Abstract
Nonalcoholic steatohepatitis (NASH) is a necro-inflammatory response that ensues when hepatocytes are injured by lipids (lipotoxicity). NASH is a potential outcome of nonalcoholic fatty liver (NAFL), a condition that occurs when lipids accumulate in hepatocytes. NASH may be reversible, but it can also result in cirrhosis and primary liver cancer. We are beginning to learn about the mechanisms of progression of NAFL and NASH. NAFL does not inevitably lead to NASH, because NAFL is a heterogeneous condition. This heterogeneity exists because different types of lipids with different cytotoxic potential accumulate in the NAFL, and individuals with NAFL differ in their ability to defend against lipotoxicity. There are no tests that reliably predict which patients with NAFL will develop lipotoxicity. However, NASH encompasses the spectrum of wound-healing responses induced by lipotoxic hepatocytes. Differences in these wound-healing responses among individuals determine whether lipotoxic livers regenerate, leading to stabilization or resolution of NASH, or develop progressive scarring, cirrhosis, and possibly liver cancer. We review concepts that are central to the pathogenesis of NASH.
Keywords: Nonalcoholic fatty liver disease, lipotoxicity, wound-healing response, misrepair
Nonalcoholic fatty liver disease comprises nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH)1, which each progress differently. NAFL rarely results in cirrhosis or liver cancer, whereas patients with NASH are at risk for these outcomes2,3. The rate of hepatocyte death is greater in NASH than in NAFLD—the key factor that differentiates NASH from NAFL4. The toxic effects of specific lipids on hepatocytes (hepatic lipotoxicity) could cause hepatocyte death in patients with NASH5. However, the risk for lipotoxicity differs according to the type of lipid that accumulates, and is modified by factors that can exacerbate or defend against their effects 6. What factors contribute to the effects of hepatic fat accumulation (steatosis)?
Steatosis Sets the Stage
Steatosis, the accumulation of fat in hepatocytes, is present in NAFL and NASH7. Steatosis occurs whenever the import or synthesis of fat exceeds fat export or degradation8 (Figure 1). Triglyceride (triacylglycerol) is the most conspicuous type of fat in fatty livers.9 So, the extent of triglyceride accumulation has been the basis for grading the severity of steatosis in NAFLD. Triglycerides per se are not hepatotoxic10, so steatosis grade or severity do not predict hepatic injury, inflammation, or fibrosis11,12. On the other hand, some of the other types of lipids that accumulate in fatty livers (e.g., fatty acids, diacylglycerol, oxysterols, cholesterol, and phospholipids)9 can injure hepatocytes. The realization that lipotoxocity is caused by lipids other than triglyceride has spurred development of strategies to prevent or treat NASH by blocking hepatic accumulation of lipotoxic lipids13. Lipotoxicity therefore initiates NASH development and is a new therapeutic target.
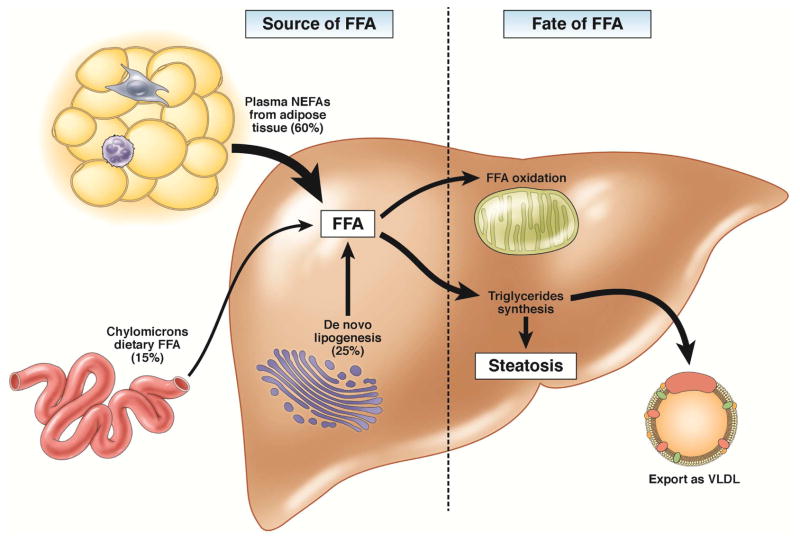
Figure 1. Mechanisms of hepatic steatosis.
Hepatic steatosis results from increased influx of lipids to the liver or decreased lipid disposal. The main sources of FA are plasma FFA (arriving mostly from the adipose tissue), de novo lipogenesis, and dietary FA. The liver discards fat by oxidation or by exporting it as VLDL. Alternatively, hepatocytes can shunt excess lipids to the synthesis of triglycerides and storage in lipid droplets. Red boxes highlight rate-limiting enzymes that regulate the main fates of fatty acids in the liver: FAS, fatty acid synthase and ACC, acetyl-CoA carboxylase, which are enzymes in fatty acids synthesis; CPT-1, carnitine palmitoyltransferase 1, enzyme that allows entry of acyl groups into the mitochondria by transferring an acyl group from CoA to carnitine and subsequent transport of acylcarnitine; SCD-1, stearoyl-CoA dessaturase, an enzyme that converts saturated in monounsaturated fatty acids the fatty acids that are preferentially incorporated in triglycerides; DGAT, diglyceride acyltransferase that catalyzes the synthesis of triglycerides from diacylglycerol and acylCoA; MTTP, microsomal triglyceride transfer protein, which controls lipoprotein assembly. Blue boxes indicate transcription factors involved in lipid metabolism: SREBP1C, sterol regulatory element-binding protein-1c; PPARA and PPARG, peroxisome proliferator-activated receptor-α and -γ.
Briefly, under conditions of chronic energy surplus, adipose tissue produces adipo-cytokines that prevent adipocytes from assimilating fatty acids and promote release of fatty acids from adipose depots. This results in increased delivery of fatty acids to the liver and fuels hepatocyte triglyceride synthesis14,15. The ability of triglyceride synthesis to compensate for increased hepatic fatty acid exposure appears to determine whether or not lipotoxicity results. For example, studies of mouse models of NASH showed that inhibiting liver triglyceride synthesis increased hepatic accumulation of free fatty acids and the severity of liver injury and fibrosis, despite reducing steatosis10. Other studies extended the evidence that fatty acids (rather than triglyceride) are hepatotoxic, demonstrating that lipotoxicity is affected by the specific types of fatty acid that accumulate. For example, Li et al showed that simply inhibiting stearoyl-CoA desaturase (an enzyme that converts saturated fatty acids into monounsaturated fatty acids) exacerbated liver injury in mouse models of NASH16. The realization that the lipotoxic potential of various types of lipids differs helps to explain why the outcomes of hepatic steatosis vary. Interventions that block accumulation of lipotoxic lipids might therefore be used to prevent or treat NASH.
Lipids can cause toxicity by diverse mechanisms. For example, lipotoxicity can result from lipid metabolism. Mitochondrial and peroxisomal fatty acid oxidation generate reactive oxygen species that may be immediately toxic or that eventually deplete antioxidant reserves, rendering hepatocytes more vulnerable to other factors that generate oxidative stress17,18. Accumulation of fatty acids within mitochondria could also dissipate the proton-motive force that typically occurs during mitochondrial respiration19,20. This makes mitochondria more vulnerable to other insults that collapse the mitochondrial membrane potential, such as tumor necrosis factor alpha (TNF) and could lead to release of mitochondrial factors that promote apoptosis21,22. Extreme depolarization of mitochondrial membranes causes complete cessation of mitochondrial electron transport and ATP synthesis, resulting in cellular necrosis23. Because damaged mitochondria cannot metabolize fatty acids efficiently, fatty acids accumulate24. In addition to its directly cytotoxic effects, fatty acid accumulation exacerbates insulin resistance and hyperinsulinemia25, which leads to further hepatic lipid accumulation26, and promotes inflammatory27 and fibrogenic responses28, as well mitogenic responses that could be carcinogenic26.
Another mechanism for lipotoxicity involves changes in cell signaling. For example, fatty acids interact with or modify other molecules, including transcription factors (hepatocyte nuclear factor-alpha)29 and innate immune receptors (toll-like receptors)30, leading to overall changes in signaling pathways that regulate metabolism and stress responses. Other types of lipids (oxysterols, diacylglycerol, cholesterol, and phospholipids) are also involved in signaling mechanisms that control cell metabolism. Aberrant accumulation of these molecules therefore disrupts hepatocyte metabolic homeostasis and compromises cell viability5. Lipotoxicity induces several different types of cellular stress, including ER stress31 and impaired autophagy32. In addition, it promotes a sterile inflammatory response that can potentiate liver cell injury and death. The role of inflammatory pathways in NASH pathogenesis is more extensively discussed by Gao and Tsukamoto.
Despite growing evidence that the risk for lipotoxicity is conveyed by lipids other than triglyceride, there are no simple methods to identify and quantify the non-triglyceride types of lipids that accumulate in fatty livers33. Clinicians must therefore assume that significant accumulation of lipid species has occurred after lipotoxicity becomes overt—once steatohepatitis is present. However, even if it were possible to quantify these other lipids, the outcomes of hepatic steatosis would remain difficult to predict, because the ability to adapt to or defend against various mechanisms for lipotoxicity differs among individuals and over the lifetime of each individual.
One example of the importance of disease-modifying factors is hepatic iron content34. Iron accumulation exacerbates hepatic oxidative stress and can therefore affect susceptibility to oxidant stress induced by fatty acid oxidation35. Hepatic iron content is sensitive to factors that differ among individuals (polymorphisms in genes such as HFE), and factors that might change during the lifetime of any individual, including sex-related factors (menstruation or pregnancy) and diet (consumption of fiber or red meat)36. The lipotoxic outcomes of identical fatty acid exposures can therefore differ based on other factors that modulate hepatic iron content.
The issue is further confounded by inter- and intra-individual differences in anti-oxidant defense37. Two individuals with identical fatty acid exposures and identical hepatic iron contents might ultimately have different levels of lipotoxicity based on differences in their capacities to defend against oxidative stress. Likewise, inter-individual differences in gut microbiota and intestinal permeability contribute to variations in liver lipotoxicity by modifying metabolic pathways that control lipid homeostasis and adipocytokine production. The intestinal microbiota and permeability can also determine hepatic exposure to toxic bacterial products such as lipopolysaccharide, ethanol, and choline metabolites. The role of the gut dysbiota in NAFLD pathogenesis is reviewed by Bajaj and Gillevet. Individuals with hepatic steatosis should therefore be carefully assessed to identify factors that might increase their susceptibility to lipotoxicity (see Table 1). These factors could identify individuals with simple steatosis (NAFL) who are at greater risk for developing lipid-related liver injury (NASH).
Table 1.
Factors That Promote Lipotoxicity in Patients With NAFLD
| Factor | Mechanism | |
|---|---|---|
| Preventable | Iron overload | Oxidant stress |
| Environmental toxins | Oxidant stress | |
| Medications | Oxidant stress | |
| Viral hepatitis | ER stress, oxidant stress, metabolic stress | |
| Non-preventable | Congenital Liver Disease | |
| Alpha 1 antitrypsin deficiency | ER stress | |
| Wilson’s disease | Oxidant stress | |
Pathogenesis
NASH occurs because lipotoxic hepatocytes release factors that initiate wound-healing responses to replace dying hepatocytes38. Wound healing is a complex multi-faceted process that can restore liver structure and function to a healthy state39. It encompasses a spectrum of responses whose intensities vary according to the extent and severity of liver cell death. Key wound-healing responses that are induced by hepatocyte lipotoxicity include activation of resident immune cells and recruitment of bone marrow-derived inflammatory cells (inflammation), matrix remodeling (fibrogenesis and fibrinolysis), angiogenesis, and mobilization of liver progenitor populations39 (Figure 2).
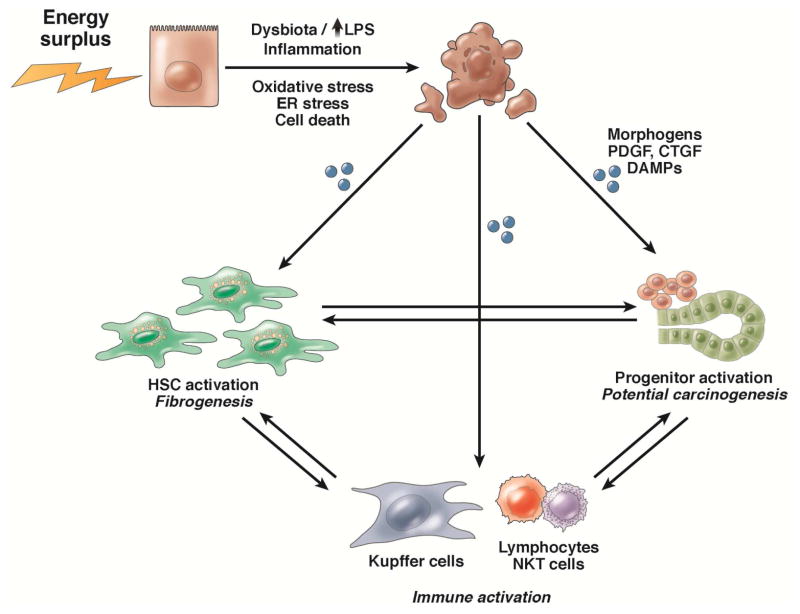
Figure 2. Lipotoxicity and the wound-healing response.
Lipid toxicity to hepatocytes causes cell stress and cell death. Dying hepatocytes release signals that induce a wound-healing response, such as damage associated molecular patterns, cytokines, and hedgehog. To promote regeneration or repair, the liver recruits and activates hepatic stellate cells to remove dying and dead cells and sustain the remaining epithelia, and progenitors to replace dead cells. If the wound-healing response cannot be restrained, it promotes inflammation, fibrogenesis, and hepatocarcinogenesis.
Each feature of wound-healing has a role in the regenerative process and is therefore necessary for effective repair of lipotoxic damage. However, aberrations in wound-healing responses can be dangerous. These can lead to defective or dysregulated repair of the injured hepatic parenchyma and promote development of liver cancer and/or progressive replacement of hepatic parenchyma by scar (cirrhosis).
The concept that NASH is no more (or less) than a wound-healing response to chronic hepatocyte lipotoxicity is unconventional and likely to provoke some controversy. We therefore provide 2 lines of evidence to support our hypothesis. A brief over-view of wound-healing biology reveals the similarities between the wound-healing process and development NASH. Hepatocyte injury or death (via lipotoxicity) is the key parameter that distinguishes NASH from NAFL4. In order for dead hepatocytes to be replaced, the wounded hepatic epithelia must be repaired. Wound-healing responses are mobilized as needed to reconstruct the hepatic parenchyma, and become more robust as hepatocyte lipotoxicity increases39. Inflammation is necessary to clear damage-related debris and stimulate local accumulation of other wound-healing cells, such as liver progenitors and myofibroblasts. However, excessive inflammation can compromise the viability of residual hepatocytes and promote over-growth of progenitors and myofibroblasts, laying the groundwork for carcinogenesis and progressive fibrosis.
Similarly, activation of liver sinusoidal endothelial cells (LSECs) is required for angiogenesis, in response to increased demand for nutrients and oxygen during liver repair. However, activated LSECs also interact with inflammatory cells and wounded hepatocytes to promote transdifferentiation of hepatic stellate cells into contractile, fibrogenic myofibroblasts. Excessive LSEC activation therefore contributes to the pathogenesis of portal hypertension and liver fibrosis. Myofibroblasts can remodel the liver matrix and are key sources of heaptotrophic factors and chemokines. Myofibroblast accumulation promotes liver repair by enriching the hepatic microenvironment with growth factors and cells that support hepatocyte regeneration. However, excessive myofibroblast accumulation perpetuates fibrogenesis and sustains inflammatory and proliferative responses that drive fibrosis progression and promote carcinogenesis38.
Finally, transient de-differentiation and proliferation of surviving hepatocytes and/or outgrowth of hepatocyte progenitors are necessary to generate new hepatocytes to replace the hepatocytes that that were killed by toxic lipids. Wound-healing responses are required to initiate hepatocyte regeneration, but must be curtailed eventually for the newly generated hepatocytes to resume the full functions of healthy hepatocytes. The liver is therefore variably repopulated with relatively immature or dysfunctional hepatocytes as long as wound-healing responses are active. This potentiates metabolic stress and increases the risk for liver cancer40. Senescent myofibroblasts that accumulate during matrix remodeling may exacerbate this risk for hepatic malignancy41. In any case, termination of the wound-healing process is the final step in successful repair because complete normalization of liver structure/function is not possible while wound-healing is in progress.
Histologic features of NASH indicate the ongoing repair responses to chronic hepatocyte lipotoxicity, and vary with the severity of lipotoxicity and success of the wound-healing process. The liver is usually able to undergo repair and regeneration after acute injury or when chronic injury causes minor increases in the rate of hepatocyte death. Therefore, there is no progressive replacement of hepatic parenchyma with scar, and the risk for liver cancer remains low in many patients with minimal hepatic lipotoxicity and mild NASH40. However, regeneration is generally less effective when chronic injury increases rates of hepatocyte death. The futile regenerative response perpetuates variable repair-related expansion of immature liver cells, inflammation, vascular remodeling, and fibrogenesis, resulting in more extreme (advanced or severe) NASH. Over time, functional hepatic parenchyma is progressively replaced by scar and the liver becomes enriched with neoplastic immature hepatocytes; this could account for the increased risk of cirrhosis and liver cancer in patients with severe NASH12. Like lipotoxicity, the efficiency or fidelity of the wound-healing response (such as during NASH) is influenced by genetic and non-genetic factors such as age, nutritional state, the intestinal microbiome, co-morbidities, and treatments—these could account for some of the heterogeneity in outcomes of NASH. Studies of NAFLD progression have found that the severity of liver fibrosis is the only feature of NASH that independently predicts liver-related morbidity and mortality11. Further research is needed to determine why progressive scarring develops in only some patients with NASH, define the mechanisms that shift effective regeneration to pathologic scarring42,43, and determine how wound-healing responses might be modulated to heal lipotoxicity without scar44.
The second line of evidence that supports the concept that NASH is a wound-healing response to chronic hepatocyte lipotoxicity comes from studies of hedgehog pathway in pre-clinical and clinical studies. Studies of the hedgehog pathway have provided important insights into NASH progression. Members of the hedgehog family of intercellular signaling molecules are produced by ballooned hepatocytes—a feature of NASH7. These hepatocytes, under various forms of stress (such as oxidative, apoptotic, and endoplasmic reticulum)45–47, release signals to neighboring cells47. Those signals include morphogens, cytokines, damaged-associated molecular patterns such as high-mobility group box-a (HMGB1)48 and microRNAs.
Hedgehog signaling
The ligand sonic hedgehog (SHH) is one of the distress factors released by ballooned hepatocytes49. It can be released directly into the extracellular matrix or incorporated into exosomes50. Exosomes are small membrane vesicles that signal stress to distant cells. Lipotoxic hepatocytes51, particularly those committed to apoptosis52, increase release of exosomes that signal other hepatocytes53, Kupffer cells52, hepatic stellate cells,54 and sinusoidal endothelial cells50. Through this process, dying hepatocytes induce an integrated regenerative response that assures their replacement53.
SHH regulates adult liver regeneration55 and is a highly-conserved morphogen that controls tissue construction during development. SHH regulates proliferation, differentiation, and viability of cells that express receptors for SHH (patched 1)56 (Figure 3). Interestingly, lipids define the activities of SHH are of its signal transduction pathway (patched 1 signals via smoothened, frizzled class receptor [SMO]). For example, fatty acids and cholesterol respectively interact with SHH to regulate its localization and extent of distribution within and among tissues57. The ability of lipid-modified SHH to interact with patched 1 is regulated by a family of lipid-sensitive adaptor proteins58. The activity of patched 1 is also affected by phospholipids59. Oxysterols in the cholesterol and bile acid synthetic pathways interact directly with SMO to affect its activity in SHH-target cells60.
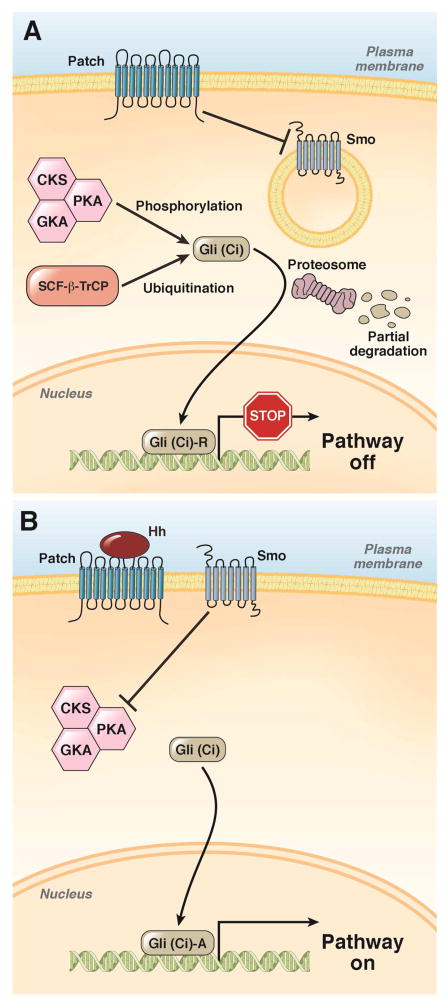
Figure 3. The hedgehog signaling pathway.
A. The SHH ligand patched 1 constitutively represses SMO, allowing the sequential phosphorylation of Gli by protein kinase A (PKA), glycogen synthase kinase 3 beta (GSK3B), and casein kinase (CK). Phosphorylation of the Gli family zinc finger proteins marks them for ubiquitination and limited proteosomal degradation. N-terminal fragments of Gli proteins translocate to the nucleus where they repress transcription.
B. Binding of SHH to patched 1 prevents its inhibition of SMO. This prevents phosphorylation and degradation of Gli proteins. Full-length Gli proteins translocate to the nucleus and promote transcription.
Activities of SHH it have been linked to outcomes of patients with NASH38. Hepatocytes in healthy adult livers do not express detectable levels of SHH. However, stressed hepatocytes quickly up-regulate expression of SHH mRNA and protein56. For example, hepatocyte expression of SHH is induced by hepatocyte-specific deletion of a gene that promotes nuclear accumulation of the transcription factor NF-kB, which regulates genes that control survival of these cells61. Similarly, exposure of hepatocytes to agents that induce ER stress up-regulates SHH expression47. Most importantly, lipotoxic injury, induced by hepatocyte accumulation of apoptotic fatty acids, stimulates SHH expression47. Conversely, hepatocytes from which casapase-2 has been deleted are protected from palmitate-induced apoptosis and do not induce SHH expression when challenged with this lipotoxic fatty acid46.
Stressed hepatocytes release SHH ligands into the extracellular space and SHH protein has been demonstrated in exosomes50. The ligand also associates with lipoproteins62. Association with membrane-bound particles and lipid particles permits SHH to initiate signaling in distant cells. Conversely, free (non-lipid particle-associated) SHH binds to matrix proteins, such as glypican 3, to increase its concentration, near producing cells in livers of patients with NASH63.
Hedgehog-responsive cells include hepatic stellate cells64, sinusoidal endothelial cells65, inflammatory cells66,67, and liver progenitors68. SHH signaling stimulates these cells to produce other factors that regulate wound-healing. For example, SHH signaling induces expression of transforming growth factor beta by hepatic stellate cells as well as connective tissue growth factor, amphiregulin, jagged, and Wnt ligands69,70. SHH stimulates sinusoidal endothelial cells to produce vascular endothelial growth factor65 and induces ductal cells to express osteopontin and chemokines that recruit various types of immune cells to the injured liver67,71. These immune cells, in turn, produce diverse cytokines, including interferon gamma, interleukins, TNF, and TNF-like cytokines. SHH also influences macrophage polarization, thereby modulating the local balance of inflammatory, anti-inflammatory, and fibrogenic cytokines67. Many of these SHH-inducible wound-healing factors are also able to regulate activity of the hedgehog signaling pathway (including the actions of SHH-regulated transcription factors) via mechanisms that do not require patched 1 and/or SMO. This relieves SHH from the sole responsibility of controlling liver repair and assures that the process can be perpetuated (or aborted) by an array of other mediators. Redundant regulation is an inherent feature of the wound-healing response, regardless of the particular factor that triggers this vital process.
Like development and neoplasia (other responses that involve the growth, differentiation, and involution of different cell types), wound-healing is regulated by a relatively small number of highly conserved signaling pathways. Hedgehog signaling also regulates animal development, carcinogenesis, and wound healing58. Increases in hedgehog signaling promote development of NASH-associated cirrhosis72 and primary liver cancers (hepatocellular cancer and intrahepatic cholangiocarcinoma)73,74. Conversely, reductions in hedgehog signaling compromise liver regeneration and compensation for hepatocyte loss 75. Hedgehog activity must therefore be tightly controlled to assure that the timing, intensity, and duration are appropriate to mediate liver repair.
Therapeutic Approaches
An interactive network of regulatory mechanisms modulates the activity of hedgehog and other pathways that mediate tissue construction. Loss of efficiency of these networks leads to defects in repair and chronic liver damage, such as development of NASH. Agents that regulate these mediators of liver repair might be developed to reduce lipotoxic tissue damage.
A recent analysis of paired liver biopsy samples from patients with NASH who responded to treatment in the Pioglitazone vs. Vitamine E NASH trial supports the concept that lipotoxic liver damage (such as NASH) can be reduced by targeting factors that regulate liver repair, such as hedgehog76. The study compared the efficacy of vitamin E with that of placebo in patients with NASH. Vitamin E was selected based on evidence that it reduced hepatocyte lipotoxicity in animal models. Lipotoxicity stimulates hepatocytes to produce SHH, and SHH-positive hepatocytes were detected in biopsy samples collected from patients before and after treatment. Analyses of biopsies demonstrated accumulation of SHH-responsive myofibroblasts, distributed in a “chicken wire” pattern around clusters of ballooned hepatocytes and portal-based inflammatory infiltrates that contained many SHH-responsive (i.e. with positive nuclear staining for Gli-2) inflammatory and progenitor cells. Effective treatment virtually eliminated hepatocyte ballooning, significantly reduced the numbers of SHH-expressing hepatocytes in biopsies. Loss of SHH-positive hepatocytes was accompanied by disappearance of SHH-responsive myofibroblasts, inflammatory cells, and progenitors, confirming that the SHH-regulated inflammatory response (NASH) was downregulated by factors that remove the stimulus for SHH production (lipotoxicity)76.
Based on these findings, risk for NASH is determined by hepatocyte susceptibility to toxic lipids and potential for repair of lipotoxic liver damage. Therapies for NASH might therefore include those that prevent hepatic lipotoxicity, by alleviating systemic metabolic stress. Examples include weight loss and insulin sensitizers. This approach has not yet been proven to be effective in patients with NASH. Most patients are unable to comply with long-term non-surgical strategies to lose or even maintain weight. Insulin-sensitizers have not consistently prevented or reduced NASH in mice or humans, despite reducing insulin resistance. Moreover, some insulin sensitizing agents promote weight gain, which is unacceptable for many patients.
Agents are also being developed to prevent lipotoxicity by manipulating lipid metabolism, restricting hepatic accumulation of lipotoxic fats. This approach could prevent development of NASH, but more research is needed to establish its efficacy and safety. These agents would be designed to target the liver and reduce the toxic effects of lipids toward hepatocytes or to optimize recovery from lipotoxicity. Examples include inhibitors of apoptosis and anti-fibrotic agents. Clinical trials are also underway to establish the safety and efficacy of these agents13.
Future Directions
Hepatic steatosis is a biomarker of metabolic stress and therefore a risk factor for lipotoxicity. The outcomes of hepatic steatosis are heterogeneous because the severity of metabolic stress is not determined by levels of triglycerides—the lipid commonly used to diagnose patients with hepatic steatosis. Other lipids can accumulate in hepatocytes in conjunction with triglyceride and could be toxic. Lipotoxicity is determined by the type and amount of lipid that accumulates, as well as the ability of hepatocytes to defend against or adapt to accumulation of that lipid. Toxic lipids induce stressed hepatocytes to release distress factors to neighboring cells and induce a wound-healing response to replace dead hepatocytes. This repair process is complex and involves responses such as recruitment of inflammatory cells and expansion of progenitor and myofibroblast populations; these are deleterious when deregulated. NASH is the histologic manifestation of the wound-healing response to hepatocyte lipotoxicity. Deregulated wound-healing responses worsen clinical outcomes by promoting development of cirrhosis and liver cancer. NASH might be prevented or treated by preventing lipotoxicity and/or by optimizing repair responses induced by lipid toxicity to hepatocytes.
Acknowledgments
Financial support: This research is supported by NIH DK0077794, DK053792 and R37 AA010154 (Diehl AM), and Duke Endowment: The Florence McAlister Professorship (Diehl AM). MVM is a receiver of a PhD grant from Fundação para a Ciência e Tecnologia, FCT, Portugal.
Abbreviations
- CK
casein kinase
- DAMP
damage associated molecular patterns
- GSK3
glycogen synthase kinase-3
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NF-κB
nuclear factor kappa B
- PKA
protein kinase A
- SHH
sonic hedgehog
- TNF
tumor necrosis factor
Footnotes
Conflict of interest: There are no conflicts of interest to state.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matteoni CA, Younossi ZM, Gramlich T, et al. NAFLD: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 2.Rafiq N, Bai C, Fang Y, et al. Long-term follow-up of patients with NAFL. Clin Gastroenterol Hepatol. 2009;7:234–8. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of NAFLD and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–49. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 4.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human NASH. Gastroenterology. 2003;125:437–43. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 5.Cusi K. Role of obesity and lipotoxicity in the development of NASH: pathophysiology and clinical implications. Gastroenterology. 2012;142:711–725. e6. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in NAFLD: not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445–51. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for NAFLD. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 8.Machado MV, Cortez-Pinto H. NAFLD: what the clinician needs to know. World J Gastroenterol. 2014;20:12956–80. doi: 10.3748/wjg.v20.i36.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of NAFLD. Hepatology. 2007;46:1081–90. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with NASH. Hepatology. 2007;45:1366–74. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 11.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, associates with long-term outcomes of patients with NAFLD. Gastroenterology. 2015;149:389–97. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richardson MM, Jonsson JR, Powell EE, et al. Progressive fibrosis in NASH: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Filozof C, Goldstein BJ, Williams RN, et al. NASH: Limited Available Treatment Options but Promising Drugs in Development and Recent Progress Towards a Regulatory Approval Pathway. Drugs. 2015;75:1373–92. doi: 10.1007/s40265-015-0437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with NAFLD. J Clin Invest. 2005;115:1343–51. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bays H. Adiposopathy, “sick fat,” Ockham’s razor, and resolution of the obesity paradox. Curr Atheroscler Rep. 2014;16:409. doi: 10.1007/s11883-014-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li ZZ, Berk M, McIntyre TM, et al. Hepatic lipid partitioning and liver damage in NAFLD: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–44. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begriche K, Massart J, Robin MA, et al. Mitochondrial adaptations and dysfunctions in NAFLD. Hepatology. 2013;58:1497–507. doi: 10.1002/hep.26226. [DOI] [PubMed] [Google Scholar]
- 18.Brady LJ, Brady PS, Romsos DR, et al. Elevated hepatic mitochondrial and peroxisomal oxidative capacities in fed and starved adult obese (ob/ob) mice. Biochem J. 1985;231:439–44. doi: 10.1042/bj2310439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavin KD, Yang S, Lin HZ, et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem. 1999;274:5692–700. doi: 10.1074/jbc.274.9.5692. [DOI] [PubMed] [Google Scholar]
- 20.Serviddio G, Bellanti F, Tamborra R, et al. Alterations of hepatic ATP homeostasis and respiratory chain during development of NASH in a rodent model. Eur J Clin Invest. 2008;38:245–52. doi: 10.1111/j.1365-2362.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 21.Cortez-Pinto H, Chatham J, Chacko VP, et al. Alterations in liver ATP homeostasis in human NASH: a pilot study. JAMA. 1999;282:1659–64. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- 22.Serviddio G, Bellanti F, Tamborra R, et al. Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of NASH liver to ischaemia-reperfusion injury. Gut. 2008;57:957–65. doi: 10.1136/gut.2007.147496. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Jiang G, Zhang P, et al. Programmed cell death and its role in inflammation. Mil Med Res. 2015;2:12. doi: 10.1186/s40779-015-0039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koliaki C, Szendroedi J, Kaul K, et al. Adaptation of hepatic mitochondrial function in humans with NAFL is lost in steatohepatitis. Cell Metab. 2015;21:739–46. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Gruben N, Shiri-Sverdlov R, Koonen DP, et al. NAFLD: A main driver of insulin resistance or a dangerous liaison? Biochim Biophys Acta. 2014;1842:2329–2343. doi: 10.1016/j.bbadis.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol. 2014;220:T1–T23. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miao H, Zhang Y, Lu Z, et al. FOXO1 involvement in insulin resistance-related pro-inflammatory cytokine production in hepatocytes. Inflamm Res. 2012;61:349–58. doi: 10.1007/s00011-011-0417-3. [DOI] [PubMed] [Google Scholar]
- 28.Svegliati-Baroni G, Ridolfi F, Di Sario A, et al. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743–51. doi: 10.1002/hep.510290632. [DOI] [PubMed] [Google Scholar]
- 29.Wisely GB, Miller AB, Davis RG, et al. Hepatocyte nuclear factor 4 is a transcription factor that constitutively binds fatty acids. Structure. 2002;10:1225–34. doi: 10.1016/s0969-2126(02)00829-8. [DOI] [PubMed] [Google Scholar]
- 30.Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in NAFLD. World J Gastroenterol. 2014;20:7381–91. doi: 10.3748/wjg.v20.i23.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashraf NU, Sheikh TA. Endoplasmic reticulum stress and Oxidative stress in the pathogenesis of NAFLD. Free Radic Res. 2015;49:1405–18. doi: 10.3109/10715762.2015.1078461. [DOI] [PubMed] [Google Scholar]
- 32.Amir M, Czaja MJ. Autophagy in NASH. Expert Rev Gastroenterol Hepatol. 2011;5:159–66. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazhar SM, Shiehmorteza M, Sirlin CB. Noninvasive assessment of hepatic steatosis. Clin Gastroenterol Hepatol. 2009;7:135–40. doi: 10.1016/j.cgh.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aigner E, Weiss G, Datz C. Dysregulation of iron and copper homeostasis in NAFL. World J Hepatol. 2015;7:177–88. doi: 10.4254/wjh.v7.i2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita N, Miyachi H, Tanaka H, et al. Iron overload is associated with hepatic oxidative damage to DNA in NASH. Cancer Epidemiol Biomarkers Prev. 2009;18:424–32. doi: 10.1158/1055-9965.EPI-08-0725. [DOI] [PubMed] [Google Scholar]
- 36.Siah CW, Ombiga J, Adams LA, et al. Normal iron metabolism and the pathophysiology of iron overload disorders. Clin Biochem Rev. 2006;27:5–16. [PMC free article] [PubMed] [Google Scholar]
- 37.Namikawa C, Shu-Ping Z, Vyselaar JR, et al. Polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in NASH. J Hepatol. 2004;40:781–6. doi: 10.1016/j.jhep.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 38.Angulo P, Machado MV, Diehl AM. Fibrosis in NAFLD: mechanisms and clinical implications. Semin Liver Dis. 2015;35:132–45. doi: 10.1055/s-0035-1550065. [DOI] [PubMed] [Google Scholar]
- 39.Machado MV, Diehl AM. Liver renewal: detecting misrepair and optimizing regeneration. Mayo Clin Proc. 2014;89:120–30. doi: 10.1016/j.mayocp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–65. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 41.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–85. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mogler C, Wieland M, Konig C, et al. Hepatic stellate cell-expressed endosialin balances fibrogenesis and hepatocyte proliferation during liver damage. EMBO Mol Med. 2015;7:332–8. doi: 10.15252/emmm.201404246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding BS, Cao Z, Lis R, et al. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pritchard MT, McCracken JM. Identifying Novel Targets for Treatment of Liver Fibrosis: What Can We Learn from Injured Tissues which Heal Without a Scar? Curr Drug Targets. 2015;16:1332–46. doi: 10.2174/1389450116666150825111439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujii H, Ikura Y, Arimoto J, et al. Expression of perilipin and adipophilin in NAFLD; relevance to oxidative injury and hepatocyte ballooning. J Atheroscler Thromb. 2009;16:893–901. doi: 10.5551/jat.2055. [DOI] [PubMed] [Google Scholar]
- 46.Machado MV, Michelotti GA, Pereira TD, et al. Reduced lipoapoptosis, hedgehog pathway activation and fibrosis in caspase-2 deficient mice with NASH. Gut. 2015;64:1148–57. doi: 10.1136/gutjnl-2014-307362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rangwala F, Guy CD, Lu J, et al. Increased production of sonic hedgehog by ballooned hepatocytes. J Pathol. 2011;224:401–10. doi: 10.1002/path.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Chen L, Hu L, et al. Nuclear factor high-mobility group box1 mediating the activation of Toll-like receptor 4 signaling in hepatocytes in the early stage of NAFLD in mice. Hepatology. 2011;54:1620–30. doi: 10.1002/hep.24552. [DOI] [PubMed] [Google Scholar]
- 49.Guy CD, Suzuki A, Zdanowicz M, et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human NAFLD. Hepatology. 2012;55:1711–21. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witek RP, Yang L, Liu R, et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320–330. e2. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Povero D, Eguchi A, Li H, et al. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One. 2014;9:e113651. doi: 10.1371/journal.pone.0113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verma VK, Li H, Wang R, et al. Alcohol stimulates macrophage activation through caspase dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nojima H, Freeman CM, Schuster RM, et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang R, Ding Q, Yaqoob U, et al. Exosome adherence and internalization by hepatic stellate cells triggers sphingosine 1-phosphate dependent migration. J Biol Chem. 2015 doi: 10.1074/jbc.M115.671735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swiderska-Syn M, Syn WK, Xie G, et al. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut. 2014;63:1333–44. doi: 10.1136/gutjnl-2013-305962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi SS, Omenetti A, Syn WK, et al. The role of Hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol. 2011;43:238–44. doi: 10.1016/j.biocel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farzan SF, Singh S, Schilling NS, et al. The adventures of Shh in development and repair. III. Hedgehog processing and biological activity. Am J Physiol Gastrointest Liver Physiol. 2008;294:G844–9. doi: 10.1152/ajpgi.00564.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–29. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 59.Yavari A, Nagaraj R, Owusu-Ansah E, et al. Role of lipid metabolism in smoothened derepression in hedgehog signaling. Dev Cell. 2010;19:54–65. doi: 10.1016/j.devcel.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nachtergaele S, Mydock LK, Krishnan K, et al. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol. 2012;8:211–20. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jung Y, Witek RP, Syn WK, et al. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–65. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Palm W, Swierczynska MM, Kumari V, et al. Secretion and signaling activities of lipoprotein-associated hedgehog and non-sterol-modified hedgehog in flies and mammals. PLoS Biol. 2013;11:e1001505. doi: 10.1371/journal.pbio.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Filmus J, Capurro M. The role of glypicans in Hedgehog signaling. Matrix Biol. 2014;35:248–52. doi: 10.1016/j.matbio.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Michelotti GA, Xie G, Swiderska M, et al. Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013;123:2380–94. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie G, Choi SS, Syn WK, et al. Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation. Gut. 2013;62:299–309. doi: 10.1136/gutjnl-2011-301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Syn WK, Agboola KM, Swiderska M, et al. NKT-associated hedgehog and osteopontin drive fibrogenesis in NAFLD. Gut. 2012;61:1323–9. doi: 10.1136/gutjnl-2011-301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pereira TA, Xie G, Choi SS, et al. Macrophage-derived Hedgehog ligands promotes fibrogenic and angiogenic responses in human schistosomiasis mansoni. Liver Int. 2013;33:149–61. doi: 10.1111/liv.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sicklick JK, Li YX, Melhem A, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290:G859–70. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 69.Choi SS, Omenetti A, Witek RP, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1093–106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xie G, Karaca G, Swiderska-Syn M, et al. Cross-talk between Notch and Hedgehog regulates hepatic stellate cell fate in mice. Hepatology. 2013;58:1801–13. doi: 10.1002/hep.26511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Omenetti A, Syn WK, Jung Y, et al. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–27. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moylan CA, Pang H, Dellinger A, et al. Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe NAFLD. Hepatology. 2014;59:471–82. doi: 10.1002/hep.26661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zheng X, Zeng W, Gai X, et al. Role of the Hedgehog pathway in hepatocellular carcinoma. Oncol Rep. 2013;30:2020–6. doi: 10.3892/or.2013.2690. [DOI] [PubMed] [Google Scholar]
- 74.Tang L, Tan YX, Jiang BG, et al. The prognostic significance and therapeutic potential of hedgehog signaling in intrahepatic cholangiocellular carcinoma. Clin Cancer Res. 2013;19:2014–24. doi: 10.1158/1078-0432.CCR-12-0349. [DOI] [PubMed] [Google Scholar]
- 75.Machado MV, Michelotti GA, de Pereira TA, et al. Accumulation of duct cells with activated YAP parallels fibrosis progression in NAFLD. J Hepatol. 2015;63:962–70. doi: 10.1016/j.jhep.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guy CD, Suzuki A, Abdelmalek MF, et al. Treatment response in the PIVENS trial is associated with decreased Hedgehog pathway activity. Hepatology. 2015;61:98–107. doi: 10.1002/hep.27235. [DOI] [PMC free article] [PubMed] [Google Scholar]