Abstract
Background
The continued presence of biofilm may be one cause of the high risk of failure observed with irrigation and débridement with component retention in acute periprosthetic joint infection (PJI). There is a poor understanding of the role of biofilm antibiotic tolerance in PJI.
Questions/purposes
(1) Do increasing doses of cefazolin result in decreased viable biofilm mass on arthroplasty materials? (2) Is cefazolin resistance phenotypic or genotypic? (3) Is biofilm viability a function of biofilm depth after treatment with cefazolin? (4) Is the toxin-antitoxin system, yoeB expression, associated with antibiotic stress?
Methods
Methicillin-sensitive Staphylococcus aureus biofilm was cultured on total knee arthroplasty (TKA) materials and exposed to increasing doses of cefazolin (control, 0.5, 1.0, 10.0, 100.0 μg/mL). Quantitative confocal microscopy and quantitative culture were used to measure viable biofilm cell density. To determine if cefazolin resistance was phenotypic or genotypic, we measured minimum inhibitory concentration (MIC) after exposure to different cefazolin concentrations; changes in MIC would suggest genotypic features, whereas unchanged MIC would suggest phenotypic behavior. Finally, quantitative reverse transcription-polymerase chain reaction was used to quantify expression of yoeB levels between biofilm and planktonic bacteria after exposure to 1 μg/mL cefazolin for 3 hours.
Results
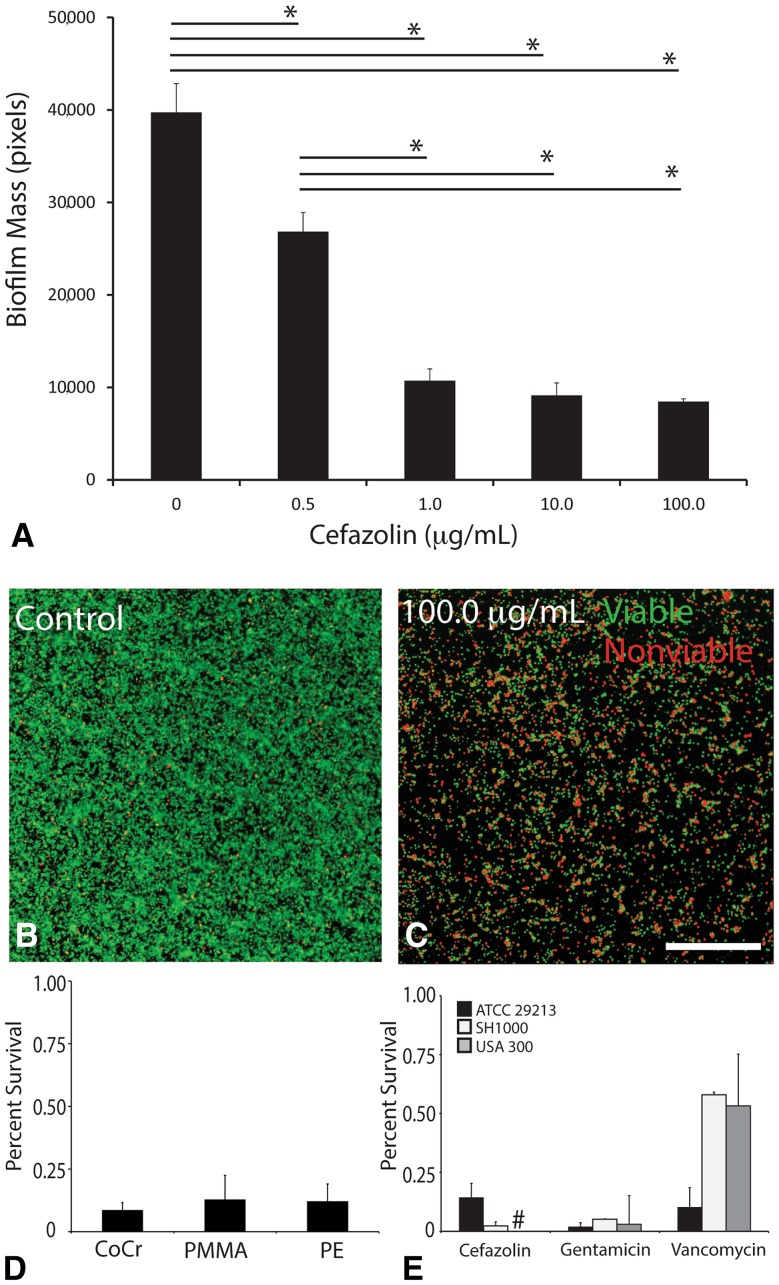
Although live biofilm mass was reduced by exposure to cefazolin when compared with biofilm mass in controls (39.2 × 103 ± 26.4 × 103 pixels), where the level after 0.5 µg/mL exposure also showed reduced mass (20.3 × 103 ± 11.9 × 103 pixels), no further reduction was seen after higher doses (mass at 1.0 µg/mL: 5.0 × 103 pixels ± 1.1 × 103 pixels; at 10.0 µg/mL: 6.4 × 103 ± 9.6 × 103 pixels; at 100.0 µg/mL: 6.4 × 103 ± 3.9 × 103). At the highest concentration tested (100 µg/mL), residual viable biofilm was present on all three materials, and there were no differences in percent biofilm survival among cobalt-chromium (18.5% ± 15.1%), polymethylmethacrylate (22.8% ± 20.2%), and polyethylene (14.7% ± 10.4%). We found that tolerance was a phenotypic phenomenon, because increasing cefazolin exposure did not result in changes in MIC as compared with controls (MIC in controls: 0.13 ± 0.02; at 0.5 µg/mL: 0.13 ± 0.001, p = 0.96; at 1.0 µg/m: 0.14 ± 0.04, p = 0.95; at 10.0 µg/m: 0.11 ± 0.016, p = 0.47; at 100.0 µg/m: 0.94 ± 0.047, p = 0.47). Expression of yoeB after 1 µg/mL cefazolin for 3 hours in biofilm cells was greater in biofilm but not in planktonic cells (biofilm: 62.3-fold change, planktonic cells: −78.8-fold change, p < 0.001).
Conclusions
Antibiotics are inadequate at complete removal of the biofilm from the surface of TKA materials. Results suggest that bacterial persisters are responsible for this phenotypic behavior allowing biofilm high tolerance to antibiotics.
Clinical Relevance
Antibiotic-tolerant biofilm suggests a mechanism behind the poor results in irrigation and débridement for acute TKA PJI.
Introduction
The high failure rate of irrigation and débridement in periprosthetic joint infection (PJI) suggests that biofilm has a high tolerance to antibiotics [3, 4, 7, 11, 14, 15, 19, 20, 23, 32]. Bacterial persisters are defined as a subpopulation present in bacterial species that have a phenotypically high tolerance to antibiotics secondary to an absence of metabolism after an environmental stress [1]. Persisters are less sensitive to antibiotics because the bacteria are not undergoing cellular activities that antibiotics can corrupt, resulting in tolerance to the antibiotics. Bacterial persisters are thought to be regulated by a series of toxin-antitoxin systems, including yoeB. Toxin-antitoxin systems consist of a toxin that disrupts an essential cellular process (translation through ribonuclease, dephosphorylation, etc) and a labile antitoxin that prevents toxin activation. In a subpopulation of the biofilm, when a bacterial cell encounters a stress, ie, antibiotics, the antitoxin is triggered to disassemble using a variety of mechanisms, and the toxin becomes activated to disrupt an essential bacterial metabolic process, inducing a state of dormancy [9, 12, 30]. After antibiotics are removed, the antitoxin binds the toxin allowing metabolic processes to resume and the sensitivity to its normal antibiotic spectrum to return. This has been hypothesized to explain the remarkable resilience of biofilms after treatment with antibiotics and is believed to serve an important role in chronic infections [30, 33].
There is a poor understanding of the role of bacterial persisters and biofilm antibiotic tolerance in PJI. This tolerance may be conferred as a result of the decreased efficacy of antibiotics on bacterial persisters in the biofilm, developed antibiotic genetic resistance by the bacteria, or the extracellular polymeric substance preventing diffusion of the antibiotic through the biofilm [12, 16].
We therefore asked: (1) Do increasing doses of cefazolin result in decreased viable biofilm mass on arthroplasty materials? (2) Is cefazolin resistance phenotypic or genotypic? (3) Is biofilm viability a function of biofilm depth after treatment with cefazolin? (4) Is the toxin-antitoxin system, yoeB, expression associated with antibiotic stress?
Materials and Methods
Coupon Fabrication
To establish an in vitro biofilm model, coupons, samples of the arthroplasty material fashioned into disks with an approximate area of 1 cm2, of cobalt-chrome, polymethylmethacrylate (PMMA), and polyethylene were prepared. Surfaces of cobalt-chrome alloy coupons were prepared to have smoothness to unused TKA implants. Surfaces underwent grinding, lapping, and polishing, and surface roughness measurements were taken using an Ambios (Santa Cruz, CA, USA) Xi-100 noncontact (interferometer type) profiler with Ambios Image Studio software. The maximum vertical resolution of the Xi-100 profiler was 0.2 nm, which was sufficient for accurately assessing orthopaedic joint replacement metallic bearing surfaces’ average roughness and maximum peak-to-valley distance per American Society for Testing and Materials standard (ASTM F2083) [2]. PMMA coupons were fashioned from medium-viscosity bone cement (SmartSet; DePuy Orthopaedics, Warsaw, IN, USA) without the addition of antibiotics in molds to match the dimensions of the cobalt-chrome coupons. Polyethylene patella implants (Sigma oval domed patella single peg; DePuy Orthopaedics) of similar dimensions were used for the polyethylene coupons.
Biofilm Culture and Antibiotic Exposure
To determine if increasing doses of cefazolin result in decreased viable biofilm mass on arthroplasty materials, biofilm mass with increasing doses of cefazolin was measured. A methicillin-sensitive strain of Staphylococcus aureus (ATCC 29213) was used for biofilm growth. Experiments were completed on three coupons in triplicate. Cobalt-chrome metal, PMMA, and polyethylene were inoculated with S aureus with an optical density of 0.1 at 660 nm. Biofilms were cultured in tryptic soy broth media for 24 hours in an agitating water bath (37 °C, 30 RPM).
After biofilm culture, coupons were gently washed three times in sterile phosphate-buffered saline (PBS) and placed in fresh tryptic soy media with doses of 0, 0.5, 1, 10, or 100 μg/mL cefazolin. Doses of cefazolin were selected to be above the minimum inhibitory concentration (MIC), 10 × MIC, and 100 × MIC. Submerged coupons were exposed to cefazolin for 3 hours in an agitating water bath (37 °C, 30 RPM). After the cefazolin exposure, coupons were washed three additional times in PBS before staining.
Confocal Microscopy
To assess biofilm mass and viability as a function of biofilm depth, confocal microscopy was used to quantify viable biofilm mass and viable bacteria as a fraction of total bacteria (viable and nonviable) on the base and surface of the biofilm. Quantitative microscopy was performed using a laser-scanning confocal imaging system (Leica TCS SP8 AOBS; Leica Microsystems Heidelberg GmbH, Heidelberg, Germany). Image stacks were collected using FITC (490 nm excitation and 525 nm emission, green) and TRITC (557 excitation and 576 nm emission, red) beam path settings using a x 40 objective lens and 0.3-mm step size across the z-axis. Laser power and pinhole settings remained constant. Live/dead stain (0.02M SYTO 9, 0.06M propidium iodide; Moleculer Probes, Eugene, OR, USA) was used according to manufacturer instructions with volumes doubled for the Syto 9 staining. After coupons were imaged at three locations, disks were irrigated as described previously and then restained using identical parameters. Tile scans captured nine adjacent planes of the biofilm, forming a three-by-three grid measuring 1 mm × 1 mm of biofilm surface. Biofilm mass was quantified using ImageJ (Version 1.45j; National Institute of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij). Viable and nonviable bacteria were identified by particles 15 to 100 pixels in area after segmentation with a manual threshold and application of watershed filters on the green (Syto9) and red (propidium iodide) channels, respectively.
Quantitative Culture
To quantify remaining viable biofilm after extended treatment with cefazolin, biofilms were cultured and exposed to cefazolin for 24 hours. Cobalt-chrome metal, PMMA, and polyethylene (ultrahigh-molecular-weight polyethylene) coupons were used for biofilm surfaces. After cefazolin exposure at 100 μg/mL, all coupons were gently washed three times in PBS and sonicated for 10 minutes in PBS with 1% Tween 20. The sonicate was centrifuged (3200 RPM, 10 minutes). The supernatant was removed, and bacterial pellets were resuspended in 1 mL PBS. Drop assays were performed on blood agar plates to determine colony-forming units of S aureus [8].
Remaining viable biofilm mass was then quantified using multiple antibiotics and multiple species of S aureus. Three strains of S aureus (ATCC 29213, USA300 FPR3757, and SH1000) were cultured using the biofilm model as described previously. Each strain was exposed to greater than 10 × MIC for three different antibiotics (cefazolin, 10 μg/mL; gentamycin, 50 μg/mL; vancomycin, 20 μg/mL) for 24 hours. USA300 FPR3757 was not exposed to cefazolin because the strain is a methicillin-resistant S aureus strain. All coupons were washed three times in PBS and sonicated for 10 minutes in PBS with 1% Tween 20. Sonicate was centrifuged (3200 RPM, 10 minutes). The supernatant was removed, and bacterial pellets were resuspended in 1 mL PBS. Drop assays were performed on blood agar plates to determine colony-forming units of S aureus [8].
Minimum Inhibitory Concentration
To determine if cefazolin resistance was phenotypic or genotypic, MIC was measured after increasing doses of cefazolin. The biofilm model with increasing doses of cefazolin for 3 hours as described previously was used. After exposure to cefazolin for 3 hours, cobalt-chrome coupons were washed three times in PBS and placed in tryptic soy broth for 24 hours. MIC was then determined by cefazolin E-test strips as described by the manufacturer protocol (Biomerieux, Craponne, France) [5].
Toxin-antitoxin Expression
To assess possible toxin-antitoxin expression after antibiotic stress, biofilm was cultured as described previously on cobalt-chrome coupons. Planktonic bacteria for comparison were collected from the same culture system as the biofilm. Cells were lysed using 0.1-mm zirconia/silica beads and a bead beater (Biospec, Bartlesville, OK, USA) and total RNA was isolated using the RNeasy Mini kit (Qiagen, Hilden, Germany) [21]. Quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) was performed to analyze expression of yoeB. Reactions were performed in duplicate for each strain/primer pair following instructions for the Power SYBR® Green RNA-to-CT™ 1-Step kit (Life Technologies, Carlsbad, CA, USA) using 100 ng of total RNA as the template. Primers were annealed at 60° C, and data were normalized against the housekeeping gene rrsA. The specificity of qRT-PCR primers was verified through standard PCR, and fold changes were calculated using the 2−ΔΔCT formula [18].
Statistical Analysis
Data are expressed as a mean ± SD except where noted. All statistical tests were completed using R (R Core Development Team, www.r-project.org). Direct comparisons between two populations were made using an unpaired Student’s t-test. Multiple group comparisons were made using one-way and two-way analysis of variance where appropriate. In both cases, significance levels were determined using the Tukey post hoc analysis for pairwise comparison. Statistical significance was determined if p < 0.05 unless otherwise noted.
Results
Antibiotics were unable to eliminate abiotic biofilm mass. After 3 hours of cefazolin exposure, live biofilm mass was reduced when compared with biofilm mass in controls (39.2 × 103 ± 26.4 × 103 pixels), where the level after 0.5 µg/mL exposure also showed reduced mass (20.3 × 103 ± 11.9 × 103 pixels); no further reduction was seen after higher doses (mass at 1.0 µg/mL: 5.0 × 103 pixels ± 1.1 × 103 pixels; at 10.0 µg/mL: 6.4 × 103 ± 9.6 × 103 pixels; at 100.0 µg/mL: 6.4 × 103 ± 3.9 × 103 pixels; Fig. 1A; Table 1). Exposure to 1.0 µg/mL, 10.0 µg/mL, and 100.0 µg/mL all resulted in reduction in biofilm mass compared with controls (mean difference: −34.2 × 103 [confidence interval {CI}, −26.7 × 103 to −11.2 × 103], p < 0.001; mean difference: −32.8 × 103 [CI, −40.1 × 103 to −25.2 × 103], p < 0.001; mean difference: −32.7 × 103 [CI, −39.4 × 103 to −25.9 × 103], p < 0.001, respectively). However, levels above 1.0 µg/mL produced no additional reduction in biofilm mass (10.0 µg/mL versus 1.0 µg/mL mean difference: mean difference: 1.3 × 103 [CI, −8.2 × 103 to 10.8 × 103], p = 0.995; 100.0 µg/mL versus 1.0 µg/mL mean difference: 1.5 × 103 [CI, −7.3 × 103 to 10.3 × 103], p = 0.990; Table 2). These differences could be directly observed on confocal microscopy images between control and the highest cefazolin dose tested, 100.0 µg/mL (Fig. 1B–C). At 24 hours and the highest concentration of cefazolin tested (100 µg/mL), residual viable biofilm was present on all three materials, and there were no differences in percent biofilm survival among cobalt-chromium (18.5% ± 15.1%), PMMA (22.8% ± 20.2%), and highly crosslinked polyethylene (14.7% ± 10.4%). One-way analysis of variance showed no difference among the means of all three groups (p = 0.679; Fig. 1D; Table 3). At 24 hours, residual biofilm of three different S aureus strains (ATCC 29213, SH1000, USA 300) remained on material after individual treatment with multiple antibiotics at a dose of greater than 10 × MIC for all three strains (cefazolin, 10 μg/mL; gentamicin, 50 μg/mL; vancomycin, 20 μg/mL; Fig. 1E; Table 4).
Fig. 1A–E.
Methicillin-sensitive S aureus biofilm is tolerant to cefazolin. (A) Biofilm mass was quantified after 3 hours of exposure to cefazolin using confocal microscopy and live-dead stain. After 1.0 μg/mL, a clinically relevant value, increasing doses of cefazolin had no increased loss of biofilm. (B) Example confocal image of control biofilm and (C) biofilm exposed to 100 μg/mL of cefazolin (Stain: live-dead; scale bar = 100 μm). (D) Quantitative agar culture was used as a viability assay demonstrating that regardless of material, approximately 10% of biofilm cells survived after exposure to 100 μg/mL of cefazolin after 24 hours. CoCr = cobalt-chromium; PE = polyethylene. (E) Quantitative agar culture was used as a viability assay demonstrating that biofilm from multiple strains of S aureus remained after treatment with multiple antibiotics (dose > 10 × MIC) after 24 hours. *p < 0.05. #The methicillin-resistant S aureus strain USA300 was not tested with cefazolin because it has known and well-documented genetic resistance.
Table 1.
Remaining Staphylococcus aureus viable biofilm mass after 3 hours of treatment with increasing doses of cefazolin
| Cefazolin (μg/mL) | Biofilm mass pixels (n = 27) Mean (SD) |
|---|---|
| 0 | 39.2 × 103 (26.4 × 103) |
| 0.5 | 20.3 × 103 (11.9 × 103) |
| 1 | 5.0 × 103 (1.1 × 103) |
| 10 | 6.4 × 103 (9.6 × 103) |
| 100 | 6.4 × 103 (3.9 × 103) |
Table 2.
Differences in means between Staphylococcus aureus viable biofilm mass at increasing doses of cefazolin after 3 hours of treatment
| Group (μg/mL) | Mean difference (95% CI) | p value |
|---|---|---|
| 0.5-0 | −1.9 × 104 (−2.7 × 104, −1.1 × 104) | < 0.001 |
| 1-0 | −3.4 × 104 (−4.3 × 104, −2.5 × 104) | < 0.001 |
| 10-0 | −3.3 × 104 (−4.0 × 104, −2.5 × 104) | < 0.001 |
| 100-0 | −3.3 × 104 (−4.0 × 104, −2.5 × 104) | < 0.001 |
| 1-0.5 | −1.5 × 104 (−2.5 × 104, −5.6 × 104) | < 0.001 |
| 10-0.5 | −1.4 × 104 (−2.2 × 104, −5.6 × 104) | < 0.001 |
| 100-0.5 | −3.4 × 104 (−2.1 × 104, −6.3 × 104) | < 0.001 |
| 10-1 | 1.4 × 104 (−8.2 × 104, 1.1 × 104) | 0.995 |
| 100-1 | 1.5 × 104 (−7.3 × 104, 1.0 × 104) | 0.990 |
| 100-10 | 1.4 × 104 (−7.2 × 104, 7.5 × 104) | 1.000 |
CI = confidence interval.
Table 3.
Staphylococcus aureus biofilm percent survival after treatment with cefazolin (100 μg/mL) for 24 hours on different arthroplasty materials
| Parameter | CoCr (n = 9) | PMMA (n = 9) | PE (n = 9) | ||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean difference versus CoCR(95% CI) | p value for PMMA versus CoCr | Mean (SD) | Mean difference versus CoCR (95% CI) | p value for PE versus CoCr | |
| Fraction survival | 0.185 (0.151) | 0.228 (0.202) | 0.4 (−0.19, 0.28) | 0.882 | 0.147 (0.104) | −0.04 (−0.27, 0.20) | 0.911 |
CoCr = cobalt-chrome; PMMA = polymethylmethacrylate; PE = polyethylene; CI = confidence interval.
Table 4.
Biofilm percent survival of different Staphylococcus aureus strains after treatment with different antibiotics for 24 hours
| Strain | Antibiotic | Percent survival (n = 3) Mean (SD) |
|---|---|---|
| ATCC 29213 | Cefazolin | 14.4% (6.0%) |
| ATCC 29213 | Vancomycin | 10.2% (8.3%) |
| ATCC 29213 | Gentamicin | 2.0% (1.7%) |
| USA 300 | Vancomycin | 53.3% (21.9%) |
| USA 300 | Gentamicin | 3.0% (1.1%) |
| SH1000 | Cefazolin | 2.3% (0.1%) |
| SH1000 | Vancomycin | 57.9% (12.2%) |
| SH1000 | Gentamicin | 5.2% (2.5%) |
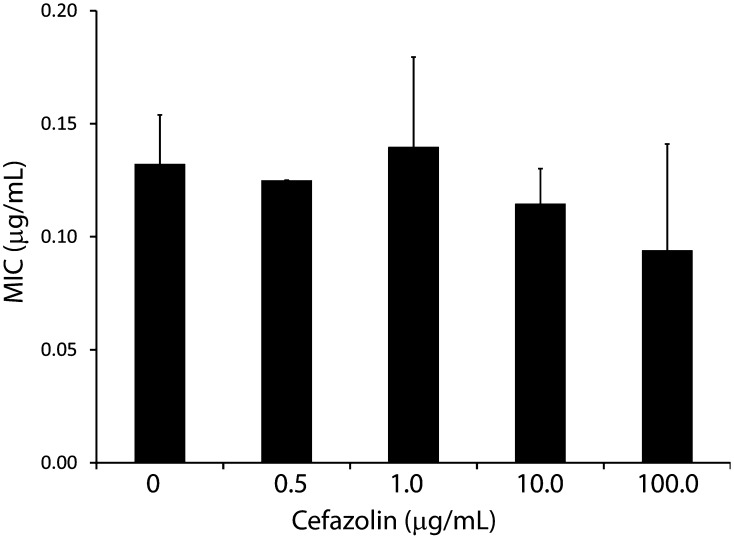
We found that tolerance was a phenotypic phenomenon, because increasing cefazolin exposure did not result in changes in MIC as compared with controls (MIC in controls: 0.13 ± 0.021; at 0.5 µg/mL: 0.13 ± 0.001, mean difference to controls: −.0072 [CI, −0.037 to 0.023], p = 0.960; at 1.0 µg/mL: 0.14 ± 0.040, mean difference to controls: 0.0075 [CI, −0.023 to 0.038], p = 0.952; at 10.0 µg/mL: 0.11 ± 0.016, mean difference to controls: −0.018 [CI, −0.048 to 0.012], p = 0.472; at 100.0 µg/mL: 0.94 ± 0.047, mean difference to controls: −0.017 [CI, −0.047 to 0.013], p = 0.472; Fig. 2; Table 5). Small colony variant morphology was not observed after exposure to antibiotics.
Fig. 2.
Biofilm antibiotic tolerance is not based on genetic resistance. The MIC of surviving biofilm was quantified at each dose of antibiotic. There was no statistical significant difference between groups. *p < 0.05.
Table 5.
MIC of Staphylococcus aureus after 3 hours of treatment with increasing doses of cefazolin compared with untreated S aureus MIC
| Cefazolin (μg/mL) | MIC (μg/mL) (n = 9) Mean (SD) | Mean difference (95% CI) | p value |
|---|---|---|---|
| 0 | 0.13 (0.021) | ||
| 0.5 | 0.13 (0.001) | −0.0072 (−0.037, 0.023) | < 0.959 |
| 1 | 0.14 (0.040) | 0.0075 (−0.023, 0.038) | < 0.952 |
| 10 | 0.11 (0.016) | −0.018 (−0.048, 0.012) | < 0.472 |
| 100 | 0.94 (0.047) | −0.017 (−0.047, 0.013) | < 0.472 |
MIC = minimum inhibitory concentration; CI = confidence interval.
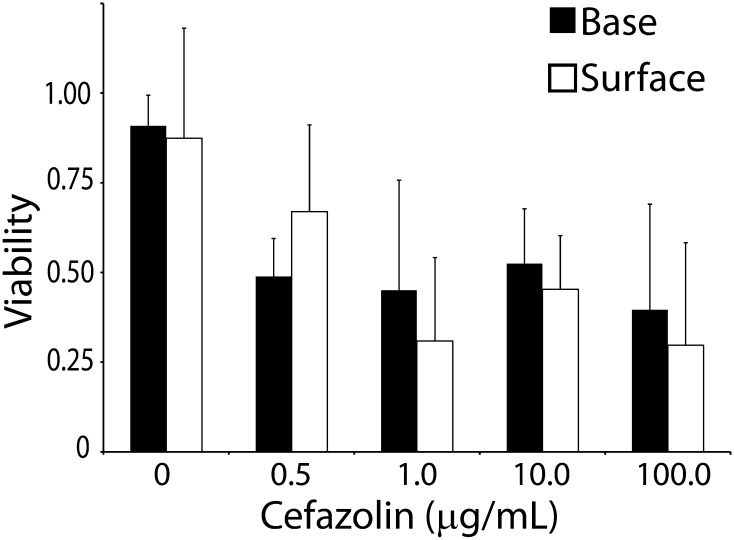
Biofilm antibiotic tolerance was not a function of the depth of the biofilm (base viability at 0 µg/mL: 0.90 ± 0.08; 0.5 µg/mL: 0.49 ± 0.11; 1.0 µg/mL: 0.45 ± 0.31; 10.0 µg/mL: 0.52 ± 0.15; 100.0 µg/mL: 0.40 ± 0.29 and surface viability at 0 µg/mL: 0.87 ± 0.31; 0.5 µg/mL: 0.67 ± 0.24; 1.0 µg/mL: 0.31 ± 0.23; 10.0 µg/mL: 0.45 ± 0.15; 100.0 µg/mL: 0.30 ± 0.29; Fig. 3; Table 6). There was no statistically significant difference between viability at the surface verses base of the biofilm (mean difference: −0.0089 [CI, −0.116 to 0.098], p = 0.868).
Fig. 3.
Biofilm antibiotic tolerance was not a function of biofilm depth. Confocal laser scanning microscopy with live-dead stain was used to quantify biofilm viability at the surface and base of the biofilm at increasing doses of cefazolin. There was no statistically significant difference between biofilm viability at the surface and base at each dose. *p < 0.05.
Table 6.
Comparison of Staphylococcus aureus biofilm percent viability at the surface and base of the biofilm after 3 hours of treatment with cefazolin (100 μg/mL)
| Cefazolin (μg/mL) | Base (n = 7) | Surface (n = 7) | ||
|---|---|---|---|---|
| Biofilm fraction survival Mean (SD) | Biofilm fraction survival Mean (SD) | Mean difference versus base (95% CI) | p value | |
| 0 | 0.90 (0.08) | 0.87 (0.31) | −0.035 (−0.390, 0.320) | 1.000 |
| 0.5 | 0.49 (0.11 | 0.67 (0.24) | 0.180 (−0.216, 0.577) | 0.891 |
| 1 | 0.45 (0.31) | 0.31 (0.23) | −0.141 (−0.496, 0.213) | 0.949 |
| 10 | 0.52 (0.15) | 0.45 (0.15) | −0.279 (−0.731, 0.173) | 0.998 |
| 100 | 0.40 (0.29) | 0.30 (0.29) | −0.099 (−0.551, 0.353) | 0.999 |
CI = confidence interval.
Expression of the toxin-antitoxin system, yoeB, is associated with antibiotic stress. Expression of yoeB is induced after 1 µg/mL cefazolin for 3 hours in biofilm as compared with planktonic cells and repressed in the absence of cefazolin as measured by change in cycling threshold values (biofilm: −5.96 ± 1.62, 62.34-fold change; planktonic cells: 6.30 ± 0.63, −78.8-fold change; p < 0.001).
Discussion
Single-stage irrigation and débridement with component retention has a high failure rate. This approach uses two separate strategies of pulse lavage to débride the bulk of the biofilm and long-term antibiotics to eradicate any remaining bacteria. Here, we questioned if the second approach in irrigation and débridement, antibiotics, is capable of eliminating biofilm in vitro.
This study has several limitations. Caution should be used when extrapolating these results to a clinical scenario. The glycocalyx structure of biofilm in vivo is likely to have a different composition as compared with biofilm cultured in vitro [6]. Furthermore, in a clinical setting antibiotic delivery occurs in cyclic episodes as compared with a consistent and constant level in vitro. Second, we used a limited number of S aureus strains with four common antibiotics. Finally, antibiotics may not reach all surfaces of the implant as compared with in vitro. There is evidence that S aureus can invade host cells, and it is possible that intracellular survival of the bacteria in the host may be advantageous in avoiding host defense and some types of antibiotics [10]. There is no clinical evidence validating or disproving this hypothesis, and it remains a subject of intense debate. Future work with a PJI animal model using multiple clinical strains will further verify these results.
S aureus biofilms cultured on TKA materials remain surprisingly tolerant to antibiotics. After doses above 1 μg/mL, slightly lower than an approximate clinical value [13, 17, 25, 26, 31], no further increase in bacterial death was observed. Surprisingly, even after 24 hours in supratherapeutic levels, cefazolin was unable to eliminate the biofilm beyond one to two orders of magnitude. Similar results were observed using multiple types of antibiotic with different strains of S aureus. This provides insight into the high failure rate of irrigation and débridement with antibiotic therapy.
The tolerance was a phenotypic behavior and not a genetic alteration. When placed in fresh culture media, surviving bacteria had an identical MIC as controls. These results are not unexpected. From a clinical microbiology perspective, an MIC between two strains is considered similar when they are within one doubling.
The antibiotic tolerance was not from limited diffusion of antibiotic through the biofilm. It has been hypothesized that mass transport limitations of the biofilm extracellular matrix prevent some antibiotics from penetrating the biofilm. In our experiments, confocal microscopy demonstrated that there were no statistically significant differences in bacteria viability at the surface as compared with the base of the biofilm. This suggests that the glycocalyx was not inhibiting diffusion of the antibiotic to the point of providing protection at the base of the biofilm. These results correlate with other models that demonstrate limited restriction in solubilized molecules [24, 28].
We demonstrate that the putative toxin-antitoxin system locus, yefM-yoeB, is repressed in unstressed biofilm cultures but induced after exposure of these biofilms to the antibiotic cefazolin. There is growing evidence that toxin-antitoxin systems are important regulators of the bacterial persistence phenotype of high antibiotic tolerance [30]. Toxin-antitoxin systems are highly redundant. In Mycobacterium tuberculosis, more than 79 different toxin-antitoxin systems have been identified [22]. We investigated one possible system, yoeB, in S aureus, but many others likely exist.
Biofilm has a surprisingly high tolerance to antibiotic on TKA materials, and this tolerance appears to be related, in part, to bacterial persisters. Two strategies are used in irrigation and débridement in PJI; biofilm is débrided secondary to the shear force during irrigation or physically surgically débrided and, in theory, any remaining biofilm is eradicated with antibiotics. Multiple groups have demonstrated that although biofilm is débrided, a substantial volume remains after irrigation [27, 29]. Here, we demonstrate that antibiotics are unable to eliminate biofilm. The combined difficulty or removing biofilm with both of these methods provides a mechanism for the high failure rates of irrigation and débridement in PJI.
Footnotes
The project described was supported by the National Institutes of Health through Grant Number KL2 TR000146 (KLU). Additional funding support was provided in part by the Woodward Family Endowment in Biomedical Engineering (CMD, TKW) and the Pellegrini Endowment to the Department of Orthopaedics and Rehabilitation at the Pennsylvania State University (KLU).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
This study was performed at the Department of Orthopaedics and Rehabilitation at the Penn State Hershey Medical Center, Hershey, PA, USA, and the Department of Orthopaedic Surgery at the University of Pittsburgh, Pittsburgh, PA, USA.
References
- 1.Amato SM, Fazen CH, Henry TC, Mok WW, Orman MA, Sandvik EL, Volzing KG, Brynildsen MP. The role of metabolism in bacterial persistence. Front Microbiol. 2014;5:70. doi: 10.3389/fmicb.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ASTM. 12 Standard Specification for Knee Replacement Prosthesis. Standard F2083. West Conshohocken, PA, USA: ASTM International; 2013.
- 3.Bradbury T, Fehring TK, Taunton M, Hanssen A, Azzam K, Parvizi J, Odum SM. The fate of acute methicillin-resistant Staphylococcus aureus periprosthetic knee infections treated by open débridement and retention of components. J Arthroplasty. 2009;24:101–104. doi: 10.1016/j.arth.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Brandt CM, Sistrunk WW, Duffy MC, Hanssen AD, Steckelberg JM, Ilstrup DM, Osmon DR. Staphylococcus aureus prosthetic joint infection treated with débridement and prosthesis retention. Clin Infect Dis. 1997;24:914–919. doi: 10.1093/clinids/24.5.914. [DOI] [PubMed] [Google Scholar]
- 5.Institute Clinical Laboratory Standards., editor. M07-A10 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. Wayne, PA, USA: CLSI; 2015. [Google Scholar]
- 6.Dastgheyb S, Parvizi J, Shapiro IM, Hickok NJ, Otto M. Effect of biofilms on recalcitrance of staphylococcal joint infection to antibiotic treatment. J Infect Dis. 2015;211:641–650. doi: 10.1093/infdis/jiu514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deirmengian C, Greenbaum J, Stern J, Braffman M, Lotke PA, Booth RE, Jr, Lonner JH. Open débridement of acute gram-positive infections after total knee arthroplasty. Clin Orthop Relat Res. 2003;416:129–134. doi: 10.1097/01.blo.0000092996.90435.35. [DOI] [PubMed] [Google Scholar]
- 8.Donegan K, Matyac C, Seidler R, Porteous A. Evaluation of methods for sampling, recovery, and enumeration of bacteria applied to the phylloplane. Appl Environ Microbiol. 1991;57:51–56. doi: 10.1128/aem.57.1.51-56.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasani RA, Savageau MA. Molecular mechanisms of multiple toxin-antitoxin systems are coordinated to govern the persister phenotype. Proc Natl Acad Sci USA. 2013;110:E2528–2537. doi: 10.1073/pnas.1301023110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garzoni C, Kelley WL. Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol. 2009;17:59–65. doi: 10.1016/j.tim.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Hartman MB, Fehring TK, Jordan L, Norton HJ. Periprosthetic knee sepsis. The role of irrigation and débridement. Clin Orthop Relat Res. 1991;273:113–118. [PubMed] [Google Scholar]
- 12.Kwan BW, Valenta JA, Benedik MJ, Wood TK. Arrested Protein synthesis increases antibiotic persistence. Antimicrob Agents Chemother. 2013;57:1468–1473. doi: 10.1128/AAC.02135-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd KC, Stover SM, Pascoe JR, Baggot JD, Kurpershoek C, Hietala S. Plasma and synovial fluid concentrations of gentamicin in horses after intra-articular administration of buffered and unbuffered gentamicin. Am J Vet Res. 1988;49:644–649. [PubMed] [Google Scholar]
- 14.Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, Osmon DR. Outcome of prosthetic joint infections treated with débridement and retention of components. Clin Infect Dis. 2006;42:471–478. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- 15.Mont MA, Waldman B, Banerjee C, Pacheco IH, Hungerford DS. Multiple irrigation, débridement, and retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12:426–433. doi: 10.1016/S0883-5403(97)90199-6. [DOI] [PubMed] [Google Scholar]
- 16.Neut D, van der Mei HC, Bulstra SK, Busscher HJ. The role of small-colony variants in failure to diagnose and treat biofilm infections in orthopedics. Acta Orthop. 2007;78:299–308. doi: 10.1080/17453670710013843. [DOI] [PubMed] [Google Scholar]
- 17.Perry CR, Hulsey RE, Mann FA, Miller GA, Pearson RL. Treatment of acutely infected arthroplasties with incision, drainage, and local antibiotics delivered via an implantable pump. Clin Orthop Relat Res. 1992;281:216–223. [PubMed] [Google Scholar]
- 18.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rand JA. Alternatives to reimplantation for salvage of the total knee arthroplasty complicated by infection. J Bone Joint Surg Am. 1993;75:282–289. doi: 10.2106/00004623-199302000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Rasul AT, Jr, Tsukayama D, Gustilo RB. Effect of time of onset and depth of infection on the outcome of total knee arthroplasty infections. Clin Orthop Relat Res. 1991;273:98–104. [PubMed] [Google Scholar]
- 21.Ren D, Bedzyk LA, Thomas SM, Ye RW, Wood TK. Gene expression in Escherichia coli biofilms. Appl Microbiol Biotechnol. 2004;64:515–524. doi: 10.1007/s00253-003-1517-y. [DOI] [PubMed] [Google Scholar]
- 22.Sala A, Bordes P, Genevaux P. Multiple toxin-antitoxin systems in Mycobacterium tuberculosis. Toxins (Basel). 2014;6:1002–1020. doi: 10.3390/toxins6031002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoifet SD, Morrey BF. Treatment of infection after total knee arthroplasty by débridement with retention of the components. J Bone Joint Surg Am. 1990;72:1383–1390. [PubMed] [Google Scholar]
- 24.Schurks N, Wingender J, Flemming HC, Mayer C. Monomer composition and sequence of alginates from Pseudomonas aeruginosa. Int J Biol Macromol. 2002;30:105–111. doi: 10.1016/S0141-8130(02)00002-8. [DOI] [PubMed] [Google Scholar]
- 25.Schurman DJ, Hirshman HP, Kajiyama G, Moser K, Burton DS. Cefazolin concentrations in bone and synovial fluid. J Bone Joint Surg Am. 1978;60:359–362. [PubMed] [Google Scholar]
- 26.Schurman DJ, Hirshman HP, Nagel DA. Antibiotic penetration of synovial fluid in infected and normal knee joints. Clin Orthop Relat Res. 1978;136:304–310. [PubMed] [Google Scholar]
- 27.Schwechter EM, Folk D, Varshney AK, Fries BC, Kim SJ, Hirsh DM. Optimal irrigation and débridement of infected joint implants: an in vitro methicillin-resistant Staphylococcus aureus biofilm model. J Arthroplasty. 2011;26:109–113. doi: 10.1016/j.arth.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart PS. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol Bioeng. 1998;59:261–272. doi: 10.1002/(SICI)1097-0290(19980805)59:3<261::AID-BIT1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Urish KL, DeMuth PW, Craft DW, Haider H, Davis CM., 3rd Pulse lavage is inadequate at removal of biofilm from the surface of total knee arthroplasty materials. J Arthroplasty. 2014;29:1128–1132. doi: 10.1016/j.arth.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Lord DM, Cheng HY, Osbourne DO, Hong SH, Sanchez-Torres V, Quiroga C, Zheng K, Herrmann T, Peti W, Benedik MJ, Page R, Wood TK. A new type V toxin-antitoxin system where mRNA for toxin GhoT is cleaved by antitoxin GhoS. Nat Chem Biol. 2012;8:855–861. doi: 10.1038/nchembio.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiteside LA, Peppers M, Nayfeh TA, Roy ME. Methicillin-resistant Staphylococcus aureus in TKA treated with revision and direct intra-articular antibiotic infusion. Clin Orthop Relat Res. 2011;469:26–33. doi: 10.1007/s11999-010-1313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72:878–883. [PubMed] [Google Scholar]
- 33.Wood TK, Knabel SJ, Kwan BW. Bacterial persister cell formation and dormancy. Appl Environ Microbiol. 2013;79:7116–7121. doi: 10.1128/AEM.02636-13. [DOI] [PMC free article] [PubMed] [Google Scholar]