Summary
Day length and ambient temperature are major stimuli controlling flowering time. To understand flowering mechanisms in more natural conditions, we explored the effect of daily light and temperature changes on Arabidopsis thaliana.
Seedlings were exposed to different day/night temperature and day-length treatments to assess expression changes in flowering genes.
Cooler temperature treatments increased CONSTANS (CO) transcript levels at night. Nighttime CO induction was diminished in flowering bhlh (fbh)-quadruple mutants. FLOWERING LOCUS T (FT) transcript levels were reduced at dusk, but increased at the end of cooler nights. The dusk suppression, which was alleviated in short vegetative phase (svp) mutants, occurred particularly in younger seedlings, while the increase during the night continued over 2 wk. Cooler temperature treatments altered the levels of FLOWERING LOCUS M-β (FLM-β) and FLM-δ splice variants. FT levels correlated strongly with flowering time across treatments.
Day/night temperature changes modulate photoperiodic flowering by changing FT accumulation patterns. Cooler nighttime temperatures enhance FBH-dependent induction of CO and consequently increase CO protein. When plants are young, cooler temperatures suppress FT at dusk through SVP function, perhaps to suppress precocious flowering. Our results suggest day length and diurnal temperature changes combine to modulate FT and flowering time.
Keywords: ambient temperature, Arabidopsis thaliana, CONSTANS, FLOWERING LOCUS T, flowering time, photoperiod, signal integration
Introduction
In nature, plants experience temperature fluctuations coinciding with day and night, and daily temperature fluctuations affect plant development (Myster & Moe, 1995). When exposed to day/night temperature cycles, flowering of rice and barley can be accelerated or delayed relative to constant-temperature conditions (Yin & Kropff, 1996; Karsai et al., 2008). Time-of-day information is important for a range of physiological responses as sensitivity to temperature is modulated by the circadian clock throughout the day (McClung, 2006). Further, plant responses to combined environmental stimuli cannot be easily predicted from the responses to each alone (Prasch & Sonnewald, 2015). Therefore, to study the mechanisms that operate in nature, it is useful to assess the combinational influence of day length and day-to-night temperature fluctuations on seasonal flowering.
The day-length dependent (photoperiodic) flowering pathway is well studied in Arabidopsis thaliana (Golembeski et al., 2014; Osugi & Izawa, 2014; Shrestha et al., 2014). Components of this pathway are implicated in ambient-temperature-dependent flowering (Song et al., 2013), indicating that light and temperature signals can be integrated into the same pathway. Photoperiod information is processed through regulation of the CONSTANS (CO) gene. CO peaks in the afternoon and evening in long days (LD) (Suárez-López et al., 2001). CO protein production follows the same pattern during the day; however, it is degraded during the night by CONSTITUTIVE PHOTOMORPHOGENIC 1 (COP1)/SUPPRESSOR OF PHYA-105 (SPA) E3 ubiquitin ligase complex (Laubinger et al., 2006; Jang et al., 2008; Liu et al., 2011). In LD afternoons, two types of blue-light photoreceptors CRYPTOCHROME (CRY) and FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 (FKF1) stabilize CO protein inducing FLOWERING LOCUS T (FT) at the end of the day to promote flowering (Zuo et al., 2011; Song et al., 2012, 2014; Tan et al., 2013).
Temperature affects flowering by modulating FT levels through CO-dependent and -independent mechanisms (Lee et al., 2007; Lazaro et al., 2012; Jung et al., 2012; Nomoto et al., 2013). In constant lower (16°C) temperatures in LD, FT levels are reduced and plants flower later compared with 23°C conditions (Blazquez et al., 2003; Lee et al., 2007). However, in short vegetative phase (svp) mutants, the FT level is high even in constant 16°C (Lee et al., 2007). In both 16°C and 23°C, svp mutants flower at similar times, indicating SVP represses FT and delays flowering under cool temperatures. SVP interacts with the FLOWERING LOCUS M (FLM) splice variant, FLM-β, to bind the FT promoter (Posé et al., 2013). Intermittent drops to cold (4°C) temperatures during the day may act through CO to repress flowering (Jung et al., 2012). A temperature drop from 23°C to 4°C from Zeitgeber time (ZT) 10 to ZT16 in LD leads to FT repression and a delay of flowering. The decline in FT mRNA strongly parallels a decline in CO protein abundance.
Colder temperatures within a day do not always reduce FT levels. In constant light, a 22°C/12°C temperature cycle over a 24-h period causes a strong induction of FT during the cool periods of the cycle, and temperature cycles are likely associated with early flowering (Schwartz et al., 2009). Under 12-h light and 12-h dark conditions, having warmer temperatures (28°C) at night but not in the day (22°C) causes up-regulation of FT (Thines et al., 2014). Therefore, depending on light conditions, temperature changes occurring at different times of the day differentially regulate FT expression patterns and do so through several mechanisms.
In Arabidopsis, small numbers of consecutive LD treatments are sufficient to induce early flowering, and FT is immediately upregulated after plants are moved from short days (SD) to LD, but declines to basal levels shortly after plants are returned to SD (Corbesier et al., 1996, 2007; Krzymuski et al., 2015). This accumulation of a discrete amount of FT correlates with early flowering and suggests that environmental cues that modulate FT levels will affect flowering time. To better assess the mechanisms that occur in natural conditions, we analyzed the combined influence of temperature and day-length changes on photoperiodic-flowering-pathway genes and tested the extent to which FT gene expression levels can explain flowering times across a range of treatments.
Materials and Methods
Plant material and growth conditions
Wild-type accessions of Arabidopsis thaliana (L.) Heynh, Columbia-0 (Col-0), Landsberg erecta (Ler), and Wassilewskija-2 (Ws-2), were used. All mutants and transgenic lines were in the Col-0 background and described previously: co-101 and ft-101 (Takada & Goto, 2003), fbh-quadruple mutant (fbh-q #2) and FBH1:FLAG-FBH1 (Ito et al., 2012), CO:HA-CO and 35S:3HA-CO (Song et al., 2012), svp-31 (Lee et al., 2007), and SVP:SVP-6HA (Shen et al., 2011). For flowering experiments, seeds were sown on soil (Sunshine #3 Mix; Sun Gro Horticulture) containing Osmocote Classic time-release fertilizer (Scotts) and Systemic Granules: Insect Control (Bionide). For gene, GUS activity, and protein expression analyses and the 14-d, LD-to-SD-transfer flowering experiment, seeds were placed on plates containing 1× Linsmaier and Skoog (LS) media (Caisson) and 3% sucrose. Plants were grown in LD (16-h light/8-h dark) or SD (8-h light/16-h dark) conditions for 7, 14, or 21 d to avoid temperature effects on germination and early development, then moved to constant 12°C or 22°C, or to temperature cycles of 22°C (light) and 12°C or 17°C (dark) in SD, 12-h light/12-h dark (MD) or LD conditions. HOBO Pendant temperature/light data loggers (Onset) were used to ensure temperature deviated 0.5°C or less from the target temperatures. Full-spectrum fluorescent light (Octron F032/950/48; Osram-Sylvania) intensities in the growth chambers averaged c. 100 μmol m−2 s−1 in SD and 60 μmol m−2 s−1 in LD. For flowering experiments, individuals from each strain were assigned random positions within 32-pot flats. The flats were repositioned at least weekly within and between chambers to avoid chamber and positional affects. For the 14-d, transfer experiment, one representative of each age (1–14 d) was randomized and put into a single flat to account for differences due to watering or fertilization. For the remaining analyses, temperature treatments were randomly assigned different incubators for each replicate. Flowering time was measured by recording the number of rosette leaves on the main stem. To assess rate of leaf production, new leaves at least 2 mm long were counted once a week.
Gene expression analysis (qPCR)
For most experiments, plants were grown for 7 d in SD conditions before being transferred to treatments and harvested every 4 h beginning at dawn (ZT0) on day 4 in treatment. For the 48-h time courses, seedlings were harvested starting at ZT12 on day three of treatment, when the seedlings were 10 d old. For the 14-d time courses, seedlings were harvested at ZT0, ZT8, and ZT16 on days 1–14 of treatment. RNA isolation, cDNA synthesis, and qPCR were described (Ito et al., 2012). All genes were normalized against ISOPENTENYL PYROPHOSPHATE/DIMETHYLALLYL PYROPHOSPHATE ISOMERASE (IPP2) (Hazen et al., 2005). We confirmed diurnal IPP2 was expressed uniformly across our temperature conditions compared to other potential internal control genes, SERINE/THREONINE PROTEIN PHOSPHATASE 2A (PP2A) (Hong et al., 2010) and ACTIN 2 (ACT2) (Sawa et al. 2007) (Supporting Information Fig. S1). Primers and PCR conditions for FT, CO, SUPPRESSOR OF OVEREXPRESSION OF CONSTANS (SOC1), and FLC (Ito et al., 2012), and ACT2 and PP2A were described previously (Sawa et al., 2007; Hong et al., 2010). The primers for SVP are 5′-ACGGAAGAGAACGAGCGACTTG-3′ and 5′-CTCGTACACAGCAGCGTTCTCC-3′. qPCR was done using the following program: 1 min 95°C denaturation, then 45 cycles of 10 s 95°C, 20 s 59°C, and 20 s 72°C.
Protein expression analysis (Western blot)
Extraction and detection of FLAG-FBH1 and SVP-6HA proteins, and of nuclear HA-CO were performed as described (Ito et al., 2012; Song et al., 2012). FLAG-FBH1 and SVP-6HA and HA-CO protein were detected by anti-FLAG antibody (Sigma) or anti-HA conjugated to horseradish-peroxidase (Roche). Actin, HSP90, and Histone H3, detected by anti-Actin, anti-HSP90, or anti-Histone H3 antibodies (Song et al., 2012), respectively, were used as loading controls. Relative expression levels were normalized by the values of loading controls.
GUS activity visualization
pFT:GUS and pCO:GUS lines were described previously (Takada & Goto 2003). Eleven-day-old seedlings were harvested at ZT24 on day 4 of the temperature and day length treatment. Tissue preparation and staining were described previously (Ito et al., 2012). Seedlings were prepared for visualization with a series of washes of 70%, 50%, and 30% ethanol, H2O, then 25% and 50% glycerol.
Statistical analyses
Statistical analyses were done using R Statistical Computing software (v. 3.1.1 and earlier, R Core Team, 2015). Statistical significance of final rosette leaf numbers at bolt was determined using ANOVA with temperature treatment and strain as main effects. Pairwise comparisons were determined using Tukey Honest Statistical Difference (R Core Team, 2015). In some cases the replicate and/or tray was introduced as a co-variant to account for pseudo-replication. Statistical significance of leaf production over time was analyzed using linear mixed-effects regression (Bates et al., 2015), which accounted for repeated measures of the same individuals, with temperature, strain, and time (weeks) included as the main effects. This was followed by Satterthwaite approximation to generate P-values (Satterthwaite, 1946). For leaf production, we considered only data recorded before the appearance of visible bolts. Data was transformed as needed based on Box-Cox analysis (Box & Cox, 1964) and visual comparisons of residuals to ensure equal variance. Most flowering experiments were replicated at least two times, with at least 10 individuals per strain and treatment in each replicate. The 14-d, LD-to-SD transfer experiment was replicated twice with five individuals per treatment per replicate.
Gene expression data displayed heteroskedastic residuals, so effects of temperature and time were compared using the Generalized Estimating Equation (Carey, 2015), which computes robust standard errors and test statistics, followed by pairwise Tukey comparisons using lsmeans (Lenth, 2015). To improve normality and equal variance among treatments, time (ZT) was transformed by finding the cosine, and, if necessary, day and night segregation was included as a covariate to account for oscillatory patterns. All gene and protein experiments were replicated at least three times, and we obtained similar results. Effects were considered significant when both of the following conditions were met: P-value ≤ significance level and the 95% Confidence Interval (CI) for the difference between a pair did not contain zero. The significance level was 0.05. For highly non-linear data, subsets (by time) were tested and the significance level was lowered using Bonferonni correction for multiple comparisons.
Results
Cool nighttime temperatures result in delay of flowering, and altered accumulation profiles of CO and FT transcripts
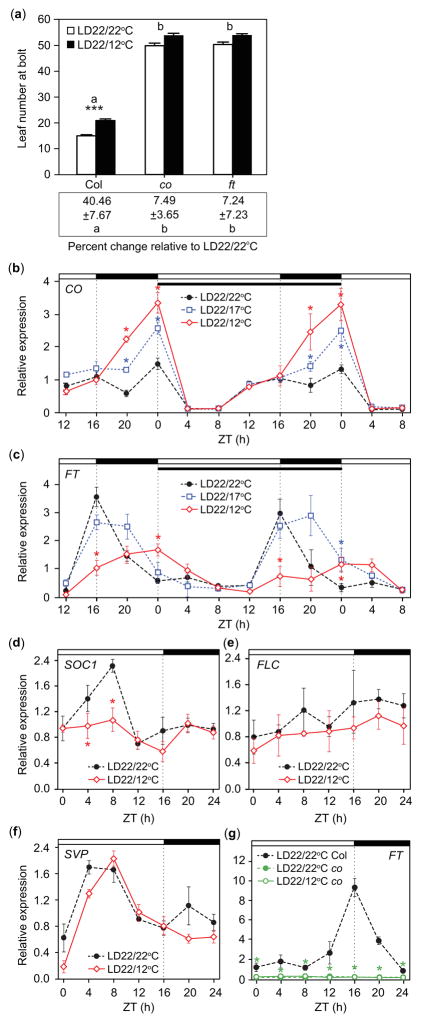
Outdoors, diurnal light and temperature cycles occur together. How do plants integrate these cues? Using as a guide the 21.1°C-high/11.7°C-low temperature range of the summer solstices in Seattle, Washington, USA (averages from 1971 to 2000, day length: 15 h 59 min), we overlaid a 22°C/12°C temperature cycle onto a 16-h-light, 8-h-dark LD cycle, such that the cool period occurred throughout the night (referred to as LD22/12°C) and assessed the timing of flowering. As temperature influences numerous plant processes (Parent et al., 2010), we mitigated its influence on early development by growing all seedlings in non-flowering-inductive SD conditions at 22°C for 7 d before moving them to temperature treatments. Growth in cool nighttime temperatures resulted in a delay in flowering (20.84 ± 0.64 leaves) and a 40.46% increase in the number of leaves produced at bolt in Col-0 plants relative to plants grown in the constant-temperature conditions usually used in studies of photoperiodic flowering in Arabidopsis (LD22/22°C) (14.96 ± 0.42 leaves, P ≤ 0.001; Fig. 1a; Table 1).
Fig. 1.
Flowering is delayed and transcript accumulation patterns of CONSTANS (CO) and FLOWERING LOCUS T (FT) are altered in response to day: night temperature fluctuations. (a) Rosette leaf number of Columbia-0 (Col-0) wild-type Arabidopsis thaliana plants, co-101, and ft-101 mutants (top) and percent change in rosette leaf number between treatment means within a replicate (bottom) in long-day treatments with 22°C daytime temperatures and 22°C or 12°C nighttime temperatures (LD22/22°C and LD22/12°C). Letters represent significant differences among strains of P<0.05. Asterisks (***) indicate significance between treatments within Col-0 (P<0.001), mean and 95% Confidence Interval (CI, 0.329, 0.232, 0.425). Treatment differences of co-101 and ft-101 (top) were not significantly different (P = 0.199 and 0.375), mean and 95% CI (0.081, −0.015, 0.177) and (0.072, −0.025, 0.169), respectively; for differences in percent change from that of Col-0 (bottom), mean and 95% CI (−3.681, −5.095, −2.267) and (−3.489, −4.903, −2.075), respectively (n = 10, replicate = 5). (b, c) CO and FT transcript accumulation patterns in LD treatments with different nighttime temperatures (LD22/22°C, LD22/17°C, and LD22/12°C). Topmost white and black bars designate day and night, respectively. Dotted vertical lines are times of lights on or off. Narrow black bar designates day 4 of treatment. (d) SUPPRESSOR OF OVEREXPRESSION OF CONSTANS (SOC1), (e) FLOWERING LOCUS C (FLC), and (f) SHORT VEGITATIVE PHASE (SVP) gene transcript accumulation patterns in Col-0, and (g) FT transcript accumulation in co-101 mutant line from seedlings harvested on day 4 in LD22/22°C and LD22/12°C. Data represent means ± SEM derived from at least three biological replicates. (b–g) Asterisks (*) represent significance (P<0.05) and 95% CI of difference between pair did not contain zero in statistical comparisons made against the LD22/22°C control. ZT, zeitgeber time.
Table 1.
Means, variances, 95% Confidence Intervals, and P-values for final rosette leaf number at bolt of Arabidopsis thaliana strains in response to constant (22°C) temperatures or warm (22°C) days and cool (12°C) nights
| Strain | Night temp. (°C) | Mean (leaf number) | Standard deviation | SE | Mean (log(leaf number)) | 95% CI
|
adjusted P* | |
|---|---|---|---|---|---|---|---|---|
| upper | lower | |||||||
| Col-0** | 22 | 14.96 | 2.91 | 0.42 | 0.3289 | 0.2323 | 0.4254 | 0.0000 |
| 12 | 20.84 | 4.51 | 0.64 | |||||
| co-101 | 22 | 49.76 | 6.99 | 1.01 | 0.0809 | −0.0152 | 0.1769 | 0.1987 |
| 12 | 53.70 | 5.98 | 0.85 | |||||
| ft-101 | 22 | 50.22 | 6.52 | 0.94 | 0.0720 | −0.0245 | 0.1686 | 0.3748 |
| 12 | 53.78 | 4.19 | 0.60 | |||||
| svp-31 | 22 | 7.30 | 1.44 | 0.23 | 0.1874 | 0.0784 | 0.2964 | 0.0000 |
| 12 | 8.84 | 1.74 | 0.29 | |||||
| Ler | 22 | 7.40 | 2.07 | 0.33 | 0.3131 | 0.2062 | 0.4199 | 0.0000 |
| 12 | 9.98 | 1.75 | 0.28 | |||||
| Ws-2 | 22 | 8.00 | 1.04 | 0.17 | 0.2902 | 0.1833 | 0.3971 | 0.0000 |
| 12 | 10.73 | 1.74 | 0.28 | |||||
|
| ||||||||
| Col-0 | 22 | 15.79 | 2.11 | 0.33 | 0.3144 | 0.2127 | 0.4162 | 0.0000 |
| 12 | 22.23 | 5.04 | 0.94 | |||||
| fbh-quadruple #2 | 22 | 22.80 | 2.99 | 0.55 | 0.2241 | 0.0972 | 0.3510 | 0.0000 |
| 12 | 27.72 | 3.71 | 0.90 | |||||
P-value adjusted to account for multiple comparisons.
Col-0 included as a wild-type control in all experiments.
To determine the mechanisms responsible for this delay, we analyzed the profiles of several flowering-regulator gene transcripts (Yant et al., 2009). We found significant increase of CO transcript accumulation at night in LD22/12°C conditions relative to plants grown in the LD22/22°C control (Fig. 1b). FT transcript levels in LD22/12°C were also significantly altered compared to the control. FT levels were reduced at dusk, but higher at dawn (Fig. 1c). A smaller (5°C) difference between day and nighttime temperatures (LD22/17°C) resulted in an intermediate response relative to the control. CO was induced at night in LD22/17°C conditions, but to a lesser extent than in LD22/12°C (Fig. 1b). FT profile in LD22/17°C conditions was similar at dusk and dawn to the control, but higher than either the control or LD22/12°C in the middle of the night (ZT20, Fig. 1c). Therefore, diurnal temperature cycles, in conjunction with day and night, influence the profiles of CO and FT transcript accumulation.
SOC1 is downstream of FT and acts at the shoot apex to induce LEAFY (LFY) to promote flowering (Lee et al., 2008). SOC1 level was reduced during the morning in LD22/12°C conditions relative to the control (Fig. 1d), consistent with a delay in flowering. The expression of strong FT repressor, FLOWERING LOCUS C (FLC) gene (Michaels & Amasino, 1999), did not respond to our temperature treatments (Fig. 1e), suggesting that if day/night temperature fluctuations affect flowering through FLC, it is not through altered mRNA levels. SVP represses FT and delays flowering time under lower temperatures (i.e. 16°C) (Lee et al., 2007). The daily patterns of SVP transcript accumulation were not significantly changed between our two conditions (Fig. 1f).
FT and CO are upstream of SOC1 and several studies suggest FT levels are predictive of flowering (Kobayashi et al., 1999; Blazquez et al., 2003; Corbesier et al., 2007; Salazar et al., 2009; Krzymuski et al., 2015; Seaton et al., 2015), therefore, we chose to explore whether FT and CO profile changes could account for the delay in flowering we observed. We tested the flowering time of co and ft mutants. The final leaf numbers at bolt of ft and co mutant plants were not significantly different between the LD22/22°C control and LD22/12°C temperature treatments (Fig. 1a; Table 1), indicating that CO and FT may be involved in the temperature-dependent delay of flowering. CO is a chief activator of daytime FT expression (Yanovsky & Kay, 2002). We tested whether CO is required for the cooler-night-temperature-induced FT changes. In the co mutant line, we found FT remained at basal levels throughout the day in both LD22/22°C and LD22/12°C conditions relative to FT in wild-type plants (Fig. 1g), indicating that nighttime expression of FT under LD22/12°C conditions still requires the CO gene.
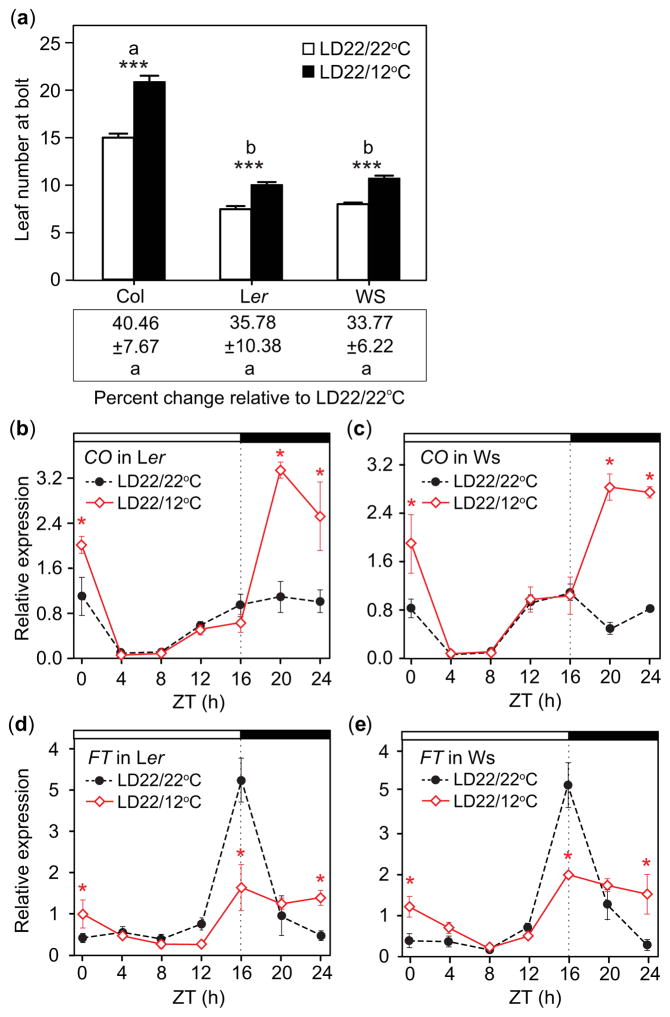
To analyze whether these nighttime-temperature-induced responses are conserved in other wild-type accessions, we measured flowering time and gene expression in two common lab strains, Ler and Ws-2. Both showed delayed flowering in LD22/12°C conditions relative to LD22/22°C that were similar to Col-0 (Fig. 2a; Table 1). As with Col-0, they both displayed nighttime induction of CO (Fig. 2b,c), suppression of FT at dusk and higher levels of FT at dawn (Fig. 2d,e) in the LD22/12°C conditions relative to the LD22/22°C control, indicating that this response was not accession-specific.
Fig. 2.
Flowering and transcript accumulation patterns of CONSTANS (CO) and FLOWERING LOCUS T (FT) in different Arabidopsis thaliana ecotypes in response to day: night temperature fluctuations are similar to those in the Columbia-0 (Col-0) Arabidopsis thaliana accession. (a) Rosette leaf number of Col-0, Landsberg erecta (Ler), and Wassilewskija-2 (Ws-2) (top) and percent change in rosette leaf number between treatment means within a replicate (bottom) in LD22/22°C (long days with day: night temperatures = 22°C: 22°C) and LD22/12°C conditions. (Top) Asterisks (***) indicate significance between treatments (P<0.001), mean and 95% Confidence Interval (CI, 0.329, 0.232, 0.425), (0.313, 0.206, 0.419), and (0.290, 0.183, 0.397), respectively. (Bottom) Differences in percent change from that of Col-0 for Ler and Ws (P = 0.949 and 0.844, respectively), mean and 95% CI (−0.408, −1.908, 1.092) and (−0.549, −2.049, 0.951), respectively, (replicate = 5, n = 10). (b, c) CO transcript accumulation and (d, e) FT transcript accumulation in Ler and Ws in LD22/22°C and LD22/12°C conditions. Seedlings were grown under the same conditions used for the experiments shown in Fig. 1. Data represent means ± SEM derived from at least three biological replicates. (b–e) Asterisks (*) indicate P<0.05 and 95% CI of difference between pair did not contain zero. ZT, zeitgeber time.
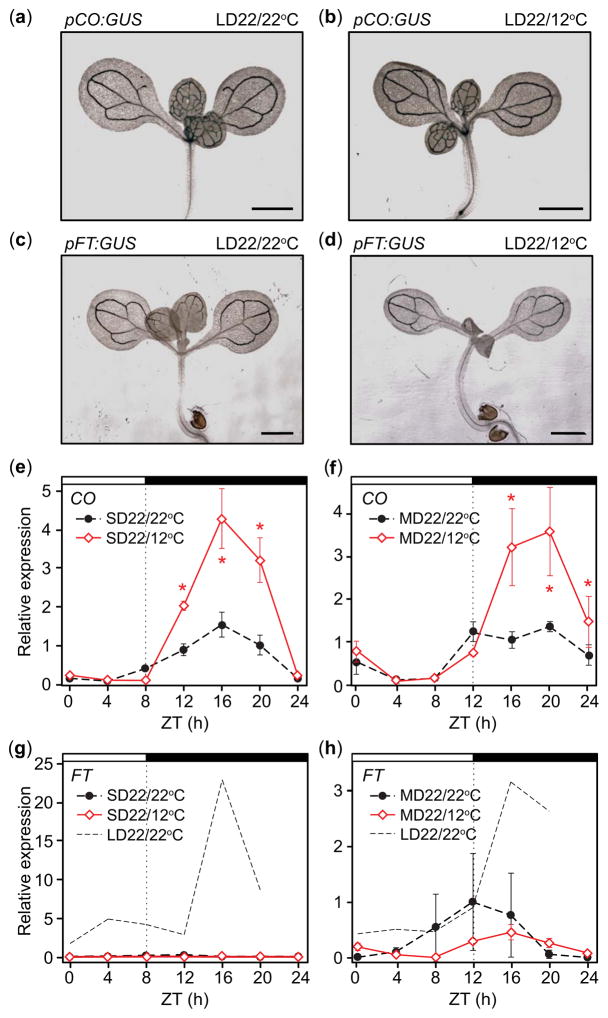
We wondered whether the changes in CO and FT transcript accumulation were due to changes in the spatial accumulation patterns of these genes, which are expressed in the leaf vasculature in the LD22/22°C conditions (Takada & Goto, 2003). We examined the spatial patterns of CO and FT and found that the tissue-specific accumulation of pFT:GUS and pCO:GUS were not affected by LD22/12°C treatments relative to the control (Fig. 3a–d), indicating that temperature fluctuations only alter the temporal accumulation patterns of these genes.
Fig. 3.
Spatial transcript accumulation patterns of CONSTANS (CO) and FLOWERING LOCUS T (FT) are not altered in response to day: night temperature fluctuations in Arabidopsis thaliana (Columbia-0 (Col-0)). Nighttime induction of CO in response to cool nighttime temperatures occurs regardless of day length; FT induction by cool temperatures does not occur in short day (SD). (a–d) Tissue specific patterns of GUS activity in pCO:GUS (a, b) and pFT:GUS (c, d) plants grown in LD22/22°C (long days with day: night temperatures = 22°C: 22°C) and LD22/12°C conditions for 5 d. Scale bars, 1 mm. (e, f) CO transcript accumulation and (g, h) FT transcript accumulation on day 4 in SD or 12-h, mid-length-day (MD) treatments with 22°C daytime temperatures and 22°C or 12°C nighttime temperatures. Replicate of FT transcript accumulation in LD22/22°C conditions is included for comparison. Data represent means ± SEM derived from at least three biological replicates. (e–h) Asterisks (*) indicate P<0.05 and 95% CI of difference between pair did not contain zero. ZT, zeitgeber time.
FT induction is dependent on the presence of light in the afternoon in constant temperatures (Yanovsky & Kay, 2002). We asked whether nighttime CO induction and the altered FT profile were day-length dependent. We tested accumulation profiles of CO and FT transcripts in SD and 12-h-light, 12-h-dark (MD) days. In both cases, the seedlings were exposed to 12°C for the duration of the night. Induction of CO in response to 12°C nighttime temperatures occurred in both day lengths (Fig. 3e,f). However, FT was not appreciably induced in SD, while FT did not differ significantly between treatments in MD (Fig. 3g,h), indicating that cool nighttime temperatures do not override the requirement of FT for afternoon light.
Induction of CO and FT in response to cool nighttime temperatures is reproduced over 2 wk of treatment
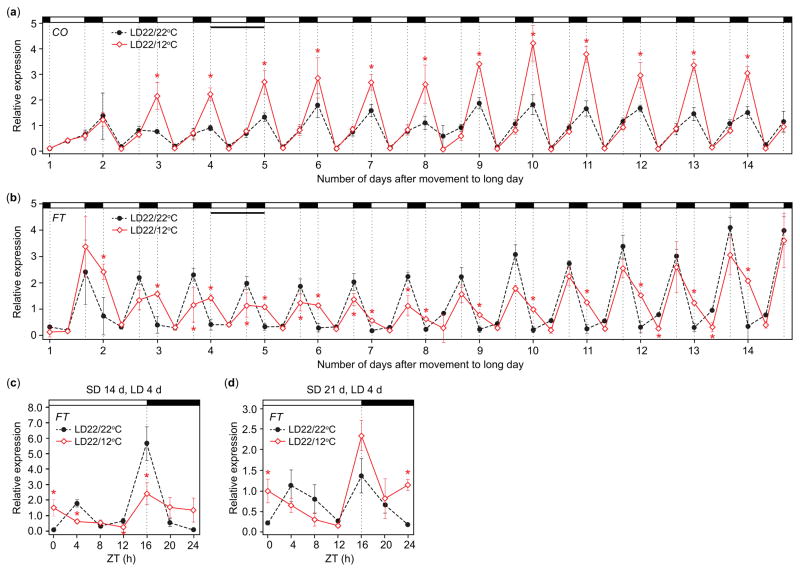
Peak expression of FT increases as plants age from 5 to 15 d (Mathieu et al., 2009). We wondered whether the rate of increase would be slower in LD22/12°C conditions, helping to explain the delay in flowering, and whether the diurnal transcript accumulation patterns we observed remained consistent over time. We extended the LD22/22°C control and LD22/12°C treatment for 14 d (from 7- to 21-d-old plants), harvesting at dawn (ZT0), during the middle of the day (ZT8) when CO and FT transcripts are at trough levels, and at dusk (ZT16) for the full 2 wk. In LD22/12°C conditions, nighttime induction of CO and upregulation of FT at dawn were reproduced for the full 14 da (Fig. 4a,b). However, dusk suppression of FT in LD22/12°C disappeared over time (Fig. 4b). Could suppression of FT at dusk be a transient response to cool nights, perhaps acting to buffer development to short-term temperature modulations? If correct, we would expect dusk suppression of FT even in plants first exposed to the LD22/12°C treatment later in development. We sampled 18- and 25-d-old seedlings, each having been exposed to treatments for four days, to determine whether we observed FT accumulation at dusk. Interestingly, in 25-d-old plants but not in 18-d-old plants, dusk levels of FT were higher than those of the control (Fig. 4c,d), indicating that dusk suppression can be a transient response, dependent on developmental age, potentially suppressing flowering in young plants when temperatures are unfavorable.
Fig. 4.
Late night/dawn inductions of CONSTANS (CO) and FLOWERING LOCUS T (FT) transcript accumulation are conserved over 2 wk, dusk suppression declines as plants age in Arabidopsis thaliana (Columbia-0 (Col-0)). (a) CO and (b) FT transcript accumulation in LD22/22°C (long days with day: night temperatures = 22°C: 22°C) and LD22/12°C conditions for two consecutive weeks. Narrow, horizontal black bar designates day 4 of treatment. Seedlings were harvested at zeitgeber time (ZT, hours after dawn) 0, ZT8, and ZT16. Seedlings were grown for 7 d in short days (SD) before being transferred to LD, temperature treatments; harvest began on day 1 in treatments. Data represent means ± SEM derived from five biological replicates. (c, d) FT transcript accumulation on day 4 of LD, temperature treatments in 18-d-old seedlings (c), and in 25-d-old (d) seedlings harvested at the same time. Seedlings were kept in short days until moved to the treatments. Data represent means ± SEM derived from at least three biological replicates. (a–d) Asterisks (*) indicate P<0.05 and 95% CI of difference between pair did not contain zero.
FT expression correlates with flowering across treatments
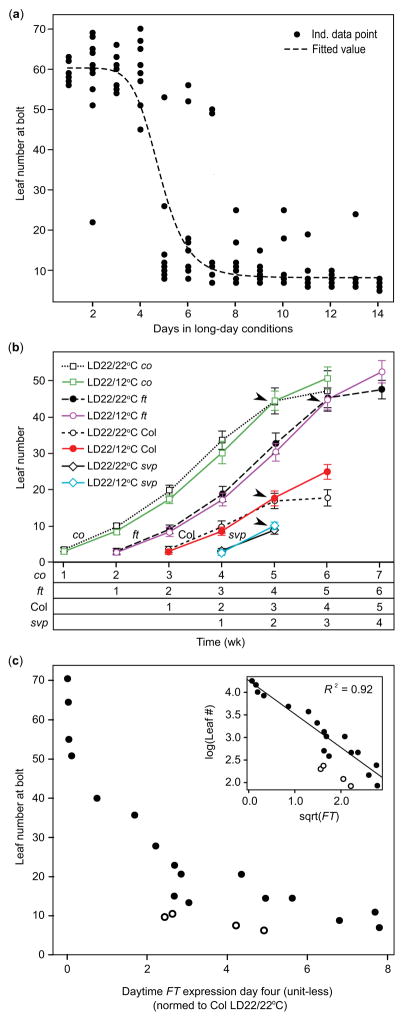
A discrete number of LDs and amount of FT expression is required to induce flowering (Krzymuski et al., 2015). We confirmed that such a distinct switch occurs in our conditions. After 7 d in SD, seedlings were moved to LD22/22°C conditions. For 2 wk, after each LD treatment, we transferred some seedlings back to SD conditions and recorded leaf number at bolt. Seedlings exposed to one to four days of LD flowered similarly to plants grown in SD (leaf number 57.7 ± 11.68 (standard deviation)) (Fig. 5a). After five consecutive LDs, most seedlings flowered early (leaf number 9.35 ± 8.03). Together with previous research, this implies that flowering occurs after FT reaches a threshold and that alterations in FT levels in response to environmental perturbations can be predictive of flowering time.
Fig. 5.
Short-term exposure to long days rapidly induces flowering in Arabidopsis thaliana (Columbia-0 (Col-0)), and FLOWERING LOCUS T (FT) transcript accumulation correlates with final rosette leaf number across treatments and mutant strains within the Col-0 background. (a) Seven-day-old seedlings were transferred to LD22/22°C (long days with day: night temperatures = 22°C: 22°C) and LD22/12°C for 14 d. After each LD, beginning on day 1, a subset of seedlings were transferred to short days (SD) and allowed to grow until bolts were observed (closed circles). A Hill function (dashed line) describing the final rosette leaf number (LfNmbr) at bolt as a function of days exposed to LD (days) was fitted to the flowering data: LfNmbr = {(Bmax(tc^n))/(tc^n + days^n)} + Bmin, where Bmax is highest fitted leaf number at bolt (= 52.17 leaves), Bmin is lowest fitted leaf number at bolt (= 8.23 leaves), tc is day at which transition occurs (= 4.86), n is modifier affecting abruptness of the transition (= 8.31) (P <0.0001 for fit of all parameters). (b) Number of rosette leaves added each week for Col-0 wild-type plants, co-101, ft-101, and svp-31 in LD22/22°C and LD22/12°C conditions. Black arrows indicate when transition to bolting occurred. Error bars; means ± standard deviation. Overall temperature effect (P<0.001), mean for difference between temperature treatments and 95% Confidence Interval (CI, 0.008, −0.134, 0.1497). The strains are plotted adjacent to the others for ease of comparison; however, all were planted at the same time. At time week 1, seedlings were 7 d old and had not yet been exposed to long-day temperature treatments. LMER was used to test for a delay in leaf production, followed by Satterthwaite approximation to generate P-values. Leaf numbers to the right of the arrow were excluded from the test as plants in the LD22/22°C treatment had begun to bolt and stopped rosette leaf production. Note that svp-31 plants began to bolt within the first week of treatment, so do not show the same pattern as the other strains, Landsberg erecta (Ler), and Wassilewskija-2 (Ws-2) had patterns similar to svp-31 and so are not shown. (c) Final rosette leaf number plotted against daytime FT transcript accumulation harvested from time points at zeitgeber time (ZT) 0, ZT8, and ZT16 across several photoperiod and temperature conditions, and strains within the Col-0 background (closed circles), and for Ler and Ws (open circles). Accumulated FT at time points ZT0, ZT8, and ZT16 (dawn, trough, and dusk) was found by integrating under the curve and is normalized to Col-0 harvested at ZT16 in LD22/22°C constant temperature conditions. Data is linearized by transforming as shown in inset for linear regression analysis (RMSE = 0.208 log(leaves)).
However, cool temperature affects several plant processes and slows leaf production (Parent et al., 2010). It is difficult to separate temperature effects mediated through CO and FT from other factors. To assess whether leaf production slowed in LD22/12°C temperature cycle conditions relative to the LD22/22°C control, we counted the number of leaves produced each week for several wild-type and mutant strains. When considering only leaves produced before production of visible bolts, there was a slightly lower overall leaf number across strains in LD22/12°C conditions relative to the LD22/22°C control (P < 0.001, Fig. 5b). However, the 95% CI (−0.134, 0.1497) of the difference between treatments contained zero and so is not significant. This was true even when considering only late flowering strains, which were exposed to cool nighttime temperatures the longest, suggesting that our temperature-cycle treatments do not appreciably affect leaf production.
We then asked how well the relative FT levels in each strain and treatment explain final leaf numbers at bolt. Because the FT transcript profiles remain relatively consistent in LD (Fig. 4b), we assumed that the pattern of FT on day 4 of our treatments was representative of the amount of FT produced before plants reaching a LD threshold. We found that leaf numbers declined with increasing levels of FT, when accumulated day 4 FT transcript was found by integrating under the curve (Fig. 5c, closed circles). After the data were linearized, FT levels explained much of the variation in flowering across day length and temperature treatments and wild-type and mutant strains within the Col-0 background. Recent work indicated that FT generated around dusk (ZT12 to ZT20, Krzymuski et al., 2015) is more effective for inducing flowering, and suppression of FT at dusk likely accounts for the delays in flowering induced by our warm-day/cool-night conditions. The correlation between FT amounts over this timeframe and flowering time was strong in our conditions as well, showing the same correlation as for total FT (R2 = 0.88, RMSE = 0.25 log(leaves)). However, the correlation was strongest when daytime FT from samples collected at ZT0, ZT8 and ZT16 were considered (Fig. 5c inset, R2 = 0.92, RMSE = 0.21 log(leaves)), indicating morning levels of FT may contribute to flowering time. Ler and Ws-2 showed a similar negative correlation between FT and flowering between the LD22/22°C control and LD22/12°C treatments; however, the intercept was different from that of Col-0, perhaps indicating a lower requirement of FT for flowering in these accessions (Fig. 5c, open circles). These data imply that alterations to FT levels in response to environmental changes can be predictive of flowering time across multiple conditions.
Induction of CO requires a decrease in temperature
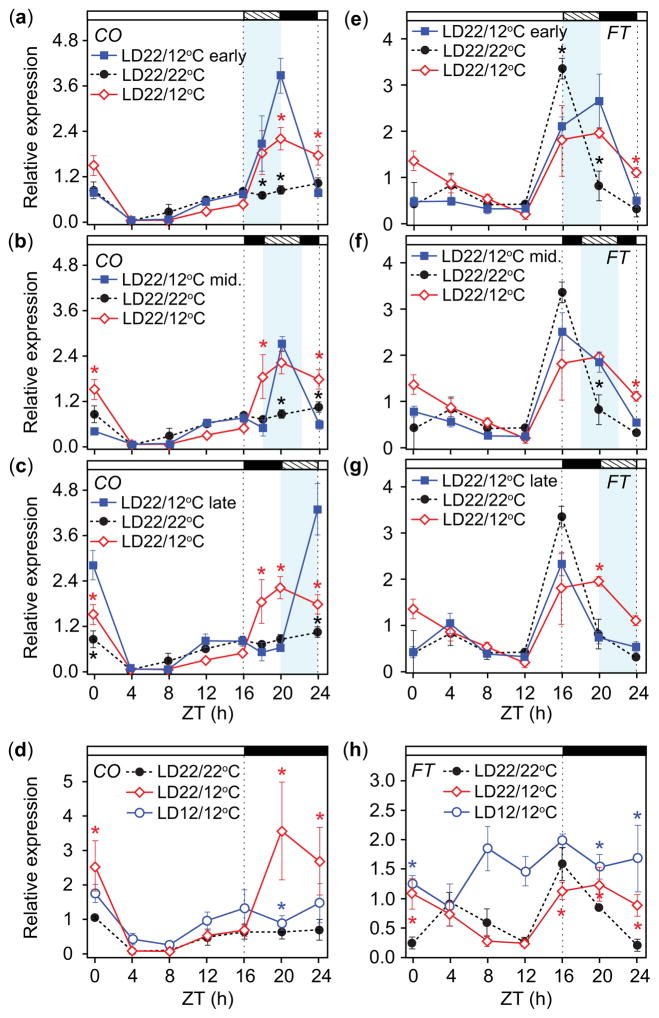
Once we determined FT transcript accumulation levels were predictive of flowering across a range of conditions, we explored the mechanisms responsible for the transcript profile we observed. As CO is a major activator of FT, we examined whether nighttime induction of CO by cool temperatures showed time-dependent differences. Seedlings were exposed to cool temperatures (12°C) for 4-h durations at the beginning, middle, or end of the night in LD. Cool temperatures induced CO regardless of when during the night they occurred, and CO declined again to control levels during the warm periods of the night (Fig. 6a–c). It appeared as if a change in temperature from 22°C to 12°C induced nighttime CO, but it was possible that CO was simply high at 12°C. Would CO would be induced in plants grown in constant 12°C (LD12/12°C) conditions? The pattern of CO for plants grown in SD22/22°C then moved to LD12/12°C for 4 d was slightly higher but similar to the LD22/22°C control and did not show strong nighttime induction (Fig. 6d). These results suggested a drop in temperature during the night results in immediate upregulation of CO, and that there is no obvious circadian-gating effect for CO induction at night.
Fig. 6.
Transcript accumulation of CONSTANS (CO) is induced regardless of when temperature drops occur at night in Arabidopsis thaliana (Columbia-0 (Col-0)); cool temperatures at the beginning of the night induce FLOWERING LOCUS T (FT). (a–c) CO transcript accumulation and (e–g) FT transcript accumulation in response to 4-h periods of 12°C at the beginning of the night from zeitgeber time (ZT) 16 to 20 (a, e early), middle of the night from ZT18 to 22 (b, f mid), and end of the night from ZT20 to 24 (c, g late) in LD. (d) CO transcript accumulation and (h) FT transcript accumulation in LD with constant 12°C conditions (LD12/12°C). All panels show CO and FT transcript accumulation patterns in LD22/22°C and LD22/12°C conditions as references. Data represent means ± SEM derived from at least three biological replicates. Topmost white and black bars designate day and night, respectively. Hashed bars on the top and light blue areas in the graphs represent 4-h cool period (a–c, e–g). Asterisks (a–c, e–g) represent significance (P<0.05) and 95% CI of difference between pair did not contain zero in statistical comparisons between the early, middle, or late night treatments and the LD22/22°C (black) or LD22/12°C controls (red). Asterisks (*) (d, f) represent significance (P<0.05) and 95% CI of difference between pair did not contain zero in statistical comparisons made against the LD22/22°C control.
By contrast, the response of FT showed time-dependent differences. Cool temperatures at the beginning of the night resulted in an FT transcript accumulation profile that was similar to the pattern observed in LD22/12°C conditions (Fig. 6e). Cool temperatures in the middle of the night resulted in peak FT level at dusk (ZT16) that declined more slowly than the LD22/22°C control such that it was higher in the middle of the night (ZT20, Fig. 6f). In both cases, FT declined to control levels during the warm period at the end of the night (ZT24). When cool temperatures occurred at the end of the night, the FT profile was similar to that in the LD22/22°C conditions (Fig. 6g), suggesting that acute induction of CO at the beginning, but not the end, of the night is sufficient to induce nighttime FT expression.
As previous work showed that FT levels were low throughout the day in 12-h-light, 12-h-dark cycles when grown from seed in constant 16°C temperatures (Blazquez et al., 2003), we anticipated that FT levels would be suppressed when cool temperatures were applied for 4 d throughout the day (LD12/12°C). Unexpectedly, FT was higher throughout the day (Fig. 6h). We wondered whether the differences in FT levels between our study and the previous work might be caused by growth conditions of younger seedlings, as we grew seedlings at constant SD22/22°C for a week before temperature treatments. Therefore, we grew seedlings in LD12/12°C conditions from seed and compared these to seedlings grown in LD22/22°C and in LD22/22°C for 7 d then switched to LD12/12°C for 4 d. Seedlings grown entirely in the LD12/12°C conditions had lower levels of FT and CO, and were delayed in development (Fig. S2), lacking true leaves by day 12 while plants in the other treatments had two true leaves that had begun to emerge. However, plants grown from seed in LD12/12°C conditions appeared stressed, so we repeated this experiment using constant 17°C (LD17/17°C) conditions. Plants grown from seed in LD17/17°C conditions were smaller than those grown in LD22/22°C, and had lower overall levels of FT similar to the previous results (Blazquez et al., 2003). In the plants moved from LD22/22°C to LD17/17°C, daytime FT levels were higher especially in the morning (Fig. S3). Previous results also showed lower day-time temperatures upregulate FT. When the daytime temperature was cooler (22°C) relative to a 28°C night, FT levels were c. 15-times higher than that in constant temperature conditions (Thines et al., 2014), and FT was drastically induced during the 12°C phase under 22/12°C daily temperature cycles in constant lights (Schwartz et al., 2009). Taken together, our results suggest that plants respond to cooler temperatures differently depending on how and when (including which part of the day) they are applied, and some conditions can upregulate FT.
FBH genes regulate the cooler night-induced CO transcription
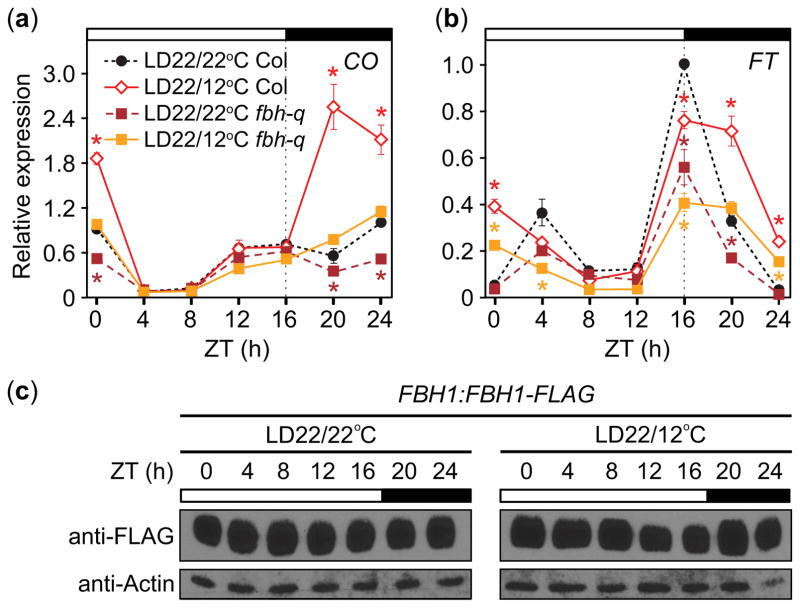
We next examined which genetic components might be involved in the lower-temperature induced changes in CO and FT transcript levels. CO transcription is regulated by both positive and negative transcription factors (Golembeski et al., 2014). CYCLING DOF FACTOR (CDF) family proteins repress CO during the daytime, while FLOWERING BHLH (FBH) family proteins induced CO especially during the night (Fornara et al., 2009; Ito et al., 2012). We examined whether FBHs are involved in the lower-temperature CO induction during night. The lower-temperature-induced accumulation of CO transcript was strongly attenuated in the fbh quadruple (fbh-q: fbh1 fbh2 fbh3 fbh4; Ito et al., 2012) mutants when compared with the level of wild-type plants grown in LD22/12°C (Fig. 7a), indicating that FBH proteins play a role in the upregulation of CO during cooler nights. In the fbh-q mutant, nighttime CO levels in LD22/12°C were still slightly higher than those of the LD22/22°C control, potentially as a result of incomplete silencing of FBH1 and FBH4 expression in this mutant (Ito et al., 2012) or due to contribution of another unknown factor. As expected, expression of FT was lower overall in the fbh-q mutant compared to the control (Fig. 7b) and the fbh-q flowered later than wild type under both conditions (Table 1). The patterns of FT were similar to those in wild type, showing suppression at dusk and upregulation at the end of the night in LD22/12°C conditions (Fig. 7b), consistent with the higher levels of CO in these conditions.
Fig. 7.
The FLOWERING BHLH (FBH) family is involved in cool-night induction of CONSTANS (CO) and FLOWERING LOCUS T (FT) transcript accumulation in Arabidopsis thaliana (Columbia-0 (Col-0)). (a) CO and (b) FT gene transcript accumulation in fbh-quadruple (fbh-q) mutant line. Data, which are normalized to the maximum value in Col-0 wild-type control, represent means ± SEM derived from three biological replicates. Asterisks (*) represent significance (P<0.05) and 95% CI of difference between pair did not contain zero in statistical comparisons made within a strain against the LD22/22°C treatment. (c) Accumulation patterns of FLAG-FBH1 protein in FBH1:FLAG-FBH1 plants grown in LD22/22°C (long days with day: night temperatures = 22°C: 22°C) and LD22/12°C conditions. Actin serves as a loading control. ZT, zeitgeber time.
As FBH function seemed higher under cooler nighttime temperature conditions, we analyzed whether lower night temperature alter the expression patterns of FBH protein. Using the FBH1:FLAG-FBH1 line (Ito et al., 2012), we found similar patterns of FBH1 protein accumulated throughout the day in both LD22/22°C and LD22/12°C (Fig. 7c), indicating that FBH1-mediated induction of CO in response to cool nighttime temperatures may not be caused by changes in FBH1 protein abundance. Taken together, our results suggest that FBHs mediate cooler night-induced increases in CO transcription and this alters FT expression.
CO protein is higher under cool nighttime temperatures
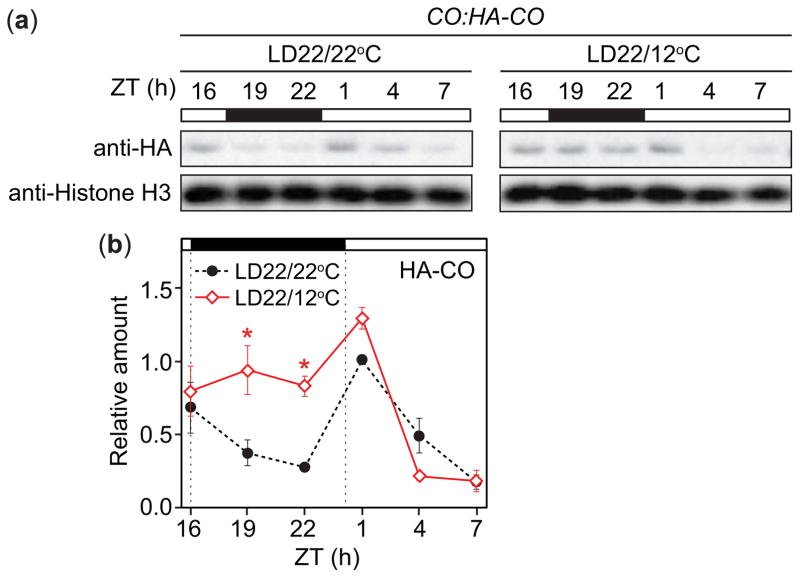
Posttranslational regulation of CO plays an important role in the induction of FT (Song et al., 2015). Under constant temperatures, CO protein is stable in the LD afternoon to induce FT (Valverde et al., 2004), and is degraded at night through the activity of the COP1-SPA1/3/4 ubiquitin ligase complex (Laubinger et al., 2006; Jang et al., 2008; Liu et al., 2011). We found that FT levels declined more slowly in cooler temperatures at night (Fig. 1b), and speculated that CO protein might become more stable under those conditions. We analyzed CO protein profiles in CO:HA-CO plants (Song et al., 2012) grown in LD22/22°C or LD22/12°C conditions. We found that HA-CO protein driven by the native CO promoter was higher at night in LD22/12°C conditions compared to the LD22/22°C control (Fig. 8a,b). As CO mRNA levels are highly induced during the cooler night (Fig. 1a), it remained uncertain whether this was caused by transcriptional or posttranscriptional changes. We measured CO protein levels driven from a constitutively expressed 35S promoter in 35S:3HA-CO plants and found no obvious difference in CO protein profiles in the plants grown under LD22/12°C and LD22/22°C conditions (Fig. S4). This indicates that CO protein is not particularly stabilized under cooler night conditions, and suggests that CO protein levels become higher during cooler nights due mainly to increases in transcription rather than protein stability regulation.
Fig. 8.
CONSTANS (CO) protein is higher during cool nights in Arabidopsis thaliana (Columbia (Col-0)). (a, b) Accumulation patterns (a) and quantification (b) of HA-CO protein in CO:HA-CO plants grown in LD22/22°C (long days with day: night temperatures = 22°C: 22°C) and LD22/12°C conditions. Histone H3 serves as a loading control. (b) Data represent means ± SEM derived from three biological replicates. Asterisks (*) indicate P<0.5 and 95% CI of difference between pair did not contain zero in statistical comparisons made to the LD22/22°C control. ZT, zeitgeber time.
Involvement of SVP in cool-nighttime-temperature induction of FT changes
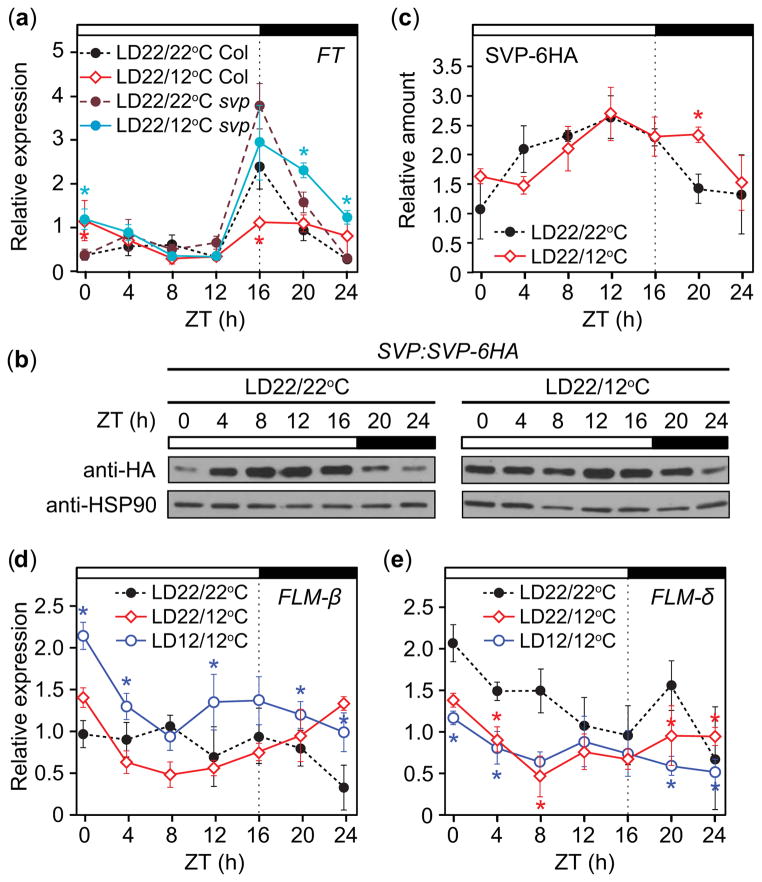
We observed suppression of FT at dusk following cool nights (Fig. 4b), which may prevent precocious flowering in young plants. One possible candidate for FT suppression was SVP, which represses FT under constant 16°C LD conditions (Blazquez et al., 2003). Are the proceeding cooler nights sufficient to facilitate FT repression by SVP at dusk the following day? We found that the dusk peak of FT in svp mutants was similar between LD22/12°C conditions and the LD22/22°C control relative to wild type (ZT16, Fig. 9a), while dawn FT levels still differed between the two conditions (ZT24, Fig. 9a), indicating that SVP is required to reduce the level of FT particularly around dusk. Consistent with a smaller difference in the amount of FT in the svp mutant between the two treatments, the svp mutants were delayed in flowering to a lesser degree than wild type in LD22/12°C conditions relative to LD22/22°C (Table 1). These results imply that SVP represses FT around dusk under our conditions and that cooler night temperatures are enough to activate SVP function.
Fig. 9. SHORT VEGITATIVE PHASE.
(SVP) is involved in cool-night induced dusk suppression of FLOWERING LOCUS T (FT) accumulation in Arabidopsis thaliana (Columbia-0 (Col-0)); suppression may be aided by a decrease in non-DNA-binding FLOWERING LOCUS M (FLM)-δ splice variant before dusk. (a) FT transcript accumulation in svp mutant lines. Accumulation patterns (b) and quantification (c) of SVP-6HA protein in SVP:SVP-6HA plants grown in LD22/22°C and LD22/12°C conditions. HEAT SHOCK PROTEIN 90 (HSP90) serves as a loading control. (d) DNA-binding FLM-β splice variant and (e) non-DNA-binding FLM-δ transcript accumulation in wild-type plants grown in LD22/22°C (long days with day: night temperatures = 22°C: 22°C), LD22/12°C, and LD12/12°C conditions. Data represent means ± SEM derived from three biological replicates. (a, c–e) Statistical comparisons are made to the LD22/22°C control; asterisks indicate P<0.5 and 95% CI of difference between pair did not contain zero. ZT, zeitgeber time.
Cooler constant temperatures stabilize SVP protein (Lee et al., 2013). Do cooler nights affect daily SVP protein profiles? SVP-6HA protein levels in SVP:SVP-6HA plants (Shen et al., 2011) in LD22/12°C conditions remained higher after dusk and into the nighttime than those in LD22/22°C (Fig. 9b,c), indicating that timing of SVP protein stability is altered when plants are exposed to lower nighttime temperatures for part of the day. Binding of SVP to the FT promoter is affected when temperature alters the amounts of FLM splicing variants (Posé et al., 2013). Under cool constant temperatures, the FLM-β variant is more prevalent, promoting DNA binding of SVP, while the FLM-δ variant, which lacks a functional MADS-box domain and inhibits DNA binding of SVP by forming a complex, is present in warm temperatures (Posé et al., 2013). The increased ratio of FLM-β to FLM-δ was proposed to facilitate SVP-mediated flowering delays (Posé et al., 2013); although a recent study indicated that FLM-β transcript levels correlate more strongly with flowering than those of FLM-δ (Lutz et al., 2015). To analyze whether cooler night temperatures change the abundance of these splice variants, we measured the accumulated levels of FLM-β and FLM-δ under LD22/22°C, LD22/12°C, and LD12/12°C conditions. Consistent with previous results, FLM-β was higher under LD12/12°C than the LD22/22°C control (Fig. 9d); FLM-β in LD22/12°C did not significantly differ from the LD22/22°C control. However, the levels of FLM-δ in LD22/12°C, were lower during the day than in LD22/22°C, similar to that of LD12/12°C, causing a higher ratio of FLM-β to FLM-δ in both conditions (Fig. 9e). Together with the observation that SVP protein is slightly more stable around dusk, this result indicates that more SVP may form a heterodimer with functional FLM (FLM-β) to repress FT around dusk under LD22/12°C.
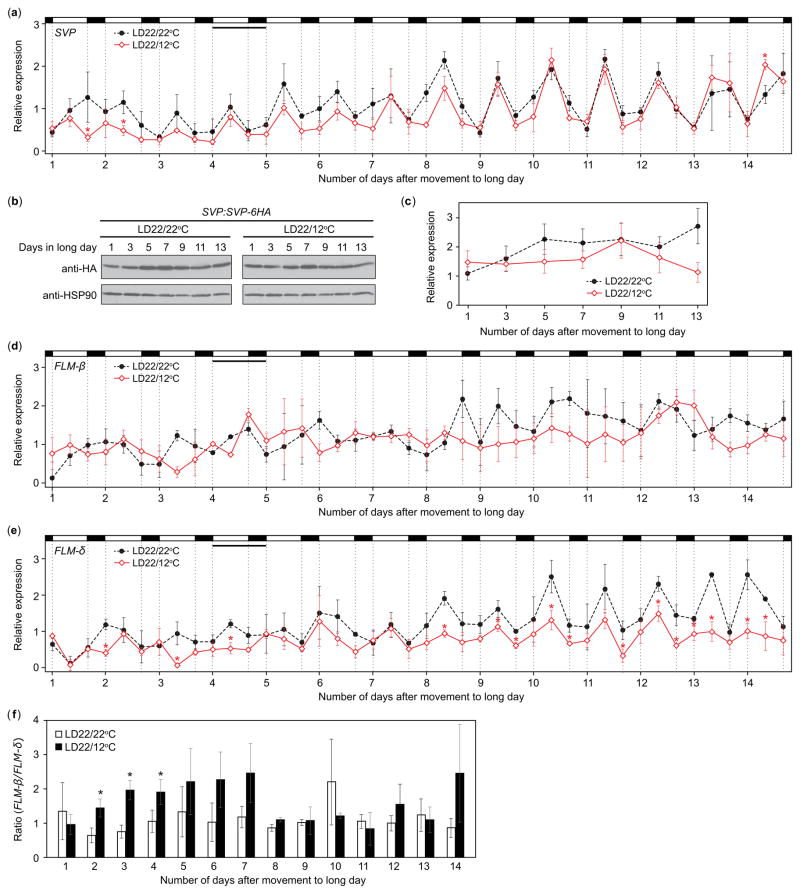
We observed dusk FT transcript levels to increase over 2 wk in LD22/12°C, becoming similar to those of LD22/22°C (Fig. 4b), and wondered whether this change might correlate with levels of SVP mRNA and/or protein over that period. SVP mRNA levels were similar between LD22/22°C and LD22/12°C treatments, although SVP transcript levels in LD22/12°C were slightly lower during week 1 (Fig. 10a). SVP protein sampled before dusk (ZT13) did not differ significantly over the 2-wk period (Fig. 10b,c). We next analyzed FLM-β and FLM-δ transcript accumulation over this timeframe. FLM-β levels did not differ significantly between the two treatments (Fig. 10d). FLM-δ levels were lower in the LD22/12°C conditions relative to the LD22/22°C control (Fig. 10e). To more clearly assess the relationship between these transcripts over developmental time, we calculated the ratio of FLM-β relative to FLM-δ at each time point and then averaged across each day. The ratio of FLM-β to FLM-δ remained consistent over 2 wk in LD22/22°C (Fig. 10f). In LD22/12°C, however, the ratio of FLM-β to FLM-δ was higher than in LD22/22°C during week 1, becoming similar to the LD22/22°C control during week 2 (Fig. 10f). The decreased ratio of FLM-β relative to FLM-δ in LD22/12°C in week 2 may facilitate FT accumulation by affecting DNA binding ability of SVP (Posé et al., 2013; Lutz et al., 2015).
Fig. 10.
Alleviation of dusk FLOWERING LOCUS T (FT) transcript suppression over 2 wk may be caused by the change in FLOWERING LOCUS M (FLM)-β and FLM-δ transcripts in Arabidopsis thaliana (Columbia-0 (Col-0)). (a) SVP transcript accumulation in wild-type plants grown in LD22/22°C (long days with day: night temperatures = 22°C: 22°C) and LD22/12°C conditions in wild type for 14 d harvested at zeitgeber time (ZT) 0, ZT8, and ZT16 starting on day 1 of treatment. Accumulation patterns (b) and quantification (c) of SVP-6HA protein in SVP:SVP-6HA plants grown in LD22/22°C and LD22/12°C conditions over the 2 wk, harvested every other day at ZT13 starting on day 1. HSP90 serves as a loading control. (d) FLM-β and (e) FLM-δ splice variant transcript accumulation patterns and (f) the ratio of FLM-β to FLM-δ in wild-type plants grown in LD22/22°C and LD22/12°C for 14 d harvested at ZT0, ZT8, and ZT16 starting on day 1 of treatment. The ratios (f) were calculated for each time point and then averaged over each day. In all cases plants were grown in short days (SD) for 1 wk before being transferred to treatments. Data represent means ± SEM derived from three biological replicates. (a, e, f) Asterisks (*) indicate P<0.05 and 95% CI of difference between pair did not contain zero.
Discussion
Diurnal temperature changes alter flowering time and CO and FT profiles
To understand flowering time regulation under natural conditions, we studied the effects of temperature alterations coincident with light changes, and demonstrate how two environmental cues can be integrated through the FT gene to influence flowering. A warm-day, cool-night temperature treatment results in upregulation of the CO gene and protein levels at night and an altered FT accumulation profile in LDs. FT accumulates during the night and at dawn, but is suppressed at dusk through the action of SVP and FLM-β (Fig. S5). We found that FT levels explained much of the variance in final leaf number across several temperature and photoperiod treatments and across several mutant lines consistent with other studies (Fig. 5c and Kobayashi et al., 1999; Blazquez et al., 2003; Corbesier et al., 2007; Salazar et al., 2009; Krzymuski et al., 2015; Seaton et al., 2015). Therefore, while temperature influences myriad processes, the delay in flowering we observed in LD22/12°C conditions is largely explained by the amount of FT produced. Interestingly, the strongest correlation was observed when ZT0, 8, and 16 were considered, and all three wild-type strains tested displayed both dusk suppression and dawn induction of FT, indicating morning levels of FT may be important in the ability of plants to integrate environmental perturbations such as temperature changes.
Although this is beyond the scope of our current research, together with previous results (Schwartz et al., 2009; Thines et al., 2014), our results indicate that plants respond to drops in temperature differently than would be expected from studies of plants grown in constant temperature conditions (Blazquez et al., 2003), and FT is frequently induced by lower temperatures that occur in conjunction with light (Figs 6h, S2c, S3c; Schwartz et al., 2009; Thines et al., 2014). The effects of cold treatment (4°C) are also more pronounced when plants are treated during the day than at night (Fowler et al., 2005). Cooler temperatures occurring under light may be perceived as a signal of the changing seasons, such as a shift from summer to fall. Alterations of FT under these conditions may be a mechanism to accelerate flowering before unfavorable weather sets in. For instance, flowering was similarly accelerated between a 22°C-day/28°C-night treatment and a constant 28°C treatment, whereas flowering occurred later in a 28°C-day/22°C-night treatment (Thines et al., 2014). Plants may have different responses to typical fluctuations (warmer day vs colder night) and changes that might associate with seasonal changes (warmer day vs colder day). If that is the case, these responses might be regulated differently for different purposes.
Roles of CO and FBH in temperature-cycle-mediated induction of FT at dawn
The degree of upregulation of CO at night is dependent upon the degree of temperature difference between day and night. The mechanism driving this phenomenon is unknown. The FBH protein family members are logical players, as we demonstrate that FBHs are required for strong nighttime induction of CO and FT. However, amounts of FLAG-FBH1 did not differ between treatments. The levels of other FBH family members may vary. Alternatively, the phosphorylation states of FBHs could be altered. FBH1 is a putative substrate of several mitogen-activated protein kinases (MPKs) (Popescu et al., 2009). In addition, FBH3 is phosphorylated by SNF1-RELATED PROTEIN KINASE 2 (SnRK2) in response to ABA signaling (Takahashi et al., 2013), and is a putative substrate for CALMODULIN DOMAIN PROTEIN KINASE 9 (CPK9) (Yang et al., 2013). CPK9 expression is altered by ethylene, which, along with ABA, is involved in perception of numerous environmental stresses (Qiao et al., 2009; Wang et al., 2013; Nakashima et al., 2014). Daily temperature changes may influence amounts/activities of these kinases and, consequently FBH phosphorylation states, and may be a mechanism by which plant stress modulates flowering time.
Age dependent suppression of FT expression at dusk by SVP
Although CO induces FT expression in response to warm day, cool night conditions, this effect may be countered by suppression of FT by SVP at dusk in LD in plants less than 3 wk old. Our analysis indicated that SVP accounts for the FT repression at the end of the warmer day, and this repression was regulated by the cooler night in a previous day. The ratio of FLM-β to FLM-δ transcript was higher during the daytime in LD22/12°C, perhaps increasing SVP binding of the FT promoter and decreasing FT accumulation at dusk, and SVP-6HA protein levels shift from daytime to around dusk and decline more slowly at night, which may also contribute to FT suppression.
Dusk suppression of FT is alleviated over 2 wk in the cool-night treatment, and we observed dusk suppression of FT in 11- and 18-d-old plants, not in 25-d-old plants. The cooler temperature treatments did not drastically alter the accumulation levels of SVP mRNA and protein over the 2 wk, indicating that SVP levels likely do not cause the age-dependent alleviation of FT suppression. We observed the changes in both FLM-β and FLM-δ transcript levels over the 2 wk. The ratio of daily FLM-β/FLM-δ levels in LD22/12°C conditions decreased after 1 wk in this treatment. A recent report indicated that the FLM-β level, more than the FLM-β/FLM-δ ratio, correlates with flowering time in the accessions examined (Lutz et al., 2015). However, as Lutz et al. also stated, and because increasing the FLM-δ level without altering the FLM-β level drastically caused early flowering (Posé et al., 2013), their ratio changes may partly account for the changes in FT repression by altering the DNA-binding-abilities of SVP under our conditions. It is possible that developmental-age-dependent suppression by SVP/FLM may serve to repress precocious flowering in younger plants.
Daytime and nighttime temperatures differentially affect leaf number as well as flowering in other plant species (Yin & Kropff, 1996; Karsai et al., 2008). Therefore, understanding the relationship between day and nighttime temperatures in conjunction to day length is important. Our study highlights an interface between environmental conditions and the mechanisms controlling flowering that may become more important as local weather regimes are altered with climate change.
Supplementary Material
Acknowledgments
We thank Dr Hao Yu for SVP:SVP-6HA seeds, Sheila Davis and Leema Singh for their assistance. This work was supported by funding from the National Science Foundation Graduate Research Fellowship Program to H.A.K-S., partly from Next-Generation BioGreen 21 Program (SSAC, PJ01117501 to Y.H.S. and PJ01117502 to T.I, Rural Development Administration, Republic of Korea), and from the National Institutes of Health (GM079712) and the University of Washington Royalty Research Fund to T.I.
Footnotes
Author contributions
H.A.K-S., S.-H.K. and T.I. planned and designed the research. H.A.K-S., X.T., J.L., Y.H.S. and S.I. performed experiments and analyzed data. H.A.K-S. and T.I. wrote the manuscript.
Additional supporting information may be found in the online version of this article.
Fig. S1 Transcript accumulation levels of ISOPENTENYL PYROPHOSPHATE/DIMETHYLALLYL PYROPHOSPHATE ISOMERASE (IPP2) remain unaltered over the day across three temperature treatments.
Fig. S2 Growth is delayed and FLOWERING LOCUS T (FT) level is depressed in seedlings exposed to constant 12°C temperatures.
Fig. S3 Growth is delayed and FLOWERING LOCUS T (FT) level is reduced in seedlings exposed to constant 17°C temperatures.
Fig. S4 CONSTANS (CO) protein stabilization is not altered during the cool night.
Fig. S5 Proposed mechanism for the transcriptional regulation of FLOWERING LOCUS T (FT) in long days with temperature changes.
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
References
- Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67:1–48. [Google Scholar]
- Blazquez M, Ahn J, Weigel D. A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nature Genetics. 2003;33:168–171. doi: 10.1038/ng1085. [DOI] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformations. Journal of the Royal Statistical Society. 1964;26:211–252. [Google Scholar]
- Carey VJ. [accessed 19 July 2015];Ported to R by Thomas Lumley (versions 3.13 and 4.4) and Brian Ripley (version 4.13). 2015. gee: Generalized Estimation Equation Solver. Version 4.13-19. (Computer program) http://CRAN.R-project.org/package=gee.
- Corbesier L, Gadisseur I, Silvestre G, Jacqmard A, Bernier G. Design in Arabidopsis thaliana of a synchronous system of floral induction by one long day. Plant Journal. 1996;9:947–952. doi: 10.1046/j.1365-313x.1996.9060947.x. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis thaliana. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- Fornara F, Panigrahi KC, Gissot L, Sauerbrunn N, Rühl M, Jarillo JA, Coupland G. Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Developmental Cell. 2009;17:75–86. doi: 10.1016/j.devcel.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Fowler SG, Cook D, Thomashow MF. Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiology. 2005;137:961–968. doi: 10.1104/pp.104.058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golembeski GS, Kinmonth-Schultz HA, Song YH, Imaizumi T. Photoperiodic flowering regulation in Arabidopsis thaliana. In: Fornara F, editor. Molecular genetics of floral transition and flower development. London, UK: Academic Press Ltd-Elsevier Science Ltd; 2014. pp. 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazen SP, Schultz TF, Pruneda-Paz JL, Borevitz JO, Ecker JR, Kay SA. LUX ARRHYTHMO encodes a Myb domain protein essential for circadian rhythms. Proceedings of the National Academy of Sciences. 2005;102:10387–10392. doi: 10.1073/pnas.0503029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SM, Bahn SC, Lyu A, Jung HS, Ahn JH. Identification and testing of superior reference genes for a starting pool of transcript normalization in Arabidopsis. Plant and Cell Physiology. 2010;51:1694–1706. doi: 10.1093/pcp/pcq128. [DOI] [PubMed] [Google Scholar]
- Ito S, Song YH, Josephson-Day AR, Miller RJ, Breton G, Olmstead RG, Imaizumi T. FLOWERING BHLH transcriptional activators control expression of the photoperiodic flowering regulator CONSTANS in Arabidopsis. Proceedings of the National Academy of Sciences. 2012;109:3582–3587. doi: 10.1073/pnas.1118876109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Marchal V, Panigrahi KCS, Wenkel S, Soppe W, Deng X-W, Valverde F, Coupland G. Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO Journal. 2008;27:1277–1288. doi: 10.1038/emboj.2008.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J-H, Seo PJ, Park C-M. The E3 ubiquitin ligase HOS1 regulates Arabidopsis flowering by mediating CONSTANS degradation under cold stress. Journal of Biological Chemistry. 2012;287:43277–43287. doi: 10.1074/jbc.M112.394338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsai I, Szucs P, Koszegi B, Hayes PM, Casas A, Bedo Z, Veisz O. Effects of photo and thermo cycles on flowering time in barley: a genetical phenomics approach. Journal of Experimental Botany. 2008;59:2707–2715. doi: 10.1093/jxb/ern131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- Krzymuski M, Andrés F, Cagnola JI, Seonghoe J, Yanovsky M, Coupland G, Casal JJ. The dynamics of FLOWERING LOCUS T expression encodes long-day information. Plant Journal. 2015;83:952–961. doi: 10.1111/tpj.12938. [DOI] [PubMed] [Google Scholar]
- Laubinger S, Marchal V, Le Gourrierec J, Gentilhomme J, Wenkel S, Adrian J, Jang S, Kulajta C, Braun H, Coupland G, et al. Arabidopsis SPA proteins regulate photoperiodic flowering and interact with the floral inducer CONSTANS to regulate its stability. Development. 2006;133:3213–3222. doi: 10.1242/dev.02481. [DOI] [PubMed] [Google Scholar]
- Lazaro A, Valverde F, Pineiro M, Jarillo JA. The Arabidopsis E3 Ubiquitin Ligase HOS1 negatively regulates CONSTANS abundance in the photoperiodic control of flowering. Plant Cell. 2012;24:982–999. doi: 10.1105/tpc.110.081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Oh M, Park H, Lee I. SOC1 translocated to the nucleus by interaction with AGL24 directly regulates LEAFY. Plant Journal. 2008;55:832–843. doi: 10.1111/j.1365-313X.2008.03552.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ryu H-S, Chung KS, Posé D, Kim S, Schmid M, Ahn JH. Regulation of temperature-responsive flowering by MADS-Box transcription factor repressors. Science. 2013;342:628–632. doi: 10.1126/science.1241097. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes & Development. 2007;21:397–402. doi: 10.1101/gad.1518407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R. [accessed 9 August 2015];lsmeans: Least-Squares Means. Version 2.21-1 (Computer program) 2015 http://CRAN.R-project.org/package=lsmeans.
- Liu B, Zuo Z, Liu H, Liu X, Lin C. Arabidopsis cryptochrome 1 interacts with SPA1 to suppress COP1 activity in response to blue light. Genes & Development. 2011;25:1029–1034. doi: 10.1101/gad.2025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz U, Posé D, Pfeifer M, Gundlach H, Hagmann J, Wang C, Weigel D, Mayer KFX, Schmid M, Schwechheimer C. Modulation of ambient temperature-dependent flowering in Arabidopsis thaliana by natural variation of FLOWERING LOCUS M. PLoS Genetics. 2015;11:e1005588. doi: 10.1371/journal.pgen.1005588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J, Yant LJ, Mürdter F, Küttner F, Schmid M. Repression of flowering by the miR172 target SMZ. PLoS Biology. 2009;7:e1000148. doi: 10.1371/journal.pbio.1000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CR. Plant circadian rhythms. Plant Cell. 2006;18:792–803. doi: 10.1105/tpc.106.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels S, Amasino R. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myster J, Moe R. Effect of diurnal temperature alternations on plant morphology in some greenhouse crops – a mini review. Scientia Horticulturae. 1995;62:205–215. [Google Scholar]
- Nakashima K, Yamaguchi-Shinozaki K, Shinozaki K. The transcriptional regulatory network in the drought response and its crosstalk in abiotic stress responses including drought, cold, and heat. Frontiers in Plant Science. 2014;5:1–7. doi: 10.3389/fpls.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto Y, Kubozono S, Miyachi M, Yamashino T, Nakamichi N, Mizuno T. A circadian clock- and PIF4-mediated double coincidence mechanism is implicated in the thermosensitive photoperiodic control of plant architectures in Arabidopsis thaliana. Plant and Cell Physiology. 2013;54:643–643. doi: 10.1093/pcp/pcs141. [DOI] [PubMed] [Google Scholar]
- Osugi A, Izawa T. Critical gates in day-length recognition to control the photoperiodic flowering. In: Fornara F, editor. Molecular genetics of floral transition and flower development. London, UK: Academic Press Ltd-Elsevier Science Ltd; 2014. pp. 103–130. [Google Scholar]
- Parent B, Turc O, Gibon Y, Stitt M, Tardieu F. Modelling temperature-compensated physiological rates, based on the co-ordination of responses to temperature of developmental processes. Journal of Experimental Botany. 2010;61:2057–2069. doi: 10.1093/jxb/erq003. [DOI] [PubMed] [Google Scholar]
- Popescu SC, Popescu GV, Bachan S, Zhang Z, Gerstein M, Snyder M, Dinesh-Kumar SP. MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes & Development. 2009;23:80–92. doi: 10.1101/gad.1740009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posé D, Verhage L, Ott F, Yant L, Mathieu J, Angenent GC, Immink RGH, Schmid M. Temperature-dependent regulation of flowering by antagonistic FLM variants. Nature. 2013;503:414–417. doi: 10.1038/nature12633. [DOI] [PubMed] [Google Scholar]
- Prasch CM, Sonnewald U. Signaling events in plants: stress factors in combination change the picture. Environmental and Experimental Botany. 2015;114:4–14. [Google Scholar]
- Qiao H, Chang KN, Yazaki J, Ecker JR. Interplay between ethylene, ETP1/ETP2 F-box proteins, and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes & Development. 2009;23:512–521. doi: 10.1101/gad.1765709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar JD, Saithong T, Brown PE, Foreman J, Locke JC, Halliday KJ, Carre IA, Rand DA, Millar AJ. Prediction of photoperiodic regulators from quantitative gene circuit models. Cell. 2009;139:1170–9. doi: 10.1016/j.cell.2009.11.029. [DOI] [PubMed] [Google Scholar]
- Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics Bulletin. 1946;2:110–114. [PubMed] [Google Scholar]
- Sawa M, Nusinow DA, Kay SA, Imaizumi T. FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science. 2007;318:261–265. doi: 10.1126/science.1146994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C, Balasubramanian S, Warthmann N, Michael TP, Lempe J, Sureshkumar S, Kobayashi Y, Maloof JN, Borevitz JO, Chory J, et al. Cis-regulatory changes at FLOWERING LOCUS T mediate natural variation in flowering responses of Arabidopsis thaliana. Genetics. 2009;183:723–732. doi: 10.1534/genetics.109.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton DD, Smith RW, Song YH, MacGregor DR, Stewart K, Steel G, Foreman J, Penfield S, Imaizumi T, Millar AJ, et al. Linked circadian outputs control elongation growth and flowering in response to photoperiod and temperature. Molecular Systems Biology. 2015;11:1–19. doi: 10.15252/msb.20145766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Kang YGG, Liu L, Yu H. The J-Domain protein J3 mediates the integration of flowering signals in Arabidopsis. Plant Cell. 2011;23:499–514. doi: 10.1105/tpc.111.083048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha R, Gomez-Ariza J, Brambilla V, Fornara F. Molecular control of seasonal flowering in rice, Arabidopsis and temperate cereals. Annals of Botany. 2014;114:1445–1458. doi: 10.1093/aob/mcu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Estrada DA, Johnson RS, Kim SK, Lee SY, MacCoss MJ, Imaizumi T. Distinct roles of FKF1, GIGANTEA, and ZEITLUPE proteins in the regulation of CONSTANS stability in Arabidopsis photoperiodic flowering. Proceedings of the National Academy of Sciences. 2014;111:17672–17677. doi: 10.1073/pnas.1415375111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Ito S, Imaizumi T. Flowering time regulation: photoperiod- and temperature-sensing in leaves. Trends in Plant Science. 2013;18:575–583. doi: 10.1016/j.tplants.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y-H, Shim J-S, Kinmonth-Schultz HA, Imaizumi T. Photoperiodic flowering: time measurement mechanisms in leaves. Annual Review of Plant Biology. 2015;66:441–464. doi: 10.1146/annurev-arplant-043014-115555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YH, Smith RW, To BJ, Millar AJ, Imaizumi T. FKF1 conveys timing information for CONSTANS stabilization in photoperiodic flowering. Science. 2012;336:1045–1049. doi: 10.1126/science.1219644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- Takada S, Goto K. TERMINAL FLOWER2, an Arabidopsis homolog of HETEROCHROMATIN PROTEIN1, counteracts the activation of FLOWERING LOCUS T by CONSTANS in the vascular tissues of leaves to regulate flowering time. Plant Cell. 2003;15:2856–2865. doi: 10.1105/tpc.016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Ebisu Y, Kinoshita T, Doi M, Okuma E, Murata Y, Shimazaki K. bHLH transcription factors that facilitate K+ uptake during stomatal opening are repressed by abscisic acid through phosphorylation. Science Signaling. 2013;6:ra48. doi: 10.1126/scisignal.2003760. [DOI] [PubMed] [Google Scholar]
- Tan S-T, Dai C, Liu H-T, Xue H-W. Arabidopsis casein kinase1 proteins CK1.3 and CK1.4 phosphorylate cryptochrome2 to regulate blue light signaling. Plant Cell. 2013;25:2618–2632. doi: 10.1105/tpc.113.114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines BC, Youn Y, Duarte MI, Harmon FG. The time of day effects of warm temperature on flowering time involve PIF4 and PIF5. Journal of Experimental Botany. 2014;65:1141–1151. doi: 10.1093/jxb/ert487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science. 2004;303:1003–1006. doi: 10.1126/science.1091761. [DOI] [PubMed] [Google Scholar]
- Wang F, Cui X, Sun Y, Dong C-H. Ethylene signaling and regulation in plant growth and stress responses. Plant Cell Reports. 2013;32:1099–1109. doi: 10.1007/s00299-013-1421-6. [DOI] [PubMed] [Google Scholar]
- Yang Z, Guo G, Zhang M, Liu C, Hu Q, Lam H, Cheng H, Xue Y, Li J, Li N. Stable isotope metabolic labeling-based quantitative phosphoproteomic analysis of Arabidopsis mutants reveals ethylene- regulated time-dependent phosphoproteins and putative substrates of Constitutive Triple Response 1 Kinase. Molecular and Cellular Proteomics. 2013;12:3559–3582. doi: 10.1074/mcp.M113.031633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky MJ, Kay SA. Molecular basis of seasonal time measurement in Arabidopsis. Nature. 2002;419:308–312. doi: 10.1038/nature00996. [DOI] [PubMed] [Google Scholar]
- Yant L, Mathieu J, Schmid M. Just say no: floral repressors help Arabidopsis bide the time. Current Opinion in Plant Biology. 2009;12:580–586. doi: 10.1016/j.pbi.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Yin X, Kropff M. The effect of temperature on leaf appearance in rice. Annals of Botany. 1996;77:215–221. [Google Scholar]
- Zuo Z, Liu H, Liu B, Liu X, Lin C. Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Current Biology. 2011;21:841–847. doi: 10.1016/j.cub.2011.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.