 0.87 | 1 | 0.35 |
| Tests of transmission parameters | |||||||
| Set all At = 1 | 8597.53 | 3463 | 1672 | 5 | 19.83 | 6 | <0.01 |
| Set At50–55 | 8578.59 | 3458 | 1663 | 5 | 0.89 | 1 | 0.35 |
| Set At55–60 | 8582.46 | 3458 | 1666 | 5 | 4.76 | 1 | 0.03 |
| Set At60–65 | 8578.47 | 3458 | 1662 | 5 | 0.77 | 1 | 0.38 |
| Set At65–70 | 8578.81 | 3458 | 1663 | 5 | 1.11 | 1 | 0.29 |
| Set At70–75 | 8585.13 | 3458 | 1669 | 5 | 7.43 | 1 | 0.01 |
| Set At75–80 | 8579.51 | 3458 | 1664 | 5 | 1.81 | 1 | 0.18 |
| Set all Et = 1 | 8587.11 | 3463 | 1661 | 5 | 9.42 | 6 | 0.15 |
| Set Et50–55 | 8577.70 | 3458 | 1662 | 5 | 0.01 | 1 | 0.99 |
| Set Et55–60 | 8579.03 | 3458 | 1663 | 5 | 1.33 | 1 | 0.25 |
| Set Et60–65 | 8577.97 | 3458 | 1662 | 5 | 0.27 | 1 | 0.60 |
| Set Et65–70 | 8577.70 | 3458 | 1662 | 5 | 0.01 | 1 | 0.99 |
| Set Et70–75 | 8579.48 | 3458 | 1663 | 5 | 1.78 | 1 | 0.18 |
| Set Et75–80 | 8581.40 | 3458 | 1665 | 5 | 3.70 | 1 | 0.05 |
Note: −2LL = negative log-likelihood; df = degrees of freedom; AIC = Akaike information criterion; Compare = the comparison model for the log-likelihood ratio test; Set transmission factors means constraining beta to equal 1.0. The Ai and Ei entries represent the tests of the genetic and environmental innovations. The At and the Et entries represent the tests of the transmission parameters.
Figure 2.
Depiction of the parameter estimates from full AE STPI simplex model. A = additive genetic effects; E = unique environmental effects; STPI= STPI for each age bucket; single headed arrows=path coefficients; double headed arrow = variance components; * represents a significant decrease in model fit when parameter is dropped/constrained to 1.0
Figure 3.
Graph of the estimated unstandardized raw variance components with 95% confidence intervals in STPI by additive genetic (A) and unique non-shared environments (E) factors. Estimates are from the full AE model estimating all genetic and environmental innovations without constraint on the autoregressive transmission factors.
The omnibus tests of the genetic (Δχ2 (6) = 7.94, p = 0.24) and unique environmental innovations (Δχ2 (6) = 2.54, p = 0.86) suggested that, with the exception of age 50–54, all genetic and unique environmental innovations could be dropped without worse model fit. Power to detect modest innovations was likely not high, thus we ran exploratory analyses examining specific genetic innovations. Beginning with ages 55–59, genetic innovations were dropped one by one in a stepwise fashion. Compared to the full AE model there was a significant deterioration in model fit when the genetic innovation at age 60–64 was dropped (Δχ2 (1) = 5.90, p = 0.02). Next, unique environmental innovations were dropped one by one in a stepwise fashion. There were no significant decreases in model fit when all unique environment innovations from ages 55–80 were removed from the model.
The additive genetic and unique environmental autoregressive transmittal factors were examined next by conducting omnibus tests of genetic and unique environmental transmission factors by constraining all transmittal betas to equal 1.0. The omnibus test for the genetic transmissions (Δχ2 (6) = 19.83, p < 0.01) was statistically significant while the test of the unique environmental (Δχ2 (6) = 9.42, p = 0.15) transmissions were not. Transmission factors were next examined in a stepwise fashion. The genetic transmission factor from age 55 to age 60 was significantly less than 1.0 (Δχ2 (1) = 4.76, p = 0.03) while the genetic transmission beta from age 70 to age 75 was significantly greater than 1.0 (Δχ2 (1) = 7.43, p = 0.01). The remaining genetic transmission betas could be constrained to 1.0 without worse model fit. The unique environmental transmission beta from age 75 to 80 was marginally less than 1.0 (Δχ2 (1) = 3.70, p = 0.054), suggesting a trend towards degradation of prior unique environmental influences at ages 80. All other unique environmental transmission betas constrained to 1.0 without worse model fit.
Sensitivity analyses of the STPI including only participants completing 3 or more assessments were run. The omnibus test of the genetic transmissions (Δχ2 (6) = 12.91, p = 0.04) remained significant. Estimates of the genetic and environmental variance at each age bucket were similar to the full model containing all participants.
APQ simplex models
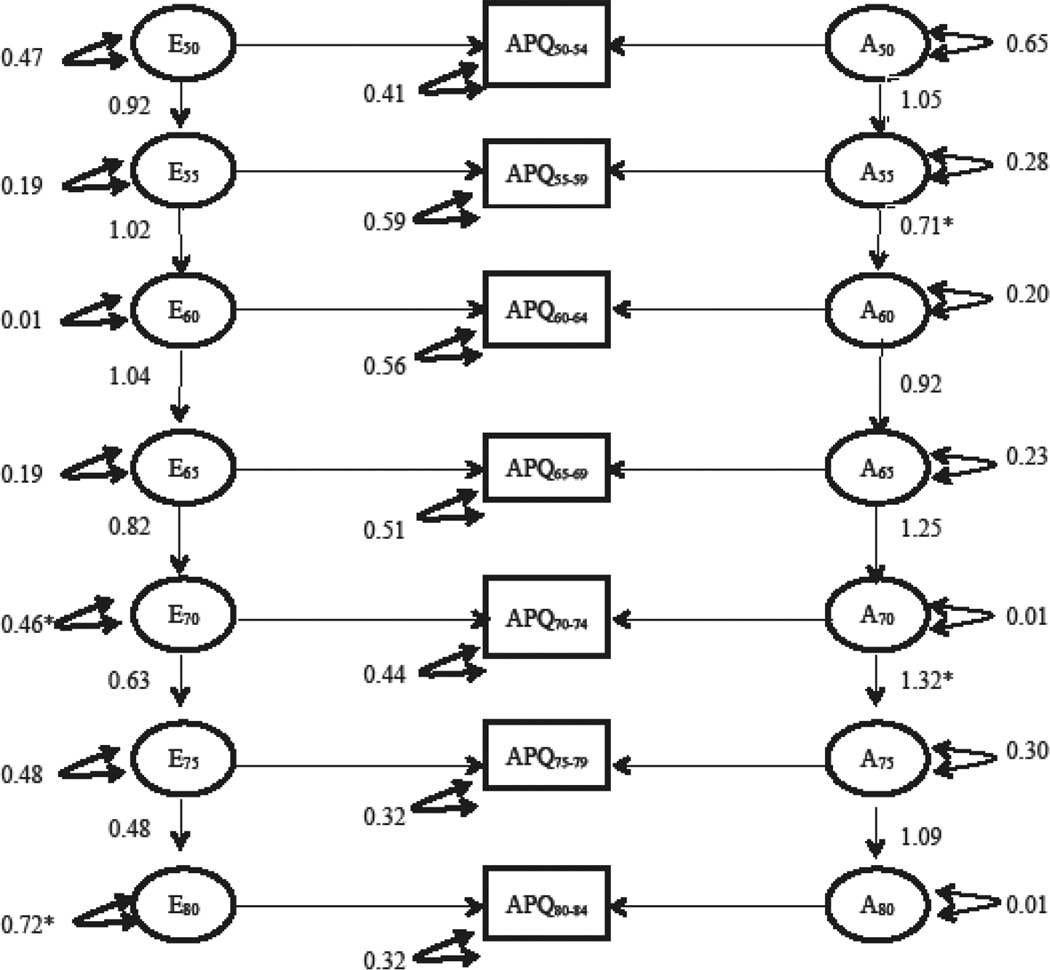
Table IV presents a summary of the model fitting results for the longitudinal simplex model of the APQ. Model comparisons suggested that dropping both the shared rearing and shared environment variance components (Δχ2 (26)= 29.76, p = 0.28) and the dominant genetic effects (Δχ2 (13) = 8.03, p = 0.84) did not result in worse fit, while dropping the additive genetic component resulted in significantly worse model fit (Δχ2 (13) = 146.84, p <0.01). Figure 4 presents the parameter estimates from the full AE model for the APQ and Figure 5 presents the estimated additive genetic and unique environmental variance by age. The estimated heritability of APQ at each age bucket were as follows: 44% ages 50–54, 44% ages 55–59, 32% ages 60–64 and 65–69, 42% ages 70–74, 65% ages 75–79, and 62% ages 80–84.
Table IV.
Model fit statistics of APQ simplex models.
| Model | −2LL | df | AIC | Compare | Δ −2LL |
Δ df | p |
|---|---|---|---|---|---|---|---|
| 1.ACSE | 12244.73 | 4694 | 2857 | - | - | - | - |
| 2.ADSE | 12254.56 | 4694 | 2867 | - | - | - | - |
| 3.ACE | 12254.27 | 4707 | 2840 | 1 | 9.53 | 13 | 0.73 |
| 4.ASE | 12262.59 | 4707 | 2849 | 1 | 17.85 | 13 | 0.16 |
| 5.AE | 12274.49 | 4720 | 2834 | 1 | 29.76 | 26 | 0.28 |
| 6.E | 12421.33 | 4733 | 2955 | 5 | 146.84 | 13 | <0.01 |
| AE simplex sub-models | |||||||
| Tests of innovation parameters | |||||||
| Drop Ai55-Ai80 | 12288.07 | 4726 | 2836 | 5 | 13.58 | 6 | 0.03 |
| Drop Ai55 | 12275.88 | 4721 | 2833 | 5 | 1.39 | 1 | 0.24 |
| Drop Ai60 | 12276.99 | 4721 | 2835 | 5 | 2.50 | 1 | 0.11 |
| Drop Ai65 | 12275.72 | 4721 | 2833 | 5 | 1.23 | 1 | 0.27 |
| Drop Ai70 | 12274.49 | 4721 | 2832 | 5 | <0.01 | 1 | 0.99 |
| Drop Ai75 | 12275.30 | 4721 | 2833 | 5 | 0.81 | 1 | 0.37 |
| Drop Ai80 | 12274.49 | 4721 | 2832 | 5 | <0.01 | 1 | 0.99 |
| Drop Ei55-Ei80 | 12293.72 | 4726 | 2842 | 5 | 19.24 | 6 | <0.01 |
| Drop Ei55 | 12274.49 | 4721 | 2832 | 5 | <0.01 | 1 | 0.99 |
| Drop Ei60 | 12274.78 | 4721 | 2833 | 5 | 0.30 | 1 | 0.59 |
| Drop Ei65 | 12274.85 | 4721 | 2833 | 5 | 0.36 | 1 | 0.55 |
| Drop Ei70 | 12280.87 | 4721 | 2839 | 5 | 6.38 | 1 | 0.01 |
| Drop Ei75 | 12276.91 | 4721 | 2835 | 5 | 2.42 | 1 | 0.12 |
| Drop Ei80 | 12285.75 | 4721 | 2844 | 5 | 11.27 | 1 | <0.01 |
| Tests of transmission parameter | |||||||
| Set all At = 1 | 12302.18 | 4726 | 2850 | 5 | 27.69 | 6 | <0.01 |
| Set At50–55 | 12274.66 | 4721 | 2832 | 5 | 0.18 | 1 | 0.67 |
| Set At55–60 | 12282.58 | 4721 | 2832 | 5 | 8.09 | 1 | <0.01 |
| Set At60–65 | 12274.77 | 4721 | 2833 | 5 | 0.28 | 1 | 0.59 |
| Set At65–70 | 12277.92 | 4721 | 2836 | 5 | 3.44 | 1 | 0.06 |
| Set At70–75 | 12283.14 | 4721 | 2841 | 5 | 8.66 | 1 | <0.01 |
| Set At75–80 | 12275.67 | 4721 | 2834 | 5 | 1.18 | 1 | 0.28 |
| Set all Et = 1 | 12293.69 | 4726 | 2842 | 5 | 19.21 | 6 | <0.01 |
| Set Et50–55 | 12274.49 | 4721 | 2833 | 5 | <0.01 | 1 | 0.99 |
| Set Et55–60 | 12274.52 | 4721 | 2833 | 5 | 0.03 | 1 | 0.87 |
| Set Et60–65 | 12274.55 | 4721 | 2833 | 5 | 0.06 | 1 | 0.80 |
| Set Et6570 | 12276.17 | 4721 | 2834 | 5 | 1.68 | 1 | 0.19 |
| Set Et7075 | 12281.32 | 4721 | 2839 | 5 | 6.83 | 1 | 0.01 |
| Set Et75–80 | 12278.28 | 4721 | 2836 | 5 | 3.79 | 1 | 0.06 |
Note: −2LL = negative log-likelihood; df = degrees of freedom; AIC = Akaike information criterion; Compare = the comparison model for the log-likelihood ratio test; Set transmission factors means constraining beta to equal 1.0. The Ai and Ei entries represent the tests of the genetic and environmental innovations. The At and the Et entries represent the tests of the transmission parameters.
Figure 4.
Depiction of parameter estimates from the full AE APQ simplex model A = additive genetic effects; E = unique environmental effects; APQ= APQ for each age bucket; single headed arrows=path coefficients; double headed arrow = variance components; * represents a significant decrease in model fit when parameter is dropped/constrained to 1.0
Figure 5.
Graph of the estimated unstandardized raw variance components with 95% confidence intervals in APQ by additive genetic (A) and unique non-shared environments (E) factors. Estimates are from the full AE model estimating all genetic and environmental innovations without constraint on the autoregressive transmission factors.
The omnibus tests of the genetic (Δχ2 (6) = 13.58, p = 0.03) and unique environmental (Δχ2 (6) = 19.24, p < 0.01) innovations were significant, suggesting these could not be dropped from the model. See subheadings for tests of innovations presented in table IV. When examining in a stepwise fashion however, no specific genetic innovation was statistically significant. Compared to the full AE model there was a significant deterioration in model fit when the unique environmental innovation at ages 70–74 (Δχ2 (1) = 6.38, p = 0.01) and 80–84 were dropped (Δχ2 (1) = 11.27, p < 0.01).
The additive genetic and unique environmental autoregressive transmittal factors were examined next by conducting omnibus tests of genetic and unique environmental transmission factors by constraining all transmittal betas to equal 1.0. The tests of transmittal factors are presented in the subheadings listed in table IV. The omnibus test for the genetic (Δχ2 (6) = 27.69, p < 0.01) and unique environmental (Δχ2 (6) = 19.21, p < 0.01) transmissions were statistically significant meaning all betas could not be constrained to 1.0. Transmission factors were next examined in a stepwise fashion. The genetic transmission factor from age 55 to age 60 was significantly less than 1.0 (Δχ2 (1) = 8.09, p < 0.01). The genetic transmission beta from age 70 to age 75 was significantly greater than 1.0 (Δχ2 (1) = 8.66, p = 0.01). The remaining genetic transmission betas were constrained to 1.0 without worse model fit. The unique environmental transmission beta from age 70 to 75 was less than 1.0 (Δχ2 (1)= 6.83, p = 0.01), suggesting a degradation of prior unique environmental influences at ages 75 and the beta from age 75–80 was marginally less than 1.0 (Δχ2 (1) = 3.79, p = 0.052). All other unique environmental transmission betas could be constrained to 1.0 without worse model fit.
Sensitivity analyses of the APQ including only participants completing 3 or more assessments were run. The omnibus test of the genetic innovations (Δχ2 (6) = 14.6, p = 0.02) and transmissions (Δχ2 (6) = 30.75, p < 0.01) remained significant as did the omnibus test of the environmental innovations (Δχ2 (6) = 27.32, p < 0.01) and transmissions (Δχ2 (6) = 17.01, p = 0.01). Estimates of the genetic and environmental variance at each age bucket were similar to the full model containing all participants.
Discussion
The aim of this study was to examine the change and stability of genetic and environmental influences on anxiety symptoms across older adulthood. Consistent with our hypothesis, we did find evidence for new genetic determinants of anxiety over age with new genetic influences arising between the ages of 60–64 for our main anxiety measure, the STPI. These genetic factors continued to contribute to anxiety symptoms up to age 85. No new genetic influences were found on our secondary measure of anxiety. On both measures, additive genetic transmission factors from ages 70–75 were greater than 1.0, suggesting the amplification of prior genetic influences in participants in their 70’s and into their 80’s. On both measures there were genetic transmissions significantly less than 1.0 from ages 55 to 60. Evidence for new unique environmental determinants of anxiety arising in participants 70’s also was found for our secondary measure but not for the STPI. These innovations were combined with a degradation of unique environmental factors at subsequent ages on both measures, either age 70–75 or age 75–80 depending on the measure. This suggests that the unique environmental innovations were relatively transient and primarily important during this age and not correlated with subsequent unique environmental contributions. Our two measures of anxiety showed remarkably similar patterns across age. Although the statistical significance of particular parameters did not always follow suit across measures, they were consistent in magnitude and direction of effect. No significant shared or correlated environmental factors were found, which is also consistent with a large body of literature (Kendler et al. 2011).
Genetic innovations at age 50–54 (and earlier in life) were the greatest contributors to genetic variance across older adulthood. This is consistent with past research (Gillespie et al., 2004). We also found evidence for new genetic contributions for anxiety in older adulthood, in the age range not well covered in the Gillespie et al. study. Perhaps also leading to disparate findings, five-year age buckets were used in the current work, while ten-year age buckets were utilized in the Gillespie et al. study. The ages at which new genetic contributions were found (age 60–64) are consistent with when late-onset anxiety may occur (Zhang et al. 2015). This finding is of particular importance due to the paucity of studies examining genetic contributions to late-life anxiety. Late-onset anxiety occurs in the context of chronic health conditions, cognitive decline, or stressful life events (Zhang et al. 2015). The new genetic contributions may reflect genes that are shared with these other phenotypes that are commonly comorbid with late life anxiety. A growing body of literature is documenting the association between anxiety and poorer cognitive performance, particularly in processing speed and executive functioning, in older adulthood (Beaudreau and O’Hara 2008). Although varying by domain, cognitive functioning typically starts to decline at a greater rate starting around the age of 60 (Salthouse 2010). It is possible that genes contributing to these physiological changes and cognitive decline may partially explain this genetic innovation in anxiety seen at age 60 as research has hypothesized genetic overlap of the phenotypes of anxiety, depression, and cognitive performance (Rodrigues et al. 2014). These explanations are speculative and future research needs to examine this further and the need for future research to investigate genetic contributions to late life anxiety is great.
In addition to increased heritability of anxiety symptoms at older ages, on our secondary measure, we found evidence for new unique environmental innovations on anxiety symptoms at age 70–74 and 80–84. The innovations may reflect some of the stressful life events that are associated with the aging process that commonly increase in frequency at these ages. Providing care for a significant other who is chronically ill or cognitively impaired may be one of these environmental events associated with anxiety at this age. Additionally, the likelihood of experiencing significant bereavement from death of a spouse, family, or close friend increases. These stressors may explain the significant unique environmental contributions to anxiety symptoms that were found starting at these ages.
Results were largely consistent for both the more extensively studied measure of anxiety, the STPI, as well as our harmonized anxiety measure, the APQ. The primary purpose of including the APQ in these analyses was to fill in the 17 year gap in administration of the STPI, as the APQ was administered at all assessment times. Although tests of significance were not consistent both measures provided similar estimates of the genetic and environmental contributions to individual differences. Both measures gave similar results for genetic and environmental transmissions. Additionally, for both measures heritability estimates and additive genetic variance increased after age 70. The similarity between these measures provides support for using the APQ as a measure of anxiety in future work. Additionally, the consistency of results between both measures provides support that the 17-year gap in the STPI did not significantly impact results.
In past research with SATSA, Pedersen and Reynolds (1998) reported on heritability of the 9-item neuroticism scale across four times of measurement, reporting a decrease from 29% heritability at the first occasion (Q1) to 7% at the last time point (Q4). These results cannot be compared directly to the current paper, as the authors used the full neuroticism scale rather than the subset of anxiety items, and they analyzed change over time rather than age.
Our study has a number of limitations. First, the limited sample size prevented examination of some important factors. Namely, the sample size prevented the examination of sex differences and prevented examination of anxiety in participants over the age of 85. Relatively small samples also may have limited our ability to detect significant innovations contributing to the differences in statistical testing between the two measures of anxiety. Schmitz, Cherney, & Fulker (1998) conducted a power analysis for a 4-wave univariate simplex. They found that with 100 MZ and DZ complete pair one can reliably detect significant variance with heritability of 0.40 and transmission parameters’ greater than 0.50. For some age buckets of the STPI analyses we are under 200 complete pairs, however for the APQ we generally exceed this number for most age buckets. Additionally, we have more than four waves of the APQ and transmission estimates are generally at or above 0.50 resulting in greater statistical power. Another limitation was measurement of anxiety. Although the APQ performed similarly to the more psychometrically established STPI, the APQ is a measure of anxiety derived from a measure of neuroticism. Anxiety measures specifically designed for use with older adults should be considered in future research. Although sensitivity analyses suggested that differential attrition did not greatly impact the results, it should be noted that participants completing more assessments had lower anxiety and were younger on average than participants completing two or more assessments. Additionally, there are limitations inherent to longitudinal simplex models. Simplex models do not enable the examination of specific trajectories over time. Additionally, Ormel and Rijsdijk (2000) highlight that genetic or environmental innovations are significant events that not only impact variance at specific time points, but all subsequent time periods. The extent to which such significant genetic or environmental contributions occur uniformly at a specific age and have enough effect to impact variance at subsequent ages is potentially rare and unlikely.
In sum, this study examined change and stability of genetic and environmental contributions to anxiety in later life. We found that genetic and environmental influences are mostly stable throughout later life. For our main anxiety measure we found evidence for new genetic factors impacting the etiology of anxiety symptoms starting at the ages of 60–64 with amplification in participants in their 70’s. New environmental factors were found in participants in their 70’s and 80’s; however, these appeared more transient. It is unclear what these new genetic influences are, although correlates of anxiety such as cognitive impairment that commonly co-occur with anxiety in later life might be contributing factors. Future studies will be able to focus on identifying the specific genetic and environmental innovations associated with anxiety in late life.
Acknowledgements
SATSA was supported by grants R01 AG04563, R01 AG10175, the MacArthur Foundation Research Network on Successful Aging, the Swedish Council For Working Life and Social Research (FAS) (97:0147:1B, 2009-0795) and Swedish Research Council (825-2007-7460, 825-2009-6141).
Dr. Petkus was partially supported by a Ruth L. Kirschstein National Research Service Award (NRSA) fellowship awarded by the National Institute on Aging (1F31AG042218-01).
Footnotes
Human Rights and Informed Consent
Participants provided written informed consent to participate in the study. This study was approved by the Institutional Review Board at the Karolinksa Institutet and the University of Southern California. All participants were treated in accordance with the Declaration of Helsinki.
References
- Beaudreau S, MacKay-Brandt A, Reynolds J. Application of a cognitive neuroscience perspective of cognitive control to late-life anxiety. Journal of Anxiety Disorders. 2013;27:559–566. doi: 10.1016/j.janxdis.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudreau SA, O’Hara R. Late-life anxiety and cognitive impairment: a review. American Journal of Geriatric Psychiatry. 2008;16:790–803. doi: 10.1097/JGP.0b013e31817945c3. [DOI] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes HH, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J. OpenMx: An open source extended structural equation modeling framework. Psychometricka. 2011 doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond TG, Fox CM. Applying the Rasch model: Fundamental measurement in the human sciences. New Jersey: Lawrence Erlbaum Associates, Mahwah; 2007. [Google Scholar]
- Boomsma DI, Martin NG, Molenaar PC. Factor and simplex models for repeated measures: Application to two psychomotor measures of alcohol sensitivity in twins. Behavior Genetics. 1989;19:79–96. doi: 10.1007/BF01065885. [DOI] [PubMed] [Google Scholar]
- Braam AW, Copeland JR, Delespaul PA, Beekman AT, Como A, Dewey M, Fichter M, Holwerda TJ, Lawlor BA, Lobo A, Magnusson H, Prince MJ, Reischies F, Wilson KC, Skoog I. Depression, subthreshold depression and comorbid anxiety symptoms in older Europeans: Results from the EURODEP concerted action. Journal of Affective Disorders. 2014;155:266–272. doi: 10.1016/j.jad.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Brenes GA, Penninx BW, Judd PH, Rockwell E, Sewell DD, Wetherell JL. Anxiety, depression and disability across the lifespan. Aging & mental health. 2008;12:158–163. doi: 10.1080/13607860601124115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Kelly-Hayes M, Wolf PA, Reed T, Miller B. Longitudinal changes in the contribution of genetic and environmental influences to symptoms of depression in older male twins. Psychology and Aging. 2000;15:505–510. doi: 10.1037//0882-7974.15.3.505. [DOI] [PubMed] [Google Scholar]
- Cederlof R, Lorich U. The Swedish twin registry. Progress in Clinical and Biological Research. 1978;24 Pt B:189–195. [PubMed] [Google Scholar]
- Chou KL. Age at onset of generalized anxiety disorder in older adults. The American Journal of Geriatic Psychiatry. 2009;17:455–464. doi: 10.1097/jgp.0b013e31818f3a93. [DOI] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8:430–457. [PubMed] [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual for the Eysenck Personality Inventory. San Diego, CA: Educational and Industrial Testing Service; 1968. [Google Scholar]
- Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging, Neuropsychology, and Cognition. 2004;11:325–345. [Google Scholar]
- Gatz M, Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. Importance of shared genes and shared environments for symptoms of depression in older adults. Journal of Abnormal Psychology. 1992;101:701–708. doi: 10.1037//0021-843x.101.4.701. [DOI] [PubMed] [Google Scholar]
- Gibbons L, Teri L, Logsdon R, McCurry S, Kukull W, Bowen J, McCormick W, Larson E. Anxiety symptoms as predictors of nursing home placement in patients with Alzheimer’s disease. Journal of Clinical Geropsychology. 2002;8:335–342. [Google Scholar]
- Gillespie NA, Kirk KM, Evans DM, Heath AC, Hickie IB, Martin NG. Do the genetic or environmental determinants of anxiety and depression change with age? A longitudinal study of Australian twins. Twin Research and Human Genetics. 2004;7:39–53. doi: 10.1375/13690520460741435. [DOI] [PubMed] [Google Scholar]
- Goncalves DC, Byrne GJ. Sooner or later: Age at onset of generalized anxiety disorder in older adults. Depression and Anxiety. 2012;29:39–46. doi: 10.1002/da.20881. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Archives of General Psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- Johnson W, McGue M, Gaist D, Vaupel JW, Christensen K. Frequency and heritability of depression symptomatology in the second half of life: evidence from Danish twins over 45. Psychological Medicine. 2002;32:1175–1185. doi: 10.1017/s0033291702006207. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Roysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. American Journal of Psychiatry. 2011;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Heath A, Martin NG, Eaves LJ. Symptoms of anxiety and depression in a volunteer twin population. The etiologic role of genetic and environmental factors. Archives of General Psychiatry. 1986;43:213–221. doi: 10.1001/archpsyc.1986.01800030023002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Martin NG, Eaves LJ. Symptoms of anxiety and symptoms of depression. Same genes, different environments? Archives of General Psychiatry. 1987;44:451–457. doi: 10.1001/archpsyc.1987.01800170073010. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Le Roux H, Gatz M, Wetherell JL. Age at onset of generalized anxiety disorder in older adults. American Journal of Geriatric Psychiatry. 2005;13:23–30. doi: 10.1176/appi.ajgp.13.1.23. [DOI] [PubMed] [Google Scholar]
- Losada A, Marquez-Gonzalez M, Pachana NA, Wetherell JL, Fernandez-Fernandez V, Nogales-Gonzalez C, Ruiz-Diaz M. Behavioral correlates of anxiety in well-functioning older adults. International Psychogeriatrics / IPA:1–12. 2014 doi: 10.1017/S1041610214001148. [DOI] [PubMed] [Google Scholar]
- McGue M, Bouchard TJ. Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14:325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- McGue M, Christensen K. The heritability of depression symptoms in elderly Danish twins: Occasion-specific versus general effects. Behavior Genetics. 2003;33:83–93. doi: 10.1023/a:1022545600034. [DOI] [PubMed] [Google Scholar]
- McGue M, Christensen K. Growing old but not growing apart: twin similarity in the latter half of the lifespan. Behavior Genetics. 2013;43:1–12. doi: 10.1007/s10519-012-9559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Rijsdijk F. Continuing change in neuroticism during adulthood- Structural modeling of a 16-year 5-wave community study. Personality and Individual Differences. 2000;28:461–478. [Google Scholar]
- Pedersen NL, McClearn GE, Plomin R, Nesselroade JR, Berg S, deFaire U. The Swedish Adoption Twin Study of Aging: An update. Acta Genet Med Gemollol. 1991;40:7–20. doi: 10.1017/s0001566000006681. [DOI] [PubMed] [Google Scholar]
- Pederson NL, Reynolds CA. Stability and change in adult personality: Genetic and environmental components. European Journal of Personality. 1998;12:365–386. [Google Scholar]
- Porensky EK, Dew MA, Karp JF, Skidmore E, Rollman BL, Shear MK, Lenze EJ. The burden of late-life generalized anxiety disorder: effects on disability, health-related quality of life, and healthcare utilization. American Journal of Geriatric Psychiatry. 2009;17:473–482. doi: 10.1097/jgp.0b013e31819b87b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin O, Bergua V, Meillon C, Le Goff M, Bouisson J, Dartigues JF, Amieva H. Norms and associated factors of the STAI-Y State anxiety inventory in older adults: Results from the PAQUID study. International psychogeriatrics / IPA:1–11. 2011 doi: 10.1017/S1041610210002358. [DOI] [PubMed] [Google Scholar]
- Rodrigues R, Petersen RB, Perry G. Parallels between major depressive disorder and Alzheimer’s disease: role of oxidative stress and genetic vulnerability. Cell and Molecular Neurobiology. 2014;34:925–949. doi: 10.1007/s10571-014-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Selective review of cognitive aging. Journal of International Neuropsychology Society. 2010;16:754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz S, Cherny SS, Fulker DW. Increase in power through multivariate analyses. Behavior Genetics. 1998;28:357–363. doi: 10.1023/a:1021669602220. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Preliminary manual for the State-Trait Personality Inventory (STPI) Tampa, FL: University of South Florida; 1979. [Google Scholar]
- Van Orden KA, Simning A, Conwell Y, Skoog I, Waern M. Characteristics and comorbid symptoms of older adults reporting death ideation. American Journal of Geriatric Psychiatry. 2013;21:803–810. doi: 10.1016/j.jagp.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherell JL, Gatz M, Pedersen NL. A longitudinal analysis of anxiety and depressive symptoms. Psychology and Aging. 2001;16:187–195. doi: 10.1037//0882-7974.16.2.187. [DOI] [PubMed] [Google Scholar]
- Wolitzky-Taylor KB, Castriotta N, Lenze EJ, Stanley MA, Craske MG. Anxiety disorders in older adults: A comprehensive review. Depression and Anxiety. 2010;27:190–211. doi: 10.1002/da.20653. [DOI] [PubMed] [Google Scholar]
- Zhang X, Norton J, Carriere I, Ritchie K, Chaudieu I, Ancelin ML. Risk factors for late-onset generalized anxiety disorder: results from a 12-year prospective cohort (the ESPRIT study) Translational Psychiatry. 2015;5:e536. doi: 10.1038/tp.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]