Abstract
Background
Frozen section histology is widely used to aid in the diagnosis of periprosthetic joint infection at the second stage of revision arthroplasty, although there are limited data regarding its utility. Moreover, there is no definitive method to assess control of infection at the time of reimplantation. Because failure of a two-stage revision can have serious consequences, it is important to identify the cases that might fail and defer reimplantation if necessary. Thus, a reliable test providing information about the control of infection and risk of subsequent failure is necessary.
Questions/purposes
(1) At second-stage reimplantation surgery, what is the diagnostic accuracy of frozen sections as compared with the Musculoskeletal Infection Society (MSIS) as the gold standard? (2) What are the diagnostic accuracy parameters for the MSIS criteria and frozen sections in predicting failure of reimplantation? (3) Do positive MSIS criteria or frozen section at the time of reimplantation increase the risk of subsequent failure?
Methods
A total of 97 patients undergoing the second stage of revision total hip arthroplasty or total knee arthroplasty in 2013 for a diagnosis of periprosthetic joint infection (PJI) were considered eligible for the study. Of these, 11 had incomplete MSIS criteria and seven lacked 1-year followup, leaving 79 patients (38 knees and 41 hips) available for analysis. At the time of reimplantation, frozen section results were compared with modified MSIS criteria as the gold standard in detecting infection. Subsequently, success or failure of reimplantation was defined by (1) control of infection, as characterized by a healed wound without fistula, drainage, or pain; (2) no subsequent surgical intervention for infection after reimplantation surgery; and (3) no occurrence of PJI-related mortality; and diagnostic parameters in predicting treatment failure were calculated for both the modified MSIS criteria and frozen sections.
Results
At the time of second-stage reimplantation surgery, frozen section is useful in ruling in infection, where the specificity is 94% (95% confidence interval [CI], 89%–99%); however, there is less utility in ruling out infection, because sensitivity is only 50% (CI, 13%–88%). Both the MSIS criteria and frozen sections have high specificity for ruling in failure of reimplantation (MSIS criteria specificity: 96% [CI, 91%–100%]; frozen section: 95% [CI, 88%–100%]), but screening capabilities are limited (MSIS sensitivity: 26% [CI, 9%–44%]; frozen section: 22% [CI, 9%–29%]). Positive MSIS criteria at the time of reimplantation were a risk factor for subsequent failure (hazard ratio [HR], 5.22 [1.64–16.62], p = 0.005), whereas positive frozen section was not (HR, 1.16 [0.15–8.86], p = 0.883).
Conclusions
On the basis of our results, both frozen section and MSIS are recommended at the time of the second stage of revision arthroplasty. Both frozen section and modified MSIS criteria had limited screening capabilities to identify failure, although both demonstrated high specificity. MSIS criteria should be evaluated at the second stage of revision arthroplasty because performing reimplantation in a joint that is positive for infection significantly increases the risk for subsequent failure.
Level of Evidence
Level III, diagnostic study.
Introduction
A number of tests used alone or in combination with clinical judgment can help with early diagnosis of hip and knee periprosthetic joint infection (PJI). However, despite the broad variety of these diagnostic tests, there is no definitive method to assess control of infection in a patient in whom infection had earlier been diagnosed and treatment such as resection arthroplasty and placement of an antibiotic-containing cement spacer had been initiated. Serologic markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are reportedly not diagnostic of persistent infection, although their values do decrease at the time of reimplantation [12, 18]. Although both Gram staining and frozen section can provide intraoperative information, the former is not recommended as a result of its very low sensitivity [2, 7, 15, 30]. Intraoperative frozen sections have been suggested for the diagnosis of infection at the time of reimplantation and are widely used despite their reportedly low sensitivity at the time of second-stage revision arthroplasty [3, 10]. In previous studies, culture results with or without permanent histology have been used as the reference standard for diagnosis [3, 10, 25]. Intraoperative culture results are not available immediately and the results may be affected by the antibiotic-loaded spacer and systemic antibiotic administration with positive cultures reported as being present in only approximately 5% to 10% of cases at reimplantation [10, 18, 19, 24]. Therefore, culture results may not be a true reflection of persistent infection.
The Musculoskeletal Infection Society (MSIS) criteria have recently gained popularity as the gold standard for PJI because they use a combination of tests to either confirm or exclude the diagnosis [28]. However, these criteria were developed to aid in the diagnosis of PJI before revision and not to evaluate the control of infection at the time of reimplantation. To the authors’ knowledge, no study has evaluated the use of frozen sections with MSIS as the reference standard for the second-stage revision arthroplasty.
We therefore aimed to answer the following questions: (1) At second-stage reimplantation surgery, what is the diagnostic accuracy of frozen sections as compared with the MSIS as the gold standard? (2) What are the diagnostic accuracy parameters for the MSIS criteria and frozen sections in predicting failure of reimplantation? (3) Do positive MSIS criteria or frozen section at the time of reimplantation increase the risk of subsequent failure?
Patients and Methods
Approval was obtained from the institutional review board before the commencement of this study. All patients who underwent the second step of a two-stage revision surgery of the knee/hip at our institute from January 2013 to December 2013 were considered for inclusion in this retrospective study. All the data were retrieved from the electronic medical records and patients were contacted by telephone to obtain information on outcomes whenever necessary.
Over the 12-month study period, 107 patients underwent a staged revision arthroplasty of the knee or hip for PJI. Of those, 10 (9%) did not have the second stage because of medical comorbidities precluding another surgery (n = 6) or death before the second stage (n = 4). The remaining 97 patients who underwent the second stage of the procedure were potentially eligible for evaluation in this study. Of these, 11 (11%) patients lacked sufficient information in the medical record to assess MSIS criteria and seven (7%) patients were lost to followup before 1 year, leaving 79 patients (38 revision TKAs, 41 revision THAs) available for analysis. The minimum followup was 1 year with a mean followup of 1.62 ± 0.45 years. The indication for revision in all patients was a confirmed PJI as diagnosed by clinical signs, serologic markers, and microbiological results. During the first stage of the revision, all patients had the implant removed followed by irrigation and débridement. An antibiotic-loaded cement spacer was then implanted.
The second step of the two-stage revision was attempted after at least 6 weeks of antibiotic therapy. The antibiotic selection was based on culture sensitivity reports when available. The decision to reimplant or to perform a spacer exchange was made on the basis of clinical and intraoperative laboratory parameters. Intraoperative samples were collected for histological and microbiological analysis.
The study population consisted of 48 (61%) men and 31 (39%) women with a mean age of 63 ± 14 years. Articulating spacers were present in 39 hips (n = 39 of 41 [95%]) and seven knees (n = seven of 38 [18%]). All other patients had been treated with static spacers (two of 41 [5%] hips and 31 of 38 [82%] knees). The mean duration to attempted reimplantation was 99.7 ± 40 days. A total of 72 procedures involved reimplantation of the prosthesis, whereas six patients had only a spacer exchange. One patient underwent an arthrodesis at the second stage as a result of extensive soft tissue defects and a history of prior recurrent infections.
For the first question regarding the diagnostic accuracy of frozen section as compared with the MSIS criteria as the gold standard, patients were classified as infected or not at the time of the surgery as per MSIS criteria [28]. A case was considered to be positive for infection when one of the following existed: two positive periprosthetic cultures with phenotypically identical organisms, a sinus tract communicating with the joint, or three of the following minor criteria: elevated serum CRP (> mg/dL) and ESR (> 30 mm/hr), presence of purulence in the affected joint, elevated synovial fluid white blood cell count (> 3000/µL), elevated synovial fluid polymorphonuclear neutrophil percentage (> 80%), or a single positive culture in periprosthetic tissue/synovial fluid. Although interpretation of purulence is subjective and there are concerns of false positivity with purulence, especially with metal-on-metal hips, we retained purulence as we evaluated second-stage procedures that are less likely to be affected by metal debris compared with the first stage of revision [4, 21]. For the frozen sections, at the time of surgery, tissue samples were routinely obtained from multiple sites to increase the detection rate. A total of 250 analyzable samples were obtained from the 79 cases with an average of 3.2 ± 1.2 samples per case. All samples were promptly sent to the laboratory and processed for frozen section and paraffin section analysis. An experienced pathologist (TWB) reviewed the slides and the results were made available to the surgeon within 30 minutes. An acute inflammation suggestive of infection was reported by the pathologist when more than five neutrophils in at least three high-power fields were seen on the frozen section slides (modified Mirra criteria) [22]. For the current study, a case was considered to be positive for PJI by frozen section when at least one of the samples demonstrated acute inflammation suggestive of infection. Diagnostic accuracy from frozen section criteria was calculated by comparing against the MSIS criteria.
Subsequently, success or failure of reimplantation was determined. A treatment failure was defined as a case in which reimplantation was aborted as a result of clinical, laboratory, or histological parameters indicating infection or a case that failed the reimplantation. The failure of reimplantation was defined using the criteria published after a Delphi based consensus [11]. Successful reimplantation was defined as (1) control of infection, as characterized by a healed wound without fistula, drainage, or pain and no infection recurrence caused by the same organism strain; (2) no subsequent surgical intervention for infection after reimplantation surgery; and (3) no occurrence of PJI-related mortality (by causes such as sepsis or necrotizing fasciitis). This information was obtained by one of two coauthors (JG, GK) from chart review or by phone interview when charts were incomplete. In the event of disagreement regarding the success of reimplantation between the two authors (JG, GK), another author (CHR) independently reviewed the patient information to reach consensus. This method allowed us to include all the presumed cases of persistent infection, the ones in which obvious infection precluded reimplantation and the ones in which the reimplantation failed perhaps as a result of an undetected infection at the time of reimplantation. Although MSIS criteria include sinus tract as a major criterion, this is usually excised at time of surgery and is assumed to be treated. Therefore, any further drainage or wound breakdown (included in the definition above) was considered as a failure of the reimplantation surgery rather than a preexisting condition.
Because this study compared MSIS criteria with frozen section analysis, the histological component of MSIS was not included in the modified MSIS criteria. Therefore, in this study, MSIS refers to the modified MSIS unless otherwise specified. The original/complete MSIS included histological component as a minor criteria with four (of six) minor criteria to be present to be considered positive for infection.
Statistical Analysis
Frozen section results were compared with permanent section and MSIS criteria as the reference standards to calculate sensitivity (true-positive/[false-negative + true-positive] and specificity (true-negative/[false-positives + true-negatives]. Also, sensitivities and specificities of both MSIS criteria and frozen section results in predicting treatment failure were determined. Because the sample size was small, knees and hips were not analyzed separately. Receiver operating characteristic (ROC) curves were used to compare the diagnostic accuracy of tests. Diagnostic accuracy of two tests was compared by testing the area under the ROC curves (AUC) with a test having a higher AUC performing better. Kaplan-Meier survival analysis and Cox proportional hazards model were used to evaluate the risk factors for failure of reimplantation. The threshold for statistical significance was p < 0.05. Statistical analysis was done using Stata statistical software, Version 12 (StataCorp, College Station, TX, USA).
Results
At the time of second-stage reimplantation surgery, frozen section is useful in ruling in infection where the specificity is 94% (95% confidence interval [CI], 89%–99%] and negative predictive value is 94% (CI, 91%–99%); however, there is less utility in ruling out infection, because sensitivity is only 50% (CI, 13%–88%) and positive predictive value is 50% (CI, 25%–86%; Table 1). The overall accuracy is 90% (CI, 84%–96%). Of the 250 samples from the 79 cases, 12 were positive for infection (n = 12 of 250 [5%]). In eight cases (n = eight of 79 [11%]), at least one frozen section sample was positive. There were no discrepancies between the results of frozen and permanent sections in all cases, thereby yielding 100% concordance. The modified MSIS criteria were positive in eight cases (n = eight of 79 [11%]; n = six of 38 [16%] knees and n = two of 41 [5%] hips). Overall, the frozen section analysis performed better in hips than in knees (hips versus knees: AUC = 0.962 ± 0.021 versus 0.651 ± 0.107, p = 0.004; Table 1).
Table 1.
Diagnostic accuracy of frozen section with MSIS as the reference standard
| Value | Combined (n = 79) | Knee revisions (n = 38) | Hip revisions (n = 41) |
|---|---|---|---|
| Sensitivity | 50% (13%–88%) | 33% (17%–67%) | 100% (100%–100%) |
| Specificity | 94% (89%–99%) | 97% (91%–100%) | 92% (82%–100%) |
| PPV | 50% (25%–86%) | 67% (33%–100%) | 40% (22%–100%) |
| NPV | 94% (91%–99%) | 89% (86%–94%) | 100% (100%–100%) |
| Accuracy | 90% (84%–96%) | 87% (79%–95%) | 93% (83%–100%) |
| AUC (± SE) | 0.722 ± 0.096 | 0.651 ± 0.107 | 0.962 ± 0.021 |
Ninety-five percent confidence intervals are shown in parentheses; MSIS = Musculoskeletal Tumor Society; PPV = positive predictive value; NPV = negative predictive value; AUC = area under the curve; SE = standard error.
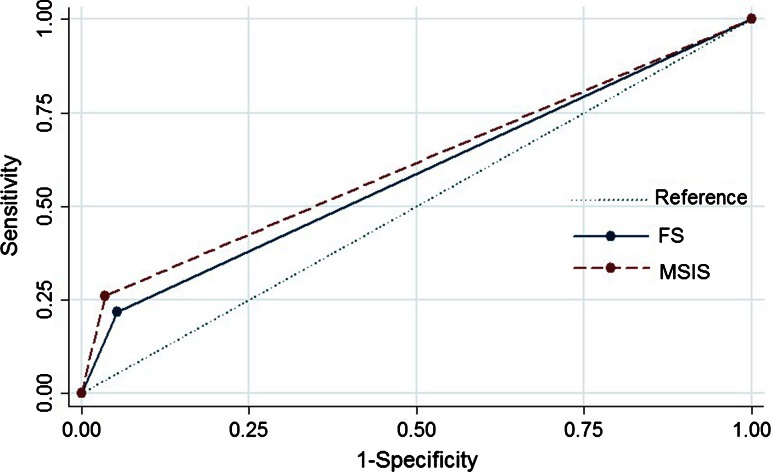
Both the MSIS criteria and frozen sections have high specificity for ruling in treatment failure (MSIS criteria specificity: 96% [CI, 91%–100%]; frozen section: 95% [CI, 88%–100%]), but screening capabilities are limited (MSIS sensitivity: 26% [CI, 9%–44%]; frozen section: 22% [CI, 9%–29%]). Both MSIS criteria and frozen section had reasonable positive (MSIS, 75% [44%–100%]; frozen section, 63% [33%–100%]) and negative predictive values (MSIS, 76% [72%–81%]; frozen section, 75% [71%–79%]; Table 2). The AUCs for MSIS criteria and frozen sections were not different and hence no difference in diagnostic value (MSIS versus frozen section: AUC = 0.613 ± 0.049 versus AUC = 0.582 ± 0.047, p = 0.554; Fig. 1). In this study 23 patients were classified as failures (seven as a result of aborted reimplantation and 16 resulting from failure of reimplantation, which are described later). In seven cases reimplantation was aborted. Of the 72 cases that underwent reimplantation, 16 (22.2%) patients had failure during the followup. The mean duration to failure of reimplantation was 24 ± 7 weeks. Failure of reimplantation was greater in revision TKA than in revision THA (n = 13 of 33 [39%] versus n = three of 39 [8%]; p = 0.002). The reason for failed reimplantation was another two-stage revision for infection (n = 11), persistent pain/wound drainage (n = 3), irrigation and débridement with polyethylene exchange (n = 1), and death from PJI-related sepsis (n = 1). Only five of the 16 failures had positive culture growth at the time of reimplantation. Of these, two patients subsequently developed failure with the same organism, two had a reinfection with a different organism, and in one case, no organism could be isolated at rerevision.
Table 2.
Accuracy of frozen section and MSIS in predicting failure
| Value | MSIS | Frozen section |
|---|---|---|
| Sensitivity | 26% (9%–44%) | 22% (9%–29%) |
| Specificity | 96% (91%–100%) | 95% (88%–100%) |
| PPV | 75% (44%–100%) | 63% (33%–100%) |
| NPV | 76% (72%–81%) | 75% (71%–79%) |
| Accuracy | 76% (70%–82%) | 73% (68%–80%) |
| AUC (± SE) | 0.613 ± 0.049 | 0.582 ± 0.047 |
Ninety-five percent confidence intervals are shown in parentheses; MSIS = Musculoskeletal Tumor Society; PPV = positive predictive value; NPV = negative predictive value; AUC = area under the curve; SE = standard error.
Fig. 1.
A ROC curve comparing MSIS criteria and frozen sections in predicting failure is shown. Both tests demonstrate similar diagnostic accuracy (DeLong test comparing AUCs, p = 0.554. FS = frozen section.
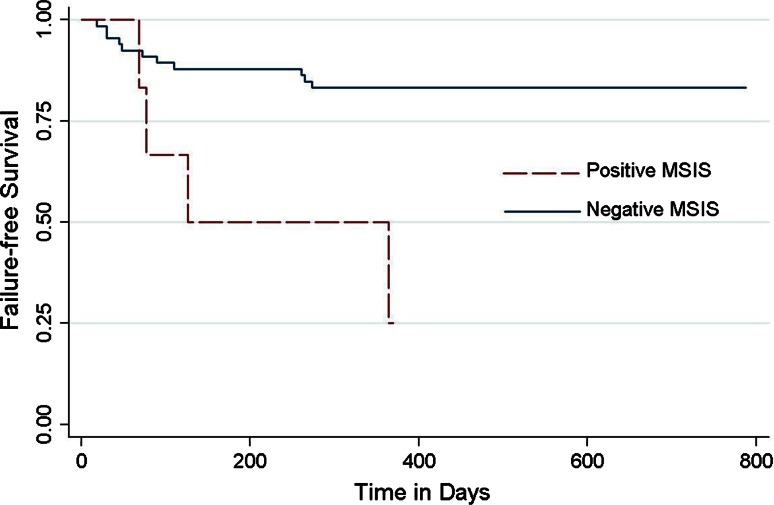
Positive MSIS criteria at the time of reimplantation was a risk factor for subsequent failure (hazard ratio [HR], 5.22 [1.64–16.62], p = 0.005; Fig. 2), whereas positive frozen section was not (HR, 1.16 [0.15–8.86], p = 0.883; Fig. 3). Of the 72 cases reimplanted, four had a positive frozen section and six had a positive MSIS. One patient with a positive frozen section (n = one of four [25%]) and four patients with a positive MSIS (n = four of six [67%]) failed during followup.
Fig. 2.
A Kaplan-Meier graph comparing the failure rates based on the MSIS criteria at the time of reimplantation is shown. There is increased risk of failure when reimplantation was performed in MSIS-positive patients, p = 0.002 (by log-rank test).
Fig. 3.
A Kaplan-Meier graph comparing the failure rates based on the frozen sections (FS) at the time of reimplantation is shown. There was no difference in failures rates based on FS, p = 0.883 (by log-rank test).
Intraoperative cultures from the periprosthetic tissue/synovial fluid were positive in 12 cases (n = 12 of 79 [15%]) at the time of the second stage, four of which had at least two positive cultures. Coagulase-negative Staphylococcus was the most common pathogen (n = five of 12) followed by Staphylococcus aureus (n = three of 12). When the original MSIS criteria were used, the sensitivity and specificity were 22% (CI, 9%–39%) and 96% (CI, 91%–100%), respectively (AUC = 0.591 ± 0.046). The AUCs for frozen section, modified MSIS, and original MSIS in predicting failure were not statistically different from each other (p = 0.596).
Discussion
Intraoperative frozen sections are routinely used to determine eradication of infection at the time of reimplantation [3, 16, 20]. Previous studies have compared the use of frozen sections using permanent section analysis or microbiological results [33]. However, no studies have assessed frozen section using MSIS criteria as a reference standard for diagnosing infection at the second stage of revision surgery. In our study, we compared frozen section results with MSIS criteria and evaluated whether any of these predicted treatment failure.
This study had a number of limitations. The most important limitation of our study is its short followup. Although most failures tended to occur within the first year after reimplantation, infections could recur after many years of apparently successful treatment. Because Clinical Orthopaedics and Related Research ® generally does not publish reconstructive series with less than 2 years of followup, this article’s estimates on diagnostic accuracies in predicting failure should be interpreted with caution as they could be inflated as a result of the shorter followup. For example, the sensitivities of frozen section (22%) and MSIS (26%) in predicting failure could diminish further as more infections develop in the long term. This study is also limited by the fact that there is no agreement about the gold standard to confirm the presence or absence of infection. Although the Delphi criterion is a reasonable approach for defining the failure of treatment, it is not without limitations. It is partly subjective and the criterion pertaining to healed wound could potentially overlap with sinus tract of the MSIS criteria, although we assume the second-stage surgery would have treated it. Another limitation of the study is the low sample size, especially when THA and TKA revisions were studied separately, because they result in larger confidence intervals of the diagnostic parameters limiting their reproducibility. All components of MSIS are not routinely available limiting the assessment of those patients. When interpreting the use of MSIS criteria and frozen section analysis in predicting failure, it should be noted that information from frozen section and components of MSIS criteria would have influenced the treatment decision in those cases in which reimplantation was deferred. Also the postoperative antibiotic regimens might have been different among the cases and its influence on failure rates was not accounted for in this study. It is also possible that the patients not included as a result of lack of followup might have been reinfected and treated at outside facilities, in which case the estimates might have been altered. Finally, this study done with an expert pathologist may not be transferrable to the community pathologist and thus the ability to rely on this test may be affected.
Our results show that frozen sections performed reasonably well at the second stage of revision with a high specificity when compared with MSIS criteria, although it had low sensitivity. The use of frozen sections has a high variability for the diagnosis of infection depending on the study design and patient population [33]. The two studies analyzing frozen section during reimplantation reported similar results as our study in terms of sensitivity and specificity, although the reference standards in those studies were cultures with or without permanent sections [3, 10]. This similarity is perhaps explained by the fact that bacterial cultures are a major part of the MSIS criteria. Bori et al. [3] also showed that when the Athanasou criterion was used (in which the number of neutrophils required for the diagnosis of infection is low), the sensitivity increased at the expense of specificity [1, 3]. False-positive frozen section results could potentially arise in patients with inflammatory arthropathies or periprosthetic fracture, thereby making conclusions from frozen section difficult in such scenarios. The disparity of results in hips and knees is difficult to interpret from the small sample size and the present lack of literature comparing tests between joints. A possible explanation for the difference could be the lower infection prevalence in the hips and/or the nature of sampling of the joints. Also, the use of dynamic spacers in hips may result in more debris, which could accentuate the inflammation seen in frozen sections.
Both MSIS criteria and frozen section had poor sensitivity in predicting treatment failure, although both demonstrated high specificity. The accuracies of both these tests were comparable in predicting treatment failure. Because the followup of the current study is short, it is important to recognize that the low sensitivities of frozen section and MSIS could decrease even further as more latent infections might unveil with longer followup. This highlights the importance of continuing to develop diagnostic strategies for this group of patients, in whom failures can be limb-threatening. We used Delphi criteria, which comprise a multitude of factors along with aborted reimplantation to define failure, whereas most other studies have considered failure as a requirement of subsequent surgery [11, 26]. The reported rate of reinfection varies from 10% to 25% for the knees [12, 14, 17, 23] and is slightly lower for the hips at 7% to 12% [6, 13, 16, 29]. When the histological component was also included in the MSIS criteria, the results did not change further. This is explained by the fact that with the addition of a histological component, four criteria were required to be met as opposed to three. Persistent infection at the time of reimplantation can be indolent and may produce little changes in the laboratory parameters. Therefore, cutoff values or the number of criteria required for infection might need to be lower.
Reimplantation of a new prosthesis to a joint, which fulfilled the MSIS criteria, increased the risk of failure. Therefore, we conclude that the MSIS criteria should guide decision-making during reimplantation. However, the cultures are generally taken only at the time of surgery, limiting the intraoperative applicability of MSIS criteria. Moreover, a positive frozen section at the time of reimplantation failed to increase the risk of failure if reimplantation was performed. In the current practice when results of frozen section guide the intraoperative decision to proceed with reimplantation, it is noteworthy that reimplantation is usually aborted after a positive frozen section unless the surgeon had reasons to believe the contrary. Therefore, the cases that were reimplanted despite a positive frozen section might have had features favoring clearance of infection or a false-positive frozen section result. Although our results support the use of MSIS criteria at reimplantation, the decision to defer reimplantation in cases with positive MSIS criteria can only be definitively evaluated by well-designed prospective studies.
In view of the low sensitivity of MSIS criteria and their inherent limitations, better tests should be considered to identify control of infection before the second stage of revision. A number of novel tests detecting alpha defensin, synovial CRP, and synovial fluid leukocyte esterase among other biomarkers have been successfully described for the diagnosis of PJI [8, 9]. These tests have been compared with different reference standards including MSIS criteria [5, 27, 31, 32]. However, none of the studies have specifically evaluated the second stage of revision arthroplasty. Therefore, further studies must be conducted to establish guidelines for infection control at the time of reimplantation and develop accurate diagnostic tests for the same. Both frozen sections and modified MSIS criteria at the time of reimplantation were comparable in predicting failure. Performing reimplantation in a joint that is positive for infection by MSIS criteria certainly increases the risk for failure. Therefore, both frozen sections and MSIS criteria are recommended for decision-making at the time of reimplantation surgery.
Acknowledgments
We thank Yaxia Zhang MD, PhD, for all the help with compiling results of frozen sections.
Footnotes
One author (WB) has received research support in the amount of USD 100,001 to USD 1,000,000 from grants from Stryker (Kalamazoo, MI, USA) and Zimmer (Warsaw, IN, USA); personal fees from Shukla Medical (Piscataway, NJ, USA), Wright Medical Technology (Memphis, TN, USA), Exactech (Gainesville, FL, USA), and KEF Healthcare (Westminster, CO, USA); other from OtisMed Corporation (Alameda, CA, USA), Custom Orthopaedic Solution (Cleveland, OH, USA), and iVHR (Cleveland, OH, USA); grants from DJO (Vista, CA, USA), Active Implants (Memphis, TN, USA), The Medicines Company (Parsippany-Troy Hills, NJ, USA), the State of Ohio (Columbus, OH, USA), Orthovita (Malvern, PA, USA), CoolSystems (Alameda, CA, USA), Orthopaedic Research and Education Foundation (Rosemont, IL, USA), and Salient Surgical Technologies (Portsmouth, NH, USA) outside the submitted work. One of the authors (CHR) received research support funding in the amount of USD 100,001 to USD 1,000,000 from Stryker (Kalamazoo, MI, USA) and KCI (San Antonio, TX, USA) outside the submitted work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Athanasou NA, Pandey R, de Steiger R, Crook D, Smith PM. Diagnosis of infection by frozen section during revision arthroplasty. J Bone Joint Surg Br. 1995;77:28–33. [PubMed] [Google Scholar]
- 2.Bauer TW, Parvizi J, Kobayashi N, Krebs V. Diagnosis of periprosthetic infection. J Bone Joint Surg Am. 2006;88:869–882. doi: 10.2106/JBJS.E.01149. [DOI] [PubMed] [Google Scholar]
- 3.Bori G, Soriano A, García S, Mallofré C, Riba J, Mensa J. Usefulness of histological analysis for predicting the presence of microorganisms at the time of reimplantation after hip resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 2007;89:1232–1237. doi: 10.2106/JBJS.F.00741. [DOI] [PubMed] [Google Scholar]
- 4.Browne JA, Bechtold CD, Berry DJ, Hanssen AD, Lewallen DG. Failed metal-on-metal hip arthroplasties: a spectrum of clinical presentations and operative findings. Clin Orthop Relat Res. 2010;468:2313–2320. doi: 10.1007/s11999-010-1419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buttaro MA, Martorell G, Quinteros M, Comba F, Zanotti G, Piccaluga F. Intraoperative synovial C-reactive protein is as useful as frozen section to detect periprosthetic hip infection. Clin Orthop Relat Res. 2015;473:3876–3881. doi: 10.1007/s11999-015-4340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S-Y, Hu C-C, Chen C-C, Chang Y-H, Hsieh P-H. Two-stage revision arthroplasty for periprosthetic hip infection: mean follow-up of ten years. Biomed Res Int. 2015;2015:345475. doi: 10.1155/2015/345475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chimento GF, Finger S, Barrack RL. Gram stain detection of infection during revision arthroplasty. J Bone Joint Surg Br. 1996;78:838–839. [PubMed] [Google Scholar]
- 8.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96:1439–1445. doi: 10.2106/JBJS.M.01316. [DOI] [PubMed] [Google Scholar]
- 9.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472:3254–3262. doi: 10.1007/s11999-014-3543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Della Valle CJ, Bogner E, Desai P, Lonner JH, Adler E, Zuckerman JD, Di Cesare PE. Analysis of frozen sections of intraoperative specimens obtained at the time of reoperation after hip or knee resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 1999;81:684–689. doi: 10.2106/00004623-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res. 2013;471:2374–2382. doi: 10.1007/s11999-013-2866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghanem E, Azzam K, Seeley M, Joshi A, Parvizi J. Staged revision for knee arthroplasty infection: what is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009;467:1699–1705. doi: 10.1007/s11999-009-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haddad FS, Muirhead-Allwood SK, Manktelow AR, Bacarese-Hamilton I. Two-stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg Br. 2000;82:689–694. doi: 10.1302/0301-620X.82B5.9668. [DOI] [PubMed] [Google Scholar]
- 14.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 15.Johnson AJ, Zywiel MG, Stroh DA, Marker DR, Mont MA. Should gram stains have a role in diagnosing hip arthroplasty infections? Clin Orthop Relat Res. 2010;468:2387–2391. doi: 10.1007/s11999-009-1216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraay MJ, Goldberg VM, Fitzgerald SJ, Salata MJ. Cementless two-staged total hip arthroplasty for deep periprosthetic infection. Clin Orthop Relat Res. 2005;441:243–249. doi: 10.1097/01.blo.0000194312.97098.0a. [DOI] [PubMed] [Google Scholar]
- 17.Kurd MF, Ghanem E, Steinbrecher J, Parvizi J. Two-stage exchange knee arthroplasty: does resistance of the infecting organism influence the outcome? Clin Orthop Relat Res. 2010;468:2060–2066. doi: 10.1007/s11999-010-1296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusuma SK, Ward J, Jacofsky M, Sporer SM, Della Valle CJ. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res. 2011;469:1002–1008. doi: 10.1007/s11999-010-1619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malekzadeh D, Osmon DR, Lahr BD, Hanssen AD, Berbari EF. Prior use of antimicrobial therapy is a risk factor for culture-negative prosthetic joint infection. Clin Orthop Relat Res. 2010;468:2039–2045. doi: 10.1007/s11999-010-1338-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGrory BJ, Becker MW. Screening for infection to determine treatment in second-stage reimplantation of total knee prostheses. J Bone Joint Surg Am. 2001;83:1433. doi: 10.2106/00004623-200109000-00032. [DOI] [PubMed] [Google Scholar]
- 21.Mikhael MM, Hanssen AD, Sierra RJ. Failure of metal-on-metal total hip arthroplasty mimicking hip infection. A report of two cases. J Bone Joint Surg Am. 2009;91:443–446. doi: 10.2106/JBJS.H.00603. [DOI] [PubMed] [Google Scholar]
- 22.Mirra JM, Marder RA, Amstutz HC. The pathology of failed total joint arthroplasty. Clin Orthop Relat Res. 1982;170:175–183. [PubMed] [Google Scholar]
- 23.Mittal Y, Fehring TK, Hanssen A, Marculescu C, Odum SM, Osmon D. Two-stage reimplantation for periprosthetic knee infection involving resistant organisms. J Bone Joint Surg Am. 2007;89:1227–1231. doi: 10.2106/JBJS.E.01192. [DOI] [PubMed] [Google Scholar]
- 24.Mont MA, Waldman BJ, Hungerford DS. Evaluation of preoperative cultures before second-stage reimplantation of a total knee prosthesis complicated by infection. A comparison-group study. J Bone Joint Surg Am. 2000;82:1552–1557. doi: 10.2106/00004623-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Moore GA, Hill MV, Kuo YF, Stephenson K, Lindsey RW. First Place: The utility of frozen sections in hip and knee reimplantation surgery. Curr Orthop Pract. 2015;26:332–335. doi: 10.1097/BCO.0000000000000244. [DOI] [Google Scholar]
- 26.Mortazavi SMJ, Vegari D, Ho A, Zmistowski B, Parvizi J. Two-stage exchange arthroplasty for infected total knee arthroplasty: predictors of failure. Clin Orthop Relat Res. 2011;469:3049–3054. doi: 10.1007/s11999-011-2030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parvizi J, McKenzie JC, Cashman JP. Diagnosis of periprosthetic joint infection using synovial C-reactive protein. J Arthroplasty. 2012;27:12–16. doi: 10.1016/j.arth.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469:2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez-Sotelo J, Berry DJ, Hanssen AD, Cabanela ME. Midterm to long-term followup of staged reimplantation for infected hip arthroplasty. Clin Orthop Relat Res. 2009;467:219–224. doi: 10.1007/s11999-008-0480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683. doi: 10.2106/00004623-199905000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Tetreault MW, Wetters NG, Moric M, Gross CE, Della Valle CJ. Is synovial C-reactive protein a useful marker for periprosthetic joint infection? Clin Orthop Relat Res. 2014;472:3997–4003. doi: 10.1007/s11999-014-3828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tischler EH, Cavanaugh PK, Parvizi J. Leukocyte esterase strip test: matched for Musculoskeletal Infection Society criteria. J Bone Joint Surg Am. 2014;96:1917–1920. doi: 10.2106/JBJS.M.01591. [DOI] [PubMed] [Google Scholar]
- 33.Tsaras G, Maduka-Ezeh A, Inwards CY, Mabry T, Erwin PJ, Murad MH, Montori VM, West CP, Osmon DR, Berbari EF. Utility of intraoperative frozen section histopathology in the diagnosis of periprosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2012;94:1700–1711. doi: 10.2106/JBJS.J.00756. [DOI] [PubMed] [Google Scholar]