Abstract
Background
Diagnosing periprosthetic joint infection (PJI) requires a combination of clinical and laboratory parameters, which may be expensive and difficult to interpret. Synovial fluid cytokines have been shown to accurately differentiate septic from aseptic failed total knee (TKA) and hip (THA) arthroplasties. However, after first-stage explantation, there is still no reliable test to rule out PJI before a second-stage reimplantation procedure.
Questions/purposes
(1) Which synovial fluid cytokines have the highest diagnostic accuracy for PJI? (2) Which cytokine shows the greatest decrease associated with the resolution of infection in the same patient between explantation and subsequent reimplantation of an infected arthroplasty? (3) What is the accuracy of synovial fluid cytokines and the Musculoskeletal Infection Society (MSIS) criteria to rule out PJI after first-stage explantation? (4) What are the most studied synovial fluid cytokines for diagnosing PJI as reported in the literature and what are their cumulative diagnostic accuracy?
Methods
Between May 2013 and March 2014, 104 patients with painful THA and TKA evaluated for possible PJI were included in our study. Of these, 90 (87%) had cytokine levels measured from synovial fluid samples collected as part of this prospective study (n = 33 hips, n = 57 knees). A second group of 35 patients (n = 36 samples) who presented during the same time period with an antibiotic spacer also had synovial cytokines measured before second-stage reimplantation. For the first group of 90 patients, the MSIS definition classified each joint at the time of surgery as infected (n = 31) or not infected (n = 59) and was used as the standard to test the accuracy in diagnosing PJI. Of the 35 patients with synovial marker data before second-stage surgery, 15 patients had cytokine measurements both at explantation and reimplantation and were used to quantify the change between stages. The reimplantation group had a minimum 1-year followup (with four [11%] patients lost to followup) and was classified into successful or failed treatment based on Delphi-based consensus data and was used to test the accuracy in detecting infection resolution at reimplantation.
Results
Interleukin (IL)-1β and interferon-γ demonstrated the highest diagnostic utility (area under the curve 0.92, 0.91, respectively); IL-1β and IL-6 had the highest sensitivities (0.90 [95% confidence interval {CI}, 0.74–0.98] and 0.81 [0.63–0.93]). As a measure of infection resolution, IL-1β had the greatest decrease (12.4-fold; level at explantation: 232.4 [range, 23.1–1545.7]; level at reimplantation: 18.8 (range 1.2–298.9); mean difference: 325.5 [95% CI, 65.0–596.0]; p = 0.0001), and IL-6 had a nearly similar decrease (11.2-fold; level at explantation: 228.1 [range, 10,158.4–182,725.0]; level at reimplantation: 2518.2 [range, 10.4–41,319.3]; mean difference: 33,176.0 [95% CI, 7543.6–58,808.3]; p < 0.0001). Cytokines and MSIS criteria had low sensitivity to rule out infection in a joint treated for PJI.
Conclusions
IL-6 and IL-1β demonstrated high sensitivities to diagnose PJI and showed the greatest decrease between first and second stages, which may potentially be used to monitor treatment response to PJI. However, cytokines and MSIS criteria had low sensitivity to rule out infection before reimplantation.
Level of Evidence
Level III, diagnostic study.
Electronic supplementary material
The online version of this article (doi:10.1007/s11999-016-4710-x) contains supplementary material, which is available to authorized users.
Introduction
Current guidelines to diagnose periprosthetic joint infection (PJI) have been proposed by the Musculoskeletal Infection Society (MSIS) criteria and require a combination of serum, synovial fluid, and histologic parameters [14]. Results can often be difficult to interpret, requiring a complex clinical algorithm to determine a diagnosis [22], which may be incorrect in up to 10% of cases of presumed aseptic joints [19]. It is not only challenging to diagnose infection, but also to determine if infection is adequately controlled after antibiotic spacer placement and is deemed ready for reimplantation. As a result of the elevated cost, time expenditure, and often lack of a specialized pathology service and/or standardized methods, orthopaedic surgeons are faced with diagnostic dilemmas when the recommended PJI criteria cannot be fulfilled.
Synovial fluid inflammatory markers have long been sought as an alternative to the current methods of diagnosis as a result of the low cost, simple interpretation, and accurate results associated with their use. Several studies have reported the clinical utility of synovial fluid inflammatory biomarkers in diagnosing PJI [4, 6, 9–12, 17]. Although inflammatory responses can also be elicited by aseptic conditions, Deirmengian et al. [7] demonstrated that a specific genomic response by neutrophils is associated with an infectious etiology. Thus, the assessment of certain inflammatory biomarkers within the synovial fluid has proven to be not only sensitive, but also highly specific to PJI. Synovial fluid biomarkers shown to have the highest diagnostic accuracy include α-defensin, C-reactive protein (CRP) and interleukins (IL)-1α, IL-1β, IL-6, and IL-8 [4, 6, 9–12]. Nevertheless, no formal systematic review has been conducted on this topic. Although synovial fluid cytokines have shown high diagnostic accuracy for detecting PJI, they have not been studied as potential markers for monitoring treatment response. There are currently no reports on the value of synovial fluid biomarkers to monitor treatment response after a first-stage explantation or to determine the optimal timing for second-stage reimplantation. We therefore asked: (1) Which synovial fluid cytokines have the highest diagnostic accuracy for PJI? (2) Which cytokine shows the greatest decrease associated with the resolution of infection in the same patient between explantation and subsequent reimplantation of an infected arthroplasty? (3) What is the accuracy of synovial fluid cytokines and the MSIS criteria to rule out PJI after first-stage explantation? (4) What are the most studied synovial fluid cytokines for diagnosing PJI as reported in the literature and what are their cumulative diagnostic accuracy?
Patients and Methods
The institutional review board approved this study and informed consent was obtained from all patients preoperatively for the collection of synovial fluid cytokines. Patients of five adult reconstruction fellowship-trained orthopaedic surgeons (WKB, CAH, RM, VK, TM) from a single academic institution were prospectively enrolled between May 2013 and March 2014. Inclusion criteria for a first subset of patients encompassed (1) evaluation for painful THA or TKA with minimum symptom duration of 30 days; and (2) sufficient synovial fluid sample for the MSIS criterion components and for cytokine assessment. Because of the increased difficulty in differentiating chronic PJI from aseptic failure, only patients with chronic symptoms (ie, more than 30 days) were included in this study. Patients who received preoperative antibiotics and patients with systemic inflammatory disease were not excluded. Exclusion criteria encompassed patients with coexisting metallosis and those who underwent muscle repair without component alteration. Synovial fluid from a second subset of patients undergoing second-stage reimplantation was also collected during the study period. This was done to investigate diagnostic parameters capable of detecting eradication of infection in a joint previously diagnosed with PJI.
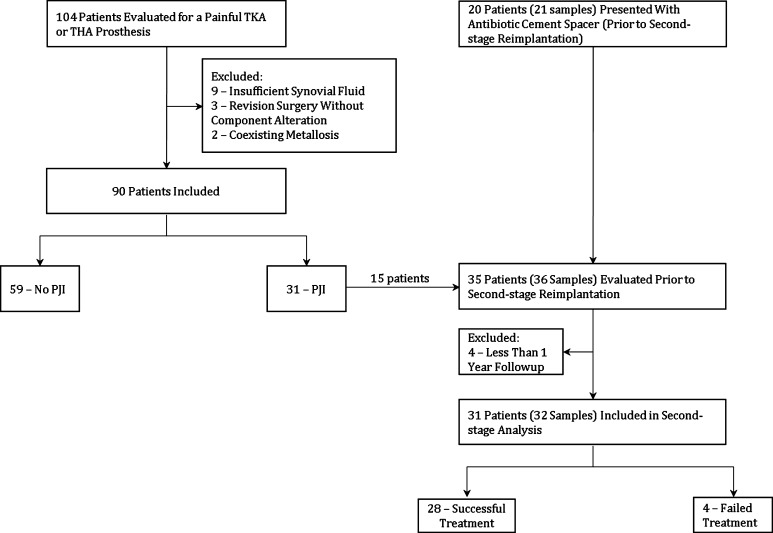
Between May 2013 and March 2014, 104 patients with painful THA or TKA were screened for enrollment. Patients with insufficient intraoperative fluid samples (n = 9), coexisting metallosis (n = 2), and revision surgery without component alteration (ie, muscle repairs and superficial wound drainages) (n = 3) were excluded. The remaining 90 patients were enrolled (Fig. 1). A second group of 36 patients (reimplantation group) also presenting between May 2013 and March 2014 with antibiotic cement spacer placed for previous PJI were also enrolled in the study before their second-stage reimplantation. All 36 patients had sufficient intraoperative synovial fluid samples to be included in the study.
Fig. 1.
Flowchart depicting the 90 patients evaluated for PJI using the MSIS criteria and the 35 patients evaluated for successful or failed treatment at 1 year.
At the time of surgery, the MSIS criteria were prospectively applied to classify the first subset of patients as infected or noninfected. Of the 90 patients with a painful prosthesis, 59 were deemed noninfected and 31 deemed infected. Synovial fluid analysis and MSIS criteria collection were done separately as to prevent bias. The researchers investigating MSIS criteria had no knowledge of the synovial fluid results. Thresholds for detecting infection and the diagnostic characteristics for each cytokine were determined. Cytokine thresholds for infection found in the first subset of patients (infected versus noninfected patients) were applied for the second subset (reimplantation group). Treatment success or failure after a minimum of 1-year followup was the reference standard for the reimplantation group. Treatment failure was determined as described by Diaz-Ledezma et al. [8] as (1) subsequent surgical intervention for infection after the index procedure (ie, second-stage reimplantation); (2) persistent fistula, drainage, or joint pain at last followup; and (3) occurrence of PJI-related mortality. Any unresolved drainage at the last followup visit was considered a failure. Pain was considered a failure only when it was severely debilitating and progressive since surgery. Patients who had less than 1-year followup (n = 4) were excluded from the reimplantation group. One patient died for causes not related to infection at 8 months and three patients were lost to followup before 1 year. Of the 31 patients (32 synovial fluid samples) included in the reimplantation group, four met criteria for treatment failure. Two knees underwent further incision and débridement at 8 and 25 days, and one hip and one knee underwent a repeat two-stage revision at 18 days and 12 months. In all four of those failures, the infecting organism was the same as the original infection. The remaining 27 patients (29 samples) were deemed successful (Fig. 1).
As a general rule, patients who did not have PJI were treated with single-stage revisions. Patients with chronic PJI were treated with component explantation and placement of an antibiotic spacer. Articulated spacers were used for hip PJI, whereas static spacers were used for knee PJI. Reimplantation was only performed after the full course of intravenous antibiotics, absence of clinical symptoms, normalization of CRP and erythrocyte sedimentation rate, and after negative cultures from aspirates. Demographic, clinical, and surgical data, including isolated organisms, were recorded. A formal power analysis for sample size calculation was not performed for this study given the lack of data concerning the cytokines evaluated in this study.
The group of patients without PJI was composed of 41 women and 18 men with a mean age and body mass index of 65 years (range, 40–89 years) and 33 kg/m2 (range, 17–54 kg/m2), respectively. There were 22 failed THAs and 37 failed TKAs. Reasons for failure included 26 patients with aseptic loosening, 17 with instability, five with polyethylene wear, three with patellar maltracking, two recurrent dislocations, two with periprosthetic fracture, one with osteolysis, one with heterotopic ossification, one as a result of squeaking, and the last one resulting from presumed infection despite negative MSIS criteria. This last patient had mildly elevated synovial fluid cell counts (1916 white blood cells with 54% polymorphonuclear leukocytes) associated with a positive frozen section. Although the patient was MSIS-negative and had negative cultures, clinical judgment favored infection with a low-virulence pathogen and thus it was elected to proceed with first-stage explantation. Two patients had one positive intraoperative culture, including one coagulase-negative Staphylococcus and one Micrococcus spp. Both results were considered to be false-positives. Six patients (10.2%) had concomitant inflammatory disease, including rheumatoid arthritis (n = 2), systemic lupus erythematosus (n = 2), and polymyalgia rheumatica (n = 2). Eight patients (14%) were receiving systemic immunomodulators and five (8.5%) were receiving antibiotics at the time of diagnostic aspiration. Antibiotic treatment for the five patients at the time of aspiration was related to positive urinary cultures and/or urinary symptoms (n = 2), rosacea (n = 1), and chronic diarrhea with stool culture positive for Aeromonas spp (n = 1). One patient had died 5 months after surgery as a result of causes not related to infection. Three patients had less than 1-year followup postoperatively. These four patients (7%) were excluded from the analysis. For all remaining patients (n = 55), after minimum followup of 1 year (mean, 38 months; range, 13–61 months), none showed any signs of recurrence of the infection.
In the group of patients with PJI, there were 10 women and 21 men with a mean age and body mass index of 63 years (range, 35–86 years) and 33 kg/m2 (range, 21–54 kg/m2), respectively. Eleven patients had a failed THA and 20 a failed TKA. All but one patient was considered infected preoperatively and underwent a first-stage explantation. Twenty-four patients were culture-positive and seven were culture-negative. Isolated pathogens included coagulase-negative Staphylococcus (n = 5), methicillin-resistant Staphylococcus aureus (n = 4), methicillin-sensitive S aureus (n = 2), Streptococcus viridans (n = 2), Propionibacterium acnes (n = 1), Propionibacterium granulosum (n = 1), Candida albicans (n = 1), Candida glabrata (n = 1), Candida tropicalis (n = 1), Staphylococcus lugdunensis (n = 1), Bacteroides thetaiotaomicron (n = 1), Diphtheroid bacilli (n = 1), Enterococcus faecium (n = 1), Enterococcus faecalis (n = 1), and Cladosporium species (n = 1). Two patients (6.5%) also had concomitant inflammatory disease (hepatitis C for both) and six were receiving systemic immunomodulators (19%). Seven patients were receiving antibiotics at the time of aspiration with a mean treatment duration of 75 days (range, 45–120 days). Only one patient underwent a single-stage revision instead of a first-stage explantation. This patient did not have any sign or symptom of infection preoperatively and was operated on as a result of a diagnosis of recurrent dislocations. However, two independent intraoperative cultures came back positive for E faecalis, yielding positive MSIS criteria. The patient was treated with 6 weeks of intravenous ampicillin and subsequent oral suppression with amoxicillin-clavulanic acid. After a 16-month followup, the patient remains asymptomatic.
The reimplantation group was composed of 32 synovial fluid samples from 31 eligible patients. Fifteen of those patients were included in the study in both first-stage explantation and second-stage reimplantation (Fig. 1). Overall, there were seven women and 24 men. Mean age and body mass index were 62 years (range, 35–80 years) and 33 kg/m2 (range, 22–66 kg/m2), respectively. There were 17 hip reimplantations and 15 knee reimplantations. One patient (3%) had a diagnosis of rheumatoid arthritis and five were receiving immunomodulators at the time of reimplantation (14%). Mean time between the explantation and reimplantation was 13.8 weeks (range, 8–58 weeks). Before reimplantation, all patients completed a full course of intravenous antibiotics for a mean of 6 weeks (range, 3–16 weeks). Nine patients (29%) had positive intraoperative cultures including coagulase-negative Staphylococcus (n = 7), P acnes (n = 1), and C albicans (n = 1). Four of those nine patients were considered to be false-positives and the remaining five were chronically suppressed with oral antibiotics. Another nine patients who were culture-negative were also chronically suppressed as a result of a high risk of recurrence of the infection based on clinical judgment. Mean followup for the reimplantation group was 17 months (range, 12–23 months). Four patients (13%) underwent further surgical intervention as a result of infection caused by the same infecting organism as the original PJI and thus met criteria for treatment failure. Organisms isolated in those four cases were coagulase-negative Staphylococcus species (n = 2), Group B Streptococcus species (n = 1) and methicillin-resistant S aureus (n = 1).
Synovial Fluid Analysis
For each operative case, synovial fluid was obtained intraoperatively for culture and cytokine analysis before arthrotomy through direct needle aspiration to mimic preoperative conditions and to avoid contamination with blood. Periprosthetic tissue specimens were obtained from soft tissue samples along the bone-implant or cement-implant interface, deep capsular or pseudocapsular tissue, synovial lining, and intramedullary tissue during stem removal. Intraoperative antibiotics were given after obtaining the synovial fluid and tissue samples. Fluid specimens were placed into anaerobic fluid vials (BBLTM Port-A-CulTM; Becton, Dickinson & Co, Sparks, MD, USA), whereas tissue specimens were transported in sterile containers and processed within 3 hours of collection. For aerobic bacterial cultures, the following media were inoculated: blood agar plate, chocolate agar plate, and thioglycollate broth. Cultures were read daily and routinely held for a 48-hour incubation period at 35°C in 5% CO2. Specimens submitted for anaerobic culture were planted on CDC and BBE agars and held for 5 days in anaerobic jars at 35°C. Tissue specimens were also sent for frozen section histologic analysis with five or more polymorphonuclear leukocytes in each of five or more high-power fields (×400) used as the institutional criteria for acute inflammation consistent with infection.
Synovial fluid for cytokine testing was placed in a BD VacutainerTM test tube containing 7.2 mg of EDTA. Samples were centrifuged at 2000 rpm for 10 minutes within 2 hours of collection to remove all cellular and particulate content. The resulting supernatant was divided into aliquots and stored in a −80°C freezer until samples were sent for testing. Cytokines evaluated in this study included IL-1, IL-2, IL-6, IL-8, IL-10, IL-12p70, interferon-γ, granulocyte-macrophage colony stimulating factor, and tumor necrosis factor-α. Cytokine levels in synovial fluid were measured using the Human Proinflammatory Ultra-Sensitive Kit on the MesoScale Discovery Multi-Array platform (MesoScale Discovery [MSD], Rockville, MD, USA). This assay is a multiplex cytokine immunoassay that measures cytokines in a plate-based format using electrochemiluminescent detection [3]. The assay was modified for use with synovial fluid as follows: 25 μL of a 1:1 or 1:10 dilution of synovial fluid with kit diluent buffer was added to preblocked plates and incubated at room temperature for 2 hours with vigorous shaking. After a wash step, 25 μL of conjugated detection antibody was added to each well and incubated for 2 hours at room temperature. After a final wash step, 150 μL of MSD Read Buffer was added to each well, and plates were read using the MSD Sector Imager 2400. Raw data were analyzed using the Discovery Workbench 3.0 software (MSD) and four-parameter logistic curve fitting was used to generate results. Assay performance was validated according to CLSI standards [23].
Statistical Analysis
To answer question 1, diagnostic accuracy for each cytokine was assessed by categorizing patients into an infection group or no infection group with the MSIS criteria as the reference standard for PJI. For each cytokine, threshold, sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were determined using a receiver operating characteristic curve (ROC) and Youden’s criterion. ROC curves were summarized using area under the curve (AUC) calculations with AUC values above 0.9 indicating excellent diagnostic strength.
To address question 2, the decrease in synovial fluid cytokine levels between first-stage explantation and second-stage reimplantation for the 15 patients who had synovial fluid measured at both stages of the two-stage revision, we used the Wilcoxon signed-rank test.
For question 3, the thresholds obtained from question 1 were applied to the reimplantation group. We calculated sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios for each cytokine and MSIS criterion measured against successful or failed treatment at 1 year as the reference standard as described by Diaz-Ledezma et al. [8]. If a cytokine level was below the inferior detection limit for the assay, the lowest reportable value was used. If a cytokine value was above the superior detection limit, the sample was diluted and the value corrected.
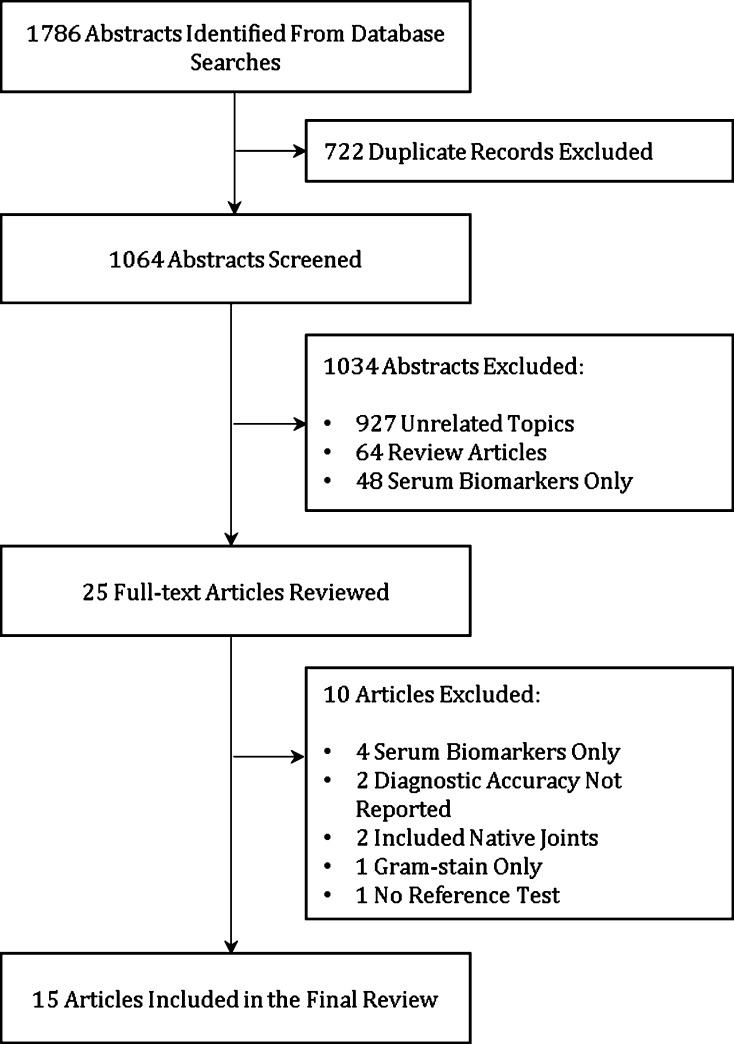
For question 4, a literature search was performed on May 2014 using the following individual search terms: total knee arthroplasty, total hip arthroplasty, total knee replacement, total hip replacement, hip prosthesis, knee prosthesis, prosthesis-related infection, bacterial infection, revision arthroplasty, sensitivity, specificity, predictive value, diagnosis, preoperative, periprosthetic joint infection, prosthetic infection, failed total joint arthroplasty, inflammatory cytokines, inflammatory peptides, synovial fluid, interleukin, and biomarkers. The following databases were searched: MEDLINE (n = 357 abstracts), EMBASE (n = 399 abstracts), Cochrane (n = 192 abstracts), Scopus (n = 53 abstracts), Biosis Citation Index (n = 12), Conference Proceedings Index (n = 1 abstract), and Science Citation Index (n = 50 abstracts). Exclusion criteria for our search encompassed (1) study population other than revision knee or hip arthroplasty; (2) studies that did not include a reference test; (3) studies that did not report diagnostic accuracy as both sensitivity and specificity or as likelihood ratios; and (4) studies examining serum biomarkers only. A total of 1064 abstracts were screened by two independent reviewers (MBPS, AS) and of those, 29 underwent full-text review. After appropriate exclusion criteria were applied, 15 articles were included in the final review [1, 4–6, 9–13, 15–18, 20, 21] (Fig. 2). The cumulative two-by-two tables were constructed for each cytokine and sensitivity, specificity, positive and negative predictive values, and accuracy were calculated for the ones that were most studied.
Fig. 2.
Flowchart depicting systematic review of the literature for studies including diagnostic parameters of synovial fluid inflammatory markers for PJI.
All analyses were done using R software (Version 3.0.2; Vienna, Austria). A significance level of 5% was used for all testing.
Results
Two cytokines, IL-1β and interferon interferon (IFN)-γ, had excellent diagnostic strength with AUC of 0.92 and 0.91, respectively. Sensitivity and specificity was 90.3% (95% confidence interval [CI], 74%–98%) and 87% (95% CI, 76%–95%) for IL-1β and 73% (95% CI, 52%–88%) and 96% (95% CI, 87%–99.5%) for IFN-γ. All other cytokines, including IL-6, tumor necrosis factor-α, IL-12p70, IL-8, IL-10, granulocyte-macrophage colony stimulating factor (GM-CSF), and IL-2, had good diagnostic strength with AUC ranging from 0.8 to 0.89 (Table 1; Appendix 1 [Supplemental materials are available with the online version of CORR ®.]).
Table 1.
Cutoff, sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios are listed for all nine biomarkers
| Cytokine | Cutoff (pg/mL) | Sensitivity | Specificity | PPV | NPV | LR+ | LR− | AUC |
|---|---|---|---|---|---|---|---|---|
| IL-1β | 8.26 | 0.90 (0.74–0.98) | 0.87 (0.76–0.95) | 0.8 | 0.94 | 7.1 | 0.11 | 0.92 |
| IFN-γ | 34 | 0.73 (0.52–0.88) | 0.96 (0.87–1.00) | 0.91 | 0.88 | 19 | 0.28 | 0.91 |
| IL-6 | 8,671 | 0.81 (0.63–0.93) | 0.96 (0.88–1.00) | 0.93 | 0.9 | 22.18 | 0.2 | 0.89 |
| TNF-α | 18.8 | 0.7 (0.51–0.85) | 0.96 (0.87–1.00) | 0.91 | 0.85 | 18.55 | 0.31 | 0.88 |
| IL-12p70 | 10.52 | 0.69 (0.49–0.85) | 0.96 (0.87–1.00) | 0.91 | 0.85 | 18.62 | 0.32 | 0.87 |
| IL-8 | 7779.5 | 0.74 (0.55–0.88) | 0.91 (0.80–0.97) | 0.82 | 0.86 | 8.16 | 0.28 | 0.86 |
| IL-10 | 48.7 | 0.76 (0.57–0.90) | 0.85 (0.72–0.93) | 0.73 | 0.87 | 5.03 | 0.28 | 0.86 |
| GM-CSF | 3.9 | 0.74 (0.55–0.88) | 0.86 (0.73–0.94) | 0.74 | 0.86 | 5.1 | 0.3 | 0.83 |
| IL-2 | 2.46 | 0.77 (0.59–0.90) | 0.8 (0.67–0.90) | 0.69 | 0.86 | 3.87 | 0.28 | 0.8 |
PPV = positive predictive value; NPV = negative predictive value; LR+ = positive likelihood ratio; LR− = negative likelihood ratio; AUC = area under the curve; IL = interleukin; IFN = interferon; TNF = tumor necrosis factor; GM-CSF = granulocyte-macrophage colony stimulating factor.
As a measure of infection resolution, IL-1β had the greatest decreases (12.4-fold; level at explantation: median 232.4 pg/mL [range, 23.1–1545.7]; level at reimplantation: median 18.8 pg/mL [range, 1.2–298.9]; mean difference: 325.5 [95% CI, 65–596]; p = 0.0001), and IL-6 had a nearly similar decrease (11.2-fold; level at explantation: median 28,228.1 pg/mL [range, 10,158.4–182,725]; level at reimplantation: median 2518.2 pg/mL [range, 10.4–41,319.3]; mean difference: 33,176 pg/mL [95% CI, 7543.6–58,808.3]; p < 0.0001). GM-CSF was the only cytokine that did not decrease from explantation to reimplantation (p = 0.290) (Table 2).
Table 2.
Comparisons of synovial fluid cytokine levels of the 15 patients who underwent both first-stage explantation and second-stage reimplantation
| Cytokine | First-stage explantation* | Second-stage reimplantation* | Mean difference (95% CI) | Fold decrease | p value† |
|---|---|---|---|---|---|
| IL-1β | 232.4 (23.1–1545.7) | 18.8 (1.2–298.9) | 325.5 (65.0–596.0) | 12.4 | 0.0001 |
| IL-6 | 28,228.1 (10,158.4–182,725.0) | 2518.2 (10.4–1,319.3) | 33,176.0 (7543.6–58,808.3) | 11.2 | < 0.0001 |
| IL-2 | 11.3 (1.2–56.8) | 1.3 (1.2–14.6) | 10.1 (1.3–19.0) | 8.7 | 0.005 |
| IL-8 | 24,487.5 (1569.9–114,627.8) | 6133.4 (36.3–72,003.8) | 28,566.4 (4549.8–52,583.1) | 4.0 | 0.012 |
| IL-10 | 122.8 (25.7–273.9) | 35.8 (10.4–96.3) | 82.8 (33.8–131.8) | 3.4 | 0.002 |
| IFN-γ | 75.5 (11.2–223.1) | 31.5 (9.3–122.2) | 45.7 (3.0–88.6) | 2.4 | 0.026 |
| TNF-α | 25.9 (8.5–41.9) | 13 (4.5–25.9) | 13.3 (5.8–20.9) | 2.0 | 0.004 |
| IL-12p70 | 18.6 (5.1–82.5) | 11.2 (1.2–39.3) | 13.9 (-1.8 to 29.6) | 1.7 | 0.044 |
| GM-CSF | 8.8 (2–32.3) | 7.1 (1.2–25.5) | 2.9 (-4.3 to 10.1) | 1.2 | 0.290 |
* Values expressed as median (range); †Wilcoxon rank-sum test; CI = confidence interval; IL = interleukin; IFN = interferon; TNF = tumor necrosis factor; GM-CSF = granulocyte-macrophage colony stimulating factor.
For the purposes of ruling out infection in a previously infected joint, IFN-γ, IL-1β, and GM-CSF showed the highest sensitivity (0.75; 95% CI, 0.19–0.99 for all). The MSIS criteria and IL-6 had poor sensitivity (0; 95% CI, 0–0.6 for both) because neither was able to detect true positives (Table 3; Appendix 2 [Supplemental materials are available with the online version of CORR ®.]).
Table 3.
Diagnostic parameters for synovial fluid cytokines and MSIS criteria applied for detecting successful or failed treatment in the second-stage reimplantation group
| Parameter | Sensitivity | Specificity | PPV | NPV | LR+ | LR− | Accuracy |
|---|---|---|---|---|---|---|---|
| MSIS criteria | 0 (0–0.60) | 0.89 (0.72–0.97) | 0 | 0.86 | 0 | 1.12 | 0.78 |
| IL-6 | 0 (0–0.60) | 0.89 (0.72–0.97) | 0 | 0.86 | 0 | 1.12 | 0.78 |
| IFN-γ | 0.75 (0.19–0.99) | 0.64 (0.44–0.81) | 0.23 | 0.95 | 2.10 | 0.39 | 0.66 |
| GM-CSF | 0.75 (0.19–0.99) | 0.46 (0.28–0.66) | 0.17 | 0.93 | 1.40 | 0.54 | 0.50 |
| IL-1β | 0.75 (0.19–0.99) | 0.29 (0.13–0.49) | 0.13 | 0.89 | 1.05 | 0.88 | 0.34 |
| IL-10 | 0.50 (0.07–0.93) | 0.79 (0.59–0.92) | 0.25 | 0.92 | 2.33 | 0.64 | 0.75 |
| TNF-α | 0.25 (0.06–0.81) | 0.75 (0.55–0.89) | 12.50 | 87.50 | 1 | 1 | 0.69 |
| IL-12p70 | 0.50 (0.07–0.93) | 0.57 (0.37–0.76) | 0.14 | 0.89 | 1.17 | 0.88 | 0.56 |
| IL-8 | 0.50 (0.07–0.93) | 0.54 (0.34–0.73) | 0.13 | 0.88 | 1.08 | 0.93 | 0.53 |
| IL-2 | 0.50 (0.07–0.93) | 0.59 (0.39–0.78) | 0.15 | 0.89 | 1.23 | 0.84 | 0.58 |
MSIS = Musculoskeletal Infection Society; PPV = positive predictive value; NPV = negative predictive value; LR+ = positive likelihood ratio; LR− = negative likelihood ratio; IL = interleukin; IFN = interferon; GM-CSF = granulocyte-macrophage colony stimulating factor; TNF = tumor necrosis factor.
After a systematic review of the literature, the four most studied synovial fluid biomarkers were CRP, IL-6, alpha-defensin, and IL-1B. α-defensin had the highest sensitivity (0.92 [CI, 0.85–0.97]) and specificity (0.99 [CI, 0.97–1.00]) (Table 4; Appendix 3 [Supplemental materials are available with the online version of CORR ®.]).
Table 4.
Cumulative accuracy of the four most studied markers for the detection of PJI
| Marker | Studies | Total number | Sensitivity* | Specificity* | PPV | NPV | Accuracy |
|---|---|---|---|---|---|---|---|
| CRP | 5,6,13,15,16, 18,20,21 | 655 | 0.78 (0.72–0.83) | 0.95 (0.93–0.97) | 0.89 | 0.89 | 0.89 |
| IL-6 | 4,6,9–12,19 | 513 | 0.87 (0.81–0.92) | 0.89 (0.86–0.92) | 0.8 | 0.94 | 0.89 |
| α-defensin | 1,5,6 | 305 | 0.92 (0.85–0.97) | 0.99 (0.93–1.00) | 0.99 | 0.91 | 0.95 |
| IL-1β | 4,6,9,12 | 259 | 0.81 (0.70–0.89) | 0.93 (0.88–0.96) | 0.83 | 0.92 | 0.89 |
* Ninety-five confidence intervals in parentheses; PJI = periprosthetic joint infection; PPV = positive predictive value; NPV = negative predictive value; CRP = C-reactive protein; IL = interleukin.
Discussion
The differentiation between a septic and aseptic failed arthroplasty is paramount for appropriate decision-making and intervention. Chronic and low-grade infections pose a challenge to current orthopaedic practice because the available diagnostic tools are often unavailable or unreliable. As a result of the lack of a single gold standard test to diagnose PJI, the International Consensus on Periprosthetic Joint Infection [24] established a complex algorithm involving clinical evaluation, laboratory parameters, and histologic criteria to reach reliable diagnostic accuracy for PJI. Studies have shown that local proinflammatory cytokines have favorable diagnostic properties for PJI and largely outperform established serum markers such as CRP and erythrocyte sedimentation rate [4, 6, 10]. As successful as this new diagnostic modality has been, there are no studies concerning markers that may adequately detect resolution of infection in a joint previously treated with component explantation and placement of an antibiotic spacer. The MSIS criteria were not designed for this purpose and thus surgeons are often faced with a difficult decision when determining optimal timing for reimplantation. The current study examined not only the ability of synovial fluid cytokines in differentiating septic from aseptic conditions, but also the ability of cytokines and MSIS criteria in detecting successful resolution of infection.
There are a number of limitations to this study. First, the heterogeneous group of patients included in this study in an attempt to faithfully represent the patient population that presents with PJI may potentially introduce confounding biases. However, restricting inclusion criteria would negatively affect generalizability of this study. In this sense, although our limited sample size precluded controlling for potential confounding variables, our results apply for the general PJI population without coexisting metallosis irrespective of the presence or absence of inflammatory diseases, use of immunomodulators, or antibiotics. Second, the sample size of patients who underwent second-stage reimplantation and subsequently failed treatment is small. Only four patients reached this endpoint. This undoubtedly limits our findings but suggests new methods of investigating what would be a useful clinical marker. Furthermore, there were four patients lost to followup in the reimplantation group (11%). Second, the accuracy of diagnostic testing is generally limited by the accuracy of the gold standard test. Although MSIS is the accepted criteria for diagnosing PJI, it remains a moving target that is continuously refined as more data become available. Techniques to enhance the sensitivity of intraoperative cultures such as sonication cultures of explanted components were not routinely performed in our institution, possibly overestimating culture-negative PJIs. Lastly, recent studies from the last 2 years have shown that both synovial fluid CRP and α-defensin are highly accurate markers of PJI [1, 5, 6]; however, neither was included in this study. The reason for this was the lack of supportive data for either of those markers at the time this study was conceptualized in 2012. Our group has ongoing projects investigating the ability of new biomarkers, including α-defensin, to monitor treatment response after a first-stage explantation.
Although many individual markers have shown excellent diagnostic strength, both IL-6 and IL-1β had high diagnostic accuracy with sensitivities above 0.8 [6, 7, 10]. However, two studies reported lower diagnostic accuracy for these cytokines. Gollwitzer et al. [9] evaluated 10 markers in their ability to specifically distinguish aseptic loosening from staphylococcal infections, and IL-1β had a sensitivity of 0.67, whereas IL-6 had a sensitivity of 0.6. Nilsdotter-Augustinsson et al. [12] attempted to differentiate septic from aseptic loosening with the use of 11 inflammatory markers; IL-1β and IL-6 had sensitivities of 0.59 and 0.69, respectively. Despite the promising clinical applicability that synovial fluid cytokines may provide for the diagnosis of PJI, there is still a lack of large multiinstitutional studies to validate this method.
All cytokines with the exception of GM-CSF showed a decrease at the time of reimplantation with the largest decreases in IL-1β and IL-6. This may have occurred as a result of the short half-life of these molecules [22] and a rapid interruption in production after antibiotic spacer placement with eradication of the infecting organism. This characteristic may allow IL-1β and IL-6 to be potentially used as markers of treatment response. GM-CSF, on the other hand, did not experience a decrease between the two procedures. Although it also has a short half-life [2], this finding suggests that the production of GM-CSF does not experience a sudden halt after eradication of the infecting pathogen and may be related to the chronic state of inflammation and remodeling.
None of the currently available diagnostic criteria and synovial markers had good sensitivity at the time of second-stage reimplantation to rule out infection in previously infected joints. The MSIS criterion was not validated to detect eradication of infection and is known to be sometimes unable to recognize PJI caused by low virulence pathogens such as P acnes [22]. In this study, we attempted to measure the ability of the MSIS criteria and of synovial fluid cytokines to detect resolution of infection based on 1-year outcomes as the reference standard. As a result of the relatively low failure rates of two-stage revisions in this study (11%), only four patients met criteria for treatment failure after 1 year. The MSIS criteria and IL-6 were not able to detect a single true-positive with a sensitivity of 0. None of the markers correctly identified all four cases of treatment failure. Thus far, no other studies have investigated markers to detect resolution of a previously treated PJI and this knowledge may potentially lead to a decrease in failure rates of two-stage revisions.
A systematic review of the literature revealed α-defensin to be the most accurate synovial fluid biomarker to differentiate septic from aseptic failed THAs and TKAs (Table 4). The four synovial fluid biomarkers with the highest patient numbers evaluated for this purpose were CRP [5, 6, 13, 15, 16, 18, 20, 21], IL-6 [4, 6, 9–12, 19], α-defensin [1, 5, 6], and IL-1β [4, 6, 9, 12], respectively. Although α-defensin clearly shows the highest diagnostic accuracy, it has only been evaluated in three studies to the date of our review, two of which emanate from the same group of researchers. The remaining three markers, CRP, IL-6, and IL-1β, had the same accuracy of 0.89, also demonstrating strong diagnostic strength. Further studies are necessary to establish the single best synovial fluid biomarker to diagnose PJI and to rule out PJI in treated joints.
This was the first study to demonstrate the downtrend of IL-6 and IL-1β between first-stage explantation and second stage-reimplantation. Although none of the cytokines analyzed appeared to be reliable to rule out infection at the time of reimplantation, the downtrend between the two stages may provide an important guide for clinicians to monitor treatment response. Our results are also in accordance with previous studies that show high diagnostic accuracy for IL-1β, IFN-γ, and IL-6 in differentiating septic from aseptic failed TKAs and THAs. None of the synovial fluid cytokines or the MSIS criteria applied for the reimplantation group were able to satisfactorily detect resolution of infection in a joint previously treated for PJI.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr Mario Farias-Kovac for assistance with data collection, Dr Xiaochun Zhang for the development and validation of the synovial fluid cytokine assay protocol, and Mr Colin O’Rourke for performing the statistical analysis. We also thank Drs Trevor Murray, Robert Molloy, and Victor Krebs for allowing patient enrollment.
Footnotes
One of the authors certifies that he (CAH), or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Stryker (Kalamazoo, MI, USA), an amount of USD 100,001 to USD 1,000,000 from KCI (San Antonio, TX, USA), an amount of USD 100,001 to USD 1,000,000 from Myoscience (Fresno, CA, USA), and an amount of USD 100,001 to USD 1,000,000 from CD Diagnostics (Claymont, DE, USA). One of the authors certifies that he (WKB), or a member of his immediate family, has or may receive payments or benefits, during the study period, an amount of USD 100,001 to USD 1,000,000 from Stryker, an amount of USD 100,000 to USD 1,000,000 from Exatech (Gainesville, FL, USA), an amount of USD 10,000 to USD 100,000 from Custom Orthopaedic Solutions (Cleveland, OH, USA), and an amount of USD 10,000 to USD 100,000 from KEF Healthcare (Dubai, UAE).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bingham J, Clarke H, Spangehl M, Schwartz A, Beauchamp C, Goldberg B. The alpha-defensin 1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin Orthop Relat Res. 2014;472:4006–4009. doi: 10.1007/s11999-014-3900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burgess AW, Metcalf D. Serum half-life and organ distribution of radiolabeled colony stimulating factor in mice. Exp Hematol. 1977;5:456–464. [PubMed] [Google Scholar]
- 3.Dabitao D, Margolick JB, Lopez J, Bream JH. Multiplex measurement of proinflammatory cytokines in human serum: comparison of the Meso Scale Discovery electrochemiluminescence assay and the Cytometric Bead Array. J Immunol Methods. 2011;372:71–77. doi: 10.1016/j.jim.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deirmengian C, Hallab N, Tarabishy A, Della Valle C, Jacobs JJ, Lonner J, Booth RE. Synovial fluid biomarkers for periprosthetic infection. Clin Orthop Relat Res. 2010;468:2017–2023. doi: 10.1007/s11999-010-1298-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid α-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am. 2014;96:1439–1745. doi: 10.2106/JBJS.M.01316. [DOI] [PubMed] [Google Scholar]
- 6.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res. 2014;472:3254–3262. doi: 10.1007/s11999-014-3543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deirmengian C, Lonner JH, Booth RE. The Mark Coventry Award: White blood cell gene expression: a new approach toward the study and diagnosis of infection. Clin Orthop Relat Res. 2005;440:38–44. doi: 10.1097/01.blo.0000185756.17401.32. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus. Clin Orthop Relat Res. 2013;471:2374–2382. doi: 10.1007/s11999-013-2866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gollwitzer H, Dombrowski Y, Prodinger PM, Peric M, Summer B, Hapfelmeier A, Saldamli B, Pankow F, von Eisenhart-Rothe R, Imhoff AB, Schauber J, Thomas P, Burgkart R, Banke IJ. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am. 2013;95:644–651. doi: 10.2106/JBJS.L.00205. [DOI] [PubMed] [Google Scholar]
- 10.Jacovides CL, Parvizi J, Adeli B, Jung KA. Molecular markers for diagnosis of periprosthetic joint infection. J Arthroplasty. 2011;26(99–103):e1. doi: 10.1016/j.arth.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Lenski M, Scherer MA. Synovial IL-6 as inflammatory marker in periprosthetic joint infections. J Arthroplasty. 2014;29:1105–1109. doi: 10.1016/j.arth.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Nilsdotter-Augustinsson A, Briheim G, Herder A, Ljunghusen O, Wahlström O, Ohman L. Inflammatory response in 85 patients with loosened hip prostheses: a prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening. Acta Orthop. 2007;78:629–639. doi: 10.1080/17453670710014329. [DOI] [PubMed] [Google Scholar]
- 13.Omar M, Ettiinger M, Reichling M, Petri M, Guenther D, Gehrke T, Krettek C, Mommsen P. Synovial C-reactive protein as a marker for chronic periprosthetic infection in total hip arthroplasty. Bone Joint J. 2015;97:173–176. doi: 10.1302/0301-620X.97B2.34550. [DOI] [PubMed] [Google Scholar]
- 14.Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge. J Bone Joint Surg Am. 2012;94:e104. doi: 10.2106/JBJS.9416edit. [DOI] [PubMed] [Google Scholar]
- 15.Parvizi J, Jacovides C, Adeli B, Jung KA, Hozack, WJ. Mark B. Coventry Award. Synovial C-reactive protein: a prospective evaluation of a molecular marker for periprosthetic knee joint infection. Clin Orthop Relat Res. 2012;470:54–60. [DOI] [PMC free article] [PubMed]
- 16.Parvizi J, McKenzie JC, Cashman JP. Diagnosis of periprosthetic joint infection using synovial fluid C-reactive protein. J Arthroplasty. 2012;27(Suppl 1):12–16. doi: 10.1016/j.arth.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Randau TM, Friedrich MJ, Wimmer MD, Reichert B, Kuberra D, Stoffel-Wagner B, Limmer A, Wirtz DC, Gravius S. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One. 2014;9:e89045. doi: 10.1371/journal.pone.0089045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ronde-Oustau C, Diesinger Y, Jeny JY, Antoni M, Gaudias J, Boeri C, Sibilia J, Lessinger JM. Diagnostic accuracy of intra-articular C-reactive protein assay in periprosthetic knee joint infection–a preliminary study. Orthop Traumatol Surg Res. 2014;100:221–224. doi: 10.1016/j.otsr.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Saleh A, Guirguis A, Klika AK, Johnson L, Higuera CA, Barsoum WK. Unexpected positive intraoperative cultures in aseptic revision arthroplasty. J Arthroplasty. 2014;29:2181–2186. doi: 10.1016/j.arth.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Tetreault MW, Wetters NG, Moric M, Gross CE, Della-Valle CJ. Is synovial C-reactive protein a useful marker for periprosthetic joint infection? Clin Orthop Relat Res. 2014;472:3997–4003. doi: 10.1007/s11999-014-3828-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderstappen C, Verhoeven N, Stuyck J, Bellemans J. Intra-articular versus C-reactive protein analysis in suspected periprosthetic knee joint infection. Acta Orthop Belg. 2013;79:326–330. [PubMed] [Google Scholar]
- 22.Wirtz DC, Heller KD, Miltner O, Zilkens KW, Wolff JM. Interleukin-6: a potential inflammatory marker after total joint replacement. Int Orthop. 2000;24:194–196. doi: 10.1007/s002640000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Gibson B, Jr, Frangiamore SJ, Saleh A, Grosso MJ, Kovac MF, Bauer TW, Ricchetti ET, Iannotti JP, Daly TM. Validation of a multiplex electrochemiluminescence assay for quantitation of synovial fluid cytokines and establishing reference interval in non-infected arthroplasty patients. Clin Chem. 2014;60:S99–S100. [Google Scholar]
- 24.Zmistowski B, Della Valle C, Bauer TW, Malizos KN, Alavi A, Bedair H, Booth RE, Choong P, Deirmengian C, Ehrlich GD, Gambir A, Huang R, Kissin Y, Kobayashi H, Kobayashi N, Krenn V, Drago L, Lorenzo D, Marston SB, Meermans G, Perez J, Ploegmakers JJ, Rosenberg A, Simpendorfer C, Thomas P, Tohtz S, Villafuerte JA, Wahl P, Wagenaar F-C, Witzo E. Diagnosis of periprosthetic joint infection. J Arthroplasty. 2014;29:77–83. doi: 10.1016/j.arth.2013.09.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.